Integration of Electrical Signals and Phytohormones in the Control of Systemic Response
Abstract
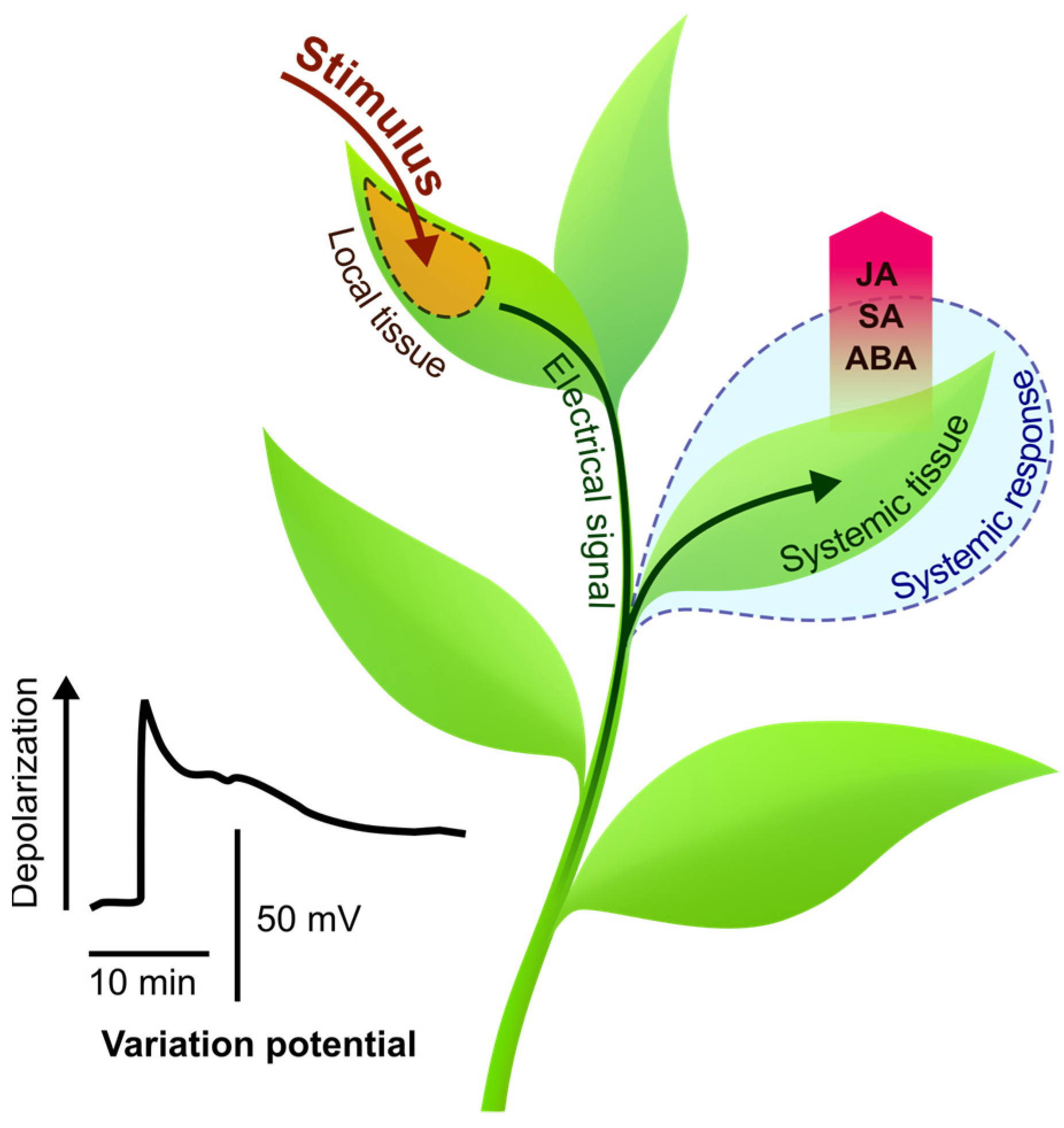
:1. Introduction
2. Electrical Signals Are Involved in the Regulation of Stimulus-Induced Changes in Phytohormone Levels
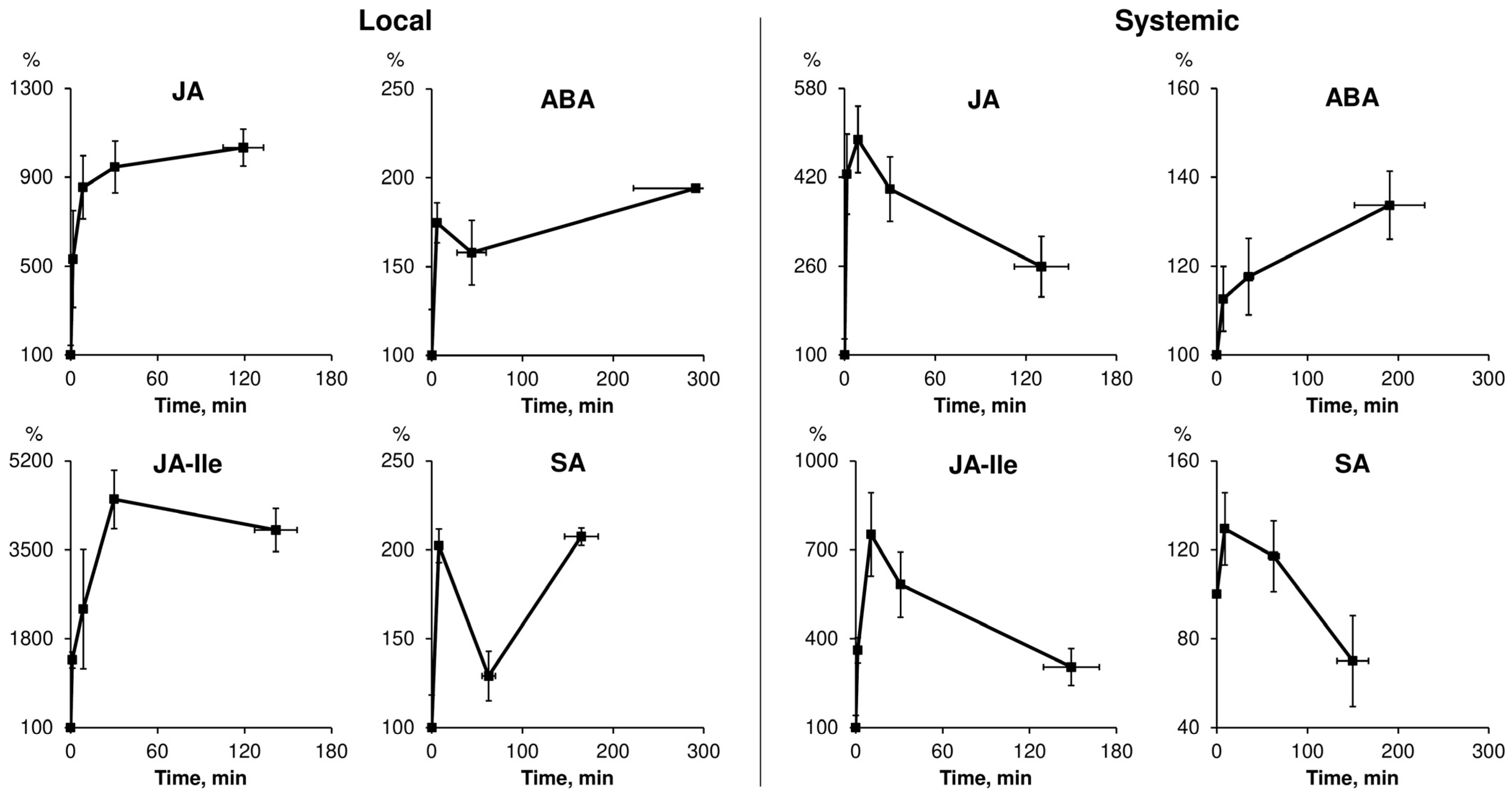
2.1. Spatiotemporal Dynamics of Phytohormones in Response to Local Stimuli
2.1.1. Specificity of Phytohormone Dynamics Induced by Local Stimuli of Different Nature
| JA | ABA | SA |
| Herbivore attack | Drought | Pathogen attack |
| Pathogen attack | Heat stress | Light stress |
| Mechanical wounding | Cold stress | Heat stress |
| Light stress | Light stress | Herbivore attack |
| Heat stress | Mechanical wounding | Salt stress |
| Drought | Pathogen attack | Cold stress |
| Cold stress | Salt stress | Drought |
| Ozone stress | Etc. | Etc. |
| Heavy metal stress | ||
| Salt stress | ||
| Etc. |
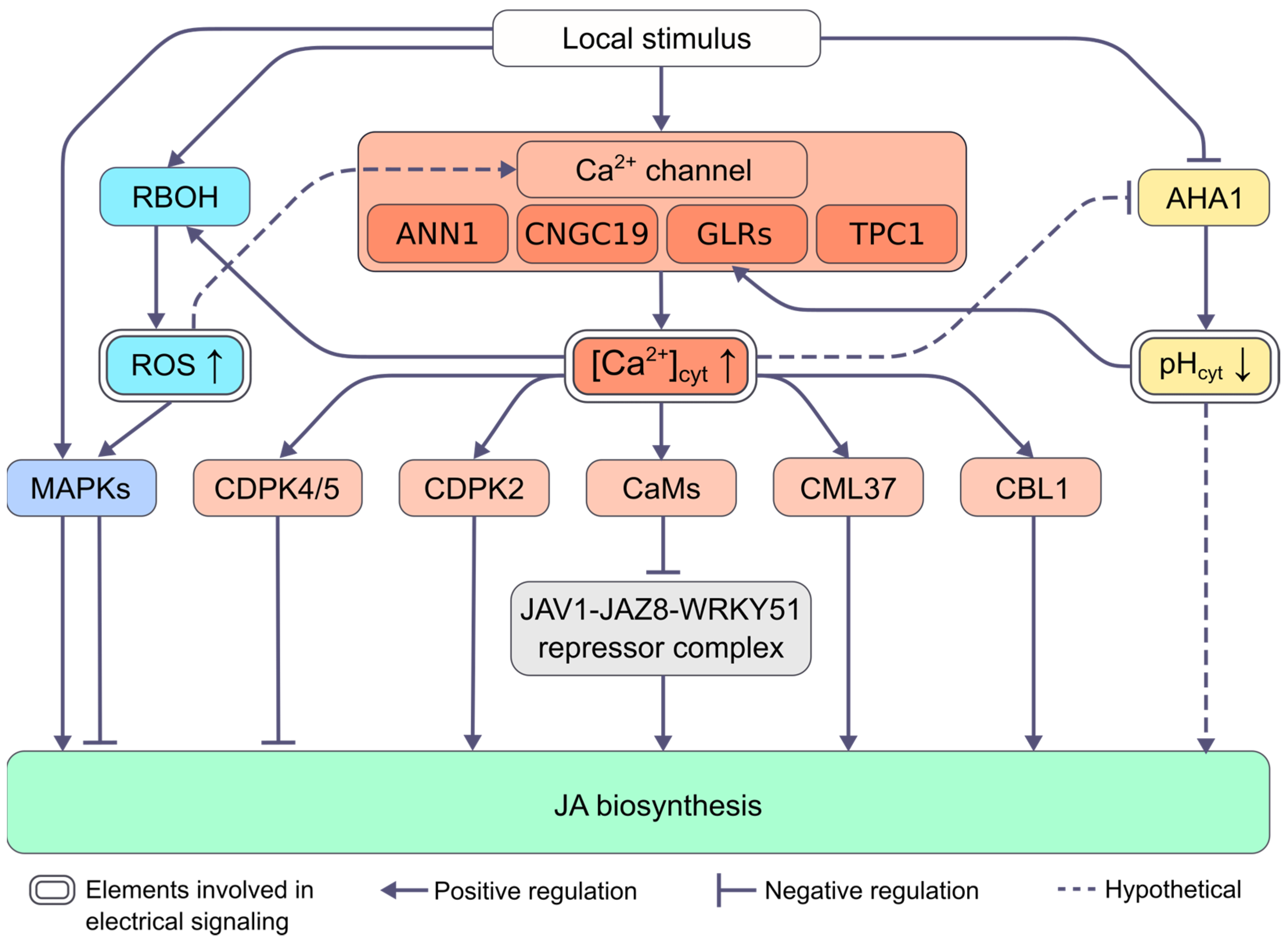
2.2. Mechanisms of Regulation of Changes in Phytohormone Levels by Electrical Signals
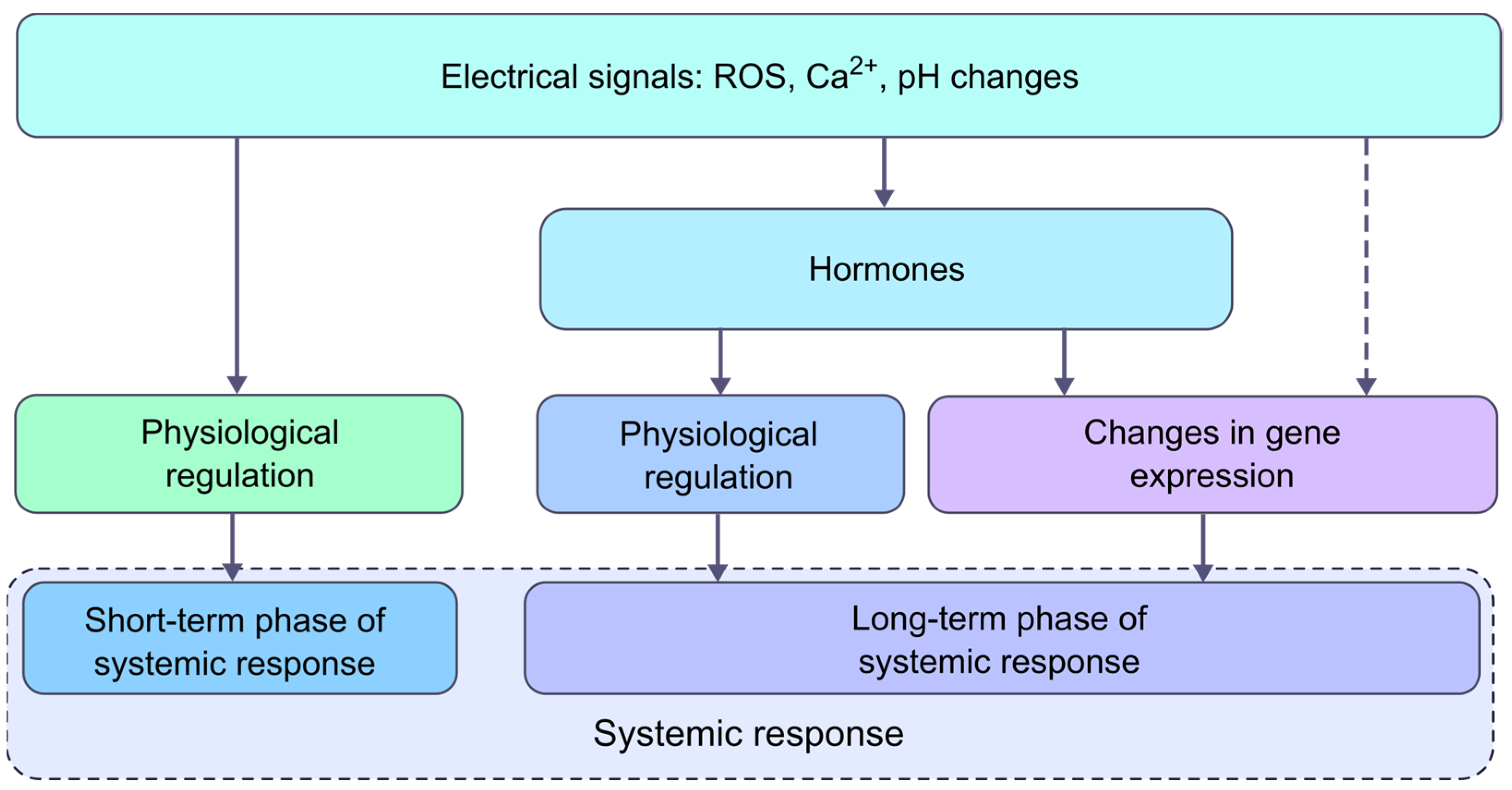
3. The Role of Electrical Signals and Phytohormones in the Formation of a Systemic Response
| photosynthesis | transpiration | respiration | movements |
| production of metabolites | phytohormone production | Transport processes | reproductive processes |
| gene expression | growth processes | morphogenesis | etc. |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huber, A.E.; Bauerle, T.L. Long-Distance Plant Signaling Pathways in Response to Multiple Stressors: The Gap in Knowledge. EXBOTJ 2016, 67, 2063–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, W.-G.; Hilleary, R.; Swanson, S.J.; Kim, S.-H.; Gilroy, S. Rapid, Long-Distance Electrical and Calcium Signaling in Plants. Annu. Rev. Plant Biol. 2016, 67, 287–307. [Google Scholar] [CrossRef]
- Farmer, E.E.; Gasperini, D.; Acosta, I.F. The Squeeze Cell Hypothesis for the Activation of Jasmonate Synthesis in Response to Wounding. New Phytol. 2014, 204, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Vodeneev, V.A.; Katicheva, L.A.; Sukhov, V.S. Electrical Signals in Higher Plants: Mechanisms of Generation and Propagation. Biophysics 2016, 61, 505–512. [Google Scholar] [CrossRef]
- Szechyńska-Hebda, M.; Lewandowska, M.; Karpiński, S. Electrical Signaling, Photosynthesis and Systemic Acquired Acclimation. Front. Physiol. 2017, 8, 684. [Google Scholar] [CrossRef]
- Sukhov, V.; Sukhova, E.; Vodeneev, V. Long-Distance Electrical Signals as a Link between the Local Action of Stressors and the Systemic Physiological Responses in Higher Plants. Prog. Biophys. Mol. Biol. 2019, 146, 63–84. [Google Scholar] [CrossRef]
- Johns, S.; Hagihara, T.; Toyota, M.; Gilroy, S. The Fast and the Furious: Rapid Long-Range Signaling in Plants. Plant Physiol. 2021, 185, 694–706. [Google Scholar] [CrossRef]
- Klejchova, M.; Silva-Alvim, F.A.L.; Blatt, M.R.; Alvim, J.C. Membrane Voltage as a Dynamic Platform for Spatiotemporal Signaling, Physiological, and Developmental Regulation. Plant Physiol. 2021, 185, 1523–1541. [Google Scholar] [CrossRef]
- Mudrilov, M.; Ladeynova, M.; Grinberg, M.; Balalaeva, I.; Vodeneev, V. Electrical Signaling of Plants under Abiotic Stressors: Transmission of Stimulus-Specific Information. IJMS 2021, 22, 10715. [Google Scholar] [CrossRef] [PubMed]
- Farmer, E.E.; Gao, Y.; Lenzoni, G.; Wolfender, J.; Wu, Q. Wound- and Mechanostimulated Electrical Signals Control Hormone Responses. New Phytol. 2020, 227, 1037–1050. [Google Scholar] [CrossRef]
- Hilleary, R.; Gilroy, S. Systemic Signaling in Response to Wounding and Pathogens. Curr. Opin. Plant Biol. 2018, 43, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Devireddy, A.R.; Zandalinas, S.I.; Fichman, Y.; Mittler, R. Integration of Reactive Oxygen Species and Hormone Signaling during Abiotic Stress. Plant J. 2021, 105, 459–476. [Google Scholar] [CrossRef] [PubMed]
- Toyota, M.; Betsuyaku, S. In Vivo Imaging Enables Understanding of Seamless Plant Defense Responses to Wounding and Pathogen Attack. Plant Cell Physiol. 2022, 63, 1391–1404. [Google Scholar] [CrossRef]
- Herde, O.; Peña-Cortés, H.; Wasternack, C.; Willmitzer, L.; Fisahn, J. Electric Signaling and Pin2 Gene Expression on Different Abiotic Stimuli Depend on a Distinct Threshold Level of Endogenous Abscisic Acid in Several Abscisic Acid-Deficient Tomato Mutants1. Plant Physiol. 1999, 119, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Hettenhausen, C.; Meldau, S.; Baldwin, I.T. Herbivory Rapidly Activates MAPK Signaling in Attacked and Unattacked Leaf Regions but Not between Leaves of Nicotiana attenuata. Plant Cell 2007, 19, 1096–1122. [Google Scholar] [CrossRef] [Green Version]
- Glauser, G.; Grata, E.; Dubugnon, L.; Rudaz, S.; Farmer, E.E.; Wolfender, J.-L. Spatial and Temporal Dynamics of Jasmonate Synthesis and Accumulation in Arabidopsis in Response to Wounding. J. Biol. Chem. 2008, 283, 16400–16407. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Welti, R.; Wang, X. Simultaneous Quantification of Major Phytohormones and Related Compounds in Crude Plant Extracts by Liquid Chromatography–Electrospray Tandem Mass Spectrometry. Phytochemistry 2008, 69, 1773–1781. [Google Scholar] [CrossRef]
- Suza, W.P.; Staswick, P.E. The Role of JAR1 in Jasmonoyl-l-Isoleucine Production during Arabidopsis Wound Response. Planta 2008, 227, 1221–1232. [Google Scholar] [CrossRef]
- Koo, A.J.K.; Gao, X.; Daniel Jones, A.; Howe, G.A. A Rapid Wound Signal Activates the Systemic Synthesis of Bioactive Jasmonates in Arabidopsis. Plant J. 2009, 59, 974–986. [Google Scholar] [CrossRef]
- Miller, G.; Schlauch, K.; Tam, R.; Cortes, D.; Torres, M.A.; Shulaev, V.; Dangl, J.L.; Mittler, R. The Plant NADPH Oxidase RBOHD Mediates Rapid Systemic Signaling in Response to Diverse Stimuli. Sci. Signal. 2009, 2, ra45. [Google Scholar] [CrossRef]
- Sato, C.; Seto, Y.; Nabeta, K.; Matsuura, H. Kinetics of the Accumulation of Jasmonic Acid and Its Derivatives in Systemic Leaves of Tobacco (Nicotiana tabacum Cv. Xanthi Nc) and Translocation of Deuterium-Labeled Jasmonic Acid from the Wounding Site to the Systemic Site. Biosci. Biotechnol. Biochem. 2009, 73, 1962–1970. [Google Scholar] [CrossRef] [PubMed]
- Kallenbach, M.; Alagna, F.; Baldwin, I.T.; Bonaventure, G. Nicotiana attenuata SIPK, WIPK, NPR1, and Fatty Acid-Amino Acid Conjugates Participate in the Induction of Jasmonic Acid Biosynthesis by Affecting Early Enzymatic Steps in the Pathway. Plant Physiol. 2009, 152, 96–106. [Google Scholar] [CrossRef] [Green Version]
- Sato, C.; Aikawa, K.; Sugiyama, S.; Nabeta, K.; Masuta, C.; Matsuura, H. Distal Transport of Exogenously Applied Jasmonoyl–Isoleucine with Wounding Stress. Plant Cell Physiol. 2011, 52, 509–517. [Google Scholar] [CrossRef] [Green Version]
- Matsuura, H.; Takeishi, S.; Kiatoka, N.; Sato, C.; Sueda, K.; Masuta, C.; Nabeta, K. Transportation of de Novo Synthesized Jasmonoyl Isoleucine in Tomato. Phytochemistry 2012, 83, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Vadassery, J.; Reichelt, M.; Hause, B.; Gershenzon, J.; Boland, W.; Mithöfer, A. CML42-Mediated Calcium Signaling Coordinates Responses to Spodoptera Herbivory and Abiotic Stresses in Arabidopsis. Plant Physiol. 2012, 159, 1159–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.-H.; Hettenhausen, C.; Baldwin, I.T.; Wu, J. Silencing Nicotiana attenuata Calcium-Dependent Protein Kinases, CDPK4 and CDPK5, Strongly Up-Regulates Wound- and Herbivory-Induced Jasmonic Acid Accumulations. Plant Physiol. 2012, 159, 1591–1607. [Google Scholar] [CrossRef] [Green Version]
- Chauvin, A.; Caldelari, D.; Wolfender, J.-L.; Farmer, E.E. Four 13-Lipoxygenases Contribute to Rapid Jasmonate Synthesis in Wounded Arabidopsis thaliana Leaves: A Role for Lipoxygenase 6 in Responses to Long-Distance Wound Signals. New Phytol. 2013, 197, 566–575. [Google Scholar] [CrossRef]
- Heyer, M.; Reichelt, M.; Mithöfer, A. A Holistic Approach to Analyze Systemic Jasmonate Accumulation in Individual Leaves of Arabidopsis Rosettes Upon Wounding. Front. Plant Sci. 2018, 9, 1569. [Google Scholar] [CrossRef] [PubMed]
- Toyota, M.; Spencer, D.; Sawai-Toyota, S.; Jiaqi, W.; Zhang, T.; Koo, A.J.; Howe, G.A.; Gilroy, S. Glutamate Triggers Long-Distance, Calcium-Based Plant Defense Signaling. Science 2018, 361, 1112–1115. [Google Scholar] [CrossRef]
- Malabarba, J.; Meents, A.K.; Reichelt, M.; Scholz, S.S.; Peiter, E.; Rachowka, J.; Konopka-Postupolska, D.; Wilkins, K.A.; Davies, J.M.; Oelmüller, R.; et al. ANNEXIN1 Mediates Calcium-dependent Systemic Defense in Arabidopsis Plants upon Herbivory and Wounding. New Phytol. 2021, 231, 243–254. [Google Scholar] [CrossRef]
- Zhang, C.; Žukauskaitė, A.; Petřík, I.; Pěnčík, A.; Hönig, M.; Grúz, J.; Široká, J.; Novák, O.; Doležal, K. In Situ Characterisation of Phytohormones from Wounded Arabidopsis Leaves Using Desorption Electrospray Ionisation Mass Spectrometry Imaging. Analyst 2021, 146, 2653–2663. [Google Scholar] [CrossRef]
- Devireddy, A.R.; Zandalinas, S.I.; Gómez-Cadenas, A.; Blumwald, E.; Mittler, R. Coordinating the Overall Stomatal Response of Plants: Rapid Leaf-to-Leaf Communication during Light Stress. Sci. Signal. 2018, 11, eaam9514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zandalinas, S.I.; Fichman, Y.; Devireddy, A.R.; Sengupta, S.; Azad, R.K.; Mittler, R. Systemic Signaling during Abiotic Stress Combination in Plants. Proc. Natl. Acad. Sci. USA 2020, 117, 13810–13820. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Miller, G.; Salazar, C.; Mondal, H.A.; Shulaev, E.; Cortes, D.F.; Shuman, J.L.; Luo, X.; Shah, J.; Schlauch, K.; et al. Temporal-Spatial Interaction between Reactive Oxygen Species and Abscisic Acid Regulates Rapid Systemic Acclimation in Plants. Plant Cell 2013, 25, 3553–3569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mudrilov, M.; Ladeynova, M.; Berezina, E.; Grinberg, M.; Brilkina, A.; Sukhov, V.; Vodeneev, V. Mechanisms of Specific Systemic Response in Wheat Plants under Different Locally Acting Heat Stimuli. J. Plant Physiol. 2021, 258–259, 153377. [Google Scholar] [CrossRef] [PubMed]
- Hlaváčková, V.; Krchňák, P.; Nauš, J.; Novák, O.; Špundová, M.; Strnad, M. Electrical and Chemical Signals Involved in Short-Term Systemic Photosynthetic Responses of Tobacco Plants to Local Burning. Planta 2006, 225, 235–244. [Google Scholar] [CrossRef]
- Hlavinka, J.; Nožková-Hlaváčková, V.; Floková, K.; Novák, O.; Nauš, J. Jasmonic Acid Accumulation and Systemic Photosynthetic and Electrical Changes in Locally Burned Wild Type Tomato, ABA-Deficient Sitiens Mutants and Sitiens Pre-Treated by ABA. Plant Physiol. Biochem. 2012, 54, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Paulmann, M.K.; Kunert, G.; Zimmermann, M.R.; Theis, N.; Ludwig, A.; Meichsner, D.; Oelmüller, R.; Gershenzon, J.; Habekuss, A.; Ordon, F.; et al. Barley Yellow Dwarf Virus Infection Leads to Higher Chemical Defense Signals and Lower Electrophysiological Reactions in Susceptible Compared to Tolerant Barley Genotypes. Front. Plant Sci. 2018, 9, 145. [Google Scholar] [CrossRef]
- Ladeynova, M.; Mudrilov, M.; Berezina, E.; Kior, D.; Grinberg, M.; Brilkina, A.; Sukhov, V.; Vodeneev, V. Spatial and Temporal Dynamics of Electrical and Photosynthetic Activity and the Content of Phytohormones Induced by Local Stimulation of Pea Plants. Plants 2020, 9, 1364. [Google Scholar] [CrossRef]
- Wang, L.; Allmann, S.; Wu, J.; Baldwin, I.T. Comparisons of LIPOXYGENASE3- and JASMONATE-RESISTANT4/6-Silenced Plants Reveal That Jasmonic Acid and Jasmonic Acid-Amino Acid Conjugates Play Different Roles in Herbivore Resistance of Nicotiana attenuata. Plant Physiol. 2008, 146, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Hettenhausen, C.; Yang, D.-H.; Baldwin, I.T.; Wu, J. Calcium-Dependent Protein Kinases, CDPK4 and CDPK5, Affect Early Steps of Jasmonic Acid Biosynthesis in Nicotiana attenuata. Plant Signal. Behav. 2013, 8, e22784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholz, S.S.; Vadassery, J.; Heyer, M.; Reichelt, M.; Bender, K.W.; Snedden, W.A.; Boland, W.; Mithöfer, A. Mutation of the Arabidopsis Calmodulin-Like Protein CML37 Deregulates the Jasmonate Pathway and Enhances Susceptibility to Herbivory. Mol. Plant 2014, 7, 1712–1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meena, M.K.; Prajapati, R.; Krishna, D.; Divakaran, K.; Pandey, Y.; Reichelt, M.; Mathew, M.K.; Boland, W.; Mithöfer, A.; Vadassery, J. The Ca2+ Channel CNGC19 Regulates Arabidopsis Defense Against Spodoptera Herbivory. Plant Cell 2019, 31, 1539–1562. [Google Scholar] [CrossRef]
- Thornburg, R.W.; Li, X. Wounding Nicotiana tabacum Leaves Causes a Decline in Endogenous Indole-3-Acetic Acid. Plant Physiol. 1991, 96, 802–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, Perception, Signal Transduction and Action in Plant Stress Response, Growth and Development. An Update to the 2007 Review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef]
- Vadassery, J.; Reichelt, M.; Jimenez-Aleman, G.H.; Boland, W.; Mithöfer, A. Neomycin Inhibition of (+)-7-Iso-Jasmonoyl-L-Isoleucine Accumulation and Signaling. J. Chem Ecol. 2014, 40, 676–686. [Google Scholar] [CrossRef]
- Shabala, S.; White, R.G.; Djordjevic, M.A.; Ruan, Y.-L.; Mathesius, U. Root-to-Shoot Signalling: Integration of Diverse Molecules, Pathways and Functions. Funct. Plant Biol. 2016, 43, 87. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, S.A.R.; Chauvin, A.; Pascaud, F.; Kellenberger, S.; Farmer, E.E. GLUTAMATE RECEPTOR-LIKE Genes Mediate Leaf-to-Leaf Wound Signalling. Nature 2013, 500, 422–426. [Google Scholar] [CrossRef] [Green Version]
- Peláez-Vico, M.Á.; Fichman, Y.; Zandalinas, S.I.; Van Breusegem, F.; Karpiński, S.M.; Mittler, R. ROS and Redox Regulation of Cell-to-Cell and Systemic Signaling in Plants during Stress. Free Radic. Biol. Med. 2022, 193, 354–362. [Google Scholar] [CrossRef]
- Fisahn, J.; Herde, O.; Willmitzer, L.; Peña-Cortés, H. Analysis of the Transient Increase in Cytosolic Ca2+ during the Action Potential of Higher Plants with High Temporal Resolution: Requirement of Ca2+ Transients for Induction of Jasmonic Acid Biosynthesis and PINII Gene Expression. Plant Cell Physiol. 2004, 45, 456–459. [Google Scholar] [CrossRef]
- Jogawat, A.; Meena, M.K.; Kundu, A.; Varma, M.; Vadassery, J. Calcium Channel CNGC19 Mediates Basal Defense Signaling to Regulate Colonization by Piriformospora indica in Arabidopsis Roots. J. Exp. Bot. 2020, 71, 2752–2768. [Google Scholar] [CrossRef]
- Wang, G.; Hu, C.; Zhou, J.; Liu, Y.; Cai, J.; Pan, C.; Wang, Y.; Wu, X.; Shi, K.; Xia, X.; et al. Systemic Root-Shoot Signaling Drives Jasmonate-Based Root Defense against Nematodes. Curr. Biol. 2019, 29, 3430–3438.e4. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, F.; Li, S.; Yu, G.; Wang, L.; Li, Q.; Zhu, X.; Li, Z.; Yuan, L.; Liu, P. Importers Drive Leaf-to-Leaf Jasmonic Acid Transmission in Wound-Induced Systemic Immunity. Mol. Plant 2020, 13, 1485–1498. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Luo, L.; Wei, J.; Chen, Q.; Yang, Y.; Hu, X.; Kong, X. The Glutamate Receptors AtGLR1.2 and AtGLR1.3 Increase Cold Tolerance by Regulating Jasmonate Signaling in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2018, 506, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Bellandi, A.; Papp, D.; Breakspear, A.; Joyce, J.; Johnston, M.G.; de Keijzer, J.; Raven, E.C.; Ohtsu, M.; Vincent, T.R.; Miller, A.J.; et al. Diffusion and Bulk Flow of Amino Acids Mediate Calcium Waves in Plants. Sci. Adv. 2022, 8, eabo6693. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Miller, G.; Wallace, I.; Harper, J.; Mittler, R.; Gilroy, S. Orchestrating Rapid Long-distance Signaling in Plants with Ca2+, ROS and Electrical Signals. Plant J. 2017, 90, 698–707. [Google Scholar] [CrossRef] [Green Version]
- Bonaventure, G.; Gfeller, A.; Proebsting, W.M.; Hörtensteiner, S.; Chételat, A.; Martinoia, E.; Farmer, E.E. A Gain-of-Function Allele of TPC1 Activates Oxylipin Biogenesis after Leaf Wounding in Arabidopsis: A Cation Channel Regulates Oxylipin Biogenesis. Plant J. 2007, 49, 889–898. [Google Scholar] [CrossRef]
- Lenglet, A.; Jaślan, D.; Toyota, M.; Mueller, M.; Müller, T.; Schönknecht, G.; Marten, I.; Gilroy, S.; Hedrich, R.; Farmer, E.E. Control of Basal Jasmonate Signalling and Defence through Modulation of Intracellular Cation Flux Capacity. New Phytol. 2017, 216, 1161–1169. [Google Scholar] [CrossRef] [Green Version]
- Ryu, S.B.; Wang, X. Activation of Phospholipase D and the Possible Mechanism of Activation in Wound-Induced Lipid Hydrolysis in Castor Bean Leaves. Biochim. Et Biophys. Acta (BBA)—Lipids Lipid Metab. 1996, 1303, 243–250. [Google Scholar] [CrossRef]
- Wang, C.; Zien, C.A.; Afitlhile, M.; Welti, R.; Hildebrand, D.F.; Wang, X. Involvement of Phospholipase D in Wound-Induced Accumulation of Jasmonic Acid in Arabidopsis. Plant Cell 2000, 12, 2237–2246. [Google Scholar] [CrossRef]
- Hunter, K.; Kimura, S.; Rokka, A.; Tran, H.C.; Toyota, M.; Kukkonen, J.P.; Wrzaczek, M. CRK2 Enhances Salt Tolerance by Regulating Callose Deposition in Connection with PLDα1. Plant Physiol. 2019, 180, 2004–2021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, C.; Fan, M.; Yang, M.; Zhao, J.; Zhang, W.; Su, Y.; Xiao, L.; Deng, H.; Xie, D. Injury Activates Ca2+/Calmodulin-Dependent Phosphorylation of JAV1-JAZ8-WRKY51 Complex for Jasmonate Biosynthesis. Mol. Cell 2018, 70, 136–149.e7. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zhang, X.; Huang, Y.; Feng, Y.; Li, Y. OsCBL1 Modulates Lateral Root Elongation in Rice via Affecting Endogenous Indole-3-Acetic Acid Biosynthesis. J. Genet. Genom. 2015, 42, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.A.; Saitoh, H.; Felix, G.; Freymark, G.; Miersch, O.; Wasternack, C.; Boller, T.; Jones, J.D.G.; Romeis, T. Ethylene-Mediated Cross-Talk between Calcium-Dependent Protein Kinase and MAPK Signaling Controls Stress Responses in Plants. Proc. Natl. Acad. Sci. USA 2005, 102, 10736–10741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, A.; Chételat, A.; Nguyen, C.T.; Farmer, E.E. Arabidopsis H+-ATPase AHA1 Controls Slow Wave Potential Duration and Wound-Response Jasmonate Pathway Activation. Proc. Natl. Acad. Sci. USA 2019, 116, 20226–20231. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Seo, P.J. Wound-Induced Systemic Responses and Their Coordination by Electrical Signals. Front. Plant Sci. 2022, 13, 880680. [Google Scholar] [CrossRef]
- Basu, D.; Haswell, E.S. The Mechanosensitive Ion Channel MSL10 Potentiates Responses to Cell Swelling in Arabidopsis Seedlings. Curr. Biol. 2020, 30, 2716–2728.e6. [Google Scholar] [CrossRef]
- Moe-Lange, J.; Gappel, N.M.; Machado, M.; Wudick, M.M.; Sies, C.S.A.; Schott-Verdugo, S.N.; Bonus, M.; Mishra, S.; Hartwig, T.; Bezrutczyk, M.; et al. Interdependence of a Mechanosensitive Anion Channel and Glutamate Receptors in Distal Wound Signaling. Sci. Adv. 2021, 7, eabg4298. [Google Scholar] [CrossRef]
- Zou, Y.; Chintamanani, S.; He, P.; Fukushige, H.; Yu, L.; Shao, M.; Zhu, L.; Hildebrand, D.F.; Tang, X.; Zhou, J.-M. A Gain-of-Function Mutation in Msl10 Triggers Cell Death and Wound-Induced Hyperaccumulation of Jasmonic Acid in Arabidopsis: Role of a Mechanosensitive Ion Channel in Wounding. J. Integr. Plant Biol. 2016, 58, 600–609. [Google Scholar] [CrossRef]
- Limami, A.; Hirel, B.; Lothier, J. Does Potassium (K+) Contribute to High-Nitrate (NO3−) Weakening of a Plant’s Defense System against Necrotrophic Fungi? IJMS 2022, 23, 15631. [Google Scholar] [CrossRef]
- Hedrich, R.; Salvador-Recatalà, V.; Dreyer, I. Electrical Wiring and Long-Distance Plant Communication. Trends Plant Sci. 2016, 21, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Cuin, T.; Dreyer, I.; Michard, E. The Role of Potassium Channels in Arabidopsis thaliana Long Distance Electrical Signalling: AKT2 Modulates Tissue Excitability While GORK Shapes Action Potentials. IJMS 2018, 19, 926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Neill, S.; Cai, W.; Tang, Z. Hydrogen Peroxide and Jasmonic Acid Mediate Oligogalacturonic Acid-Induced Saponin Accumulation in Suspension-Cultured Cells of Panax ginseng. Physiol. Plant. 2003, 118, 414–421. [Google Scholar] [CrossRef]
- Hu, X.; Neill, S.J.; Yang, Y.; Cai, W. Fungal Elicitor Pep-25 Increases Cytosolic Calcium Ions, H2O2 Production and Activates the Octadecanoid Pathway in Arabidopsis thaliana. Planta 2009, 229, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.; Li, S.; Feng, J.; Liu, P.; Gao, Z.; Yang, Y.; Xu, Y.; Wei, J. Hydrogen Peroxide Burst Triggers Accumulation of Jasmonates and Salicylic Acid Inducing Sesquiterpene Biosynthesis in Wounded Aquilaria sinesis. J. Plant Physiol. 2019, 234–235, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Hettenhausen, C.; Schuman, M.C.; Wu, J. MAPK Signaling: A Key Element in Plant Defense Response to Insects: MAPK Signaling in Plant-Insect Interactions. Insect Sci. 2015, 22, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Seo, S.; Okamoto, M.; Seto, H.; Ishizuka, K.; Sano, H.; Ohashi, Y. Tobacco MAP Kinase: A Possible Mediator in Wound Signal Transduction Pathways. Science 1995, 270, 1988–1992. [Google Scholar] [CrossRef]
- Seo, S.; Sano, H.; Ohashi, Y. Jasmonate-Based Wound Signal Transduction Requires Activation of WIPK, a Tobacco Mitogen-Activated Protein Kinase. Plant Cell 1999, 11, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Seo, S.; Katou, S.; Seto, H.; Gomi, K.; Ohashi, Y. The Mitogen-Activated Protein Kinases WIPK and SIPK Regulate the Levels of Jasmonic and Salicylic Acids in Wounded Tobacco Plants: Roles of WIPK and SIPK in Wound Signalling. Plant J. 2007, 49, 899–909. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Hu, L.; Zhang, T.; Zhang, G.; Lou, Y. OsMPK3 Positively Regulates the JA Signaling Pathway and Plant Resistance to a Chewing Herbivore in Rice. Plant Cell Rep. 2013, 32, 1075–1084. [Google Scholar] [CrossRef]
- Vodeneev, V.A.; Opritov, V.A.; Pyatygin, S.S. Reversible Changes of Extracellular pH during Action Potential Generation in a Higher Plant Cucurbita Pepo. Russ. J. Plant Physiol. 2006, 53, 481–487. [Google Scholar] [CrossRef]
- Duby, G.; Boutry, M. The Plant Plasma Membrane Proton Pump ATPase: A Highly Regulated P-Type ATPase with Multiple Physiological Roles. Pflug. Arch-Eur. J. Physiol. 2009, 457, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Ookura, T.; Komatsu, S.; Kawamura, Y.; Kasamo, K. A 55-KDa Calcium Dependent Protein Kinase Phosphorylated Thr Residues from the Auto-Regulatory Domain of Plasma Membrane H+-ATPase in Rice. JARQ 2005, 39, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Behera, S.; Xu, Z.; Luoni, L.; Bonza, M.C.; Doccula, F.G.; De Michelis, M.I.; Morris, R.J.; Schwarzländer, M.; Costa, A. Cellular Ca2+ Signals Generate Defined pH Signatures in Plants. Plant Cell 2018, 30, 2704–2719. [Google Scholar] [CrossRef] [Green Version]
- Shao, Q.; Gao, Q.; Lhamo, D.; Zhang, H.; Luan, S. Two Glutamate- and pH-Regulated Ca2+ Channels Are Required for Systemic Wound Signaling in Arabidopsis. Sci. Signal. 2020, 13, eaba1453. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V. ROS-Activated Ion Channels in Plants: Biophysical Characteristics, Physiological Functions and Molecular Nature. IJMS 2018, 19, 1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcec, M.J.; Gilroy, S.; Poovaiah, B.W.; Tanaka, K. Mutual Interplay of Ca2+ and ROS Signaling in Plant Immune Response. Plant Sci. 2019, 283, 343–354. [Google Scholar] [CrossRef]
- Xia, X.-J.; Zhou, Y.-H.; Shi, K.; Zhou, J.; Foyer, C.H.; Yu, J.-Q. Interplay between Reactive Oxygen Species and Hormones in the Control of Plant Development and Stress Tolerance. J. Exp. Bot. 2015, 66, 2839–2856. [Google Scholar] [CrossRef] [Green Version]
- Pandey, G.K.; Cheong, Y.H.; Kim, K.-N.; Grant, J.J.; Li, L.; Hung, W.; D’Angelo, C.; Weinl, S.; Kudla, J.; Luan, S. The Calcium Sensor Calcineurin B-Like 9 Modulates Abscisic Acid Sensitivity and Biosynthesis in Arabidopsis. Plant Cell 2004, 16, 1912–1924. [Google Scholar] [CrossRef] [Green Version]
- Xiong, L.; Zhu, J.-K. Regulation of Abscisic Acid Biosynthesis. Plant Physiol. 2003, 133, 29–36. [Google Scholar] [CrossRef]
- Hartung, W.; Radin, J.W.; Hendrix, D.L. Abscisic Acid Movement into the Apoplastic Solution of Water-Stressed Cotton Leaves: Role of Apoplastic pH. Plant Physiol. 1988, 86, 908–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Netting, A.G. pH, Abscisic Acid and the Integration of Metabolism in Plants under Stressed and Non-Stressed Conditions. II. Modifications in Modes of Metabolism Induced by Variation in the Tension on the Water Column and by Stress. J. Exp. Bot. 2002, 53, 151–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffield, P.H.; Netting, A.G. Methods for the Quantitation of Abscisic Acid and Its Precursors from Plant Tissues. Anal. Biochem. 2001, 289, 251–259. [Google Scholar] [CrossRef]
- Finkelstein, R. Abscisic Acid Synthesis and Response. Arab. Book 2013, 11, e0166. [Google Scholar] [CrossRef] [Green Version]
- Burla, B.; Pfrunder, S.; Nagy, R.; Francisco, R.M.; Lee, Y.; Martinoia, E. Vacuolar Transport of Abscisic Acid Glucosyl Ester Is Mediated by ATP-Binding Cassette and Proton-Antiport Mechanisms in Arabidopsis. Plant Physiol. 2013, 163, 1446–1458. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.-Y.; Gehring, C.; Zhu, J.; Li, F.-M.; Zhu, J.-K.; Xiong, L. The Arabidopsis Vacuolar Sorting Receptor1 Is Required for Osmotic Stress-Induced Abscisic Acid Biosynthesis. Plant Physiol. 2014, 167, 137–152. [Google Scholar] [CrossRef] [Green Version]
- McAdam, S.A.M.; Brodribb, T.J. Linking Turgor with ABA Biosynthesis: Implications for Stomatal Responses to Vapor Pressure Deficit across Land Plants. Plant Physiol. 2016, 171, 2008–2016. [Google Scholar] [CrossRef] [Green Version]
- Sussmilch, F.C.; Brodribb, T.J.; McAdam, S.A.M. Up-Regulation of NCED3 and ABA Biosynthesis Occur within Minutes of a Decrease in Leaf Turgor but AHK1 Is Not Required. J. Exp. Bot. 2017, 68, 2913–2918. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, A.A.; Gori, A.; Da-Silva, C.J.; Brunetti, C. Abscisic Acid Biosynthesis and Signaling in Plants: Key Targets to Improve Water Use Efficiency and Drought Tolerance. Appl. Sci. 2020, 10, 6322. [Google Scholar] [CrossRef]
- Romeis, T.; Herde, M. From Local to Global: CDPKs in Systemic Defense Signaling upon Microbial and Herbivore Attack. Curr. Opin. Plant Biol. 2014, 20, 1–10. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Li, X.; Zhang, Y. Biosynthesis and Regulation of Salicylic Acid and N-Hydroxypipecolic Acid in Plant Immunity. Mol. Plant 2020, 13, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Gallé, A.; Lautner, S.; Flexas, J.; Fromm, J. Environmental Stimuli and Physiological Responses: The Current View on Electrical Signalling. Environ. Exp. Bot. 2015, 114, 15–21. [Google Scholar] [CrossRef]
- Fabricant, A.; Iwata, G.Z.; Scherzer, S.; Bougas, L.; Rolfs, K.; Jodko-Władzińska, A.; Voigt, J.; Hedrich, R.; Budker, D. Action Potentials Induce Biomagnetic Fields in Carnivorous Venus Flytrap Plants. Sci. Rep. 2021, 11, 1438. [Google Scholar] [CrossRef]
- Harmer, S.L.; Brooks, C.J. Growth-Mediated Plant Movements: Hidden in Plain Sight. Curr. Opin. Plant Biol. 2018, 41, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Fromm, J.; Hajirezaei, M.-R.; Becker, V.K.; Lautner, S. Electrical Signaling along the Phloem and Its Physiological Responses in the Maize Leaf. Front. Plant Sci. 2013, 4, 00239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krausko, M.; Perutka, Z.; Šebela, M.; Šamajová, O.; Šamaj, J.; Novák, O.; Pavlovič, A. The Role of Electrical and Jasmonate Signalling in the Recognition of Captured Prey in the Carnivorous Sundew Plant Drosera Capensis. New Phytol. 2017, 213, 1818–1835. [Google Scholar] [CrossRef] [Green Version]
- Vian, A.; Davies, E. Two Different Wound Signals Evoke Very Rapid, Systemic CMBP Transcript Accumulation in Tomato. Plant Signal. Behav. 2006, 1, 261–264. [Google Scholar] [CrossRef] [Green Version]
- Koziolek, C.; Grams, T.E.E.; Schreiber, U.; Matyssek, R.; Fromm, J. Transient Knockout of Photosynthesis Mediated by Electrical Signals. New Phytol. 2004, 161, 715–722. [Google Scholar] [CrossRef]
- Yudina, L.; Gromova, E.; Grinberg, M.; Popova, A.; Sukhova, E.; Sukhov, V. Influence of Burning-Induced Electrical Signals on Photosynthesis in Pea Can Be Modified by Soil Water Shortage. Plants 2022, 11, 534. [Google Scholar] [CrossRef]
- Khokon, M.A.R.; Okuma, E.; Hossain, M.A.; Munemasa, S.; Uraji, M.; Nakamura, Y.; Mori, I.C.; Murata, Y. Involvement of Extracellular Oxidative Burst in Salicylic Acid-Induced Stomatal Closure in Arabidopsis: Extracellular ROS Mediate SA-Induced Stomatal Closure. Plant Cell Environ. 2011, 34, 434–443. [Google Scholar] [CrossRef]
- Prodhan, M.Y.; Munemasa, S.; Nahar, M.N.-E.-N.; Nakamura, Y.; Murata, Y. Guard Cell Salicylic Acid Signaling Is Integrated into Abscisic Acid Signaling via the Ca2+/CPK-Dependent Pathway. Plant Physiol. 2018, 178, 441–450. [Google Scholar] [CrossRef] [Green Version]
- Surova, L.; Sherstneva, O.; Vodeneev, V.; Katicheva, L.; Semina, M.; Sukhov, V. Variation Potential-Induced Photosynthetic and Respiratory Changes Increase ATP Content in Pea Leaves. J. Plant Physiol. 2016, 202, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Gallé, A.; Lautner, S.; Flexas, J.; Ribas-Carbo, M.; Hanson, D.; Roesgen, J.; Fromm, J. Photosynthetic Responses of Soybean (Glycine max L.) to Heat-Induced Electrical Signalling Are Predominantly Governed by Modifications of Mesophyll Conductance for CO2: Electrical Signalling and Mesophyll Conductance in Soybean. Plant Cell Environ. 2013, 36, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Grams, T.E.E.; Lautner, S.; Felle, H.H.; Matyssek, R.; Fromm, J. Heat-Induced Electrical Signals Affect Cytoplasmic and Apoplastic pH as Well as Photosynthesis during Propagation through the Maize Leaf. Plant Cell Environ. 2009, 32, 319–326. [Google Scholar] [CrossRef]
- Vuralhan-Eckert, J.; Lautner, S.; Fromm, J. Effect of Simultaneously Induced Environmental Stimuli on Electrical Signalling and Gas Exchange in Maize Plants. J. Plant Physiol. 2018, 223, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Białasek, M.; Górecka, M.; Mittler, R.; Karpiński, S. Evidence for the Involvement of Electrical, Calcium and ROS Signaling in the Systemic Regulation of Non-Photochemical Quenching and Photosynthesis. Plant Cell Physiol. 2017, 58, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Lautner, S.; Grams, T.E.E.; Matyssek, R.; Fromm, J. Characteristics of Electrical Signals in Poplar and Responses in Photosynthesis. Plant Physiol. 2005, 138, 2200–2209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukhov, V.; Sherstneva, O.; Surova, L.; Katicheva, L.; Vodeneev, V. Proton Cellular Influx as a Probable Mechanism of Variation Potential Influence on Photosynthesis in Pea: VP Influences on Photosynthesis Due to H+ Influx. Plant Cell Environ. 2014, 37, 2532–2541. [Google Scholar] [CrossRef]
- Sukhov, V. Electrical Signals as Mechanism of Photosynthesis Regulation in Plants. Photosynth. Res. 2016, 130, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Sherstneva, O.N.; Surova, L.M.; Vodeneev, V.A.; Plotnikova, Y.I.; Bushueva, A.V.; Sukhov, V.S. The Role of the Intra- and Extracellular Protons in the Photosynthetic Response Induced by the Variation Potential in Pea Seedlings. Biochem. Mosc. Suppl. Ser. A 2016, 10, 60–67. [Google Scholar] [CrossRef]
- Sherstneva, O.N.; Vodeneev, V.A.; Katicheva, L.A.; Surova, L.M.; Sukhov, V.S. Participation of Intracellular and Extracellular pH Changes in Photosynthetic Response Development Induced by Variation Potential in Pumpkin Seedlings. Biochem. Mosc. 2015, 80, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Attaran, E.; Major, I.T.; Cruz, J.A.; Rosa, B.A.; Koo, A.J.K.; Chen, J.; Kramer, D.M.; He, S.Y.; Howe, G.A. Temporal Dynamics of Growth and Photosynthesis Suppression in Response to Jasmonate Signaling. Plant Physiol. 2014, 165, 1302–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herde, O.; Pena-Cortes, H.; Willmitzer, L.; Fisahn, J. Stomatal Responses to Jasmonic Acid, Linolenic Acid and Abscisic Acid in Wild-Type and ABA-Deficient Tomato Plants. Plant Cell Env. 1997, 20, 136–141. [Google Scholar] [CrossRef]
- Nabity, P.D.; Zavala, J.A.; DeLucia, E.H. Herbivore Induction of Jasmonic Acid and Chemical Defences Reduce Photosynthesis in Nicotiana attenuata. J. Exp. Bot. 2013, 64, 685–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorecka, M.; Alvarez-Fernandez, R.; Slattery, K.; McAusland, L.; Davey, P.A.; Karpinski, S.; Lawson, T.; Mullineaux, P.M. Abscisic Acid Signalling Determines Susceptibility of Bundle Sheath Cells to Photoinhibition in High Light-Exposed Arabidopsis Leaves. Phil. Trans. R. Soc. B 2014, 369, 20130234. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Li, G.; Bressan, R.A.; Song, C.; Zhu, J.; Zhao, Y. Abscisic Acid Dynamics, Signaling, and Functions in Plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of Jasmonic Acid in Plant Regulation and Response to Abiotic Stress. IJMS 2020, 21, 1446. [Google Scholar] [CrossRef] [Green Version]
- Buckley, T.N. Modeling Stomatal Conductance. Plant Physiol. 2017, 174, 572–582. [Google Scholar] [CrossRef] [Green Version]
- Jezek, M.; Blatt, M.R. The Membrane Transport System of the Guard Cell and Its Integration for Stomatal Dynamics. Plant Physiol. 2017, 174, 487–519. [Google Scholar] [CrossRef] [Green Version]
- Bright, J.; Desikan, R.; Hancock, J.T.; Weir, I.S.; Neill, S.J. ABA-Induced NO Generation and Stomatal Closure in Arabidopsis Are Dependent on H2O2 Synthesis. Plant J. 2006, 45, 113–122. [Google Scholar] [CrossRef]
- Jahan, M.S.; Nozulaidi, M.; Khairi, M.; Mat, N. Light-Harvesting Complexes in Photosystem II Regulate Glutathione-Induced Sensitivity of Arabidopsis Guard Cells to Abscisic Acid. J. Plant Physiol. 2016, 195, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.M. NADPH Oxidase AtrbohD and AtrbohF Genes Function in ROS-Dependent ABA Signaling in Arabidopsis. EMBO J. 2003, 22, 2623–2633. [Google Scholar] [CrossRef] [PubMed]
- Munemasa, S.; Hossain, M.A.; Nakamura, Y.; Mori, I.C.; Murata, Y. The Arabidopsis Calcium-Dependent Protein Kinase, CPK6, Functions as a Positive Regulator of Methyl Jasmonate Signaling in Guard Cells. Plant Physiol. 2011, 155, 553–561. [Google Scholar] [CrossRef] [Green Version]
- Pei, Z.-M.; Murata, Y.; Benning, G.; Thomine, S.; Klüsener, B.; Allen, G.J.; Grill, E.; Schroeder, J.I. Calcium Channels Activated by Hydrogen Peroxide Mediate Abscisic Acid signalling in Guard Cells. Nature 2000, 406, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Peña-Cortés, H.; Fisahn, J.; Willmitzer, L. Signals Involved in Wound-Induced Proteinase Inhibitor II Gene Expression in Tomato and Potato Plants. Proc. Natl. Acad. Sci. USA 1995, 92, 4106–4113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savchenko, T.; Kolla, V.A.; Wang, C.-Q.; Nasafi, Z.; Hicks, D.R.; Phadungchob, B.; Chehab, W.E.; Brandizzi, F.; Froehlich, J.; Dehesh, K. Functional Convergence of Oxylipin and Abscisic Acid Pathways Controls Stomatal Closure in Response to Drought. Plant Physiol. 2014, 164, 1151–1160. [Google Scholar] [CrossRef] [Green Version]
- Suhita, D.; Raghavendra, A.S.; Kwak, J.M.; Vavasseur, A. Cytoplasmic Alkalization Precedes Reactive Oxygen Species Production during Methyl Jasmonate- and Abscisic Acid-Induced Stomatal Closure. Plant Physiol. 2004, 134, 1536–1545. [Google Scholar] [CrossRef] [Green Version]
- Gehring, C. Jasmonates Induce Intracellular Alkalinization and Closure Of Paphiopedilum Guard Cells. Ann. Bot. 1997, 80, 485–489. [Google Scholar] [CrossRef] [Green Version]
- Geng, S.; Misra, B.B.; de Armas, E.; Huhman, D.V.; Alborn, H.T.; Sumner, L.W.; Chen, S. Jasmonate-Mediated Stomatal Closure under Elevated CO2 Revealed by Time-Resolved Metabolomics. Plant J. 2016, 88, 947–962. [Google Scholar] [CrossRef] [Green Version]
- Förster, S.; Schmidt, L.K.; Kopic, E.; Anschütz, U.; Huang, S.; Schlücking, K.; Köster, P.; Waadt, R.; Larrieu, A.; Batistič, O.; et al. Wounding-Induced Stomatal Closure Requires Jasmonate-Mediated Activation of GORK K+ Channels by a Ca2+ Sensor-Kinase CBL1-CIPK5 Complex. Dev. Cell 2019, 48, 87–99.e6. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, Y.; Yang, Y.; Du, M.; Zhang, X.; Guo, Y.; Li, C.; Zhou, J.-M. An Arabidopsis Plasma Membrane Proton ATPase Modulates JA Signaling and Is Exploited by the Pseudomonas syringae Effector Protein AvrB for Stomatal Invasion. Plant Cell 2015, 27, 2032–2041. [Google Scholar] [CrossRef] [Green Version]
- De Ollas, C.; Arbona, V.; Gómez-Cadenas, A.; Dodd, I.C. Attenuated Accumulation of Jasmonates Modifies Stomatal Responses to Water Deficit. J. Exp. Bot. 2018, 69, 2103–2116. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.A.; Munemasa, S.; Uraji, M.; Nakamura, Y.; Mori, I.C.; Murata, Y. Involvement of Endogenous Abscisic Acid in Methyl Jasmonate-Induced Stomatal Closure in Arabidopsis. Plant Physiol. 2011, 156, 430–438. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.Y. Methyl Jasmonate Reduces Water Stress in Strawberry. J. Plant Growth Regul 1999, 18, 127–134. [Google Scholar] [CrossRef]
- Yan, S.; McLamore, E.S.; Dong, S.; Gao, H.; Taguchi, M.; Wang, N.; Zhang, T.; Su, X.; Shen, Y. The Role of Plasma Membrane H+-ATPase in Jasmonate-induced Ion Fluxes and Stomatal Closure in Arabidopsis thaliana. Plant J. 2015, 83, 638–649. [Google Scholar] [CrossRef]
- Murata, Y.; Pei, Z.-M.; Mori, I.C.; Schroeder, J. Abscisic Acid Activation of Plasma Membrane Ca2+ Channels in Guard Cells Requires Cytosolic NAD(P)H and Is Differentially Disrupted Upstream and Downstream of Reactive Oxygen Species Production in Abi1-1 and Abi2-1 Protein Phosphatase 2C Mutants. Plant Cell 2001, 13, 2513–2523. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Waadt, R.; Nuhkat, M.; Kollist, H.; Hedrich, R.; Roelfsema, M.R.G. Calcium Signals in Guard Cells Enhance the Efficiency by Which Abscisic Acid Triggers Stomatal Closure. New Phytol. 2019, 224, 177–187. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.; Panzeri, D.; Okuma, E.; Tõldsepp, K.; Wang, Y.-Y.; Louh, G.-Y.; Chin, T.-C.; Yeh, Y.-H.; Yeh, H.-L.; Yekondi, S.; et al. STRESS INDUCED FACTOR 2 Regulates Arabidopsis Stomatal Immunity through Phosphorylation of the Anion Channel SLAC1. Plant Cell 2020, 32, 2216–2236. [Google Scholar] [CrossRef]
- Munemasa, S.; Oda, K.; Watanabe-Sugimoto, M.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. The Coronatine—Insensitive 1 Mutation Reveals the Hormonal Signaling Interaction between Abscisic Acid and Methyl Jasmonate in Arabidopsis Guard Cells. Specific Impairment of Ion Channel Activation and Second Messenger Production. Plant Physiol. 2007, 143, 1398–1407. [Google Scholar] [CrossRef] [Green Version]
- Melotto, M.; Underwood, W.; Koczan, J.; Nomura, K.; He, S.Y. Plant Stomata Function in Innate Immunity against Bacterial Invasion. Cell 2006, 126, 969–980. [Google Scholar] [CrossRef]
- MacRobbie, E.A.C. Signalling in Guard Cells and Regulation of Ion Channel Activity. J. Exp. Bot. 1997, 48, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Manthe, B.; Schulz, M.; Schnabl, H. Effects of Salicylic Acid on Growth and Stomatal Movements of Vicia faba L.: Evidence for Salicylic Acid Metabolization. J. Chem. Ecol. 1992, 18, 1525–1539. [Google Scholar] [CrossRef] [PubMed]
- Mori, I.C.; Pinontoan, R.; Kawano, T.; Muto, S. Involvement of Superoxide Generation in Salicylic Acid-Induced Stomatal Closure in Vicia faba. Plant Cell Physiol. 2001, 42, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Khokon, M.A.R.; Salam, M.A.; Jammes, F.; Ye, W.; Hossain, M.A.; Okuma, E.; Nakamura, Y.; Mori, I.C.; Kwak, J.M.; Murata, Y. MPK9 and MPK12 Function in SA-Induced Stomatal Closure in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2017, 81, 1394–1400. [Google Scholar] [CrossRef] [Green Version]
- Zottini, M.; Costa, A.; De Michele, R.; Ruzzene, M.; Carimi, F.; Lo Schiavo, F. Salicylic Acid Activates Nitric Oxide Synthesis in Arabidopsis. J. Exp. Bot. 2007, 58, 1397–1405. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Pang, Q.; Dai, S.; Wang, Y.; Chen, S.; Yan, X. Proteomic Identification of Differentially Expressed Proteins in Arabidopsis in Response to Methyl Jasmonate. J. Plant Physiol. 2011, 168, 995–1008. [Google Scholar] [CrossRef]
- Sirhindi, G.; Mushtaq, R.; Gill, S.S.; Sharma, P.; Abd_Allah, E.F.; Ahmad, P. Jasmonic Acid and Methyl Jasmonate Modulate Growth, Photosynthetic Activity and Expression of Photosystem II Subunit Genes in Brassica oleracea L. Sci. Rep. 2020, 10, 9322. [Google Scholar] [CrossRef]
- Böhm, J.; Scherzer, S.; Krol, E.; Kreuzer, I.; von Meyer, K.; Lorey, C.; Mueller, T.D.; Shabala, L.; Monte, I.; Solano, R.; et al. The Venus Flytrap Dionaea muscipula Counts Prey-Induced Action Potentials to Induce Sodium Uptake. Curr. Biol. 2016, 26, 286–295. [Google Scholar] [CrossRef] [Green Version]
- Vian, A.; Henry-Vian, C.; Schantz, R.; Ledoigt, G.; Frachisse, J.-M.; Desbiez, M.-O.; Julien, J.-L. Is Membrane Potential Involved in Calmodulin Gene Expression after External Stimulation in Plants? FEBS Lett. 1996, 380, 93–96. [Google Scholar] [CrossRef] [Green Version]
- Major, I.T.; Yoshida, Y.; Campos, M.L.; Kapali, G.; Xin, X.; Sugimoto, K.; Oliveira Ferreira, D.; He, S.Y.; Howe, G.A. Regulation of Growth–Defense Balance by the JASMONATE ZIM-DOMAIN (JAZ)-MYC Transcriptional Module. New Phytol. 2017, 215, 1533–1547. [Google Scholar] [CrossRef]
- Guo, Q.; Yoshida, Y.; Major, I.T.; Wang, K.; Sugimoto, K.; Kapali, G.; Havko, N.E.; Benning, C.; Howe, G.A. JAZ Repressors of Metabolic Defense Promote Growth and Reproductive Fitness in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, E10768–E10777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chehab, E.W.; Yao, C.; Henderson, Z.; Kim, S.; Braam, J. Arabidopsis Touch-Induced Morphogenesis Is Jasmonate Mediated and Protects against Pests. Curr. Biol. 2012, 22, 701–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erb, M.; Reymond, P. Molecular Interactions Between Plants and Insect Herbivores. Annu. Rev. Plant Biol. 2019, 70, 527–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, H.S.; Koo, A.J.K.; Gao, X.; Jayanty, S.; Thines, B.; Jones, A.D.; Howe, G.A. Regulation and Function of Arabidopsis JASMONATE ZIM-Domain Genes in Response to Wounding and Herbivory. Plant Physiol. 2008, 146, 952–964. [Google Scholar] [CrossRef] [Green Version]
- Reymond, P.; Weber, H.; Damond, M.; Farmer, E.E. Differential Gene Expression in Response to Mechanical Wounding and Insect Feeding in Arabidopsis. Plant Cell 2000, 12, 707–719. [Google Scholar] [CrossRef] [Green Version]
- Clarke, S.M.; Cristescu, S.M.; Miersch, O.; Harren, F.J.M.; Wasternack, C.; Mur, L.A.J. Jasmonates Act with Salicylic Acid to Confer Basal Thermotolerance in Arabidopsis thaliana. New Phytol. 2009, 182, 175–187. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, L.; Wang, F.; Yu, D. Jasmonate Regulates the INDUCER OF CBF EXPRESSION-C-REPEAT BINDING FACTOR/DRE BINDING FACTOR1 Cascade and Freezing Tolerance in Arabidopsis. Plant Cell 2013, 25, 2907–2924. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Guo, Z.; Li, H.; Wang, M.; Onac, E.; Zhou, J.; Xia, X.; Shi, K.; Yu, J.; Zhou, Y. Phytochrome A and B Function Antagonistically to Regulate Cold Tolerance via Abscisic Acid-Dependent Jasmonate Signaling. Plant Physiol. 2016, 170, 459–471. [Google Scholar] [CrossRef] [Green Version]
- Blanco, F.; Salinas, P.; Cecchini, N.M.; Jordana, X.; Van Hummelen, P.; Alvarez, M.E.; Holuigue, L. Early Genomic Responses to Salicylic Acid in Arabidopsis. Plant Mol. Biol. 2009, 70, 79–102. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Vásquez, A.; Salinas, P.; Holuigue, L. Salicylic Acid and Reactive Oxygen Species Interplay in the Transcriptional Control of Defense Genes Expression. Front. Plant Sci. 2015, 6, 00171. [Google Scholar] [CrossRef]
- Lukan, T.; Coll, A. Intertwined Roles of Reactive Oxygen Species and Salicylic Acid Signaling Are Crucial for the Plant Response to Biotic Stress. IJMS 2022, 23, 5568. [Google Scholar] [CrossRef]
- Mateo, A. Controlled Levels of Salicylic Acid Are Required for Optimal Photosynthesis and Redox Homeostasis. J. Exp. Bot. 2006, 57, 1795–1807. [Google Scholar] [CrossRef] [Green Version]
- Fryer, M.J.; Ball, L.; Oxborough, K.; Karpinski, S.; Mullineaux, P.M.; Baker, N.R. Control of Ascorbate Peroxidase 2 Expression by Hydrogen Peroxide and Leaf Water Status during Excess Light Stress Reveals a Functional Organisation of Arabidopsis Leaves. Plant J. 2003, 33, 691–705. [Google Scholar] [CrossRef]
- Caarls, L.; Pieterse, C.M.J.; Van Wees, S.C.M. How Salicylic Acid Takes Transcriptional Control over Jasmonic Acid Signaling. Front. Plant Sci. 2015, 6, 00170. [Google Scholar] [CrossRef]
- Ndamukong, I.; Abdallat, A.A.; Thurow, C.; Fode, B.; Zander, M.; Weigel, R.; Gatz, C. SA-Inducible Arabidopsis Glutaredoxin Interacts with TGA Factors and Suppresses JA-Responsive PDF1.2 Transcription: Interaction of Glutaredoxin with TGA Factors. Plant J. 2007, 50, 128–139. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ladeynova, M.; Kuznetsova, D.; Mudrilov, M.; Vodeneev, V. Integration of Electrical Signals and Phytohormones in the Control of Systemic Response. Int. J. Mol. Sci. 2023, 24, 847. https://doi.org/10.3390/ijms24010847
Ladeynova M, Kuznetsova D, Mudrilov M, Vodeneev V. Integration of Electrical Signals and Phytohormones in the Control of Systemic Response. International Journal of Molecular Sciences. 2023; 24(1):847. https://doi.org/10.3390/ijms24010847
Chicago/Turabian StyleLadeynova, Maria, Darya Kuznetsova, Maxim Mudrilov, and Vladimir Vodeneev. 2023. "Integration of Electrical Signals and Phytohormones in the Control of Systemic Response" International Journal of Molecular Sciences 24, no. 1: 847. https://doi.org/10.3390/ijms24010847




