Effect of Quercetin on mitoBKCa Channel and Mitochondrial Function in Human Bronchial Epithelial Cells Exposed to Particulate Matter
Abstract
1. Introduction
2. Results
2.1. Quercetin Improves Transepithelial Resistance in HBE Cells
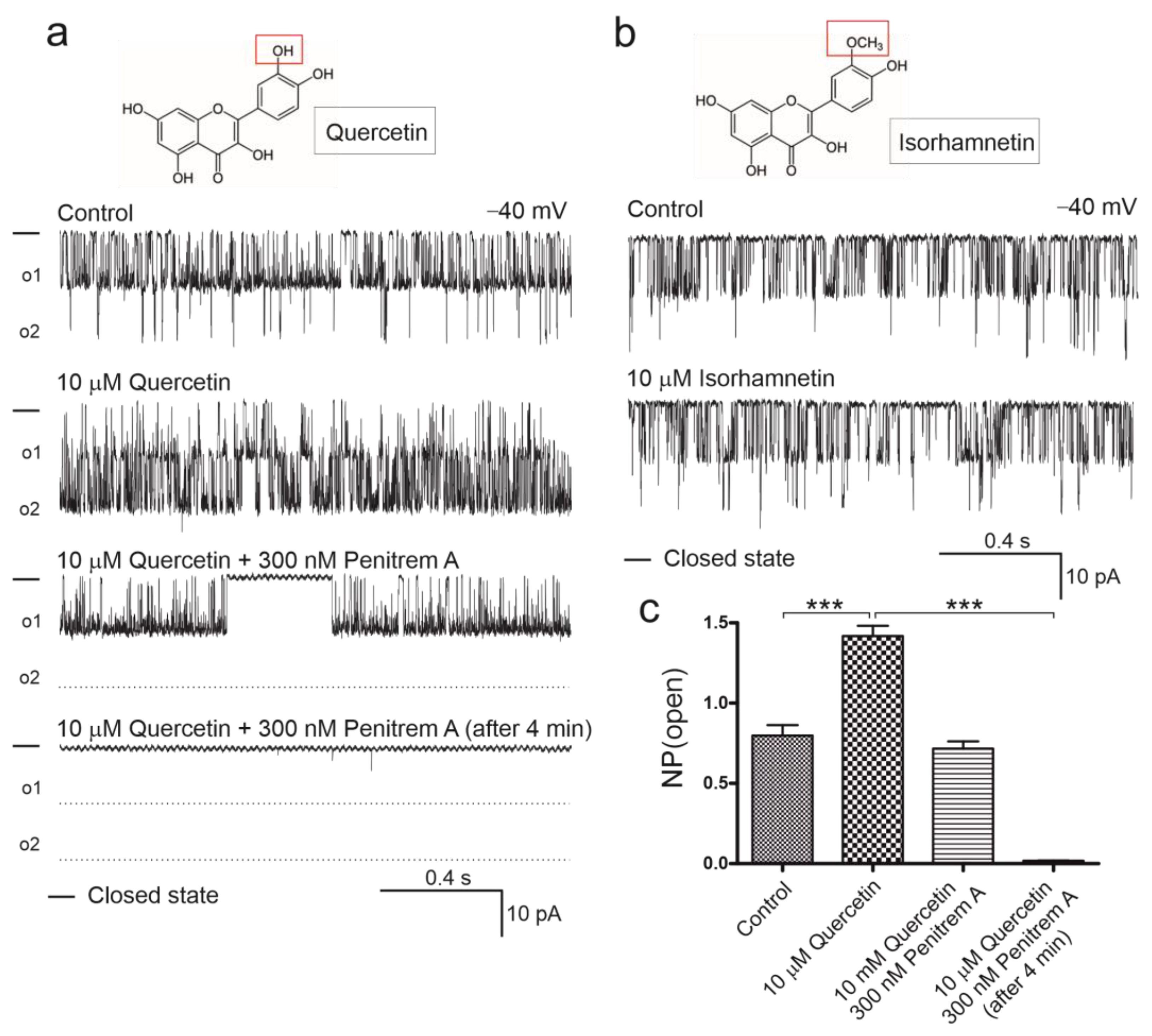
2.2. Quercetin, but Not Isorhamnetin, Activates Mitochondrial BKCa Channel
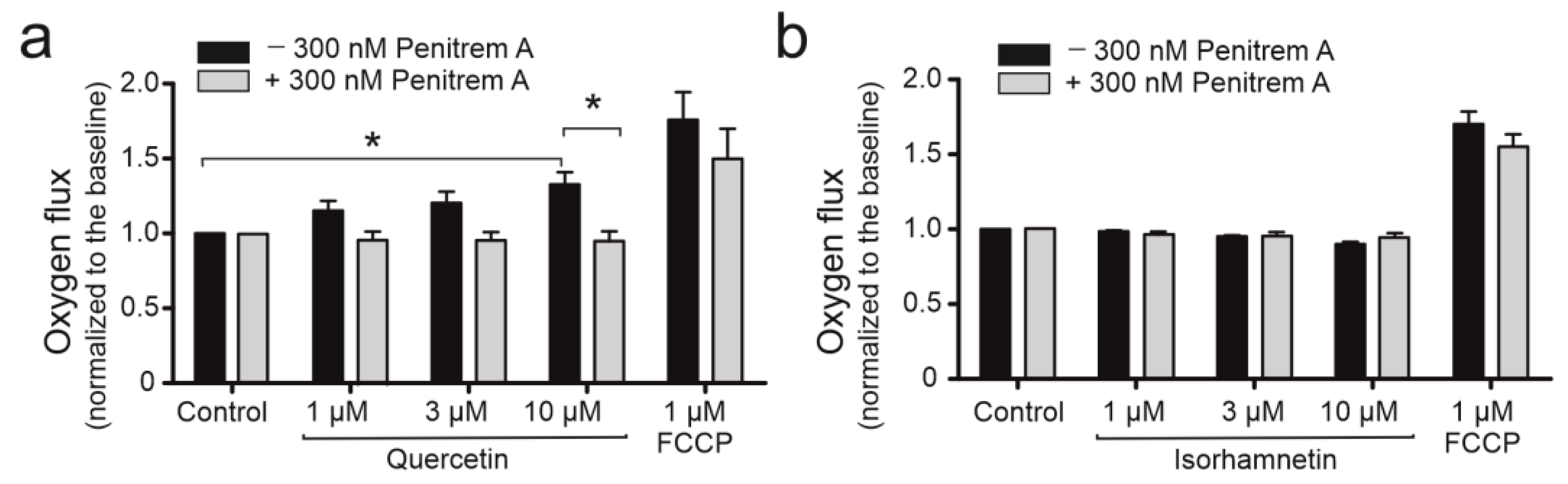
2.3. Quercetin Impacts Mitochondrial Respiration Rate in BKCa Channel Dependent Way
2.4. The Mitochondrial Potential of HBE Cells Is Affected by Quercetin
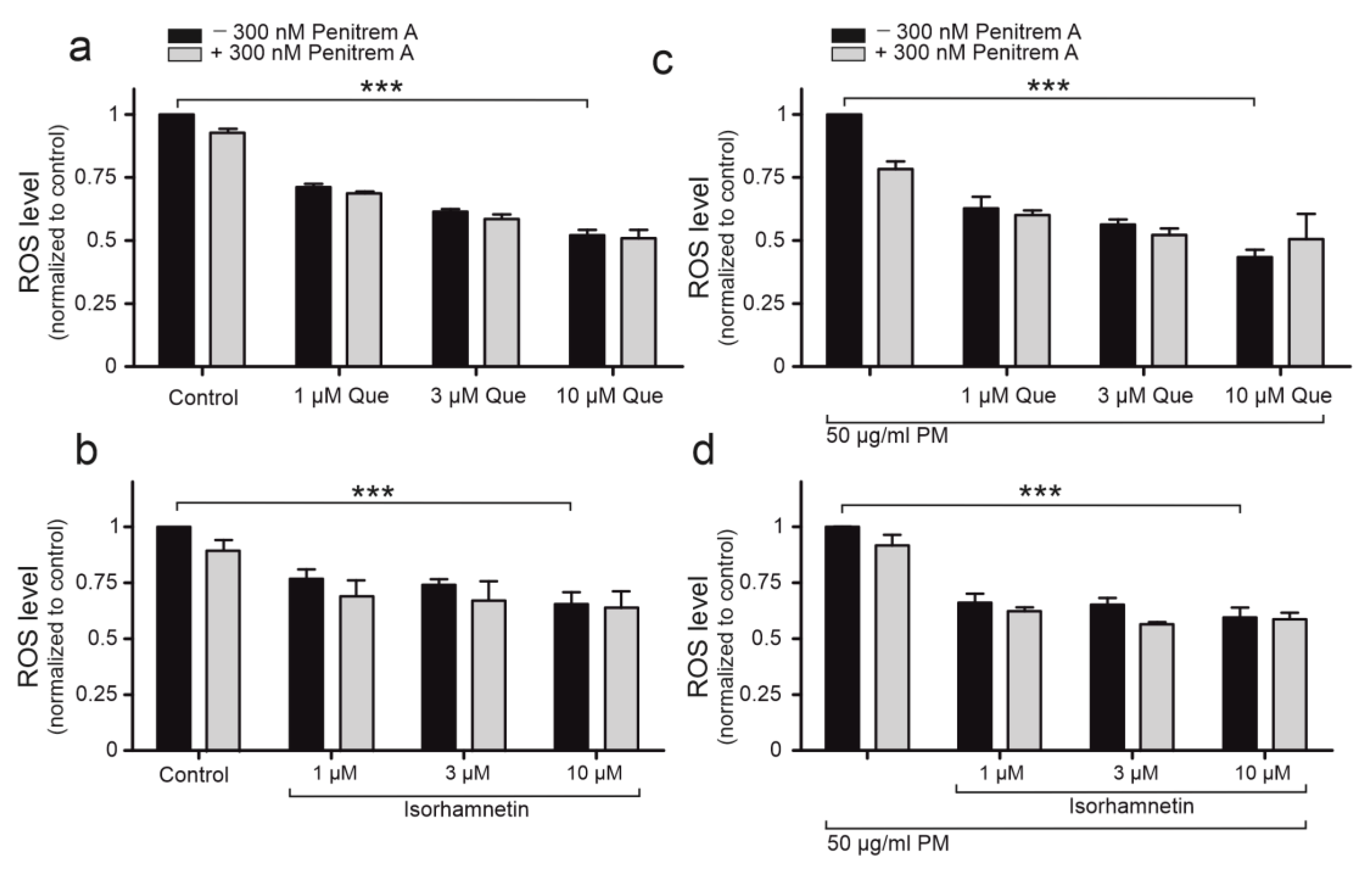
2.5. Quercetin Demonstrates Antioxidant Properties on Cellular Level
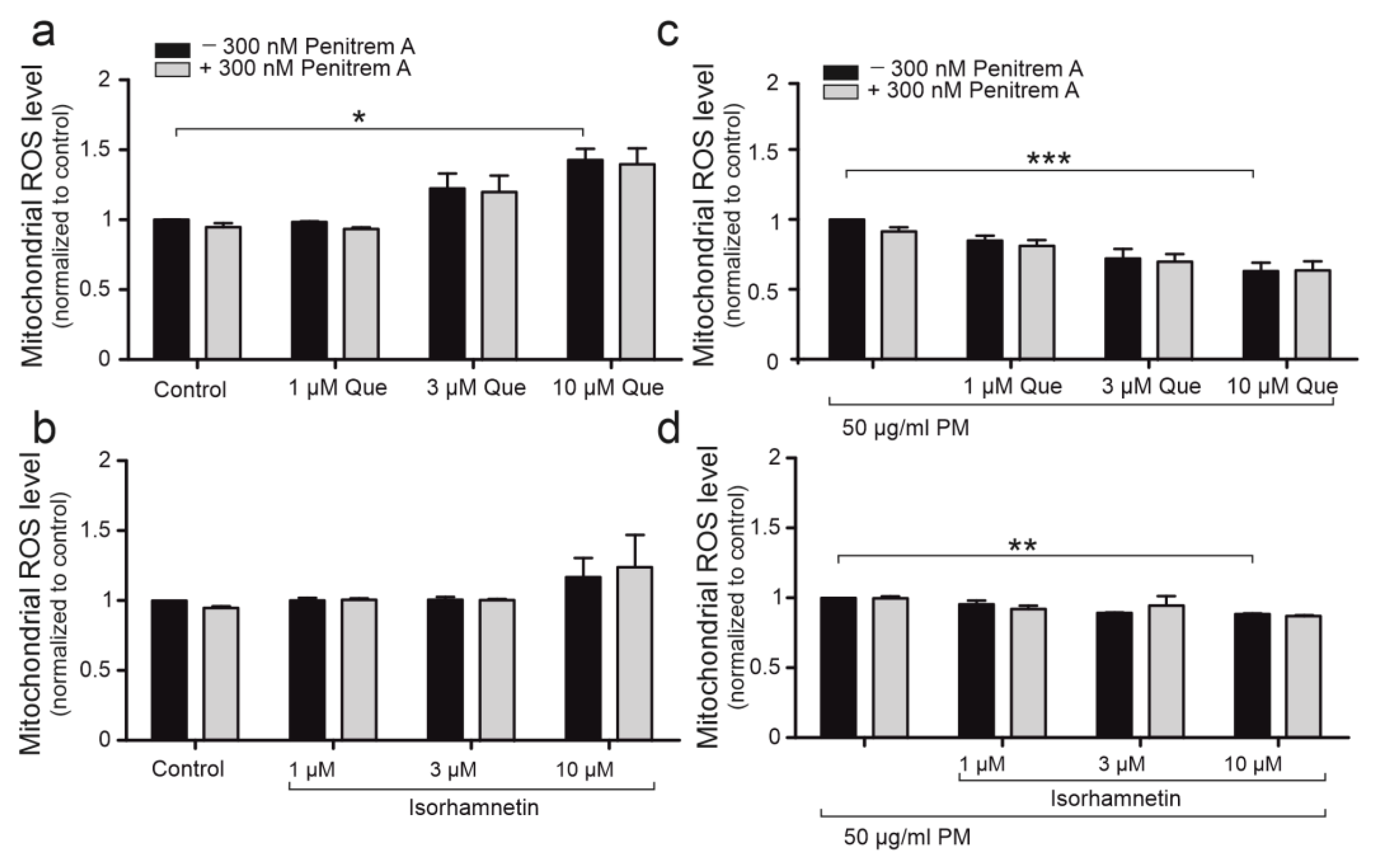
2.6. Quercetin Demonstrates Antioxidant Properties on Mitochondrial Level
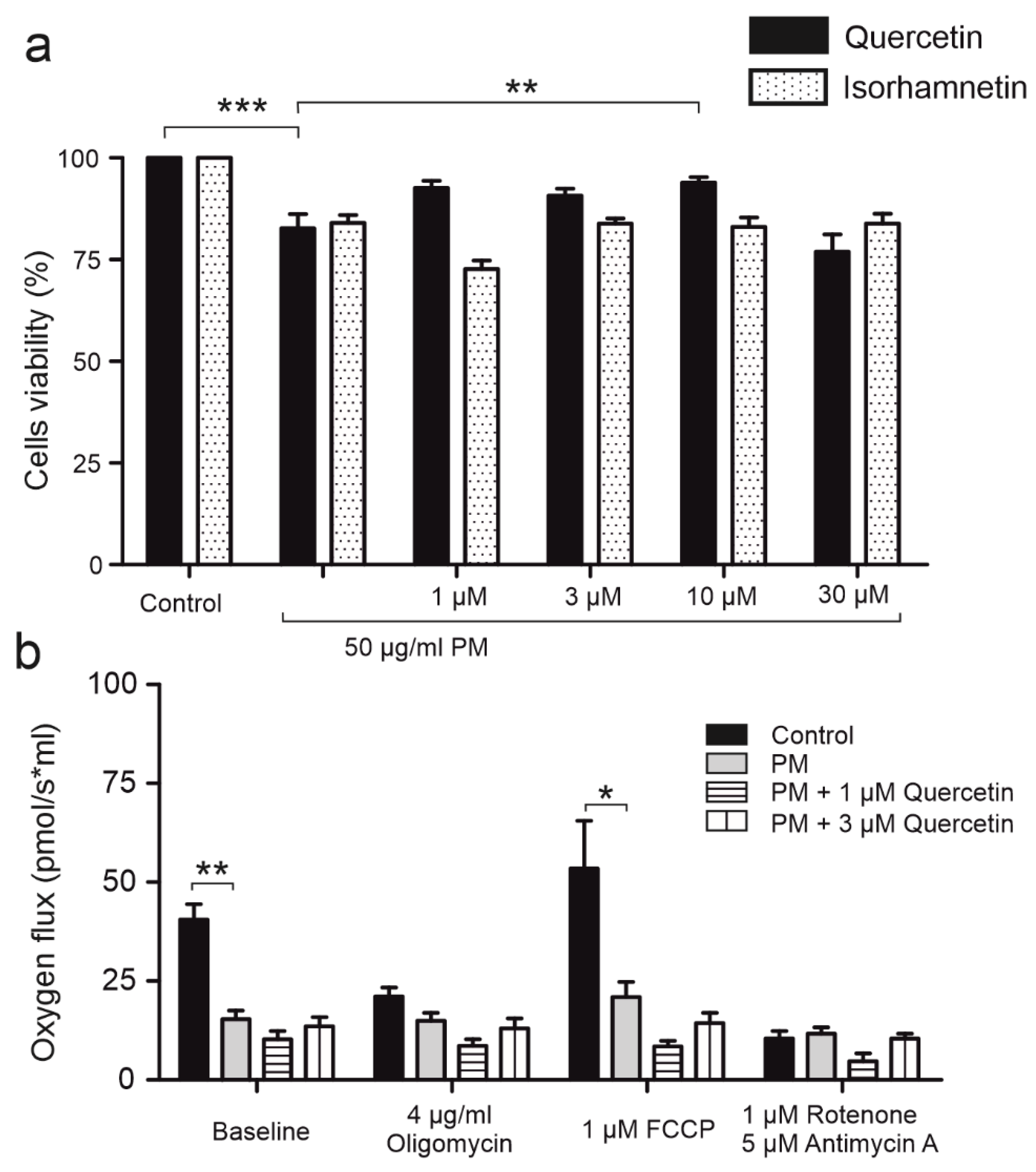
2.7. Long Term Application of PM Impairs Mitochondrial Function and Cell Viability
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Chemicals
4.3. Transepithelial Electrical Resistance (TEER) Measurements
4.4. Mitoplast Preparation for Electrophysiology
4.5. Patch-Clamp Experiments
4.6. High-Resolution Respirometry
4.7. Potential Measurements
4.8. Measurement of Reactive Oxygen Species
4.9. Trypan Blue Staining for Cell Viability Assessment
4.10. Mitochondrial Dysfunction Assessment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szabo, I.; Zoratti, M. Mitochondrial channels: Ion fluxes and more. Physiol. Rev. 2014, 94, 519–608. [Google Scholar] [CrossRef] [PubMed]
- Pereira, O.J.; Kowaltowski, A.J. Mitochondrial K(+) transport: Modulation and functional consequences. Molecules 2021, 26, 2935. [Google Scholar] [CrossRef] [PubMed]
- Tano, J.-Y.; Gollasch, M. Calcium-activated potassium channels in ischemia reperfusion: A brief update. Front. Physiol. 2014, 5, 381. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Wang, X.; Wu, H.; Chen, T.; Li, X.; Zhang, L.; Li, X.; Er, L.; Du, R. Cardioprotective effect of anisodamine against ischemia/reperfusion injury through the mitochondrial ATP-sensitive potassium channel. Eur. J. Pharmacol. 2021, 901, 174095. [Google Scholar] [CrossRef]
- Frankenreiter, S.; Bednarczyk, P.; Kniess, A.; Bork, N.I.; Straubinger, J.; Koprowski, P.; Wrzosek, A.; Mohr, E.; Logan, A.; Murphy, M.P.; et al. CGMP-elevating compounds and ischemic conditioning provide cardioprotection against ischemia and reperfusion injury via cardiomyocyte-specific BK channels. Circulation 2017, 136, 2337–2355. [Google Scholar] [CrossRef]
- Da Pozzo, E.; Costa, B.; Cavallini, C.; Testai, L.; Martelli, A.; Calderone, V.; Martini, C. The citrus flavanone naringenin protects myocardial cells against age-associated damage. Oxid. Med. Cell. Longev. 2017, 2017, 9536148. [Google Scholar] [CrossRef]
- Szteyn, K.; Singh, H. BK(Ca) channels as targets for cardioprotection. Antioxidants 2020, 9, 760. [Google Scholar] [CrossRef]
- Peng, K.; Hu, J.; Xiao, J.; Dan, G.; Yang, L.; Ye, F.; Zou, Z.; Cao, J.; Sai, Y. Mitochondrial ATP-sensitive potassium channel regulates mitochondrial dynamics to participate in neurodegeneration of Parkinson’s Disease. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864 Pt A, 1086–1103. [Google Scholar] [CrossRef]
- Su, F.; Yang, H.; Guo, A.; Qu, Z.; Wu, J.; Wang, Q. Mitochondrial BK(Ca) mediates the protective effect of low-dose ethanol preconditioning on oxygen-glucose deprivation and reperfusion-induced neuronal apoptosis. Front. Physiol. 2021, 12, 719753. [Google Scholar] [CrossRef]
- Checchetto, V.; Teardo, E.; Carraretto, L.; Leanza, L.; Szabo, I. Physiology of intracellular potassium channels: A unifying role as mediators of counterion fluxes? Biochim. Biophys. Acta 2016, 1857, 1258–1266. [Google Scholar] [CrossRef]
- Akopova, O.; Kolchinskaya, L.; Nosar, V.; Mankovska, I.; Sagach, V. Diazoxide affects mitochondrial bioenergetics by the opening of MKATP channel on submicromolar scale. BMC Mol. Cell Biol. 2020, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Kampa, R.P.; Gliździńska, A.; Szewczyk, A.; Bednarczyk, P.; Filipek, S. Flavonoid Quercetin Abolish Paxilline Inhibition of the Mitochondrial BK(Ca) Channel. Mitochondrion 2022, 65, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Testai, L.; Martelli, A.; Marino, A.; D’Antongiovanni, V.; Ciregia, F.; Giusti, L.; Lucacchini, A.; Chericoni, S.; Breschi, M.C.; Calderone, V. The activation of mitochondrial BK potassium channels contributes to the protective effects of naringenin against myocardial ischemia/reperfusion injury. Biochem. Pharmacol. 2013, 85, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Hannigan, K.I.; Large, R.J.; Bradley, E.; Hollywood, M.A.; Sergeant, G.P.; McHale, N.G.; Thornbury, K.D. Effect of a novel BKCa opener on BKCa currents and contractility of the rabbit corpus cavernosum. Am. J. Physiol. Cell Physiol. 2016, 310, C284–C292. [Google Scholar] [CrossRef] [PubMed]
- Leanza, L.; Checchetto, V.; Biasutto, L.; Rossa, A.; Costa, R.; Bachmann, M.; Zoratti, M.; Szabo, I. Pharmacological modulation of mitochondrial ion channels. Br. J. Pharmacol. 2019, 176, 4258–4283. [Google Scholar] [CrossRef] [PubMed]
- Kampa, R.P.; Sęk, A.; Szewczyk, A.; Bednarczyk, P. Cytoprotective effects of the flavonoid quercetin by activating mitochondrial BK(Ca) channels in endothelial cells. Biomed. Pharmacother. 2021, 142, 112039. [Google Scholar] [CrossRef]
- Pocernich, C.B.; Lange, M.L.B.; Sultana, R.; Butterfield, D.A. Nutritional approaches to modulate oxidative stress in Alzheimer’s Disease. Curr. Alzheimer Res. 2011, 8, 452–469. [Google Scholar] [CrossRef]
- Jagtap, S.; Meganathan, K.; Wagh, V.; Winkler, J.; Hescheler, J.; Sachinidis, A. Chemoprotective mechanism of the natural compounds, Epigallocatechin-3-O-Gallate, quercetin and curcumin against cancer and cardiovascular diseases. Curr. Med. Chem. 2009, 16, 1451–1462. [Google Scholar] [CrossRef]
- Liu, H.; Guo, X.; Chu, Y.; Lu, S. Heart protective effects and mechanism of quercetin preconditioning on anti-myocardial Ischemia Reperfusion (IR) injuries in rats. Gene 2014, 545, 149–155. [Google Scholar] [CrossRef]
- Sek, A.; Kampa, R.P.; Kulawiak, B.; Szewczyk, A.; Bednarczyk, P. Identification of the large-conductance Ca2+-regulated potassium channel in mitochondria of human bronchial epithelial cells. Molecules 2021, 26, 3233. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Kovochich, M.; Nel, A.E. Impairment of mitochondrial function by Particulate Matter (PM) and their toxic components: Implications for PM-induced cardiovascular and lung disease. Front. Biosci. 2007, 12, 1238–1246. [Google Scholar] [CrossRef]
- Aghapour, M.; Raee, P.; Moghaddam, S.J.; Hiemstra, P.S.; Heijink, I.H. Airway epithelial barrier dysfunction in chronic obstructive pulmonary disease: Role of cigarette smoke exposure. Am. J. Respir. Cell Mol. Biol. 2018, 58, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Georas, S.N.; Rezaee, F. Epithelial barrier function: At the front line of asthma immunology and allergic airway inflammation. J. Allergy Clin. Immunol. 2014, 134, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.A.; Puett, R.C.; Hart, J.E.; Camargo, C.A.J.; Varraso, R.; Yanosky, J.D.; Laden, F. Particulate matter exposures and adult-onset asthma and COPD in the nurses’ health study. Eur. Respir. J. 2016, 46, 1113–1130. [Google Scholar] [CrossRef]
- Zhao, R.; Guo, Z.; Zhang, R.; Deng, C.; Xu, J.; Dong, W.; Hong, Z.; Yu, H.; Situ, H.; Liu, C.; et al. Nasal epithelial barrier disruption by particulate matter ≤2.5 μm via tight junction protein degradation. J. Appl. Toxicol. 2018, 38, 678–687. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Dou, M.; He, H.; Ju, M.; Ji, S.; Zhou, J.; Chen, C.; Zhang, D.; Miao, C.; et al. Particulate matter disrupts airway epithelial barrier via oxidative stress to promote pseudomonas aeruginosa infection. J. Thorac. Dis. 2019, 11, 2617–2627. [Google Scholar] [CrossRef]
- Körper, S.; Nolte, F.; Rojewski, M.T.; Thiel, E.; Schrezenmeier, H. The K+ channel openers diazoxide and NS1619 induce depolarization of mitochondria and have differential effects on cell Ca2+ in CD34+ cell line KG-1a. Exp. Hematol. 2003, 31, 815–823. [Google Scholar] [CrossRef]
- Javadinia, S.S.; Abbaszadeh-Goudarzi, K.; Mahdian, D.; Hosseini, A.; Ghalenovi, M.; Javan, R. A review of the protective effects of quercetin-rich natural compounds for treating ischemia-reperfusion injury. Biotech. Histochem. 2022, 97, 237–246. [Google Scholar] [CrossRef]
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of neuroprotection by quercetin: Counteracting oxidative stress and more. Oxid. Med. Cell. Longev. 2016, 2016, 2986796. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Neuroprotective effects of quercetin in Alzheimer’s Disease. Biomolecules 2019, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-M.; Deng, X.-T.; Zhou, J.; Li, Q.-P.; Ge, X.-X.; Miao, L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother. 2020, 121, 109604. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.-Y.; Ima-Nirwana, S. Quercetin as an agent for protecting the bone: A review of the current evidence. Int. J. Mol. Sci. 2020, 21, 6448. [Google Scholar] [CrossRef]
- Jin, X.; Su, R.; Li, R.; Song, L.; Chen, M.; Cheng, L.; Li, Z. Amelioration of particulate matter-induced oxidative damage by vitamin C and quercetin in human bronchial epithelial cells. Chemosphere 2016, 144, 459–466. [Google Scholar] [CrossRef]
- Durga, M.; Vijayakumar, M.; Priya, K.; Vidhya Kanagarajan, S.; Banu, B.B.; Abraham, V.S.M.; Devasena, T.; Abdelaziz, M.A.; Mohideen, A.P.; Bahakim, N.O.; et al. Effects of quercetin on ultrafine petrol exhaust nanoparticles induced DNA damage, oxidative stress and inflammation in different sections of rat brain. J. King Saud Univ. Sci. 2022, 34, 101813. [Google Scholar] [CrossRef]
- Wang, T.; Shimizu, Y.; Wu, X.; Kelly, G.T.; Xu, X.; Wang, L.; Qian, Z.; Chen, Y.; Garcia, J.G.N. Particulate matter disrupts human lung endothelial cell barrier integrity via rho-dependent pathways. Pulm. Circ. 2017, 7, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Chen, W.; Zhang, Y.; Wang, X.; Dong, Y.; Zhang, W.; Lin, X. Shp2 regulates PM2.5-induced airway epithelial barrier dysfunction by modulating ERK1/2 signaling pathway. Toxicol. Lett. 2021, 350, 62–70. [Google Scholar] [CrossRef]
- Xu, W.; Liu, Y.; Wang, S.; McDonald, T.; Van Eyk, J.E.; Sidor, A.; O’Rourke, B. Cytoprotective role of Ca2+-activated K+ channels in the cardiac inner mitochondrial membrane. Science 2002, 298, 1029–1033. [Google Scholar] [CrossRef]
- Dorta, D.J.; Pigoso, A.A.; Mingatto, F.E.; Rodrigues, T.; Prado, I.M.R.; Helena, A.F.C.; Uyemura, S.A.; Santos, A.C.; Curti, C. The interaction of flavonoids with mitochondria: Effects on energetic processes. Chem. Biol. Interact. 2005, 152, 67–78. [Google Scholar] [CrossRef]
- Rodríguez, L.; Badimon, L.; Méndez, D.; Padró, T.; Vilahur, G.; Peña, E.; Carrasco, B.; Vogel, H.; Palomo, I.; Fuentes, E. Antiplatelet activity of isorhamnetin via mitochondrial regulation. Antioxidants 2021, 10, 666. [Google Scholar] [CrossRef]
- Thangavel, P.; Park, D.; Lee, Y.-C. Recent insights into Particulate Matter (PM2.5)-mediated toxicity in humans: An overview. Int. J. Environ. Res. Public Health 2022, 19, 7511. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Hua, S.; Song, L. PM2.5 exposure and asthma development: The key role of oxidative stress. Oxid. Med. Cell. Longev. 2022, 2022, 3618806. [Google Scholar] [CrossRef] [PubMed]
- Quezada-Maldonado, E.M.; Sánchez-Pérez, Y.; Chirino, Y.I.; García-Cuellar, C.M. Airborne particulate matter induces oxidative damage, DNA adduct formation and alterations in DNA repair pathways. Environ. Pollut. 2021, 287, 117313. [Google Scholar] [CrossRef]
- Dorta, D.J.; Pigoso, A.A.; Mingatto, F.E.; Rodrigues, T.; Pestana, C.R.; Uyemura, S.A.; Santos, A.C.; Curti, C. Antioxidant activity of flavonoids in isolated mitochondria. Phytother. Res. 2008, 22, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Metodiewa, D.; Jaiswal, A.K.; Cenas, N.; Dickancaité, E.; Segura-Aguilar, J. Quercetin may act as a cytotoxic prooxidant after its metabolic activation to semiquinone and quinoidal product. Free Radic. Biol. Med. 1999, 26, 107–116. [Google Scholar] [CrossRef]
- De Marchi, U.; Biasutto, L.; Garbisa, S.; Toninello, A.; Zoratti, M. Quercetin can act either as an inhibitor or an inducer of the mitochondrial permeability transition pore: A demonstration of the ambivalent redox character of polyphenols. Biochim. Biophys. Acta 2009, 1787, 1425–1432. [Google Scholar] [CrossRef]
- Fukaya, M.; Sato, Y.; Kondo, S.; Adachi, S.-I.; Yoshizawa, F.; Sato, Y. Quercetin enhances fatty acid β-oxidation by inducing lipophagy in AML12 hepatocytes. Heliyon 2021, 7, e07324. [Google Scholar] [CrossRef]
- Zhao, F.; Ma, Q.; Yue, Q.; Chen, H. SARS-CoV-2 infection and lung regeneration. Clin. Microbiol. Rev. 2022, 35, e0018821. [Google Scholar] [CrossRef]
- Zheng, J.; Ramirez, V.D. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Br. J. Pharmacol. 2000, 130, 1115–1123. [Google Scholar] [CrossRef]
- Lagoa, R.; Graziani, I.; Lopez-Sanchez, C.; Garcia-Martinez, V.; Gutierrez-Merino, C. Complex I and cytochrome C are molecular targets of flavonoids that inhibit hydrogen peroxide production by mitochondria. Biochim. Biophys. Acta 2011, 1807, 1562–1572. [Google Scholar] [CrossRef]
- Sandoval-Acuña, C.; Lopez-Alarcón, C.; Aliaga, M.E.; Speisky, H. Inhibition of mitochondrial complex I by various non-steroidal anti-inflammatory drugs and its protection by quercetin via a coenzyme Q-like action. Chem. Biol. Interact. 2012, 199, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Zajac, M.; Lewenstam, A.; Bednarczyk, P.; Dolowy, K. Measurement of multi ion transport through human bronchial epithelial cell line provides an insight into the mechanism of defective water transport in cystic fibrosis. Membranes 2020, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk, P.; Koziel, A.; Jarmuszkiewicz, W.; Szewczyk, A. Large-conductance Ca2+-activated potassium channel in mitochondria of endothelial EA.Hy926 cells. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1415–H1427. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk, P.; Wieckowski, M.R.; Broszkiewicz, M.; Skowronek, K.; Siemen, D.; Szewczyk, A. Putative structural and functional coupling of the mitochondrial BKCa channel to the respiratory chain. PLoS ONE 2013, 8, e68125. [Google Scholar] [CrossRef]
- Hall, A.; Moghimi, S.M. Determination of polycation-mediated perturbation of mitochondrial respiration in intact cells by high-resolution respirometry (Oxygraph-2k, OROBOROS). Methods Mol. Biol. 2019, 1943, 313–322. [Google Scholar] [CrossRef]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabrowska, A.; Zajac, M.; Bednarczyk, P.; Lukasiak, A. Effect of Quercetin on mitoBKCa Channel and Mitochondrial Function in Human Bronchial Epithelial Cells Exposed to Particulate Matter. Int. J. Mol. Sci. 2023, 24, 638. https://doi.org/10.3390/ijms24010638
Dabrowska A, Zajac M, Bednarczyk P, Lukasiak A. Effect of Quercetin on mitoBKCa Channel and Mitochondrial Function in Human Bronchial Epithelial Cells Exposed to Particulate Matter. International Journal of Molecular Sciences. 2023; 24(1):638. https://doi.org/10.3390/ijms24010638
Chicago/Turabian StyleDabrowska, Adrianna, Miroslaw Zajac, Piotr Bednarczyk, and Agnieszka Lukasiak. 2023. "Effect of Quercetin on mitoBKCa Channel and Mitochondrial Function in Human Bronchial Epithelial Cells Exposed to Particulate Matter" International Journal of Molecular Sciences 24, no. 1: 638. https://doi.org/10.3390/ijms24010638
APA StyleDabrowska, A., Zajac, M., Bednarczyk, P., & Lukasiak, A. (2023). Effect of Quercetin on mitoBKCa Channel and Mitochondrial Function in Human Bronchial Epithelial Cells Exposed to Particulate Matter. International Journal of Molecular Sciences, 24(1), 638. https://doi.org/10.3390/ijms24010638





