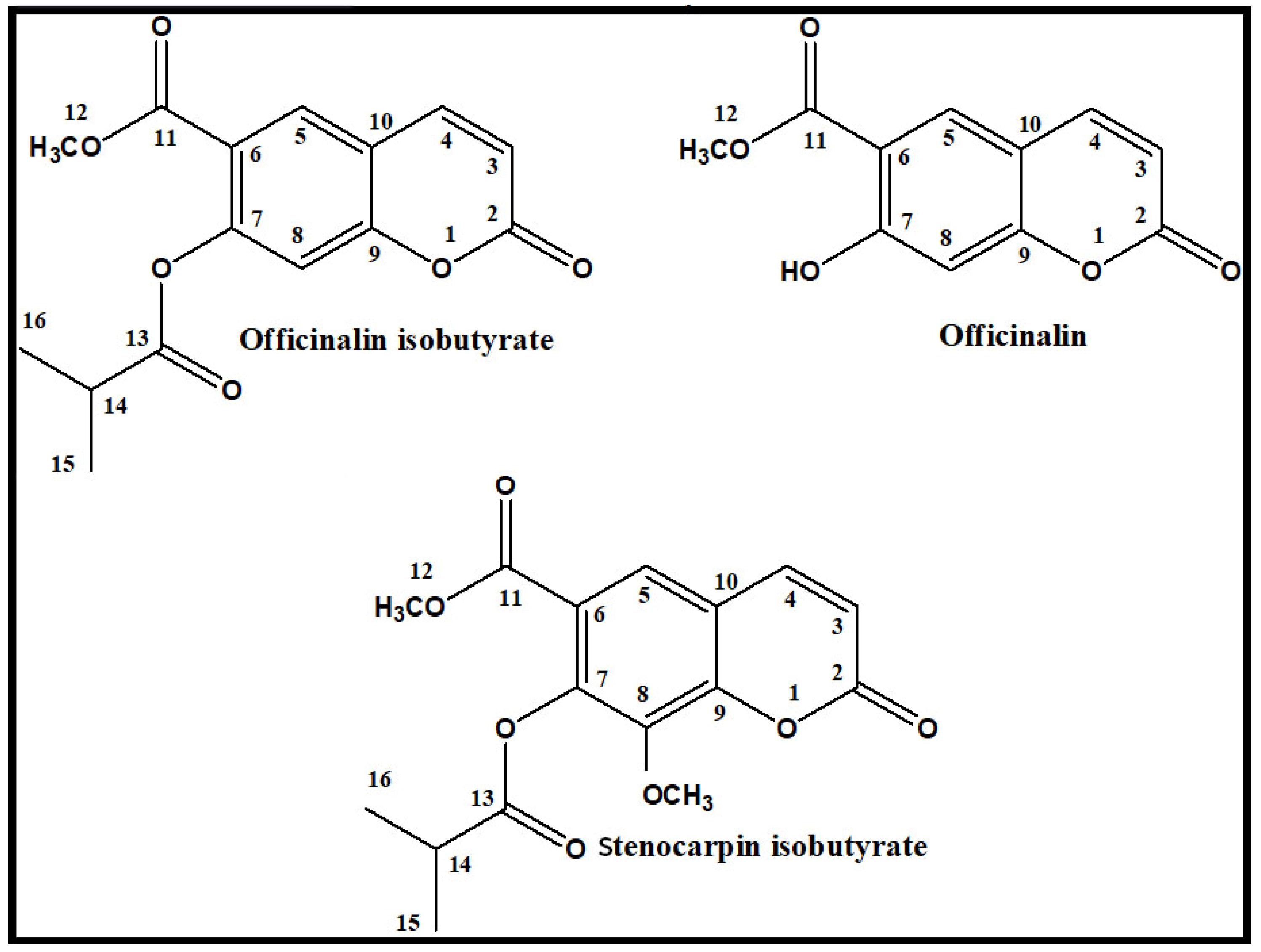
Simple Coumarins from Peucedanum luxurians Fruits: Evaluation of Anxiolytic Activity and Influence on Gene Expression Related to Anxiety in Zebrafish Model
Abstract
:1. Introduction
2. Results
2.1. The Effects of Isolated Coumarins on Thigmotaxis Behaviors of the Zebrafish Larvae during Light–Dark Changes
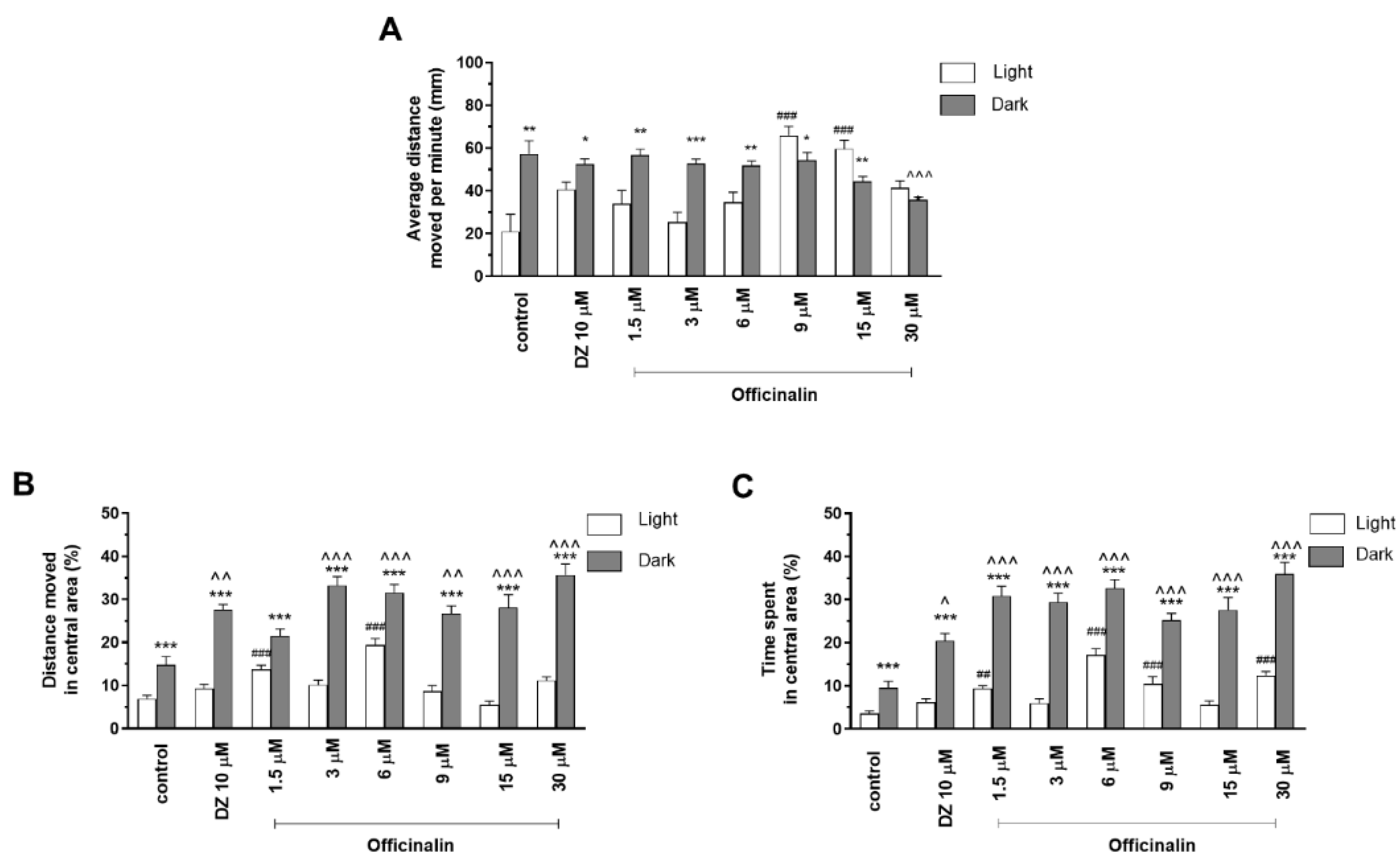
2.1.1. The Effects of Officinalin on Locomotor Activity and Thigmotaxis Behaviors of the Zebrafish Larvae during Light–Dark Changes
2.1.2. The Effects of Stenocarpin Isobutyrate on Locomotor Activity and Thigmotaxis Behaviors of the Zebrafish Larvae during Light–Dark Changes
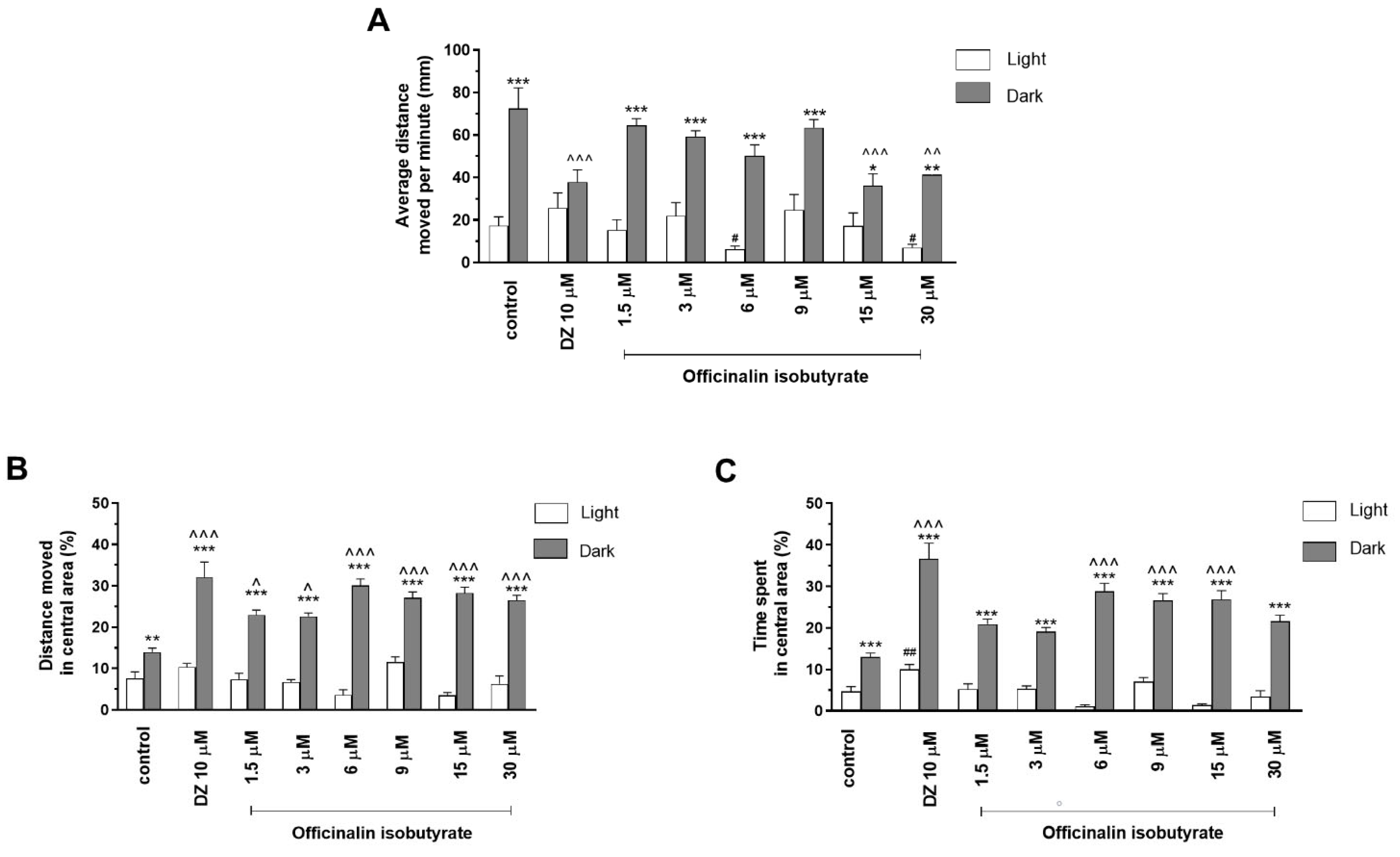
2.1.3. The Effects of Officinalin Isobutyrate on Locomotor Activity and Thigmotaxis Behaviors of the Zebrafish Larvae during Light–Dark Changes
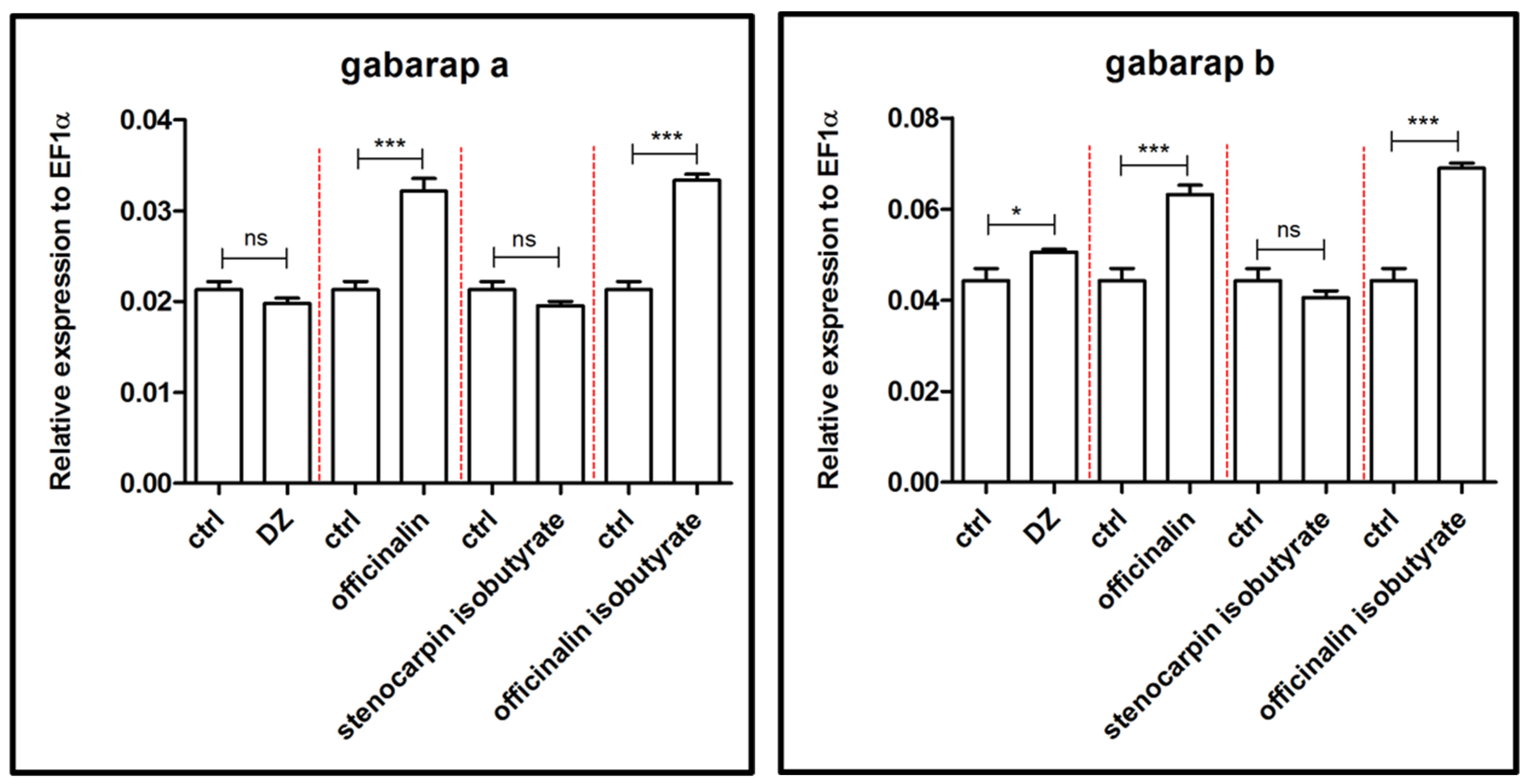
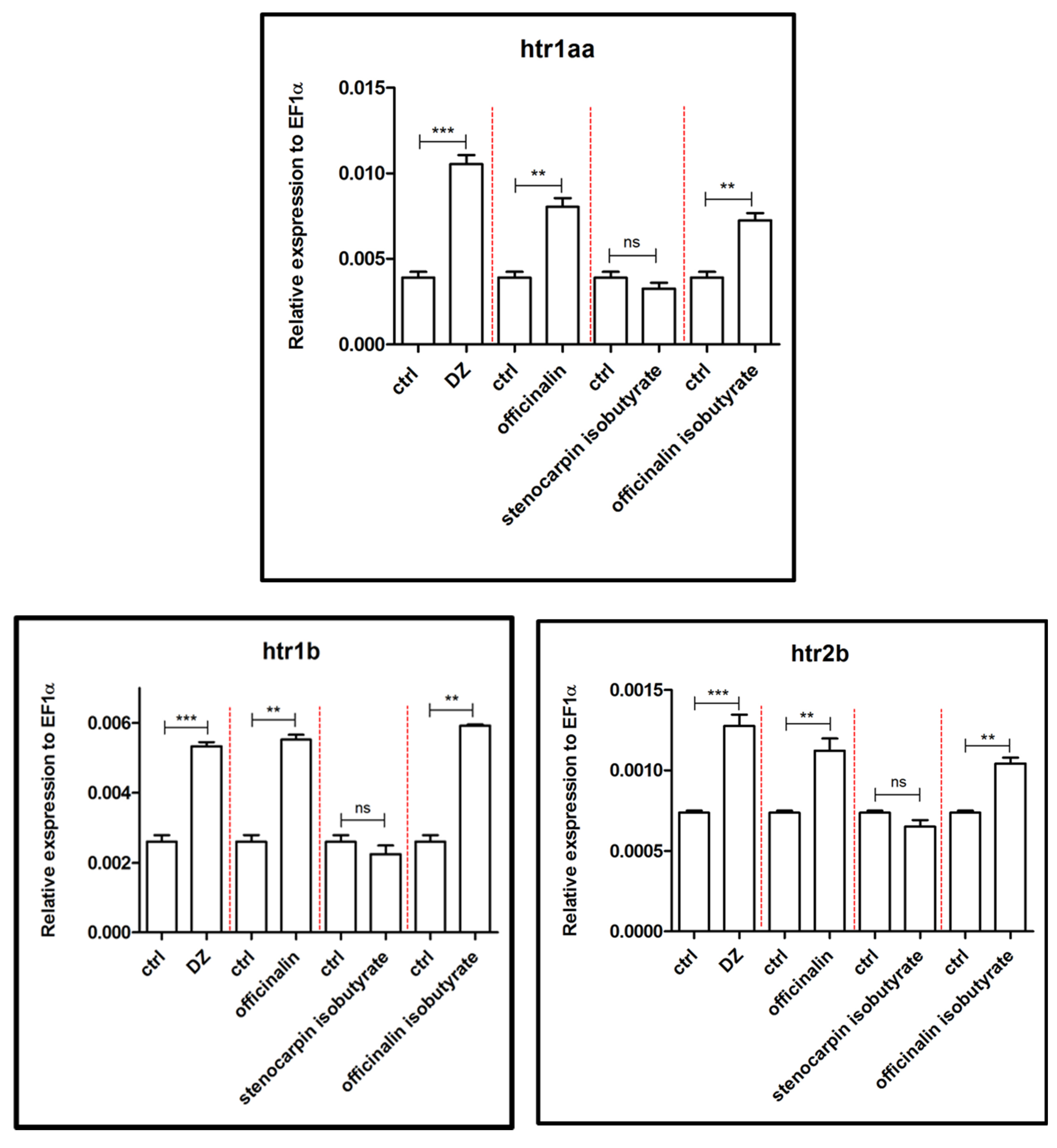
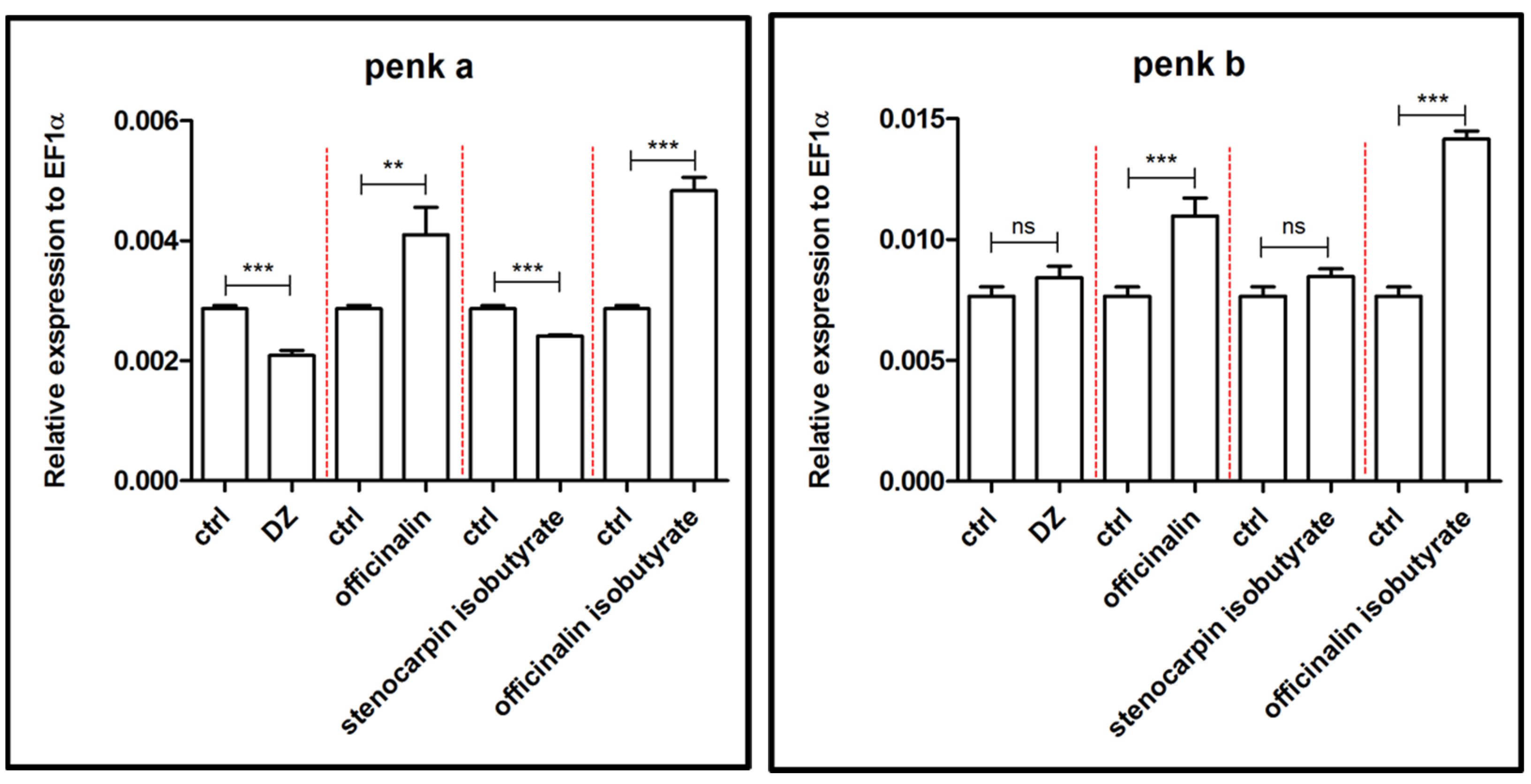
2.2. Influence of Isolated Coumarins on the Expression of Genes Associated with the Etiology and Extinction of Anxiety
3. Discussion
4. Materials and Methods
4.1. Drugs
4.2. Zebrafish Model for Evaluation of Anxiety-Like Behaviors
4.2.1. Animal Husbandry
4.2.2. Determination of the Maximum Tolerated Concentration
4.2.3. Anxiolytic Activity Assay
4.3. RNA Isolation and Quantitative PCR
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arya, A.; Sindhwani, G. A review on the neural circuits in anxiety disorders. Asian J. Pharm. Clin. Res. 2016, 9, 26–31. [Google Scholar] [CrossRef]
- Gołyszny, M.; Obuchowicz, E. Medicinal plant materials in the treatment of anxiety disorders: Neurobiological aspects. Altern. Ther. Health Med. 2018, 24, 44–57. [Google Scholar]
- Santomauro, D.F.; Mantilla Herrera, A.M.; Shadid, J.; Zheng, P.; Ashbaugh, C.; Pigott, D.M.; Abbafati, C.; Adolph, C.; Amlag, J.O.; Aravkin, A.Y.; et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef]
- Muniandy, Y. The use of larval zebrafish (Danio rerio) model for identifying new anxiolytic drugs from herbal medicine. Zebrafish 2018, 15, 321–339. [Google Scholar] [CrossRef]
- Ressler, K.J.; Nemeroff, C.B. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress. Anxiety 2000, 12 (Suppl. S1), 2–19. [Google Scholar] [CrossRef]
- Griebel, G.; Perrault, G.; Sanger, D.J. A Comparative Study of the Effects of Selective and Non-Selective 5-HT2 Receptor Subtype Antagonists in Rat and Mouse Models of Anxiety. Neuropharmacology 1997, 36, 793–802. [Google Scholar] [CrossRef]
- McCorvy, J.D.; Roth, B.L. Structure and function of serotonin G protein-coupled receptors. Pharmacol. Ther. 2015, 150, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Spiacci, A.; Pobbe, R.L.H.; Matthiesen, M.; Zangrossi, H. 5-HT1A receptors of the rat dorsal raphe lateral wings and dorsomedial subnuclei differentially control anxiety- and panic-related defensive responses. Neuropharmacology 2016, 107, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.; Gainetdinov, R.R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef] [PubMed]
- DeGroot, S.R.; Zhao-Shea, R.; Chung, L.; Klenowski, P.M.; Sun, F.; Molas, S.; Gardner, P.D.; Li, Y.; Tapper, A.R. Midbrain dopamine controls anxiety-like behavior by engaging unique interpeduncular nucleus microcircuitry. Biol. Psychiatry 2020, 88, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Koda-Kimble, M.A.; Alldredge, B.K.; Corelli, R.L.; Ernst, M.E.; Gugliemo, B.J.; Jacobson, P.A.; Kradjan, W.A.; Williams, B.R. Koda-Kimble and Young’s Applied Therapeutics: The Clinical Use of Drugs; Lippincott Williams&Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Nuss, P. Anxiety disorders and GABA neurotransmission: A disturbance of modulation. Neuropsychiatr. Dis. Treat. 2015, 11, 165–175. [Google Scholar] [CrossRef]
- Shader, R.I.; Greenblatt, D.J. Use of benzodiazepines in anxiety disorders. N. Engl. J. Med. 1993, 328, 1398–1405. [Google Scholar] [CrossRef]
- Arbanas, G.; Arbanas, D.; Dujam, K. Adverse effects of benzodiazepines in psychiatric outpatients. Psychiatr. Danub. 2009, 21, 103–107. [Google Scholar]
- Starcevic, V. The reappraisal of benzodiazepines in the treatment of anxiety and related disorders. Expert. Rev. Neurother. 2014, 14, 1275–1286. [Google Scholar] [CrossRef]
- Annunziata, F.; Pinna, C.; Dallavalle, S.; Tamborini, L.; Pinto, A. An overview of coumarin as a versatile and readily accessible scaffold with broad-ranging biological activities. Int. J. Mol. Sci. 2020, 21, 4618. [Google Scholar] [CrossRef]
- Borges, F.; Roleira, F.; Milhanzes, N.; Santana, L.; Uriarte, E. Simple coumarins and analogues in medicinal chemistry: Occurrence, synthesis and biological activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef]
- Zhu, J.J.; Jiang, J.G. Pharmacological and Nutritional Efects of Natural Coumarins and Their Structure–Activity Relationships. Mol. Nutr. Food Res. 2018, 62, 1701073. [Google Scholar] [CrossRef]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef]
- Skalicka-Woźniak, K.; Orhan, I.E.; Cordell, G.A.; Nabavi, S.M.; Budzyńska, B. Implication of coumarins towards central nervous system disorders. Pharmacol. Res. 2016, 103, 188–203. [Google Scholar] [CrossRef]
- Champagne, D.L.; Hoefnagels, C.C.M.; de Kloet, R.E.; Richardson, M.K. Translating rodent behavioral repertoire to zebrafish (Danio rerio): Relevance for stress research. Behav. Brain Res. 2010, 214, 332–342. [Google Scholar] [CrossRef]
- Guo, S. Linking genes to brain, behavior and neurological diseases: What can we learn from zebrafish? Genes Brain Behav. 2004, 3, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Ellis, L.D.; Soanes, K.H. A larval zebrafish model of bipolar disorder as a screening platform for neuro-therapeutics. Behav. Brain Res. 2012, 233, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Chiang, C.; Tsai, H. Zebrafish and medaka: New model organisms for modern biomedical research. J. Biomed. Sci. 2016, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Schnorr, S.J.; Steenberger, P.J.; Richardson, M.K.; Champagne, D.L. Measuring thigmotaxis in larval zebrafish. Behav. Brain Res. 2012, 228, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Widelski, J.; Luca, S.V.; Skiba, A.; Maciąg, M.; Budzyńska, B.; Marcourt, L.; Wolfender, J.L.; Skalicka-Woźniak, K. Coumarins from seseli devenyense simonk.: Isolation by liquid-liquid chromatography and potential anxiolytic activity using an in vivo zebrafish larvae model. Int. J. Mol. Sci. 2021, 22, 1829. [Google Scholar] [CrossRef] [PubMed]
- Widelski, J.; Luca, S.V.; Skiba, A.; Chinou, I.; Marcourt, L.; Wolfender, J.L.; Skalicka-Woźniak, K. Isolation, and antimicrobial activity o coumarin derivatives from fruits of Peucedanum luxurians Tamamsch. Molecules 2018, 23, 1222. [Google Scholar] [CrossRef]
- Monsef-Esfahani, H.R.; Amini, M.; Goodarzi, N.; Saiedmohammadi, F.; Hajiaghaee, R.; Faramarzi, M.A.; Tofighi, Z.; Ghahremani, M.H. Coumarin compounds of Biebesteinia multifida roots show potential anxiolytic effects in mice. DARU J. Pharm. Sci. 2013, 21, 51. [Google Scholar] [CrossRef]
- Joseph, A.; Thuy, T.T.T.; Thanh, L.T.; Okada, M. Antidepressive and anxiolytic effects of ostruthis, a TREK-1 channel activator. PLoS ONE 2018, 13, e0201092. [Google Scholar] [CrossRef]
- Herrera-Ruiz, M.; González-Carranza, A.; Zamilpa, A.; Jiménez-Ferrer, E.; Huerta-Reyes, M.; Navarro-García, V.M. The standardized extract of loeselia mexicana possesses anxiolytic activity through the γ-amino butyric acid mechanism. J. Ethnopharmacol. 2011, 138, 261–267. [Google Scholar] [CrossRef]
- Cocco, A.; Ronnenberg, C.A.M.; Jin, Z.; Andre, G.I.; Vossen, L.E.; Bhandage, A.K.; Thornqvist, P.O.; Birnir, B.; Winberg, S. Characterization of the γ-aminobutyric acid signaling system in the zebrafish (Danio rerio Hamilton) central nervous system by reverse transcription-quantitative polymerase chain reaction. Neuroscience 2017, 343, 300–321. [Google Scholar] [CrossRef]
- Klee, E.W.; Schneider, H.; Clark, K.J.; Cousin, M.A.; Ebbert, J.O.; Hooten, W.M.; Karpyak, V.M.; Warner, D.O.; Ekker, S.C. Zebrafish: A model for the study of addiction genetics. Hum. Genet. 2012, 131, 977–1008. [Google Scholar] [CrossRef]
- Chen, L.; Wang, H.; Vicini, S.; Olsen, R.W. The γ-aminobutyric acid type A (GABAA) receptor-associated protein (GABARAP) promotes GABAA receptor clustering and modulates the channel kinetics. Proc. Natl. Acad. Sci. USA 2000, 97, 11557–11562. [Google Scholar] [CrossRef]
- Everitt, A.B.; Luu, T.; Cromer, B.; Tierney, M.L.; Birnir, B.; Olsen, R.W.; Gage, P.W. The conductance of recombinant GABAA channels is increased in cells co-expressing GABAA receptor-associated protein. J. Biol. Chem. 2004, 279, 21701–21706. [Google Scholar] [CrossRef]
- Rajarao, S.J.R.; Platt, B.; Sukoff, S.J.; Lin, Q.; Bender, C.N.; Nieuwenhuijsen, B.W.; Ring, R.H.; Schechter, L.E.; Rosenzweig-Lipson, S.; Beyer, C.E. Anxiolytic-like activity of the non-selective galanin receptor agonist, galnon. Neuropeptides 2007, 41, 307–320. [Google Scholar] [CrossRef]
- Podlasz, P.; Jakimiuk, A.; Kasica-Jarosz, N.; Czaja, K.; Wasowicz, K. Neuroanatomical localization of galanin in zebrafish telencephalon and anticonvulsant effect of galanin overexpression. ACS Chem. Neurosci. 2018, 9, 3049–3059. [Google Scholar] [CrossRef]
- Barnabas, K.; Zhang, L.; Wang, H.; Kirouac, G.; Vrontakis, M. Changes in galanin systems in a rat model of post-traumatic stress disorder (PTSD). PLoS ONE 2016, 11, e0167569. [Google Scholar] [CrossRef]
- Overstreet, D.H.; Commissaris, R.C.; De La Garza, R.; File, S.E.; Knapp, D.J.; Seiden, L.S. Involvement of 5-HT1A receptors in animal tests of anxiety and depression: Evidence from genetic models. Stress 2003, 6, 101–110. [Google Scholar] [CrossRef]
- Konig, M.; Zimmer, A.M.; Steiner, H.; Holmes, P.V.; Crawley, J.N.; Brownstein, M.J.; Zimmer, A. Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature 1996, 383, 535–538. [Google Scholar] [CrossRef]
- Demin, K.A.; Meshalkina, D.A.; Kysil, E.V.; Antonova, K.A.; Volgin, A.D.; Yakovlev, O.A.; Kalueff, A.V. Zebrafish models relevant to studying central opioid and endocannabinoid systems. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 86, 301–312. [Google Scholar] [CrossRef]
- Przewlocki, R. Stress, opioid peptides, and their receptors. In Hormones, Brain and Behavior Online; Elsevier Academic Press: Cambridge, MA, USA, 2009; pp. 289–332. [Google Scholar] [CrossRef]
- Colasanti, A.; Rabiner, E.; Lingford-Hughes, A.; Nutt, D. Opioids and anxiety. J. Psychopharmacol. 2010, 25, 1415–1433. [Google Scholar] [CrossRef]
- Tu, X.; Li, Y.; Chen, Q.; Shen, Y.; Liu, Z. Tributyltin enhanced anxiety of adult male zebrafish through elevating cortisol level and disruption in serotonin, dopamine and gamma-aminobutyric acid neurotransmitter pathways. Ecotoxicol. Environ. Saf. 2020, 203, 111014. [Google Scholar] [CrossRef] [PubMed]
- Bozas, E.; Tritos, N.; Phillipidis, H.; Stylianopoulou, F. At least three neurotransmitter systems mediate a stress-induced increase in c-fos mRNA in different rat brain areas. Cell. Mol. Neurobiol. 1997, 17, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Baxendale, S.; Holdsworth, C.J.; Meza Santoscoy, P.L.; Harrison, M.R.M.; Fox, J.; Parkin, C.A.; Cunliffe, V.T. Identification of compounds with anti-convulsant properties in a zebrafish model of epileptic seizures. Dis. Models Mech. 2012, 5, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xue, X.; Shi, H.; Qi, L.; Gong, D. Osthole upregulates BDNF to enhance adult hippocampal neurogenesis in APP/PS1 transgenic mice. Biol. Pharm. Bull. 2015, 38, 1439–1449. [Google Scholar] [CrossRef]
- Miyazaki, S.; Oikawa, H.; Takekoshi, H.; Hoshizaki, M.; Ogata, M.; Fujikawa, T. Anxiolytic effects of acanthopanax senticosus HARMS occur via regulation of autonomic function and activate hippocampal BDNF-TrkB signaling. Molecules 2019, 24, 132. [Google Scholar] [CrossRef]
- Maciąg, M.; Michalak, A.; Skalicka-Woźniak, K.; Zykubek, M.; Ciszewski, A.; Budzyńska, B. Zebrafish and mouse models for anxiety evaluation—A comparative study with xanthotoxin as a model compound. Brain Res. Bull. 2020, 165, 139–145. [Google Scholar] [CrossRef]
- Peng, X.; Lin, J.; Zhu, Y.; Liu, X.; Zhang, Y.; Ji, Y.; Yang, X.; Zhang, Y.; Guo, N.; Li, Q. Anxiety-related behavioral responses of pentylenetetrazole-treated zebrafish larvae to light-dark transitions. Pharmacol. Biochem. Behav. 2016, 145, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Kasica-Jarosz, N.; Podlasz, P.; Kaleczyc, J. Pituitary adenylate cyclase–activating polypeptide (PACAP-38) plays an inhibitory role against inflammation induced by chemical damage to zebrafish hair cells. PLoS ONE 2018, 13, e0198180. [Google Scholar] [CrossRef]




| Gene | Forward 5′-3′ | Reverse 5′-3′ | Accession No/Source |
|---|---|---|---|
| c-fos | GTGCAGCACGGCTTCACCGA | TTGAGCTGCGCCGTTGGAGG | NM_205569.1 |
| th1 | GACGGAAGATGATCGGAGACA | CCGCCATGTTCCGATTTCT | NM_131149.1/ |
| gal | GACCAACTGATACTCAGGATGCA | ATCCCGAGTGTTTCTGTCAGAA | NM_001346239.1 |
| gabrapa | AGGATCCACTTGAGGGCTGA | CTTCTTCGTGATGCTCCTGGT | NM_001013260.1/ |
| gabarapb | TGTAAACAACGTCATTCCCCCT | ATCTTCTTCGTGATGTTCCTGGT | NM_001386387.1 |
| penka | CTTTGAGCGCCTGTCTCGTG | TTCTAATGTGCAGGCCAGTGTG | NM_200083.2 |
| penkb | TGGTGGCAGGAGTCTAAACG | TTGGACATCGCGGACATCAT | NM_182883.1 |
| htr1aa | CAGAGCAGAGCAGCACAAG | TGGTCTGAGAGTTCTGGTCTAATC | NM_001123321.1 |
| htr1b | GTGTCGGTGCTCGTGATG | CAGCCAGATGTCGCAGATG | NM_001128709.1 |
| htr2b | GCTGCTCATTCTTCTGGTCAT | GTTAGTGGCGTTCTGGAGTT | NM_001044743.1 |
| bdnf | GGCGAAGAGCGGACGAATATC | AAGGAGACCATTCAGCAGGACAG | NM_131595.2 |
| Ef1α | CTGGAGGCCAGCTCAAACAT | ATCAAGAAGAGTAGTACCGCTAGCATTAC | NM_131263.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widelski, J.; Kasica, N.; Maciąg, M.; Luca, S.V.; Budzyńska, B.; Fondai, D.; Podlasz, P.; Skalicka-Woźniak, K. Simple Coumarins from Peucedanum luxurians Fruits: Evaluation of Anxiolytic Activity and Influence on Gene Expression Related to Anxiety in Zebrafish Model. Int. J. Mol. Sci. 2023, 24, 8693. https://doi.org/10.3390/ijms24108693
Widelski J, Kasica N, Maciąg M, Luca SV, Budzyńska B, Fondai D, Podlasz P, Skalicka-Woźniak K. Simple Coumarins from Peucedanum luxurians Fruits: Evaluation of Anxiolytic Activity and Influence on Gene Expression Related to Anxiety in Zebrafish Model. International Journal of Molecular Sciences. 2023; 24(10):8693. https://doi.org/10.3390/ijms24108693
Chicago/Turabian StyleWidelski, Jarosław, Natalia Kasica, Monika Maciąg, Simon Vlad Luca, Barbara Budzyńska, Dafina Fondai, Piotr Podlasz, and Krystyna Skalicka-Woźniak. 2023. "Simple Coumarins from Peucedanum luxurians Fruits: Evaluation of Anxiolytic Activity and Influence on Gene Expression Related to Anxiety in Zebrafish Model" International Journal of Molecular Sciences 24, no. 10: 8693. https://doi.org/10.3390/ijms24108693






