Increasing Stress to Induce Apoptosis in Pancreatic Cancer via the Unfolded Protein Response (UPR)
Abstract
1. Introduction
2. Mechanisms of Cellular Homeostasis
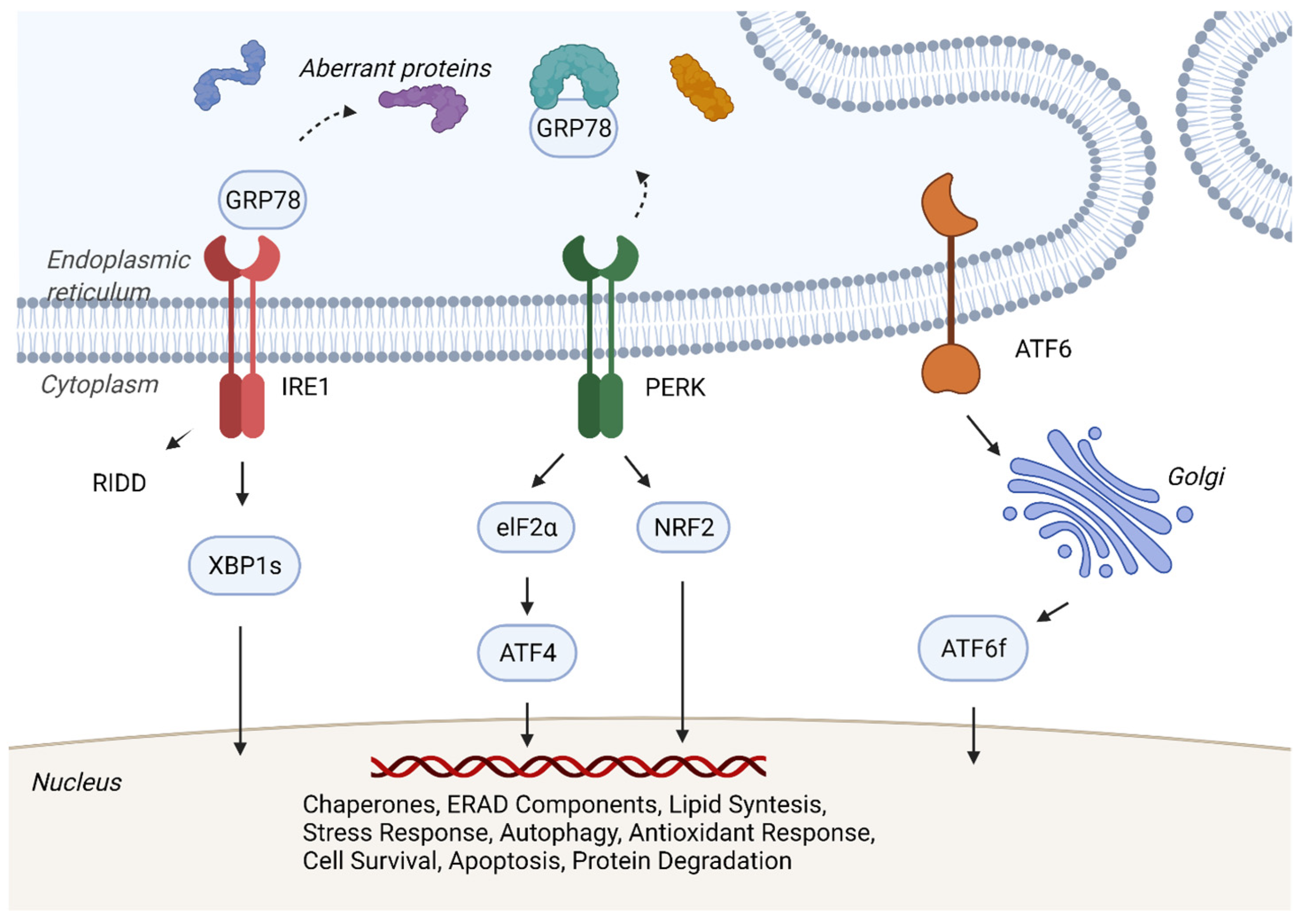
3. Mechanisms of Unfolded Protein Response and Cancer
4. Evidence for UPR Being Important in Pancreatic Cancer
5. Molecular Induction of ER Stress and Apoptosis
6. Gambogenic Acid (GNA)
7. Tigatuzumab
8. Minnelide
9. BOLD-100/KP1339
10. Radiation Therapy to Activate UPR
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Cancer Society. Facts & Figures 2022. American Cancer Society. Atlanta, Ga. 2022. Available online: https://www.cancer.org/cancer/pancreatic-cancer/about/key-statistics.html (accessed on 10 October 2022).
- Sarantis, P.; Koustas, E.; Papadimitropoulou, A.; Papavassiliou, A.G.; Karamouzis, M.V. Pancreatic ductal adenocarcinoma: Treatment hurdles, tumor microenvironment and immunotherapy. World J. Gastrointest. Oncol. 2020, 12, 173–181. [Google Scholar] [CrossRef]
- Uson, P.L.S., Jr.; Samadder, N.J.; Riegert-Johnson, D.; Boardman, L.; Borad, M.J.; Ahn, D.; Sonbol, M.B.; Faigel, D.O.; Fukami, N.; Pannala, R.; et al. Clinical Impact of Pathogenic Germline Variants in Pancreatic Cancer: Results from a Multicenter, Prospective, Universal Genetic Testing Study. Clin. Transl. Gastroenterol. 2021, 12, e00414. [Google Scholar] [CrossRef]
- Golan, T.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.-O.; Hochhauser, D.; Arnold, D.; Oh, D.-Y.; et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019, 381, 317–327. [Google Scholar] [CrossRef]
- O’Reilly, E.M.; Lee, J.W.; Zalupski, M.; Capanu, M.; Park, J.; Golan, T.; Tahover, E.; Lowery, M.A.; Chou, J.F.; Sahai, V.; et al. Randomized, Multicenter, Phase II Trial of Gemcitabine and Cisplatin with or Without Veliparib in Patients With Pancreas Adenocarcinoma and a Germline BRCA/PALB2 Mutation. J. Clin. Oncol. 2020, 38, 1378–1388. [Google Scholar] [CrossRef]
- Leidner, R.; Silva, N.S.; Huang, H.; Sprott, D.; Zheng, C.; Shih, Y.-P.; Leung, A.; Payne, R.; Sutcliffe, K.; Cramer, J.; et al. Neoantigen T-Cell Receptor Gene Therapy in Pancreatic Cancer. N. Engl. J. Med. 2022, 386, 2112–2119. [Google Scholar] [CrossRef]
- Robinson, C.M.; Talty, A.; Logue, S.E.; Mnich, K.; Gorman, A.M.; Samali, A. An Emerging Role for the Unfolded Protein Response in Pancreatic Cancer. Cancers 2021, 13, 261. [Google Scholar] [CrossRef]
- Arnold, F.; Gout, J.; Wiese, H.; Weissinger, S.E.; Roger, E.; Perkhofer, L.; Walter, K.; Scheible, J.; Bozzo, C.P.; Lechel, A.; et al. RINT1 Regulates SUMOylation and the DNA Damage Response to Preserve Cellular Homeostasis in Pancreatic Cancer. Cancer Res. 2021, 81, 1758–1774. [Google Scholar] [CrossRef]
- Dong, L.; Khoonkari, M.; Avril, T.; Chevet, E.; Kruyt, F.A.E. The unfolded protein response as regulator of cancer stemness and differentiation: Mechanisms and implications for cancer therapy. Biochem. Pharmacol. 2021, 192, 114737. [Google Scholar]
- Shah, V.M.; Sheppard, B.C.; Sears, R.C.; Alani, A.W.G. Hypoxia: Friend or Foe for drug delivery in Pancreatic Cancer. Cancer Lett. 2020, 492, 63–70. [Google Scholar] [CrossRef]
- Keener, J.; Sneyd, J. Cellular Homeostasis. In Mathematical Physiology. Interdisciplinary Applied Mathematics; Keener, J., Sneyd, J., Eds.; Springer: New York, NY, USA, 2009; Volume 8/1. [Google Scholar] [CrossRef]
- Liput, M.; Magliaro, C.; Kuczynska, Z.; Zayat, V.; Ahluwalia, A.; Buzanska, L. Tools and approaches for analyzing the role of mitochondria in health, development and disease using human cerebral organoids. Dev. Neurobiol. 2021, 81, 591–607. [Google Scholar] [CrossRef]
- Osellame, L.D.; Blacker, T.S.; Duchen, M.R. Cellular and molecular mechanisms of mitochondrial function. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 711–723. [Google Scholar] [CrossRef]
- Konstantinos, P.; Lionaki, E.; Tavernarakis, N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol. 2018, 20, 1013–1022. [Google Scholar]
- Hoseki, J.; Ushioda, R.; Nagata, K. Mechanism and components of endoplasmic reticulum-associated degradation. J. Biochem. 2010, 147, 19–25. [Google Scholar] [CrossRef]
- Ellgaard, L.; Helenius, A. Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 2003, 4, 181191. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, J.D.; Kaufman, R.J. The endoplasmic reticulum and the unfolded protein response. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2007; Volume 18. [Google Scholar]
- Tian, X.; Zhang, S.; Zhou, L.; Seyhan, A.A.; Borrero, L.H.; Zhang, Y.; El-Deiry, W.S. Targeting the integrated stress response in cancer therapy. Front. Pharmacol. 2021, 12, 747837. [Google Scholar] [CrossRef]
- Bertolotti, A.; Zhang, Y.; Hendershot, L.M.; Harding, H.P.; Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000, 2, 326–332. [Google Scholar] [CrossRef]
- Shen, J.; Chen, X.; Hendershot, L.; Prywes, R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell 2002, 3, 99–111. [Google Scholar] [CrossRef]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef]
- Han, J.; Kaufman, R.J. Physiological/pathological ramifications of transcription factors in the unfolded protein response. Genes 2017, 31, 1417–1438. [Google Scholar] [CrossRef]
- Pattabiraman, D.R.; Weinberg, R.A. Tackling the cancer stem cells—What challenges do they pose? Nat. Rev. Drug Discov. 2014, 13, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Caras, I.W. Two cancer stem cell-targeted therapies in clinical trials as viewed from the standpoint of the cancer stem cell model. Stem Cells Transl. Med. 2020, 9, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Imaizumi, K. Unfolded Protein Response-Dependent Communication and Contact among Endoplasmic Reticulum, Mitochondria, and Plasma Membrane. Int. J. Mol. Sci. 2018, 19, 3215. [Google Scholar] [CrossRef] [PubMed]
- Frakes, A.E.; Dillin, A. The UPR(ER): Sensor and Coordinator of Organismal Homeostasis. Mol. Cell 2017, 66, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Kaufman, R.J. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 2016, 529, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hendershot, L.M. The role of the unfolded protein response in tumour development: Friend or foe? Nat. Rev. Cancer 2004, 4, 966–977. [Google Scholar] [CrossRef]
- Chevet, E.; Hetz, C.; Samali, A. Endoplasmic reticulum stress-activated cell reprogramming in oncogenesis. Cancer Discov. 2015, 5, 586–597. [Google Scholar] [CrossRef]
- Siwecka, N.; Rozpędek, W.; Pytel, D.; Wawrzynkiewicz, A.; Dziki, A.; Diehl, J.A.; Majsterek, I. Dual role of endoplasmic reticulum stress-mediated unfolded protein response signaling pathway in carcinogenesis. Int. J. Mol. Sci. 2019, 20, 4354. [Google Scholar] [CrossRef] [PubMed]
- Urra, H.; Dufey, E.; Avril, T.; Chevet, E.; Hetz, C. Endoplasmic reticulum stress and the hallmarks of cancer. Trends Cancer 2016, 2, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Holtrup, F.; Bauer, A.; Fellenberg, K.; Hilger, R.A.; Wink, M.; Hoheisel, J.D. Microarray analysis of nemorosone-induced cytotoxic effects on pancreatic cancer cells reveals activation of the unfolded protein response (UPR). Br. J. Pharmacol. 2011, 162, 1045–1059. [Google Scholar] [CrossRef]
- Lowenfels, A.B.; Maisonneuve, P.; Cavallini, G.; Ammann, R.W.; Lankisch, P.G.; Andersen, J.R.; DiMagno, E.P.; Andren-Sandberg, A.; Domellof, L. Pancreatitis and the Risk of Pancreatic Cancer. N. Engl. J. Med. 1993, 328, 1433–1437. [Google Scholar] [CrossRef]
- Kirkegård, J.; Mortensen, F.V.; Cronin-Fenton, D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2017, 112, 1366–1372. [Google Scholar] [CrossRef]
- Raimondi, S.; Lowenfels, A.B.; Morselli-Labate, A.M.; Maisonneuve, P.; Pezzilli, R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 349–358. [Google Scholar] [CrossRef]
- Tong, G.X.; Geng, Q.Q.; Chai, J.; Cheng, J.; Chen, P.L.; Liang, H.; Shen, X.R.; Wang, D.B. Association between pancreatitis and subsequent risk of pancreatic cancer: A systematic review of epidemiological studies. Asian Pac. J. Cancer Prev. 2014, 15, 5029–5034. [Google Scholar] [CrossRef]
- Umans, D.S.; Hoogenboom, S.A.; Sissingh, N.J.; Lekkerkerker, S.J.; Verdonk, R.C.; van Hooft, J.E. Pancreatitis and pancreatic cancer: A case of the chicken or the egg. World J. Gastroenterol. 2021, 27, 3148–3157. [Google Scholar] [CrossRef]
- Wang, L.; Xie, D.; Wei, D. Pancreatic Acinar-to-Ductal Metaplasia and Pancreatic Cancer. Methods Mol. Biol. 2019, 1882, 299–308. [Google Scholar] [CrossRef]
- Hingorani, S.R.; Petricoin, E.F.; Maitra, A.; Rajapakse, V.; King, C.; Jacobetz, M.A.; Ross, S.; Conrads, T.P.; Veenstra, T.D.; Hitt, B.A.; et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003, 4, 437–450. [Google Scholar] [CrossRef]
- Carrière, C.; Young, A.L.; Gunn, J.R.; Longnecker, D.S.; Korc, M. Acute pancreatitis markedly accelerates pancreatic cancer progression in mice expressing oncogenic Kras. Biochem. Biophys. Res. Commun. 2009, 382, 561–565. [Google Scholar] [CrossRef]
- Kong, B.; Cheg, T.; Wu, W.; Regel, I.; Raulefs, R.; Friess, H.; Erkan, M.; Esposito, I.; Kleeff, J.; Michalski, C.W. Hypoxia-induced endoplasmic reticulum stress characterizes a necrotic phenotype of pancreatic cancer. Oncotarget 2015, 6, 32154. [Google Scholar] [CrossRef]
- Lu, G.; Luo, H.; Zhu, X. Targeting the GRP78 pathway for cancer therapy. Front. Med. 2020, 7, 351. [Google Scholar] [CrossRef]
- Niu, Z.; Wang, M.; Zhou, L.; Yao, L.; Liao, Q.; Zhao, Y. Elevated GRP78 expression is associated with poor prognosis in patients with pancreatic cancer. Sci. Rep. 2015, 5, 16067. [Google Scholar] [CrossRef]
- Dauer, P.; Sharma, N.S.; Gupta, V.K.; Durden, B.; Hadad, R.; Banerjee, S.; Dudeja, V.; Saluja, A.; Banerjee, S. ER stress sensor, glucose regulatory protein 78 (GRP78) regulates redox status in pancreatic cancer thereby maintaining “stemness”. Cell Death Dis. 2019, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Ha, D.P.; Zhu, G.; Rangel, D.F.; Kobielak, A.; Gill, P.S.; Groshen, S.; Dubeau, L.; Lee, A.S. GRP78 haploinsufficiency suppresses acinar-to-ductal metaplasia, signaling, and mutant Kras-driven pancreatic tumorigenesis in mice. Proc. Natl. Acad. Sci. USA 2017, 114, E4020–E4029. [Google Scholar] [CrossRef] [PubMed]
- Gifford, J.B.; Huang, W.; Zeleniak, A.E.; Hindoyan, A.; Wu, H.; Donahue, T.R.; Hill, R. Expression of GRP78, Master Regulator of the Unfolded Protein Response, Increases Chemoresistance in Pan-creatic Ductal Adenocarcinoma. Mol. Cancer Ther. 2016, 15, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Chien, W.; Ding, L.-W.; Sun, Q.-Y.; Torres-Fernandez, L.A.; Tan, S.Z.; Xiao, J.; Lim, S.L.; Garg, M.; Lee, K.L.; Kitajima, S.; et al. Selective inhibition of unfolded protein response induces apoptosis in pancreatic cancer cells. Oncotarget 2014, 5, 4881. [Google Scholar] [CrossRef] [PubMed]
- Thakur, P.C.; Miller-Ocuin, J.L.; Kguyen, K.; Matsuda, R.; Singhi Ad Zeh, H.J.; Bahary, N. Inhibition of endoplasmic-reticulum-stress-mediated autophagy enhances the effectiveness of chemotherapeutics on pancreatic cancer. J. Transl. Med. 2018, 16, 190. [Google Scholar] [CrossRef] [PubMed]
- Atkins, C.; Liu, Q.; Minthorn, E.; Zhang, S.-Y.; Figueroa, D.J.; Moss, K.; Stanley, T.B.; Sanders, B.; Goetz, A.; Gaul, N.; et al. Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2013, 73, 1993–2002. [Google Scholar] [CrossRef] [PubMed]
- Terrab, L.; Wipf, P. Hsp70 and the unfolded protein response as a challenging drug target and an inspiration for probe molecule development. ACS Med. Chem. Lett. 2020, 11, 232–236. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhong, J.; Bi, Y.; Liu, Y.; Liu, Y.; Guo, J.; Pan, L.; Tan, Y.; Yu, X. Gambogenic acid induces Noxa-mediated apoptosis in colorectal cancer through ROS-dependent activation of IRE1α/JNK. Phytomedicine 2020, 78, 153306. [Google Scholar] [CrossRef]
- Liu, C.; Xu, J.; Guo, C.; Chen, X.; Qian, C.; Zhang, X.; Zhou, P.; Yang, Y. Gambogenic Acid Induces Endoplasmic Reticulum Stress in Colorectal Cancer via the Aurora A Pathway. Front. Cell Dev. Biol. 2021, 9, 736350. [Google Scholar] [CrossRef]
- Su, J.; Xu, T.; Jiang, G.; Hou, M.; Liang, M.; Cheng, H.; Li, Q. Gambogenic acid triggers apoptosis in human nasopharyngeal carcinoma CNE-2Z cells by activating volume-sensitive outwardly rectifying chloride channel. Fitoterapia 2019, 133, 150–158. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Kang, Y.-K.; He, A.R.; Lim, H.Y.; Ryoo, B.-Y.; Hung, C.-H.; Sheen, I.-S.; Izumi, N.; Austin, T.; Wang, Q.; et al. Safety and efficacy of tigatuzumab plus sorafenib as first-line therapy in subjects with advanced hepatocellular carcinoma: A phase 2 randomized study. J. Hepatol. 2015, 63, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Forero-Torres, A.; Shah, J.; Wood, T.; Posey, J.; Carlisle, R.; Copigneaux, C.; Luo, F.; Wojtowicz-Praga, S.; Percent, I.; Saleh, M. Phase I trial of weekly tigatuzumab, an agonistic humanized monoclonal antibody targeting death receptor 5 (DR5). Cancer Biother. Radiopharm. 2010, 25, 13–19. [Google Scholar] [CrossRef] [PubMed]
- DeRosier, L.C.; Vickers, S.M.; Zinn, K.R.; Huang, Z.; Wang, W.; Grizzle, W.E.; Sellers, J.; Stockard, C.R.; Zhou, T.; Oliver, P.G.; et al. TRA-8 anti-DR5 monoclonal antibody and gemcitabine induce apoptosis and inhibit radiologically validated orthotopic pancreatic tumor growth. Mol. Cancer Ther. 2007, 6, 3198–3207. [Google Scholar] [CrossRef]
- Forero-Torres, A.; Infante, J.R.; Waterhouse, D.; Wong, L.; Vickers, S.; Arrowsmith, E.; He, A.R.; Hart, L.; Trent, D.; Wade, J.; et al. Phase 2, multicenter, open-label study of tigatuzumab (CS-1008), a humanized monoclonal antibody targeting death receptor 5, in combination with gemcitabine in chemotherapy-naive patients with unresectable or metastatic pancreatic cancer. Cancer Med. 2013, 2, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Forero-Torres, A.; Varley, K.E.; Abramson, V.G.; Li, Y.; Vaklavas, C.; Lin, N.U.; Liu, M.C.; Rugo, H.S.; Nanda, R.; Storniolo, A.M.; et al. Translational Breast Cancer Research Consortium (TBCRC). TBCRC 019: A Phase II Trial of Nanoparticle Albumin-Bound Paclitaxel with or without the Anti-Death Receptor 5 Monoclonal Antibody Tigatuzumab in Patients with Triple-Negative Breast Cancer. Clin. Cancer Res. 2015, 21, 2722–2729. [Google Scholar] [CrossRef]
- Mujumdar, N.; Banerjee, S.; Chen, Z.; Sangwan, V.; Chugh, R.; Dudeja, V.; Yamamoto, M.; Vickers, S.M.; Saluja, A.K. Triptolide activates unfolded protein response leading to chronic ER stress in pancreatic cancer cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G1011–G1020. [Google Scholar] [CrossRef] [PubMed]
- Greeno, E.; Borazanci, E.; Gockerman, J.; Korn, R.; Saluja, A.; Von Hoff, D. Phase I dose escalation and pharmokinetic study of 14-O phosphonooxymethyltriptolide. In Proceedings of the 106th Annual Meeting of the American Association for Cancer Research, 18–22 April 2015; Philadelphia, P.A., Ed.; AACR. Cancer Res 2015, 75 (Suppl. S15), nr CT207. [Google Scholar] [CrossRef]
- Neuditschko, B.; Legin, A.A.; Baier, D.; Schintlmeinster, A.; Reipert, S.; Wagner, M.; Keppler, K.K.; Berger, W.; Meier-Menches, S.M.; Gerner, C. Interaction with Ribosomal Proteins Accompanies Stress Induction of the Anticancer Metallodrug BOLD-100/KP1339 in the Endoplasmic Reticulum. Angew. Chem. Int. Ed. Engl. 2021, 60, 5063–5068. [Google Scholar] [CrossRef]
- Flocke, L.S.; Trondl, R.; Jakupec, M.A.; Keppler, B.K. Molecular mode of action of NKP-1339—A clinically investigated ruthenium-based drug—Involves ER- and ROS-related effects in colon carcinoma cell lines. Investig. New Drugs. 2016, 34, 261–268. [Google Scholar] [CrossRef]
- Burris, H.A.; Bakewell, S.; Bendell, J.C.; Infante, J.; Jones, S.F.; Spigel, D.R.; Weiss, G.J.; Ramanathan, R.K.; Ogden, A.; Von Hoff, D. Safety and activity of IT-139, a ruthenium-based compound, in patients with advanced solid tumours: A first-in-human, open-label, dose-escalation phase I study with expansion cohort. ESMO Open. 2017, 1, e000154. [Google Scholar] [CrossRef]
- Ekinci, E.; Rohondia, S.; Khan, R.; Dou, Q.P. Repurposing Disulfiram as An Anti-Cancer Agent: Updated Review on Literature and Patents. Recent Pat. Anticancer. Drug Discov. 2019, 14, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Cardenes, H.R.; Moore, A.M.; Johnson, C.S.; Yu, M.; Helft, P.; Chiorean, E.G.; Vinson, J.; Howard, T.J.; Stephens, A.W.; Tai, D.F.; et al. A phase II study of gemcitabine in combination with radiation therapy in patients with localized, unresectable, pancreatic cancer: A Hoosier Oncology Group study. Am. J. Clin. Oncol. 2011, 34, 460–465. [Google Scholar] [CrossRef] [PubMed]
- McGinn, C.J.; Zalupski, M.M.; Shureiqi, I.; Robertson, J.M.; Eckhauser, F.E.; Smith, D.; Brown, D.; Hejna, G.; Strawderman, M.; Normolle, D.; et al. Phase I trial of radiation dose escalation with concurrent weekly full-dose gemcitabine in patients with advanced pancreatic cancer. J. Clin. Oncol. 2001, 19, 4202–4208. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.D.; Adusumilli, S.; Griffith, K.A.; Ray, M.E.; Zalupski, M.M.; Lawrence, T.S.; Ben-Josef, E. Full-dose gemcitabine and concurrent radiotherapy for unresectable pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Wolff, R.A.; Evans, D.B.; Gravel, D.M.; Lenzi, R.; Pisters, P.W.; ELee, J.; Janjan, N.A.; Charnsangavej, C.; Abbruzzese, J.L. Phase I trial of gemcitabine combined with radiation for the treatment of locally advanced pancreatic adenocarcinoma. Clin. Cancer Res. 2001, 7, 2246–2253. [Google Scholar]
- Nguyen, L.; Dobiasch, S.; Schneider, G.; Schmid, R.M.; Azimzadeh, O.; Kanev, K.; Buschmann, D.; Pfaffl, M.W.; Bartzsch, S.; Schmid, T.E.; et al. Impact of DNA repair and reactive oxygen species levels on radioresistance in pancreatic cancer. Radiother. Oncol. 2021, 159, 265–276. [Google Scholar] [CrossRef]
- Shah, S.S.; Rodriguez, G.A.; Musick, A.; Walters, W.M.; Cordoba, N.; Barbarite, E.; Marlow, M.; Marples, B.; Prince, J.; Komotar, R.; et al. Targeting Glioblastoma Stem Cells with 2-Deoxy-D-Glucose (2-DG) Potentiates Radiation-Induced Unfolded Protein Response (UPR). Cancers 2019, 11, 159. [Google Scholar] [CrossRef]
- Shah, S.S.; Rodriguez, G.A.; Musick, A.; Walters, W.M.; de Cordoba, N.; Barbarite, E.; Marlow, M.M.; Marples, B.; Prince, J.S.; Komotar, R.J.; et al. KRN5500, a spicamycin derivative, exerts anti-myeloma effects through impairing both myeloma cells and osteoclasts. Br. J. Haematol. 2011, 155, 328–339. [Google Scholar] [CrossRef]
- Supko, J.G.; Eder JPJr Ryan, D.P.; Seiden, M.V.; Lynch, T.J.; Amrein, P.C.; Kufe, D.W.; Clark, J.W. Phase I clinical trial and pharmacokinetic study of the spicamycin analog KRN5500 administered as a 1-hour intravenous infusion for five consecutive days to patients with refractory solid tumors. Clin. Cancer Res. 2003, 9, 5178–5186. [Google Scholar]
- Ashton, T.M.; McKenna, W.G.; Kunz-Schughart, L.A.; Higgins, G.S. Oxidative Phosphorylation as an Emerging Target in Cancer Therapy. Clin. Cancer Res. 2018, 24, 2482–2490. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, B.; Fu, B.; Zhu, H. Atovaquone at clinically relevant concentration overcomes chemoresistance in ovarian cancer via inhibiting mitochondrial respiration. Pathol. Res. Pract. 2021, 224, 153529. [Google Scholar] [CrossRef] [PubMed]
- Abdalbari, F.H.; Telleria, C.M. The gold complex auranofin: New perspectives for cancer therapy. Discov. Oncol. 2021, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, J.; Wu, S.; Wang, L.; Cao, X.; Zhang, X.; Dai, B.; Cao, M.; Shao, R.; Zhang, R.; et al. Auranofin-mediated inhibition of PI3K/AKT/mTOR axis and anticancer activity in non-small cell lung cancer cells. Oncotarget 2016, 7, 3548–3558. [Google Scholar] [CrossRef] [PubMed]

| Intrinsic Factors | Extrinsic |
|---|---|
| Oncogenic activation | Hypoxia |
| Altered Ploidy | Acidosis |
| Exacerbated Secretors | Nutrient depletion |
| Genomic Instability | VEGF |
| Redox imbalance | |
| Inward mutation |
| Agent | Mechanism of Action | Stage of Development | Clinical Trials | Comments |
|---|---|---|---|---|
| Gambogenic Acid (GNA) | ROS-dependent activation of IRE1 leads to prolonged ER stress; PERK activation → eIF2 phosphorylation inhibits protein synthesis [51,52,53] | Preclinical | No first-in-human trials | |
| Tigatuzumab | Death Receptor (DR)5 monoclonal antibody agonist; Induces TRAIL to bind DR5 initiating downstream caspase activation in tumors [54,55,56,57,58] | Phase 2 | NCT01307891 NCT01220999 | Combination with gemcitabine, sorafenib, nab-PAC |
| Minnelide | Water-soluble analog of triptolide; Inhibits GRP78; Upregulates IRE1 and PERK pathways to increase ER stress [59,60] | Phase 1/2 | NCT03129139 NCT04896073 | Single-agent and combination trials with Paclitaxel |
| BOLD-100 | Ruthenium (III) anticancer agent; inhibits GRP78 and increases ROS production; leads to ER stress [61,62,63] | Phase 1B | NCT04421820 | Combination with FOLFOX |
| Disulfram (Antabuse) | Binds Copper to form DSF-Cu complex; Induces ROS production → Increased levels of oxidized proteins → ER stress and UPR activation [64] | Phase 2 Completed, Phase 1 (recruiting) | NCT03714555 NCT02671890 | Combination with nab-PAC-gemcitabine, FOLFIRINOX, Gemcitabine |
| Radiation therapy | Radiation → ROS/RNS production → ER stress induction and UPR activation [65,66,67,68,69,70] | ER stress inducers sensitize cancer cells to radiation treatment | ||
| KRN5500 (Spicamycin analog) | Anti-Golgi drug; Inhibits protein synthesis and glycoprotein processing via altered Golgi (dilated cisternae) → Accumulation of unfolded proteins in ER lumen → apoptosis via intrinsic pathway [71,72] | Phase 1 completed | NCT00017238 NCT00002923 | No tumor response, three disease stabilizations observed in in-human trials. |
| Atovaquone | Ubiquinone analogue: Inhibits Complex III of ETC and oxidative phosphorylation → leads to oxidative and ER stress [73,74] | Phase 1 | NCT04648033 | Investigated in NSCLC, ovarian cancer |
| Auranofin | Gold (I) complex; inhibits thioredoxin reductases (TrxRs) → increased ROS species leading to ER stress; also targets PI3K/AKT/mTOR pathway [75,76] | Phase 1 | NCT01747798 NCT03456700 | Investigated in NSCLC, ovarian cancer |
| Trial Phase | Combination Agents | Type of Cancer | Progression-Free Survival (PFS) | Overall Survival (OS) | Conclusions |
|---|---|---|---|---|---|
| Phase II | Tigatuzumab + Gemcitabine | Unresectable or metastatic pancreatic cancer | 52.5% PFS at 16 weeks; not significant from historical data at 44% seen with gemcitabine alone [57] | 8.2 months; comparable to 3.6–6.8 months (gemcitabine alone), 3.8–11.1 months (gemcitabine + other agents), 11.1 months (FOLFIRINOX trial) [57] | Marginal increase in overall survival with TIG compared to gemcitabine alone suggests possible contribution of TIG to anti-tumor effects of gemcitabine; TIG may be clinically active [57] |
| Phase II | Tigatuzumab + Sorafenib | Advanced hepatocellular carcinoma | Time to progression (TTP): 3.9 months in 6/6 mg/kg TIG + SOR; 2.8 months in SOR alone (small sample size p = 0.988) [54] | 12.2 months in TIG + SOR; 8.2 months in SOR alone (small sample size p = 0.737) [54] | TIG + SOR failed to meet primary efficacy endpoint of TTP. However, combination was well tolerated and suggests possible increase in OS for TIG [54] |
| Phase II | Tigatuzumab + nab-PAC/Abraxane | Metastatic triple-negative breast cancer (TNBC) | 2.8 months overall in TIG + nab-PAC, 3.8 months in patients with objective response; 3.7 months in nab-PAC arm [58] | Overall response rate (ORR): 28% (CI 14.9–45.0% in TIG + nab-PAC; 38% (CI 18.0–61.1%) in nab-PAC arm [58] | 3 complete responses (CR) + 1 near CR in TIG + nab-PAC arm; no CR in nab-PAC arm; does not support further research of TIG + nab-PAC; however, notable increase in complete responses suggests further investigation of anti-DR5 agents [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botrus, G.; Miller, R.M.; Uson Junior, P.L.S.; Kannan, G.; Han, H.; Von Hoff, D.D. Increasing Stress to Induce Apoptosis in Pancreatic Cancer via the Unfolded Protein Response (UPR). Int. J. Mol. Sci. 2023, 24, 577. https://doi.org/10.3390/ijms24010577
Botrus G, Miller RM, Uson Junior PLS, Kannan G, Han H, Von Hoff DD. Increasing Stress to Induce Apoptosis in Pancreatic Cancer via the Unfolded Protein Response (UPR). International Journal of Molecular Sciences. 2023; 24(1):577. https://doi.org/10.3390/ijms24010577
Chicago/Turabian StyleBotrus, Gehan, Richard M. Miller, Pedro Luiz Serrano Uson Junior, Geoffrey Kannan, Haiyong Han, and Daniel D. Von Hoff. 2023. "Increasing Stress to Induce Apoptosis in Pancreatic Cancer via the Unfolded Protein Response (UPR)" International Journal of Molecular Sciences 24, no. 1: 577. https://doi.org/10.3390/ijms24010577
APA StyleBotrus, G., Miller, R. M., Uson Junior, P. L. S., Kannan, G., Han, H., & Von Hoff, D. D. (2023). Increasing Stress to Induce Apoptosis in Pancreatic Cancer via the Unfolded Protein Response (UPR). International Journal of Molecular Sciences, 24(1), 577. https://doi.org/10.3390/ijms24010577






