Detection of Early Endothelial Dysfunction by Optoacoustic Tomography
Abstract
:1. Introduction
2. Results
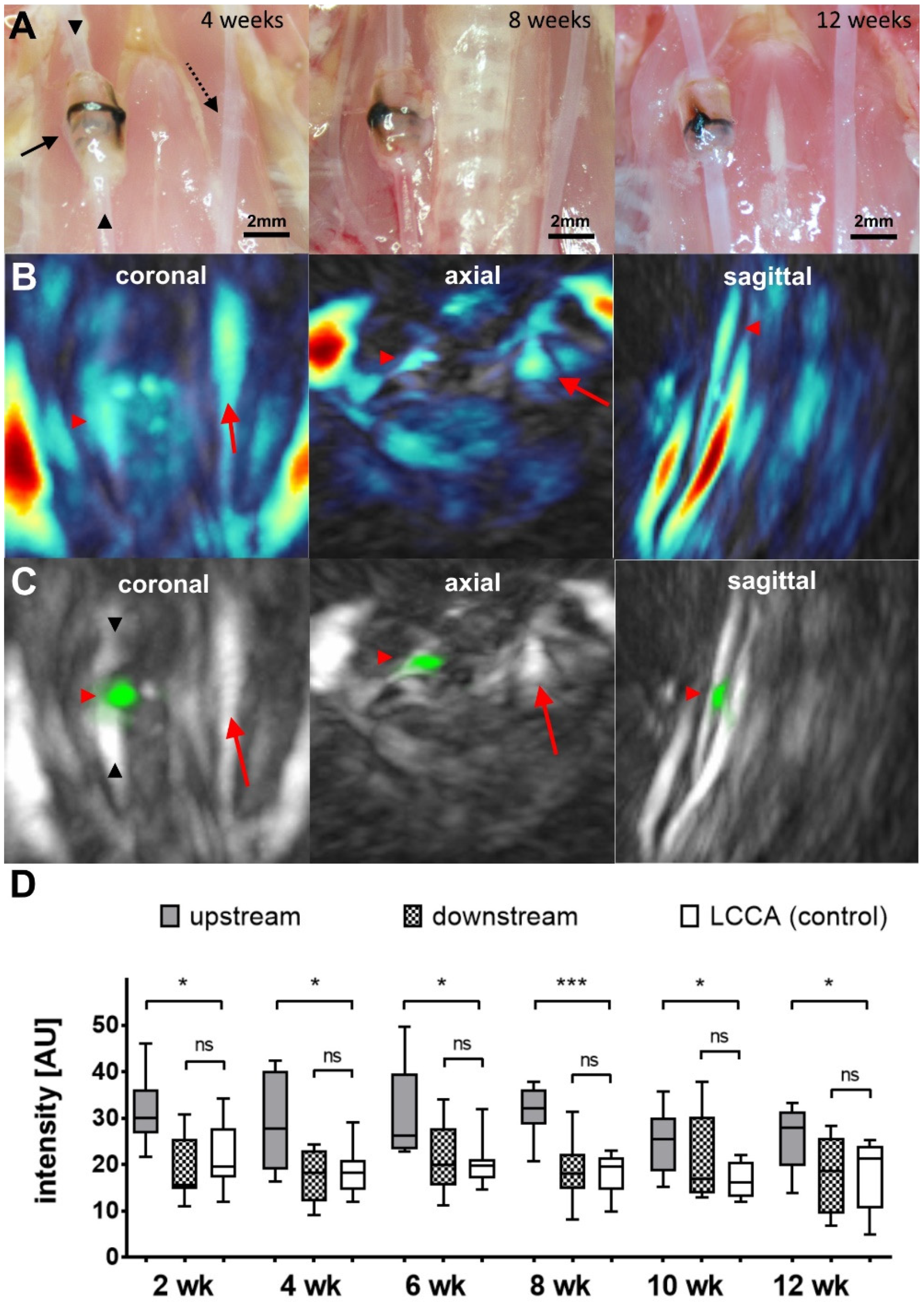
2.1. MSOT Imaging
2.2. CIMT Evaluation
2.3. Immunohistochemistry
3. Discussion
4. Methods
4.1. Animals
4.2. Fluorescent Probe
4.3. Multispectral Optoacoustic Tomography Imaging
4.4. Multispectral Optoacoustic Tomography Data Analysis
4.5. Immunohistochemistry
4.6. Carotid Intima–Media Thickness (CIMT)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Chatzizisis, Y.S.; Coskun, A.U.; Jonas, M.; Edelman, E.R.; Feldman, C.L.; Stone, P.H. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: Molecular, cellular, and vascular behavior. J. Am. Coll. Cardiol. 2007, 49, 2379–2393. [Google Scholar] [CrossRef] [PubMed]
- Chiu, J.-J.; Chien, S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiol. Rev. 2011, 91, 327–387. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, G.; Coscioni, E.; Napoli, C. Cardiovascular risk factors and molecular routes underlying endothelial dysfunction: Novel opportunities for primary prevention. Biochem. Pharmacol. 2022, 202, 115108. [Google Scholar] [CrossRef]
- Jain, M.; Chauhan, A.K. Role of Integrins in Modulating Smooth Muscle Cell Plasticity and Vascular Remodeling: From Expression to Therapeutic Implications. Cells 2022, 11, 646. [Google Scholar] [CrossRef]
- Mo, F.-E. Shear-Regulated Extracellular Microenvironments and Endothelial Cell Surface Integrin Receptors Intertwine in Atherosclerosis. Front. Cell Dev. Biol. 2021, 9, 640781. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, R.; Zhai, R.; Yang, S.; Peng, T.; Zheng, F.; Shen, Y.; Li, M.; Li, L. Matrix stiffness regulates macrophage polarization in atherosclerosis. Pharmacol. Res. 2022, 179, 106236. [Google Scholar] [CrossRef]
- Mezu-Ndubuisi, O.J.; Maheshwari, A. The role of integrins in inflammation and angiogenesis. Pediatr. Res. 2021, 89, 1619–1626. [Google Scholar] [CrossRef]
- Finney, A.C.; Stokes, K.Y.; Pattillo, C.B.; Orr, A.W. Integrin signaling in atherosclerosis. Cell Mol. Life Sci. 2017, 74, 2263–2282. [Google Scholar] [CrossRef]
- Stupack, D.G.; Cheresh, D.A. ECM remodeling regulates angiogenesis: Endothelial integrins look for new ligands. Sci. STKE 2002, 119, pe7. [Google Scholar] [CrossRef]
- Matsuno, H.; Stassen, J.M.; Vermylen, J.; Deckmyn, H. Inhibition of integrin function by a cyclic RGD-containing peptide prevents neointima formation. Circulation 1994, 90, 2203–2206. [Google Scholar] [CrossRef]
- Budatha, M.; Zhang, J.; Schwartz, M.A. Fibronectin-Mediated Inflammatory Signaling Through Integrin α5 in Vascular Remodeling. J. Am. Heart Assoc. 2021, 10, e021160. [Google Scholar] [CrossRef]
- Cheng, C.; De Crom, R.; Van Haperen, R.; Helderman, F.; Gourabi, B.M.; Van Damme, L.C.A.; Kirschbaum, S.W.; Slager, C.J.; Van Der Steen, A.F.W.; Krams, R. The role of shear stress in atherosclerosis: Action through gene expression and inflammation? Cell Biochem. Biophys. 2004, 41, 279–294. [Google Scholar] [CrossRef]
- Cheng, C.; Tempel, D.; Van Haperen, R.; Van Der Baan, A.; Grosveld, F.; Daemen, M.; Krams, R.; De Crom, R. Atherosclerotic Lesion Size and Vulnerability Are Determined by Patterns of Fluid Shear Stress. Circulation 2006, 113, 2744–2753. [Google Scholar] [CrossRef]
- Jarr, K.-U.; Ye, J.; Kojima, Y.; Nanda, V.; Flores, A.M.; Tsantilas, P.; Wang, Y.; Hosseini-Nassab, N.; Eberhard, A.V.; Lotfi, M.; et al. 18 F-Fluorodeoxyglucose-Positron Emission Tomography Imaging Detects Response to Therapeutic Intervention and Plaque Vulnerability in a Murine Model of Advanced Atherosclerotic Disease—Brief Report. Arter. Thromb. Vasc. Biol. 2020, 40, 2821–2828. [Google Scholar] [CrossRef]
- Seifert, R.; Kuhlmann, M.T.; Eligehausen, S.; Kiefer, F.; Hermann, S.; Schäfers, M. Molecular imaging of MMP activity discriminates unstable from stable plaque phenotypes in shear-stress induced murine atherosclerosis. PLoS ONE 2018, 13, e0204305. [Google Scholar] [CrossRef]
- Kwiatkowski, G.; Bar, A.; Jasztal, A.; Chlopicki, S. MRI-based in vivo detection of coronary microvascular dysfunction before alterations in cardiac function induced by short-term high-fat diet in mice. Sci. Rep. 2021, 11, 18915. [Google Scholar] [CrossRef]
- Wenning, C.; Kloth, C.; Kuhlmann, M.T.; Jacobs, A.H.; Schober, O.; Hermann, S.; Schäfers, M.A. Serial F-18-FDG PET/CT distinguishes inflamed from stable plaque phenotypes in shear-stress induced murine atherosclerosis. Atherosclerosis 2014, 234, 276–282. [Google Scholar] [CrossRef]
- Alsibai, W.; Hahnenkamp, A.; Eisenblätter, M.; Riemann, B.; Schäfers, M.; Bremer, C.; Haufe, G.; Höltke, C. Fluorescent non-peptidic RGD mimetics with high selectivity for alphaVbeta3 vs. alphaIIbbeta3 integrin receptor: Novel probes for in vivo optical imaging. J. Med. Chem. 2014, 57, 9971–9982. [Google Scholar] [CrossRef]
- Fernández-Alvarez, V.; Sánchez, M.L.; Alvarez, F.L.; Nieto, C.S.; Mäkitie, A.A.; Olsen, K.D.; Ferlito, A. Evaluation of Intima-Media Thickness and Arterial Stiffness as Early Ultrasound Biomarkers of Carotid Artery Atherosclerosis. Cardiol. Ther. 2022, 11, 231–247. [Google Scholar] [CrossRef]
- Becker, A.; Masthoff, M.; Claussen, J.; Ford, S.J.; Roll, W.; Burg, M.; Barth, P.J.; Heindel, W.; Schäfers, M.; Eisenblätter, M.; et al. Multispectral optoacoustic tomography of the human breast: Characterisation of healthy tissue and malignant lesions using a hybrid ultrasound-optoacoustic approach. Eur. Radiol. 2018, 28, 602–609. [Google Scholar] [CrossRef]
- Buehler, A.; Kacprowicz, M.; Taruttis, A.; Ntziachristos, V. Real-time handheld multispectral optoacoustic imaging. Opt. Lett. 2013, 38, 1404–1406. [Google Scholar] [CrossRef] [PubMed]
- Karlas, A.; Kallmayer, M.; Bariotakis, M.; Fasoula, N.-A.; Liapis, E.; Hyafil, F.; Pelisek, J.; Wildgruber, M.; Eckstein, H.-H.; Ntziachristos, V. Multispectral optoacoustic tomography of lipid and hemoglobin contrast in human carotid atherosclerosis. Photoacoustics 2021, 23, 100283. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.S.; Jin, P.S.; Ng, M.; Garnell, J.; Ying, C.W.; Tec, C.T.; Bhakoo, K. Development of Molecular Magnetic Resonance Imaging Tools for Risk Stratification of Carotid Atherosclerotic Disease Using Dual-Targeted Microparticles of Iron Oxide. Transl. Stroke Res. 2022, 13, 245–256. [Google Scholar] [CrossRef]
- Meletta, R.; Steier, L.; Borel, N.; Mu, L.; Keller, C.; Chiotellis, A.; Russo, E.; Halin, C.; Ametamey, S.M.; Schibli, R.; et al. CD80 Is Upregulated in a Mouse Model with Shear Stress-Induced Atherosclerosis and Allows for Evaluating CD80-Targeting PET Tracers. Mol. Imaging Biol. 2017, 19, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, M.-J.; Abran, M.; Maafi, F.; Busseuil, D.; Merlet, N.; Mihalache-Avram, T.; Geoffroy, P.; Tardif, P.-L.; Abulrob, A.; Arbabi-Ghahroudi, M.; et al. In Vivo Near-Infrared Fluorescence Imaging of Atherosclerosis Using Local Delivery of Novel Targeted Molecular Probes. Sci. Rep. 2019, 9, 1–12. [Google Scholar]
- Yao, Y.; Jiang, Y.; Sheng, Z.; Zhang, Y.; An, Y.; Yan, F.; Ma, G.; Liu, N.; Teng, G.; Cheng, Z. Analysis of in situ and ex vivo alphaVbeta3 integrin expression during experimental carotid atherogenesis. Int. J. Nanomed. 2012, 7, 641–649. [Google Scholar]
- Maniotis, A.J.; Chen, C.S.; Ingber, D.E. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc. Natl. Acad. Sci. USA 1997, 94, 849–854. [Google Scholar] [CrossRef]
- Shyy, J.Y.; Chien, S. Role of integrins in cellular responses to mechanical stress and adhesion. Curr. Opin. Cell Biol. 1997, 9, 707–713. [Google Scholar] [CrossRef]
- Kock, L.M.; Schulz, R.M.; van Donkelaar, C.C.; Thümmler, C.B.; Bader, A.; Ito, K. RGD-dependent integrins are mechanotransducers in dynamically compressed tissue-engineered cartilage constructs. J. Biomech. 2009, 42, 2177–2182. [Google Scholar] [CrossRef]
- Millward-Sadler, S.J.; Salter, D.M. Integrin-Dependent Signal Cascades in Chondrocyte Mechanotransduction. Ann. Biomed. Eng. 2004, 32, 435–446. [Google Scholar] [CrossRef]
- Ross, T.D.; Coon, B.G.; Yun, S.; Baeyens, N.; Tanaka, K.; Ouyang, M.; Schwartz, M.A. Integrins in mechanotransduction. Curr. Opin. Cell Biol. 2013, 25, 613–618. [Google Scholar] [CrossRef]
- Hamrangsekachaee, M.; Wen, K.; Bencherif, S.A.; Ebong, E.E. Atherosclerosis and endothelial mechanotransduction: Current knowledge and models for future research. Am. J. Physiol. Physiol. 2023, 324, C488–C504. [Google Scholar] [CrossRef]
- Bachmann, M.; Kukkurainen, S.; Hytönen, V.P.; Wehrle-Haller, B. Cell Adhesion by Integrins. Physiol. Rev. 2019, 99, 1655–1699. [Google Scholar] [CrossRef]
- Al-Yafeai, Z.; Yurdagul, A., Jr.; Peretik, J.M.; Alfaidi, M.; Murphy, P.A.; Orr, A.W. Endothelial FN (Fibronectin) Deposition by alpha5beta1 Integrins Drives Atherogenic Inflammation. Arter. Thromb. Vasc. Biol. 2018, 38, 2601–2614. [Google Scholar] [CrossRef]
- Lee, B.H.; Bae, J.S.; Park, R.W.; Kim, J.E.; Park, J.Y.; Kim, I.S. betaig-h3 triggers signaling pathways mediating adhesion and migration of vascular smooth muscle cells through alphavbeta5 integrin. Exp. Mol. Med. 2006, 38, 153–161. [Google Scholar] [CrossRef]
- Urbich, C.; Walter, D.H.; Zeiher, A.M.; Dimmeler, S. Laminar shear stress upregulates integrin expression: Role in endothelial cell adhesion and apoptosis. Circ. Res. 2000, 87, 683–689. [Google Scholar] [CrossRef]
- Ivankovic, I.; Merčep, E.; Schmedt, C.-G.; Deán-Ben, X.L.; Razansky, D. Real-time Volumetric Assessment of the Human Carotid Artery: Handheld Multispectral Optoacoustic Tomography. Radiology 2019, 291, 45–50. [Google Scholar] [CrossRef]
- Kuhlmann, M.T.; Cuhlmann, S.; Hoppe, I.; Krams, R.; Evans, P.C.; Strijkers, G.J.; Nicolay, K.; Hermann, S.; Schäfers, M. Implantation of a carotid cuff for triggering shear-stress induced atherosclerosis in mice. J. Vis. Exp. 2012, 59, e3308. [Google Scholar]
- Höltke, C.; Alsibai, W.; Grewer, M.; Stölting, M.; Geyer, C.; Eisenblätter, M.; Wildgruber, M.; Helfen, A. How Different Albumin-Binders Drive Probe Distribution of Fluorescent RGD Mimetics. Front. Chem. 2021, 9, 689850. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Höltke, C.; Enders, L.; Stölting, M.; Geyer, C.; Masthoff, M.; Kuhlmann, M.T.; Wildgruber, M.; Helfen, A. Detection of Early Endothelial Dysfunction by Optoacoustic Tomography. Int. J. Mol. Sci. 2023, 24, 8627. https://doi.org/10.3390/ijms24108627
Höltke C, Enders L, Stölting M, Geyer C, Masthoff M, Kuhlmann MT, Wildgruber M, Helfen A. Detection of Early Endothelial Dysfunction by Optoacoustic Tomography. International Journal of Molecular Sciences. 2023; 24(10):8627. https://doi.org/10.3390/ijms24108627
Chicago/Turabian StyleHöltke, Carsten, Leonie Enders, Miriam Stölting, Christiane Geyer, Max Masthoff, Michael T. Kuhlmann, Moritz Wildgruber, and Anne Helfen. 2023. "Detection of Early Endothelial Dysfunction by Optoacoustic Tomography" International Journal of Molecular Sciences 24, no. 10: 8627. https://doi.org/10.3390/ijms24108627
APA StyleHöltke, C., Enders, L., Stölting, M., Geyer, C., Masthoff, M., Kuhlmann, M. T., Wildgruber, M., & Helfen, A. (2023). Detection of Early Endothelial Dysfunction by Optoacoustic Tomography. International Journal of Molecular Sciences, 24(10), 8627. https://doi.org/10.3390/ijms24108627






