High-Throughput Strategies for the Design, Discovery, and Analysis of Bismuth-Based Photocatalysts
Abstract
1. Introduction
2. Background
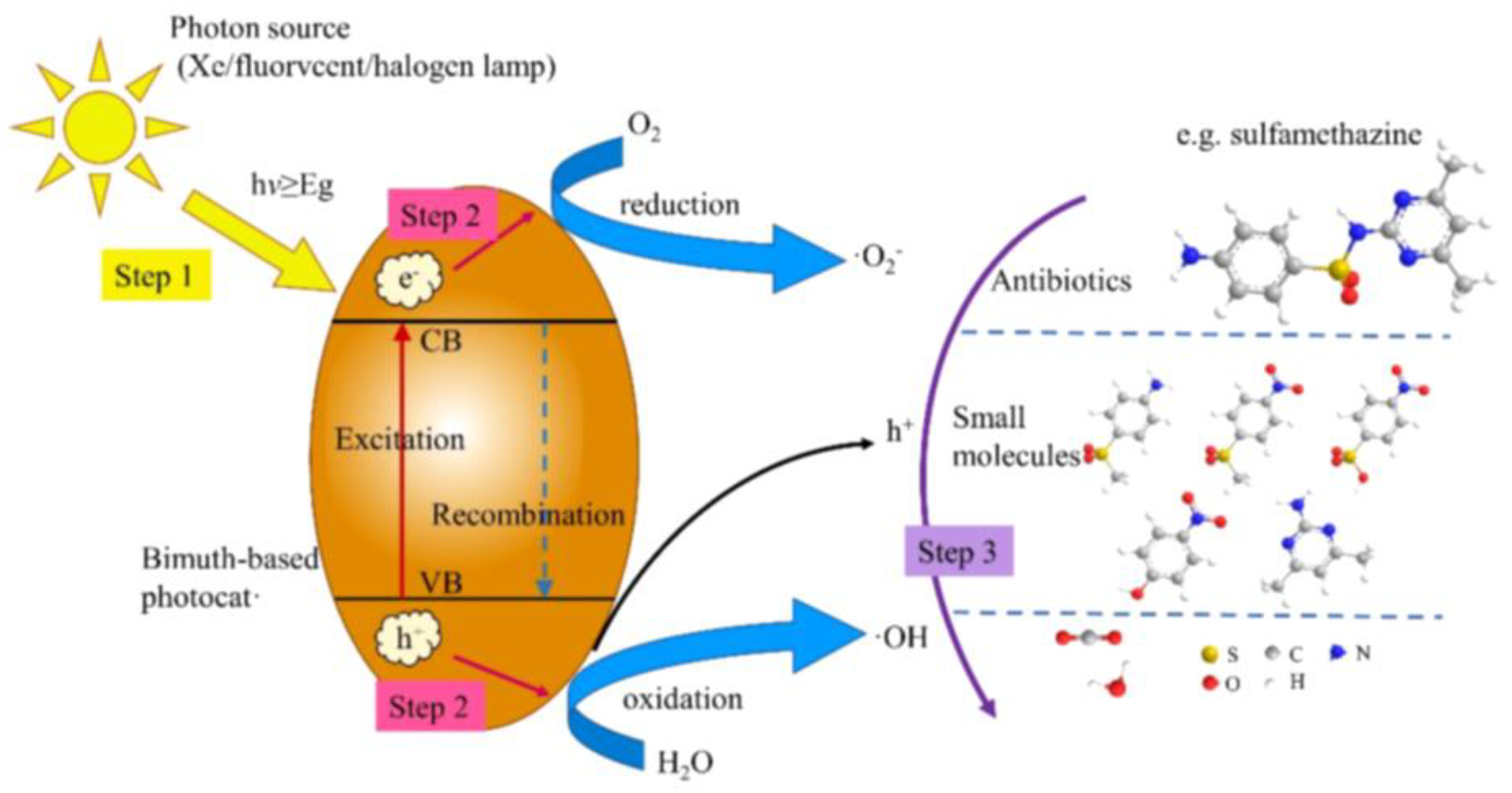
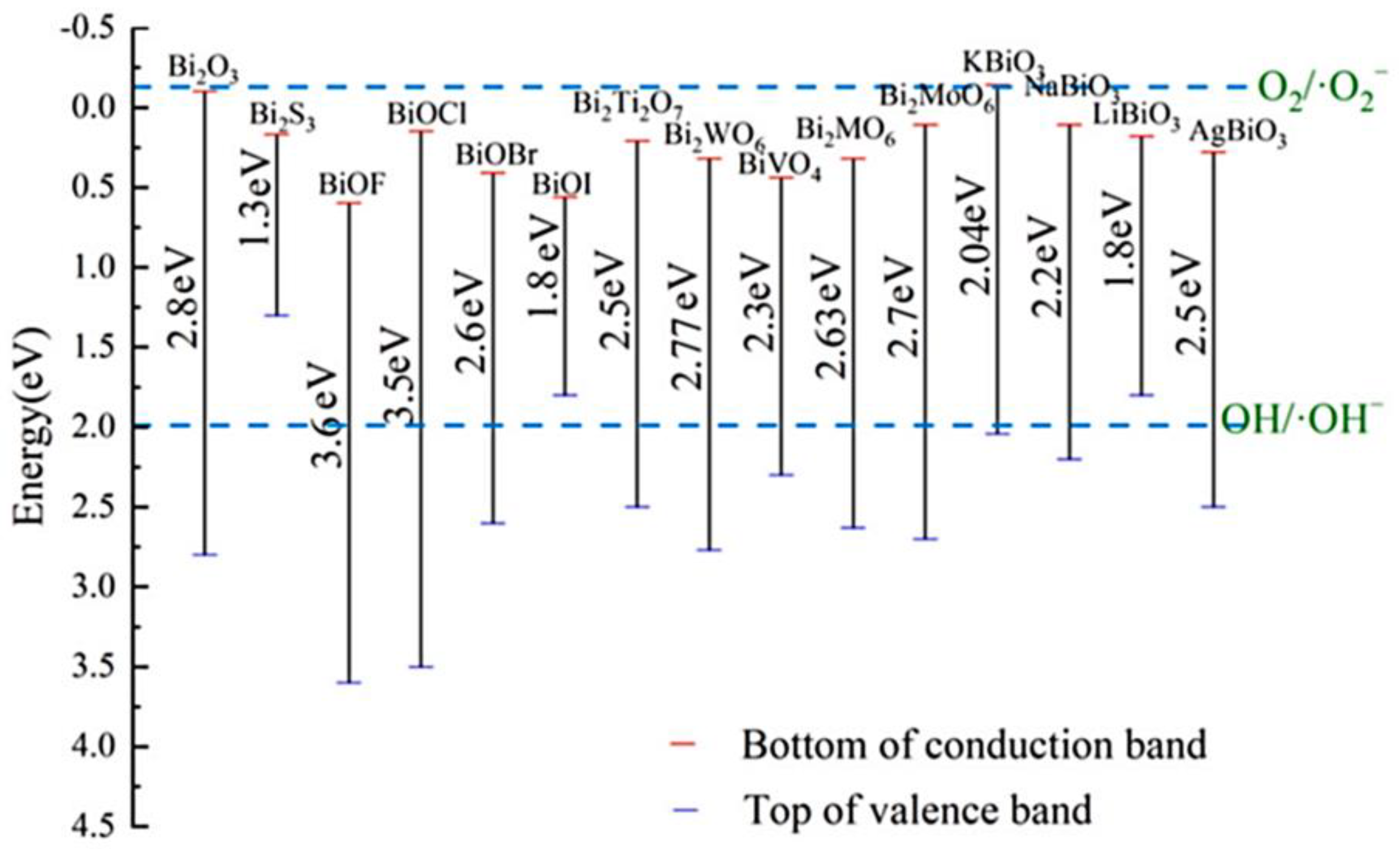
2.1. Fundamental Mechanism and Main Active Species of Bismuth-Based Photocatalysts
2.2. Significance of Nanostructure or Heterostructure or Heterojunction or Nanointerfaces
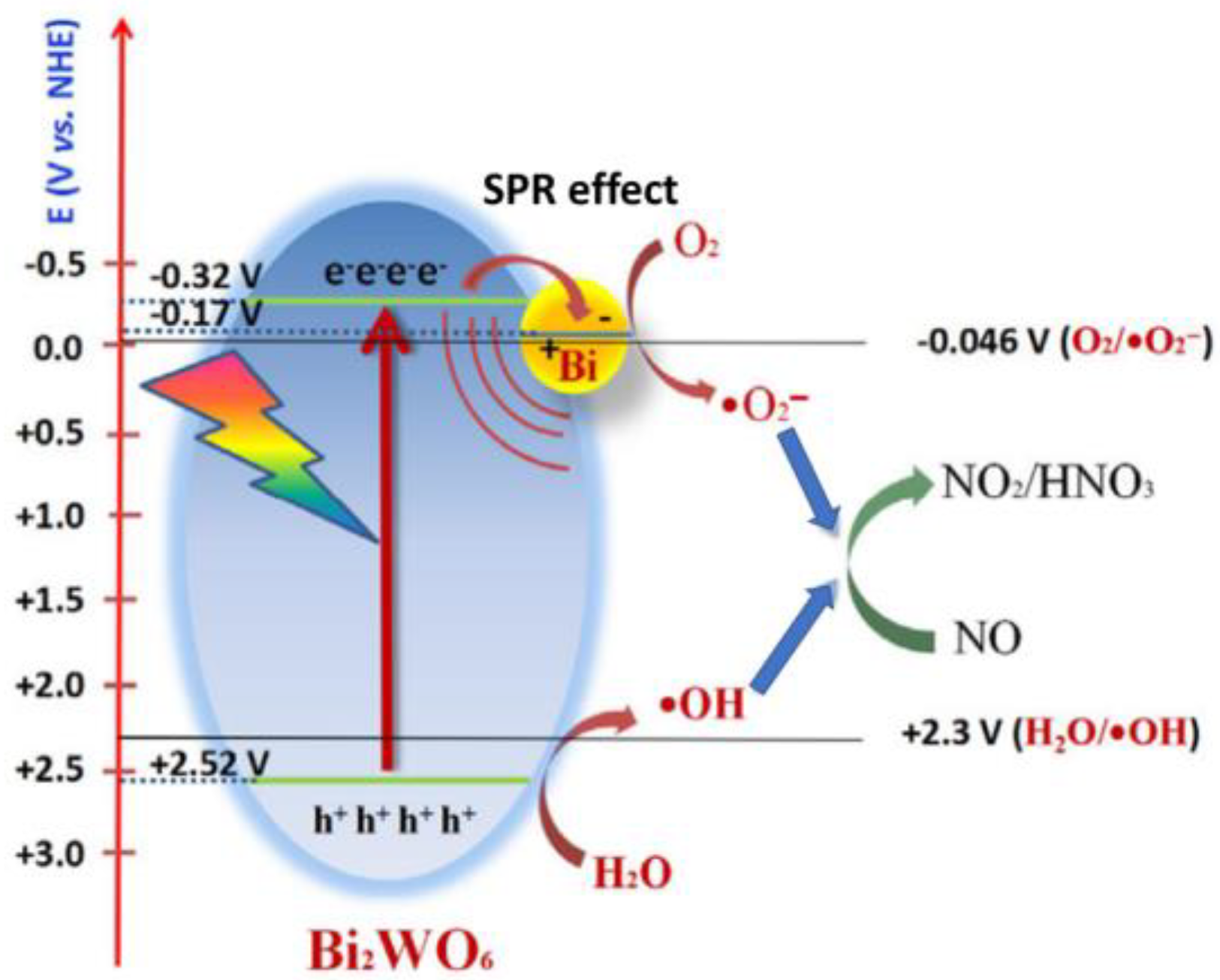
2.3. Surface Plasmon Resonance Effects in Bismuth-Based Photocatalysts
3. Synthesis Strategies of Bismuth-Based Photocatalysts
3.1. Synthesis Strategies of Bismuth Oxides
3.2. Synthesis Strategies of Bismuth Ferrites
4. Recent Developments in Bi-Based Photocatalysts
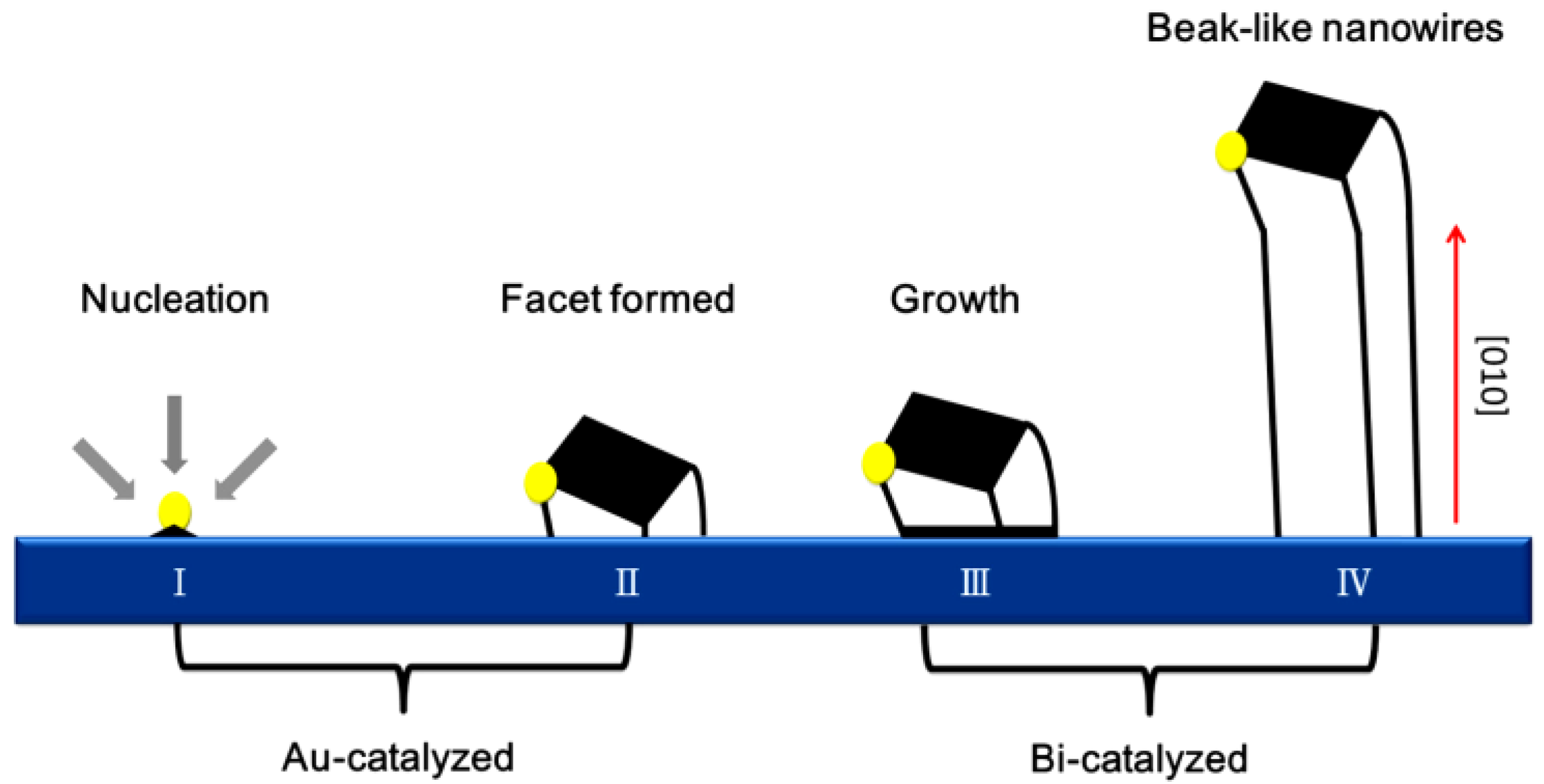
4.1. Bi-Oxide Nanostructures
4.2. Bismuth Vanadate (BiVO4)
4.3. Silver-Bismuth Photocatalysts
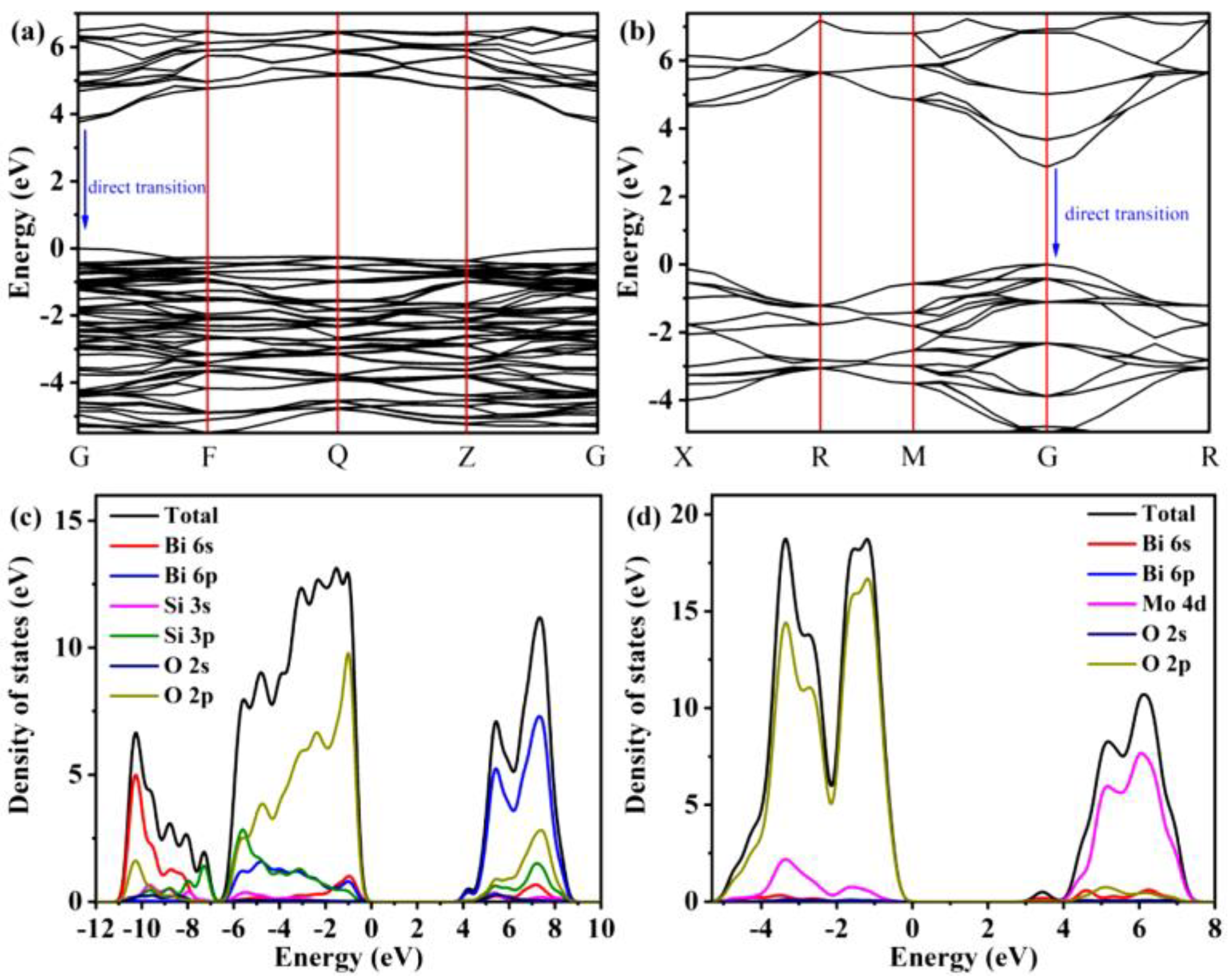
4.4. Bismuth Oxide Silicate-Based Photocatalysts
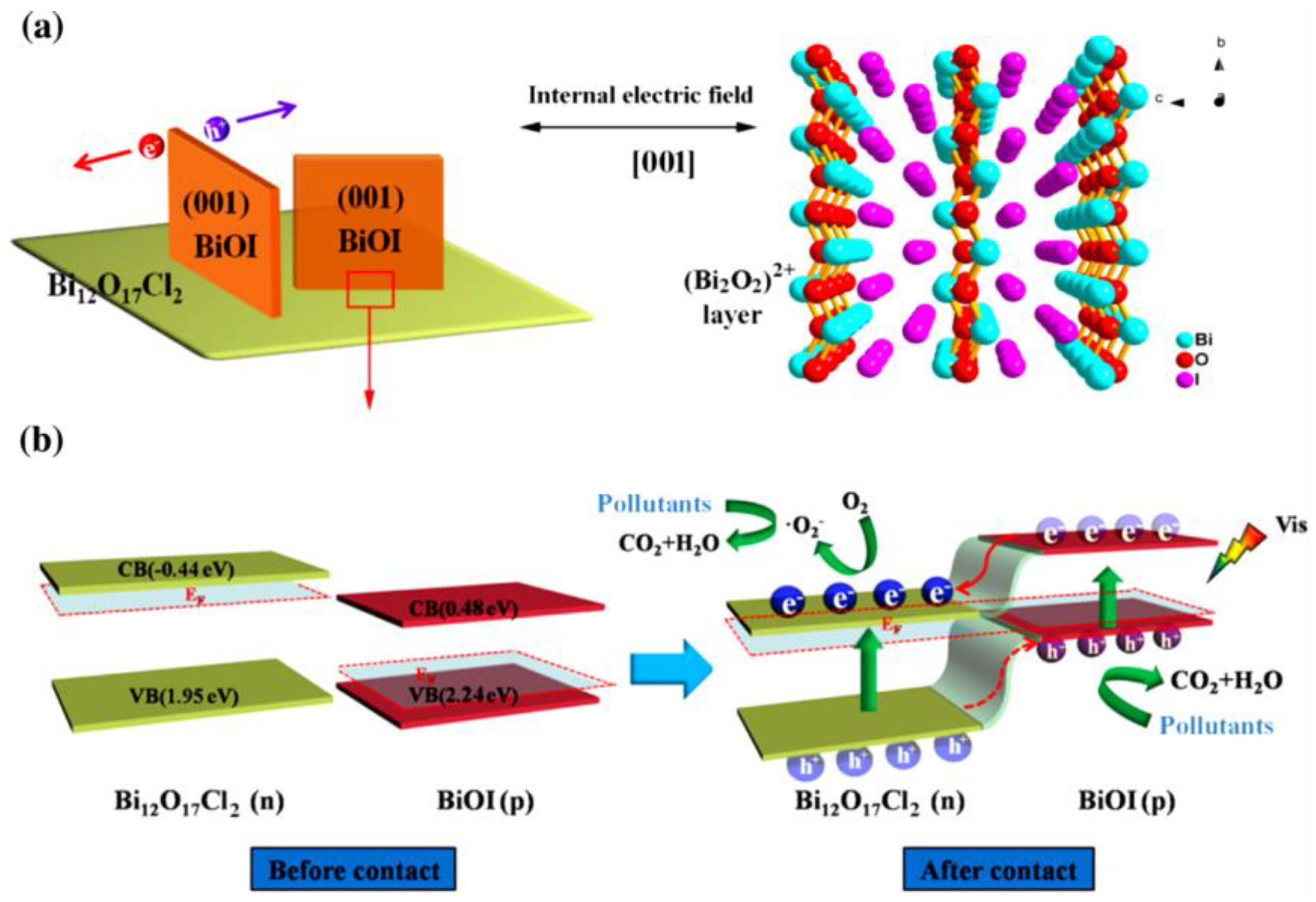
4.5. Bismuth and Bismuth-Rich Oxyhalides
5. Type of Photocatalytic Mechanism of Bismuth-Based Photocatalysts
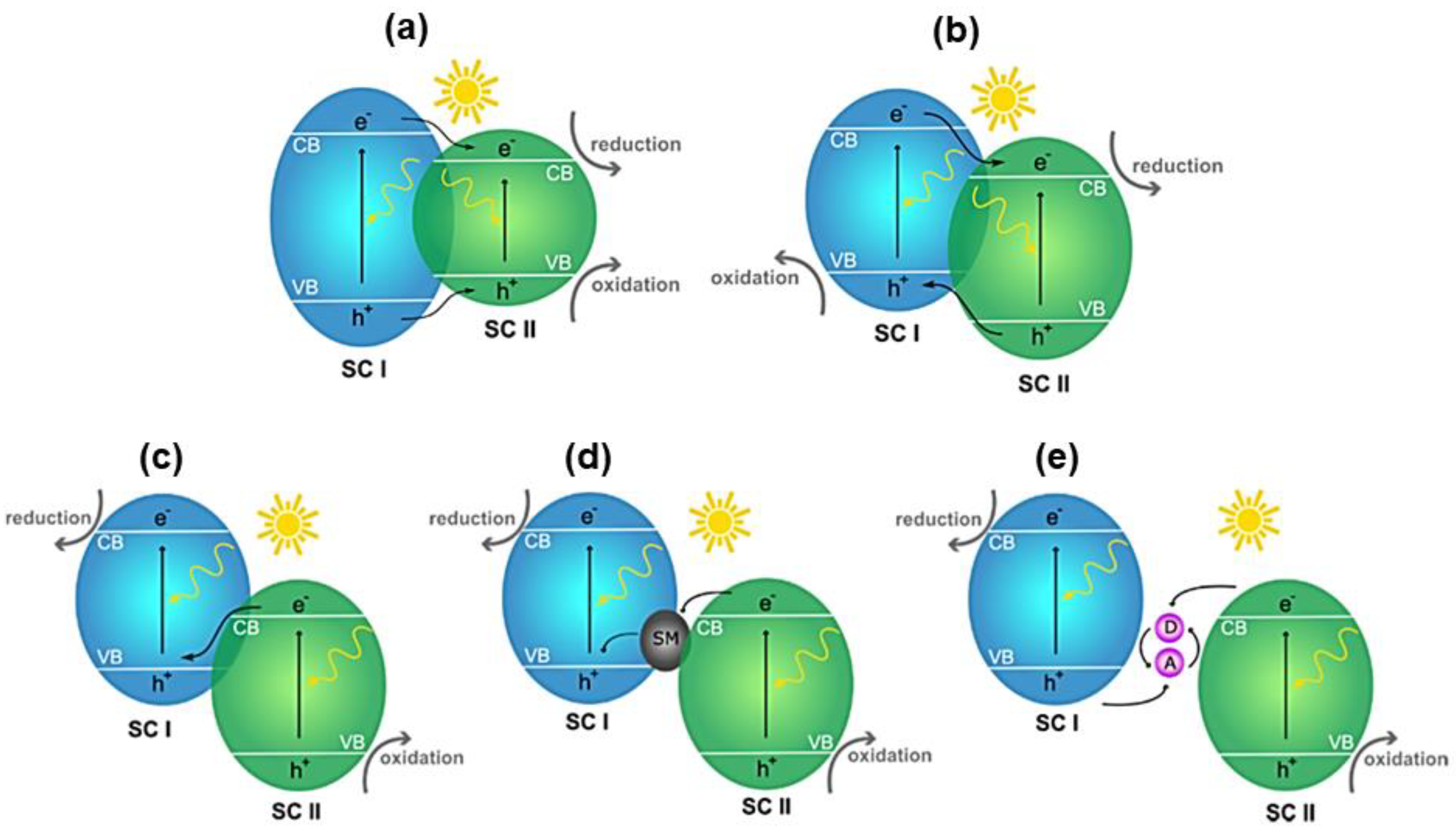
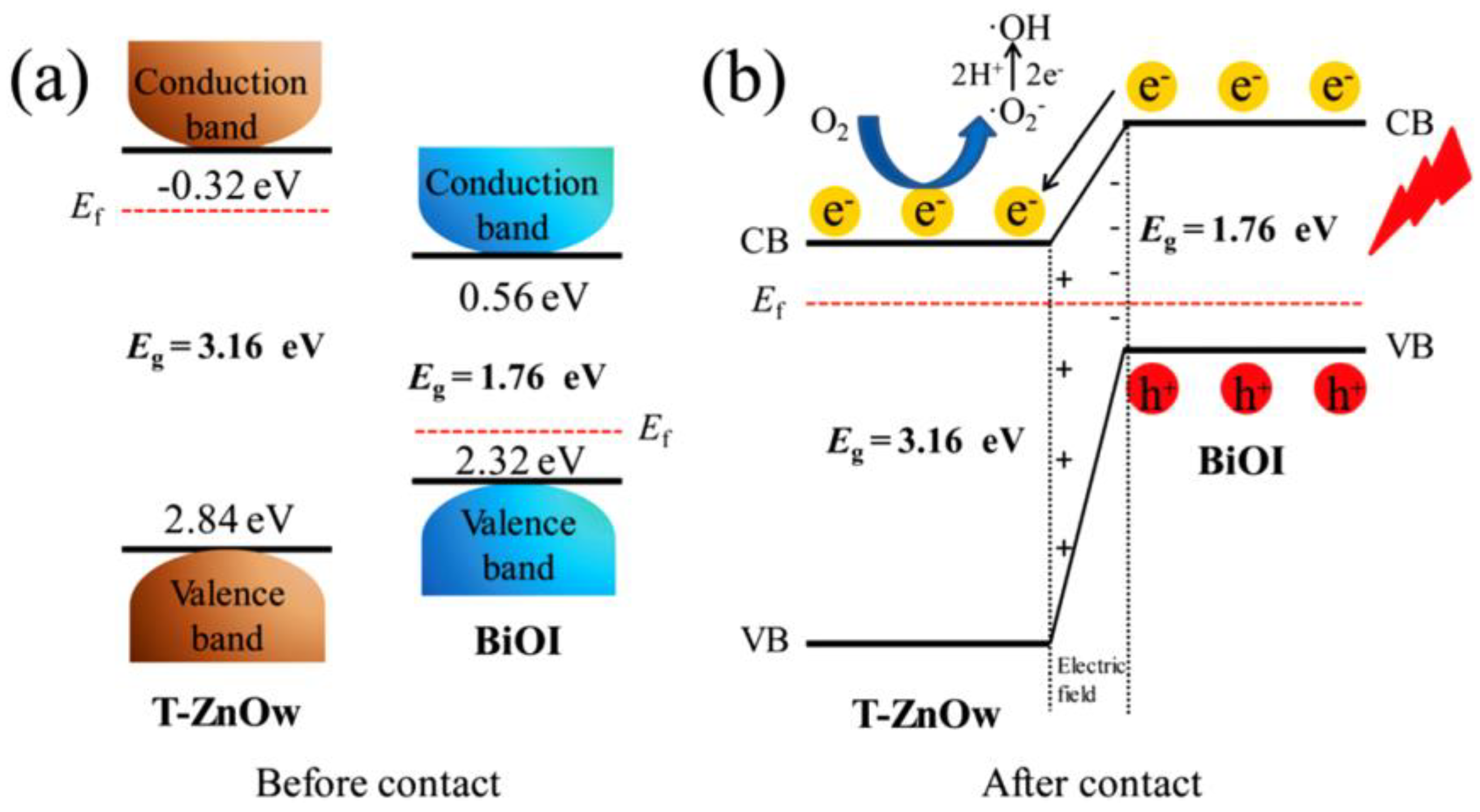
5.1. p–n Junction
5.2. n–n Junction
5.3. Z-Scheme
5.4. S-Scheme
6. Other Strategies for Enhance the Photocatalytic Activity
6.1. Doped-Bismuth-Based Photocatalyst
6.2. Ligand Modification Strategy
7. Future Prospects and Expectations
| Catalyst | Dosage (g L−1) | Pollutant | Dye Concentration (mg L−1) | Light Conditions | Degradation Time and Efficiency | Ref. |
|---|---|---|---|---|---|---|
| CNFs/g-C3N4/BiOBr | 3.0 | TC | 20 | 300 W, Xe lamp, λ > 400 nm | 120 min/86.1% | [182] |
| Bi2MoO6/CQDs/Bi2S3 | 0.3 | TC | 20 | 300 W, Xe lamp, λ > 420 nm | 120 min/92.5% | [183] |
| Bi2WO6/ZnIn2S4 | 0.2 | MO | 10 | 300 W, Xe lamp, λ > 420 nm | 60 min/97.5% | [184] |
| OVs-Bi2O3/Bi2SiO5 | 1.0 | MO | - | 500 W, Xe lamp, λ > 420 nm | 7 h/71.8% | [164] |
| BiVO4/Bi2S3 | - | Cr(VI) | - | 500 W, Xe lamp, 420 nm filters | - | [185] |
| BiVO4/Bi2O2CO3 | - | RhB | - | - | - | [186] |
| Bi25FeO40/Bi2Fe4O9 | 0.1 | RhB | 10 | - | - | [187] |
| Bi2WO6/BiOBr | - | RhB | - | 300 W, Xe lamp, λ > 420 nm | - | [188] |
| Bi2WO6/ZnFe2O4 | 0.05 | RhB | 50 | - | 300 min | [189] |
| BiFeO3/TiO2 | 0.5 | MB | - | 300 W, Xe lamp | 120 min/96% | [190] |
| BiFeO3/carbon | 0.01 | MB | - | 300 W, Xe lamp | 54 min/54% | [191] |
| BiFeO3-GdFeO3 | 0.01 | MB | - | Sunlight | 120 min/56% | [192] |
| BiFeO3/Fe3O4 | 0.02 | MB | - | 500 W, halogen lamp | 40 min/100% | [56] |
| MOF-BiFeO3 | 0.02 | MB | - | 300 W, Xe lamp | 120 min/93% | [193] |
| CuO–BiVO4 | 0.01 | MB | - | 150 W, Xe lamp | 150 min/100% | [194] |
| Bi2MoO6–ZnSnO3 | 0.01 | MB | - | 300 W, Xe lamp | 60 min/95% | [195] |
| BiOBr/Bi2O3 | 0.01 | MB | - | 300 W, Xe lamp | 50 min/87% | [196] |
| BiFeO3/Bi2WO6 | 0.06 | MB | - | 500 W, halogen lamp | 54 min/75% | [197] |
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1D | One-dimensional |
| 2,4-DCP | 2,4-dichlorophenol |
| 4-CP | 4-chlorophenol |
| APAP | Acetaminophen |
| BBNs | Bismuth-based nanostructures |
| BG | Brilliant green |
| BPA | Bisphenol A |
| CB | Conduction band |
| CIP | Ciprofloxacin |
| CQDs | Carbon quantum dots |
| CTAB | Cetyltrimethylammonium bromide |
| CTC | Chlortetracycline |
| CV | Crystal violet |
| IC | Indigo carmine |
| LED | Light-emitting diode |
| MB | Methylene blue |
| MO | Methyl orange |
| NHE | Normal hydrogen electrode |
| NO | Nitric oxide |
| OTC | Oxytetracycline |
| OVs | Oxygen vacancies |
| PTBP | 4-tert-butylphenol |
| rGO | Reduced graphene oxide |
| RhB | Rhodamine B |
| SPR | Surface plasmon resonance |
| TC | Tetracycline |
| TCHC | Tetracycline hydrochloride |
| TEOS | Tetraethoxysilane |
| TOC | Total organic carbon |
| UV | Ultraviolet |
| VB | Valence band |
References
- Li, K.; Li, B. Impacts of urbanization and industrialization on energy consumption/CO2 emissions: Does the level of development matter? Renew. Sustain. Energy Rev. 2015, 52, 1107–1122. [Google Scholar] [CrossRef]
- Andersson, J.; Grönkvist, S. Large-scale storage of hydrogen. Int. J. Hydrogen Energy 2019, 44, 11901–11919. [Google Scholar] [CrossRef]
- International Renewable Energy Agency (IRENA). Hydrogen: A Renewable Energy Perspective. Report Prepared for the 2nd Hydrogen Energy Ministerial Meeting in Tokyo, Japan (September 2019). Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2019/Sep/IRENA_Hydrogen_2019.pdf (accessed on 10 September 2019).
- Colón, G. Towards the hydrogen production by photocatalysis. Appl. Catal. A Gen. 2016, 518, 48–59. [Google Scholar] [CrossRef]
- Reischauer, S.; Pieber, B. Emerging concepts in photocatalytic organic synthesis. iScience 2021, 24, 102209. [Google Scholar] [CrossRef]
- Sudha, D.; Sivakumar, P. review on the photocatalytic activity of various composite catalysts. Chem. Eng. Proc. 2015, 97, 112–133. [Google Scholar] [CrossRef]
- Xiao, M.; Wang, Z.; Lyu, M.; Luo, B.; Wang, S.; Liu, G.; Cheng, H.-M.; Wang, L. Hollow nanostructures for photocatalysis: Advantages and challenges. Adv. Mater. 2019, 31, 1801369. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, N.; Wu, W.; He, Q. Recent progress on photocatalytic heterostructures with full solar spectral responses. Chem. Eng. J. 2020, 393, 124719. [Google Scholar] [CrossRef]
- Pereira, A.L.J.; Errandonea, D.; Beltrán, A.; Gracia, L.; Gomis, O.; Sans, J.A.; Garcia-Domene, B.; Miquel-Veyrat, A.; Manjón, F.J.; Muñoz, A.; et al. Structural study of α-Bi2O3 under pressure. J. Phys. Condens. Matter 2013, 25, 475402. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, W.; Xu, H.; Sun, S.; Shang, M. Bi₂O3 hierarchical nanostructures: Controllable synthesis, growth mechanism, and their application in photocatalysis. Chem. Eur. J. 2009, 15, 1776–1782. [Google Scholar] [CrossRef]
- Ke, J.; Liu, J.; Sun, H.; Zhang, H.; Duan, X.; Liang, P.; Li, X.; Tade, M.O.; Liu, S.; Wang, S. Facile assembly of Bi2O3/Bi2S3/MoS2 n-p heterojunction with layered n-Bi2O3 and p-MoS2 for enhanced photocatalytic water oxidation and pollutant degradation. Appl. Catal. B Environ. 2017, 200, 47–55. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Zeng, G.; Liang, J.; Chen, X.; Yu, H.; Wang, H.; Wu, Z.; Zhang, J.; Xionga, T. In-situ synthesis of direct solid-state dual Z-scheme WO3/g-C3N4/Bi2O3 photocatalyst for the degradation of refractory pollutant. Appl. Catal. B Environ. 2018, 227, 376–385. [Google Scholar] [CrossRef]
- Riente, P.; Foianchini, M.; Llanrs, P.; Pericas, M.A.; Noël, T. Shedding light on the nature of the catalytically active species in photocatalytic reaction using Bi2O3 semiconductor. Nat. Commun. 2021, 12, 625. [Google Scholar] [CrossRef] [PubMed]
- Khairnar, S.D.; Kulkarni, A.N.; Shinde, S.G.; Marathe, S.D.; Marathe, Y.V.; Dhole, S.D.; Shrivastava, V.S. Synthesis and characterization of 2-D La-doped Bi2O3 for photocatalytic degradation of organic dye and pesticide. J. Photochem. Photobiol. 2021, 6, 100030. [Google Scholar] [CrossRef]
- Najafian, H.; Manteghi, F.; Beshkar, F.; Niasari, M.S. Enhanced photocatalytic activity of a novel NiO/Bi2O3/Bi3ClO4 nanocomposite for the degradation of azo dye pollutants under visible light irradiation. Separ. Purif. Technol. 2019, 209, 6–17. [Google Scholar] [CrossRef]
- Muruganandham, M.; Amutha, R.; Lee, G.-J.; Hsieh, S.-H.; Wu, J.J.; Sillanpää, M. Facile fabrication of tunable Bi2O3 self-assembly and its visible light photocatalytic activity. J. Phys. Chem. C 2012, 116, 12906–12915. [Google Scholar] [CrossRef]
- Riente, P.; Matas, A.A.; Albero, J.; Palomares, E.; Pericàs, M.A. Light-driven organocatalysis using inexpensive, nontoxic Bi2O3 as the photocatalyst. Angew. Chem. Int. Ed. 2014, 53, 9613–9616. [Google Scholar] [CrossRef]
- Fadeyi, O.O.; Mousseau, J.J.; Feng, Y.; Allais, C.; Nuhant, P.; Chen, M.Z.; Pierce, B.; Robinson, R. Visible-light-driven photocatalytic initiation of radical thiol-ene reactions using bismuth oxide. Org. Lett. 2015, 17, 5756–5759. [Google Scholar] [CrossRef]
- Hakobyan, K.; Gegenhuber, T.; McErlean, C.S.P.; Müllner, M. Visible-light driven MADIX polymerisation via a reusable, low-cost, and non-toxic bismuth oxide photocatalyst. Angew. Chem. Int. Ed. 2019, 58, 1828–1832. [Google Scholar] [CrossRef]
- Nicewicz, D.A.; MacMillan, D.W.C. Merging photoredox catalysis with organocatalysis: The direct asymmetric alkylation of aldehydes. Science 2008, 322, 77–80. [Google Scholar] [CrossRef]
- Dong, F.; Xiong, T.; Sun, Y.; Zhao, Z.; Zhou, Y.; Feng, X.; Wu, Z. A semimetal bismuth element as a direct plasmonic photocatalyst. Chem. Commun. 2014, 50, 10386–10389. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Z.; Zhang, W.; Gao, C.; Zhang, Y.; Dong, F. Plasmonic Bi metal as cocatalyst and photocatalyst: The case of Bi/(BiO)2CO3 and Bi particles. J. Coll. Interface Sci. 2017, 485, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Boutinaud, P. Revisiting the spectroscopy of the Bi3+ ion in oxide compounds. Inorg. Chem. 2013, 52, 6028–6038. [Google Scholar] [CrossRef] [PubMed]
- Bainglass, E.; Walsh, A.; Huda, M.N. BiSbWO6: Properties of a mixed 5s/6s lone-pair-electron system. Chem. Phys. 2021, 544, 111117. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, G.; Gao, J.; Zhu, R.; Hojamberdiev, M.; Wang, C.; Wei, X.; Liu, P. A novel synergy of Er3+/Fe3+ co-doped porous Bi5O7I microspheres with enhanced photocatalytic activity under visible-light irradiation. Appl. Catal. B Environ. 2017, 205, 421–432. [Google Scholar] [CrossRef]
- Ganeshbabu, M.; Kannan, N.; Sundara Venkatesh, P.; Paulraj, G.; Jeganathan, K.; Mubarak Ali, D. Synthesis and characterization of BiVO4 nanoparticles for environmental applications. RSC Adv. 2020, 10, 18315–18322. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, L.; Li, H.; Mu, Y.; Yang, Z. Synthesis and application of Bi2WO6 for the photocatalytic degradation of two typical fluoroquinolones under visible light irradiation. RSC Adv. 2019, 9, 27768–27779. [Google Scholar] [CrossRef]
- Kumar, R.; Raizada, P.; Parwaz Khan, A.A.; Nguyen, V.-H.; Le, Q.V.; Ghotekar, S.; Selvasembian, R.; Gandhi, V.; Singh, A.; Singh, P. Recent progress in emerging BiPO4-based photocatalysts: Synthesis, properties, modification strategies, and photocatalytic applications. J. Mater. Sci. Technol. 2022, 108, 208–225. [Google Scholar] [CrossRef]
- Zhao, G.; Zheng, Y.; He, Z.; Lu, Z.; Wang, L.; Li, C.; Jiao, F.; Deng, C. Synthesis of Bi2S3 microsphere and its efficient photocatalytic activity under visible-light irradiation. Trans. Nonferrous Met. Soc. China 2018, 28, 2002–2010. [Google Scholar] [CrossRef]
- Arumugam, M.; Choi, M.Y. Recent progress on bismuth oxyiodide (BiOI) photocatalyst for environmental remediation. J. Ind. Eng. Chem. 2020, 81, 237–268. [Google Scholar] [CrossRef]
- Imam, S.S.; Adnan, R.; Mohd Kaus, N.H. The photocatalytic potential of BiOBr for wastewater treatment: A mini-review. J. Environ. Chem. Eng. 2021, 9, 105404. [Google Scholar] [CrossRef]
- Wang, Q.; Hui, J.; Huang, Y.; Ding, Y.; Cai, Y.; Yin, S.; Li, Z.; Su, B. The preparation of BiOCl photocatalyst and its performance of photodegradation on dyes. Mater. Sci. Semicond. Process. 2014, 17, 87–93. [Google Scholar] [CrossRef]
- Ai, L.H.; Zeng, Y.; Jiang, J. Hierarchical porous BiOI architectures: Facile microwave nonaqueous synthesis, characterization and application in the removal of Congo red from aqueous solution. Chem. Eng. J. 2014, 235, 331–339. [Google Scholar] [CrossRef]
- He, R.; Xu, D.; Cheng, B.; Yu, J.; Ho, W. Review on nanoscale Bi-based photocatalysts. Nanoscale Horiz. 2018, 3, 464–504. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Xing, Z.; Zhao, H.; Li, Z.; Zhou. Recent advances in bismuth-based photocatalysts: Environment and energy applications. Green Energy Environ. 2022, in press. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, X.; Li, G.; Cao, Y.; Shao, Y.; Li, D. Highly efficient Bi2O2CO3/BiOCl photocatalyst based on heterojunction with enhanced dye-sensitization under visible light. Appl. Catal. B Environ. 2016, 187, 301–309. [Google Scholar]
- Qiu, F.; Li, W.; Wang, F.; Li, H.; Liu, X.; Ren, C. Preparation of novel p-n heterojunction Bi2O2CO3/BiOBr photocatalysts with enhanced visible light photocatalytic activity. Coll. Surf. A 2017, 517, 25–32. [Google Scholar] [CrossRef]
- Huang, Y.; Fan, W.; Long, B.; Li, H.; Zhao, F.; Liu, Z.; Tong, Y.; Ji, H. Visible light Bi2S3/Bi2O3/Bi2O2CO3 photocatalyst for effective degradation of organic pollutions. Appl. Catal. B Environ. 2016, 185, 68–76. [Google Scholar] [CrossRef]
- Ding, J.; Dai, Z.; Qin, F.; Zhao, H.; Zhao, S.; Chen, R. Z-scheme BiO1-xBr/Bi2O2CO3 photocatalyst with rich oxygen vacancy as electron mediator for highly efficient degradation of antibiotics. Appl. Catal. B Environ. 2017, 205, 281–291. [Google Scholar] [CrossRef]
- Peng, D.Y.; Zeng, H.Y.; Xiong, J.; Liu, F.-Y.; Wang, L.-H.; Xu, S.; Yang, Z.-L.; Liu, S.-G. Tuning oxygen vacancy in Bi2WO6 by heteroatom doping for enhanced photooxidation-reduction properties. J. Colloid Interface Sci. Part B 2023, 629, 133–146. [Google Scholar] [CrossRef]
- Ding, J.; Dai, Z.; Tian, F.; Zhou, B.; Zhao, B.; Zhao, H.P.; Chen, Z.Q.; Liu, Y.L.; Chen, R. Generation of V•BrV’’’BiV••O defect clusters for 1O2 production for molecular oxygen activation in photocatalysis. J. Mater. Chem. A 2017, 5, 23909–23918. [Google Scholar] [CrossRef]
- Zhao, S.; Dai, Z.; Guo, W.; Chen, F.; Liu, Y.; Chen, R. Highly selective oxidation of glycerol over Bi/Bi3.64Mo0.36O6.55 heterostructure: Dual reaction pathways induced by photogenerated 1O2 and holes. Appl. Catal. B Environ. 2019, 244, 206–214. [Google Scholar] [CrossRef]
- Bai, S.; Li, X.Y.; Kong, Q.; Long, R.; Wang, C.M.; Jiang, J.; Xiong, Y.J. Toward enhanced photocatalytic oxygen evolution: Synergetic utilization of Plasmonic effect and Schottky junction via interfacing facet selection. Adv. Mater. 2015, 27, 3444–3452. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hai, X.; Ding, X.; Chang, K.; Xiang, Y.; Meng, X.; Yang, Z.; Chen, H.; Ye, J. Light-switchable oxygen vacancies in ultrafine Bi5O7Br nanotubes for boosting solar-driven nitrogen fixation in pure water. Adv. Mater. 2017, 29, 1701774. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.; Zhao, Q.; Yu, H.; Xia, X.; Li, J.; He, S.; Wei, L.; An, T. A review of bismuth-based photocatalysts for antibiotic degradation: Insight into the photocatalytic degradation performance, pathways and relevant mechanisms. Environ. Res. 2021, 199, 111360. [Google Scholar] [CrossRef]
- Asghar, A.; Raman, A.A.A.; Wan Daud, W.M.A. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: A review. J. Clean. Prod. 2015, 87, 826–838. [Google Scholar] [CrossRef]
- Subhiksha, V.; Kokilavani, S.; Sudheer Khan, S. Recent advances in degradation of organic pollutant in aqueous solutions using bismuth based photocatalysts: A review. Chemosphere 2022, 290, 133228. [Google Scholar] [CrossRef]
- Huang, C.-H.; Wu, T.; Huang, C.-W.; Lai, C.-Y.; Wu, M.-Y.; Lin, Y.-W. Enhanced photocatalytic performance of BiVO4 in aqueous AgNO3 solution under visible light irradiation. Appl. Surf. Sci. 2017, 399, 10–19. [Google Scholar] [CrossRef]
- Wang, S.; Huang, H.; Zhang, Y. A novel layered bismuth-based photocatalytic material LiBi3O4Cl2 with –OH and h+ as the active species for efficient photodegradation applications. Solid State Sci. 2016, 62, 43–49. [Google Scholar] [CrossRef]
- He, S.; Shen, M.; Wu, E.; Yin, R.; Zhu, M.; Zeng, L. Molecular structure on the detoxification of fluorinated liquid crystal monomers with reactive oxidation species in the photocatalytic process. Environ. Sci. Ecothechnol. 2022, 9, 100141. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Hong, Y.; Li, C.; Meng, Y.; Huang, C.; Shi, W. In situ synthesis of a nanoplate-like Bi-based heterojunction for photocatalytic degradation of ciprofloxacin. Mater. Sci. Eng. B 2017, 224, 69–77. [Google Scholar] [CrossRef]
- Shafiq, F.; Tahir, M.B.; Hussain, A.; Sagir, M.; Rehman, J.; Kebaili, I.; Alrobei, H.; Alzaid, M. The construction of a highly efficient p-n heterojunction Bi2O3/BiVO4 for hydrogen evolution through solar water splitting. Int. J. Hydrogen Energy 2022, 47, 4594–4600. [Google Scholar] [CrossRef]
- Stelo, F.; Kublik, N.; Ullah, S.; Wender, H. Recent advances in Bi2MoO6 based Z-scheme heterojunctions for photocatalytic degradation of pollutants. J. Alloys Compd. 2020, 829, 154591. [Google Scholar] [CrossRef]
- Shan, L.; Wang, G.; Liu, L.; Wu, Z. Band alignment and enhanced photocatalytic activation for α-Bi2O3/BiOCl (001) core–shell heterojunction. J. Mol. Catal. A Chem. 2015, 406, 145–151. [Google Scholar] [CrossRef]
- Volnistem, E.A.; Bini, R.D.; Silva, D.M.; Rosso, J.M.; Dias, G.S.; Cótica, L.F.; Santos, I.A. Intensifying the photocatalytic degradation of methylene blue by the formation of BiFeO3/Fe3O4 nanointerfaces. Ceram. Int. 2020, 46, 18768–18777. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Cheng, Z.; Peng, Y.; Shen, M.; Yang, S.; Zhang, Y. Crystal-face-selective Bi4Ti3O12/BiOI heterojunctions constructed for enhanced visible-light-driven photocatalytic activity. Appl. Surf. Sci. 2021, 552, 149507. [Google Scholar] [CrossRef]
- Bera, S.; Ghosh, S.; Maiyalagan, T.; Basu, R.N. Band edge engineering of BiOX/CuFe2O4 heterostructures for efficient water splitting. ACS Appl. Energy Mater. 2022, 5, 3821–3833. [Google Scholar] [CrossRef]
- Christopher, P.; Xin, H.L.; Marimuthu, A.; Linic, S. Singular characteristics and unique chemical bond activation mechanisms of photocatalytic reactions on plasmonic nanostructures. Nat. Mater. 2012, 11, 1044–1050. [Google Scholar] [CrossRef]
- Dong, X.A.; Zhang, W.D.; Sun, Y.J.; Li, J.Y.; Cen, W.L.; Cui, Z.H.; Huang, H.W.; Dong, F. Visible-light-induced charge transfer pathway and photocatalysis mechanism on Bi semimetal@defective BiOBr hierarchical microspheres. J. Catal. 2018, 357, 41–50. [Google Scholar] [CrossRef]
- He, W.J.; Sun, Y.J.; Jiang, G.M.; Li, Y.H.; Zhang, X.M.; Zhang, Y.X.; Zhou, Y.; Dong, F. Defective Bi4MoO9/Bi metal core/shell heterostructure: Enhanced visible light photocatalysis and reaction mechanism. Appl. Catal. B 2018, 239, 619–627. [Google Scholar] [CrossRef]
- Li, J.R.; Zhang, W.D.; Ran, M.X.; Sun, Y.J.; Huang, H.W.; Dong, F. Synergistic integration of Bi metal and phosphate defects on hexagonal and monoclinic BiPO4: Enhanced photocatalysis and reaction mechanism. Appl. Catal. B 2019, 243, 313–321. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, C.; Lv, K.; Lu, Y.; Li, Q.; Wu, X.; Li, Y.; Li, X.; Fan, J.; Li, M. SPR effect of bismuth enhanced visible photoreactivity of Bi2WO6 for NO abatement. Chin. J. Catal. 2019, 40, 755–764. [Google Scholar] [CrossRef]
- Han, Q. Advances in preparation methods of bismuth-based photocatalysts. Chem. Eng. J. 2021, 414, 127877. [Google Scholar] [CrossRef]
- Wu, L.; Li, Z.; Li, Y.; Hu, H.; Liu, Y.; Zhang, Q. Mechanochemical syntheses of bismuth oxybromides BixOyBrz as visible-light responsive photocatalyts for the degradation of bisphenol A. J. Solid State Chem. 2019, 270, 458–462. [Google Scholar] [CrossRef]
- Maleki, H. Characterization and photocatalytic activity of Y-doped BiFeO3 ceramics prepared by solid-state reaction method. Adv. Powder Technol. 2019, 30, 2832–2840. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, K.; Zhang, G.; Yin, S. Facile preparation of BiOX (X = Cl, Br, I) nanoparticles and up-conversion phosphors/BiOBr composites for efficient degradation of NO gas: Oxygen vacancy effect and near infrared light responsive mechanism. Chem. Eng. J. 2017, 325, 59–70. [Google Scholar] [CrossRef]
- Siao, C.-W.; Chen, H.-L.; Chen, L.-W.; Chang, J.-L.; Yeh, T.-W.; Chen, C.-C. Controlled hydrothermal synthesis of bismuth oxychloride/bismuth oxybromide/bismuth oxyiodide composites exhibiting visible-light photocatalytic degradation of 2-hydroxybenzoic acid and crystal violet. J. Colloid Inter. Sci. 2018, 526, 322–336. [Google Scholar] [CrossRef]
- Cai, J.; Huang, J.; Lai, Y. 3D Au-decorated BiMoO6 nanosheet/TiO2 nanotube array heterostructure with enhanced UV and visible-light photocatalytic activity. J. Mater. Chem. A 2017, 5, 16412–16421. [Google Scholar] [CrossRef]
- Lin, H.; Ye, H.; Li, X.; Cao, J.; Chen, S. Facile anion-exchange synthesis of BiOI/BiOBr composite with enhanced photoelectrochemical and photocatalytic properties. Ceram. Int. 2014, 40, 9743–9750. [Google Scholar] [CrossRef]
- Xiao, X.; Hao, R.; Zuo, X.; Nan, J.; Li, L.; Zhang, W. Microwave-assisted synthesis of hierarchical Bi7O9I3 microsheets for efficient photocatalytic degradation of bisphenol-A under visible light irradiation. Chem. Eng. J. 2012, 209, 293–300. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, X.; Fang, J.; Zhu, X.; Chu, J.; Li, B. Microemulsion synthesis, characterization of bismuth oxyiodine/titanium dioxide hybrid nanoparticles with outstanding photocatalytic performance under visible light irradiation. Appl. Surf. Sci. 2012, 258, 3771–3778. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, Y.; Zhaon, J.; Song, Y.; Huang, Z.; Gao, F.; Li, N.; Li, Y. Photoactive β-Bi2O3 architectures prepared by a simple solution crystallization method. Ceram. Int. 2014, 40, 15057–15063. [Google Scholar] [CrossRef]
- Qin, F.; Li, G.; Wang, R.; Wu, J.; Sun, H.; Chen, R. Template-free fabrication of Bi2O3 and (BiO)2CO3 nanotubes and their application in water treatment. Chem. Eur. J. 2012, 18, 16491–16497. [Google Scholar] [CrossRef]
- Hou, J.; Yang, C.; Wang, Z.; Zhou, W.; Jiao, S.; Zhu, H. In situ synthesis of α−β phase heterojunction on Bi2O3 nanowires with exceptional visible-light photocatalytic performance. Appl. Catal. B Environ. 2013, 142–143, 504–511. [Google Scholar] [CrossRef]
- Liang, Y.-C.; Li, T.-H. Controllable morphology of Bi2S3 nanostructures formed via hydrothermal vulcanization of Bi2O3 thin-film layer and their photoelectrocatalytic performances. Nanotechnol. Rev. 2022, 11, 284–297. [Google Scholar] [CrossRef]
- Sood, S.; Umar, A.; Mehta, S.K.; Kansal, S. α−Bi2O3 nanorods: An efficient sunlight active photocatalyst for degradation of Rhodamine B and 2,4,6-trichlorophenol. Ceram. Int. 2015, 41, 3355–3364. [Google Scholar] [CrossRef]
- Tien, L.-C.; Lai, Y.-C. Nucleation control and growth mechanism of pure α-Bi2O3 nanowires. Appl. Surf. Sci. 2014, 290, 131–136. [Google Scholar] [CrossRef]
- Xiao, X.; Hu, R.; Liu, C.; Xing, C.; Qian, C.; Zuo, X.; Nan, J.; Wang, L. Facile large-scale synthesis of β-Bi2O3 nanospheres as a highly efficient photocatalyst for the degradation of acetaminophen under visible light irradiation. Appl. Catal. B Environ. 2013, 140–141, 433–443. [Google Scholar] [CrossRef]
- Verma, M.K.; Kumar, A.; Das, T.; Kumar, V.; Singh, S.; Rai, V.S.; Prajapati, D.; Sonwani, R.K.; Sahoo, K.; Mandal, K.D. BiFeO3 perovskite as an efficient photocatalyst synthesized by soft chemical route. Mater. Technol. 2021, 36, 594–602. [Google Scholar] [CrossRef]
- Soltani, T.; Lee, B.-K. Novel and facile synthesis of Ba-doped BiFeO3 nanoparticles and enhancement of their magnetic and photocatalytic activities for complete degradation of benzene in aqueous solution. J. Hazard. Mater. 2016, 316, 122–133. [Google Scholar] [CrossRef]
- Ponraj, C.; Vinitha, G.; Daniel, J. A review on the visible light active BiFeO3 nanostructures as suitable photocatalyst in the degradation of different textile dyes. Environ. Nanotechnol. Monitor. Manag. 2017, 7, 110–120. [Google Scholar] [CrossRef]
- Skiker, R.; Zouraibi, M.; Saidi, M.; Ziat, K. Facile co-precipitation synthesis of novel Bi12TiO20/BiFeO3 heterostructure series with enhanced photocatalytic activity for removal of methyl orange from water. J. Phys. Chem. Solids 2018, 119, 265–275. [Google Scholar] [CrossRef]
- Duan, Q.; Kong, F.; Han, X.; Jiang, Y.; Liu, T.; Chang, Y.; Zhou, L.; Qin, G.; Zhang, X. Synthesis and characterization of morphology-controllable BiFeO3 particles with efficient photocatalytic activity. Mater. Res. Bull. 2019, 112, 104–108. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Chen, L.; Sun, H.; Zhang, H.; Si, W.; Wang, W.; Wang, L.; Li, J. Enhanced magnetic and photocatalytic properties of BiFeO3 nanotubes with ultrathin wall thickness. Vacuum 2021, 184, 109867. [Google Scholar] [CrossRef]
- Wu, T.; Liu, L.; Pi, M.; Zhang, D.; Chen, S. Enhanced magnetic and photocatalytic properties of Bi2Fe4O9 semiconductor with large exposed (001) surface. Appl. Surf. Sci. 2016, 377, 253–261. [Google Scholar] [CrossRef]
- Wang, G.; Liu, S.; He, T.; Liu, X.; Deng, Q.; Mao, Y.; Wang, S. Enhanced visible-light-driven photocatalytic activities of Bi2Fe4O9/g-C3N4 composite photocatalysts. Mater. Res. Bull. 2018, 104, 104–111. [Google Scholar] [CrossRef]
- Ma, M.; Chen, Y.; Liu, Y.; Jiang, J.; Jiao, Z.; Ma, Y. Highly efficient photocatalytic organic dyes degradation based on 1D magnetic Bi2Fe4O9/C@AgBr composite. Appl. Organomet. Chem. 2022, 36, e6619. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, B.; Lu, J.; Wang, Z.; Xu, B.; Qin, X.; Zhang, X.; Dai, Y. Synergistic effect of crystal and electronic structures on the visible-light-driven photocatalytic performances of Bi2O3 polymorphs. Phys. Chem. Chem. Phys. 2010, 12, 15468–15475. [Google Scholar] [CrossRef]
- Bano, K.; Mittal, S.K.; Singh, P.P.; Kaushal, S. Sunlight driven photocatalytic degradation of organic pollutants using a MnV2O6/BiVO4 heterojunction: Mechanistic perception and degradation pathways. Nanoscale Adv. 2021, 3, 6446–6458. [Google Scholar] [CrossRef]
- Guan, X.; Tian, L.; Zhang, Y.; Shi, J.; Shen, S. Photocatalytic water splitting on BiVO4: Balanced charge-carrier consumption and selective redox reaction. Nano Res. 2022. [Google Scholar] [CrossRef]
- Tian, S.; Bello, I.A.; Peng, J.; Ren, H.; Ma, Q.; Zhu, M.; Li, K.; Wang, H.; Wu, J.; Zhu, G. Fabricating Mn3O4/β-Bi2O3 heterojunction microspheres with enhanced photocatalytic activity for organic pollutants degradation and NO removal. J. Alloys Compd. 2021, 854, 157223. [Google Scholar] [CrossRef]
- He, G.; Xing, C.; Xiao, X.; Hu, R.; Zuo, X.; Nan, J. Facile synthesis of flower-like Bi12O17Cl2/β-Bi2O3 composites with enhanced visible light photocatalytic performance for the degradation of 4-tert-butylphenol. Appl. Catal. B Environ. 2015, 170–171, 1–9. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, W.; Zhang, L.; Zhang, Z. Design and controllable synthesis of α-/γ-Bi2O3 homojunctions have synergistic effects on photocatalytic activity. J. Chem. Eng. 2012, 211–212, 161–167. [Google Scholar] [CrossRef]
- Gadhi, T.A.; Hernández-Gordillo, A.; Bizarro, M.; Jagdale, P.; Tagliaferro, A.; Rodil, S.E. Efficient α/β-Bi2O3 composite for the sequential photodegradation of two-dyes mixture. Ceram. Int. 2016, 42, 13065–13073. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Liu, T.; Jia, Y.; Deng, H.; Wang, W.; Zhang, F. Alkali-mediated dissolution-recrystallization strategy for in situ construction of a BiVO4/Bi25VO40 heterojunction with promoted interfacial charge transfer: Formation mechanism and photocatalytic tetracycline degradation studies. Chem. Eng. J. 2022, 431, 134181. [Google Scholar] [CrossRef]
- Duan, A.; Hou, X.; Yang, M.; Yu, H.; Yang, Y.; Ma, Q.; Dong, X. EDTA-2Na assisted facile synthesis of monoclinic bismuth vanadate (m-BiVO4) and m-BiVO4/rGO as a highly efficient visible-light-driven photocatalyst. Mater. Lett. 2022, 311, 131498. [Google Scholar] [CrossRef]
- El-Hakam, S.A.; Al-Shorifi, F.T.; Salama, R.S.; Gamal, S.; El-Yazeed, W.S.A.; Ibrahim, A.A.; Ahmed, A.I. Application of nanostructured mesoporous silica/ bismuth vanadate composite catalysts for the degradation of methylene blue and brilliant green. J. Mater. Res. Technol. 2022, 18, 1963–1976. [Google Scholar] [CrossRef]
- Wu, W.; Sun, Y.; Zhou, H. In-situ construction of β-Bi2O3/Ag2O photocatalyst from deactivated AgBiO3 for tetracycline degradation under visible light. Chem. Eng. J. 2022, 432, 134316. [Google Scholar] [CrossRef]
- Wu, W.; Xu, C.; Shi, X.; Zhao, J.; An, X.; Ma, H.; Tian, Y.; Zhou, H. Effective degradation of organic pollutants and reaction mechanism with flower-like AgBiO3/g-C3N4 composite. Colloids Surf. A 2021, 599, 124901. [Google Scholar] [CrossRef]
- Bautista-Ruiz, J.; Chaparro, A.; Bautista, W. Characterization of bismuth-silicate soles. J. Phys. Conf. Ser. 2019, 1386, 012020. [Google Scholar] [CrossRef]
- Pirovano, C.; Islam, M.S.; Vannier, R.-N.; Nowogrocki, G.; Mairesse, G. Modelling the crystal structures of Aurivillius phases. Solid State Ion. 2001, 140, 115–123. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J.; Fujiwara, A.; Taniguchi, H.; Kim, S.; Tanaka, H.; Sugimoto, K.; Kato, K.; Itoh, M.; Hosonog, H.; et al. Hierarchical dielectric order in hierarchical ferroelectric Bi2SiO5. IUCrJ 2014, 1, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Kuwabara, A.; Kim, J.; Kim, Y.; Moriwake, H.; Kim, S.; Hoshiyama, T.; Koyama, T.; Mori, S.; Takata, M.; et al. Ferroelectricity driven by twisting of silicate tetrahedral chains. Angew. Chem. Int. Ed. 2013, 52, 8088–8092. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Zhang, G. Synthesis and facet-dependent enhanced photocatalytic activity of Bi2SiO5/AgI nanoplate photocatalysts. J. Mater. Chem. A 2015, 3, 16737–16745. [Google Scholar] [CrossRef]
- Liu, D.; Cai, W.; Wang, Y.; Zhu, Y. Constructing a novel Bi2SiO5/BiPO4 heterostructure with extended light response range and enhanced photocatalytic performance. Appl. Catal. B Environ. 2018, 236, 205–211. [Google Scholar] [CrossRef]
- Zou, C.; Liang, M.; Yang, Z.; Zhou, X.; Yang, Y.; Yang, S. Flower-like Bi2SiO5/Bi4MoO9 heterostructures for enhanced photocatalytic degradation of ciprofloxacin. Nanotechnology 2020, 31, 345604. [Google Scholar] [CrossRef]
- Veber, A.; Kunej, S.; Korosec, R.C.; Suvorov, D. The effects of solvents on the formation of sol–gel-derived Bi12SiO20 thin films. J. Eur. Ceram. Soc. 2010, 30, 2475–2480. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.; Huang, Y.; Ji, M.; Fan, W.; Chen, Z.; Li, H. Constructing carbon quantum dots/Bi2SiO5 ultrathin nanosheets with enhanced photocatalytic activity and mechanism investigation. Chem. Eng. J. 2016, 302, 334–343. [Google Scholar] [CrossRef]
- Yang, C.-T.; Lee, W.W.; Lin, H.-P.; Dai, Y.-M.; Chia, H.-T.; Chen, C.-C. A novel heterojunction photocatalyst, Bi2SiO5/g-C3N4: Synthesis, characterization, photocatalytic activity, and mechanism. RSC Adv. 2016, 6, 40664–40675. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, J.; Li, M.; Yuan, J.; Wu, P.; Chang, X.; Liu, C.; Wang, X. Bismuth silicate photocatalysts with enhanced light harvesting efficiency by photonic crystal. J. Alloys Compd. 2019, 810, 151839. [Google Scholar] [CrossRef]
- Jia, K.-L.; Qu, J.; Hao, S.-M.; An, F.; Jing, Y.-Q.; Yu, Z.-Z. One-pot synthesis of bismuth silicate heterostructures with tunable morphology and excellent visible light photodegradation performances. J. Colloid Interface Sci. 2017, 506, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yao, W.; Wang, J.; Liu, Y.; Zhang, M.; Zhu, Y. Enhanced visible light photocatalytic performance of a novel heterostructured Bi4O5Br2/Bi24O31Br10/Bi2SiO5 photocatalyst. Appl. Catal. B Environ. 2015, 172–173, 100–107. [Google Scholar] [CrossRef]
- Wu, Z.; Shen, J.; Ma, N.; Li, Z.; Wu, M.; Xu, D.; Zhang, S.; Feng, W.; Zhu, Y. Bi4O5Br2 nanosheets with vertical aligned facets for efficient visible-light-driven photodegradation of BPA. Appl. Catal. B Environ. 2021, 286, 119937. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, Y.; Jia, S.; Jiang, D.; Zhan, Q. Enhancing the photocatalytic performance of Bi12SiO20 by in situ grown Bi2O2CO3 and Bi through two-step light irradiation method. Appl. Surf. Sci. 2020, 520, 146355. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, M.; Huang, D.; Zeng, G.; Xu, P.; Zhou, C.; Lai, C.; Wang, H.; Cheng, M.; Wang, W. Multiply structural optimized strategies for bismuth oxyhalide photocatalysis and their environmental application. Chem. Eng. J. 2019, 374, 1025–1045. [Google Scholar] [CrossRef]
- Wu, P.; Feng, L.; Liang, Y.; Zhang, X.; Li, X.; Tian, S.; Hu, H.; Yin, G.; Khan, S. Large-scale synthesis of 2D bismuth-enriched bismuth oxyiodides at low temperatures for high-performance supercapacitor and photocatalytic applications. J. Mater. Sci. Mater. Electron. 2020, 31, 5385–5401. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, C.; Hu, R.; Zuo, X.; Nan, J.; Li, L.; Wang, L. Oxygen-rich bismuth oxyhalides: Generalized one-pot synthesis, band structures and visible-light photocatalytic properties. J. Mater. Chem. 2012, 22, 22840–22843. [Google Scholar] [CrossRef]
- Xiao, X.; Xing, C.; He, G.; Zuo, X.; Nan, J.; Wang, L. Solvothermal synthesis of novel hierarchical Bi4O5I2 nanoflakes with highly visible light photocatalytic performance for the degradation of 4-tert-butylphenol. Appl. Catal. B Environ. 2014, 148–149, 154–163. [Google Scholar] [CrossRef]
- Peng, H.; Wang, L.; Xu, J.; Jiang, S.; Xu, X.; Zhang, Q. Novel Bi4O5I2/Bi4O5I2 hybrids: Synthesis, characterization, performance test. Mater. Lett. 2019, 256, 126694. [Google Scholar] [CrossRef]
- Ding, C.; Ye, L.; Zhao, Q.; Zhong, Z.; Liu, K.; Xie, H.; Bao, K.; Zhang, X.; Huang, Z. Synthesis of BixOyIz from molecular precursor and selective photoreduction of CO2 into CO. J. CO2 Utiliz. 2016, 14, 135–142. [Google Scholar] [CrossRef]
- Yin, R.; Li, Y.; Zhong, K.; Yao, H.; Zhang, Y.; Lai, K. Multifunctional property exploration: Bi4O5I2 with visible light photocatalytic performance and a large non-linear optical effect. RSC Adv. 2019, 9, 4539–4544. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, H.; Bagwasi, S.; Shen, X.; Zhang, J. Photocatalytic study of BiOCl for degradation of organic pollutants under UV irradiation. J. Photochem. Photobio. A 2010, 215, 76–80. [Google Scholar] [CrossRef]
- Zhang, L.; Yuhan, L.; Li, Q.; Fan, J.; Carabineiro, S.A.C.; Lv, K. Recent advances on bismuth-based photocatalysts: Strategies and mechanisms. Chem. Eng. J 2021, 419, 129484. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L. Photocatalytic performance of different exposed crystal facets of BiOCl. Cur. Opin. Green Sust. Chem. 2017, 6, 48–56. [Google Scholar] [CrossRef]
- Liu, W.-W.; Peng, R.-F. Recent advances of bismuth oxychloride photocatalytic material: Property, preparation and performance enhancement. J. Electron. Sci. Technol. 2020, 18, 100020. [Google Scholar] [CrossRef]
- Li, F.; Wang, Q.; Wang, X.; Li, B.; Hao, Y.; Liu, R.; Zhao, D. In-situ one-step synthesis of novel BiOCl/Bi24O31Cl10 heterojunctions via self-combustion of ionic liquid with enhanced visible-light photocatalytic activities. Appl. Catal. B Environ. 2014, 150–151, 574–584. [Google Scholar] [CrossRef]
- Bai, Y.; Chen, T.; Wang, P.; Wang, L.; Ye, L. Bismuth-rich Bi4O5X2 (X=Br, I) nanosheets with dominant {1,0,1} facets exposure for photocatalytic H2 evolution. Chem. Eng. J. 2016, 304, 454–460. [Google Scholar] [CrossRef]
- Jiang, J.; Zhao, K.; Xiao, X.; Zhang, L. Synthesis and facet-dependent photoreactivity of BiOCl single-crystalline nanosheets. J. Am. Chem. Soc. 2012, 134, 4473–4476. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, X.; Dong, Y.; Xia, Y.; Wang, H. A special synthesis of BiOCl photocatalyst for efficient pollutants removal: New insight into the band structure regulation and molecular oxygen activation. Appl. Catal. B Environ. 2019, 256, 117872. [Google Scholar] [CrossRef]
- Dong, X.; Cui, W.; Wang, H.; Li, J.; Sun, Y.; Wang, H.; Zhang, Y.; Huang, H.; Dong, F. Promoting ring-opening efficiency for suppressing toxic intermediates during photocatalytic toluene degradation via surface oxygen vacancies. Sci. Bull. 2019, 64, 669–678. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zhang, Y.J.; Wang, W.K.; Pei, D.N.; Huang, G.X.; Chen, J.J.; Zhang, X.; Yu, H.Q. Enhanced photocatalytic degradation of bisphenol A by Co-doped BiOCl nanosheets under visible light irradiation. Appl. Catal. B Environ. 2018, 221, 320–328. [Google Scholar] [CrossRef]
- Jia, Z.; Li, T.; Zheng, Z.; Zhang, J.; Liu, J.; Li, R.; Wang, Y.; Zhang, X.; Wang, Y.; Fan, C. The BiOCl/diatomite composites for rapid photocatalytic degradation of ciprofloxacin: Efficiency, toxicity evaluation, mechanisms and pathways. Chem. Eng. J. 2020, 380, 122422. [Google Scholar] [CrossRef]
- Alansi, A.M.; Qahtan, T.F.; Al-Abass, N.; Al-Qunaibit, M.; Saleh, T.A. In-situ sunlight-driven tuning of photo-induced electron-hole generation and separation rates in bismuth oxychlorobromide for highly efficient water decontamination under visible light irradiation. J. Colloid Interface Sci. 2022, 614, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Di, J.; Chen, C.; Zhu, C.; Song, P.; Xiong, J.; Ji, M.; Zhou, J.; Fu, Q.; Xu, M.; Hao, W.; et al. Bismuth vacancy-tuned bismuth oxybromide ultrathin nanosheets toward photocatalytic CO2 reduction. ACS Appl. Mater. Interfaces 2019, 11, 30786–30792. [Google Scholar] [CrossRef]
- Sun, Y.F.; Wu, J.; Li, X.D.; Shi, W.; Ling, P.Q.; Jiao, X.C.; Gao, S.; Liang, L.; Xu, J.Q.; Yan, W.S.; et al. Efficient visible-light-driven CO2 Reduction realized by defect-mediated BiOBr atomic layers. Angew. Chem. Int. Ed. 2018, 57, 8719–8723. [Google Scholar]
- Wang, Q.; Liu, Z.; Liu, D.; Wang, W.; Zhao, Z.; Cui, F.; Li, G. Oxygen vacancy-rich ultrathin sulfur-doped bismuth oxybromide nanosheet as a highly efficient visible-light responsive photocatalyst for environmental remediation. Chem. Eng. J. 2019, 360, 838–847. [Google Scholar] [CrossRef]
- Yang, J.; Liang, Y.J.; Li, K.; Zhu, Y.L.; Liu, S.Q.; Xu, R.; Zhou, W. Design of 3D flowerlike BiOClxBr 1–x nanostructure with high surface area for visible light photocatalytic activities. J. Alloys Compd. 2017, 725, 1144–1157. [Google Scholar] [CrossRef]
- Liu, G.; Wang, T.; Ouyang, S.; Liu, L.; Jiang, H.; Yu, Q.; Kako, T.; Ye, J. Band-structure-controlled BiO(ClBr)(1–x)/2Ix solid solutions for visible-light photocatalysis. J. Mater. Chem. A 2015, 3, 8123–8132. [Google Scholar] [CrossRef]
- Dong, X.-D.; Zhang, Y.M.; Zhao, Z.Y. Role of the polar electric field in bismuth oxyhalides for photocatalytic water splitting. Inorg. Chem. 2021, 60, 8461–8474. [Google Scholar] [CrossRef]
- Wang, W.; Huang, B.; Ma, X.; Wang, Z.; Qin, X.; Zhang, X.; Dai, Y.; Whangbo, M.H. Efficient separation of photogenerated electron-hole pairs by the combination of a heterolayered structure and internal polar field in pyroelectric BiOIO3 nanoplates. Chem.-Eur. J. 2013, 19, 14777–14780. [Google Scholar] [CrossRef]
- Dong, X.-D.; Yao, G.-Y.; Liu, Q.-L.; Zhao, Q.-M.; Zhao, Z.-Y. Spontaneous polarization effect and photocatalytic activity of layered compound of BiOIO3. Inorg. Chem. 2019, 58, 15344–15353. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ma, Z.; Ye, L.; Ma, T.; Zhang, T.; Zhang, Y.; Huang, H. Macroscopic spontaneous polarization and surface oxygen vacancies collaboratively boosting CO2 photoreduction on BiOIO3 single crystals. Adv. Mater. 2020, 32, 1908350. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Tu, S.; Zeng, C.; Zhang, T.; Reshak, A.H.; Zhang, Y. Macroscopic Polarization Enhancement Promoting Photo- and Piezoelectric-Induced Charge Separation and Molecular Oxygen Activation. Angew. Chem. Int. Ed. 2017, 56, 11860–11864. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shi, Q.; Cheng, X.; Yang, J.; Liu, Z.; Chen, T.; Qu, Y.; Wang, J.; Xie, M.; Han, W. Accelerated generation of hydroxyl radical through surface polarization on BiVO4 microtubes for efficient chlortetracycline degradation. Chem. Eng. J. 2020, 400, 125871. [Google Scholar] [CrossRef]
- Cai, G.; Xu, L.; Wei, B.; Che, J.; Gao, H.; Sun, W. Facile synthesis of β-Bi2O3/Bi2O2CO3 nanocomposite with high visible-light photocatalytic activity. Mater. Lett. 2014, 120, 1–4. [Google Scholar] [CrossRef]
- Huang, H.; Xiao, K.; He, Y.; Zhang, T.; Dong, F.; Du, X.; Zhang, Y. In situ assembly of BiOI@Bi12O17Cl2 p-n junction: Charge induced unique front-lateral surfaces coupling heterostructure with high exposure of BiOI {001} active facets for robust and non selective photocatalysis. Appl. Catal. B Environ. 2016, 199, 75–86. [Google Scholar] [CrossRef]
- Yin, H.Y.; Zheng, Y.F.; Song, X.C. Synthesis and enhanced visible light photocatalytic CO2 reduction of BiPO4–BiOBrxI1−x p–n heterojunctions with adjustable energy band. RSC Adv. 2019, 9, 11005–11012. [Google Scholar] [CrossRef]
- Tang, J.; Xue, Y.; Ma, C.; Zhang, S.; Li, Q. Facile preparation of BiOI/T-ZnOw p–n heterojunction photocatalysts with enhanced removal efficiency for rhodamine B and oxytetracycline. New J. Chem. 2022, 46, 13010–13020. [Google Scholar] [CrossRef]
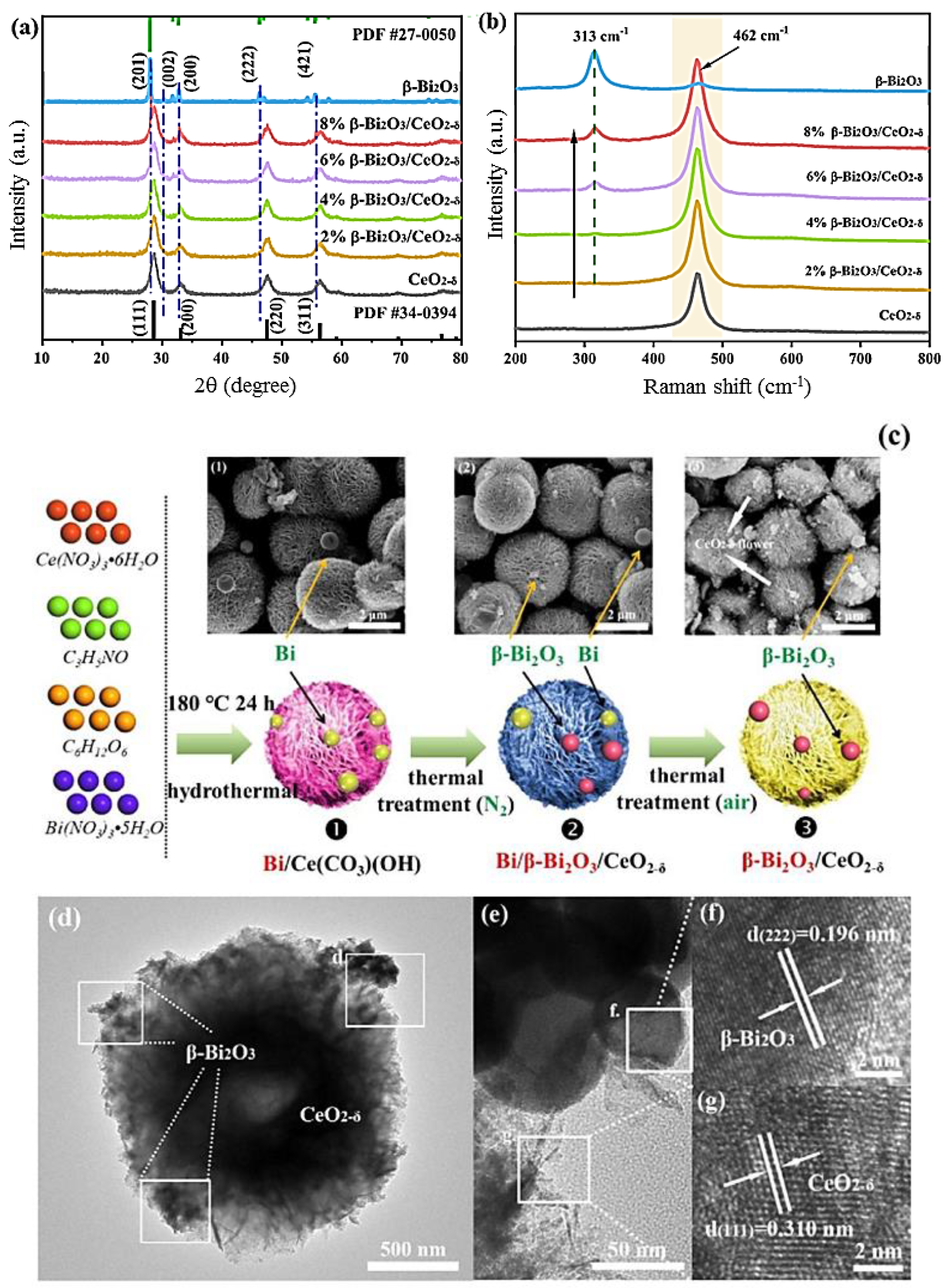
- Nie, J.; Zhu, G.; Zhang, W.; Gao, J.; Zhong, P.; Xie, X.; Huang, Y.; Hojamberdiev, M. Oxygen vacancy defects-boosted deep oxidation of NO by β-Bi2O3/CeO2-δ p-n heterojunction photocatalyst in situ synthesized from Bi/Ce(CO3) (OH) precursor. Chem. Eng. J. 2021, 424, 130327. [Google Scholar] [CrossRef]
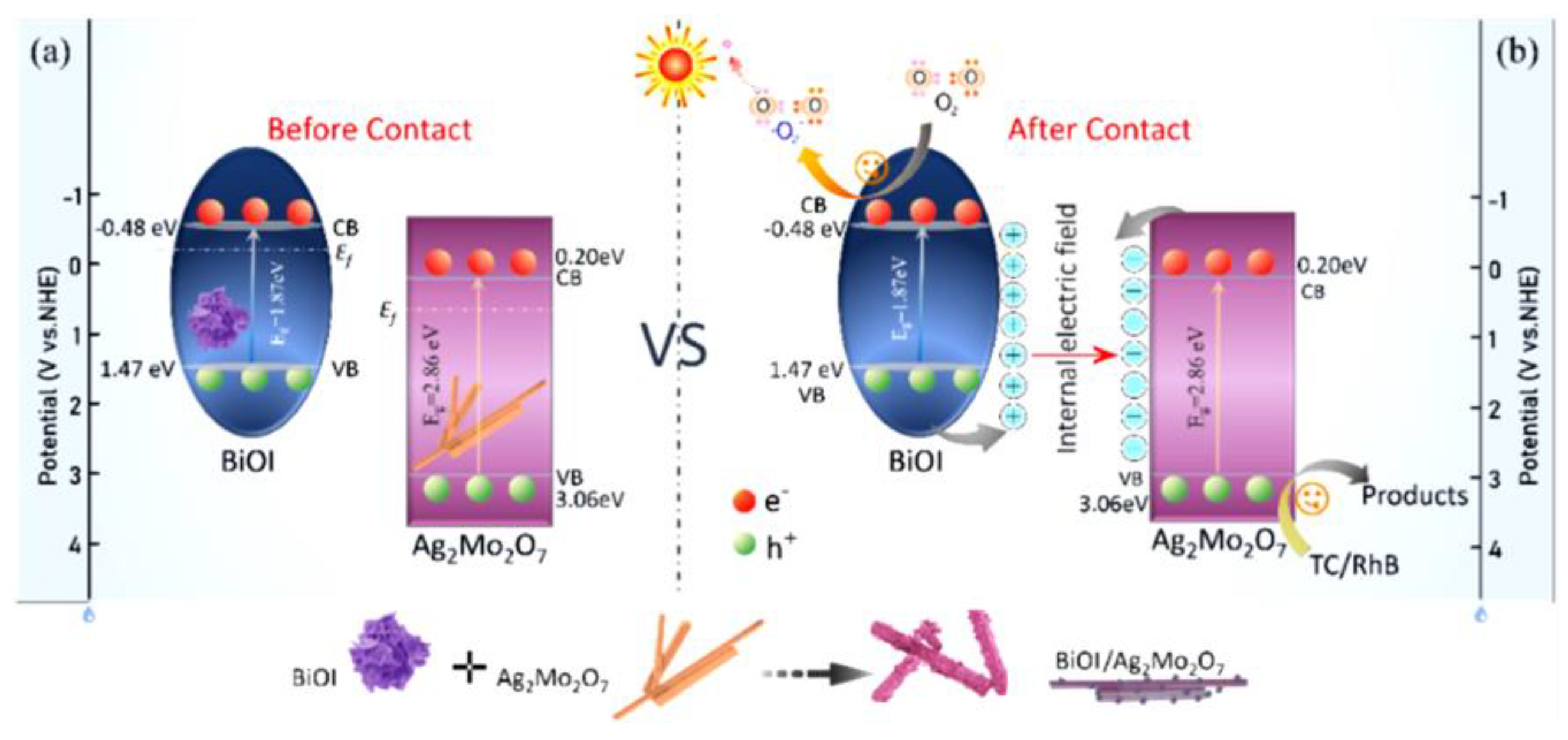
- Su, X.; Fan, D.; Sun, H.; Yang, J.; Yu, Z.; Zhang, D.; Pu, X.; Li, H.; Cai, P. One-dimensional rod-shaped Ag2Mo2O7/BiOI n-n junctions for efficient photodegradation of tetracycline and rhodamine B under visible light. J. Alloys Compd. 2022, 912, 165184. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Xia, Y.; Gao, L. AgBr/BiOI/g-C3N4 Photocatalyst with enhanced photocatalytic activity under visible-light irradiation via the formation of double Z-type heterojunction with the synergistic effect of metal Ag. Ind. Eng. Chem. Res. 2022, 61, 12918–12930. [Google Scholar] [CrossRef]
- Sahu, R.S.; Shih, Y.; Chen, W. New insights of metal free 2D graphitic carbon nitride for photocatalytic degradation of bisphenol A. J. Hazard. Mater. 2021, 402, 123509. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; He, M.; Xie, M.; Song, Y.; Wei, W.; Xu, Y.; Xu, H.; Li, H. Realizing the synergistic effect of electronic modulation over graphitic carbon nitride for highly efficient photodegradation of bisphenol A and 2-mercaptobenzothiazole: Mechanism, degradation pathway and density functional theory calculation. J. Colloid Interface Sci. 2021, 583, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Hu, Y. Effectively enhanced photodegradation of bisphenol A by in-situ g-C3N4-Zn/Bi2WO6 heterojunctions and mechanism study. Chemosphere 2020, 246, 125782. [Google Scholar] [CrossRef]
- Du, F.; Lai, Z.; Tang, H.; Wang, H.; Zhao, C. Construction of dual Z-scheme Bi2WO6/g-C3N4/black phosphorus quantum dots composites for effective bisphenol A degradation. J. Environ. Sci. 2023, 124, 617–629. [Google Scholar] [CrossRef]
- Deng, F.; Zhang, Q.; Yang, L.X.; Luo, X.B.; Wang, A.J.; Luo, S.L.; Dionysiou, D.D. Visible-light-responsive graphene-functionalized Bi-bridge Z-scheme black BiOCl/Bi2O3 heterojunction with oxygen vacancy and multiple charge transfer channels for efficient photocatalytic degradation of 2-nitrophenol and industrial wastewater treatment. Appl. Catal. B Environ. 2018, 238, 61–69. [Google Scholar] [CrossRef]
- Liu, J.; Shu, S.; Li, Y.; Liu, J.; Yao, J.; Liu, S.; Zhu, M.; Huang, L.; Huang, L. Ternary hybrid Ag/SnO2-X/Bi4O5I2 photocatalysts: Impressive efficiency for photocatalytic degradation of antibiotics and inactivation of bacteria. Appl. Surf. Sci. 2022, 606, 154610. [Google Scholar] [CrossRef]
- Zhang, Q.; Ravindra, X.H.; Zhang, L.; Zeng, K.; Xu, Y.; Xin, C. Microwave hydrothermal synthesis of a Bi2SiO5/Bi12SiO20 heterojunction with oxygen vacancies and multiple charge transfer for enhanced photocatalytic activity. Appl. Surf. Sci. 2022, 581, 152337. [Google Scholar] [CrossRef]
- Weber, M.; Rodriguez, R.D.; Zahn, D.R.T.; Mehring, M. γ-Bi2O3—Presence or absence? Comparison of sillimanite γ-Bi2O3 and isocrystalline Bi12SiO20. Inorg. Chem. 2018, 57, 8540–8549. [Google Scholar] [CrossRef]
- Guo, J.H.; Shi, L.; Zhao, J.Y.; Wang, Y.; Tang, K.B.; Zhang, W.Q.; Xie, C.Z.; Yuan, X.Y. Enhanced visible-light photocatalytic activity of Bi2MoO6 nanoplates with heterogeneous Bi2MoO6-x@Bi2MoO6 core-shell structure. Appl. Catal. B Environ. 2018, 224, 692–704. [Google Scholar] [CrossRef]
- Yuan, S.; Zhao, Y.; Chen, W.; Wu, C.; Wang, X.; Zhang, L.; Wang, Q. Self-assembled 3D hierarchical porous Bi2MoO6 microspheres toward high capacity and ultra-long-life anode material for Li-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 21781–21790. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.J.; Hao, Q.; Chen, T.; Zhang, L.H.; Chen, D.M.; Ma, C.; Yao, W.Q.; Zhu, Y.F. A high-performance Bi2O3 /Bi2SiO5 p-n heterojunction photocatalyst induced by phase transition of Bi2O3. Appl. Catal. B Environ. 2018, 237, 59–67. [Google Scholar] [CrossRef]
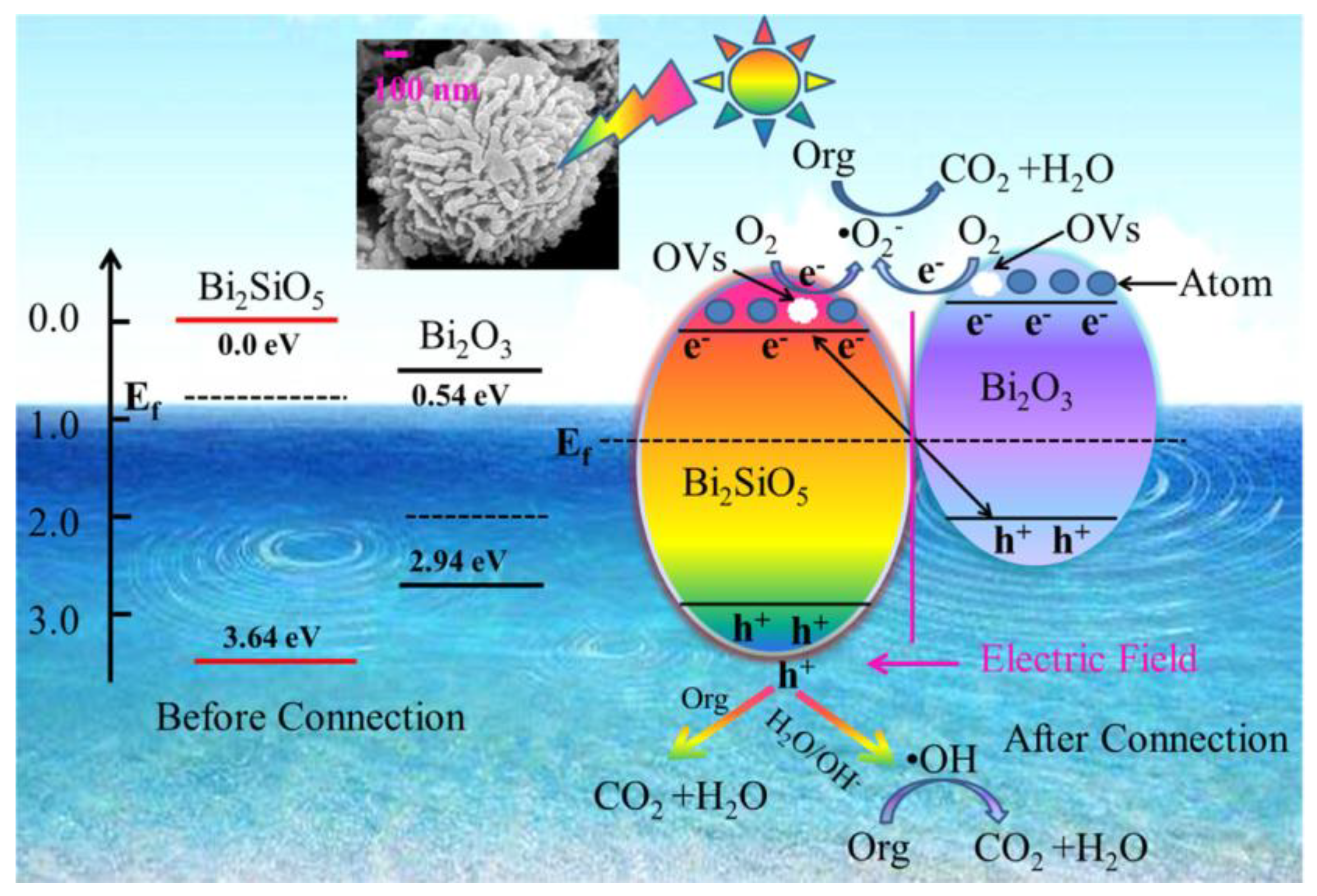
- Dou, L.; Jin, X.; Chen, J.; Zhong, J.; Li, J.; Zeng, Y.; Duan, R. One-pot solvothermal fabrication of S-scheme OVs-Bi2O3/Bi2SiO5 microsphere heterojunctions with enhanced photocatalytic performance toward decontamination of organic pollutants. Appl. Surf. Sci. 2020, 527, 146775. [Google Scholar] [CrossRef]
- He, R.; Liu, H.; Liu, H.; Xu, D.; Zhang, L. S-scheme photocatalyst Bi2O3/TiO2 nanofiber with improved photocatalytic performance. J. Mater. Sci. Technol. 2020, 52, 145–151. [Google Scholar]
- Xu, Y.F.; Zhou, Y.; Deng, Y.H.; Xiang, Y.; Tan, Y.W.; Tang, H.Q.; Zou, H. Synthesis of Bi2WO6@NH2-MIL-125(Ti): A S-scheme photocatalyst with enhanced visible light catalytic activity. Green Energy Environ. 2020, 5, 203–213. [Google Scholar] [CrossRef]
- Li, X.B.; Xiong, J.; Gao, X.M.; Ma, J.; Chen, Z.; Kang, B.B.; Liu, J.Y.; Li, H.; Feng, Z.J.; Huang, J.T. Novel BP/BiOBr S-scheme nano-heterojunction for enhanced visible-light photocatalytic tetracycline removal and oxygen evolution activity. J. Hazard. Mater. 2020, 387, 121690. [Google Scholar] [CrossRef]
- Xie, Q.; He, W.; Liu, S.; Li, C.; Zhang, J.; Wong, P.K. Bifunctional S-scheme g-C3N4/Bi/BiVO4 hybrid photocatalysts toward artificial carbon cycling. Chin. J. Catal. 2020, 41, 140–153. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, W.; Sun, Y.; Yu, J.; Zhang, Y.; Wang, H.; Dong, F.; Bi, Z.W. Cocatalyst/Bi2MoO6 microspheres nanohybrid with SPR-Promoted visible-light photocatalysis. J. Phys. Chem. C 2016, 120, 11889–11898. [Google Scholar] [CrossRef]
- Weng, S.; Chen, B.; Xie, L.; Zheng, Z.; Liu, P. Facile in situ synthesis of a Bi/BiOCl nanocomposite with high photocatalytic activity. J. Mater. Chem. A 2013, 1, 3068. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.D.; Li, W.D.; Li, J.Y.; Cen, W.L.; Li, Q.Y.; Dong, F. Highly enhanced visible light photocatalysis and in situ FT-IR studies on Bi metal@defective BiOCl hierarchical microspheres. Appl. Catal. B Environ. 2018, 225, 218–227. [Google Scholar] [CrossRef]
- Jiang, G.M.; Li, X.W.; Lan, M.N.; Shen, T.; Lv, X.S.; Dong, F.; Zhang, S. Monodisperse bismuth nanoparticles decorated graphitic carbon nitride: Enhanced visible-light-response photocatalytic NO removal and reaction pathway. Appl. Catal. B Environ. 2017, 205, 532–540. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Y.; Sun, H.; Shi, W.; Guo, F. Fabrication of Bi4Ti3O12/ZnIn2S4 S-scheme heterojunction for achieving efficient photocatalytic hydrogen production. J. Alloys Compd. 2023, 930, 167450. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Luo, L.; Shi, Y.; Wang, S.; Li, L.; Long, Y.; Jiang, F. A novel templated synthesis of C/N-doped β-Bi2O3 nanosheets for synergistic rapid removal of 17α-ethynylestradiol by adsorption and photocatalytic degradation. Ceram. Int. 2018, 44, 2178–2185. [Google Scholar] [CrossRef]
- Shahid, M.; Bashir, S.; Afzal, A.; Ibn Shamsah, S.M.; Jamil, A. Synergistic impacts of composite formation and doping techniques to boost the photocatalytic aptitude of the BiFeO3 nanostructure. Ceram. Int. 2022, 48, 2566–2576. [Google Scholar] [CrossRef]
- Sharmin, F.; Basith, M.A. Simple low temperature technique to synthesize sillenite bismuth ferrite with promising photocatalytic performance. ACS Omega 2022, 7, 34901–34911. [Google Scholar] [CrossRef]
- Tien, L.-C.; Lin, Y.-L.; Chen, S.-Y. Synthesis and characterization of Bi12O17Cl2 nanowires obtained by chlorination of α-Bi2O3 nanowires. Mater. Lett. 2013, 113, 30–33. [Google Scholar] [CrossRef]
- Prabhakar Vattikuti, S.V.; Bach, L.X.; Devarayapalli, K.C.; Reddy, A.N.R.; Shim, J.; Julien, C.M. Morphology-dependent photocatalytic and photoelectrochemical performance of bismuth oxybromide crystals applied to malachite green dye degradation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130267. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, B.T.; Xiang, D.; Zhu, Y. Effect of solvents on morphology and photocatalytic activity of BiOBr synthesized by solvothermal method. Mater. Res. Bull. 2012, 47, 3753–3757. [Google Scholar] [CrossRef]
- Patnam, H.; Bharat, L.K.; Hussain, S.K.; Yu, J.S. Effect of solvents on the morphology and optical properties of rare-earth ions doped BiOBr 3D flower-like microparticles via solvothermal method. J. Alloys Compd. 2018, 763, 478–485. [Google Scholar] [CrossRef]
- Liu, Z.; Lv, F.; Xiao, Y.; Chen, B.; Qiu, J.; Guo, W.; Wen, Y.; Li, Z.; Liu, Z. Morphology controllable synthesis of BiOBr architectures and their visible light photocatalytic activities. Mater. Technol. 2019, 34, 683–688. [Google Scholar] [CrossRef]
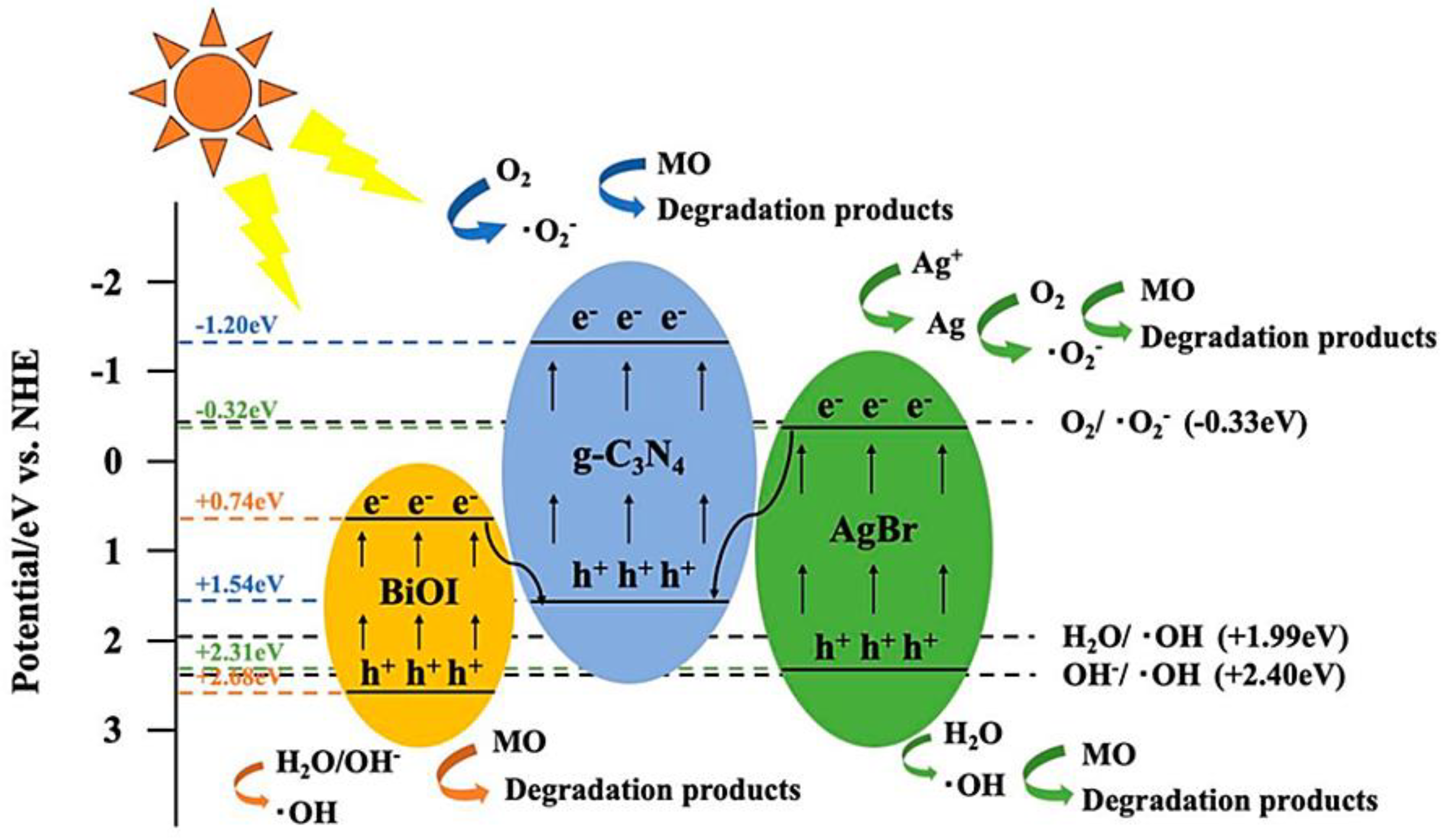
- Shi, Z.; Zhang, Y.; Shen, X.F.; Duoerkun, G.; Zhu, B.; Zhang, L.S.; Li, M.Q.; Chen, Z.G. Fabrication of g-C3N4/BiOBr heterojunctions on carbon fibers as weaveable photocatalyst for degrading tetracycline hydrochloride under visible light. Chem. Eng. J. 2020, 386, 124010. [Google Scholar] [CrossRef]
- Peng, P.; Chen, Z.; Li, X.M.; Wu, Y.; Xia, Y.T.; Duan, A.B.; Wang, D.B.; Yang, Q. Biomass-derived carbon quantum dots modified Bi2MoO6/Bi2S3 heterojunction for efficient photocatalytic removal of organic pollutants and Cr (VI). Sep. Purif. Technol. 2022, 291, 120901. [Google Scholar] [CrossRef]
- Bi, H.F.; Liu, J.S.; Wu, Z.Y.; Zhu, K.J.; Suo, H.; Lv, X.L.; Fu, Y.L.; Jian, R.; Sun, Z.B. Construction of Bi2WO6/ZnIn2S4 with Z-scheme structure for efficient photocatalytic performance. Chem. Phys. Lett. 2021, 769, 138449. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, Y.; Lv, K.; Wu, X.; Li, Q.; Luo, J. Fabrication of walnut-like BiVO4@Bi2S3 heterojunction for efficient visible photocatalytic reduction of Cr(VI). Mater. Sci. Semicond. Process. 2018, 75, 334–341. [Google Scholar] [CrossRef]
- Madhusudan, P.; Ran, J.; Zhang, J.; Yu, J.; Liu, G. Novel urea assisted hydrothermal synthesis of hierarchical BiVO4/Bi2O2CO3 nanocomposites with enhanced visible-light photocatalytic activity. Appl. Catal. B Environ. 2011, 110, 286–295. [Google Scholar] [CrossRef]
- Wang, G.; Cheng, D.; He, T.; Hu, Y.; Deng, Q.; Mao, Y.; Wang, S. Enhanced visible- light responsive photocatalytic activity of Bi25FeO40/Bi2Fe4O9 composites and mechanism investigation. J. Mater. Sci. Mater. Electron. 2019, 30, 10923–10933. [Google Scholar] [CrossRef]
- Ren, X.; Wu, K.; Qin, Z.; Zhao, X.; Yang, H. The Construction of type II heterojunction of Bi2WO6/BiOBr photocatalyst with improved photocatalytic performance. J. Alloys Compd. 2019, 788, 102–109. [Google Scholar] [CrossRef]
- Guo, S.; Luo, H.; Li, Y.; Chen, J.; Mou, B.; Shi, X.; Sun, G. Structure-controlled three-dimensional BiOI/MoS2 microspheres for boosting visible-light photocatalytic degradation of tetracycline. J. Alloys Compd. 2021, 852, 157026. [Google Scholar] [CrossRef]
- Sun, Q.; Hong, Y.; Liu, Q.; Dong, L. Synergistic operation of photocatalytic degradation and Fenton process by magnetic Fe3O4 loaded TiO2. Appl. Surf. Sci. 2018, 430, 399–406. [Google Scholar] [CrossRef]
- Jiao, S.; Zhao, Y.; Li, C.; Wang, B.; Qu, Y. Recyclable adsorbent of BiFeO3/Carbon for purifying industrial dye wastewater via photocatalytic reproducible. Green Energy Environ. 2019, 4, 66–74. [Google Scholar] [CrossRef]
- Subramanian, Y.; Ramasamy, V.; Karthikeyan, R.J.; Srinivasan, G.R.; Arulmozhi, D.; Gubendiran, R.K.; Sriramalu, M. Investigations on the enhanced dye degradation activity of heterogeneous BiFeO3–GdFeO3 nanocomposite photocatalyst. Heliyon 2019, 5, e01831. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Li, Y.; Zou, J.; Xiong, X.; Zeng, X.; Zhou, J. Photocatalytic performance of a novel MOF/BiFeO3 composite. Materials 2017, 10, 1161. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, Y.; Yang, Y.; Tang, J.; Yang, Y. Carbon spheres supported visible light-driven CuO-BiVO4 heterojunction: Preparation, characterization, and photocatalytic properties. Appl. Catal. B Environ. 2012, 115–116, 90–99. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.-H.; Song, P.-P.; Xu, R.; Wang, H. Facile synthesis of Bi2MoO6/ZnSnO3 heterojunction with enhanced visible light photocatalytic degradation of methylene blue. Appl. Surf. Sci. 2018, 430, 561–570. [Google Scholar] [CrossRef]
- Wang, Q.; Jiao, D.; Lian, J.; Ma, Q.; Yu, J.; Huang, H.; Zhong, J.; Li, J. Preparation of efficient visible-light-driven BiOBr/Bi2O3 heterojunction composite with enhanced photocatalytic activities. J. Alloys Compd. 2015, 649, 474–482. [Google Scholar] [CrossRef]
- Chaiwichian, S.; Wetchakun, K.; Kangwansupamonkon, W.; Wetchakun, N. Novel visible-light-driven BiFeO3-Bi2WO6 nanocomposites toward degradation of dyes. J. Photochem. Photobiol. A Chem. 2017, 349, 183–192. [Google Scholar] [CrossRef]
- Shtarev, D.S.; Serpone, N. A new generation of visible-light-active photocatalysts—The alkaline earth metal bismuthates: Syntheses, compositions, structures, and properties. J. Photochem. Photobiol. C Photochem. Rev. 2022, 50, 100501. [Google Scholar] [CrossRef]
- Shtarev, S.; Kevorkyants, R.; Molokeev, M.S.; Shtareva, A.V. The effect of composition on optical and photocatalytic properties of visible light response materials Bi26-xMgxO40. Inorg. Chem. 2020, 59, 8173–8183. [Google Scholar] [CrossRef]
- Li, W.; Kong, D.; Cui, X.; Du, D.; Yan, T.; You, J. Hydrothermal synthesis of Ca3Bi8O15 rods and their visible light photocatalytic properties. Mater. Res. Bull. 2014, 51, 69–73. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Qu, J. Preparation and photocatalytic degradation of malachite green by photocatalyst SrBi4O7 under visible light irradiation. Appl. Mech. Mater. 2014, 522–524, 411–415. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prabhakar Vattikuti, S.V.; Zeng, J.; Ramaraghavulu, R.; Shim, J.; Mauger, A.; Julien, C.M. High-Throughput Strategies for the Design, Discovery, and Analysis of Bismuth-Based Photocatalysts. Int. J. Mol. Sci. 2023, 24, 663. https://doi.org/10.3390/ijms24010663
Prabhakar Vattikuti SV, Zeng J, Ramaraghavulu R, Shim J, Mauger A, Julien CM. High-Throughput Strategies for the Design, Discovery, and Analysis of Bismuth-Based Photocatalysts. International Journal of Molecular Sciences. 2023; 24(1):663. https://doi.org/10.3390/ijms24010663
Chicago/Turabian StylePrabhakar Vattikuti, Surya V., Jie Zeng, Rajavaram Ramaraghavulu, Jaesool Shim, Alain Mauger, and Christian M. Julien. 2023. "High-Throughput Strategies for the Design, Discovery, and Analysis of Bismuth-Based Photocatalysts" International Journal of Molecular Sciences 24, no. 1: 663. https://doi.org/10.3390/ijms24010663
APA StylePrabhakar Vattikuti, S. V., Zeng, J., Ramaraghavulu, R., Shim, J., Mauger, A., & Julien, C. M. (2023). High-Throughput Strategies for the Design, Discovery, and Analysis of Bismuth-Based Photocatalysts. International Journal of Molecular Sciences, 24(1), 663. https://doi.org/10.3390/ijms24010663









