Multiparametric Dynamic Ultrasound Approach for Differential Diagnosis of Primary Liver Tumors
Abstract
:1. Introduction
2. Results
2.1. Patients Clinical Data
2.2. US Imaging Characteristics
| Parameter | Entire Cohort n = 82 | HCC n = 38 | ICC n = 44 | p Value |
|---|---|---|---|---|
| Arterial phase contrast enhancement | <0.01 | |||
| hyper- omogeneous | 36 (43.9) | 26 (68.5) | 10 (22.7) | |
| aspecific | 30 (36.6) | 11 (28.9) | 19 (43.2) | |
| rim | 16 (19.5) | 1 (2.6) | 15 (34.1) | |
| Wash-out (yes) | 73 (89.0) | 31 (81.6) | 42 (95.5) | 0.04 |
| marked wash-out (yes) | 34 (41.5) | 11 (28.9) | 23 (52.3) | 0.03 |
| Wash-out time (s) | 51.4 (22.8) | 59.4 ± 26.9 | 45.4 ± 17.2 | 0.01 |
| Stiffness lesion (kPa) | 41.2 ± 15.1 | 35.7 ± 13.8 | 47.1 ± 14.4 | <0.01 |
| PE lesion | 872 (314–1805) | 1269 (636–2149) | 487 (249–1751) | 0.03 |
| PE Ratio | 0.27 (0.31–1.44) | 0.66 (−0.08–1.74) | −0.04 (−0.53–1.33) | <0.01 |
| WiAUC lesion | 5802 (2670–12202) | 9399 (5060–16057) | 4145 (1493–9146) | <0.01 |
| WiAUC Ratio | −0.35 (−0.67–−0.09) | −0.22 (−0.51–0.46) | −0.52 (−0.74–−0.26) | 0.01 |
| RT lesion | 9.1 (7.1–12.7) | 10.9 (7.9–13.0) | 8.1 (6.9–12.4) | 0.14 |
| mTTI lesion | 51.2 (26.9–98.7) | 67.0 (47.8–118.0) | 42.9 (32.2–94.4) | 0.04 |
| TTP lesion | 11.4 (7.9–15.2) | 12.5 (8.7–14.5) | 9.1 (7.8–15.6) | 0.27 |
| WiR lesion | 185 (63–522) | 208 (77–625) | 133 (62–333) | 0.08 |
| WiR ratio | 1.75 (0.60–4.50) | 2.41 (1.32–6.67) | 1.32 (0.13–2.71) | <0.01 |
| WoAUC lesion | 19019 (5944–42896) | 32048 (15406–62715) | 12138 (3815–30984) | <0.01 |
| WoAUC Ratio | −0.23 (−0.59–0.57) | −0.11 (−0.38–0.62) | −0.41 (−0.68–0.03) | 0.08 |
| WiWoAUC lesion | 24894 (9085–55002) | 42243 (20351–78362) | 15779 (5489 −41443) | <0.01 |
| WiWoAUC Ratio | −0.26 (−0.58–0.22) | −0.13 (−0.43–0.52) | −0.39 (−0.64–−0.11) | 0.06 |
| Univariate Model | Multivariate Model | |||
|---|---|---|---|---|
| OR (CI 95%) | p Value | OR (CI 95%) | p Value | |
| Gender (male) | 0.57 (0.22–1.46) | 0.24 | ||
| Age (years) | 0.97 (0.93–1.02) | 0.25 | ||
| BMI (Kg/m2) | 0.96 (0.87–1.05) | 0.34 | ||
| Liver cirrhsosis | 0.17 (0.07–0.45) | <0.01 | 0.17 (0.05–0.56) | <0.01 |
| Target nodule diameter (mm) | 1.02 (1.00–1.04) | 0.02 | ||
| aFP | 1.00 (0.99–1.01) | 0.26 | ||
| Ca 19.9 | 1.00 (0.99–1.01) | 0.26 | ||
| Stiffness lesion (kPa) | 1.05 (1.02–1.09) | <0.01 | 1.05 (1.01–1.09) | 0.01 |
| PE lesion | 1.00 (0.99–1.01) | 0.13 | ||
| PE Ratio | 0.62 (0.43–0.91) | 0.01 | 0.54 (0.32–0.90) | 0.02 |
| WiAUC lesion | 1.00 (0.99–1.01) | 0.13 | ||
| WiAUC Ratio | 0.73 (0.46–1.15) | 0.18 | ||
| RT lesion | 0.98 (0.91–1.06) | 0.70 | ||
| mTTI lesion | 1.00 (0.99–1.01) | 0.87 | ||
| TTP lesion | 1.01 (0.97–1.06) | 0.55 | ||
| WiR lesion | 0.99 (0.99–1.00) | 0.06 | ||
| WiR ratio | 0.87 (0.78–0.98) | 0.02 | ||
| WoAUC lesion | 1.00 (0.99–1.01) | 0.12 | ||
| WoAUC Ratio | 0.69 (0.42–1.12) | 0.13 | ||
| WiWoAUC lesion | 1.00 (0.99–1.01) | 0.11 | ||
| WiWoAUC Ratio | 0.65 (0.38–1.10) | 0.11 | ||
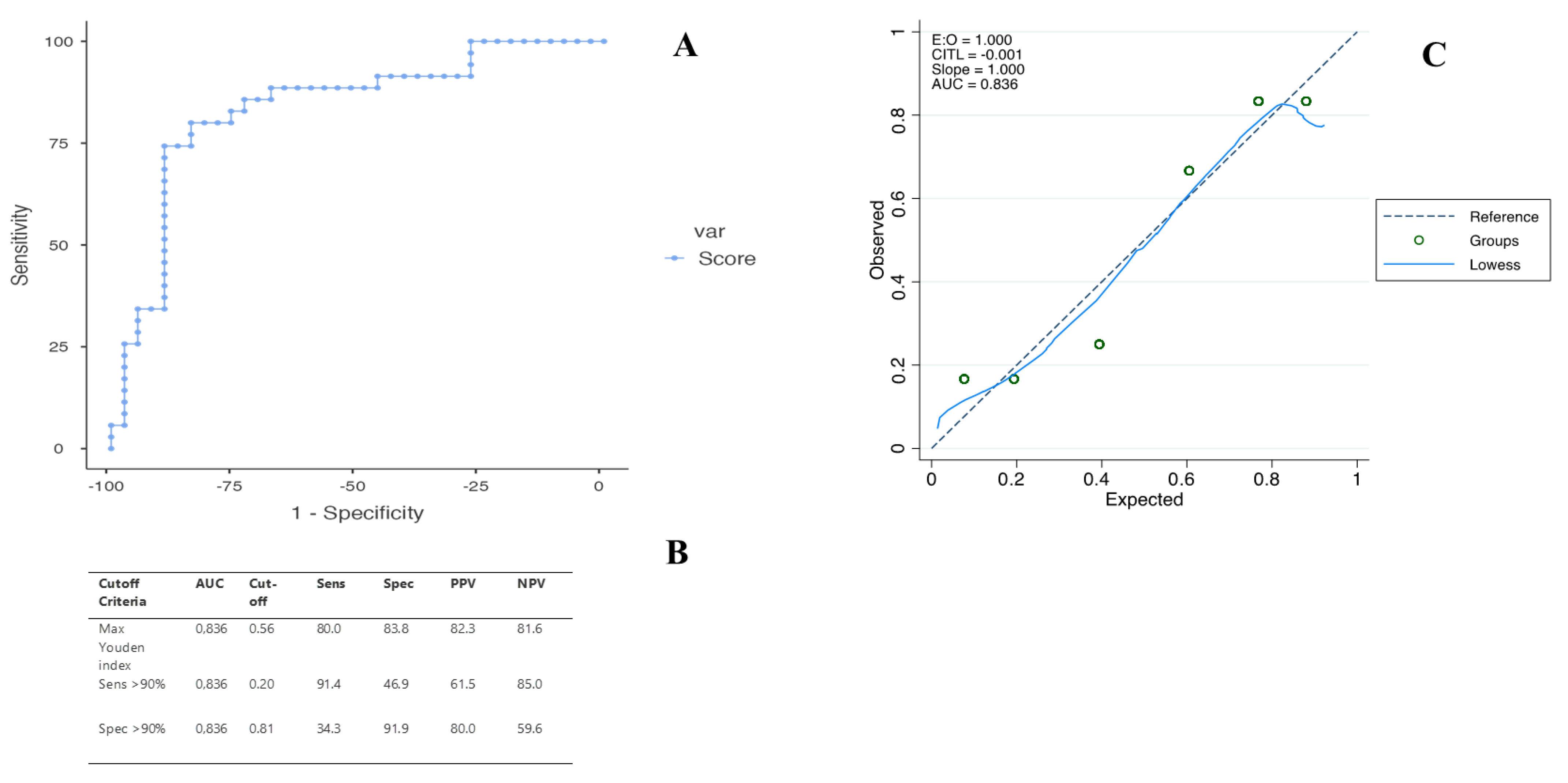
2.3. Diagnostic Performance of the US Score in Predicting ICC
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. Patients
4.2. Study Protocol
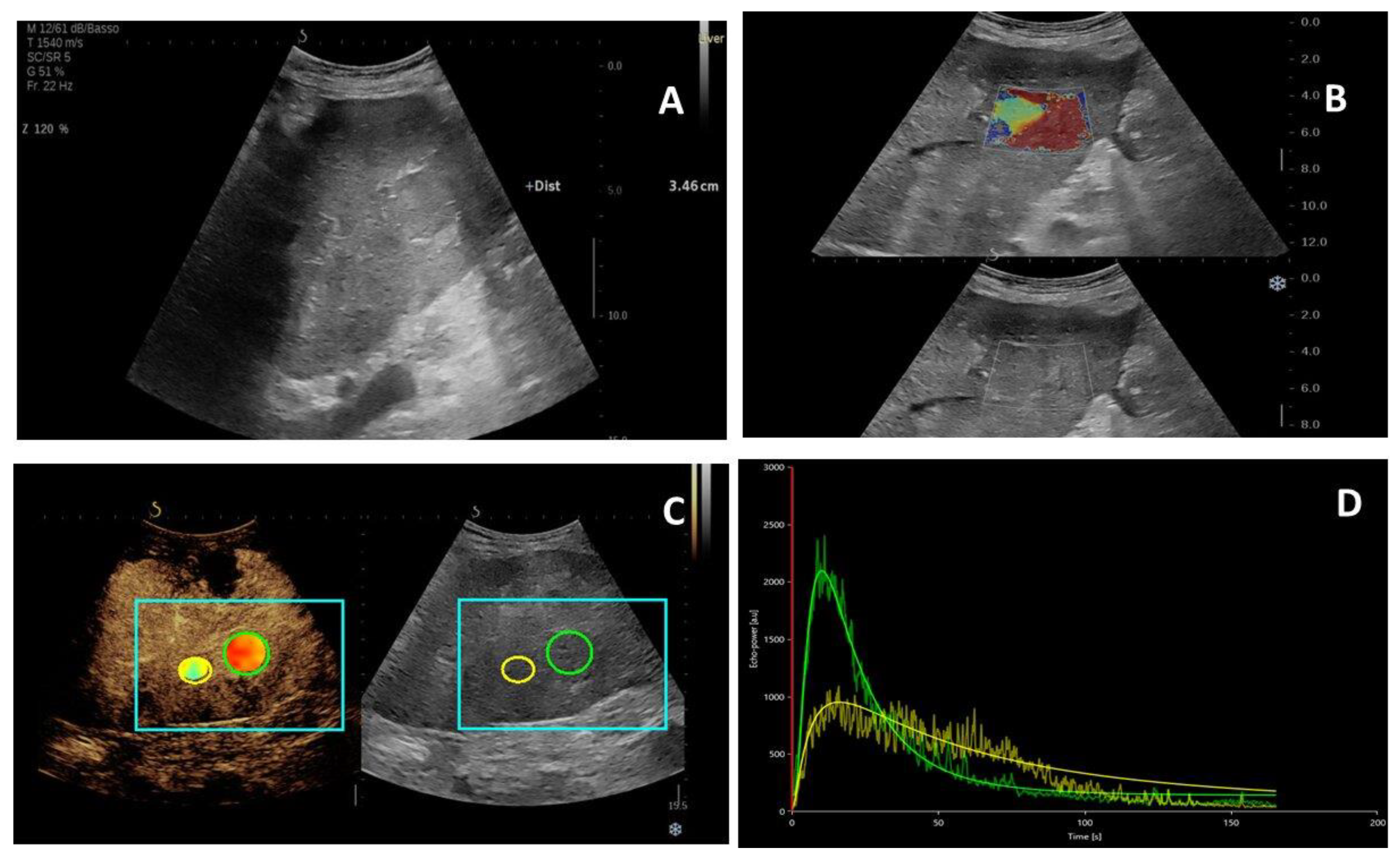
4.3. Multiparametric US Examination
4.4. Image Analysis
4.5. Reference Standard
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Lampertico, P.; Nahon, P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J. Hepatol. 2020, 72, 250–261. [Google Scholar] [CrossRef]
- Massarweh, N.N.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control 2017, 24, 1073274817729245. [Google Scholar] [CrossRef]
- Mavros, M.N.; Economopoulos, K.P.; Alexiou, V.G.; Pawlik, T.M. Treatment and Prognosis for Patients with Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg. 2014, 149, 565–574. [Google Scholar] [CrossRef]
- Bridgewater, J.; Galle, P.R.; Khan, S.A.; Llovet, J.M.; Park, J.-W.; Patel, T.; Pawlik, T.M.; Gores, G.J. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J. Hepatol. 2014, 60, 1268–1289. [Google Scholar] [CrossRef] [PubMed]
- Hahn, F.; Müller, L.; Jungmann, F.; Mähringer-Kunz, A.; Tanyildizi, Y.; Düber, C.; Galle, P.R.; Weinmann, A.; Kloeckner, R. Survival prediction for patients with non-resectable intrahepatic cholangiocarcinoma undergoing chemotherapy: A retrospective analysis comparing the tumor marker CA 19-9 with cross-sectional imaging. J. Cancer Res. Clin. Oncol. 2020, 146, 1883–1890. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Nolsøe, C.P.; Barr, R.G.; Berzigotti, A.; Burns, P.N.; Cantisani, V.; Chammas, M.C.; Chaubal, N.; Choi, B.I.; Clevert, D.-A.; et al. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver-Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med. Biol. 2020, 46, 2579–2604. [Google Scholar] [CrossRef]
- Schellhaas, B.; Bernatik, T.; Bohle, W.; Borowitzka, F.; Chang, J.; Dietrich, C.F.; Dirks, K.; Donoval, R.; Drube, K.; Friedrich-Rust, M.; et al. Contrast-Enhanced Ultrasound Algorithms (CEUS-LIRADS/ESCULAP) for the Noninvasive Diagnosis of Hepatocellular Carcinoma—A Prospective Multicenter DEGUM Study. Ultraschall Med. 2021, 42, 178–186. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, Y.; Chen, K.; Wang, H.; Zhang, W.; Bao, J.; Wang, W. Differentiation between hepatocellular carcinoma and intrahepatic cholangiocarcinoma using contrast-enhanced ultrasound: A systematic review and meta-analysis. Clin. Hemorheol. Microcirc. 2021, 79, 293–309. [Google Scholar] [CrossRef]
- Putz, F.J.; Verloh, N.; Erlmeier, A.; Schelker, R.C.; Schreyer, A.G.; Hautmann, M.G.; Stroszczynski, C.; Banas, B.; Jung, E.M. Influence of limited examination conditions on contrast-enhanced sonography for characterising liver lesions. Clin. Hemorheol. Microcirc. 2019, 71, 267–276. [Google Scholar] [CrossRef]
- Shin, J.; Lee, S.; Bae, H.; Chung, Y.E.; Choi, J.-Y.; Huh, Y.-M.; Park, M.-S. Contrast-enhanced ultrasound liver imaging reporting and data system for diagnosing hepatocellular carcinoma: A meta-analysis. Liver Int. 2020, 40, 2345–2352. [Google Scholar] [CrossRef]
- Li, F.; Li, Q.; Liu, Y.; Han, J.; Zheng, W.; Huang, Y.; Zheng, X.; Cao, L.; Zhou, J.-H. Distinguishing intrahepatic cholangiocarcinoma from hepatocellular carcinoma in patients with and without risks: The evaluation of the LR-M criteria of contrast-enhanced ultrasound liver imaging reporting and data system version 2017. Eur. Radiol. 2020, 30, 461–470. [Google Scholar] [CrossRef]
- Kim, T.K.; Noh, S.Y.; Wilson, S.R.; Kono, Y.; Piscaglia, F.; Jang, H.-J.; Lyshchik, A.; Dietrich, C.F.; Willmann, J.K.; Vezeridis, A.; et al. Contrast-enhanced ultrasound (CEUS) liver imaging reporting and data system (LI-RADS) 2017—A review of important differences compared to the CT/MRI system. Clin. Mol. Hepatol. 2017, 23, 280–289. [Google Scholar] [CrossRef]
- Terzi, E.; Iavarone, M.; Pompili, M.; Veronese, L.; Cabibbo, G.; Fraquelli, M.; Riccardi, L.; De Bonis, L.; Sangiovanni, A.; Leoni, S.; et al. Contrast ultrasound LI-RADS LR-5 identifies hepatocellular carcinoma in cirrhosis in a multicenter restropective study of 1,006 nodules. J. Hepatol. 2018, 68, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Averkiou, M.A.; Correas, J.-M.; Lassau, N.; Leen, E.; Piscaglia, F. An EFSUMB introduction into Dynamic Contrast-Enhanced Ultrasound (DCE-US) for quantification of tumour perfusion. Ultraschall Med. 2012, 33, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.M.; Clevert, D.A.; Schreyer, A.G.; Schmitt, S.; Rennert, J.; Kubale, R.; Feuerbach, S.; Jung, F. Evaluation of quantitative contrast harmonic imaging to assess malignancy of liver tumors: A prospective controlled two-center study. World J. Gastroenterol. 2007, 13, 6356–6364. [Google Scholar] [CrossRef]
- Li, R.; Zhang, X.; Ma, K.-S.; Li, X.-W.; Xia, F.; Zhong, H.; Tang, C.-L.; Guo, Y.; Yan, X.-C. Dynamic enhancing vascular pattern of intrahepatic peripheral cholangiocarcinoma on contrast-enhanced ultrasound: The influence of chronic hepatitis and cirrhosis. Abdom. Imaging 2013, 38, 112–119. [Google Scholar] [CrossRef]
- Anaye, A.; Perrenoud, G.; Rognin, N.; Arditi, M.; Mercier, L.; Frinking, P.; Ruffieux, C.; Peetrons, P.; Meuli, R.; Meuwly, J.-Y. Differentiation of focal liver lesions: Usefulness of parametric imaging with contrast-enhanced US. Radiology 2011, 261, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Ohno, N.; Miyati, T.; Yamashita, M.; Narikawa, M. Quantitative Assessment of Tissue Perfusion in Hepatocellular Carcinoma Using Perflubutane Dynamic Contrast-Enhanced Ultrasonography: A Preliminary Study. Diagnostics 2015, 5, 210–218. [Google Scholar] [CrossRef]
- Schacherer, D.; Girlich, C.; Wiest, R.; Büttner, R.; Schölmerich, J.; Feuerbach, S.; Zorger, N.; Hamer, O.W.; Jung, E.M. Semiquantitative characterization of hepatocellular carcinoma (HCC)--perfusion with contrast-enhanced ultrasound and perfusion analysis. Clin. Hemorheol. Microcirc. 2010, 44, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Wildner, D.; Pfeifer, L.; Goertz, R.S.; Bernatik, T.; Sturm, J.; Neurath, M.F.; Strobel, D. Dynamic contrast-enhanced ultrasound (DCE-US) for the characterization of hepatocellular carcinoma and cholangiocellular carcinoma. Ultraschall Med. 2014, 35, 522–527. [Google Scholar] [CrossRef]
- Zhao, Y.-J.; Chen, W.-X.; Wu, D.-S.; Zhang, W.-Y.; Zheng, L.-R. Differentiation of mass-forming intrahepatic cholangiocarcinoma from poorly differentiated hepatocellular carcinoma: Based on the multivariate analysis of contrast-enhanced computed tomography findings. Abdom. Radiol. N. Y. 2016, 41, 978–989. [Google Scholar] [CrossRef]
- Guo, H.-L.; Zheng, X.; Cheng, M.-Q.; Zeng, D.; Huang, H.; Xie, X.-Y.; Lu, M.-D.; Kuang, M.; Wang, W.; Xian, M.-F.; et al. Contrast-Enhanced Ultrasound for Differentiation Between Poorly Differentiated Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J. Ultrasound Med. 2022, 41, 1213–1225. [Google Scholar] [CrossRef]
- Zhang, P.; Zhou, P.; Tian, S.-M.; Qian, Y.; Li, J.-L.; Li, R.-Z. Diagnostic performance of contrast-enhanced sonography and acoustic radiation force impulse imaging in solid liver lesions. J. Ultrasound Med. 2014, 33, 205–214. [Google Scholar] [CrossRef] [PubMed]
- da Silva, N.P.B.; Hornung, M.; Beyer, L.P.; Hackl, C.; Brunner, S.; Schlitt, H.J.; Wiggermann, P.; Jung, E.M. Intraoperative Shear Wave Elastography vs. Contrast-Enhanced Ultrasound for the Characterization and Differentiation of Focal Liver Lesions to Optimize Liver Tumor Surgery. Ultraschall Med. 2019, 40, 205–211. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.-C.; Tian, W.-S.; Chen, L.-D.; Zheng, Q.; Hu, H.-T.; Wu, S.-S.; Guo, Y.; Xie, X.-Y.; Lu, M.-D.; et al. Shear wave elastography-based ultrasomics: Differentiating malignant from benign focal liver lesions. Abdom. Radiol. N. Y. 2021, 46, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-P.; Gu, J.-Y.; Bai, M.; Li, F.; Zhou, Y.-Q.; Du, L.-F. Value of shear wave elastography with maximal elasticity in differentiating benign and malignant solid focal liver lesions. World J. Gastroenterol. 2020, 26, 7416–7424. [Google Scholar] [CrossRef]
- Park, H.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Chon, C.Y.; Han, K.-H.; Kim, S.U. Characterization of focal liver masses using acoustic radiation force impulse elastography. World J. Gastroenterol. 2013, 19, 219–226. [Google Scholar] [CrossRef]
- Gerber, L.; Fitting, D.; Srikantharajah, K.; Weiler, N.; Kyriakidou, G.; Bojunga, J.; Schulze, F.; Bon, D.; Zeuzem, S.; Friedrich-Rust, M. Evaluation of 2D- Shear Wave Elastography for Characterisation of Focal Liver Lesions. J. Gastrointest. Liver Dis. 2017, 26, 283–290. [Google Scholar] [CrossRef]
- Seitz, K.; Greis, C.; Schuler, A.; Bernatik, T.; Blank, W.; Dietrich, C.F.; Strobel, D. Frequency of tumor entities among liver tumors of unclear etiology initially detected by sonography in the noncirrhotic or cirrhotic livers of 1349 patients. Results of the DEGUM multicenter study. Ultraschall Med. 2011, 32, 598–603. [Google Scholar] [CrossRef]
- Leoni, S.; Piscaglia, F.; Granito, A.; Borghi, A.; Galassi, M.; Marinelli, S.; Terzi, E.; Bolondi, L. Characterization of primary and recurrent nodules in liver cirrhosis using contrast-enhanced ultrasound: Which vascular criteria should be adopted? Ultraschall Med. 2013, 34, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, A.; Montesarchio, L.; Gatti, P.; Amendola, F.; Matteucci, P.; Santoro, B.; Merola, M.G.; Merola, F.; Coppola, C.; Giorgio, V. Contrast-Enhanced Ultrasound: A Simple and Effective Tool in Defining a Rapid Diagnostic Work-up for Small Nodules Detected in Cirrhotic Patients during Surveillance. J. Gastrointest. Liver Dis. 2016, 25, 205–211. [Google Scholar] [CrossRef]
- Greten, T.F.; Malek, N.P.; Schmidt, S.; Arends, J.; Bartenstein, P.; Bechstein, W.; Bernatik, T.; Bitzer, M.; Chavan, A.; Dollinger, M.; et al. Diagnosis of and therapy for hepatocellular carcinoma. Z. Gastroenterol. 2013, 51, 1269–1326. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Cui, X.W.; Boozari, B.; Hocke, M.; Ignee, A. Contrast-enhanced ultrasound (CEUS) in the diagnostic algorithm of hepatocellular and cholangiocellular carcinoma, comments on the AASLD guidelines. Ultraschall Med. 2012, 33 (Suppl. S1), S57–S66. [Google Scholar] [CrossRef]
- Gupta, A.; Dixon, E. Epidemiology and risk factors: Intrahepatic cholangiocarcinoma. Hepatobiliary Surg. Nutr. 2017, 6, 101–104. [Google Scholar] [CrossRef]
- Shin, S.K.; Kim, Y.S.; Choi, S.J.; Shim, Y.S.; Jung, D.H.; Kwon, O.S.; Choi, D.J.; Kim, J.H. Contrast-enhanced ultrasound for the differentiation of small atypical hepatocellular carcinomas from dysplastic nodules in cirrhosis. Dig. Liver Dis. 2015, 47, 775–782. [Google Scholar] [CrossRef]
- Wilson, S.R.; Kim, T.K.; Jang, H.-J.; Burns, P.N. Enhancement patterns of focal liver masses: Discordance between contrast-enhanced sonography and contrast-enhanced CT and MRI. AJR Am. J. Roentgenol. 2007, 189, W7–W12. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-D.; Ruan, S.-M.; Liang, J.-Y.; Yang, Z.; Shen, S.-L.; Huang, Y.; Li, W.; Wang, Z.; Xie, X.-Y.; Lu, M.-D.; et al. Differentiation of intrahepatic cholangiocarcinoma from hepatocellular carcinoma in high-risk patients: A predictive model using contrast-enhanced ultrasound. World J. Gastroenterol. 2018, 24, 3786–3798. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-J.; Wang, W.; Lu, M.-D.; Xie, X.-Y.; Xu, H.-X.; Xu, Z.-F.; Chen, L.-D.; Wang, Z.; Liang, J.-Y.; Huang, Y.; et al. Contrast-Enhanced Ultrasound for the Characterization of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Liver Cancer 2015, 4, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Schellhaas, B.; Waldner, M.J.; Görtz, R.S.; Vitali, F.; Kielisch, C.; Pfeifer, L.; Strobel, D.; Janka, R.; Neurath, M.F.; Wildner, D. Diagnostic accuracy and interobserver variability of Dynamic Vascular Pattern (DVP) in primary liver malignancies—A simple semiquantitative tool for the analysis of contrast enhancement patterns. Clin. Hemorheol. Microcirc. 2017, 66, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Beyer, L.P.; Pregler, B.; Wiesinger, I.; Stroszczynski, C.; Wiggermann, P.; Jung, E.-M. Continuous dynamic registration of microvascularization of liver tumors with contrast-enhanced ultrasound. Radiol. Res. Pract. 2014, 2014, 347416. [Google Scholar] [CrossRef]
- Li, R.; Yuan, M.-X.; Ma, K.; Li, X.-W.; Tang, C.-L.; Zhang, X.-H.; Guo, D.-Y.; Yan, X.-C. Detailed analysis of temporal features on contrast enhanced ultrasound may help differentiate intrahepatic cholangiocarcinoma from hepatocellular carcinoma in cirrhosis. PLoS ONE 2014, 9, e98612. [Google Scholar] [CrossRef]
- Wildner, D.; Bernatik, T.; Greis, C.; Seitz, K.; Neurath, M.F.; Strobel, D. CEUS in hepatocellular carcinoma and intrahepatic cholangiocellular carcinoma in 320 patients—Early or late washout matters: A subanalysis of the DEGUM multicenter trial. Ultraschall Med. 2015, 36, 132–139. [Google Scholar] [CrossRef]
- Yue, W.-W.; Wang, S.; Xu, H.-X.; Sun, L.-P.; Guo, L.-H.; Bo, X.-W.; Li, X.-L.; Zhao, C.-K.; Wang, D.; Liu, B.-J. Parametric imaging with contrast-enhanced ultrasound for differentiating hepatocellular carcinoma from metastatic liver cancer. Clin. Hemorheol. Microcirc. 2016, 64, 177–188. [Google Scholar] [CrossRef]
- Wildner, D.; Schellhaas, B.; Strack, D.; Goertz, R.S.; Pfeifer, L.; Fiessler, C.; Neurath, M.F.; Strobel, D. Differentiation of malignant liver tumors by software-based perfusion quantification with dynamic contrast-enhanced ultrasound (DCEUS). Clin. Hemorheol. Microcirc. 2019, 71, 39–51. [Google Scholar] [CrossRef]
- Schaible, J.; Stroszczynski, C.; Beyer, L.P.; Jung, E.M. Quantitative perfusion analysis of hepatocellular carcinoma using dynamic contrast enhanced ultrasound (CEUS) to determine tumor microvascularization. Clin. Hemorheol. Microcirc. 2019, 73, 95–104. [Google Scholar] [CrossRef]
- Galassi, M.; Iavarone, M.; Rossi, S.; Bota, S.; Vavassori, S.; Rosa, L.; Leoni, S.; Venerandi, L.; Marinelli, S.; Sangiovanni, A.; et al. Patterns of appearance and risk of misdiagnosis of intrahepatic cholangiocarcinoma in cirrhosis at contrast enhanced ultrasound. Liver Int. 2013, 33, 771–779. [Google Scholar] [CrossRef]
- Lu, Q.; Xue, L.-Y.; Wang, W.-P.; Huang, B.-J.; Li, C.-X. Dynamic enhancement pattern of intrahepatic cholangiocarcinoma on contrast-enhanced ultrasound: The correlation with cirrhosis and tumor size. Abdom. Imaging 2015, 40, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Guibal, A.; Boularan, C.; Bruce, M.; Vallin, M.; Pilleul, F.; Walter, T.; Scoazec, J.Y.; Boublay, N.; Dumortier, J.; Lefort, T. Evaluation of shearwave elastography for the characterisation of focal liver lesions on ultrasound. Eur. Radiol. 2013, 23, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Ronot, M.; Di Renzo, S.; Gregoli, B.; Duran, R.; Castera, L.; Van Beers, B.E.; Vilgrain, V. Characterization of fortuitously discovered focal liver lesions: Additional information provided by shearwave elastography. Eur. Radiol. 2015, 25, 346–358. [Google Scholar] [CrossRef]
- Lee, J.I.; Campbell, J.S. Role of desmoplasia in cholangiocarcinoma and hepatocellular carcinoma. J. Hepatol. 2014, 61, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Lurie, Y.; Webb, M.; Cytter-Kuint, R.; Shteingart, S.; Lederkremer, G.Z. Non-invasive diagnosis of liver fibrosis and cirrhosis. World J. Gastroenterol. 2015, 21, 11567–11583. [Google Scholar] [CrossRef] [PubMed]
- Claudon, M.; Dietrich, C.F.; Choi, B.I.; Cosgrove, D.O.; Kudo, M.; Nolsøe, C.P.; Piscaglia, F.; Wilson, S.R.; Barr, R.G.; Chammas, M.C.; et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver--update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med. 2013, 34, 11–29. [Google Scholar] [CrossRef]

| Parameter | Entire Cohort n = 82 | HCC N = 38 | ICC N = 44 | p Value |
|---|---|---|---|---|
| Gender (male) | 55 (67.1) | 28 (73.7) | 27 (61.4) | 0.23 |
| Age (years) | 68 ± 11 | 70 ± 10 | 67 ± 11 | 0.25 |
| BMI (Kg/m2) | 25.9 ± 4.9 | 26.5 ± 4.8 | 25.4 ± 5.0 | 0.34 |
| Liver disease | <0.01 | |||
| normal liver | 33 (40.2) | 6 (15.8) | 27 (61.4) | |
| chronic hepatitis | 4 (4.9) | 2 (5.3) | 2 (4.5) | |
| steatosis | 7 (8.5) | 4 (10.5) | 3 (6.8) | |
| cirrhosis | 38 (46.3) | 26 (68.4) | 12 (27.3) | |
| Nodules | 0.18 | |||
| single | 42 (51.2) | 23 (60.5) | 19 (43.2) | |
| 2 | 12 (14.6) | 4 (10.5) | 8 (18.2) | |
| 3 | 6 (7.3) | 4 (10.5) | 2 (4.5) | |
| >3 | 22 (26.8) | 7 (18.4) | 15 (34.1) | |
| Target nodule diameter (mm) | 40 (30–60) | 25 (23–45) | 54 (35–74) | |
| Echogenicity | 0.66 | |||
| hypo- | 58 (70.7) | 26 (68.4) | 32 (72.7) | |
| hyper- | 18 (22.0) | 10 (26.3) | 8 (18.2) | |
| iso- | 1 (1.2) | 0 (0) | 1 (2.3) | |
| mixed | 5 (6.1) | 2 (5.3) | 3 (6.8) | |
| Nodule margins | 0.06 | |||
| regular | 61 (74.4) | 32 (84.2) | 29 (65.9) | |
| irregular | 21 (25.6) | 6 (15.8) | 15 (34.1) | |
| aFP | 4.78 (2.22–51.0) | 8 (3.25–85) | 3.7 (2–13.5) | 0.09 |
| Ca 19.9 | 84.0 (12.1–576.0) | 8 (4–32) | 141 (33–3621) | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ainora, M.E.; Cerrito, L.; Liguori, A.; Mignini, I.; De Luca, A.; Galasso, L.; Garcovich, M.; Riccardi, L.; Ponziani, F.; Santopaolo, F.; et al. Multiparametric Dynamic Ultrasound Approach for Differential Diagnosis of Primary Liver Tumors. Int. J. Mol. Sci. 2023, 24, 8548. https://doi.org/10.3390/ijms24108548
Ainora ME, Cerrito L, Liguori A, Mignini I, De Luca A, Galasso L, Garcovich M, Riccardi L, Ponziani F, Santopaolo F, et al. Multiparametric Dynamic Ultrasound Approach for Differential Diagnosis of Primary Liver Tumors. International Journal of Molecular Sciences. 2023; 24(10):8548. https://doi.org/10.3390/ijms24108548
Chicago/Turabian StyleAinora, Maria Elena, Lucia Cerrito, Antonio Liguori, Irene Mignini, Angela De Luca, Linda Galasso, Matteo Garcovich, Laura Riccardi, Francesca Ponziani, Francesco Santopaolo, and et al. 2023. "Multiparametric Dynamic Ultrasound Approach for Differential Diagnosis of Primary Liver Tumors" International Journal of Molecular Sciences 24, no. 10: 8548. https://doi.org/10.3390/ijms24108548
APA StyleAinora, M. E., Cerrito, L., Liguori, A., Mignini, I., De Luca, A., Galasso, L., Garcovich, M., Riccardi, L., Ponziani, F., Santopaolo, F., Pompili, M., Gasbarrini, A., & Zocco, M. A. (2023). Multiparametric Dynamic Ultrasound Approach for Differential Diagnosis of Primary Liver Tumors. International Journal of Molecular Sciences, 24(10), 8548. https://doi.org/10.3390/ijms24108548







