Toward Quantitative Models in Safety Assessment: A Case Study to Show Impact of Dose–Response Inference on hERG Inhibition Models
Abstract
1. Introduction
2. Results
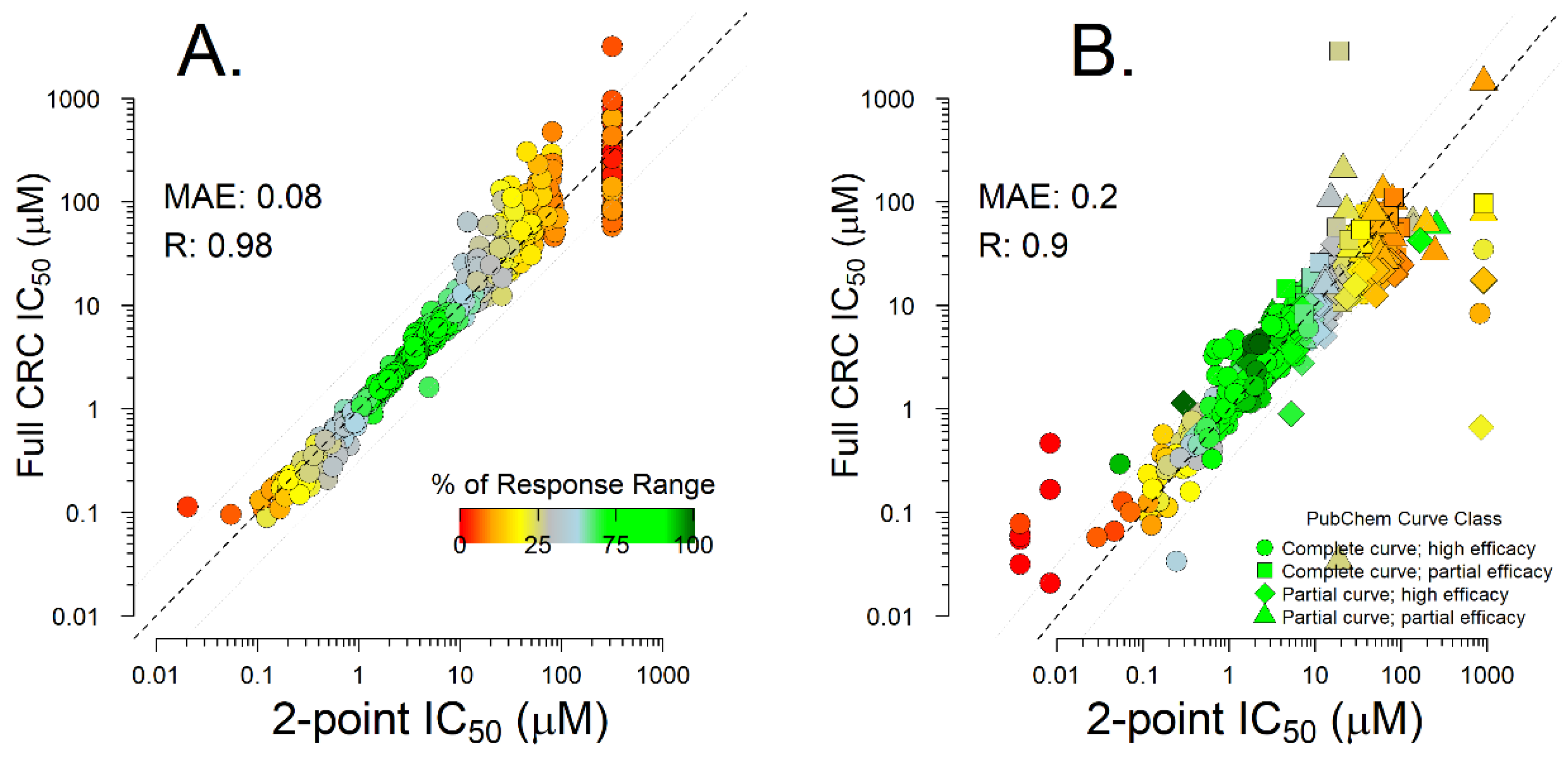
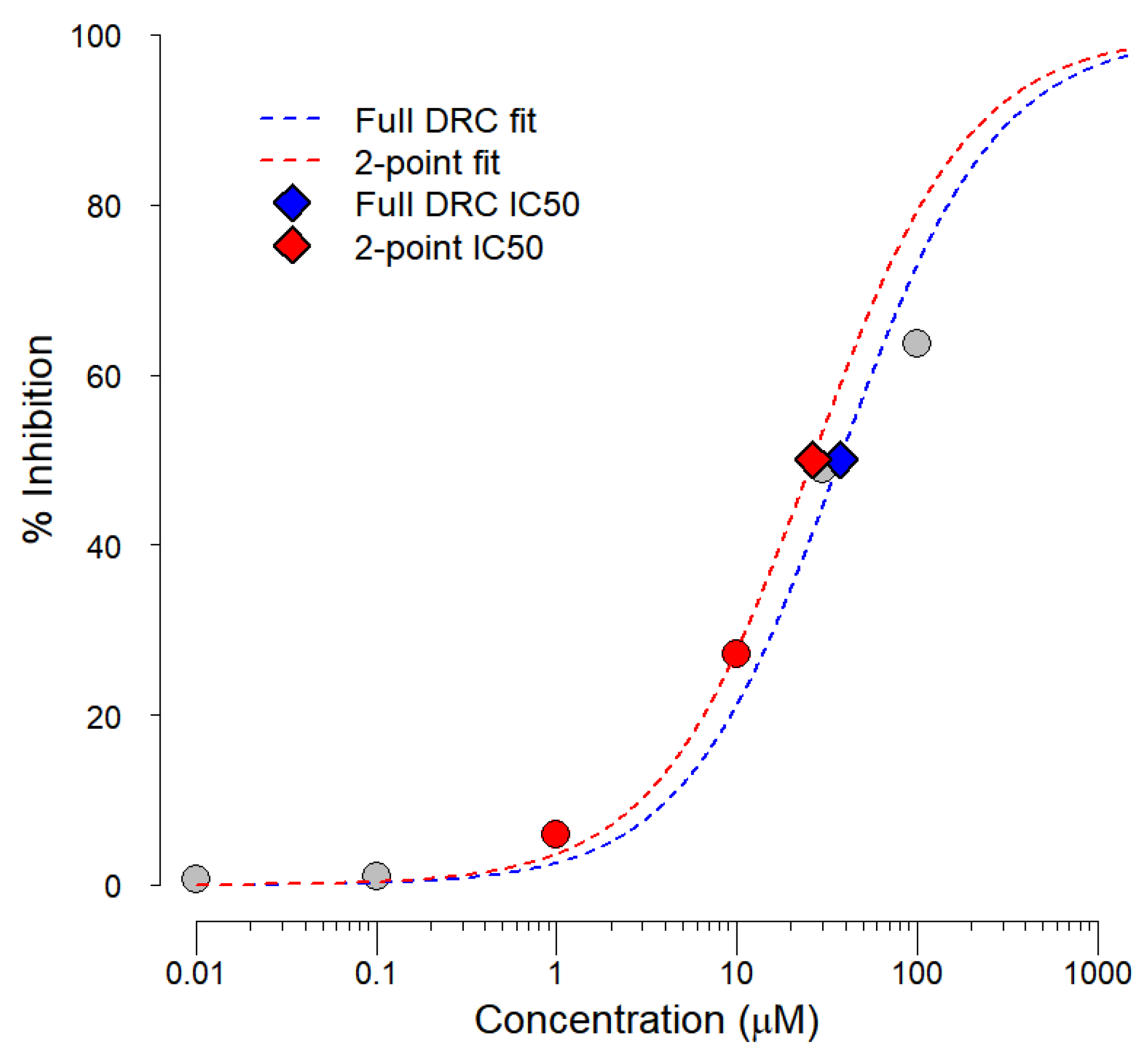
2.1. Two-Point Data Inference
2.2. hERG Model Performance
2.3. Alternative Decision Thresholds
3. Discussion
4. Materials and Methods
4.1. Chemical Data Sets
4.2. Chemical Descriptors
4.3. Dose–Response Inference for Genentech Data
4.4. hERG Modeling Strategies
4.5. Statistical Methods
4.6. Model Performance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villoutreix, B.O.; Taboureau, O. Computational Investigations of HERG Channel Blockers: New Insights and Current Predictive Models. Adv. Drug Deliv. Rev. 2015, 86, 72–82. [Google Scholar] [CrossRef]
- Sanguinetti, M.C.; Tristani-Firouzi, M. HERG Potassium Channels and Cardiac Arrhythmia. Nature 2006, 440, 463–469. [Google Scholar] [CrossRef]
- Smith, P.L.; Baukrowitz, T.; Yellen, G. The Inward Rectification Mechanism of the HERG Cardiac Potassium Channel. Nature 1996, 379, 833–836. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, J.I.; Perry, M.D.; Perrin, M.J.; Mann, S.A.; Ke, Y.; Hill, A.P. HERG K+ Channels: Structure, Function, and Clinical Significance. Physiol. Rev. 2012, 92, 1393–1478. [Google Scholar] [CrossRef]
- Brugada, R.; Hong, K.; Dumaine, R.; Cordeiro, J.; Gaita, F.; Borggrefe, M.; Menendez, T.M.; Brugada, J.; Pollevick, G.D.; Wolpert, C.; et al. Sudden Death Associated With Short-QT Syndrome Linked to Mutations in HERG. Circulation 2004, 109, 30–35. [Google Scholar] [CrossRef]
- Curran, M.E.; Splawski, I.; Timothy, K.W.; Vincen, G.M.; Green, E.D.; Keating, M.T. A Molecular Basis for Cardiac Arrhythmia: HERG Mutations Cause Long QT Syndrome. Cell 1995, 80, 795–803. [Google Scholar] [CrossRef]
- Redfern, W.S.; Carlsson, L.; Davis, A.S.; Lynch, W.G.; MacKenzie, I.; Palethorpe, S.; Siegl, P.K.S.; Strang, I.; Sullivan, A.T.; Wallis, R.; et al. Relationships between Preclinical Cardiac Electrophysiology, Clinical QT Interval Prolongation and Torsade de Pointes for a Broad Range of Drugs: Evidence for a Provisional Safety Margin in Drug Development. Cardiovasc. Res. 2003, 58, 32–45. [Google Scholar] [CrossRef]
- Brown, A.M. Drugs, HERG and Sudden Death. Cell Calcium 2004, 35, 543–547. [Google Scholar] [CrossRef]
- Giacomini, K.M.; Krauss, R.M.; Roden, D.M.; Eichelbaum, M.; Hayden, M.R.; Nakamura, Y. When Good Drugs Go Bad. Nature 2007, 446, 975–977. [Google Scholar] [CrossRef]
- Laverty, H.G.; Benson, C.; Cartwright, E.J.; Cross, M.J.; Garland, C.; Hammond, T.; Holloway, C.; McMahon, N.; Milligan, J.; Park, B.K.; et al. How Can We Improve Our Understanding of Cardiovascular Safety Liabilities to Develop Safer Medicines? Br. J. Pharmacol. 2011, 163, 675–693. [Google Scholar] [CrossRef]
- Sun, H.; Huang, R.; Xia, M.; Shahane, S.; Southall, N.; Wang, Y. Prediction of HERG Liability—Using SVM Classification, Bootstrapping and Jackknifing. Mol. Inform. 2017, 36, 1600126. [Google Scholar] [CrossRef] [PubMed]
- Witchel, H.J. The HERG Potassium Channel as a Therapeutic Target. Expert Opin. Ther. Targets 2007, 11, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Kratz, J.M.; Schuster, D.; Edtbauer, M.; Saxena, P.; Mair, C.E.; Kirchebner, J.; Matuszczak, B.; Baburin, I.; Hering, S.; Rollinger, J.M. Experimentally Validated HERG Pharmacophore Models as Cardiotoxicity Prediction Tools. J. Chem. Inf. Model. 2014, 54, 2887–2901. [Google Scholar] [CrossRef] [PubMed]
- Raschi, E.; Vasina, V.; Poluzzi, E.; De Ponti, F. The HERG K+ Channel: Target and Antitarget Strategies in Drug Development. Pharmacol. Res. 2008, 57, 181–195. [Google Scholar] [CrossRef]
- Rodolpho, C.B.; Vinicius, M.A.; Meryck, F.B.S.; Eugene, M.; Denis, F.; Alexander, T.; Carolina, H.A. Tuning HERG Out: Antitarget QSAR Models for Drug Development. Curr. Top. Med. Chem. 2014, 14, 1399–1415. [Google Scholar]
- Siramshetty, V.B.; Nguyen, D.-T.; Martinez, N.J.; Southall, N.T.; Simeonov, A.; Zakharov, A.V. Critical Assessment of Artificial Intelligence Methods for Prediction of HERG Channel Inhibition in the “Big Data” Era. J. Chem. Inf. Model. 2020, 60, 6007–6019. [Google Scholar] [CrossRef]
- Kiss, L.; Bennett, P.B.; Uebele, V.N.; Koblan, K.S.; Kane, S.A.; Neagle, B.; Schroeder, K. High Throughput Ion-Channel Pharmacology: Planar-Array-Based Voltage Clamp. ASSAY Drug Dev. Technol. 2003, 1, 127–135. [Google Scholar] [CrossRef]
- Polonchuk, L. Toward a New Gold Standard for Early Safety: Automated Temperature-Controlled HERG Test on the PatchLiner®. Front. Pharmacol. 2012, 102–111. [Google Scholar] [CrossRef]
- Wen, D.; Liu, A.; Chen, F.; Yang, J.; Dai, R. Validation of Visualized Transgenic Zebrafish as a High Throughput Model to Assay Bradycardia Related Cardio Toxicity Risk Candidates. J. Appl. Toxicol. 2012, 32, 834–842. [Google Scholar] [CrossRef]
- Polak, S.; Wiśniowska, B.; Brandys, J. Collation, Assessment and Analysis of Literature in Vitro Data on HERG Receptor Blocking Potency for Subsequent Modeling of Drugs’ Cardiotoxic Properties. J. Appl. Toxicol. 2009, 29, 183–206. [Google Scholar] [CrossRef]
- Cai, C.; Guo, P.; Zhou, Y.; Zhou, J.; Wang, Q.; Zhang, F.; Fang, J.; Cheng, F. Deep Learning-Based Prediction of Drug-Induced Cardiotoxicity. J. Chem. Inf. Model. 2019, 59, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Chavan, S.; Abdelaziz, A.; Wiklander, J.G.; Nicholls, I.A. A K-Nearest Neighbor Classification of HERG K+ Channel Blockers. J. Comput. Aided Mol. Des. 2016, 30, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Czodrowski, P. HERG Me Out. J. Chem. Inf. Model. 2013, 53, 2240–2251. [Google Scholar] [CrossRef] [PubMed]
- Konda, L.S.K.; Keerthi Praba, S.; Kristam, R. HERG Liability Classification Models Using Machine Learning Techniques. Comput. Toxicol. 2019, 12, 100089. [Google Scholar] [CrossRef]
- Korotcov, A.; Tkachenko, V.; Russo, D.P.; Ekins, S. Comparison of Deep Learning With Multiple Machine Learning Methods and Metrics Using Diverse Drug Discovery Data Sets. Mol. Pharm. 2017, 14, 4462–4475. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-L.; Aliagas, I.; Feng, J.A.; Gabriel, T.; O’Donnell, T.J.; Sellers, B.D.; Wiswedel, B.; Gobbi, A. Chemalot and Chemalot_knime: Command Line Programs as Workflow Tools for Drug Discovery. J. Cheminformatics 2017, 9, 38. [Google Scholar] [CrossRef]
- Ogura, K.; Sato, T.; Yuki, H.; Honma, T. Support Vector Machine Model for HERG Inhibitory Activities Based on the Integrated HERG Database Using Descriptor Selection by NSGA-II. Sci. Rep. 2019, 9, 12220. [Google Scholar] [CrossRef]
- Sharifi, M.; Buzatu, D.; Harris, S.; Wilkes, J. Development of Models for Predicting Torsade de Pointes Cardiac Arrhythmias Using Perceptron Neural Networks. BMC Bioinform. 2017, 18, 497. [Google Scholar] [CrossRef]
- Siramshetty, V.B.; Chen, Q.; Devarakonda, P.; Preissner, R. The Catch-22 of Predicting HERG Blockade Using Publicly Accessible Bioactivity Data. J. Chem. Inf. Model. 2018, 58, 1224–1233. [Google Scholar] [CrossRef]
- Wang, S.; Sun, H.; Liu, H.; Li, D.; Li, Y.; Hou, T. ADMET Evaluation in Drug Discovery. 16. Predicting HERG Blockers by Combining Multiple Pharmacophores and Machine Learning Approaches. Mol. Pharm. 2016, 13, 2855–2866. [Google Scholar] [CrossRef]
- Cianchetta, G.; Li, Y.; Kang, J.; Rampe, D.; Fravolini, A.; Cruciani, G.; Vaz, R.J. Predictive Models for HERG Potassium Channel Blockers. Bioorganic Med. Chem. Lett. 2005, 15, 3637–3642. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Easter, A.; Peters, D.; Kim, N.; Enyedy, I.J. In Silico Prediction of HERG Inhibition. Future Med. Chem. 2015, 7, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Sheridan, R.P.; Liaw, A.; Dahl, G.E.; Svetnik, V. Deep Neural Nets as a Method for Quantitative Structure–Activity Relationships. J. Chem. Inf. Model. 2015, 55, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Cronin, M.T.D.; Schultz, T.W. Pitfalls in QSAR. J. Mol. Struct. THEOCHEM 2003, 622, 39–51. [Google Scholar] [CrossRef]
- Bowes, J.; Brown, A.J.; Hamon, J.; Jarolimek, W.; Sridhar, A.; Waldron, G.; Whitebread, S. Reducing Safety-Related Drug Attrition: The Use of in Vitro Pharmacological Profiling. Nat. Rev. Drug Discov. 2012, 11, 909–922. [Google Scholar] [CrossRef]
- Obergrussberger, A.; Brüggemann, A.; Goetze, T.A.; Rapedius, M.; Haarmann, C.; Rinke, I.; Becker, N.; Oka, T.; Ohtsuki, A.; Stengel, T.; et al. Automated Patch Clamp Meets High-Throughput Screening: 384 Cells Recorded in Parallel on a Planar Patch Clamp Module. J. Lab. Autom. 2016, 21, 779–793. [Google Scholar] [CrossRef]
- Asmild, M.; Oswald, N.; Krzywkowski, K.M.; Friis, S.; Jacobsen, R.B.; Reuter, D.; Taboryski, R.; Kutchinsky, J.; Vestergaard, R.K.; Schrøder, R.L.; et al. Upscaling and Automation of Electrophysiology: Toward High Throughput Screening in Ion Channel Drug Discovery. Recept. Channels 2003, 9, 49–58. [Google Scholar] [CrossRef]
- Stoelzle, S.; Obergrussberger, A.; Brüggemann, A.; Haarmann, C.; George, M.; Kettenhofen, R.; Fertig, N. State-of-the-Art Automated Patch Clamp Devices: Heat Activation, Action Potentials, and High Throughput in Ion Channel Screening. Front. Pharm. 2011, 2, 76. [Google Scholar] [CrossRef]
- Titus, S.A.; Beacham, D.; Shahane, S.A.; Southall, N.; Xia, M.; Huang, R.; Hooten, E.; Zhao, Y.; Shou, L.; Austin, C.P.; et al. A New Homogeneous High-Throughput Screening Assay for Profiling Compound Activity on the Human Ether-a-Go-Go-Related Gene Channel. Anal. Biochem. 2009, 394, 30–38. [Google Scholar] [CrossRef]
- Couso, I.; Sánchez, L. Harnessing the Information Contained in Low-Quality Data Sources. Int. J. Approx. Reason. 2014, 55, 1485–1486. [Google Scholar] [CrossRef]
- Melnikov, F.; Hsieh, J.-H.; Sipes, N.S.; Anastas, P.T. Channel Interactions and Robust Inference for Ratiometric β-Lactamase Assay Data: A Tox21 Library Analysis. ACS Sustain. Chem. Eng. 2018, 6, 3233–3241. [Google Scholar] [CrossRef] [PubMed]
- Hüllermeier, E. Learning from Imprecise and Fuzzy Observations: Data Disambiguation through Generalized Loss Minimization. Int. J. Approx. Reason. 2014, 55, 1519–1534. [Google Scholar] [CrossRef]
- Bajorath, J.; Chávez-Hernández, A.L.; Duran-Frigola, M.; Fernández-de Gortari, E.; Gasteiger, J.; López-López, E.; Maggiora, G.M.; Medina-Franco, J.L.; Méndez-Lucio, O.; Mestres, J.; et al. Chemoinformatics and Artificial Intelligence Colloquium: Progress and Challenges in Developing Bioactive Compounds. J. Cheminformatics 2022, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- López-López, E.; Fernández-de Gortari, E.; Medina-Franco, J.L. Yes SIR! On the Structure–Inactivity Relationships in Drug Discovery. Drug Discov. Today 2022, 27, 2353–2362. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, R.; Vogt, M.; Bajorath, J. Influence of Varying Training Set Composition and Size on Support Vector Machine-Based Prediction of Active Compounds. J. Chem. Inf. Model. 2017, 57, 710–716. [Google Scholar] [CrossRef]
- Radchenko, E.V.; Rulev, Y.A.; Safanyaev, A.Y.; Palyulin, V.A.; Zefirov, N.S. Computer-Aided Estimation of the HERG-Mediated Cardiotoxicity Risk of Potential Drug Components. Dokl. Biochem. Biophys. 2017, 473, 128–131. [Google Scholar] [CrossRef]
- Sun, H. An Accurate and Interpretable Bayesian Classification Model for Prediction of HERG Liability. ChemMedChem 2006, 1, 315–322. [Google Scholar] [CrossRef]
- Ford, K.A. Refinement, Reduction, and Replacement of Animal Toxicity Tests by Computational Methods. ILAR J. 2016, 57, 226–233. [Google Scholar] [CrossRef]
- Yang, T.; Snyders, D.; Roden, D.M. Drug Block of I Kr : Model Systems and Relevance to Human Arrhythmias. J. Cardiovasc. Pharmacol. 2001, 38, 737–744. [Google Scholar] [CrossRef]
- Park, J.-S.; Jeon, J.-Y.; Yang, J.-H.; Kim, M.-G. Introduction to in Silico Model for Proarrhythmic Risk Assessment under the CiPA Initiative. Transl. Clin. Pharm. 2019, 27, 12–18. [Google Scholar] [CrossRef]
- Cruciani, G.; Milletti, F.; Storchi, L.; Sforna, G.; Goracci, L. In Silico PKa Prediction and ADME Profiling. Chem. Biodivers. 2009, 6, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, A.; Lee, M.-L. Handling of Tautomerism and Stereochemistry in Compound Registration. J. Chem. Inf. Model. 2012, 52, 285–292. [Google Scholar] [CrossRef]
- Heller, S.; McNaught, A.; Stein, S.; Tchekhovskoi, D.; Pletnev, I. InChI—The Worldwide Chemical Structure Identifier Standard. J. Cheminformatics 2013, 5, 7. [Google Scholar] [CrossRef]
- Landrum, G. RDKit: Open-Source Cheminformatics Software. Available online: https://www.rdkit.org/2021 (accessed on 2 August 2022).
- Manchester, J.; Walkup, G.; Rivin, O.; You, Z. Evaluation of PKa Estimation Methods on 211 Druglike Compounds. J. Chem. Inf. Model. 2010, 50, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Milletti, F.; Vulpetti, A. Tautomer Preference in PDB Complexes and Its Impact on Structure-Based Drug Discovery. J. Chem. Inf. Model. 2010, 50, 1062–1074. [Google Scholar] [CrossRef] [PubMed]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021.
- Filer, D.L.; Kothiya, P.; Setzer, R.W.; Judson, R.S.; Martin, M.T. Tcpl: The ToxCast Pipeline for High-Throughput Screening Data. Bioinformatics 2017, 33, 618–620. [Google Scholar] [CrossRef]
- Du, Y.; Days, E.; Romaine, I.; Abney, K.K.; Kaufmann, K.; Sulikowski, G.; Stauffer, S.; Lindsley, C.W.; Weaver, C.D. Development and Validation of a Thallium Flux-Based Functional Assay for the Sodium Channel NaV1.7 and Its Utility for Lead Discovery and Compound Profiling. ACS Chem. Neurosci. 2015, 6, 871–878. [Google Scholar] [CrossRef]
- Weaver, C.D.; Harden, D.; Dworetzky, S.I.; Robertson, B.; Knox, R.J. A Thallium-Sensitive, Fluorescence-Based Assay for Detecting and Characterizing Potassium Channel Modulators in Mammalian Cells. J. Biomol. Screen 2004, 9, 671–677. [Google Scholar] [CrossRef]
- Huang, R.; Xia, M.; Cho, M.-H.; Sakamuru, S.; Shinn, P.; Houck, K.A.; Dix, D.J.; Judson, R.S.; Witt, K.L.; Kavlock, R.J.; et al. Chemical Genomics Profiling of Environmental Chemical Modulation of Human Nuclear Receptors. Environ. Health Perspect. 2011, 119, 1142–1148. [Google Scholar] [CrossRef]
- Doddareddy, M.R.; Klaasse, E.C.; Shagufta; IJzerman, A.P.; Bender, A. Prospective Validation of a Comprehensive In Silico HERG Model and Its Applications to Commercial Compound and Drug Databases. ChemMedChem 2010, 5, 716–729. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining; Association for Computing Machinery: New York, NY, USA, 2016; pp. 785–794. [Google Scholar]
- Ji, X.; Tong, W.; Liu, Z.; Shi, T. Five-Feature Model for Developing the Classifier for Synergistic vs. Antagonistic Drug Combinations Built by XGBoost. Front. Genet. 2019, 10, 600. [Google Scholar] [CrossRef] [PubMed]
- McInnes, L.; Healy, J.; Melville, J. UMAP: Uniform Manifold Approximation and Projection for Dimension Reduction. arXiv preprint 2020, arXiv:1802.03426. [Google Scholar]
- Konopka, T. _umap: Uniform Manifold Approximation and Projection_. R Package Version 0.2.9.0. 2022. Available online: https://CRAN.R-Project.Org/Package=umap (accessed on 2 August 2022).
- Maaten, L.V.D.; Hinton, G.E. Visualizing Data Using T-SNE. J. Mach. Learn. Res. 2008, 9, 11. [Google Scholar]
- Krijthe, J. Rtsne: T-Distributed Stochastic Neighbor Embedding Using a Barnes-Hut Implementation. 2015. Available online: https://github.com/jkrijthe/rtsne (accessed on 2 August 2022).
- Batista, J.; Vikić-Topić, D.; Lučić, B. The Difference Between the Accuracy of Real and the Corresponding Random Model Is a Useful Parameter for Validation of Two-State Classification Model Quality. Croat. Chem. Acta 2016, 89, 527–534. [Google Scholar] [CrossRef]

| Model | Type | N | Data Included |
|---|---|---|---|
| Expanded continuous data (ECD) | Continuous | 4081 | All pIC50s from all experiments, including pIC50s extrapolated outside of the tested concentration ranges |
| Limited continuous data (LCD) | Continuous | 1686 | pIC50s from traditional dose–response experiments, calculated without extrapolation |
| All binary data (ABD) | Categorical | 3903 | All compounds for which consistent classification at the 10 μM threshold; prevalence = 40.9% |
| High confidence binary data (HCBD) | Categorical | 2812 | All compounds with % inhibition < 30% or >70% at 10 μM; prevalence = 35.9% |
| Model | Ac.Thr. | Test Set | Sens | Spec | PPV | NPV | Prev | BA | Q2 | Q2,rnd | ΔQ2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ECD | 10 | All | 0.69 | 0.90 | 0.90 | 0.67 | 0.58 | 0.79 | 0.77 | 0.49 | 0.28 |
| LCD | 10 | All | 0.96 | 0.29 | 0.65 | 0.82 | 0.58 | 0.62 | 0.68 | 0.56 | 0.12 |
| ABD | 10 | All | 0.58 | 0.83 | 0.83 | 0.59 | 0.58 | 0.71 | 0.69 | 0.48 | 0.21 |
| HCBD | 10 | All | 0.54 | 0.90 | 0.88 | 0.58 | 0.58 | 0.72 | 0.69 | 0.48 | 0.21 |
| ECD | 10 | HC | 0.74 | 0.95 | 0.95 | 0.74 | 0.56 | 0.84 | 0.83 | 0.49 | 0.34 |
| LCD | 10 | HC | 0.96 | 0.32 | 0.65 | 0.87 | 0.56 | 0.64 | 0.68 | 0.54 | 0.14 |
| ABD | 10 | HC | 0.64 | 0.85 | 0.85 | 0.65 | 0.56 | 0.75 | 0.73 | 0.49 | 0.24 |
| HCBD | 10 | HC | 0.58 | 0.93 | 0.91 | 0.63 | 0.56 | 0.76 | 0.73 | 0.48 | 0.25 |
| ECD | 1 | All | 0.08 | 0.98 | 0.33 | 0.90 | 0.10 | 0.53 | 0.89 | 0.88 | 0.01 |
| ECD | 3 | All | 0.40 | 0.98 | 0.88 | 0.79 | 0.30 | 0.69 | 0.80 | 0.64 | 0.16 |
| ECD | 5 | All | 0.51 | 0.88 | 0.75 | 0.72 | 0.41 | 0.70 | 0.73 | 0.54 | 0.19 |
| ECD | 30 | All | 0.88 | 0.62 | 0.87 | 0.64 | 0.75 | 0.75 | 0.82 | 0.63 | 0.19 |
| Model | Metric 1 | Iteration | Value |
|---|---|---|---|
| ECD | MAE | 1 | 0.334 |
| ECD | MAE | 2 | 0.347 |
| ECD | MAE | 3 | 0.353 |
| ECD | MAE | 4 | 0.338 |
| ECD | MAE | 5 | 0.341 |
| ECD | MAE | avg | 0.343 |
| LCD | MAE | 1 | 0.253 |
| LCD | MAE | 2 | 0.243 |
| LCD | MAE | 3 | 0.253 |
| LCD | MAE | 4 | 0.259 |
| LCD | MAE | 5 | 0.257 |
| LCD | MAE | avg | 0.253 |
| ABD | BA | 1 | 0.814 |
| ABD | BA | 2 | 0.824 |
| ABD | BA | 3 | 0.838 |
| ABD | BA | 4 | 0.846 |
| ABD | BA | 5 | 0.835 |
| ABD | BA | avg | 0.831 |
| HCBD | BA | 1 | 0.882 |
| HCBD | BA | 2 | 0.915 |
| HCBD | BA | 3 | 0.881 |
| HCBD | BA | 4 | 0.911 |
| HCBD | BA | 5 | 0.899 |
| HCBD | BA | avg | 0.898 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melnikov, F.; Anger, L.T.; Hasselgren, C. Toward Quantitative Models in Safety Assessment: A Case Study to Show Impact of Dose–Response Inference on hERG Inhibition Models. Int. J. Mol. Sci. 2023, 24, 635. https://doi.org/10.3390/ijms24010635
Melnikov F, Anger LT, Hasselgren C. Toward Quantitative Models in Safety Assessment: A Case Study to Show Impact of Dose–Response Inference on hERG Inhibition Models. International Journal of Molecular Sciences. 2023; 24(1):635. https://doi.org/10.3390/ijms24010635
Chicago/Turabian StyleMelnikov, Fjodor, Lennart T. Anger, and Catrin Hasselgren. 2023. "Toward Quantitative Models in Safety Assessment: A Case Study to Show Impact of Dose–Response Inference on hERG Inhibition Models" International Journal of Molecular Sciences 24, no. 1: 635. https://doi.org/10.3390/ijms24010635
APA StyleMelnikov, F., Anger, L. T., & Hasselgren, C. (2023). Toward Quantitative Models in Safety Assessment: A Case Study to Show Impact of Dose–Response Inference on hERG Inhibition Models. International Journal of Molecular Sciences, 24(1), 635. https://doi.org/10.3390/ijms24010635






