Development of Procathepsin L (pCTS-L)-Inhibiting Lanosterol-Carrying Liposome Nanoparticles to Treat Lethal Sepsis
Abstract
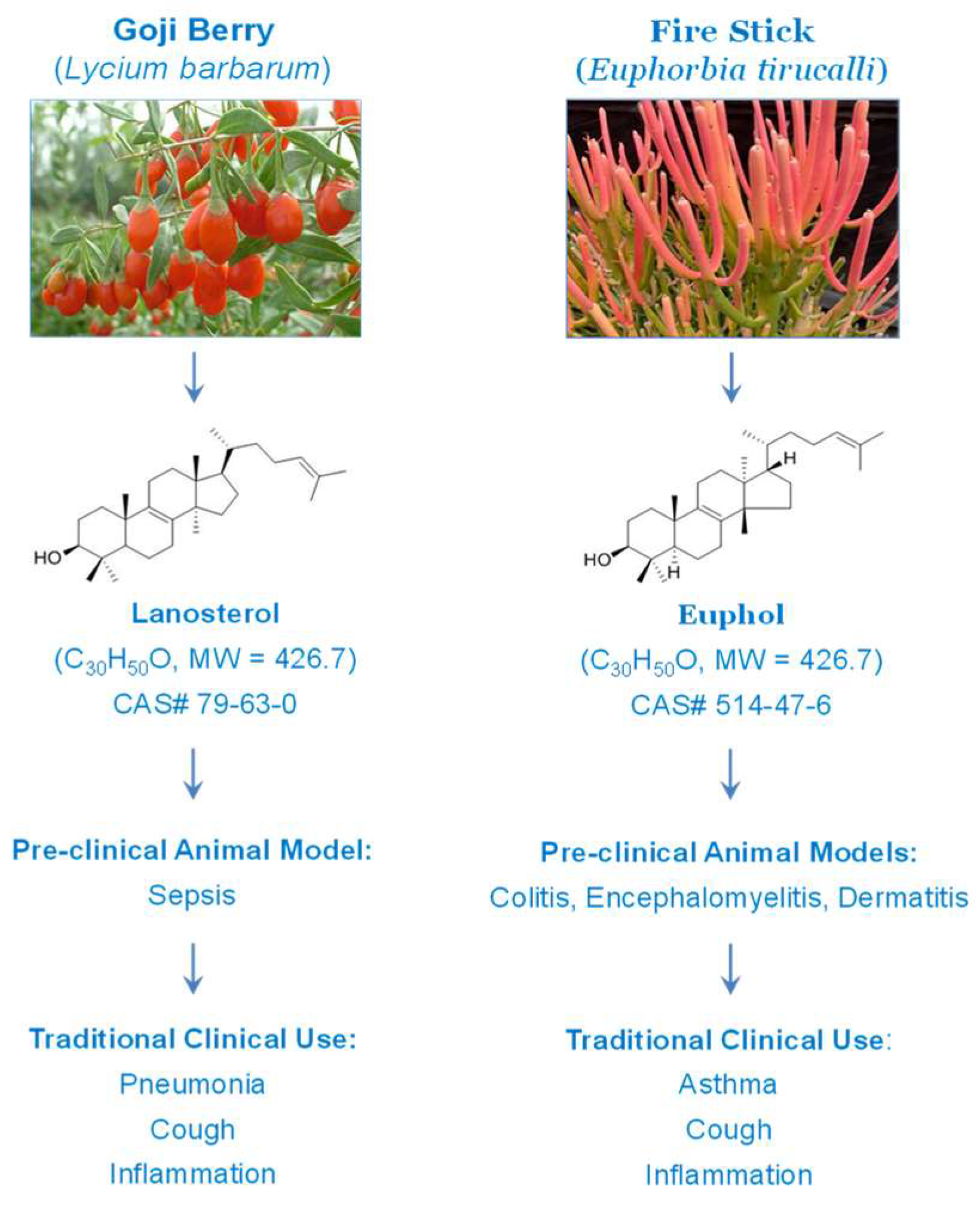
1. Introduction
2. Results
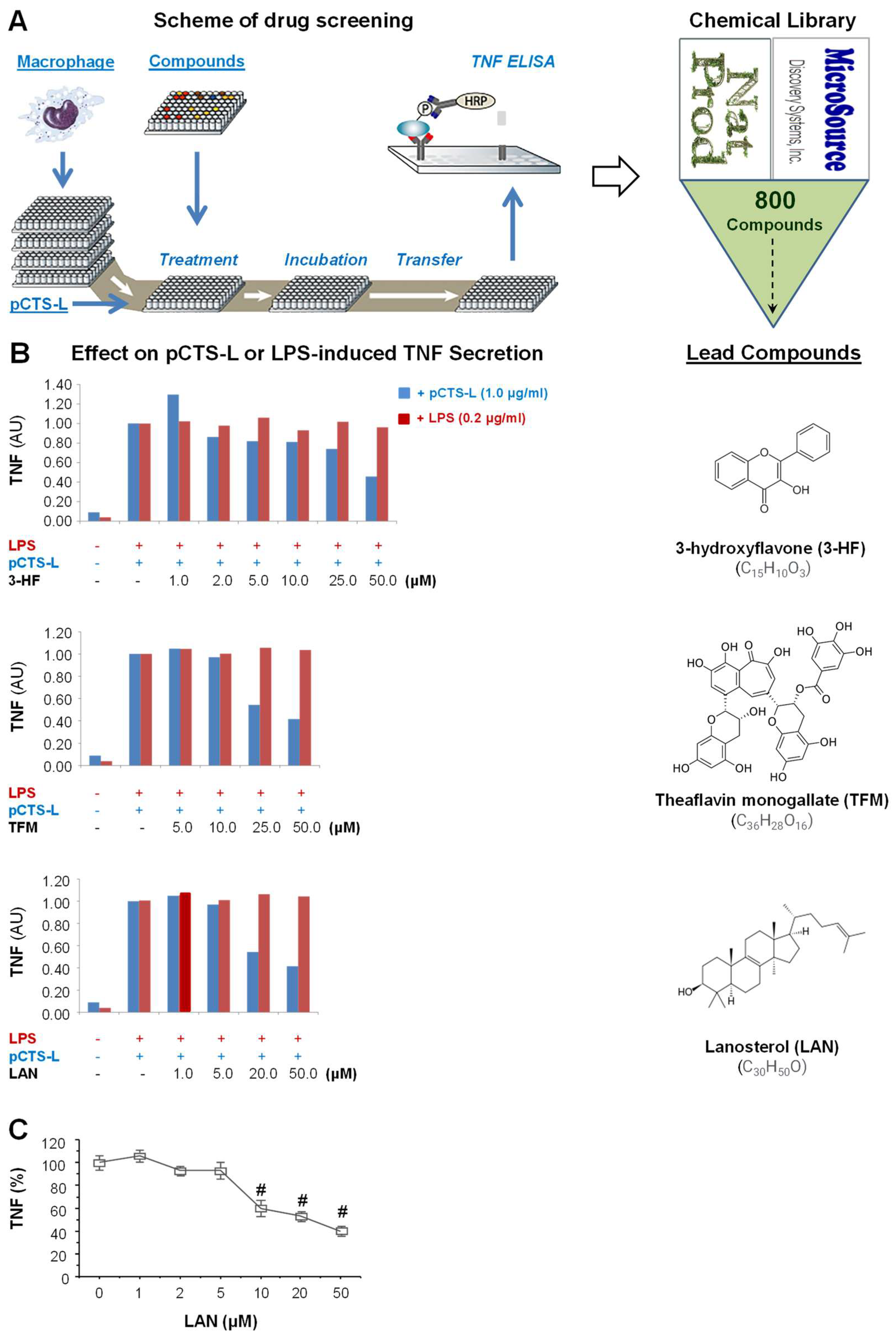
2.1. Identification of Lanosterol as a Selective Inhibitor of pCTS-L-Induced TNF Secretion
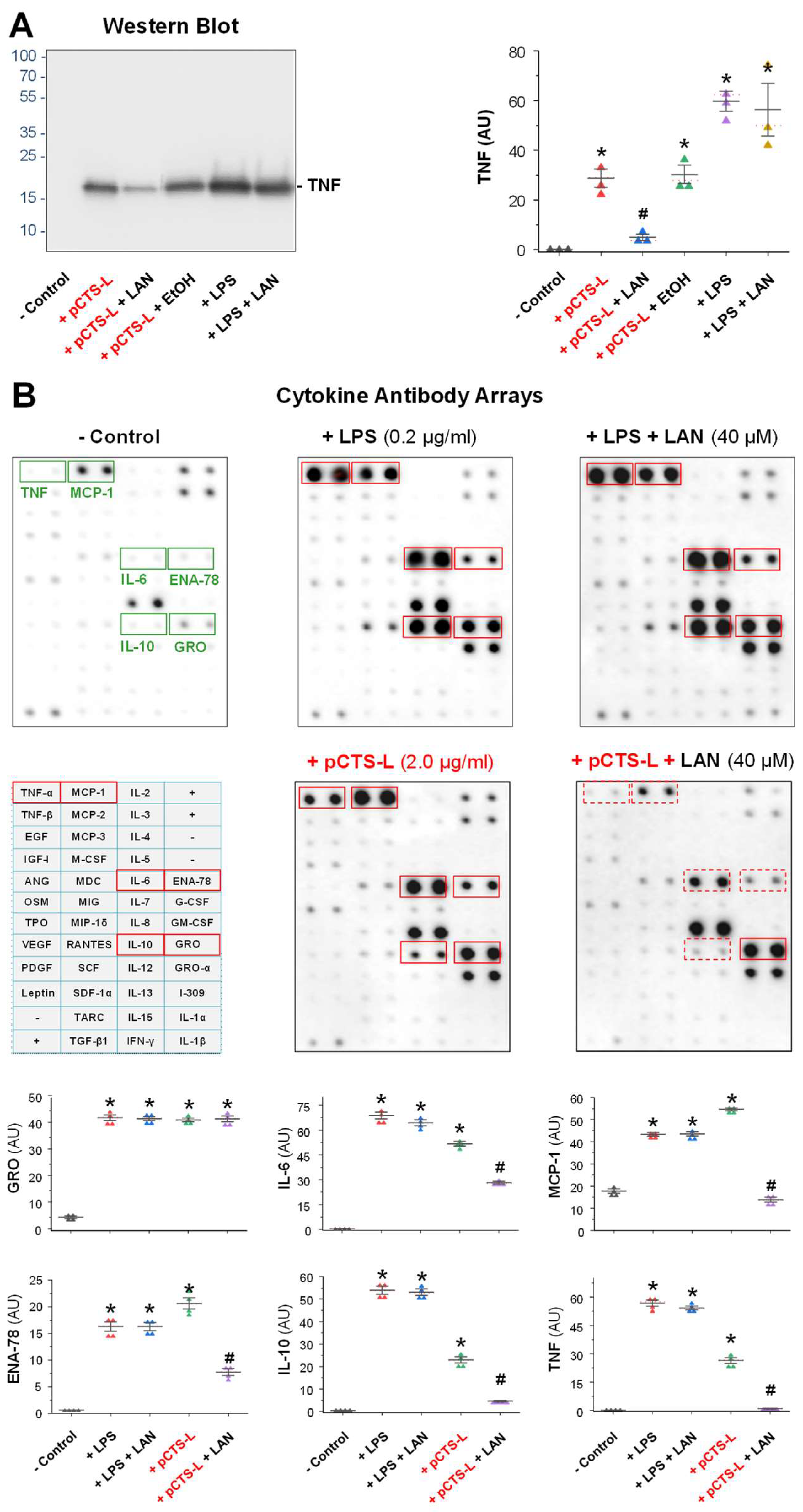
2.2. LAN Significantly Suppressed pCTS-L-Induced Production of TNF and Other Chemokines in Primary Human Peripheral Blood Mononuclear Cells (PBMCs)
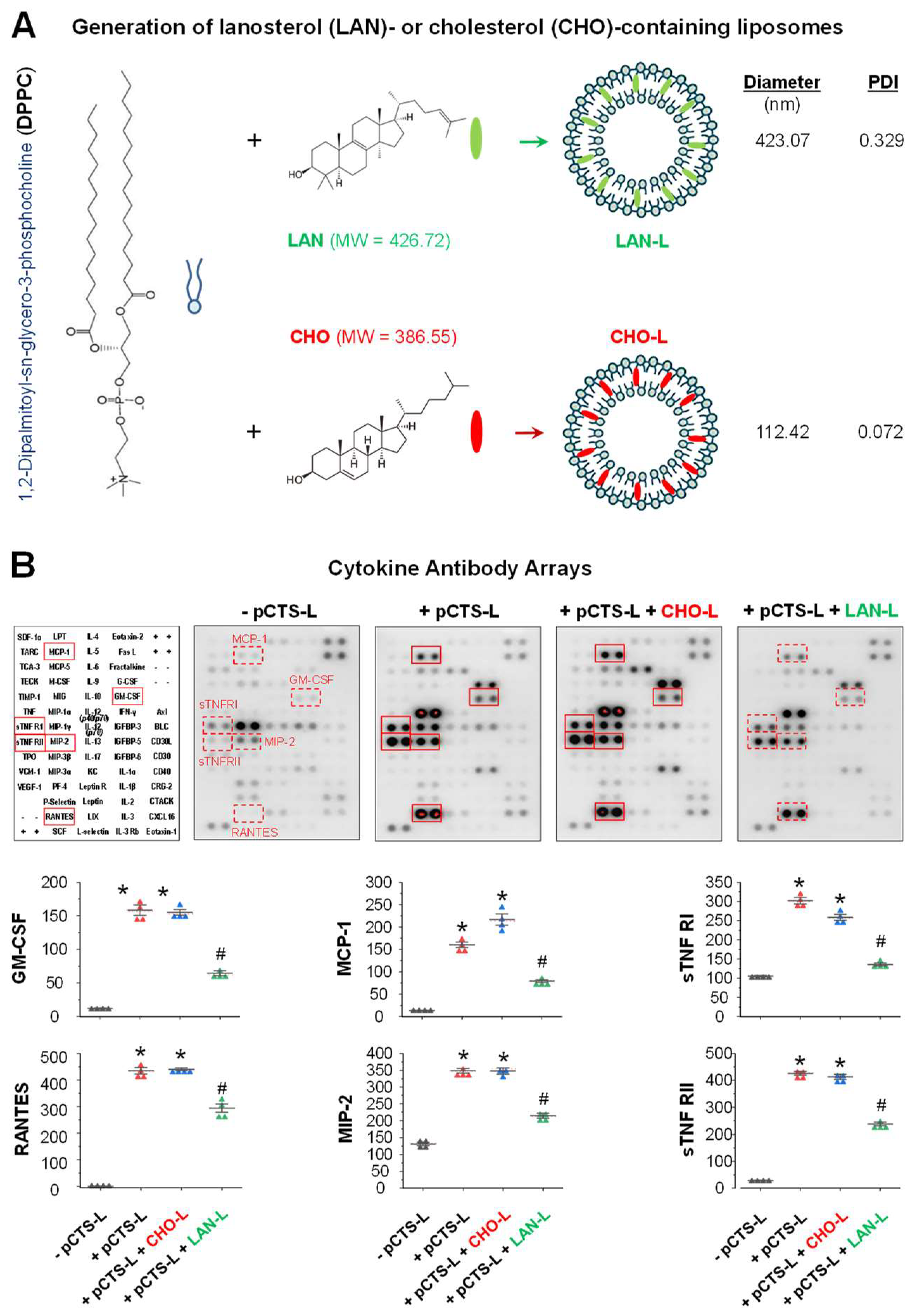
2.3. LAN-Carrying Liposomes Similarly Suppressed pCTS-L-Induced Inflammation
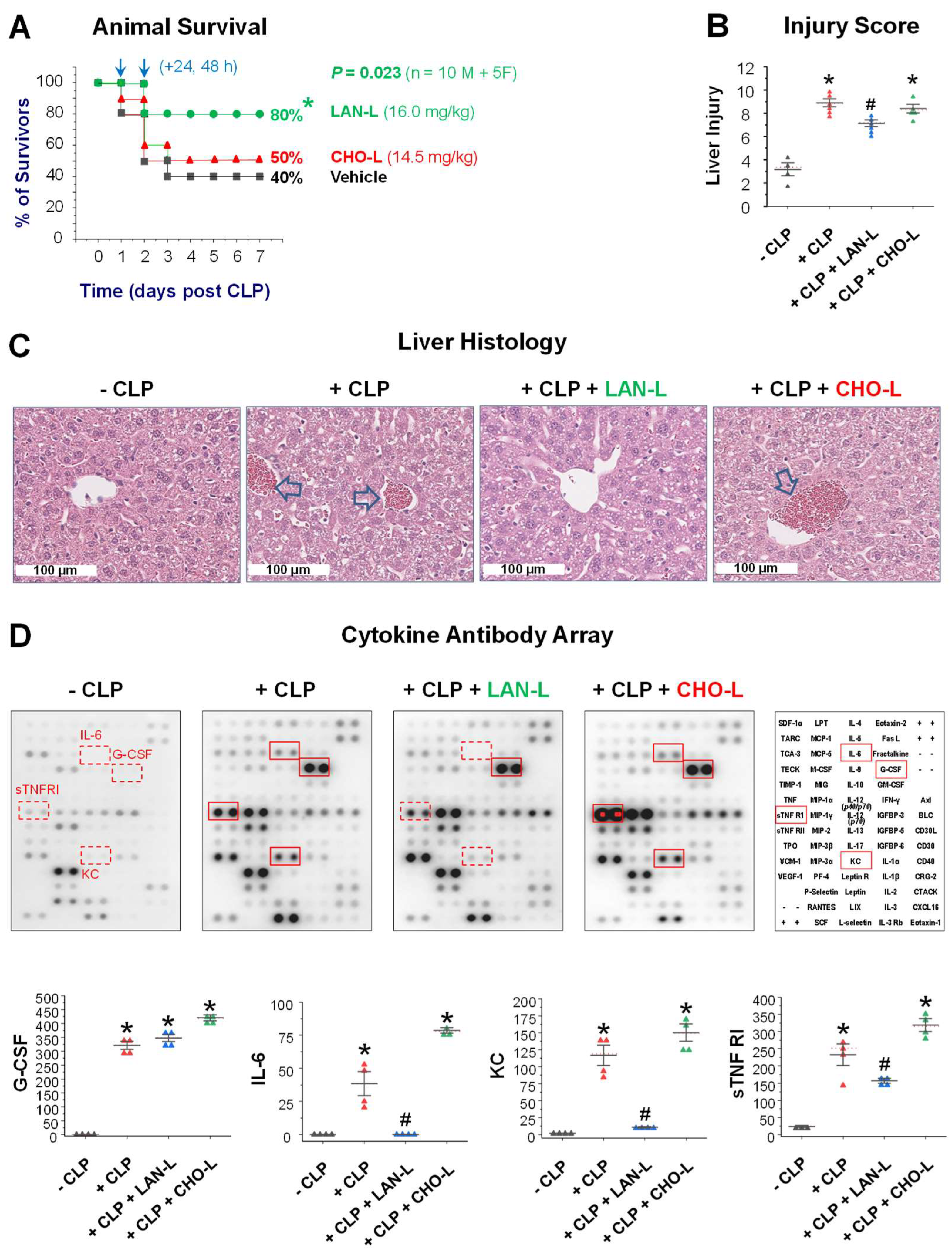
2.4. LAN-Carrying Liposome Rescued Mice from Lethal Sepsis
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Preparation of Recombinant Human and Murine pCTS-L Proteins
4.3. High-Throughput Screening of Natural Product Chemical Library for pCTS-L Inhibitors
4.4. Western Blotting
4.5. Generation of Lanosterol- or Cholesterol-Carrying Liposomes
4.6. Cytokine Antibody Arrays
4.7. Animal Model of Lethal Sepsis
4.8. Tissue Injury
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Tindal, E.W.; Armstead, B.E.; Monaghan, S.F.; Heffernan, D.S.; Ayala, A. Emerging therapeutic targets for sepsis. Expert Opin. Ther. Targets 2021, 25, 175–189. [Google Scholar] [CrossRef]
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.Y.; Huffel, C.V.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C.; et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 1998, 282, 2085–2088. [Google Scholar] [CrossRef]
- Tracey, K.J.; Fong, Y.; Hesse, D.G.; Manogue, K.R.; Lee, A.T.; Kuo, G.C.; Lowry, S.F.; Cerami, A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 1987, 330, 662–664. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Biologic basis for interleukin-1 in disease. Blood 1996, 87, 2095–2147. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Shi, Y.; Ao, L.H.; Harken, A.H.; Meng, X.Z. TLR4 mediates LPS-induced HO-1 expression in mouse liver: Role of TNF-alpha and IL-1beta. World J. Gastroenterol. 2003, 9, 1799–1803. [Google Scholar] [CrossRef]
- Fan, M.; Yang, K.; Wang, X.; Chen, L.; Gill, P.S.; Ha, T.; Liu, L.; Lewis, N.H.; Williams, D.L.; Li, C. Lactate promotes endothelial-to-mesenchymal transition via Snail1 lactylation after myocardial infarction. Sci. Adv. 2023, 9, eadc9465. [Google Scholar] [CrossRef]
- Yang, K.; Fan, M.; Wang, X.; Xu, J.; Wang, Y.; Gill, P.S.; Ha, T.; Liu, L.; Hall, J.V.; Williams, D.L.; et al. Lactate induces vascular permeability via disruption of VE-cadherin in endothelial cells during sepsis. Sci. Adv. 2022, 8, eabm8965. [Google Scholar] [CrossRef]
- Yang, L.; Xie, M.; Yang, M.; Yu, Y.; Zhu, S.; Hou, W.; Kang, R.; Lotze, M.T.; Billiar, T.R.; Wang, H.; et al. PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat. Commun. 2014, 5, 4436. [Google Scholar] [CrossRef]
- Wang, H.; Bloom, O.; Zhang, M.; Vishnubhakat, J.M.; Ombrellino, M.; Che, J.; Frazier, A.; Yang, H.; Ivanova, S.; Borovikova, L.; et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999, 285, 248–251. [Google Scholar] [CrossRef]
- Zhu, C.S.; Qiang, X.; Chen, W.; Li, J.; Lan, X.; Yang, H.; Gong, J.; Becker, L.; Wang, P.; Tracey, K.J.; et al. Identification of procathepsin L (pCTS-L)-neutralizing monoclonal antibodies to treat potentially lethal sepsis. Sci. Adv. 2023, 9, eadf4313. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ochani, M.; Li, J.; Qiang, X.; Tanovic, M.; Harris, H.E.; Susarla, S.M.; Ulloa, L.; Wang, H.; DiRaimo, R.; et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc. Natl. Acad. Sci. USA 2004, 101, 296–301. [Google Scholar] [CrossRef]
- Zhang, M.M.; Qiao, Y.; Ang, E.L.; Zhao, H. Using natural products for drug discovery: The impact of the genomics era. Expert Opin. Drug Discov. 2017, 12, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M. Recent advances in target identification of bioactive natural products. Biosci. Biotechnol. Biochem. 2019, 83, 1–9. [Google Scholar] [CrossRef]
- Zhang, M.; Caragine, T.; Wang, H.; Cohen, P.S.; Botchkina, G.; Soda, K.; Bianchi, M.; Ulrich, P.; Cerami, A.; Sherry, B.; et al. Spermine inhibits proinflammatory cytokine synthesis in human mononuclear cells: A counterregulatory mechanism that restrains the immune response. J. Exp. Med. 1997, 185, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Wang, H.; Yuan, R.; Li, H.; Ochani, M.; Ochani, K.; Rosas-Ballina, M.; Czura, C.J.; Huston, J.M.; Miller, E.; et al. Role of HMGB1 in apoptosis-mediated sepsis lethality. J. Exp. Med. 2006, 203, 1637–1642. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, J.; Sama, A.E.; Wang, H. Carbenoxolone Blocks Endotoxin-Induced Protein Kinase R (PKR) Activation and High Mobility Group Box 1 (HMGB1) Release. Mol. Med. 2013, 19, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhu, S.; Li, J.; Assa, A.; Jundoria, A.; Xu, J.; Fan, S.; Eissa, N.T.; Tracey, K.J.; Sama, A.E.; et al. EGCG stimulates autophagy and reduces cytoplasmic HMGB1 levels in endotoxin-stimulated macrophages. Biochem. Pharmacol. 2011, 81, 1152–1163. [Google Scholar] [CrossRef]
- Buchman, T.G.; Simpson, S.Q.; Sciarretta, K.L.; Finne, K.P.; Sowers, N.; Collier, M.; Chavan, S.; Oke, I.; Pennini, M.E.; Santhosh, A.; et al. Sepsis Among Medicare Beneficiaries: 1. The Burdens of Sepsis, 2012–2018. Crit. Care Med. 2020, 48, 276–288. [Google Scholar] [CrossRef]
- Dutra, R.C.; Claudino, R.F.; Bento, A.F.; Marcon, R.; Schmidt, E.C.; Bouzon, Z.L.; Pianowski, L.F.; Calixto, J.B. Preventive and therapeutic euphol treatment attenuates experimental colitis in mice. PLoS ONE 2011, 6, e27122. [Google Scholar] [CrossRef]
- Dutra, R.C.; de Souza, P.R.; Bento, A.F.; Marcon, R.; Bicca, M.A.; Pianowski, L.F.; Calixto, J.B. Euphol prevents experimental autoimmune encephalomyelitis in mice: Evidence for the underlying mechanisms. Biochem. Pharmacol. 2012, 83, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Dutra, R.C.; Simao da Silva, K.A.; Bento, A.F.; Marcon, R.; Paszcuk, A.F.; Meotti, F.C.; Pianowski, L.F.; Calixto, J.B. Euphol, a tetracyclic triterpene produces antinociceptive effects in inflammatory and neuropathic pain: The involvement of cannabinoid system. Neuropharmacology 2012, 63, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Passos, G.F.; Medeiros, R.; Marcon, R.; Nascimento, A.F.; Calixto, J.B.; Pianowski, L.F. The role of PKC/ERK1/2 signaling in the anti-inflammatory effect of tetracyclic triterpene euphol on TPA-induced skin inflammation in mice. Eur. J. Pharmacol. 2013, 698, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Araldi, E.; Fernández-Fuertes, M.; Canfrán-Duque, A.; Tang, W.; Cline, G.W.; Madrigal-Matute, J.; Pober, J.S.; Lasunción, M.A.; Wu, D.; Fernández-Hernando, C.; et al. Lanosterol Modulates TLR4-Mediated Innate Immune Responses in Macrophages. Cell Rep. 2017, 19, 2743–2755. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Vlase, L.; Vodnar, D.C.; Gheldiu, A.M.; Oprean, R.; Crișan, G. Antioxidant, Antimicrobial Effects and Phenolic Profile of Lycium barbarum L. Flowers. Molecules 2015, 20, 15060–15071. [Google Scholar] [CrossRef]
- Chen, C.L.; Chen, Y.P.; Lin, M.W.; Huang, Y.B.; Chang, F.R.; Duh, T.H.; Wu, D.C.; Wu, W.C.; Kao, Y.C.; Yang, P.H. Euphol from Euphorbia tirucalli Negatively Modulates TGF-β Responsiveness via TGF-β Receptor Segregation inside Membrane Rafts. PLoS ONE 2015, 10, e0140249. [Google Scholar] [CrossRef]
- Alvarez-Sala, A.; Garcia-Llatas, G.; Cilla, A.; Barberá, R.; Sánchez-Siles, L.M.; Lagarda, M.J. Impact of Lipid Components and Emulsifiers on Plant Sterols Bioaccessibility from Milk-Based Fruit Beverages. J. Agric. Food Chem. 2016, 64, 5686–5691. [Google Scholar] [CrossRef]
- Salen, G.; Starc, T.; Sisk, C.M.; Patel, S.B. Intestinal cholesterol absorption inhibitor ezetimibe added to cholestyramine for sitosterolemia and xanthomatosis. Gastroenterology 2006, 130, 1853–1857. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Gbian, D.L.; Omri, A. Lipid-Based Drug Delivery Systems for Diseases Managements. Biomedicines 2022, 10, 2137. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Li, Y.; Fan, G.C. Tissue-Resident Macrophages in the Control of Infection and Resolution of Inflammation. Shock 2021, 55, 14–23. [Google Scholar] [CrossRef]
- Miao, L.; Nielsen, M.; Thewalt, J.; Ipsen, J.H.; Bloom, M.; Zuckermann, M.J.; Mouritsen, O.G. From lanosterol to cholesterol: Structural evolution and differential effects on lipid bilayers. Biophys. J. 2002, 82, 1429–1444. [Google Scholar] [CrossRef]
- Pencer, J.; Nieh, M.P.; Harroun, T.A.; Krueger, S.; Adams, C.; Katsaras, J. Bilayer thickness and thermal response of dimyristoylphosphatidylcholine unilamellar vesicles containing cholesterol, ergosterol and lanosterol: A small-angle neutron scattering study. Biochim. Biophys. Acta 2005, 1720, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Bernsdorff, C.; Winter, R. Differential Properties of the Sterols Cholesterol, Ergosterol, β-Sitosterol, trans-7-Dehydrocholesterol, Stigmasterol and Lanosterol on DPPC Bilayer Order. J. Phys. Chem. B 2003, 107, 10658–10664. [Google Scholar] [CrossRef]
- Hung, W.C.; Lee, M.T.; Chen, F.Y.; Huang, H.W. The condensing effect of cholesterol in lipid bilayers. Biophys. J. 2007, 92, 3960–3967. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, X.J.; Zhu, J.; Xi, Y.B.; Yang, X.; Hu, L.D.; Ouyang, H.; Patel, S.H.; Jin, X.; Lin, D.; et al. Lanosterol reverses protein aggregation in cataracts. Nature 2015, 523, 607–611. [Google Scholar] [CrossRef]
- Osuchowski, M.F.; Welch, K.; Siddiqui, J.; Remick, D.G. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J. Immunol. 2006, 177, 1967–1974. [Google Scholar] [CrossRef]
- Li, W.; Ashok, M.; Li, J.; Yang, H.; Sama, A.E.; Wang, H. A Major Ingredient of Green Tea Rescues Mice from Lethal Sepsis Partly by Inhibiting HMGB1. PLoS ONE 2007, 2, e1153. [Google Scholar] [CrossRef]
- Chen, W.; Brenner, M.; Aziz, M.; Chavan, S.S.; Deutschman, C.S.; Diamond, B.; Pavlov, V.A.; Sherry, B.; Wang, P.; Tracey, K.J.; et al. Buprenorphine Markedly Elevates a Panel of Surrogate Markers in a Murine Model of Sepsis. Shock 2019, 52, 550–553. [Google Scholar] [CrossRef]
- Wartmann, T.; Mayerle, J.; Kahne, T.; Sahin-Toth, M.; Ruthenburger, M.; Matthias, R.; Kruse, A.; Reinheckel, T.; Peters, C.; Weiss, F.U.; et al. Cathepsin L inactivates human trypsinogen, whereas cathepsin L-deletion reduces the severity of pancreatitis in mice. Gastroenterology 2010, 138, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Kitamoto, S.; Sukhova, G.K.; Sun, J.; Yang, M.; Libby, P.; Love, V.; Duramad, P.; Sun, C.; Zhang, Y.; Yang, X.; et al. Cathepsin L deficiency reduces diet-induced atherosclerosis in low-density lipoprotein receptor-knockout mice. Circulation 2007, 115, 2065–2075. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, X.; Li, Y.; Lu, Y.; Zhong, H.; Jiang, W.; Chen, A.F.; Billiar, T.R.; Yuan, H.; Cai, J. Cathepsin L activity correlates with proteinuria in chronic kidney disease in humans. Int. Urol. Nephrol. 2017, 49, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhong, H.; Wu, J.; Chen, R.F.; Yang, H.; Al-Abed, Y.; Li, Y.; Li, X.; Jiang, W.; Montenegro, M.F.; et al. Cathepsin L promotes Vascular Intimal Hyperplasia after Arterial Injury. Mol. Med. 2017, 23, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Schurigt, U.; Eilenstein, R.; Gajda, M.; Leipner, C.; Sevenich, L.; Reinheckel, T.; Peters, C.; Wiederanders, B.; Brauer, R. Decreased arthritis severity in cathepsin L-deficient mice is attributed to an impaired T helper cell compartment. Inflamm. Res. 2012, 61, 1021–1029. [Google Scholar] [CrossRef]
- Bauer, C.; Duewell, P.; Mayer, C.; Lehr, H.A.; Fitzgerald, K.A.; Dauer, M.; Tschopp, J.; Endres, S.; Latz, E.; Schnurr, M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 2010, 59, 1192–1199. [Google Scholar] [CrossRef]
- Muus, C.; Luecken, M.D.; Eraslan, G.; Sikkema, L.; Waghray, A.; Heimberg, G.; Kobayashi, Y.; Vaishnav, E.D.; Subramanian, A.; Smillie, C.; et al. Single-cell meta-analysis of SARS-CoV-2 entry genes across tissues and demographics. Nat. Med. 2021, 27, 546–559. [Google Scholar] [CrossRef]
- Dhiman, N.; Sarvaiya, J.; Mohindroo, P. A drift on liposomes to proliposomes: Recent advances and promising approaches. J. Liposome Res. 2022, 32, 317–331. [Google Scholar] [CrossRef]
- Nekkanti, V.; Venkatesan, N.; Betageri, G.V. Proliposomes for oral delivery: Progress and challenges. Curr. Pharm. Biotechnol. 2015, 16, 303–312. [Google Scholar] [CrossRef]
- Willis, L.; Hayes, D., Jr.; Mansour, H.M. Therapeutic liposomal dry powder inhalation aerosols for targeted lung delivery. Lung 2012, 190, 251–262. [Google Scholar] [CrossRef]
- Choudhary, M.; Chaurawal, N.; Barkat, M.A.; Raza, K. Proliposome-Based Nanostrategies: Challenges and Development as Drug Delivery Systems. AAPS PharmSciTech 2022, 23, 293. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Qiang, X.; Wang, Y.; Zhu, S.; Li, J.; Babaev, A.; Yang, H.; Gong, J.; Becker, L.; Wang, P.; et al. Identification of tetranectin-targeting monoclonal antibodies to treat potentially lethal sepsis. Sci. Transl. Med. 2020, 12, eaaz3833. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Bao, G.; Chen, W.; Qiang, X.; Zhu, S.; Wang, S.; He, M.; Ma, G.; Ochani, M.; Al-Abed, Y.; et al. Connexin 43 Hemichannel as a Novel Mediator of Sterile and Infectious Inflammatory Diseases. Sci. Rep. 2018, 8, 166–18452. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhu, S.; Wang, Y.; Li, J.; Qiang, X.; Zhao, X.; Yang, H.; D’Angelo, J.; Becker, L.; Wang, P.; et al. Enhanced Macrophage Pannexin 1 Expression and Hemichannel Activation Exacerbates Lethal Experimental Sepsis. Sci. Rep. 2019, 9, 160–37232. [Google Scholar] [CrossRef]
- Qiang, X.; Li, J.; Zhu, S.; He, M.; Chen, W.; Al-Abed, Y.; Brenner, M.; Tracey, K.J.; Wang, P.; Wang, H. Human Dermcidin Protects Mice Against Hepatic Ischemia-Reperfusion-Induced Local and Remote Inflammatory Injury. Front. Immunol. 2021, 12, 821154. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Zhu, C.S.; Qiang, X.; Chen, S.; Li, J.; Wang, P.; Tracey, K.J.; Wang, H. Development of Procathepsin L (pCTS-L)-Inhibiting Lanosterol-Carrying Liposome Nanoparticles to Treat Lethal Sepsis. Int. J. Mol. Sci. 2023, 24, 8649. https://doi.org/10.3390/ijms24108649
Chen W, Zhu CS, Qiang X, Chen S, Li J, Wang P, Tracey KJ, Wang H. Development of Procathepsin L (pCTS-L)-Inhibiting Lanosterol-Carrying Liposome Nanoparticles to Treat Lethal Sepsis. International Journal of Molecular Sciences. 2023; 24(10):8649. https://doi.org/10.3390/ijms24108649
Chicago/Turabian StyleChen, Weiqiang, Cassie Shu Zhu, Xiaoling Qiang, Shujin Chen, Jianhua Li, Ping Wang, Kevin J. Tracey, and Haichao Wang. 2023. "Development of Procathepsin L (pCTS-L)-Inhibiting Lanosterol-Carrying Liposome Nanoparticles to Treat Lethal Sepsis" International Journal of Molecular Sciences 24, no. 10: 8649. https://doi.org/10.3390/ijms24108649
APA StyleChen, W., Zhu, C. S., Qiang, X., Chen, S., Li, J., Wang, P., Tracey, K. J., & Wang, H. (2023). Development of Procathepsin L (pCTS-L)-Inhibiting Lanosterol-Carrying Liposome Nanoparticles to Treat Lethal Sepsis. International Journal of Molecular Sciences, 24(10), 8649. https://doi.org/10.3390/ijms24108649







