Anti-Inflammatory Effects of Adiponectin Receptor Agonist AdipoRon against Intervertebral Disc Degeneration
Abstract
:1. Introduction
2. Results
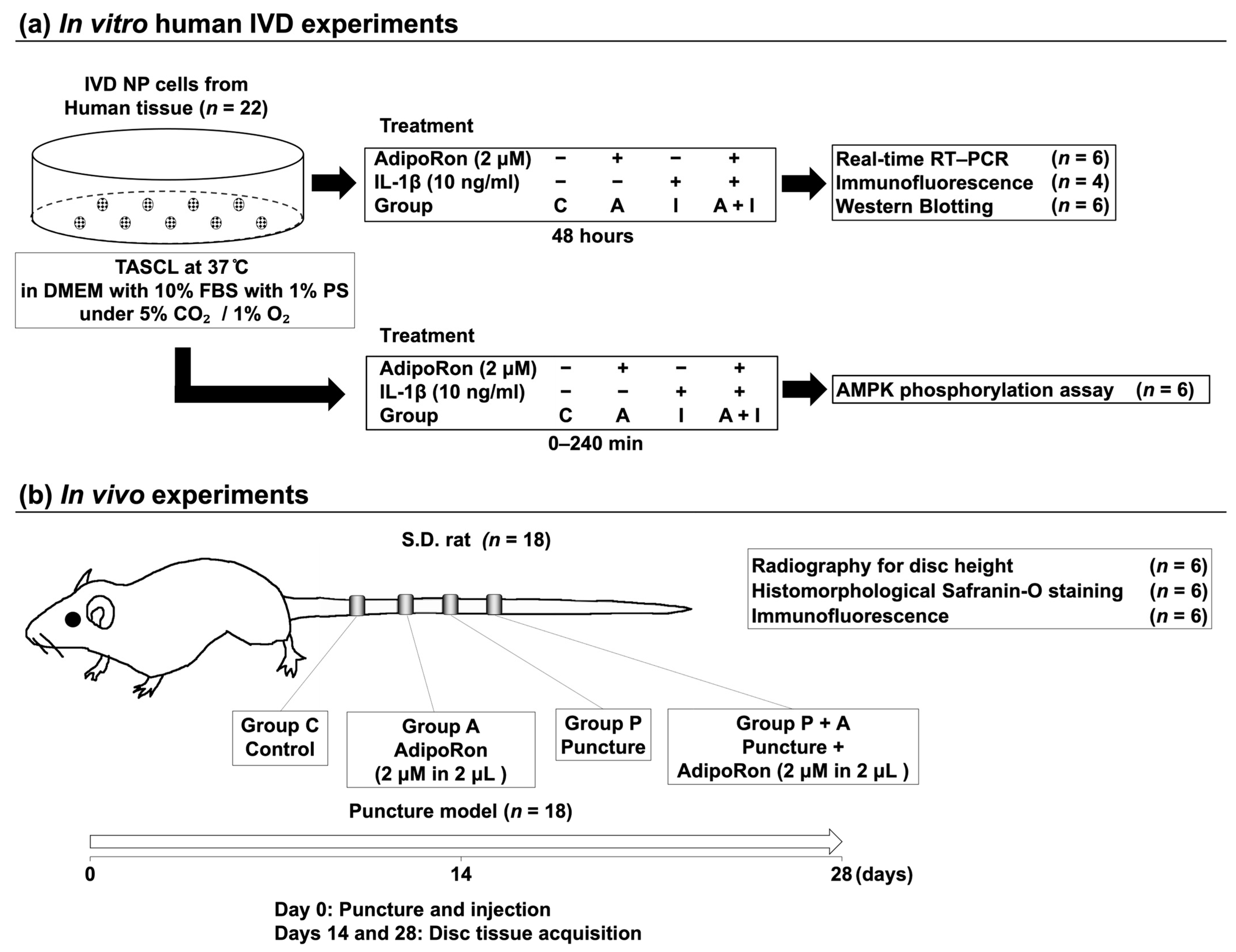
2.1. In Vitro IVD Experiments
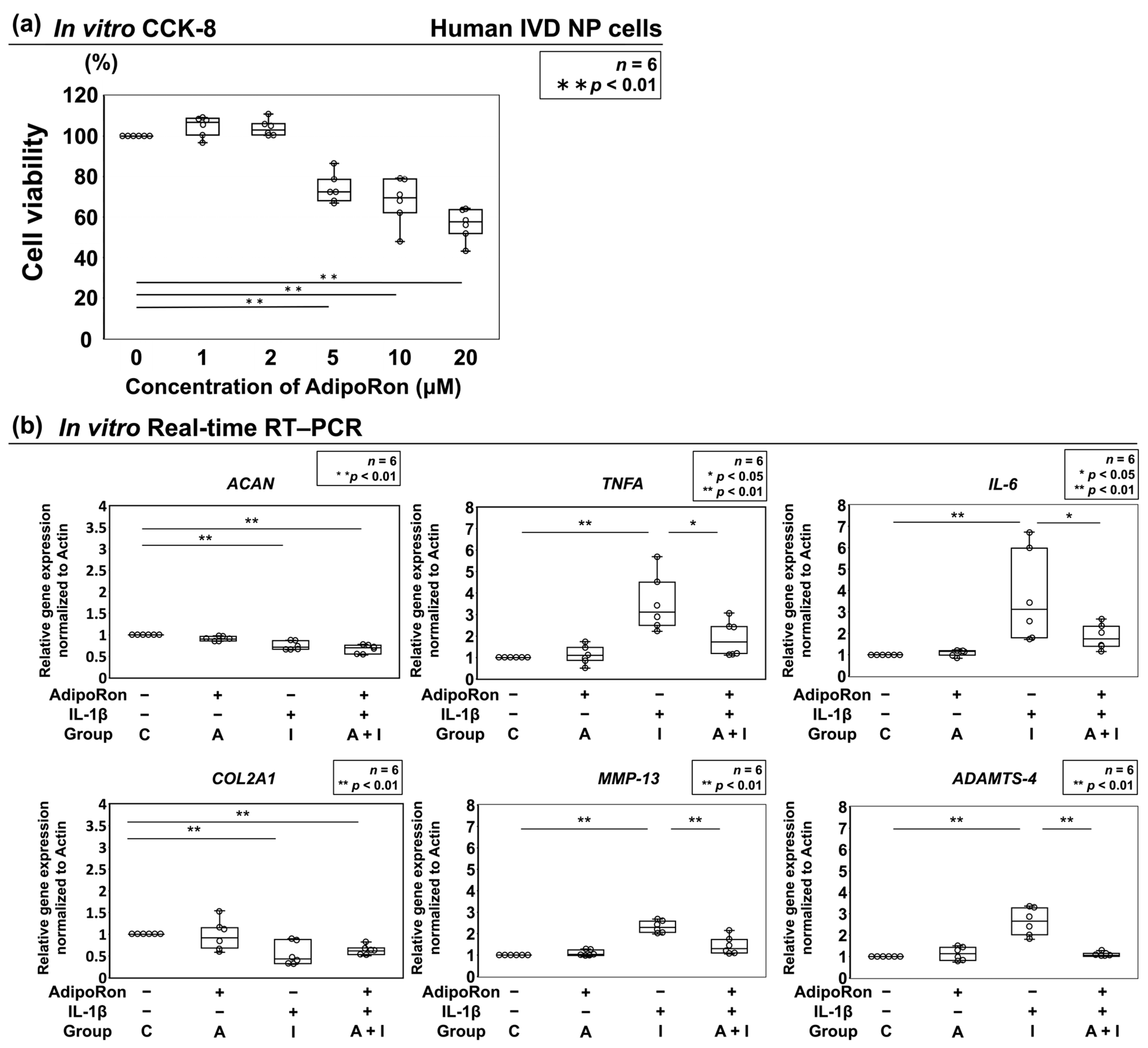
2.1.1. Effect of AdipoRon on Cell Viability
2.1.2. Effects of AdipoRon on the Expression of ACAN, COL2A1, TNF-α, IL-6, MMP-13, and ADAMTS-4
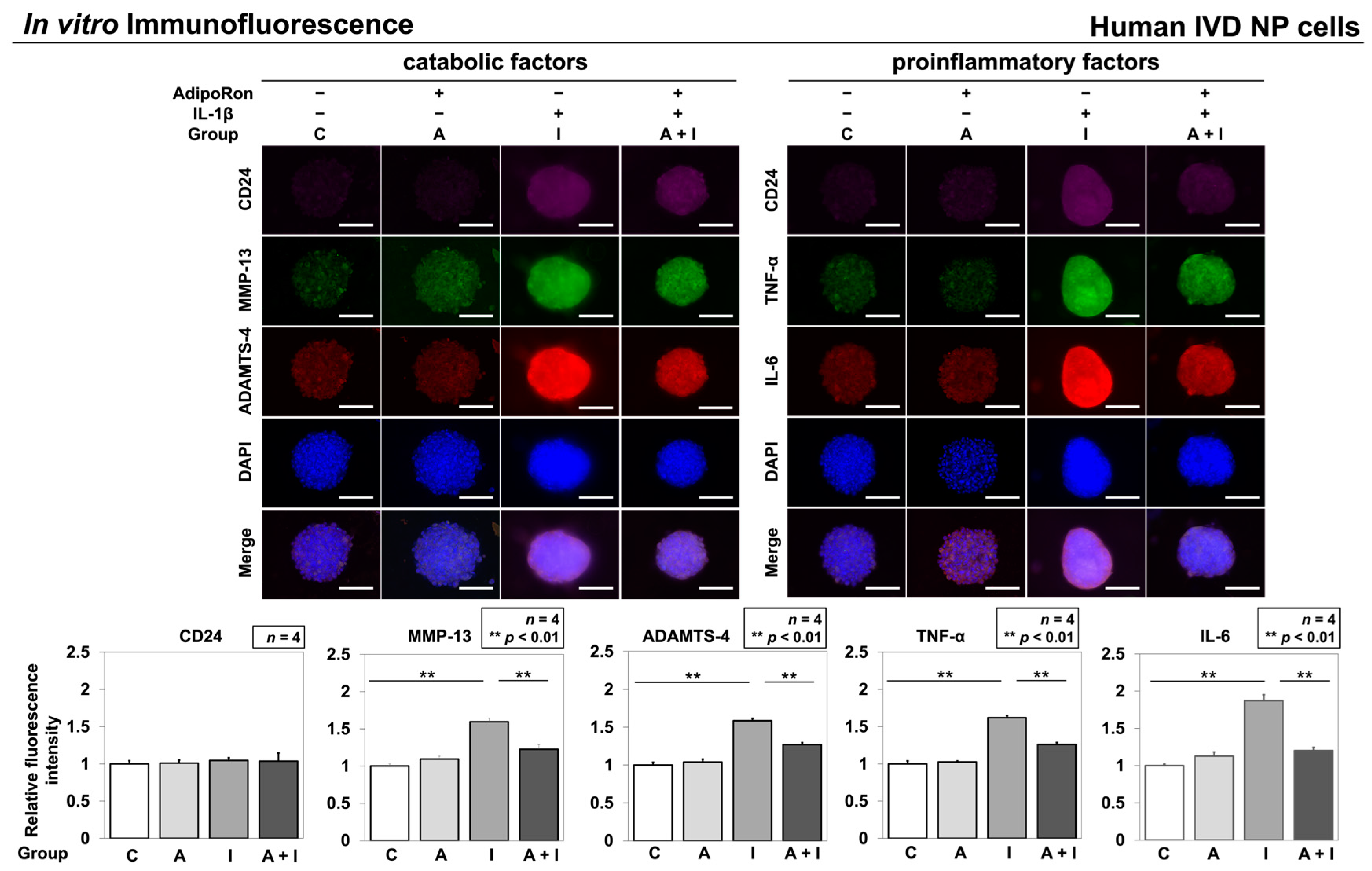
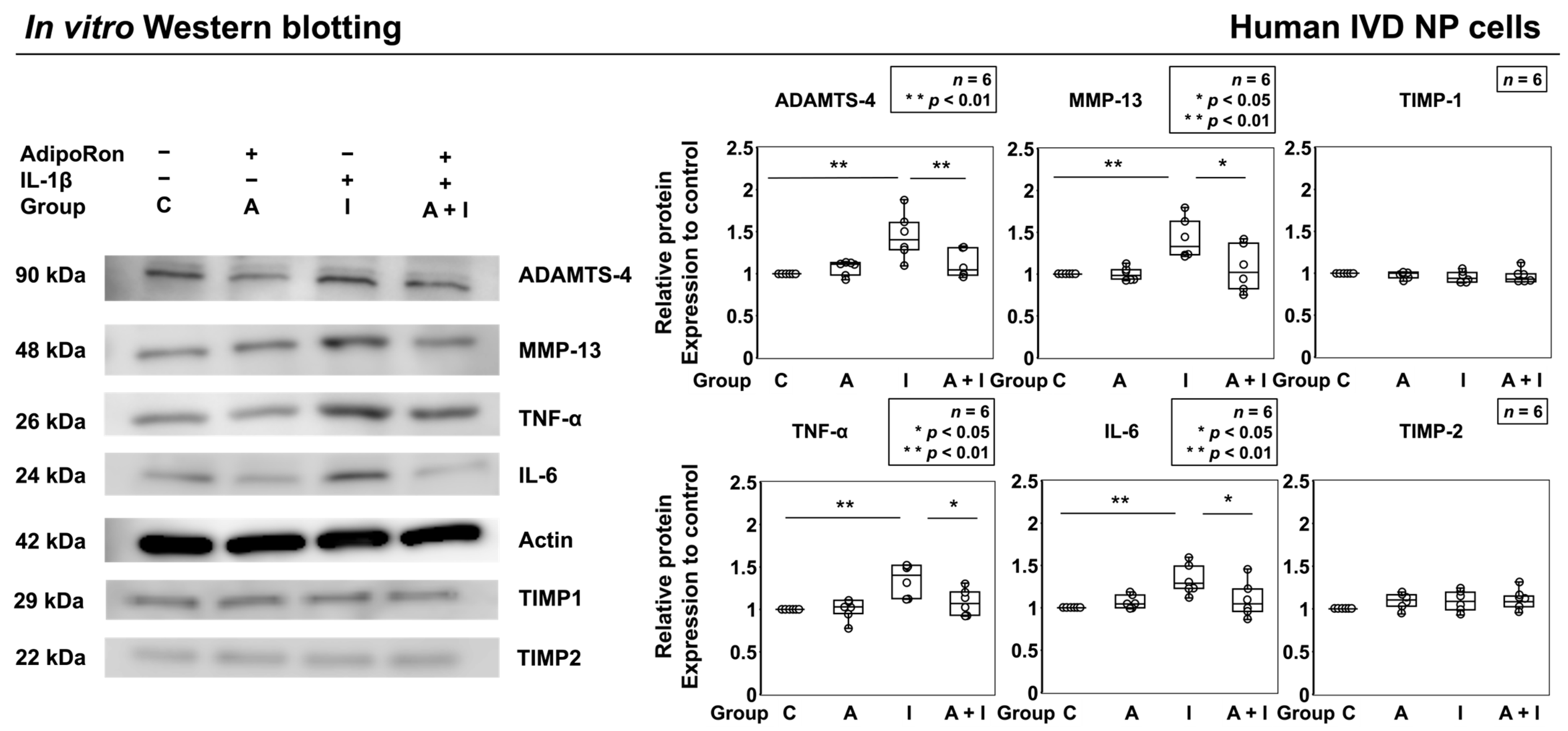
2.1.3. Effects of AdipoRon on the Expression of TNF-α, IL-6, MMP-13, and ADAMTS-4
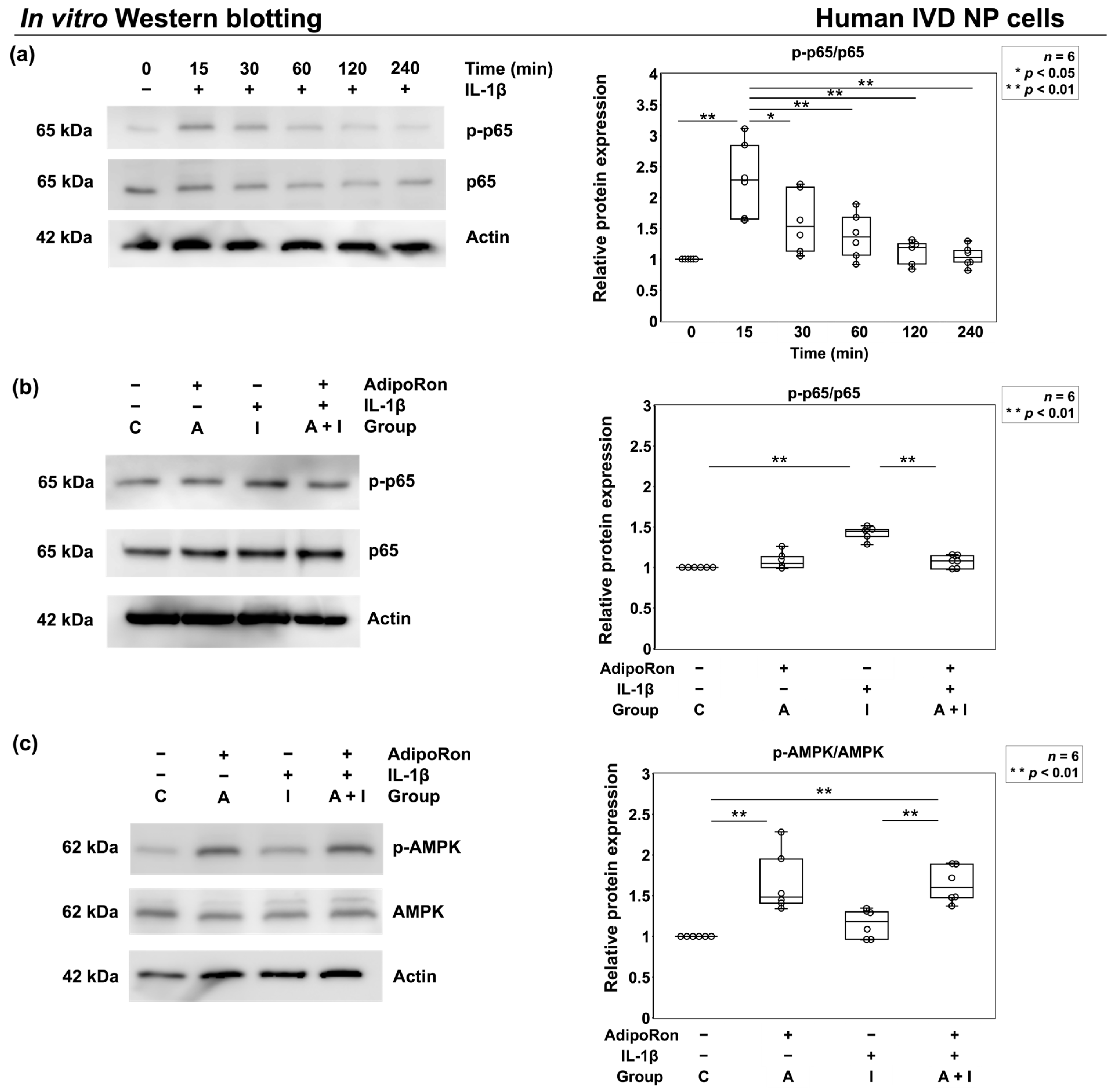
2.1.4. Effects of AdipoRon on p65 Phosphorylation under Proinflammatory Conditions
2.2. In Vivo Experiments
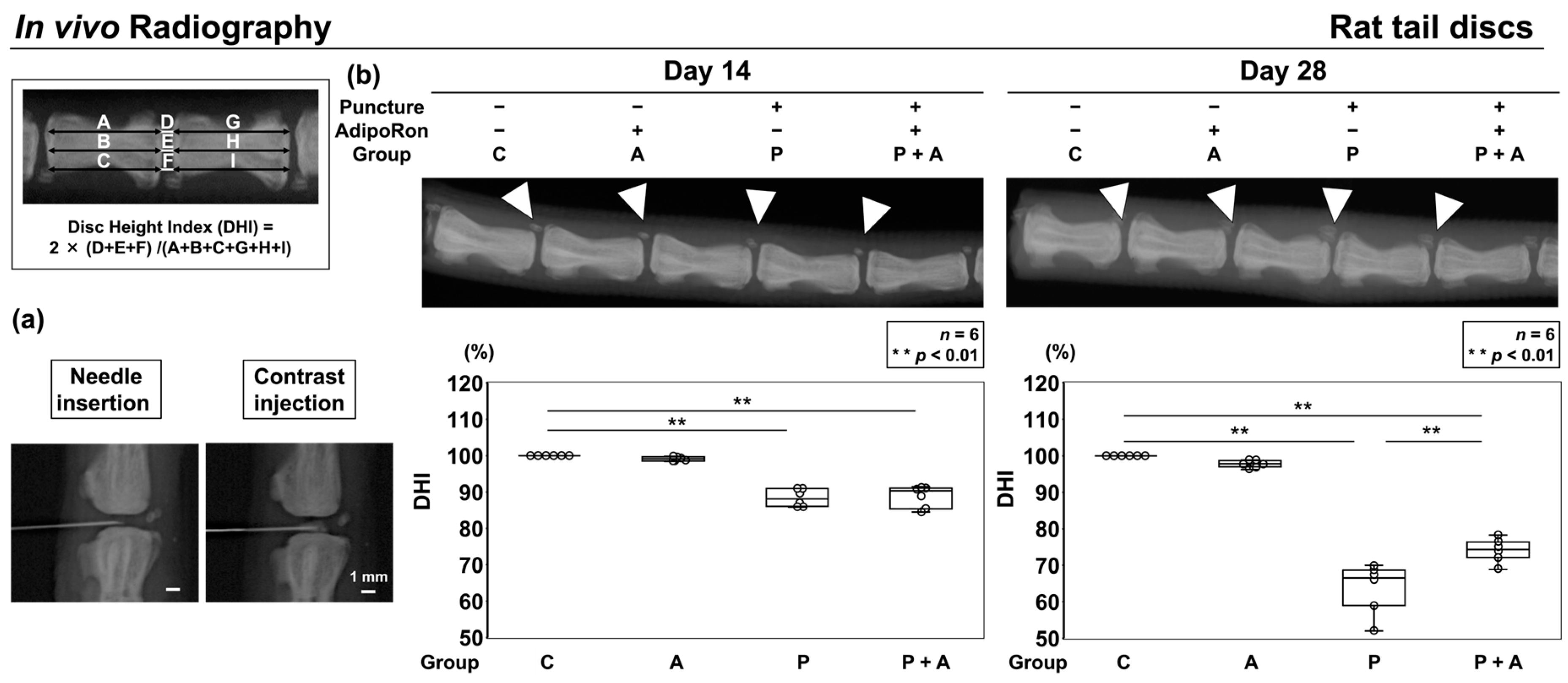
2.2.1. Effect of AdipoRon on the Height of Radiographic Disc in an Annular Puncture Model of Rat Tail IVD
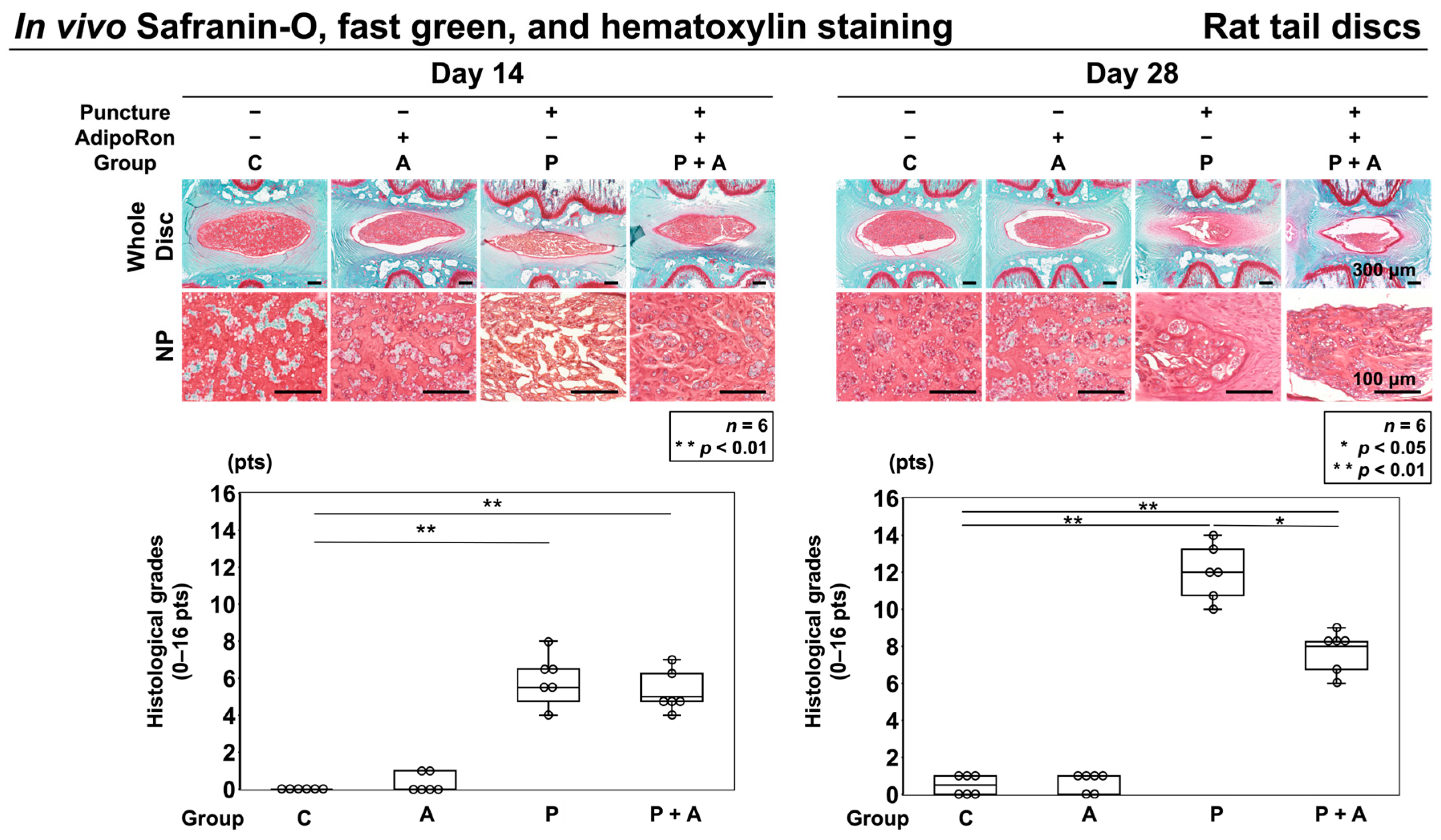
2.2.2. Effect of AdipoRon on Histological IVD Degeneration in a Rat Model of Tail Puncture Model
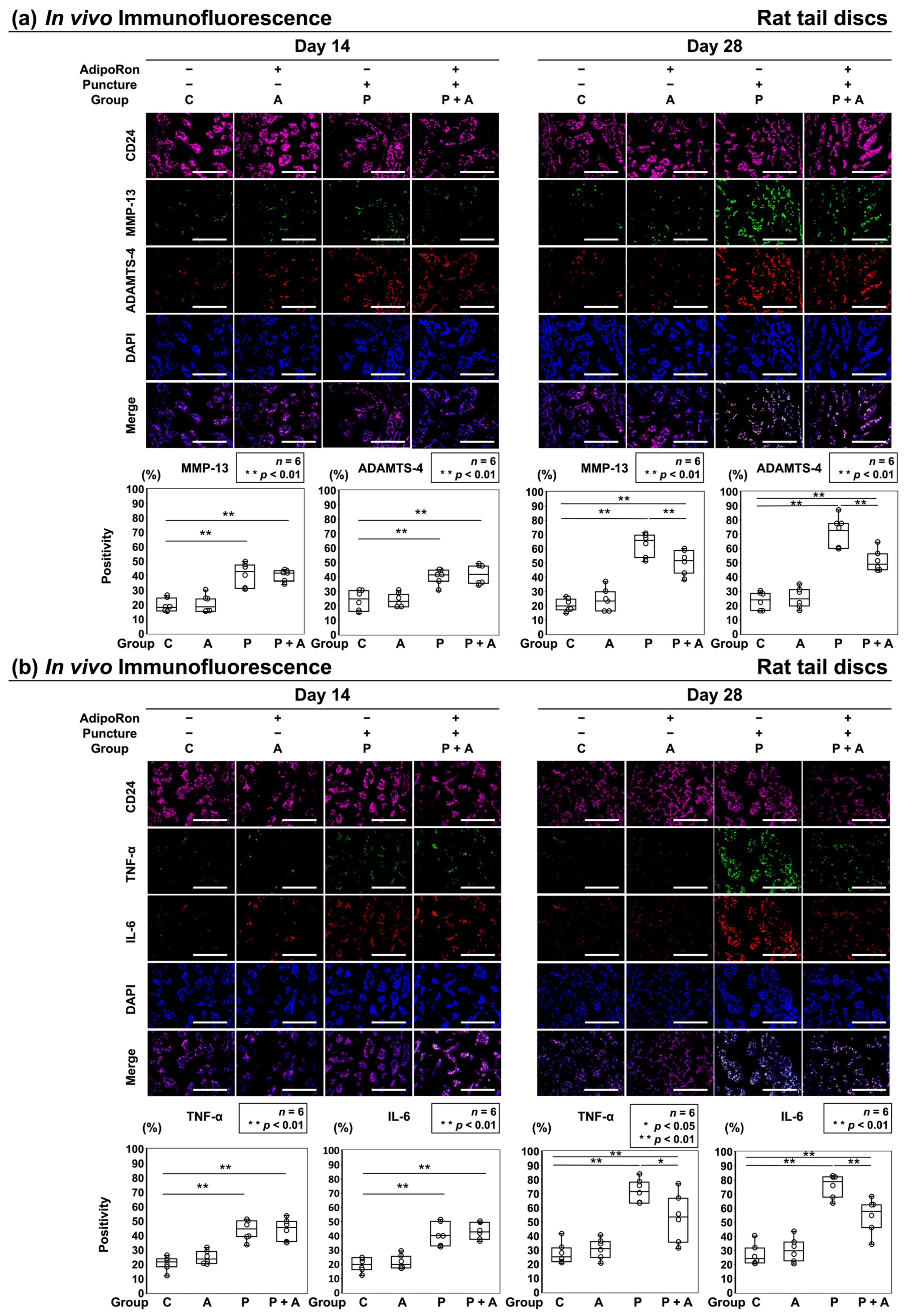
2.2.3. Effects of AdipoRon on the Expression of ECM Catabolic Factors and Proinflammatory Cytokines in a Rat Model of Tail Puncture
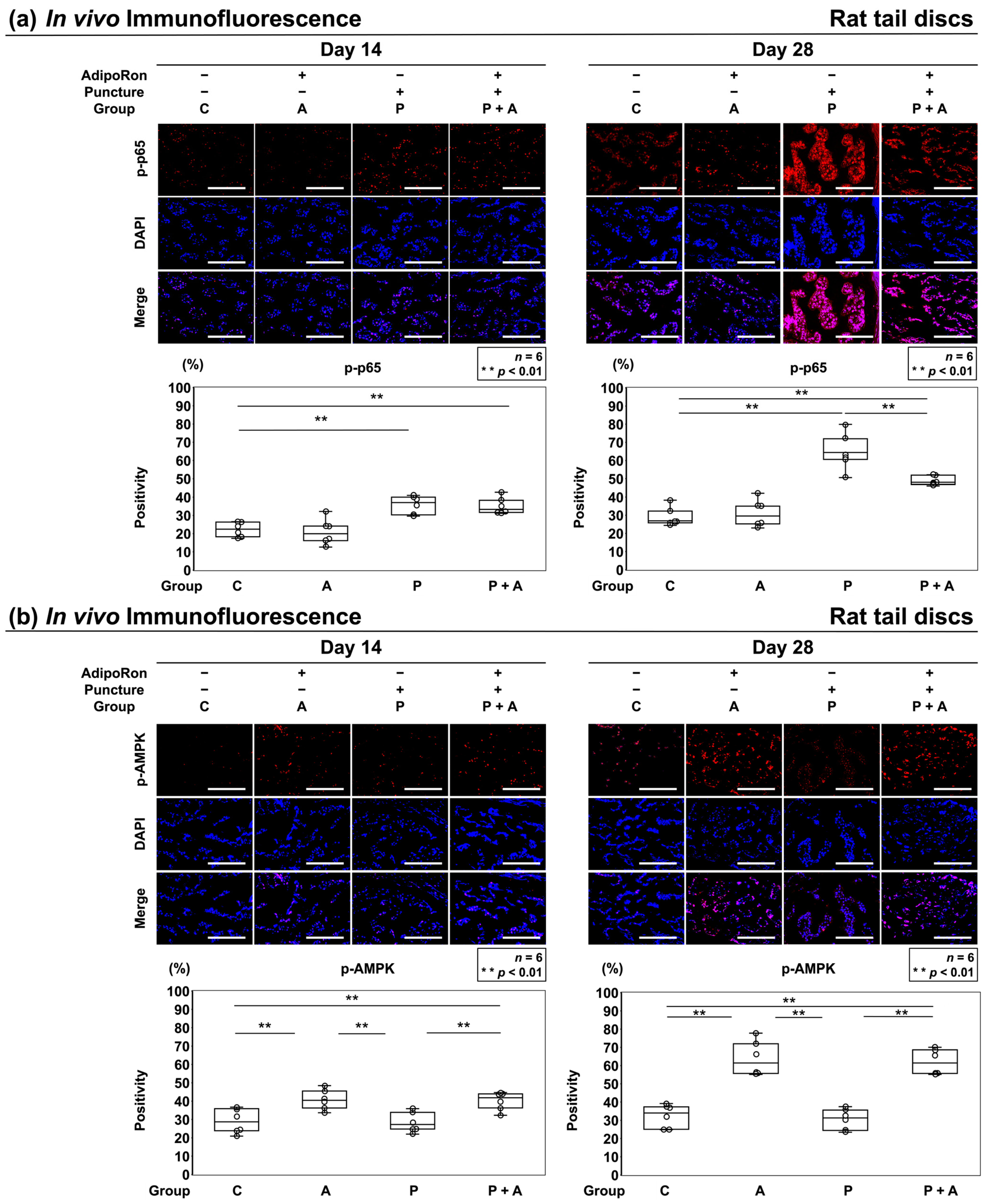
2.2.4. Effects of AdipoRon on the AMPK Pathway in a Rat Model of Tail Puncture
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Human IVD Experiments
4.2.1. Tissues
4.2.2. Cells
4.2.3. Assessment of Cell Viability
4.2.4. Protein Extraction
4.2.5. RNA Isolation and RT–PCR
4.2.6. In Vitro Immunofluorescence
4.2.7. Western Blotting
4.2.8. p65 and AMPK Phosphorylation Assay
4.3. Rat IVD Experiments
4.3.1. Animals
4.3.2. Radiography
4.3.3. Paraffin-Embedded Tissue Preparation
4.3.4. Histomorphology
4.3.5. In Vivo Immunofluorescence
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andersson, G.B.J. Epidemiological features of chronic low-back pain. Lancet 1999, 354, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.I.; Deyo, R.A.; Mirza, S.K.; Turner, J.A.; Comstock, B.A.; Hollingworth, W.; Sullivan, S.D. Expenditures and health status among adults with back and neck problems. JAMA 2008, 299, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Livshits, G.; Popham, M.; Malkin, I.; Sambrook, P.N.; Macgregor, A.J.; Spector, T.; Williams, F.M.K. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: The UK Twin Spine Study. Ann. Rheum. Dis. 2011, 70, 1740–1745. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.P.G.; Roberts, S. Degeneration of the intervertebral disc. Arthritis Res. Ther. 2003, 5, 120–130. [Google Scholar] [CrossRef]
- Crock, H.V.; Goldwasser, M.; Yoshizawa, H. Vascular anatomy related to the intervertebral disc. In Biology of the Intervertebral Disc, 1st ed.; Ghosh, P., Ed.; CRC Press: Boca Raton, FL, USA, 1988; pp. 109–133. [Google Scholar]
- Urban, J.P.G.; Smith, S.; Fairbank, J.C.T. Nutrition of the Intervertebral Disc. Spine 2004, 29, 2700–2709. [Google Scholar] [CrossRef]
- Adams, M.A.; Roughley, P.J. What is intervertebral disc degeneration, and what causes it? Spine 2006, 31, 2151–2161. [Google Scholar] [CrossRef]
- Shiri, R.; Karppinen, J.; Leino-Arjas, P.; Solovieva, S.; Viikari-Juntura, E. The association between obesity and low back pain: A meta-analysis. Am. J. Epidemiol. 2010, 171, 135–154. [Google Scholar] [CrossRef]
- Singla, P.; Bardoloi, A.; Parkash, A.A. Metabolic effects of obesity: A review. World J. Diabetes 2010, 1, 76–88. [Google Scholar] [CrossRef]
- Risbud, M.V.; Shapiro, I.M. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 2014, 10, 44–56. [Google Scholar] [CrossRef]
- Wang, S.Z.; Rui, Y.F.; Lu, J.; Wang, C. Cell and molecular biology of intervertebral disc degeneration: Current understanding and implications for potential therapeutic strategies. Cell Prolif. 2014, 47, 381–390. [Google Scholar] [CrossRef]
- Molinos, M.; Almeida, C.R.; Caldeira, J.; Cunha, C.; Goncalves, R.M.; Barbosa, M.A. Inflammation in intervertebral disc degeneration and regeneration. J. R. Soc. Interface 2015, 12, 20141191. [Google Scholar] [CrossRef]
- Tian, Y.; Yuan, W.; Fujita, N.; Wang, J.; Wang, H.; Shapiro, I.M.; Risbud, M.V. Inflammatory cytokines associated with degenerative disc disease control aggrecanase-1 (ADAMTS-4) expression in nucleus pulposus cells through MAPK and NF-kappaB. Am. J. Pathol. 2013, 182, 2310–2321. [Google Scholar] [CrossRef]
- Miyazaki, K.; Miyazaki, S.; Yurube, T.; Takeoka, Y.; Kanda, Y.; Zhang, Z.; Kakiuchi, Y.; Tsujimoto, R.; Ohnishi, H.; Matsuo, T.; et al. Protective effects of growth differentiation factor-6 on the intervertebral disc: An in vitro and in vivo study. Cells 2022, 11, 1174. [Google Scholar] [CrossRef]
- Wolf, A.M.; Wolf, D.; Rumpold, H.; Enrich, B.; Tilg, H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem. Biophys. Res. Commun. 2004, 323, 630–635. [Google Scholar] [CrossRef]
- Fang, H.; Judd, R.L. Adiponectin regulation and function. Compr. Physiol. 2018, 8, 1031–1063. [Google Scholar]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef]
- Yamauchi, T.; Nio, Y.; Maki, T.; Kobayashi, M.; Takazawa, T.; Iwabu, M.; Okada-Iwabu, M.; Kawamoto, S.; Kubota, N.; Kubota, T.; et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 2007, 13, 332–339. [Google Scholar] [CrossRef]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef]
- Chen, T.H.; Chen, L.; Hsieh, M.S.; Chang, C.P.; Chou, D.T.; Tsai, S.H. Evidence for a protective role for adiponectin in osteoarthritis. Biochim. Biophys. Acta 2006, 1762, 711–718. [Google Scholar] [CrossRef]
- Pattappa, G.; Li, Z.; Peroglio, M.; Wismer, N.; Alini, M.; Grad, S. Diversity of intervertebral disc cells: Phenotype and function. J. Anat. 2012, 221, 480–496. [Google Scholar] [CrossRef]
- Terashima, Y.; Kakutani, K.; Yurube, T.; Takada, T.; Maeno, K.; Hirata, H.; Miyazaki, S.; Ito, M.; Kakiuchi, Y.; Takeoka, Y.; et al. Expression of adiponectin receptors in human and rat intervertebral disc cells and changes in receptor expression during disc degeneration using a rat tail temporary static compression model. J. Orthop. Surg. Res. 2016, 11, 147. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Huang, L.; Yan, M.; Zhang, S.; Zhang, Y.; Jin, B.; Ma, Y.; Luo, Z. Adiponectin downregulates TNF-alpha expression in degenerated intervertebral discs. Spine 2018, 43, E381–E389. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Qiu, W.; Hu, Z.; Lian, J.; Liu, Y.; Zhu, X.; Tu, M.; Fang, F.; Yu, Y.; Valverde, P.; et al. An adiponectin receptor agonist reduces type 2 diabetic periodontitis. J. Dent. Res. 2019, 98, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Okada-Iwabu, M.; Yamauchi, T.; Iwabu, M.; Honma, T.; Hamagami, K.; Matsuda, K.; Yamaguchi, M.; Tanabe, H.; Kimura-Someya, T.; Shirouzu, M.; et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 2013, 503, 493–499. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, L.; Zhang, K.; Zhang, G.; Hu, Y.; Sun, X.; Zhao, C.; Li, H.; Li, Y.M.; Zhao, J. Circular RNA VMA21 protects against intervertebral disc degeneration through targeting miR-200c and X linked inhibitor-of-apoptosis protein. Ann. Rheum. Dis. 2018, 77, 770–779. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Z.; Wang, J.; Shen, S.; Yao, T.; Xu, Y.; Chen, Z.; Fang, B.; Ma, J. circSPG21 protects against intervertebral disc disease by targeting miR-1197/ATP1B3. Exp. Mol. Med. 2021, 53, 1547–1558. [Google Scholar] [CrossRef]
- Nunes, A.K.S.; Raposo, C.; Rocha, S.W.S.; de Sousa Barbosa, K.P.; de Almeida Luna, R.L. Involvement of AMPK, IKbetaalpha-NFkappaB and eNOS in the sildenafil anti-inflammatory mechanism in a demyelination model. Brain Res. 2015, 1627, 119–133. [Google Scholar] [CrossRef]
- Guma, M.; Wang, Y.; Viollet, B.; Liu-Bryan, R. AMPK Activation by A-769662 Controls IL-6 Expression in Inflammatory Arthritis. PLoS ONE 2015, 10, e0140452. [Google Scholar] [CrossRef]
- Tang, S.N.; Walter, B.A.; Heimann, M.K.; Gantt, C.C.; Khan, S.N.; Kokiko-Cochran, O.N.; Askwith, C.C.; Purmessur, D. In vivo mouse intervertebral disc degeneration models and their utility as translational models of clinical discogenic back pain: A comparative review. Front. Pain Res. 2022, 3, 894651. [Google Scholar] [CrossRef]
- Shi, C.; Das, V.; Li, X.; Kc, R.; Qiu, S.; O-Sullivan, I.; Ripper, R.L.; Kroin, J.S.; Mwale, F.; Wallace, A.A.; et al. Development of an in vivo mouse model of discogenic low back pain. J. Cell Physiol. 2018, 233, 6589–6602. [Google Scholar] [CrossRef]
- Colombini, A.; Lombardi, G.; Corsi, M.M.; Banfi, G. Pathophysiology of the human intervertebral disc. Int. J. Biochem. Cell Biol. 2008, 40, 837–842. [Google Scholar] [CrossRef]
- Kepler, C.K.; Ponnappan, R.K.; Tannoury, C.A.; Risbud, M.V.; Anderson, D.G. The molecular basis of intervertebral disc degeneration. Spine J. 2013, 13, 318–330. [Google Scholar] [CrossRef]
- Ruffilli, A.; Viroli, G.; Neri, S.; Traversari, M.; Barile, F.; Manzetti, M.; Assirelli, E.; Ialuna, M.; Vita, F.; Faldini, C. Mechanobiology of the Human Intervertebral Disc: Systematic Review of the Literature and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 2728. [Google Scholar] [CrossRef]
- Wang, C.; Yu, X.; Yan, Y.; Yang, W.; Zhang, S.; Xiang, Y.; Zhang, J.; Wang, W. Tumor necrosis factor-alpha: A key contributor to intervertebral disc degeneration. Acta Biochim. Biophys. Sin. 2017, 49, 1–13. [Google Scholar] [CrossRef]
- Pan, H.; Li, H.; Guo, S.; Wang, C.; Long, L.; Wang, X.; Shi, H.; Zhang, K.; Chen, H.; Li, S. The mechanisms and functions of TNF-alpha in intervertebral disc degeneration. Exp. Gerontol. 2023, 174, 112119. [Google Scholar] [CrossRef]
- Tang, C.H.; Chiu, Y.C.; Tan, T.W.; Yang, R.S.; Fu, W.M. Adiponectin enhances IL-6 production in human synovial fibroblast via an AdipoR1 receptor, AMPK, p38, and NF-kappa B pathway. J. Immunol. 2007, 179, 5483–5492. [Google Scholar] [CrossRef]
- Lee, Y.A.; Ji, H.I.; Lee, S.H.; Hong, S.J.; Yang, H.I.; Yoo, M.C.; Kim, K.S. The role of adiponectin in the production of IL-6, IL-8, VEGF and MMPs in human endothelial cells and osteoblasts: Implications for arthritic joints. Exp. Mol. Med. 2014, 46, e72. [Google Scholar] [CrossRef]
- Wong, H.L.; Wang, M.X.; Cheung, P.T.; Yao, K.M.; Chan, B.P. A 3D collagen microsphere culture system for GDNF-secreting HEK293 cells with enhanced protein productivity. Biomaterials 2007, 28, 5369–5380. [Google Scholar] [CrossRef]
- Yukawa, H.; Ikeuchi, M.; Noguchi, H.; Miyamoto, Y.; Ikuta, K.; Hayashi, S. Embryonic body formation using the tapered soft stencil for cluster culture device. Biomaterials 2011, 32, 3729–3738. [Google Scholar] [CrossRef]
- Vo, N.V.; Hartman, R.A.; Yurube, T.; Jacobs, L.J.; Sowa, G.A.; Kang, J.D. Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J. 2013, 13, 331–341. [Google Scholar] [CrossRef]
- Millward-Sadler, S.J.; Costello, P.W.; Freemont, A.J.; Hoyland, J.A. Regulation of catabolic gene expression in normal and degenerate human intervertebral disc cells: Implications for the pathogenesis of intervertebral disc degeneration. Arthritis Res. Ther. 2009, 11, R65. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Markova, D.; Anderson, D.G.; Zheng, Z.; Shapiro, I.M.; Risbud, M.V. TNF-alpha and IL-1beta promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc. J. Biol. Chem. 2011, 286, 39738–39749. [Google Scholar] [CrossRef] [PubMed]
- Scotece, M.; Perez, T.; Conde, J.; Abella, V.; Lopez, V.; Pino, J.; Gonzalez-Gay, M.A.; Gomez-Reino, J.J.; Mera, A.; Gomez, R.; et al. Adipokines induce pro-inflammatory factors in activated Cd4+ T cells from osteoarthritis patient. J. Orthop. Res. 2017, 35, 1299–1303. [Google Scholar] [CrossRef] [PubMed]
- Napetschnig, J.; Wu, H. Molecular basis of NF-kappaB signaling. Annu. Rev. Biophys. 2013, 42, 443–468. [Google Scholar] [CrossRef]
- Salminen, A.; Hyttinen, J.M.; Kaarniranta, K. AMP-activated protein kinase inhibits NF-kappaB signaling and inflammation: Impact on healthspan and lifespan. J. Mol. Med. 2011, 89, 667–676. [Google Scholar] [CrossRef]
- Nasto, L.A.; Seo, H.Y.; Robinson, A.R.; Tilstra, J.S.; Clauson, C.L.; Sowa, G.A.; Ngo, K.; Dong, Q.; Pola, E.; Lee, J.Y.; et al. ISSLS prize winner: Inhibition of NF-kappaB activity ameliorates age-associated disc degeneration in a mouse model of accelerated aging. Spine 2012, 37, 1819–1825. [Google Scholar] [CrossRef]
- Pfirrmann, C.W.; Metzdorf, A.; Zanetti, M.; Hodler, J.; Boos, N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 2001, 26, 1873–1878. [Google Scholar] [CrossRef]
- Yurube, T.; Takada, T.; Hirata, H.; Kakutani, K.; Maeno, K.; Zhang, Z.; Yamamoto, J.; Doita, M.; Kurosaka, M.; Nishida, K. Modified house-keeping gene expression in a rat tail compression loading-induced disc degeneration model. J. Orthop. Res. 2011, 29, 1284–1290. [Google Scholar] [CrossRef]
- Jimbo, K.; Park, J.S.; Yokosuka, K.; Sato, K.; Nagata, K. Positive feedback loop of interleukin-1β upregulating production of inflammatory mediators in human intervertebral disc cells in vitro. J. Neurosurg. Spine 2005, 2, 589–595. [Google Scholar] [CrossRef]
- Wang, L.; Gu, Y.; Zhao, H.; Chen, R.; Chen, W.; Qi, H.; Gao, W. Dioscin Attenuates Interleukin 1beta (IL-1beta)-Induced Catabolism and Apoptosis via Modulating the Toll-Like Receptor 4 (TLR4)/Nuclear Factor kappa B (NF-kappaB) Signaling in Human Nucleus Pulposus Cells. Med. Sci. Monit. 2020, 26, e923386. [Google Scholar] [CrossRef]
- Kameyama, K.; Motoyama, K.; Tanaka, N.; Yamashita, Y.; Higashi, T.; Arima, H. Induction of mitophagy-mediated antitumor activity with folate-appended methyl-beta-cyclodextrin. Int. J. Nanomed. 2017, 12, 3433–3446. [Google Scholar] [CrossRef]
- Han, B.; Zhu, K.; Li, F.-C.; Xiao, Y.-X.; Feng, J.; Shi, Z.-L.; Lin, M.; Wang, J.; Chen, Q.-X. A simple disc degeneration model induced by percutaneous needle puncture in the rat tail. Spine 2008, 33, 1925–1934. [Google Scholar] [CrossRef]
- Masuda, K.; Aota, Y.; Muehleman, C.; Imai, Y.; Okuma, M.; Thonar, E.J.; Andersson, G.B.; An, H.S. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: Correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine 2005, 30, 5–14. [Google Scholar] [CrossRef]
- Nishida, K.; Doita, M.; Takada, T.; Kakutani, K.; Miyamoto, H.; Shimomura, T.; Maeno, K.; Kurosaka, M. Sustained transgene expression in intervertebral disc cells in vivo mediated by microbubble-enhanced ultrasound gene therapy. Spine 2006, 31, 1415–1419. [Google Scholar] [CrossRef]
- Suzuki, T.; Nishida, K.; Kakutani, K.; Maeno, K.; Yurube, T.; Takada, T.; Kurosaka, M.; Doita, M. Sustained long-term RNA interference in nucleus pulposus cells in vivo mediated by unmodified small interfering RNA. Eur. Spine J. 2009, 18, 263–270. [Google Scholar] [CrossRef]
- Duan, Z.X.; Tu, C.; Liu, Q.; Li, S.Q.; Li, Y.H.; Xie, P.; Li, Z.H. Adiponectin receptor agonist AdipoRon attenuates calcification of osteoarthritis chondrocytes by promoting autophagy. J. Cell Biochem. 2020, 121, 3333–3344. [Google Scholar] [CrossRef]
- Shinohara, I.; Kataoka, T.; Mifune, Y.; Inui, A.; Sakata, R.; Nishimoto, H.; Yamaura, K.; Mukohara, S.; Yoshikawa, T.; Kato, T.; et al. Influence of adiponectin and inflammatory cytokines in fatty degenerative atrophic muscle. Sci. Rep. 2022, 12, 1557. [Google Scholar] [CrossRef]
- Lai, A.; Gansau, J.; Gullbrand, S.E.; Crowley, J.; Cunha, C.; Dudli, S.; Engiles, J.B.; Fusellier, M.; Goncalves, R.M.; Nakashima, D.; et al. Development of a standardized histopathology scoring system for intervertebral disc degeneration in rat models: An initiative of the ORS spine section. JOR Spine 2021, 4, e1150. [Google Scholar] [CrossRef]
| Gene Name | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| ACAN | GTCAGATACCCCATCCACACTC | CATAAAAGACCTCACCCTCCAT |
| COL2A1 | CTCAAGTCGCTGAACAACCA | GTCTCCGCTCTTCCACTCTG |
| TNFA | AGGCGGTGCTTGTTCCTCA | GTTCGAGAAGATGATCTGACTGCC |
| IL-6 | ATGAACTCCTTCTCCACAAGCGC | GAAGAGCCCTCAGGCTGGACTG |
| MMP-13 | CCAGGCATCACCATTCAAG | ATCATCTTCATCACCACCACTG |
| ADAMTS-4 | CCTGGCAAGGACTATGATGCTGA | GGGCGAGTGTTTGGTCTGG |
| GAPDH | GTTCGACAGTCAGCCGCAT | GGAATTTGCCATGGGTGGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohnishi, H.; Zhang, Z.; Yurube, T.; Takeoka, Y.; Kanda, Y.; Tsujimoto, R.; Miyazaki, K.; Matsuo, T.; Ryu, M.; Kumagai, N.; et al. Anti-Inflammatory Effects of Adiponectin Receptor Agonist AdipoRon against Intervertebral Disc Degeneration. Int. J. Mol. Sci. 2023, 24, 8566. https://doi.org/10.3390/ijms24108566
Ohnishi H, Zhang Z, Yurube T, Takeoka Y, Kanda Y, Tsujimoto R, Miyazaki K, Matsuo T, Ryu M, Kumagai N, et al. Anti-Inflammatory Effects of Adiponectin Receptor Agonist AdipoRon against Intervertebral Disc Degeneration. International Journal of Molecular Sciences. 2023; 24(10):8566. https://doi.org/10.3390/ijms24108566
Chicago/Turabian StyleOhnishi, Hiroki, Zhongying Zhang, Takashi Yurube, Yoshiki Takeoka, Yutaro Kanda, Ryu Tsujimoto, Kunihiko Miyazaki, Tomoya Matsuo, Masao Ryu, Naotoshi Kumagai, and et al. 2023. "Anti-Inflammatory Effects of Adiponectin Receptor Agonist AdipoRon against Intervertebral Disc Degeneration" International Journal of Molecular Sciences 24, no. 10: 8566. https://doi.org/10.3390/ijms24108566





