Hematological Parameters and Procalcitonin as Discriminants between Bacterial Pneumonia-Induced Sepsis and Viral Sepsis Secondary to COVID-19: A Retrospective Single-Center Analysis
Abstract
:1. Introduction
2. Results
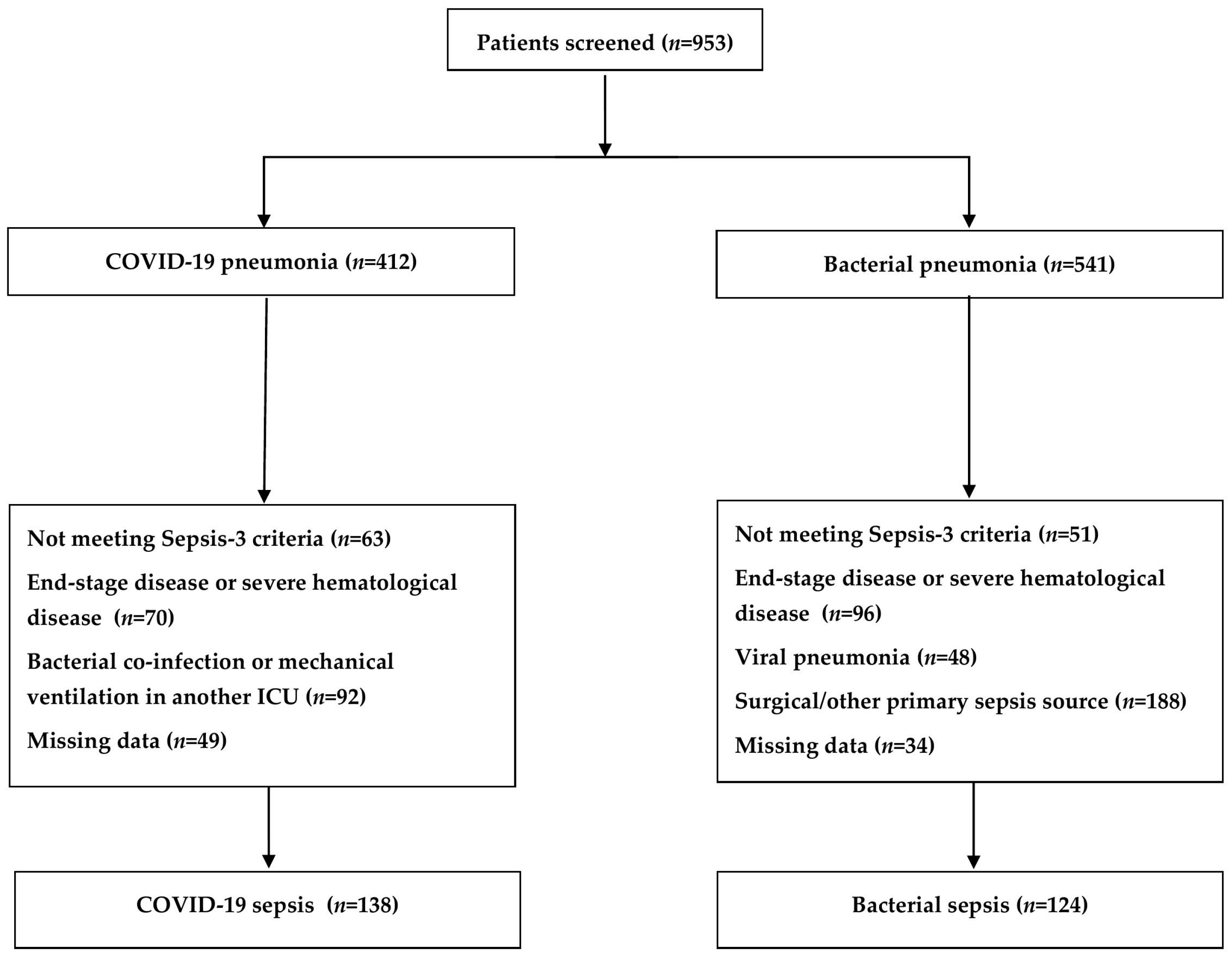
2.1. Baseline Characteristics of the Study Population
2.2. Hematological Parameters and Procalcitonin Analysis at the Moment of Diagnosis
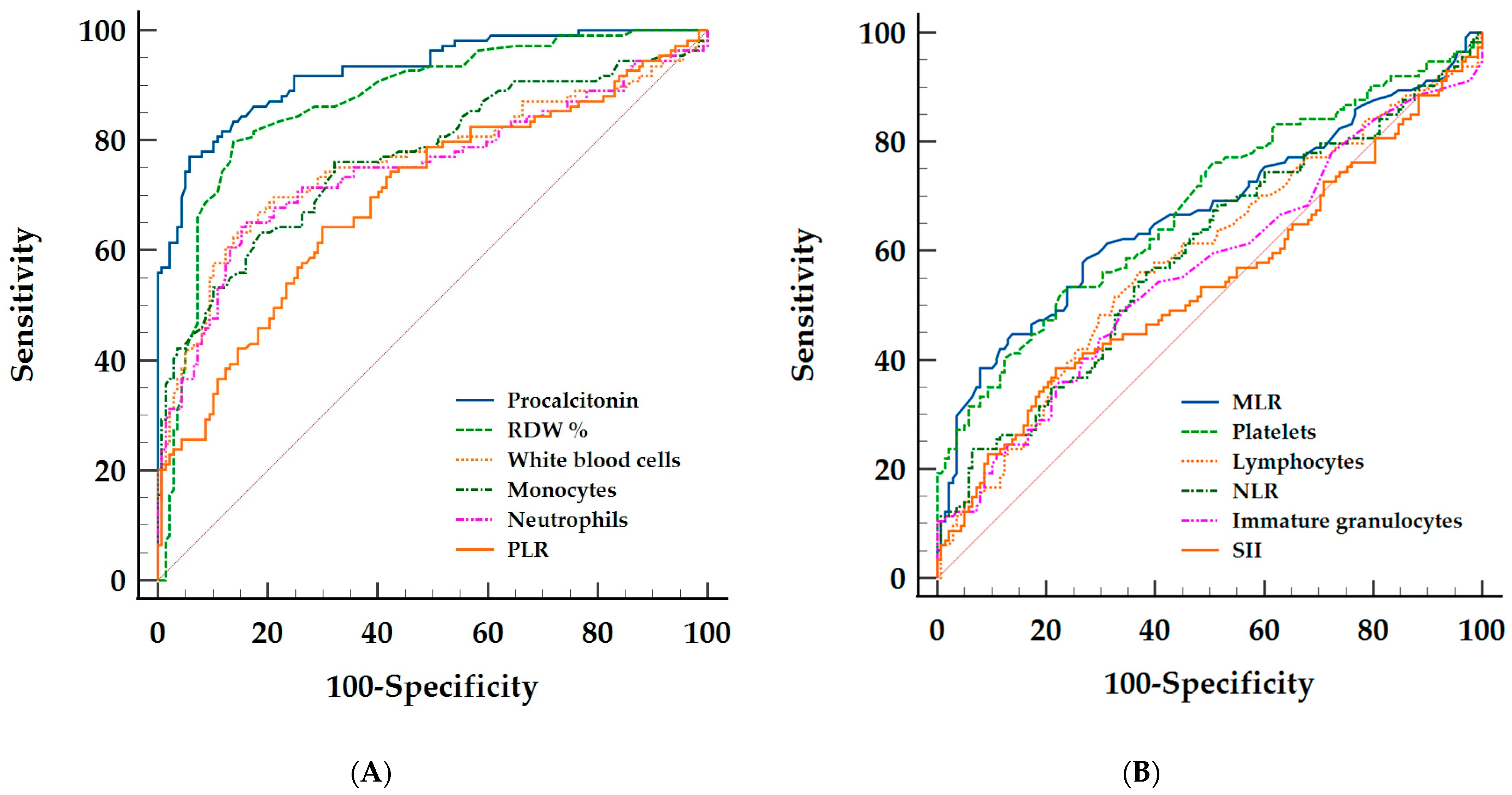
2.3. Discriminative Ability of the Hematological Parameters and Procalcitonin between Bacterial and Viral Sepsis
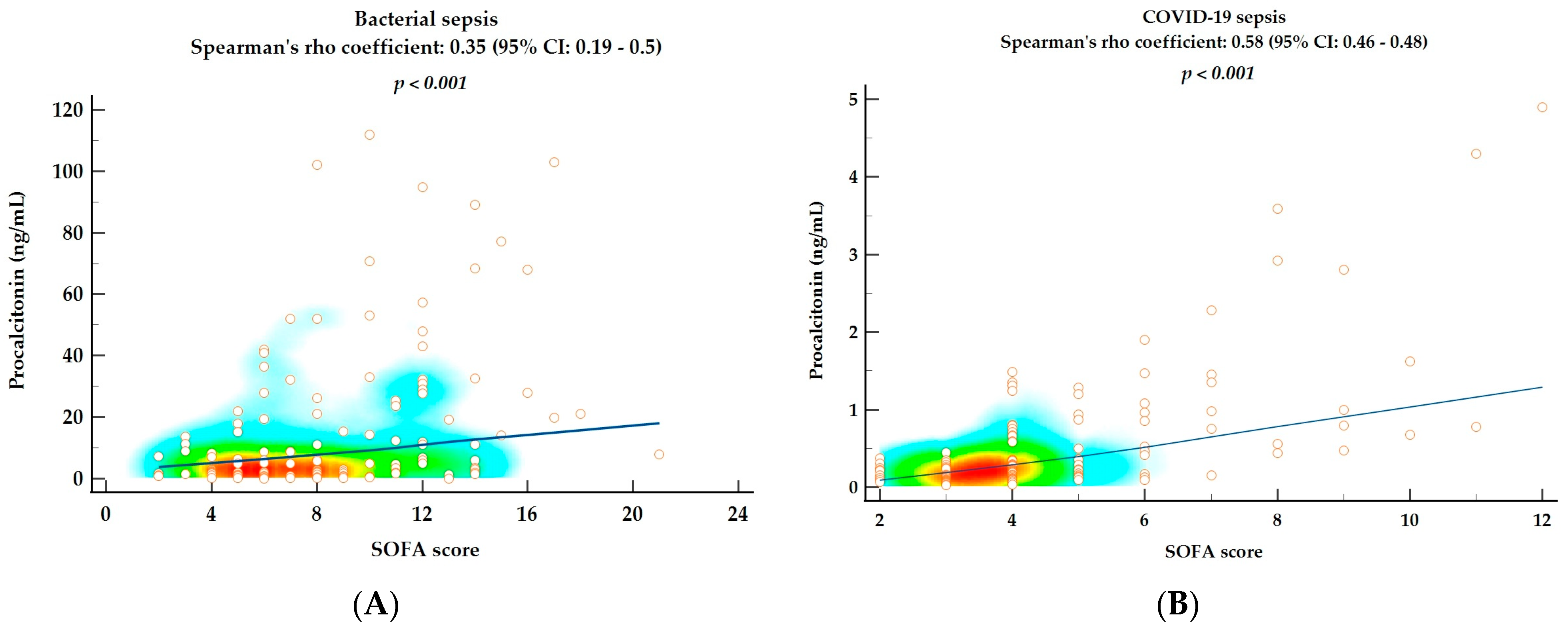
2.4. Correlations between SOFA Score and Hematological Parameters and Procalcitonin as Markers of Disease Severity
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Data Collection and Analysis
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock(Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.-L.; McGinley, J.P.; Drysdale, S.B.; Pollard, A.J. Epidemiology and Immune Pathogenesis of Viral Sepsis. Front. Immunol. 2018, 9, 2147. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L. International Study of the Prevalence and Outcomes of Infection in Intensive Care Units. JAMA 2009, 302, 2323. [Google Scholar] [CrossRef] [Green Version]
- Moser, D.; Feuerecker, M.; Biere, K.; Han, B.; Hoerl, M.; Schelling, G.; Kaufmann, I.; Choukér, A.; Woehrle, T. SARS-CoV-2 Pneumonia and Bacterial Pneumonia Patients Differ in a Second Hit Immune Response Model. Sci. Rep. 2022, 12, 15485. [Google Scholar] [CrossRef] [PubMed]
- Delano, M.J.; Ward, P.A. The Immune System’s Role in Sepsis Progression, Resolution and Long-Term Outcome. Immunol. Rev. 2016, 274, 330–353. [Google Scholar] [CrossRef] [Green Version]
- Moisa, E.; Corneci, D.; Negoita, S.; Filimon, C.R.; Serbu, A.; Negutu, M.I.; Grintescu, I.M. Dynamic Changes of the Neutrophil-To-Lymphocyte Ratio, Systemic Inflammation Index, and Derived Neutrophil-To-Lymphocyte Ratio Independently Predict Invasive Mechanical Ventilation Need and Death in Critically Ill COVID-19 Patients. Biomedicines 2021, 9, 1656. [Google Scholar] [CrossRef]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.H.; Sibbald, W.J. Definitions for Sepsis and Organ Failure and Guidelines for the Use of Innovative Therapies in Sepsis. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef] [Green Version]
- Van der Geest, P.J.; Mohseni, M.; Brouwer, R.; van der Hoven, B.; Steyerberg, E.W.; Groeneveld, A.B.J. Immature Granulocytes Predict Microbial Infection and Its Adverse Sequelae in the Intensive Care Unit. J. Crit. Care 2014, 29, 523–527. [Google Scholar] [CrossRef]
- Metzemaekers, M.; Cambier, S.; Blanter, M.; Vandooren, J.; Carvalho, A.C.; Malengier-Devlies, B.; Vanderbeke, L.; Jacobs, C.; Coenen, S.; Martens, E.; et al. Kinetics of Peripheral Blood Neutrophils in Severe Coronavirus Disease 2019. Clin. Transl. Immunol. 2021, 10, e1271. [Google Scholar] [CrossRef]
- Song, L.; Liang, E.-Y.; Wang, H.-M.; Shen, Y.; Kang, C.-M.; Xiong, Y.-J.; He, M.; Fu, W.-J.; Ke, P.-F.; Huang, X.-Z. Differential Diagnosis and Prospective Grading of COVID-19 at the Early Stage with Simple Hematological and Biochemical Variables. Diagn. Microbiol. Infect. Dis. 2021, 99, 115169. [Google Scholar] [CrossRef]
- Du, R.-H.; Liang, L.-R.; Yang, C.-Q.; Wang, W.; Cao, T.-Z.; Li, M.; Guo, G.-Y.; Du, J.; Zheng, C.-L.; Zhu, Q.; et al. Predictors of Mortality for Patients with COVID-19 Pneumonia Caused by SARS-CoV-2: A Prospective Cohort Study. Eur. Respir. J. 2020, 55, 2000524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feuerecker, M.; Sudhoff, L.; Crucian, B.; Pagel, J.-I.; Sams, C.; Strewe, C.; Guo, A.; Schelling, G.; Briegel, J.; Kaufmann, I.; et al. Early Immune Anergy towards Recall Antigens and Mitogens in Patients at Onset of Septic Shock. Sci. Rep. 2018, 8, 1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Döcke, W.-D.; Randow, F.; Syrbe, U.; Krausch, D.; Asadullah, K.; Reinke, P.; Volk, H.-D.; Kox, W. Monocyte Deactivation in Septic Patients: Restoration by IFN-γ Treatment. Nat. Med. 1997, 3, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Idiz, U.O.; Yurttas, T.T.; Degirmencioglu, S.; Orhan, B.; Erdogan, E.; Sevik, H.; Sevinc, M.M. Immunophenotyping of Lymphocytes and Monocytes and the Status of Cytokines in the Clinical Course of Covid-19 Patients. J. Med. Virol. 2022, 94, 4744–4753. [Google Scholar] [CrossRef]
- Crouser, E.D.; Parrillo, J.E.; Seymour, C.W.; Angus, D.C.; Bicking, K.; Esguerra, V.G.; Peck-Palmer, O.M.; Magari, R.T.; Julian, M.W.; Kleven, J.M.; et al. Monocyte Distribution Width. Crit. Care Med. 2019, 47, 1018–1025. [Google Scholar] [CrossRef] [Green Version]
- Alsuwaidi, L.; Al Heialy, S.; Shaikh, N.; Al Najjar, F.; Seliem, R.; Han, A.; Hachim, M. Monocyte Distribution Width as a Novel Sepsis Indicator in COVID-19 Patients. BMC Infect. Dis. 2022, 22, 27. [Google Scholar] [CrossRef]
- Ito, H.; Ishikawa, M.; Matsumoto, H.; Sugihara, F.; Okuzaki, D.; Hirata, H.; Ogura, H. Transcriptional Differences between Coronavirus Disease 2019 and Bacterial Sepsis. Virol. J. 2022, 19, 198. [Google Scholar] [CrossRef]
- Huang, Z.; Fu, Z.; Huang, W.; Huang, K. Prognostic Value of Neutrophil-To-Lymphocyte Ratio in Sepsis: A Meta-Analysis. Am. J. Emerg. Med. 2020, 38, 641–647. [Google Scholar] [CrossRef]
- Ng, W.W.-S.; Lam, S.-M.; Yan, W.-W.; Shum, H.-P. NLR, MLR, PLR and RDW to Predict Outcome and Differentiate between Viral and Bacterial Pneumonia in the Intensive Care Unit. Sci. Rep. 2022, 12, 15974. [Google Scholar] [CrossRef]
- Farkas, J.D. The Complete Blood Count to Diagnose Septic Shock. J. Thorac. Dis. 2020, 12 (Suppl. S1), S16–S21. [Google Scholar] [CrossRef]
- Cai, J.; Li, H.; Zhang, C.; Chen, Z.; Liu, H.; Lei, F.; Qin, J.-J.; Liu, Y.-M.; Zhou, F.; Song, X.; et al. The Neutrophil-To-Lymphocyte Ratio Determines Clinical Efficacy of Corticosteroid Therapy in Patients with COVID-19. Cell Metab. 2021, 33, 258–269.e3. [Google Scholar] [CrossRef] [PubMed]
- Powell, N.; Howard, P.; Llewelyn, M.J.; Szakmany, T.; Albur, M.; Bond, S.E.; Euden, J.; Brookes-Howell, L.; Dark, P.; Hellyer, T.P.; et al. Use of Procalcitonin during the First Wave of COVID-19 in the Acute NHS Hospitals: A Retrospective Observational Study. Antibiotics 2021, 10, 516. [Google Scholar] [CrossRef] [PubMed]
- May, M.; Chang, M.; Dietz, D.; Shoucri, S.; Laracy, J.; Sobieszczyk, M.E.; Uhlemann, A.-C.; Zucker, J.; Kubin, C.J. Limited Utility of Procalcitonin in Identifying Community-Associated Bacterial Infections in Patients Presenting with Coronavirus Disease 2019. Antimicrob. Agents Chemother. 2021, 65, e02167-20. [Google Scholar] [CrossRef]
- Heer, R.S.; Mandal, A.K.; Kho, J.; Szawarski, P.; Csabi, P.; Grenshaw, D.; Walker, I.A.; Missouris, C.G. Elevated Procalcitonin Concentrations in Severe Covid-19 May Not Reflect Bacterial Co-Infection. Ann. Clin. Biochem. Int. J. Lab. Med. 2021, 58, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Pierrakos, C.; Velissaris, D.; Bisdorff, M.; Marshall, J.C.; Vincent, J.-L. Biomarkers of Sepsis: Time for a Reappraisal. Crit. Care 2020, 24, 287. [Google Scholar] [CrossRef]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and Treatment of Adults with Community-Acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Y.; Lin, S.; Jiang, L.; Sang, L.; Zheng, X.; Zhong, M. Severe COVID-19 Has a Distinct Phenotype from Bacterial Sepsis: A Retrospective Cohort Study in Deceased Patients. Ann. Transl. Med. 2021, 9, 1054. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zou, Z.-Y.; Chen, Y.-H.; Wang, C.-L.; Feng, Y.-W.; Liu, Z.-F. Severe COVID-19-Associated Sepsis Is Different from Classical Sepsis Induced by Pulmonary Infection with Carbapenem-Resistant Klebsiella Pneumonia(CrKP). Chin. J. Traumatol. 2022, 25, 17–24. [Google Scholar] [CrossRef]
- Perschinka, F.; Mayerhöfer, T.; Lehner, G.F.; Hasslacher, J.; Klein, S.J.; Joannidis, M. Immunologic Response in Bacterial Sepsis Is Different from That in COVID-19 Sepsis. Infection 2022, 50, 1035–1037. [Google Scholar] [CrossRef]
- Raschke, R.A.; Agarwal, S.; Rangan, P.; Heise, C.W.; Curry, S.C. Discriminant Accuracy of the SOFA Score for Determining the Probable Mortality of Patients with COVID-19 Pneumonia Requiring Mechanical Ventilation. JAMA 2021, 325, 1469–1470. [Google Scholar] [CrossRef]
- Cillóniz, C.; Dominedò, C.; Magdaleno, D.; Ferrer, M.; Gabarrús, A.; Torres, A. Pure Viral Sepsis Secondary to Community-Acquired Pneumonia in Adults: Risk and Prognostic Factors. J. Infect. Dis. 2019, 220, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Tuculeanu, G.; Barbu, E.C.; Lazar, M.; Chitu-Tisu, C.E.; Moisa, E.; Negoita, S.I.; Ion, D.A. Coagulation Disorders in Sepsis and COVID-19—Two Sides of the Same Coin? A Review of Inflammation–Coagulation Crosstalk in Bacterial Sepsis and COVID-19. J. Clin. Med. 2023, 12, 601. [Google Scholar] [CrossRef]
- Gentile, L.F.; Moldawer, L.L. DAMPs, PAMPs, and the Origins of SIRS in Bacterial Sepsis. Shock 2013, 39, 113–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leligdowicz, A.; Chun, L.F.; Jauregui, A.; Vessel, K.; Liu, K.D.; Calfee, C.S.; Matthay, M.A. Human Pulmonary Endothelial Cell Permeability after Exposure to LPS-Stimulated Leukocyte Supernatants Derived from Patients with Early Sepsis. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 315, L638–L644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherren, P.B.; Ostermann, M.; Agarwal, S.; Meadows, C.I.S.; Ioannou, N.; Camporota, L. COVID-19-Related Organ Dysfunction and Management Strategies on the Intensive Care Unit: A Narrative Review. Br. J. Anaesth. 2020, 125, 912–925. [Google Scholar] [CrossRef]
- Helms, J.; Kremer, S.; Merdji, H.; Schenck, M.; Severac, F.; Clere-Jehl, R.; Studer, A.; Radosavljevic, M.; Kummerlen, C.; Monnier, A.; et al. Delirium and Encephalopathy in Severe COVID-19: A Cohort Analysis of ICU Patients. Crit. Care 2020, 24, 491. [Google Scholar] [CrossRef]
- Zhao, W.; Li, H.; Li, J.; Xu, B.; Xu, J. The Mechanism of Multiple Organ Dysfunction Syndrome in Patients with COVID-19. J. Med. Virol. 2022, 94, 1886–1892. [Google Scholar] [CrossRef]
- Moisa, E.; Corneci, D.; Negutu, M.I.; Filimon, C.R.; Serbu, A.; Popescu, M.; Negoita, S.; Grintescu, I.M. Development and Internal Validation of a New Prognostic Model Powered to Predict 28-Day All-Cause Mortality in ICU COVID-19 Patients—The COVID-SOFA Score. J. Clin. Med. 2022, 11, 4160. [Google Scholar] [CrossRef]
- Bateman, R.; Sharpe, M.; Singer, M.; Ellis, C. The Effect of Sepsis on the Erythrocyte. Int. J. Mol. Sci. 2017, 18, 1932. [Google Scholar] [CrossRef] [Green Version]
- Qadri, S.M.; Bissinger, R.; Solh, Z.; Oldenborg, P.-A. Eryptosis in Health and Disease: A Paradigm Shift towards Understanding the (Patho)Physiological Implications of Programmed Cell Death of Erythrocytes. Blood Rev. 2017, 31, 349–361. [Google Scholar] [CrossRef]
- Piagnerelli, M.; Zouaoui Boudjeltia, K.; Brohee, D.; Piro, P.; Carlier, E.; Vincent, J.-L.; Lejeune, P.; Vanhaeverbeek, M. Alterations of Red Blood Cell Shape and Sialic Acid Membrane Content in Septic Patients. Crit. Care Med. 2003, 31, 2156–2162. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, S. Oxidative Stress in the Regulation of Normal and Neoplastic Hematopoiesis. Antioxid. Redox Signal. 2008, 10, 1923–1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramachandran, P.; Gajendran, M.; Perisetti, A.; Elkholy, K.O.; Chakraborti, A.; Lippi, G.; Goyal, H. Red Blood Cell Distribution Width in Hospitalized COVID-19 Patients. Front. Med. 2022, 8, 582403. [Google Scholar] [CrossRef] [PubMed]
- Foy, B.H.; Carlson, J.C.T.; Reinertsen, E.; Padros, I.; Valls, R.; Pallares Lopez, R.; Palanques-Tost, E.; Mow, C.; Westover, M.B.; Aguirre, A.D.; et al. Association of Red Blood Cell Distribution Width with Mortality Risk in Hospitalized Adults with SARS-CoV-2 Infection. JAMA Netw. Open 2020, 3, e2022058. [Google Scholar] [CrossRef] [PubMed]
- Salvagno, G.L.; Sanchis-Gomar, F.; Picanza, A.; Lippi, G. Red Blood Cell Distribution Width: A Simple Parameter with Multiple Clinical Applications. Crit. Rev. Clin. Lab. Sci. 2014, 52, 86–105. [Google Scholar] [CrossRef]
- Moisă, E.; Negoiţă, S.; Corneci, D. Understanding Red Blood Cell Rheology in Sepsis and Its Role in Clinical Practice. From Biomolecular Aspects to Possible Therapeutic Interventions. Cent. Eur. J. Clin. Res. 2018, 1, 40–58. [Google Scholar] [CrossRef] [Green Version]
- Moisă, E.; Negoiţă, S.; Corneci, D. The Clinical Value of Red Blood Cell Distribution Width as a Prognosis Factor and Severity Marker in Sepsis and Septic Shock. Cent. Eur. J. Clin. Res. 2019, 2, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Self, W.H.; Balk, R.A.; Grijalva, C.G.; Williams, D.J.; Zhu, Y.; Anderson, E.J.; Waterer, G.W.; Courtney, D.M.; Bramley, A.M.; Trabue, C.; et al. Procalcitonin as a Marker of Etiology in Adults Hospitalized with Community-Acquired Pneumonia. Clin. Infect. Dis. 2017, 65, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Atallah, N.J.; Warren, H.M.; Roberts, M.B.; Elshaboury, R.H.; Bidell, M.R.; Gandhi, R.G.; Adamsick, M.; Ibrahim, M.K.; Sood, R.; Bou Zein Eddine, S.; et al. Baseline Procalcitonin as a Predictor of Bacterial Infection and Clinical Outcomes in COVID-19: A Case-Control Study. PLoS ONE 2022, 17, e0262342. [Google Scholar] [CrossRef]
- Pink, I.; Raupach, D.; Fuge, J.; Vonberg, R.-P.; Hoeper, M.M.; Welte, T.; Rademacher, J. C-Reactive Protein and Procalcitonin for Antimicrobial Stewardship in COVID-19. Infection 2021, 49, 935–943. [Google Scholar] [CrossRef]
- Lippi, G.; Plebani, M. Procalcitonin in Patients with Severe Coronavirus Disease 2019(COVID-19): A Meta-Analysis. Clin. Chim. Acta 2020, 505, 190–191. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Karn, E.; Trivedi, K.; Kumar, P.; Chauhan, G.; Kumari, A.; Pant, P.; Munisamy, M.; Prakash, J.; Sarkar, P.G.; et al. Procalcitonin as a Predictive Marker in COVID-19: A Systematic Review and Meta-Analysis. PLoS ONE 2022, 17, e0272840. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Torres, V.; Royuela, A.; Múñez-Rubio, E.; Gutierrez-Rojas, Á.; Mills-Sánchez, P.; Ortega, A.; Tejado-Bravo, S.; García-Sanz, J.; Muñoz-Serrano, A.; Calderón-Parra, J.; et al. Red Blood Cell Distribution Width as Prognostic Factor in Sepsis: A New Use for a Classical Parameter. J. Crit. Care 2022, 71, 154069. [Google Scholar] [CrossRef] [PubMed]
- Yunus, I.; Fasih, A.; Wang, Y. The Use of Procalcitonin in the Determination of Severity of Sepsis, Patient Outcomes and Infection Characteristics. PLoS ONE 2018, 13, e0206527. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837. [Google Scholar] [CrossRef]
| n (%) or Median [IQR: Q1–Q3] | Total Sample n = 262 | Bacterial Sepsis n = 124 | Viral Sepsis n = 138 | p Value |
|---|---|---|---|---|
| Age | 68 [58–77] | 73 [66–81] | 64 [53–72] | <0.001 * |
| Gender (male) | 158 (60.3%) | 74 (59.7%) | 84 (60.9%) | 0.844 * |
| Obesity | 158 (60.3%) | 67 (54%) | 91 (66%) | 0.062 * |
| Cardiac disease | 195 (74.4%) | 77.4% (96) | 71.7% (99) | 0.293 * |
| Respiratory disease | 32 (12.2%) | 20 (16.1%) | 12 (8.7%) | 0.067 * |
| Chronic kidney disease | 41 (15.7%) | 31 (25.2%) | 10 (7.2%) | <0.001 * |
| Diabetes mellitus | 104 (39.8%) | 50 (40.7%) | 54 (39.1%) | 0.802 * |
| Hepatic disease | 23 (8.8%) | 15 (12%) | 8 (5.8%) | 0.113 * |
| Charlson comorbidity index | 5 [3–7] | 7 [5.3–9] | 4 [2–5] | <0.001 ** |
| Hemoglobin (g/dL) | 11.8 [10.18–13.6] | 10.5 [9.1–12.3] | 12.8 [11.3–14.1] | <0.001 ** |
| RDW% | 14.6 [13.3–15.8] | 15.7 [15–17.2] | 13.6 [12.8–14.5] | <0.001 ** |
| Platelets × 103/mm3 | 226 [169–295] | 188 [127–260] | 253 [193–316] | <0.001 ** |
| PDW% | 12.9 [11.7–14.7] | 13.1 [11.8–15] | 12.8 [11.4–14.5] | 0.215 ** |
| White blood cells × 103/mm3 | 13.11 [9.14–18.91] | 17.7 [12.7–23] | 10.2 [7.85–13.8] | <0.001 ** |
| Neutrophils × 103/mm3 | 11.48 [7.55–16.49] | 15.5 [10.7–20.2] | 8.9 [6.6–12.4] | <0.001 ** |
| Lymphocytes × 103/mm3 | 0.8 [0.54–1.17] | 0.95 [0.58–1.4] | 0.74 [0.52–1.06] | 0.005 ** |
| Monocytes × 103/mm3 | 0.59 [0.33–0.87] | 0.82 [0.56–1.16] | 0.45 [0.28–0.66] | <0.001 ** |
| Eosinophils × 103/mm3 | 0.001 [0.00–0.02] | 0.01 [0.001–0.07] | 0.001 [0.00–0.001] | <0.001 ** |
| Basophils × 103/mm3 | 0.02 [0.01–0.03] | 0.02 [0.01–0.038] | 0.01 [0.01–0.02] | <0.001 ** |
| NLR | 14.07 [8.75–21.41] | 15.6 [9.4–26.7] | 12.2 [8.4–19.3] | 0.01 ** |
| dNLR | 7.25 [5.22–11] | 7.3 [4.5–10.5] | 7.2 [5.7–11.6] | 0.584 ** |
| MLR | 0.64 [0.42–1.16] | 0.92 [0.5–1.4] | 0.55 [0.39–0.8] | <0.001 ** |
| PLR | 268 [166–433] | 213 [122–311] | 350.4 [227–471] | <0.001 ** |
| SII | 2871 [1575–5070] | 2770 [1348–5151] | 2991 [2012–5039] | 0.314 ** |
| iGr × 103/mm3 | 0.11 [0.06–0.34] | 0.15 [0.06–0.44] | 0.1 [0.05–0.23] | 0.023 ** |
| Procalcitonin ng/mL | 0.8 [0.22–5] | 5.5 [1.6–23.2] | 0.25 [0.13–0.6] | <0.001 ** |
| Mechanical ventilation | 192 (73.3%) | 89 (71.8%) | 103 (74.6%) | 0.601 * |
| P/F ratio | 142 [89–230] | 214 [155–321] | 100 [75–133] | <0.001 ** |
| Vasopressor use | 90 (34.4%) | 76 (61.3%) | 14 (10.1%) | <0.001 * |
| SOFA score | 5 [4–9] | 8 [6–12] | 4 [3–5] | <0.001 ** |
| AUC 95% CI | p Value | Youden Index 95% CI | Cut–Off 95% CI | Sn% 95% CI | Sp% 95% CI | +LR 95% CI | –LR 95% CI | |
|---|---|---|---|---|---|---|---|---|
| Procalcitonin | 0.92 0.87–0.95 | <0.001 | 0.71 0.61–0.77 | >1.49 1.28–1.9 | 76.6% 68.2–83.7 | 94.2 88.9–97.5 | 13.22 6.7–21.1 | 0.25 0.18–0.35 |
| RDW% | 0.87 0.82–0.91 | <0.001 | 0.66 0.57–0.75 | >14.8 14.6–15.2 | 80.7% 72.6–87.2 | 85.5% 78.5–90.9 | 5.56 3.68–8.42 | 0.23 0.16–0.33 |
| Leukocytes | 0.78 0.72–0.83 | <0.001 | 0.5 0.4–0.58 | >16 14.4–17.3 | 64.5% 55.4–72.9 | 85.5% 78.5–90.9 | 4.45 2.91–6.81 | 0.41 0.32–0.53 |
| Monocytes | 0.77 0.71–0.82 | <0.001 | 0.44 0.32–0.52 | >0.69 0.55–0.9 | 63.2% 53.6–72 | 81.2% 73.6–87.3 | 3.35 2.31–4.87 | 0.45 0.35–0.58 |
| Neutrophils | 0.76 0.7–0.82 | <0.001 | 0.49 0.37–0.57 | >14.1 10.9–14.9 | 64.5% 55.4–72.9 | 84.1% 76.9–89.7 | 4.05 2.7–6.07 | 0.42 0.33–0.54 |
| Eosinophils | 0.72 0.6–0.7 | <0.001 | 0.43 0.32–0.54 | >0.001 0.00–0.001 | 66.1% 57.1–74.4 | 76.8% 68.9–83.6 | 2.85 2.1–4 | 0.44 0.34–0.57 |
| PLR | 0.71 0.64–0.76 | <0.001 | 0.35 0.23–0.43 | ≤259 226–392 | 65.3% 56.3–73.6 | 69.6% 61.2–77.1 | 2.15 1.62–2.85 | 0.5 0.38–0.65 |
| MLR | 0.69 0.61–0.75 | <0.001 | 0.34 0.19–0.4 | >0.73 0.59–1.17 | 61% 51.8–69.6 | 72.9% 64.3–80.3 | 2.25 1.64–3.08 | 0.54 0.42–0.68 |
| Basophils | 0.68 0.6–0.72 | <0.001 | 0.27 0.17–0.39 | >0.01 0.001–0.02 | 67.7% 58.8–75.9 | 59.4% 50.7–67.7 | 1.67 1.32–2.11 | 0.54 0.41–0.73 |
| Platelets | 0.67 0.6–0.73 | <0.001 | 0.28 0.16–0.36 | ≤189 169–281 | 51.6% 42.5–60.7 | 76.8% 68.9–83.6 | 2.23 1.57–3.15 | 0.63 0.51–0.77 |
| Lymphocytes | 0.6 0.53–0.7 | 0.005 | 0.21 0.09–0.3 | >0.85 0.33–1.04 | 58.1% 48.9–66.9 | 63% 54.4–71.1 | 1.57 1.21–2.05 | 0.67 0.52–0.85 |
| NLR | 0.59 0.52–0.66 | 0.01 | 0.18 0.09–0.25 | >27.24 24.5–38.4 | 25% 17.7–33.6 | 93.5% 88–97 | 3.83 1.9–7.73 | 0.8 0.72–0.9 |
| iGr | 0.58 0.52–0.67 | 0.023 | 0.17 0.08–0.24 | >0.14 0.01–0.41 | 50.8% 41.7–59.9 | 66% 57.4–73.8 | 1.49 1.12–1.99 | 0.75 0.6–0.93 |
| SII | 0.54 0.46–0.6 | 0.32 | – | – | – | – | – | – |
| dNLR | 0.52 0.47–0.58 | 0.59 | – | – | – | – | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moisa, E.; Dutu, M.; Corneci, D.; Grintescu, I.M.; Negoita, S. Hematological Parameters and Procalcitonin as Discriminants between Bacterial Pneumonia-Induced Sepsis and Viral Sepsis Secondary to COVID-19: A Retrospective Single-Center Analysis. Int. J. Mol. Sci. 2023, 24, 5146. https://doi.org/10.3390/ijms24065146
Moisa E, Dutu M, Corneci D, Grintescu IM, Negoita S. Hematological Parameters and Procalcitonin as Discriminants between Bacterial Pneumonia-Induced Sepsis and Viral Sepsis Secondary to COVID-19: A Retrospective Single-Center Analysis. International Journal of Molecular Sciences. 2023; 24(6):5146. https://doi.org/10.3390/ijms24065146
Chicago/Turabian StyleMoisa, Emanuel, Madalina Dutu, Dan Corneci, Ioana Marina Grintescu, and Silvius Negoita. 2023. "Hematological Parameters and Procalcitonin as Discriminants between Bacterial Pneumonia-Induced Sepsis and Viral Sepsis Secondary to COVID-19: A Retrospective Single-Center Analysis" International Journal of Molecular Sciences 24, no. 6: 5146. https://doi.org/10.3390/ijms24065146






