From Coffee Waste to Active Ingredient for Cosmetic Applications
Abstract
1. Introduction
2. Results
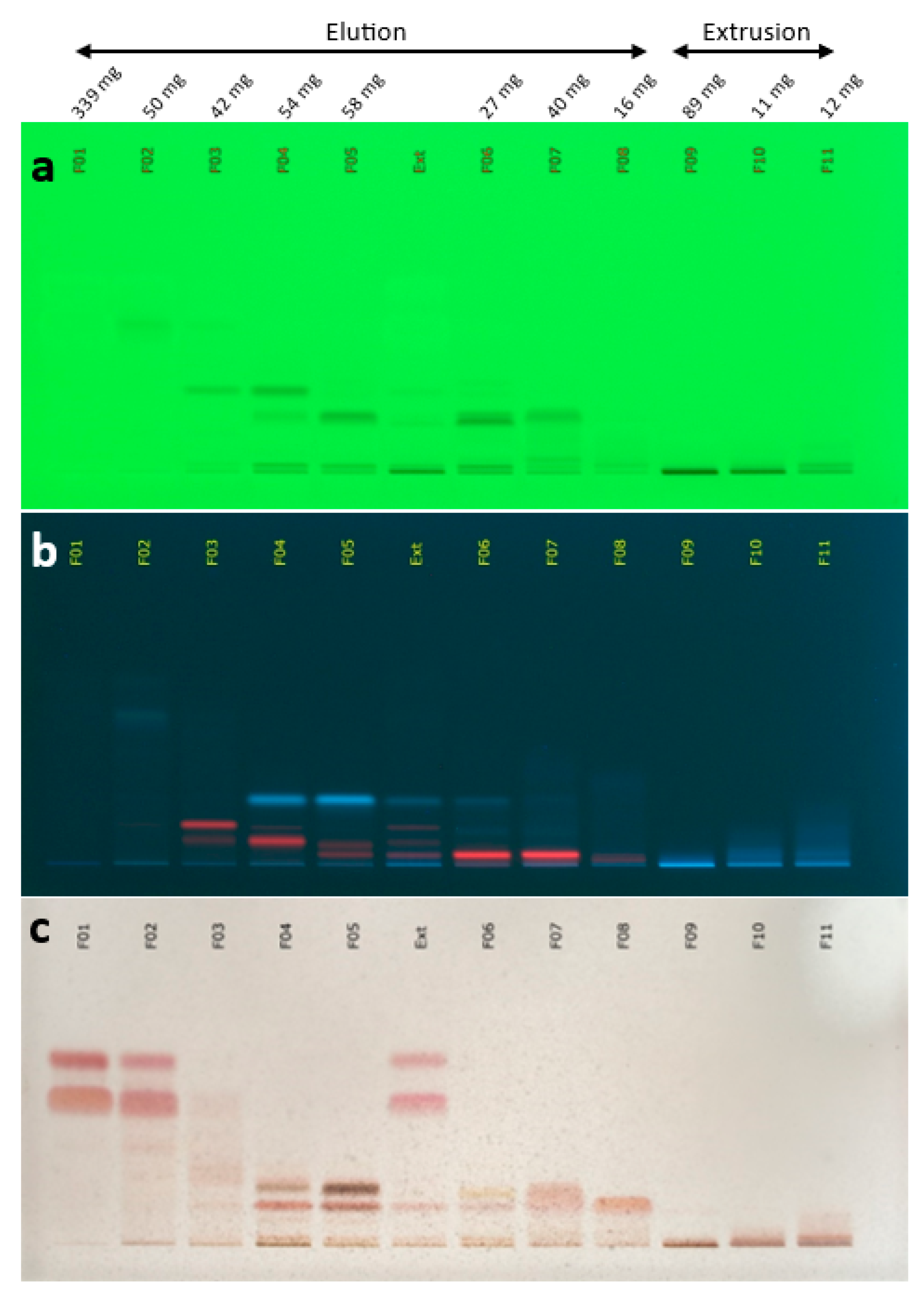
2.1. Coffee Silverskin Sourcing and Supercritical CO2 Extraction
2.2. Chemical Profiling of the Coffee Silverskin Extract
2.3. Gene Expression Analysis on Keratinocytes
2.4. Coffee Silverskin Active (CSA) Reduces Skin Sensitivity
2.5. Coffee Silverskin Active Rehydrates Dry Legs
2.6. Coffee Silverskin Active Protects against SLS Irritation
2.7. Coffee Silverskin Active Induces Faster Skin Regeneration after SLS Irritation
3. Discussion
4. Materials and Methods
4.1. Coffee Silverskin Supercritical CO2 Extraction
4.2. Centrifugal Partition Chromatography (CPC)
4.3. NMR Analyses and Metabolite Identification
4.4. High Resolution Liquid Chromatography Mass Spectrometry
4.5. Keratinocyte Culture and Treatment
4.6. Differential Expression Analysis
4.7. RNA Extraction and Reverse Transcription
4.8. Quantitative PCR
- -
- 2.5 μL of cDNA,
- -
- primers (forward and reverse),
- -
- reagent mix (Ozyme) containing taq DNA polymerase, SYBR Green I and MgCl2.
4.9. Data Management of Quantitative PCR
4.10. In Vivo Skin Protection and Regeneration Test
4.11. Lactic Acid Stinging Test
4.12. In Vivo Hydration Measurement on Dry Legs
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bessada, S.M.F.; Alves, R.C.; Oliveira, M.B.P.P. Coffee Silverskin: A Review on Potential Cosmetic Applications. Cosmetics 2018, 5, 5. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Brand, D.; Mohan, R.; Roussos, S. Biotechnological potential of coffee pulp and coffee husk for bioprocesses. Biochem. Eng. J. 2000, 6, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; Palmeira-De-Oliveira, A.; Das Neves, J.; Sarmento, B.; Amaral, M.H.; Oliveira, M.B.P.P. Coffee silverskin: A possible valuable cosmetic ingredient. Pharm. Biol. 2015, 53, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Saenger, M.; Hartge, E.U.; Werther, J.; Ogada, T.; Siagi, Z. Combustion of coffee husks. Renew. Energy 2001, 23, 103–121. [Google Scholar] [CrossRef]
- Barbero-López, A.; Monzó-Beltrán, J.; Virjamo, V.; Akkanen, J.; Haapala, A. Revalorization of coffee silverskin as a potential feedstock for antifungal chemicals in wood preservation. Int. Biodeterior. Biodegrad. 2020, 152, 105011. [Google Scholar] [CrossRef]
- Kristanto, G.A.; Wijaya, H. Assessment of spent coffee ground (SCG) and coffee silverskin (CS) as refuse derived fuel (RDF). IOP Conf. Ser. Earth Environ. Sci. 2018, 195, 012056. [Google Scholar] [CrossRef]
- Nzekoue, F.K.; Borsetta, G.; Navarini, L.; Abouelenein, D.; Xiao, J.; Sagratini, G.; Vittori, S.; Caprioli, G.; Angeloni, S. Coffee silverskin: Characterization of B-vitamins, macronutrients, minerals and phytosterols. Food Chem. 2022, 372, 131188. [Google Scholar] [CrossRef]
- Borrelli, R.C.; Esposito, F.; Napolitano, A.; Ritieni, A.; Fogliano, V. Characterization of a new potential functional ingredient: Coffee silverskin. J. Agric. Food Chem. 2004, 52, 1338–1343. [Google Scholar] [CrossRef]
- Narita, Y.; Inouye, K. High antioxidant activity of coffee silverskin extracts obtained by the treatment of coffee silverskin with subcritical water. Food Chem. 2012, 135, 943–949. [Google Scholar] [CrossRef]
- Rodrigues, F.; Pereira, C.; Pimentel, F.B.; Alves, R.C.; Ferreira, M.; Sarmento, B.; Oliveira, M.B.P. Are coffee silverskin extracts safe for topical use? An in vitro and in vivo approach. Ind. Crops Prod. 2015, 63, 167–174. [Google Scholar] [CrossRef]
- Pappas, A. Epidermal surface lipids. Derm.-Endocrinol. 2009, 1, 72–76. [Google Scholar] [CrossRef] [PubMed]
- van Smeden, J.; Janssens, M.; Kaye, E.C.; Caspers, P.J.; Lavrijsen, A.P.; Vreeken, R.J.; Bouwstra, J.A. The importance of free fatty acid chain length for the skin barrier function in atopic eczema patients. Exp. Dermatol. 2014, 23, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Speer, K.; Kölling-Speer, I. The lipid fraction of the coffee bean. Braz. J. Plant Physiol. 2006, 18, 201–216. [Google Scholar] [CrossRef]
- Patay, E.B.; Bencsik, T.; Papp, N. Phytochemical overview and medicinal importance of Coffea species from the past until now. Asian Pac. J. Trop. Med. 2016, 9, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, B.; Villarreal, D.; Laffargue, A.; Posada, H.; Lashermes, P.; Dussert, S. Comparison of the effectiveness of fatty acids, chlorogenic acids, and elements for the chemometric discrimination of coffee (Coffea arabica L.) varieties and growing origins. J. Agric. Food Chem. 2008, 56, 2273–2280. [Google Scholar] [CrossRef]
- Panusa, A.; Petrucci, R.; Lavecchia, R.; Zuorro, A. UHPLC-PDA-ESI-TOF/MS metabolic profiling and antioxidant capacity of arabica and robusta coffee silverskin: Antioxidants vs. phytotoxins. Food Res. Int. 2017, 99, 155–165. [Google Scholar] [CrossRef]
- Zorić, M.; Banožić, M.; Aladić, K.; Vladimir-Knežević, S.; Jokić, S. Supercritical CO2 extracts in cosmetic industry: Current status and future perspectives. Sustain. Chem. Pharm. 2022, 27, 100688. [Google Scholar] [CrossRef]
- Radner, F.P.; Fischer, J. The important role of epidermal triacylglycerol metabolism for maintenance of the skin permeability barrier function. Biochim. Biophys. Acta 2014, 1841, 409–415. [Google Scholar] [CrossRef]
- Kim, S.-J. The Ameliorative Effect of β-sitosterol on DNCB-induced Atopic Dermatitis in Mice. Biomed. Sci. Lett. 2017, 23, 303–309. [Google Scholar] [CrossRef]
- Haiyuan, Y.U.; Shen, X.; Liu, D.; Hong, M.; Lu, Y. The protective effects of beta-sitosterol and vermicularin from Thamnolia vermicularis (Sw.) Ach. against skin aging in vitro. An. Acad. Bras. Ciênc. 2019, 91, e20181088. [Google Scholar] [CrossRef]
- Imai, T.; Takase, Y.; Iwase, H.; Hashimoto, M. Involvement of Carboxylesterase in Hydrolysis of Propranolol Prodrug during Permeation across Rat Skin. Pharmaceutics 2013, 5, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Acosta, F.; Planas, L.; Penneys, N.S. Lipase expression in human skin. J. Dermatol. Sci. 1990, 1, 195–200. [Google Scholar] [CrossRef]
- Stinchcomb, A.L.; Swaan, P.W.; Ekabo, O.; Harris, K.K.; Browe, J.; Hammell, D.C.; Cooperman, T.A.; Pearsall, M. Straight-chain naltrexone ester prodrugs: Diffusion and concurrent esterase biotransformation in human skin. J. Pharm. Sci. 2002, 91, 2571–2578. [Google Scholar] [CrossRef] [PubMed]
- Batz, F.M.; Klipper, W.; Korting, H.C.; Henkler, F.; Landsiedel, R.; Luch, A.; von Fritschen, U.; Weindl, G.; Schafer-Korting, M. Esterase activity in excised and reconstructed human skin--biotransformation of prednicarbate and the model dye fluorescein diacetate. Eur. J. Pharm. Biopharm. 2013, 84, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Sadgrove, M.; Marson, L.; Jay, M. Biotransformation Capacity of Carboxylesterase in Skin and Keratinocytes for the Penta-Ethyl Ester Prodrug of DTPA. Drug Metab. Dispos. 2016, 44, 1313–1318. [Google Scholar] [CrossRef]
- De Roos, B.; Van Tol, A.; Urgert, R.; Scheek, L.M.; Van Gent, T.; Buytenhek, R.; Princen, H.M.; Katan, M.B. Consumption of French-press coffee raises cholesteryl ester transfer protein activity levels before LDL cholesterol in normolipidaemic subjects. J. Intern. Med. 2000, 248, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Pluchinotta, F.R.; Marventano, S.; Buscemi, S.; Li Volti, G.; Galvano, F.; Grosso, G. Coffee components and cardiovascular risk: Beneficial and detrimental effects. Int. J. Food Sci. Nutr. 2014, 65, 925–936. [Google Scholar] [CrossRef]
- Cardenas, C.; Quesada, A.R.; Medina, M.A. Anti-angiogenic and anti-inflammatory properties of kahweol, a coffee diterpene. PLoS ONE 2011, 6, e23407. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, K.S.; Jeong, H.G. Suppressive effects of the kahweol and cafestol on cyclooxygenase-2 expression in macrophages. FEBS Lett. 2004, 569, 321–326. [Google Scholar] [CrossRef]
- Choi, M.J.; Park, E.J.; Oh, J.H.; Min, K.J.; Yang, E.S.; Kim, Y.H.; Lee, T.J.; Kim, S.H.; Choi, Y.H.; Park, J.W.; et al. Cafestol, a coffee-specific diterpene, induces apoptosis in renal carcinoma Caki cells through down-regulation of anti-apoptotic proteins and Akt phosphorylation. Chem. Biol. Interact. 2011, 190, 102–108. [Google Scholar] [CrossRef]
- Mellbye, F.B.; Jeppesen, P.B.; Hermansen, K.; Gregersen, S. Cafestol, a Bioactive Substance in Coffee, Stimulates Insulin Secretion and Increases Glucose Uptake in Muscle Cells: Studies in Vitro. J. Nat. Prod. 2015, 78, 2447–2451. [Google Scholar] [CrossRef] [PubMed]
- Fumimoto, R.; Sakai, E.; Yamaguchi, Y.; Sakamoto, H.; Fukuma, Y.; Nishishita, K.; Okamoto, K.; Tsukuba, T. The coffee diterpene kahweol prevents osteoclastogenesis via impairment of NFATc1 expression and blocking of Erk phosphorylation. J. Pharmacol. Sci. 2012, 118, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hossain, M.A.; Kim, J.H.; Cho, J.Y. Kahweol Exerts Skin Moisturizing Activities by Upregulating STAT1 Activity. Int. J. Mol. Sci. 2021, 22, 8864. [Google Scholar] [CrossRef]
- Lang, T.; Lang, R.; Di Pizio, A.; Mittermeier, V.K.; Schlagbauer, V.; Hofmann, T.; Behrens, M. Numerous Compounds Orchestrate Coffee’s Bitterness. J. Agric. Food Chem. 2020, 68, 6692–6700. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.; Mansfield, C.; Colquitt, L.; Lin, C.; Ferreira, J.; Emmetsberger, J.; Reed, D.R. Personalized expression of bitter ‘taste’ receptors in human skin. PLoS ONE 2018, 13, e0205322. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.G.; Kim, Y.; Cha, Y.K.; Park, T.H.; Kim, Y. Bitter taste receptors protect against skin aging by inhibiting cellular senescence and enhancing wound healing. Nutr. Res. Pract. 2022, 16, 1–13. [Google Scholar] [CrossRef]
- Li, Y.F.; Ouyang, S.H.; Tu, L.F.; Wang, X.; Yuan, W.L.; Wang, G.E.; Wu, Y.P.; Duan, W.J.; Yu, H.M.; Fang, Z.Z.; et al. Caffeine Protects Skin from Oxidative Stress-Induced Senescence through the Activation of Autophagy. Theranostics 2018, 8, 5713–5730. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Caffeine’s mechanisms of action and its cosmetic use. Ski. Pharmacol. Physiol. 2013, 26, 8–14. [Google Scholar] [CrossRef]
- Saint-Martory, C.; Roguedas-Contios, A.M.; Sibaud, V.; Degouy, A.; Schmitt, A.M.; Misery, L. Sensitive skin is not limited to the face. Br. J. Dermatol. 2008, 158, 130–133. [Google Scholar] [CrossRef]
- Effendy, I.; Loeffler, H.; Maibach, H.I. Baseline transepidermal water loss in patients with acute and healed irritant contact dermatitis. Contact Dermat. 1995, 33, 371–374. [Google Scholar] [CrossRef]
- Goffin, V.; Pierard-Franchimont, C.; Pierard, G.E. Sensitive skin and stratum corneum reactivity to household cleaning products. Contact Dermat. 1996, 34, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Misery, L.; Loser, K.; Stander, S. Sensitive skin. J. Eur. Acad. Dermatol. Venereol. 2016, 30 (Suppl. S1), 2–8. [Google Scholar] [CrossRef] [PubMed]
- Gutowska-Owsiak, D.; de La Serna, J.B.; Fritzsche, M.; Naeem, A.; Podobas, E.I.; Leeming, M.; Colin-York, H.; O’Shaughnessy, R.; Eggeling, C.; Ogg, G.S. Orchestrated control of filaggrin-actin scaffolds underpins cornification. Cell Death Dis. 2018, 9, 412. [Google Scholar] [CrossRef] [PubMed]
- Hoober, J.K.; Eggink, L.L. The Discovery and Function of Filaggrin. Int. J. Mol. Sci. 2022, 23, 1455. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.; Oliveira, M.B.P.P.; Alves, R.C. Chlorogenic Acids and Caffeine from Coffee By-Products: A Review on Skincare Applications. Cosmetics 2023, 10, 12. [Google Scholar] [CrossRef]
- Kezic, S.; Kemperman, P.M.; Koster, E.S.; de Jongh, C.M.; Thio, H.B.; Campbell, L.E.; Irvine, A.D.; McLean, W.H.; Puppels, G.J.; Caspers, P.J. Loss-of-function mutations in the filaggrin gene lead to reduced level of natural moisturizing factor in the stratum corneum. J. Investig. Dermatol. 2008, 128, 2117–2119. [Google Scholar] [CrossRef]






| Gene Name | Gene Expression Compared to Control (%) |
|---|---|
| HMOX1 | 137% |
| CALML5 | 124% |
| FLG | 150% |
| SPRR1A | 125% |
| % Satisfaction Placebo | % Satisfaction CSA | |
|---|---|---|
| My skin seems smoothed | 81 | 86 |
| My skin is less red | 81 | 90 |
| My skin looks younger | 67 | 71 |
| My skin is more hydrated | 81 | 100 |
| My skin is less dry | 86 | 100 |
| My skin seems firmer | 71 | 86 |
| My skin seems more elastic (flexible) | 76 | 86 |
| My skin seems more radiant | 67 | 71 |
| My skin is less scaly | 90 | 95 |
| % Satisfaction Placebo | % Satisfaction CSA | |
|---|---|---|
| The treatment hydrates the skin | 85.7 | 95.2 |
| The treatment makes my skin smoother | 81.0 | 95.2 |
| The treatment reduces skin redness | 81.0 | 85.7 |
| The treatment prepares my skin for irritation | 71.4 | 76.2 |
| The treatment calms my skin after irritation | 85.7 | 90.5 |
| The treatment helps my skin to recover faster from irritation | 85.7 | 90.5 |
| The treatment reduces the itchiness after irritation | 85.7 | 90.5 |
| The treatment reduces the stinging after irritation | 85.7 | 90.5 |
| The treatment homogenizes my skin tone after irritation | 71.4 | 81.0 |
| The treatment reduces skin dryness after irritation | 81.0 | 90.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigolon, G.; Nowak, K.; Poigny, S.; Hubert, J.; Kotland, A.; Waldschütz, L.; Wandrey, F. From Coffee Waste to Active Ingredient for Cosmetic Applications. Int. J. Mol. Sci. 2023, 24, 8516. https://doi.org/10.3390/ijms24108516
Grigolon G, Nowak K, Poigny S, Hubert J, Kotland A, Waldschütz L, Wandrey F. From Coffee Waste to Active Ingredient for Cosmetic Applications. International Journal of Molecular Sciences. 2023; 24(10):8516. https://doi.org/10.3390/ijms24108516
Chicago/Turabian StyleGrigolon, Giovanna, Kathrin Nowak, Stéphane Poigny, Jane Hubert, Alexis Kotland, Laura Waldschütz, and Franziska Wandrey. 2023. "From Coffee Waste to Active Ingredient for Cosmetic Applications" International Journal of Molecular Sciences 24, no. 10: 8516. https://doi.org/10.3390/ijms24108516
APA StyleGrigolon, G., Nowak, K., Poigny, S., Hubert, J., Kotland, A., Waldschütz, L., & Wandrey, F. (2023). From Coffee Waste to Active Ingredient for Cosmetic Applications. International Journal of Molecular Sciences, 24(10), 8516. https://doi.org/10.3390/ijms24108516






