The Effect of Milling on the Ethanolic Extract Composition of Dried Walnut (Juglans regia L.) Shells
Abstract
:1. Introduction
2. Results and Discussions
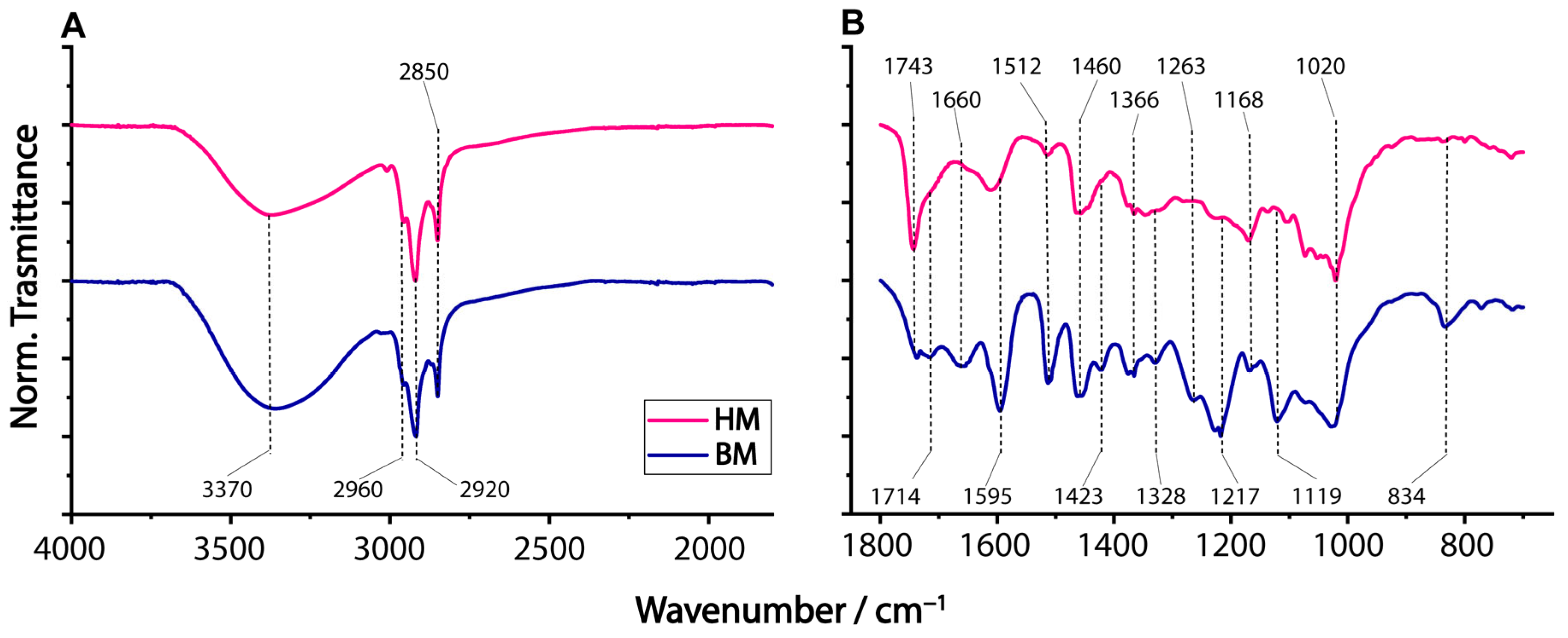
2.1. ATR-FTIR Characterization of Ball Milling Ethanolic Extracts of Walnut Shells
2.2. Separation and Detection of Fatty Acids by RPLC-ESI-MS
2.3. Double Bond Position by Tandem MS of Epoxidized FAs
2.4. LC-MS Characterization of Phenolic Compounds in Walnut Shell Extracts
2.5. Total Phenol Content and Radical Scavenger Activity (RSA)
3. Materials and Methods
3.1. Chemicals
3.2. Ethanol Extraction of Dried Walnut Shells
3.3. Sample Preparation
3.4. Attenuated Total Reflection (ATR) Fourier-Transformed Infrared Spectroscopy (FTIR)
3.5. RPLC-ESI-MS Instrumentation and Operating Conditions
3.6. Determination of Total Polyphenol Content (TPC) by Folin–Ciocalteu Assay
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F.; et al. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2013, 6, 426. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Jiang, P.; Hu, L.; Zhi, Z.; Chen, J.; Ding, T.; Ye, X.; Liu, D. Characterization of pectin from grapefruit peel: A comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocoll. 2016, 61, 730–739. [Google Scholar] [CrossRef]
- Ayrilmis, N.; Kaymakci, A.; Ozdemir, F. Physical, mechanical, and thermal properties of polypropylene composites filled with walnut shell flour. J. Ind. Eng. Chem. 2013, 19, 908–914. [Google Scholar] [CrossRef]
- Nazari, G.; Abolghasemi, H.; Esmaieli, M.; Sadeghi Pouya, E. Aqueous phase adsorption of cephalexin by walnut shell-based activated carbon: A fixed-bed column study. Appl. Surf. Sci. 2016, 375, 144–153. [Google Scholar] [CrossRef]
- Altun, T.; Pehlivan, E. Removal of Cr(VI) from aqueous solutions by modified walnut shells. Food Chem. 2012, 132, 693–700. [Google Scholar] [CrossRef]
- da Silva, C.F.; Stefanowski, B.; Maskell, D.; Ormondroyd, G.A.; Ansell, M.P.; Dengel, A.C.; Ball, R.J. Improvement of indoor air quality by MDF panels containing walnut shells. Build. Environ. 2017, 123, 427–436. [Google Scholar] [CrossRef]
- Xu, X.; Gao, J.; Tian, Q.; Zhai, X.; Liu, Y. Walnut shell derived porous carbon for a symmetric all-solid-state supercapacitor. Appl. Surf. Sci. 2017, 411, 170–176. [Google Scholar] [CrossRef]
- Nishide, R.N.; Truong, J.H.; Abu-Omar, M.M. Organosolv Fractionation of Walnut Shell Biomass to Isolate Lignocellulosic Components for Chemical Upgrading of Lignin to Aromatics. ACS Omega 2021, 6, 8142–8150. [Google Scholar] [CrossRef]
- Zijlstra, D.S.; Lahive, C.W.; Analbers, C.A.; Figueirêdo, M.B.; Wang, Z.; Lancefield, C.S.; Deuss, P.J. Mild Organosolv Lignin Extraction with Alcohols: The Importance of Benzylic Alkoxylation. ACS Sustain. Chem. Eng. 2020, 8, 5119–5131. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Amarowicz, R. Walnut (Juglans regia L.) shell pyroligneous acid: Chemical constituents and functional applications. RSC Adv. 2018, 8, 22376–22391. [Google Scholar] [CrossRef]
- Rodríguez-Padrón, D.; Zhao, D.; Garín Ortega, R.N.; Len, C.; Balu, A.M.; García, A.; Luque, R. Characterization and Antioxidant Activity of Microwave-Extracted Phenolic Compounds from Biomass Residues. ACS Sustain. Chem. Eng. 2020, 8, 1513–1519. [Google Scholar] [CrossRef]
- Blasi, D.; Mesto, D.; Cotugno, P.; Calvano, C.D.; Lo Presti, M.; Farinola, G.M. Revealing the effects of the ball milling pretreatment on the ethanosolv fractionation of lignin from walnut and pistachio shells. Green Chem. Lett. Rev. 2022, 15, 893–902. [Google Scholar] [CrossRef]
- Shen, D.; Yuan, X.; Zhao, Z.; Wu, S.; Liao, L.; Tang, F.; Bi, L.; Liu, Y. Determination of Phenolic Compounds in Walnut Kernel and Its Pellicle by Ultra-high-Performance Liquid Chromatography-Tandem Mass Spectrometry. Food Anal. Methods 2021, 14, 2408–2419. [Google Scholar] [CrossRef]
- Slatnar, A.; Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Solar, A. Identification and quantification of phenolic compounds in kernels, oil and bagasse pellets of common walnut (Juglans regia L.). Food Res. Int. 2015, 67, 255–263. [Google Scholar] [CrossRef]
- Regueiro, J.; Sánchez-González, C.; Vallverdú-Queralt, A.; Simal-Gándara, J.; Lamuela-Raventós, R.; Izquierdo-Pulido, M. Comprehensive identification of walnut polyphenols by liquid chromatography coupled to linear ion trap-Orbitrap mass spectrometry. Food Chem. 2014, 152, 340–348. [Google Scholar] [CrossRef]
- Herrera, R.; Hemming, J.; Smeds, A.; Gordobil, O.; Willför, S.; Labidi, J. Recovery of bioactive compounds from hazelnuts and walnuts shells: Quantitative–qualitative analysis and chromatographic purification. Biomolecules 2020, 10, 1363. [Google Scholar] [CrossRef]
- Queirós, C.S.G.P.; Cardoso, S.; Lourenço, A.; Ferreira, J.; Miranda, I.; Lourenço, M.J.V.; Pereira, H. Characterization of walnut, almond, and pine nut shells regarding chemical composition and extract composition. Biomass Convers. Biorefinery 2020, 10, 175–188. [Google Scholar] [CrossRef]
- Vecka, M.; Staňková, B.; Kutová, S.; Tomášová, P.; Tvrzická, E.; Žák, A. Comprehensive sterol and fatty acid analysis in nineteen nuts, seeds, and kernel. SN Appl. Sci. 2019, 1, 1531. [Google Scholar] [CrossRef]
- Heidari, M.; Talebpour, Z.; Abdollahpour, Z.; Adib, N.; Ghanavi, Z.; Aboul-Enein, H.Y. Discrimination between vegetable oil and animal fat by a metabolomics approach using gas chromatography–mass spectrometry combined with chemometrics. J. Food Sci. Technol. 2020, 57, 3415–3425. [Google Scholar] [CrossRef]
- Ventura, G.; Calvano, C.D.; Blasi, D.; Coniglio, D.; Losito, I.; Cataldi, T.R.I. Uncovering heterogeneity of anacardic acids from pistachio shells: A novel approach for structural characterization. Food Chem. 2023, 426, 136636. [Google Scholar] [CrossRef]
- Coniglio, D.; Ventura, G.; Calvano, C.D.; Losito, I.; Cataldi, T.R.I. Positional Assignment of C−C Double Bonds in Fatty Acyl Chains of Intact Arsenosugar Phospholipids Occurring in Seaweed Extracts by Epoxidation Reactions. J. Am. Soc. Mass Spectrom. 2022, 33, 823–831. [Google Scholar] [CrossRef] [PubMed]
- El Hage, R.; Brosse, N.; Chrusciel, L.; Sanchez, C.; Sannigrahi, P.; Ragauskas, A. Characterization of milled wood lignin and ethanol organosolv lignin from miscanthus. Polym. Degrad. Stab. 2009, 94, 1632–1638. [Google Scholar] [CrossRef]
- Xu, F.; Jiang, J.-X.; Sun, R.-C.; Tang, J.-N.; Sun, J.-X.; Su, Y.-Q. Fractional isolation and structural characterization of mild ball-milled lignin in high yield and purity from Eucommia ulmoides Oliv. Wood Sci. Technol. 2008, 42, 211–226. [Google Scholar] [CrossRef]
- Hansen, B.; Kusch, P.; Schulze, M.; Kamm, B. Qualitative and Quantitative Analysis of Lignin Produced from Beech Wood by Different Conditions of the Organosolv Process. J. Polym. Environ. 2016, 24, 85–97. [Google Scholar] [CrossRef]
- Lu, X.; Wang, J.; Al-Qadiri, H.M.; Ross, C.F.; Powers, J.R.; Tang, J.; Rasco, B.A. Determination of total phenolic content and antioxidant capacity of onion (Allium cepa) and shallot (Allium oschaninii) using infrared spectroscopy. Food Chem. 2011, 129, 637–644. [Google Scholar] [CrossRef]
- Murphy, R.C. Tandem Mass Spectrometry of Lipids; Royal Society of Chemistry: Cambridge, UK, 2015. [Google Scholar] [CrossRef]
- Liebisch, G.; Vizcaíno, J.A.; Köfeler, H.; Trötzmüller, M.; Griffiths, W.J.; Schmitz, G.; Spener, F.; Wakelam, M.J.O.; Vizcaino, J.A.; Kofeler, H.; et al. Shorthand notation for lipid structures derived from mass spectrometry. J. Lipid Res. 2013, 54, 1523–1530. [Google Scholar] [CrossRef]
- Calvano, C.D.; Bianco, M.; Ventura, G.; Losito, I.; Palmisano, F.; Cataldi, T.R.I. analysis of phospholipids, lysophospholipids, and their linked fatty acyl chains in yellow lupin seeds (Lupinus luteus L.) by liquid chromatography and tandem mass spectrometry. Molecules 2020, 25, 805. [Google Scholar] [CrossRef]
- Coniglio, D.; Calvano, C.D.; Masiello, M.; Losito, I.; Sabbatini, L.; Cataldi, T.R.I. The combination of RPLC-ESI-FTMS/MS and m-CPBA epoxidation for the location and geometry assignment of double bonds in unsaturated fatty acyl chains of drying oils. J. Phys. Conf. Ser. 2022, 2204, 012091. [Google Scholar] [CrossRef]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Saturated fatty acids and risk of coronary heart disease: Modulation by replacement nutrients. Curr. Atheroscler. Rep. 2010, 12, 384–390. [Google Scholar] [CrossRef]
- Basiricò, L.; Morera, P.; Dipasquale, D.; Tröscher, A.; Bernabucci, U. Comparison between conjugated linoleic acid and essential fatty acids in preventing oxidative stress in bovine mammary epithelial cells. J. Dairy Sci. 2017, 100, 2299–2309. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, B.; Yu, Q.; Li, L. Identification of Double Bond Position Isomers in Unsaturated Lipids by m-CPBA Epoxidation and Mass Spectrometry Fragmentation. Anal. Chem. 2019, 91, 1791–1795. [Google Scholar] [CrossRef] [PubMed]
- Abbattista, R.; Losito, I.; Basile, G.; Castellaneta, A.; Ventura, G.; Calvano, C.D.; Cataldi, T.R.I. Hydrogen/Deuterium Exchange Mass Spectrometry for Probing the Isomeric Forms of Oleocanthal and Oleacin in Extra Virgin Olive Oils. Molecules 2023, 28, 2066. [Google Scholar] [CrossRef]
- Rashmi, H.B.; Negi, P.S. Phenolic acids from vegetables: A review on processing stability and health benefits. Food Res. Int. 2020, 136, 109298. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Faried, A.; Kurnia, D.; Faried, L.; Usman, N.; Miyazaki, T.; Kato, H.; Kuwano, H. Anticancer effects of gallic acid isolated from Indonesian herbal medicine, Phaleria macrocarpa (Scheff.) Boerl, on human cancer cell lines. Int. J. Oncol. 2007, 30, 605–613. [Google Scholar] [CrossRef]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A Review on Protocatechuic Acid and Its Pharmacological Potential. ISRN Pharmacol. 2014, 2014, 952943. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Castillo, C.M.S.; Caroca, R.; Lazo-Vélez, M.A.; Antonyak, H.; Polishchuk, A.; Lysiuk, R.; Oliinyk, P.; De Masi, L.; et al. Ellagic Acid: A Review on Its Natural Sources, Chemical Stability, and Therapeutic Potential. Oxid. Med. Cell Longev. 2022, 2022, 3848084. [Google Scholar] [CrossRef]
- Vattem, D.A.; Shetty, K. Biological functionality of ellagic acid: A review. J. Food Biochem. 2005, 29, 234–266. [Google Scholar] [CrossRef]
- Quideau, S. Chemistry and Biology of Ellagitannins: An Underestimated Class of Bioactive Plant Polyphenols; World Scientific: Singapore, 2009. [Google Scholar] [CrossRef]
- Haslam, E.; Cai, Y. Plant polyphenols (Vegetable tannins): Gallic acid metabolism. Nat. Prod. Rep. 1994, 11, 41–66. [Google Scholar] [CrossRef]
- Quideau, S.; Feldman, K.S. Ellagitannin chemistry. Chem. Rev. 1996, 96, 475–503. [Google Scholar] [CrossRef]
- Khanbabaee, K.; van Ree, T. Tannins: Classification and definition. Nat. Prod. Rep. 2001, 18, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, C.; Ciudad, C.J.; Noé, V.; Izquierdo-Pulido, M. Health benefits of walnut polyphenols: An exploration beyond their lipid profile. Crit. Rev. Food Sci. Nutr. 2017, 57, 3373–3383. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Maróstica Junior, M.R. Phenolic Compounds. In Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–50. [Google Scholar] [CrossRef]
- Abbattista, R.; Ventura, G.; Calvano, C.D.; Cataldi, T.R.I.; Losito, I. Bioactive compounds in waste by-products from olive oil production: Applications and structural characterization by mass spectrometry techniques. Foods 2021, 10, 1236. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.M.; Abouelenein, D.; Acquaticci, L.; Alessandroni, L.; Abd-Allah, R.H.; Borsetta, G.; Sagratini, G.; Maggi, F.; Vittori, S.; Caprioli, G. Effect of Roasting, Boiling, and Frying Processing on 29 Polyphenolics and Antioxidant Activity in Seeds and Shells of Sweet Chestnut (Castanea sativa Mill.). Plants 2021, 10, 2192. [Google Scholar] [CrossRef]
- Luque de Castro, M.D.; García Ayuso, L.E. Environmental Applications Soxhlet Extraction. In Encyclopedia of Separation Science; Elsevier: Amsterdam, The Netherlands, 2000; pp. 2701–2709. [Google Scholar] [CrossRef]
- Dewanto, V.; Xianzhong, W.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]







| N. | FA a | RT (min) | [M-H]− m/z | Relative Abundance (%) | epo-FA | RT (min) | [epoM-H]− m/z | DB Location b | Diagnostic Ions (m/z) | |
|---|---|---|---|---|---|---|---|---|---|---|
| HM | BM | |||||||||
| 1 | 14:0 | 35.4 | 227.2017 | 0.49 | 0.55 | - | - | - | - | - |
| 2 | 15:0 | 39.0 | 241.2173 | 0.39 | 0.45 | - | - | - | - | - |
| 3 | 16:0 | 41.1 | 255.2330 | 14.27 | 15.65 | - | - | - | - | - |
| 4 | 16:1 | 37.6 | 253.2173 | 0.67 | 0.76 | epo-FA 16:1 | 31.0 | 269.2111 | Δ9 | 155.108/171.103 |
| 5 | 17:0 | 44.7 | 269.2486 | 0.87 | 1.17 | - | - | - | - | - |
| 6 | 18:0 | 47.0 | 283.2643 | 9.83 | 10.49 | - | - | - | - | - |
| 7 | 18:1 | 43.5 | 281.2486 | 18.30 | 16.84 | epo-FA 18:1 | 35.9 36.5 | 297.2424 | Δ11 Δ9 | 183.139/199.134 155.108/171.103 |
| 8 | 18:2 | 40.1 | 279.2330 | 42.38 | 40.96 | epo2-FA 18:2 | 21.2 | 311.2216 | Δ9, 12 | 155.108/171.103 211.134/227.129 193.123/209.118 |
| Δ11, 14 | 183.139/199.134 | |||||||||
| 9 | 18:3 | 36.7 | 277.2173 | 9.60 | 9.90 | epo3-FA 18:3 | 4.50 | 325.2009 | Δ9, 12, 15 | 155.108/171.103 211.134/227.129 193.123/209.118 249.149, 267.160 |
| 10 | 19:0 | 49.1 | 297.2799 | 0.17 | 0.16 | - | - | - | - | - |
| 11 | 20:0 | 50.8 | 311.2956 | 0.50 | 0.51 | - | - | - | - | - |
| 12 | 20:1 | 47.9 | 309.2799 | 0.16 | 0.17 | epo-FA 20:1 | 41.5 | 325.2009 | Δ11 | 183.139/199.134 |
| 13 | 21:0 | 52.4 | 325.3112 | 0.20 | 0.23 | - | - | - | - | - |
| 14 | 22:0 | 53.8 | 339.3269 | 0.58 | 0.61 | - | - | - | - | - |
| 15 | 23:0 | 55.0 | 353.3425 | 0.57 | 0.58 | - | - | - | - | - |
| 16 | 24:0 | 56.2 | 367.3582 | 1.02 | 0.97 | - | - | - | - | - |
| n | m/z [M-H]− | RT | Formula | MS/MS Fragments | Putative Identification a | Classification | HM | BM |
|---|---|---|---|---|---|---|---|---|
| 1 | 191.0561 | 1.5 | C7H12O6 | 191.056 (100)–85.029 (10)–173.040 (5) | Quinic acid | Hydrolyzable Tannins | 0.18 | 0.43 |
| 2 | 331.0671 | 1.5 | C13H16O10 | 169.014 (100)–211.025 (30)–271.046 (25) | Monogalloyl-glucose (1) | Hydrolyzable Tannins | 0.05 | 1.02 |
| 3 | 481.0624 | 1.5 | C20H18O14 | 300.999 (100)–275.020 (60)–249.040 (10) | HHDP-glucose (1) | Hydrolyzable Tannins | n.d. | 4.63 |
| 4 | 331.0671 | 2.0 | C13H16O10 | 169.014 (100)–211.025 (50)–271.046 (20) | Monogalloyl-glucose (2) | Hydrolyzable Tannins | 0.03 | 0.6 |
| 5 | 481.0624 | 2.0 | C20H18O14 | 300.999 (100)–275.020 (60)–249.040 (10) | HHDP-glucose (2) | Hydrolyzable Tannins | n.d. | 2.8 |
| 6 | 169.0143 | 2.2 | C7H6O5 | 125.024 (100)–169.0142 (40) | Gallic Acid | Phenolic acid | 0.1 | 2.93 |
| 7 | 331.0671 | 2.3 | C13H16O10 | 169.014 (100)–125.024 (15)–271.046 (2) | Monogalloyl-glucose (3) | Hydrolyzable Tannins | n.d. | 0.26 |
| 8 | 181.0507 | 3.4 | C9H10O4 | 151.040 (100)–123.045 (50) | Syringaldehyde | Phenolic acid | 13.13 | 4.76 |
| 9 | 783.0687 391.0312 b | 3.9 | C34H24O22 | 300.999 (100)–275.020 (45)–481.063 (5) | Bis-HHDP-glucose (1) | Hydrolyzable Tannins | n.d. | 4.36 |
| 10 | 153.0194 | 4.5 | C7H6O4 | 109.029 (100)–153.019 (40) | Protocatechuic acid | Phenolic acid | 1.91 | 2.22 |
| 11 | 137.0244 | 8.0 | C7H6O3 | 137.023 (100)–93.035 (10) | Hydroxybenzoic acid | Phenolic acid | 8.37 | 5.6 |
| 12 | 783.0687 391.0312 b | 10.9 | C34H24O22 | 300.999 (100)–275.020 (45)–481.063 (5) | Bis-HHDP-glucose (2) | Hydrolyzable Tannins | n.d. | 7.28 |
| 13 | 633.0734 | 12.2 | C27H22O18 | 300.999 (100)–275.020 (15)–463.0525 (10) | Galloyl-HHDP- glucose | Hydrolyzable Tannins | n.d. | 1.14 |
| 14 | 785.0843 392.0392 b | 12.3 | C34H26O22 | 300.999 (100)–275.020 (45)–249.040 (30) | Digalloyl-HHDP- glucose | Hydrolyzable Tannins | n.d. | 1.54 |
| 15 | 121.0295 | 12.6 | C7H6O2 | 121.029 (100) | Benzoic acid | Phenolic acid | 58.33 | 5.89 |
| 16 | 463.0518 | 12.7 | C20H16O13 | 299.992 (100)–300.999 (80) | Ellagic acid hexoside | Ellagic Acid derivatives | n.d. | 0.66 |
| 17 | 785.0843 392.0392 b | 13.0 | C34H26O22 | 300.999 (100)–275.020 (45)–249.040 (30) | Digalloyl-HHDP- glucose | Hydrolyzable Tannins | n.d. | 4.07 |
| 18 | 433.0412 | 13.1 | C19H14O12 | 299.992 (100)–300.999 (80) | Ellagic acid pentoside | Ellagic Acid derivatives | 0.01 | 2.55 |
| 19 | 391.1035 | 13.4 | C19H20O9 | 137.024 (100)–281.067 (90)–109.029 (20) | Lanceoloside A isomer | Benzoylglucoside | 9.98 | 4.38 |
| 20 | 405.1192 | 13.4 | C20H22O9 | 137.024 (100)–281.067 (90)–123.040 (80) | Benzoyl derivate of Lanceoloside A isomer | Benzoylglucoside | 4.07 | 2.69 |
| 21 | 197.0455 | 13.5 | C9H10O5 | 167.035 (100)–139.039 (20)–182.021 (12) | Syringic acid | Phenolic acid | 0.09 | 0.05 |
| 22 | 197.0455 | 13.5 | C9H10O5 | 197.046 (100)–169.014 (35)–125.024 (12) | Ethyl gallate | Phenolic acid | 0.4 | 5.87 |
| 23 | 463.0883 | 13.5 | C21H20O12 | 300.028 (100)–301.035 (50)–161.043 (10) | Quercetin glucoside | Flavonoid | 0.14 | 0.28 |
| 24 | 300.9990 | 13.6 | C14H6O8 | 300.999 (100)–283.995 (1)–257.010 (1) | Ellagic Acid | Ellagic Acid derivatives | 0.1 | 8.37 |
| 25 | 433.0780 | 13.7 | C20H18O11 | 300.028 (100)–301.035 (50) | Quercetin pentoside | Flavonoid | 0.12 | 0.18 |
| 26 | 447.0935 | 13.8 | C21H20O11 | 300.028 (100)–301.035 (80)–271.024 (10) | Quercitrin | Flavonoid | 1.89 | 2.7 |
| 27 | 181.0506 | 14.6 | C9H10O4 | 181.051 (100)–151.019 (40)–109.029 (12) | Ethyl protocatechuate | Phenolic acid | 0.19 | 1.91 |
| 28 | 937.0953 468.0440 b | 15.4 | C41H30O26 | 169.014 (100)–300.999 (75)–275.020 (25) | Drigalloil-HHDP- glucose | Hydrolyzable Tannins | n.d. | 5.35 |
| 29 | 271.0613 | 15.7 | C15H12O5 | 151.004 (100)–119.004 (40)–93.034 (20) | Naringenin | Flavanone | 0.91 | 0.67 |
| 30 | 935.0796 467.0365 b | 16.5 | C41H28O26 | 300.999 (100)–275.020 (20)–249.040 (5) | Digalloyl-bis-HHDP-glucose | Hydrolyzable tannins | n.d. | 14.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ventura, G.; Mesto, D.; Blasi, D.; Cataldi, T.R.I.; Calvano, C.D. The Effect of Milling on the Ethanolic Extract Composition of Dried Walnut (Juglans regia L.) Shells. Int. J. Mol. Sci. 2023, 24, 13059. https://doi.org/10.3390/ijms241713059
Ventura G, Mesto D, Blasi D, Cataldi TRI, Calvano CD. The Effect of Milling on the Ethanolic Extract Composition of Dried Walnut (Juglans regia L.) Shells. International Journal of Molecular Sciences. 2023; 24(17):13059. https://doi.org/10.3390/ijms241713059
Chicago/Turabian StyleVentura, Giovanni, Davide Mesto, Davide Blasi, Tommaso R. I. Cataldi, and Cosima Damiana Calvano. 2023. "The Effect of Milling on the Ethanolic Extract Composition of Dried Walnut (Juglans regia L.) Shells" International Journal of Molecular Sciences 24, no. 17: 13059. https://doi.org/10.3390/ijms241713059
APA StyleVentura, G., Mesto, D., Blasi, D., Cataldi, T. R. I., & Calvano, C. D. (2023). The Effect of Milling on the Ethanolic Extract Composition of Dried Walnut (Juglans regia L.) Shells. International Journal of Molecular Sciences, 24(17), 13059. https://doi.org/10.3390/ijms241713059







