Exploring the Potential of Royal-Jelly-Incorporated Hydrogel Dressings as Innovative Wound Care Materials
Abstract
:1. Introduction
2. Results and Discussion
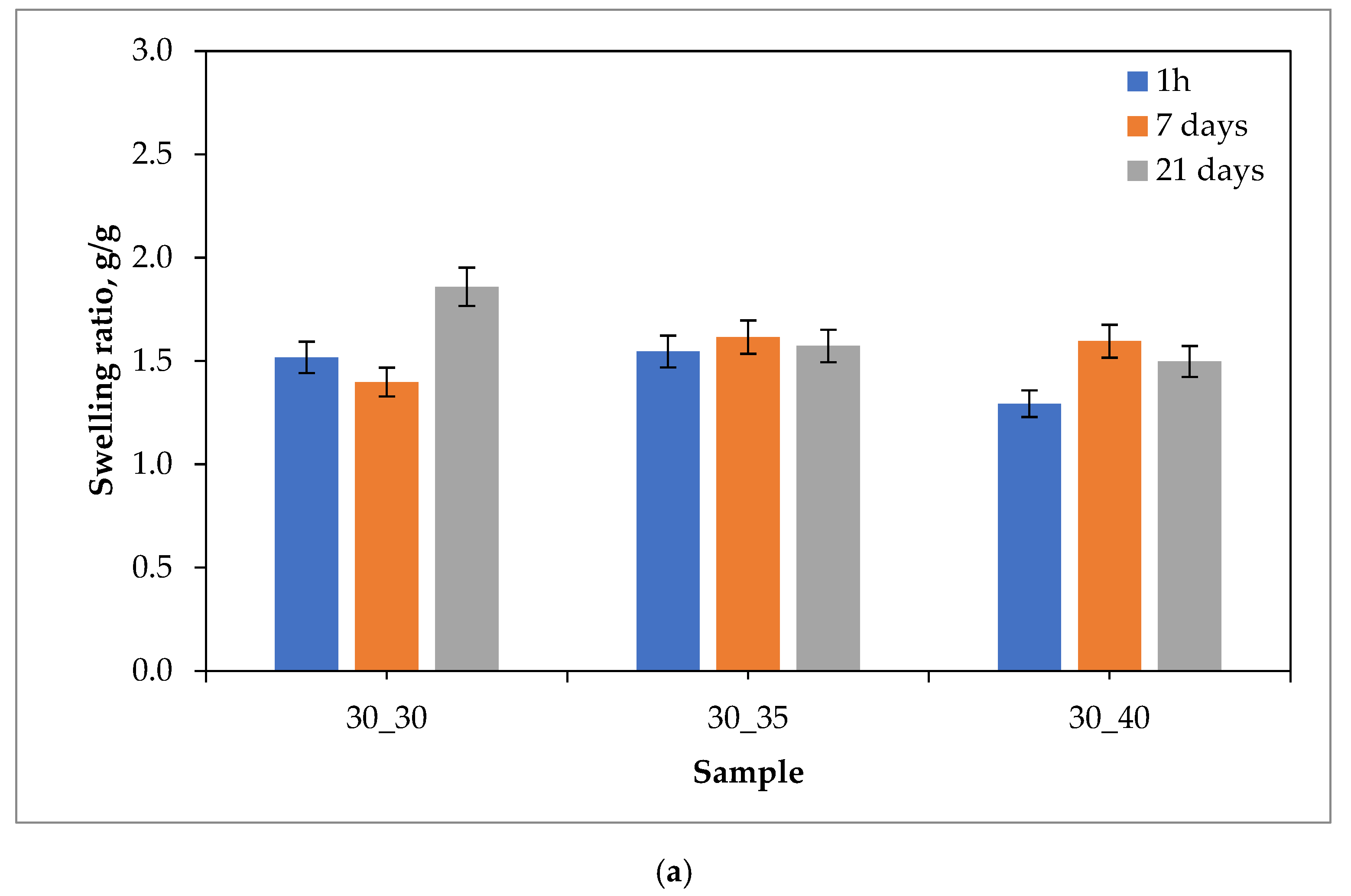
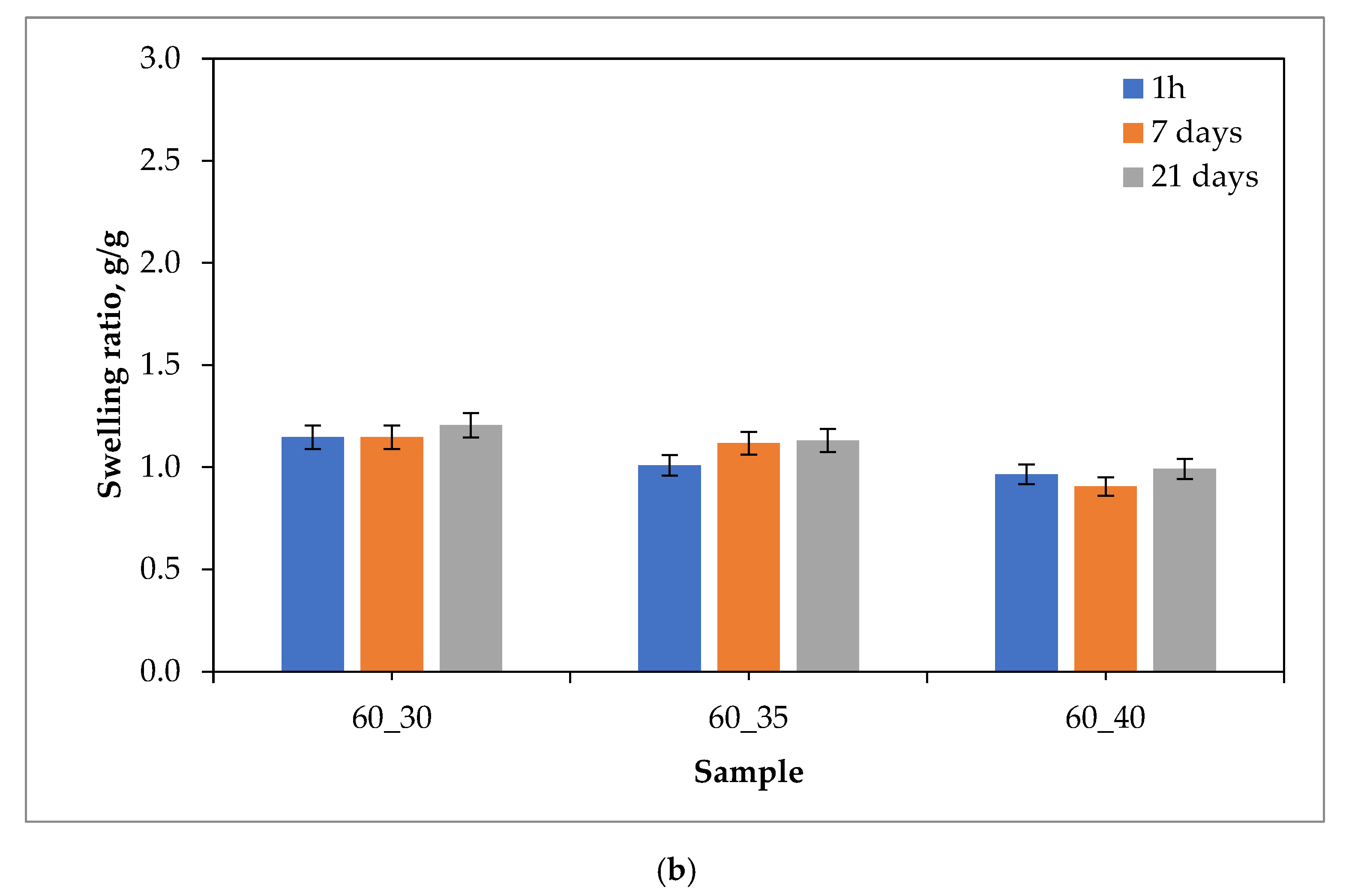
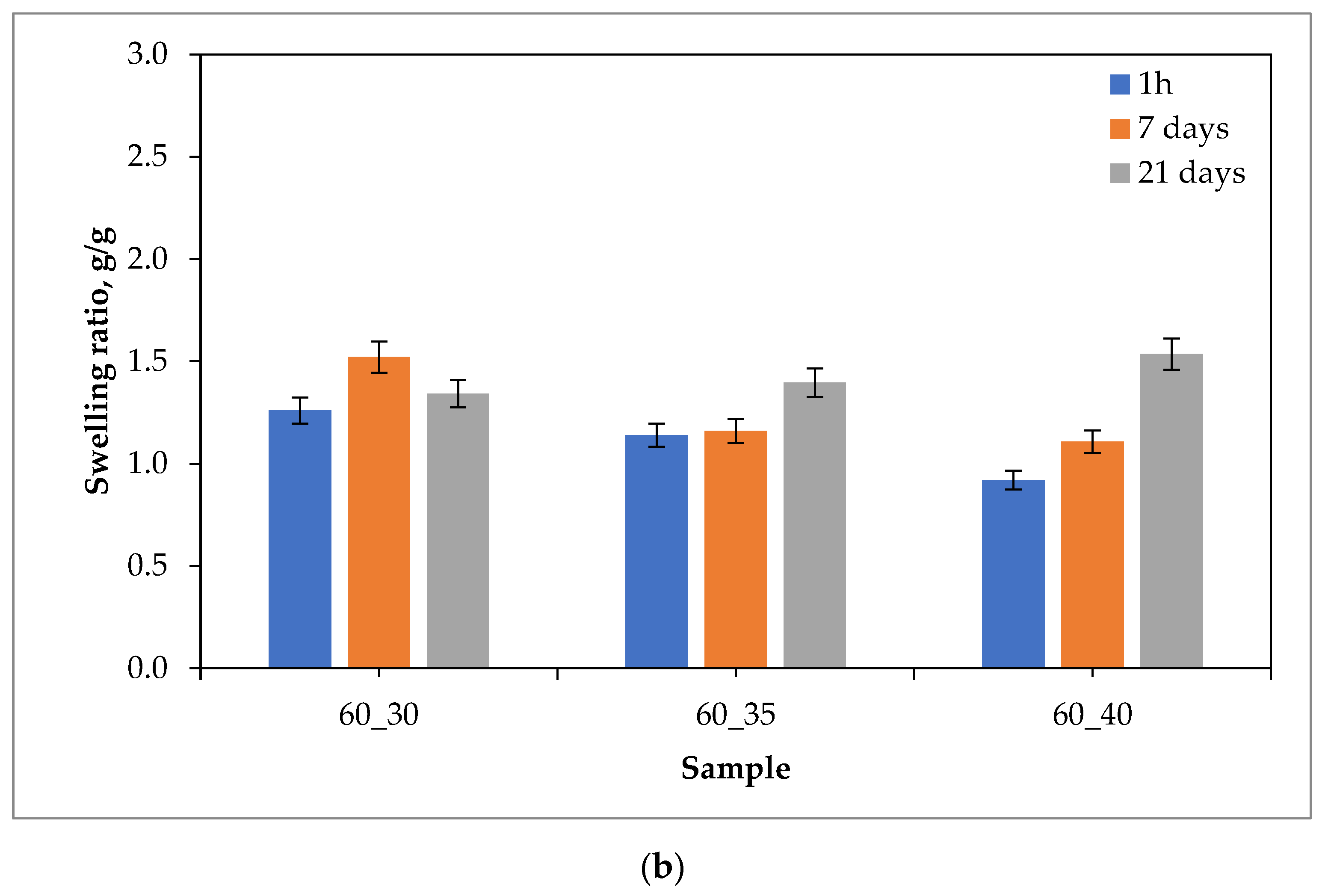
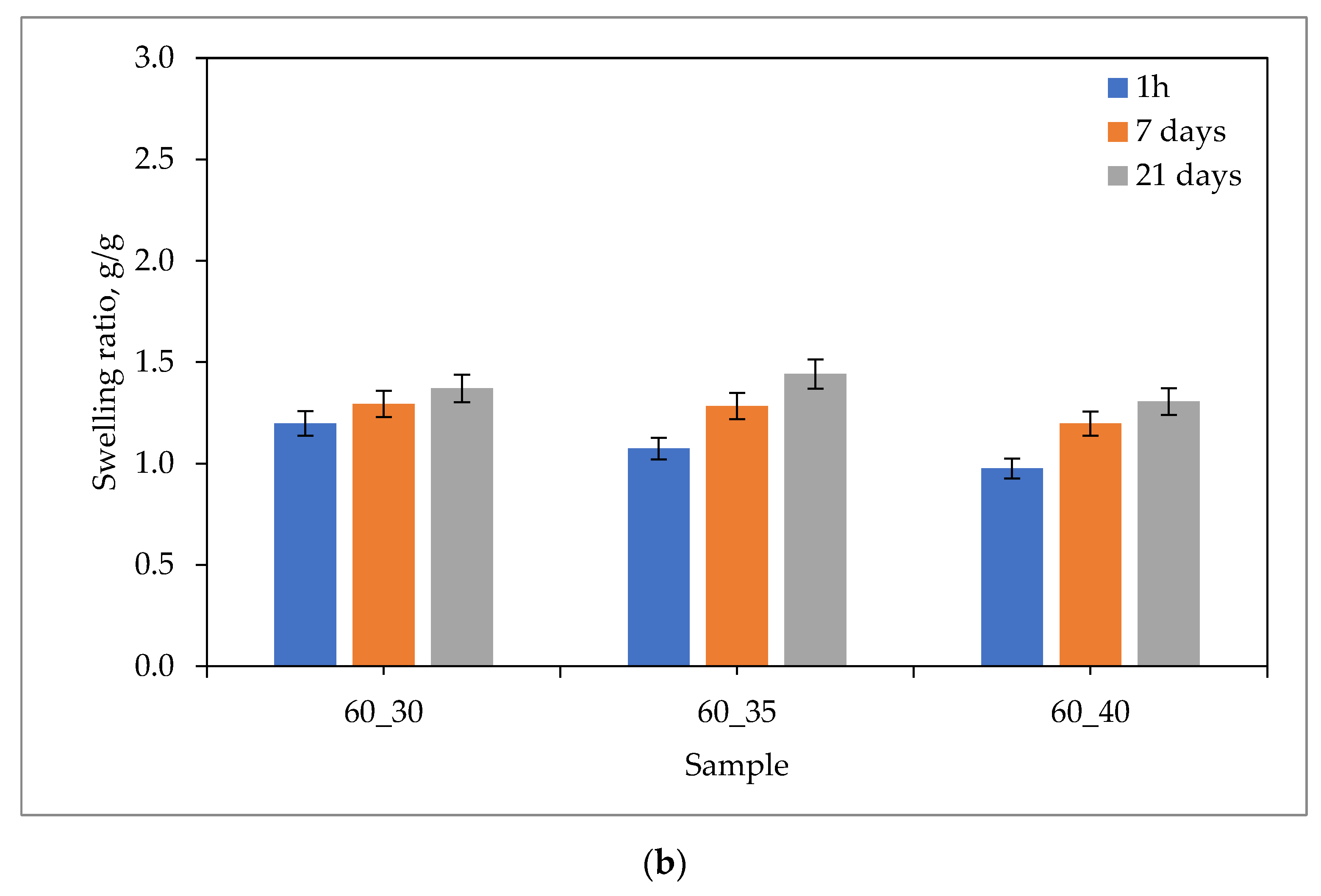
2.1. Sorption Capacity of Hydrogels
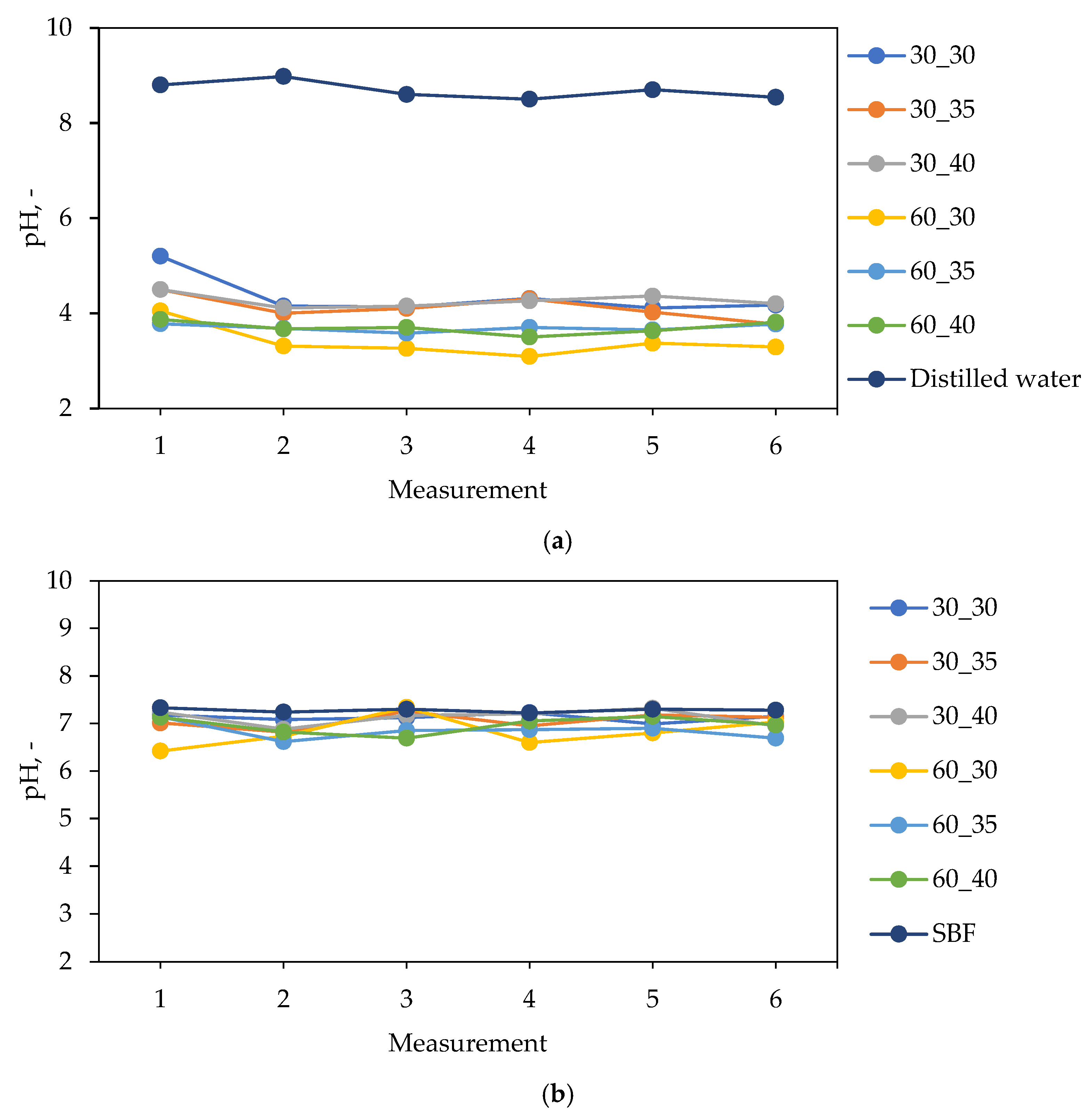
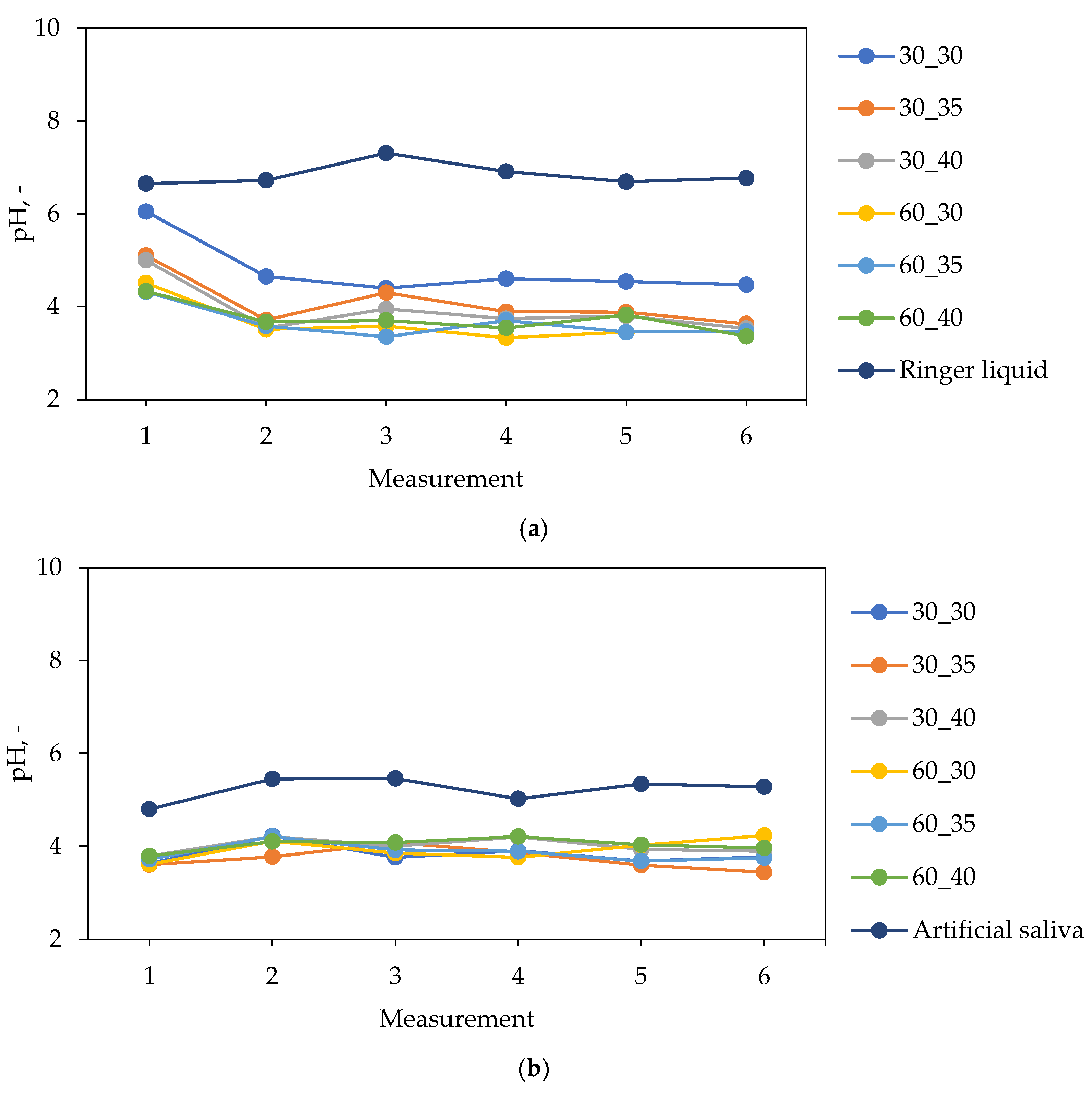
2.2. Characterization of Hydrogels in the Environment of Simulated Body Fluids
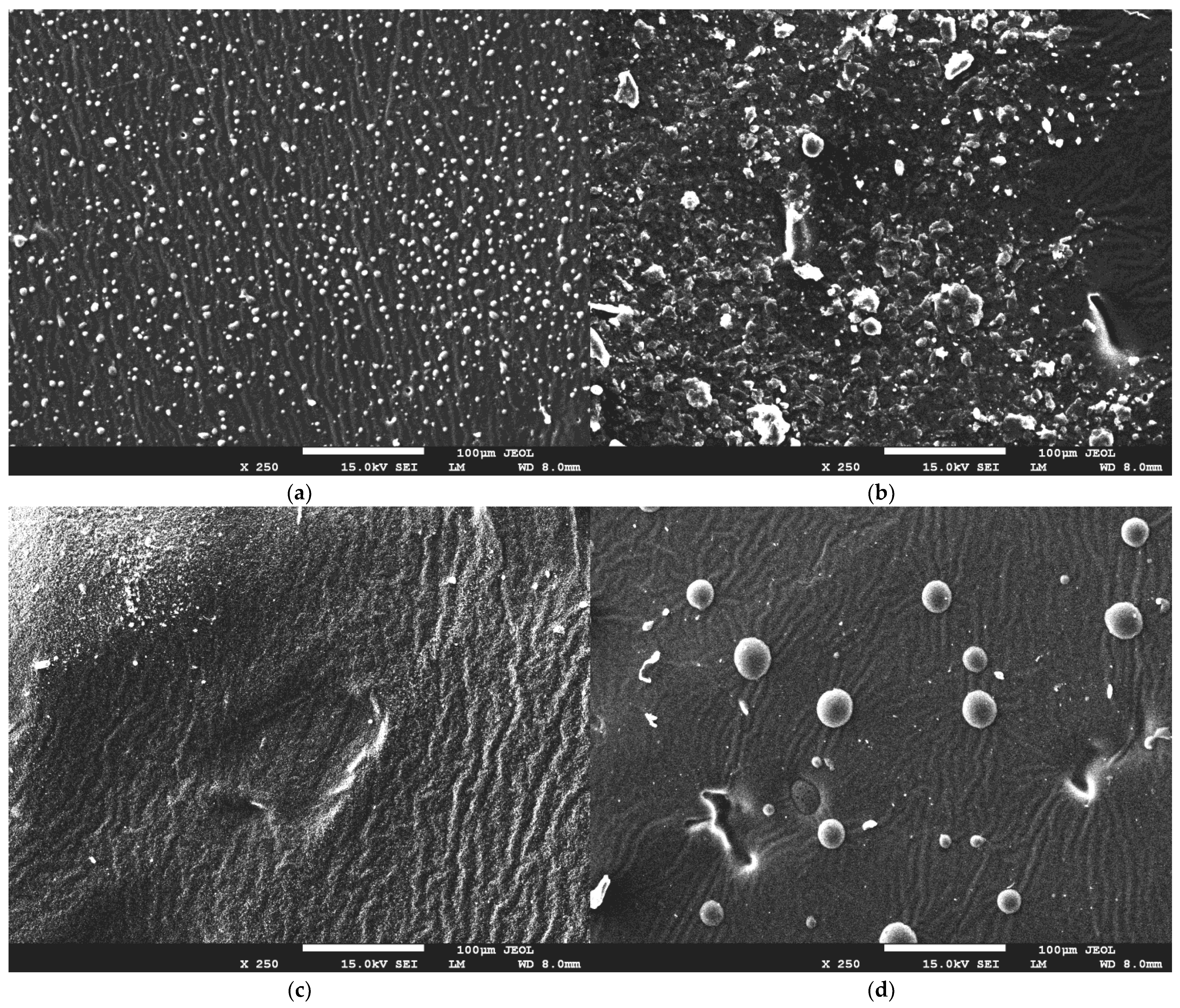
2.3. Evaluation of Hydrogel Surface Morphology Using SEM Microscopy
2.4. Wettability of Hydrogel Materials
2.5. Study of Mechanical Properties of Hydrogel Materials
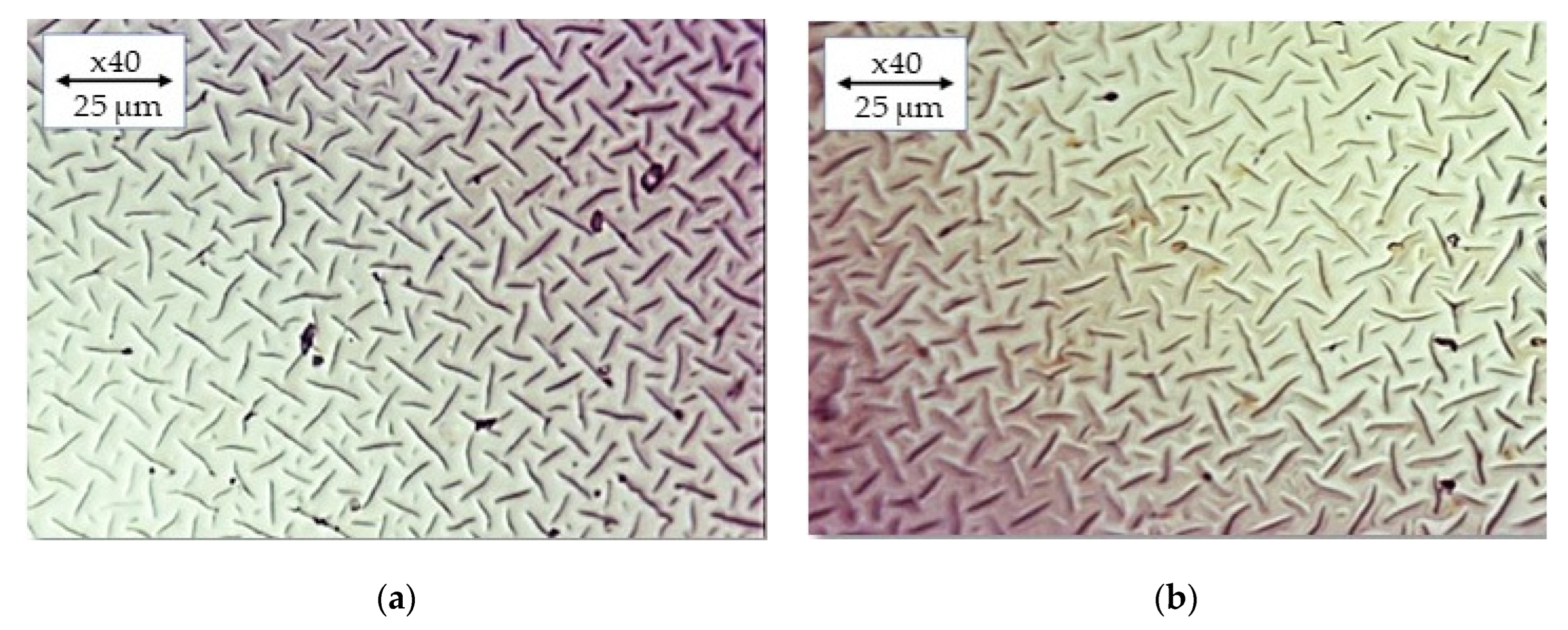
2.6. Imaging of Hydrogels by Means of Optical Microscopy
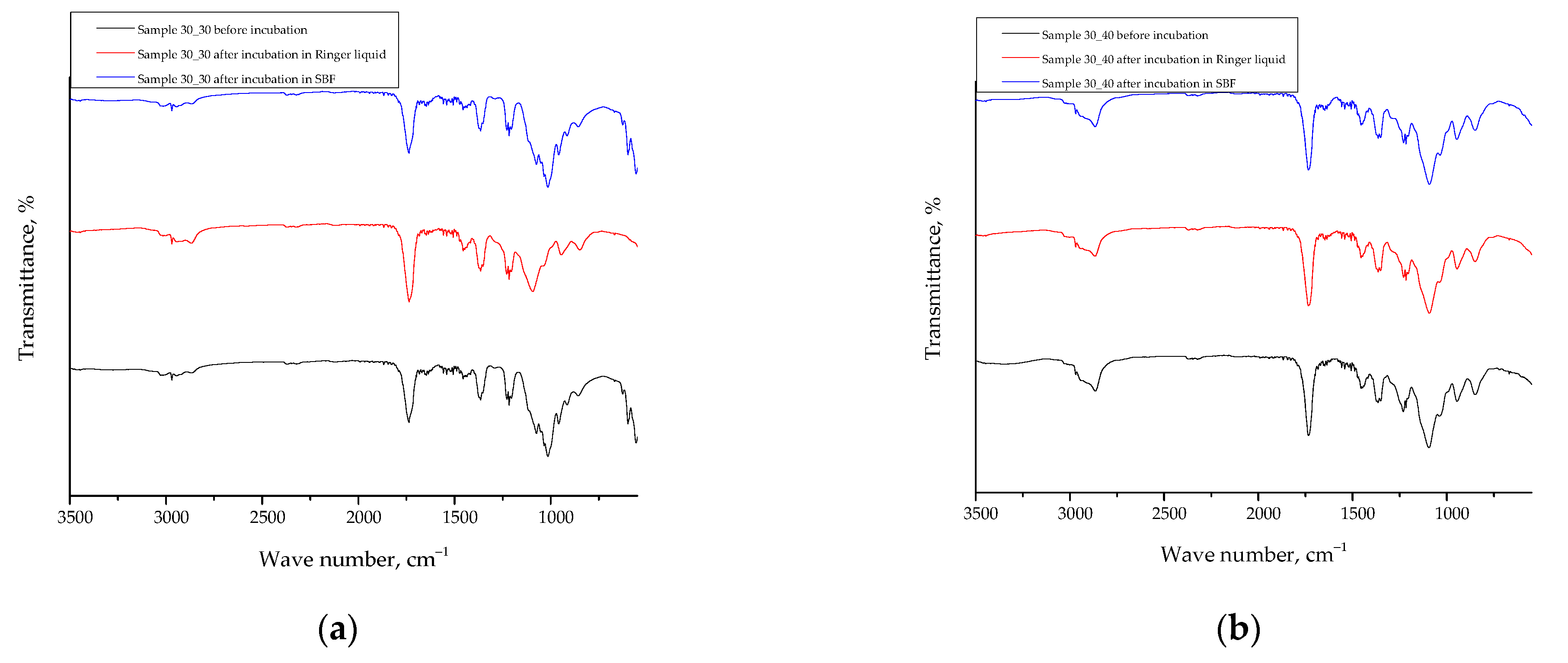
2.7. Results of the FT-IR Spectroscopy
3. Materials and Methods
3.1. Materials
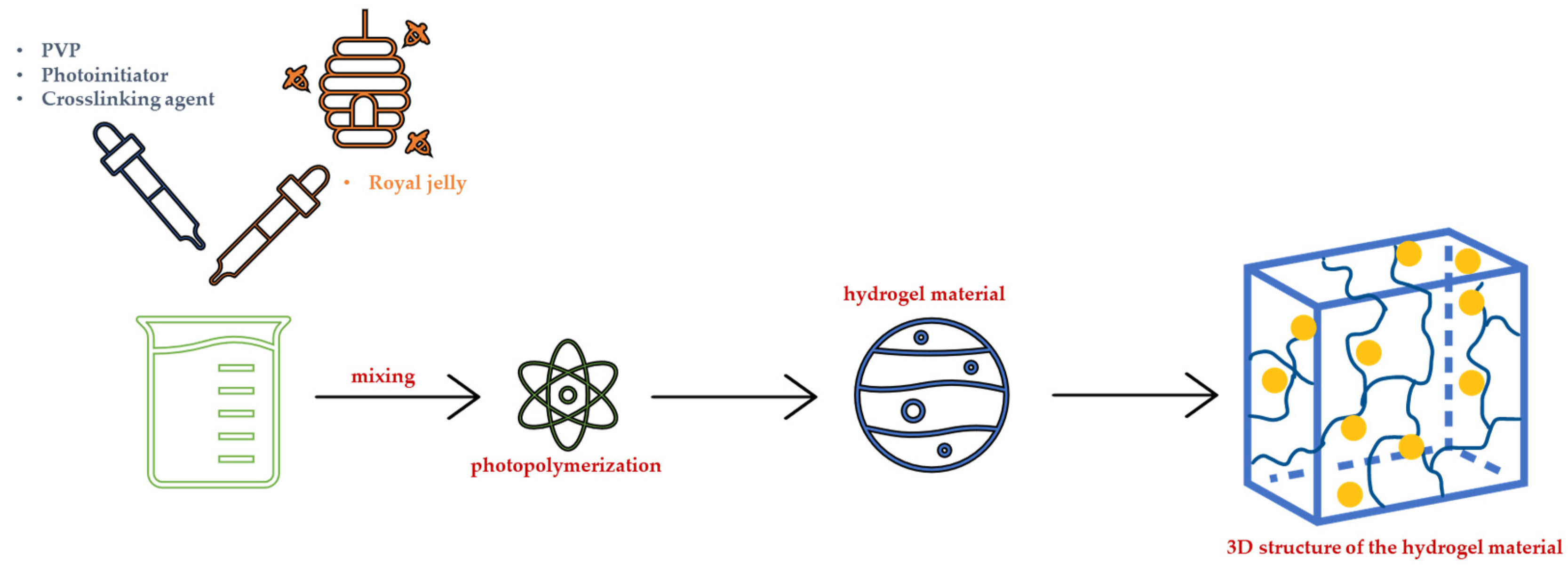
3.2. Synthesis of Hydrogel Dressings under the Influence of UV Radiation
3.3. Sorption Capacity of Hydrogel Dressings
3.4. Characterization of Hydrogels in the Environment of Simulated Physiological Body Fluids
3.5. Evaluation of Hydrogel Surface Morphology Using SEM Technique
3.6. Wettability of Hydrogel Materials
3.7. Characterization of the Mechanical Properties of Hydrogel Materials
3.8. Analysis of the Impact of the Incubation in Simulated Physiological Liquids on the Structure of Hydrogel Materials via Fourier Transform Infrared (FT-IR) Spectroscopy
3.9. Analysis of the Hydrogels via the Optical Microscope
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, Y.; Pan, S.; Jiang, F.; Wang, E.; Miinea, L.; Marchant, N.; Cakmak, M. Anisotropic swelling wound dressings with vertically aligned water absorptive particles. RSC Adv. 2018, 8, 8173–8180. [Google Scholar]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef]
- Fathil, M.A.M.; Katas, H. Antibacterial, Anti-Biofilm and Pro-Migratory Effects of Double Layered Hydrogels Packaged with Lactoferrin-DsiRNA-Silver Nanoparticles for Chronic Wound Therapy. Pharmaceutics 2023, 15, 991. [Google Scholar] [PubMed]
- Abdelghafour, M.M.; Deák, Á.; Kiss, T.; Budai-Szűcs, M.; Katona, G.; Ambrus, R.; Lőrinczi, B.; Keller-Pintér, A.; Szatmári, I.; Szabó, D.; et al. Self-Assembling Injectable Hydrogel for Controlled Drug Delivery of Antimuscular Atrophy Drug Tilorone. Pharmaceutics 2022, 14, 2723. [Google Scholar] [CrossRef] [PubMed]
- Psarrou, M.; Mitraki, A.; Vamvakaki, M.; Kokotidou, C. Stimuli-Responsive Polysaccharide Hydrogels and Their Composites for Wound Healing Applications. Polymers 2023, 15, 986. [Google Scholar] [CrossRef]
- Dong, R.; Guo, B. Smart wound dressings for wound healing. Nano Today 2021, 41, 101290. [Google Scholar]
- Bril, M.; Fredrich, S.; Kurniawan, N.A. Stimuli-responsive materials: A smart way to study dynamic cell responses. Smart Mater. Med. 2022, 3, 257–273. [Google Scholar]
- Fahimirad, S.; Ajalloueian, F. Naturally-derived electrospun wound dressings for target delivery of bio-active agents. Int. J. Pharm. 2019, 566, 307–328. [Google Scholar] [PubMed]
- Wang, W.; Ummartyotin, S.; Narain, R. Advances and challenges on hydrogels for wound dressing. Curr. Opin. Biomed. Eng. 2023, 26, 100443. [Google Scholar] [CrossRef]
- Fan, C.; Xu, Q.; Hao, R.; Wang, C.; Que, Y.; Chen, Y.; Yang, C.; Chang, J. Multi-functional wound dressings based on silicate bioactive materials. Biomaterials 2022, 287, 121652. [Google Scholar] [CrossRef]
- Hasan, S.; Hasan, M.A.; Hassan, M.U.; Amin, M.; Javed, T.; Fatima, L. Biopolymers in diabetic wound care management: A potential substitute to traditional dressings. Eur. Polym. J. 2023, 189, 111979. [Google Scholar] [CrossRef]
- Rani Raju, N.; Silina, E.; Stupin, V.; Manturova, N.; Chidambaram, S.B.; Achar, R.R. Multifunctional and Smart Wound Dressings—A Review on Recent Research Advancements in Skin Regenerative Medicine. Pharmaceutics 2022, 14, 1574. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yi, W.; Zhang, Y.; Wu, H.; Fan, H.; Zhao, J.; Wang, S. Sodium alginate hydrogel containing platelet-rich plasma for wound healing. Colloids Surf. B 2023, 222, 113096. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Zhao, J.; Wang, S. Multifunctional hydrogels based on chitosan, hyaluronic acid and other biological macromolecules for the treatment of inflammatory bowel disease: A review. Int. J. Biol. Macromol. 2023, 227, 505–523. [Google Scholar] [CrossRef]
- Jing, Y.; Ruan, L.; Jiang, G.; Nie, L.; Shavandi, A.; Sun, Y.; Xu, J.; Shao, X.; Zhu, J. Regenerated silk fibroin and alginate composite hydrogel dressings loaded with curcumin nanoparticles for bacterial-infected wound closure. Biomater. Adv. 2023, 149, 213405. [Google Scholar] [CrossRef]
- Azam, F.; Ahmad, F.; Ahmad, S.; Zafar, M.S.; Ulker, Z. Synthesis and characterization of natural fibers reinforced alginate hydrogel fibers loaded with diclofenac sodium for wound dressings. Int. J. Biol. Macromol. 2023, 241, 124623. [Google Scholar] [CrossRef]
- Mirhaji, S.S.; Soleimanpour, M.; Derakhshankhah, H.; Jafari, S.; Mamashli, F.; Rooki, M.; Karimi, M.R.; Nedaei, H.; Pirhaghi, M.; Motasadizadeh, H.; et al. Design, optimization and characterization of a novel antibacterial chitosan-based hydrogel dressing for promoting blood coagulation and full-thickness wound healing: A biochemical and biophysical study. Int. J. Biol. Macromol. 2023, 241, 124529. [Google Scholar] [CrossRef]
- Rac, V.; Lević, S.; Balanč, B.; Graells, B.O.; Bijelić, G. PVA Cryogel as model hydrogel for iontophoretic transdermal drug delivery investigations. Comparison with PAA/PVA and PAA/PVP interpenetrating networks. Colloids. Surf. B 2019, 180, 441–448. [Google Scholar] [CrossRef]
- Ionescu, O.M.; Mignon, A.; Minsart, M.; Caruntu, I.-D.; Giusca, S.; Gardikiotis, I.; Vlierberghe, S.V.; Profire, L. Acrylate-endcapped urethane-based hydrogels: An in vivo study on wound healing potential. Mater. Sci. Eng. C 2021, 130, 112436. [Google Scholar] [CrossRef]
- Contardi, M.; Kossyvaki, D.; Picone, P.; Summa, M.; Guo, X.; Heredia-Guerrero, J.A.; Giacomazza, D.; Carzino, R.; Goldoni, L.; Scoponi, G.; et al. Electrospun polyvinylpyrrolidone (PVP) hydrogels containing hydroxycinnamic acid derivatives as potential wound dressings. Chem. Eng. J. 2021, 409, 128144. [Google Scholar] [CrossRef]
- Chen, T.; Xu, G.; Bao, J.; Huang, Y.; Yang, W.; Hao, W. One-pot preparation of hydrogel wound dressings from Bletilla Striata polysaccharide and polyurethane with dual network structure. Eur. Polym. J. 2022, 181, 111648. [Google Scholar] [CrossRef]
- Deng, H.; Sun, J.; Yu, Z.; Guo, Z.; Xu, C. Low-intensity near-infrared light-triggered spatiotemporal antibiotics release and hyperthermia by natural polysaccharide-based hybrid hydrogel for synergistic wound disinfection. Mater. Sci. Eng. C 2021, 118, 111530. [Google Scholar] [CrossRef]
- Abazari, M.; Akbari, T.; Hasani, M.; Sharifikolouei, E.; Raoufi, M.; Foroumadi, A.; Sharifzadeh, M.; Firoozpour, L.; Khoobi, M. Polysaccharide-based hydrogels containing herbal extracts for wound healing applications. Carbohydr. Polym. 2022, 294, 119808. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, A.; Ehtesabi, H.; Ebrahimi, S. Incorporation of Saqez essential oil into polyvinyl alcohol/chitosan bilayer hydrogel as a potent wound dressing material. Int. J. Biol. Macromol. 2023, 226, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Atila, D.; Karataş, A.; Keskin, D.; Tezcaner, A. Pullulan hydrogel-immobilized bacterial cellulose membranes with dual-release of vitamin C and E for wound dressing applications. Int. J. Biol. Macromol. 2022, 218, 760–774. [Google Scholar] [CrossRef]
- Phonrachom, O.; Charoensuk, P.; Kiti, K.; Saichana, N.; Kakumyan, P.; Suwantong, O. Potential use of propolis-loaded quaternized chitosan/pectin hydrogel films as wound dressings: Preparation, characterization, antibacterial evaluation, and in vitro healing assay. Int. J. Biol. Macromol. 2023, 241, 124633. [Google Scholar] [CrossRef]
- Abraham, S.A.; Yashavanth, G.; Deveswaran, R.; Bharath, S.; Azamathulla, M.; Shanmuganathan, S. Honey based hydrogel as delivery system for wound healing. Mater. Today Proc. 2022, 49, 1709–1718. [Google Scholar] [CrossRef]
- Yupanqui Mieles, J.; Vyas, C.; Aslan, E.; Humphreys, G.; Diver, C.; Bartolo, P. Honey: An Advanced Antimicrobial and Wound Healing Biomaterial for Tissue Engineering Applications. Pharmaceutics 2022, 14, 1663. [Google Scholar] [CrossRef]
- Dumitru, C.D.; Neacsu, I.A.; Grumezescu, A.M.; Andronescu, E. Bee-Derived Products: Chemical Composition and Applications in Skin Tissue Engineering. Pharmaceutics 2022, 14, 750. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.; Chen, Y.; Cao, J.; Tian, W.; Ma, B.; Dong, Y. Active components and biological functions of royal jelly. J. Funct. Foods 2021, 82, 104514. [Google Scholar] [CrossRef]
- Lee, S.; Lee, K.S.; Ok, M.; Kim, B.Y.; Jin, B.R. Antimicrobial activity of major royal jelly protein 8 and 9 of honeybee (Apis mellifera) venom. J. Asia Pac. Entomol. 2022, 25, 101964. [Google Scholar] [CrossRef]
- Bagameri, L.; Baci, G.-M.; Dezmirean, D.S. Royal Jelly as a Nutraceutical Natural Product with a Focus on Its Antibacterial Activity. Pharmaceutics 2022, 14, 1142. [Google Scholar] [CrossRef] [PubMed]
- Rizki, A.M.F.; Usman, A.N.; Raya, I.; Aliyah; Dirpan, A.; Arsyad, A.; Fendi, F.; Sumidarti, A. Effect of royal jelly to deal with stress oxidative in preconception women: A literature review. Gac. Sanit. 2021, 35, S288–S290. [Google Scholar] [CrossRef] [PubMed]
- Siavash, M.; Shokri, S.; Haghighi, S.; Mohammadi, M.; Shahtalebi, M.A.; Farajzadehgan, Z. The efficacy of topical Royal Jelly on diabetic foot ulcers healing: A case series. J. Res. Med. Sci. 2011, 16, 904–909. [Google Scholar]
- Li, Y.; Wang, Y.; Xu, W.; Zhang, X.; Ren, X. Fabrication and properties of chitosan/royal jelly hydrogels for wound dressing. Int. J. Biol. Macromol. 2017, 105, 352–358. [Google Scholar]
- Khattab, M.F.; El-Shaer, A. Evaluation of royal jelly hydrogel wound dressing in rats. J. Wound Care. 2018, 27, 151–157. [Google Scholar]
- Dziewulska, A.; Kaczmarek, B.; Gerszberg, A. Effect of royal jelly on the properties of chitosan-based hydrogels. J. Appl. Polym. Sci. 2019, 136, 47476. [Google Scholar]
- Cieśla, M.; Łukowski, G.; Mertas, A.; Komosinska-Vassev, K. Influence of royal jelly on the properties of sodium alginate and gelatin hydrogels. Mater. Sci. Eng. C 2020, 108, 110464. [Google Scholar]
- Li, L.; Li, J.; Li, Y.; Li, S. Royal jelly incorporated gelatin-based hydrogels with enhanced mechanical properties for tissue engineering applications. Mater. Sci. Eng. C 2020, 110, 110690. [Google Scholar]
- Liu, C.; Wu, Y.; Cao, M.; Guo, J.; Yang, X.; Yuan, W. Preparation and characterization of royal jelly/polyvinyl alcohol hydrogels for potential wound dressing applications. Mater. Sci. Eng. C 2021, 120, 111610. [Google Scholar]
- Sun, Y.; You, J.; Dong, Y.; Sun, C. The potential of royal jelly in promoting wound healing: Review. J. Food Sci. Technol. 2019, 56, 3139–3146. [Google Scholar]
- Vargas, A.; Zecca, I.B.; Antelo, D.S.; Rocha-Filho, P.A. Fabrication and characterization of chitosan/alginate hydrogels containing royal jelly for wound healing applications. Int. J. Biol. Macromol. 2020, 153, 405–415. [Google Scholar]
- Yu, L.; Guo, J.; Yang, X.; Yuan, W. Fabrication of chitosan-based hydrogel dressings with royal jelly for wound healing. Int. J. Biol. Macromol. 2020, 151, 957–967. [Google Scholar]
- Vargas, A.; Zecca, I.B.; Antelo, D.S.; Rocha-Filho, P.A. Influence of royal jelly on the properties of chitosan hydrogels for wound healing applications. Int. J. Biol. Macromol. 2021, 172, 1029–1038. [Google Scholar]
- Yao, M.; Chen, Y.; Zhang, B.; Zhu, X. Royal jelly-chitosan hydrogel for enhanced wound healing. Int. J. Biol. Macromol. 2021, 169, 92–99. [Google Scholar]
- Zhang, S.; Yan, H.; Wang, Z.; Yang, Y. Preparation and characterization of PVA/PEG hydrogels containing royal jelly. Mater. Sci. Eng. C 2018, 85, 57–63. [Google Scholar]
- Górecka, A.; Puchalski, M.; Kaczmarek, M.; Świątek, P.; Włodarczyk, M.; Królicka, A. Effect of royal jelly addition on properties of hydrogels based on poly(vinyl alcohol). Mater. Sci. Eng. C 2019, 99, 654–663. [Google Scholar]
- Ahmed, E.M.; Abdel-Raheem, E.M.; Aljaeid, B.M. Honey as a natural additive to enhance the mechanical and biological properties of chitosan-based hydrogels. Int. J. Biol. Macromol. 2019, 132, 50–58. [Google Scholar]
- Zhao, Y.; Shao, Y.; Cai, Y.; Li, Z.; Liu, Y.; Li, Y.; Li, J. Preparation of chitosan hydrogels and their properties for wound healing. J. Biomater. Appl. 2019, 33, 409–417. [Google Scholar]
- Ren, X.; Zhang, S.; Wang, H. Preparation and characterization of glutaraldehyde crosslinked polyvinyl alcohol hydrogel. Adv. Polym. Technol. 2018, 37, 3079–3085. [Google Scholar]
- Li, H.; Zhang, L.; Wang, Y.; Zhou, J.; Guo, X. Effect of silver nanoparticles on morphology and properties of polyvinylpyrrolidone hydrogels. Polym. Com. 2020, 41, 4124–4131. [Google Scholar]
- Wang, J.; Li, C.; Zhang, X.; Wang, J.; Huang, J. Preparation and characterization of chitosan hydrogel based on waterborne polyurethane. J. Wuh. Uni. Tech. Mater. Sci. Ed. 2018, 33, 59–64. [Google Scholar]
- Sánchez-Sánchez, S.; Palza, S.; Quijada, R. FTIR Spectroscopic Study of Polymer-Matrix Nanocomposites Based on PVP and Halloysite Nanotubes. Appl. Spectr. 2018, 72, 867–875. [Google Scholar]
- Dey, N.; Chattopadhyay, S.; Bhattacharya, A.C.; Datta, S. Poly(ethylene glycol) Diacrylate Hydrogel: Characterization and Biocompatibility Evaluation for Tissue Engineering Applications. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 438–449. [Google Scholar]
- Şimşek, S.P.; Gürbüz, G.; Yılmaz, Y.; Özkalay, A.M.; Kara, A. Preparation and Characterization of the Hydrogels Based on 2-hydroxy-2-methylpropiophenone. Polym. Plast. Technol. Eng. 2019, 58, 803–811. [Google Scholar]
- Tóth-Soma, L.; Kónya, Z.; Kiricsi, Á.; Szécsényi, G.M.; Tóth, E.M.; Bajza, J. Fourier-transform infrared (FT-IR) and Raman spectroscopic study of royal jelly and beeswax. J. Apic. Res. 2016, 55, 322–330. [Google Scholar]
- Khalil, S.; Bhutto, I.A.; Qazi, M.A.; Channa, A.W.; Kandhro, A.A.; Nizamani, M.H.; Gadhi, M.A.; Nizamani, M.A. Characterization of Pure and Adulterated Honey Samples from Pakistan. Food Anal. Methods 2019, 12, 758–766. [Google Scholar]
- Zhang, Y.; Zhang, L.; Cao, C.; Wang, H.; Wang, Y.; Yang, H.; Cao, S. Chemical Composition and Antioxidant Activity of Chinese Propolis Extracts. J. Agric. Food Chem. 2019, 67, 6532–6541. [Google Scholar]
- Chen, S.; Wu, Z.; Wu, Y.; Xu, G.; Chen, X. Stability of sodium alginate/chitosan hydrogel in simulated body fluid: Effects of crosslinking and adsorption of bone morphogenetic protein-2. Int. J. Biol. Macromol. 2019, 136, 1181–1187. [Google Scholar]
- Kim, Y.J.; Kim, K.; Yang, H.S. In vitro degradation and swelling behavior of hyaluronic acid-gelatin hydrogels for soft tissue engineering applications. Polym. Test. 2018, 67, 210–217. [Google Scholar]
- Constable, P.D.; Hinchcliff, K.W.; Done, S.H.; Grünberg, W. Disturbances of Free Water, Electrolytes, Acid-Base Balance, and Oncotic Pressure. In Veterinary Medicine, 11th ed.; Constable, P.D., Hinchcliff, K.W., Done, S.H., Grünberg, W., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 113–152. [Google Scholar]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Kudłacik-Kramarczyk, S.; Głab, M.; Drabczyk, A.; Kordyka, A.; Godzierz, M.; Wróbel, P.S.; Krzan, M.; Uthayakumar, M.; Kędzierska, M.; Tyliszczak, B. Physicochemical Characteristics of Chitosan-Based Hydrogels Containing Albumin Particles and Aloe vera Juice as Transdermal Systems Functionalized in the Viewpoint of Potential Biomedical Applications. Materials 2021, 14, 5832. [Google Scholar] [CrossRef] [PubMed]
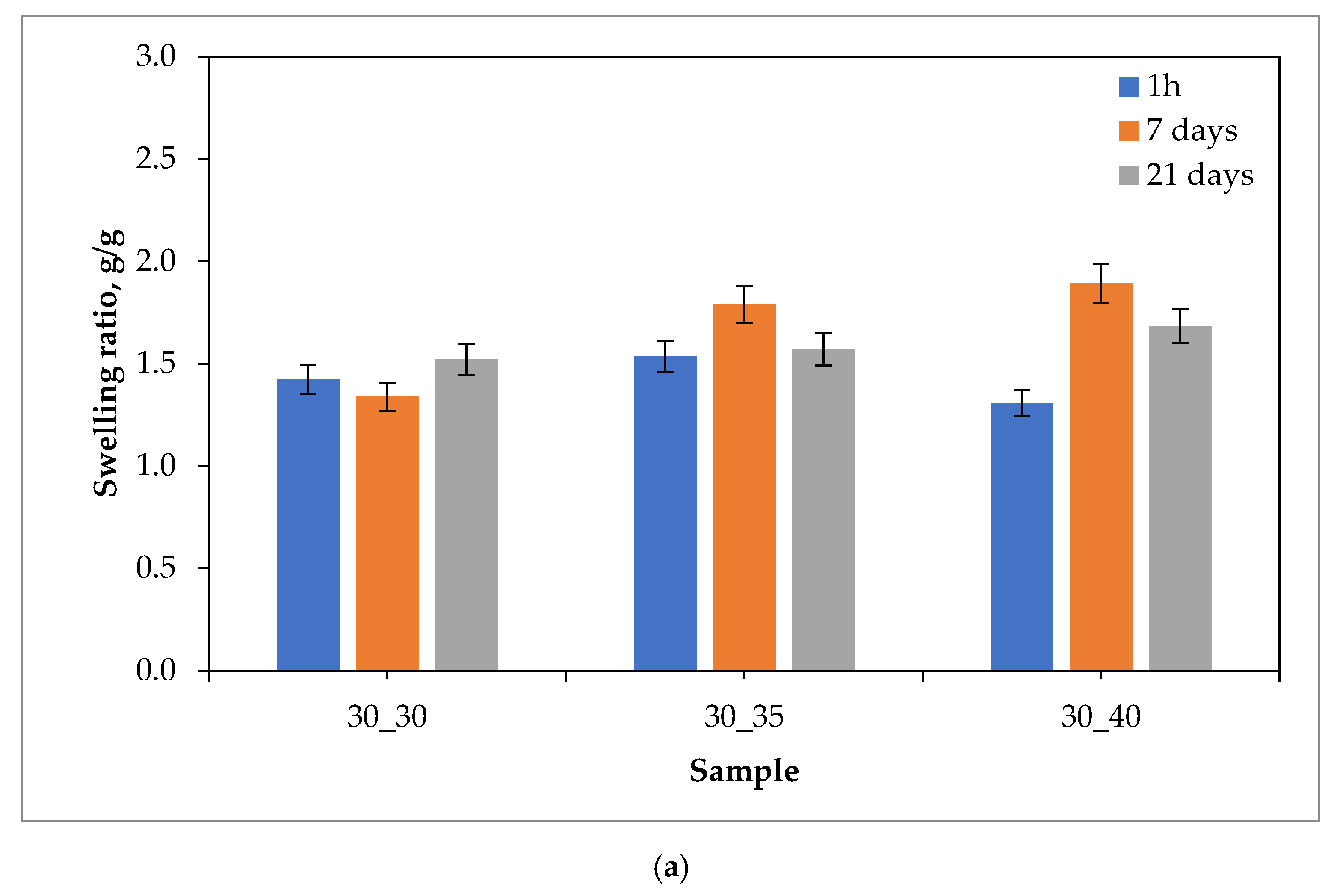


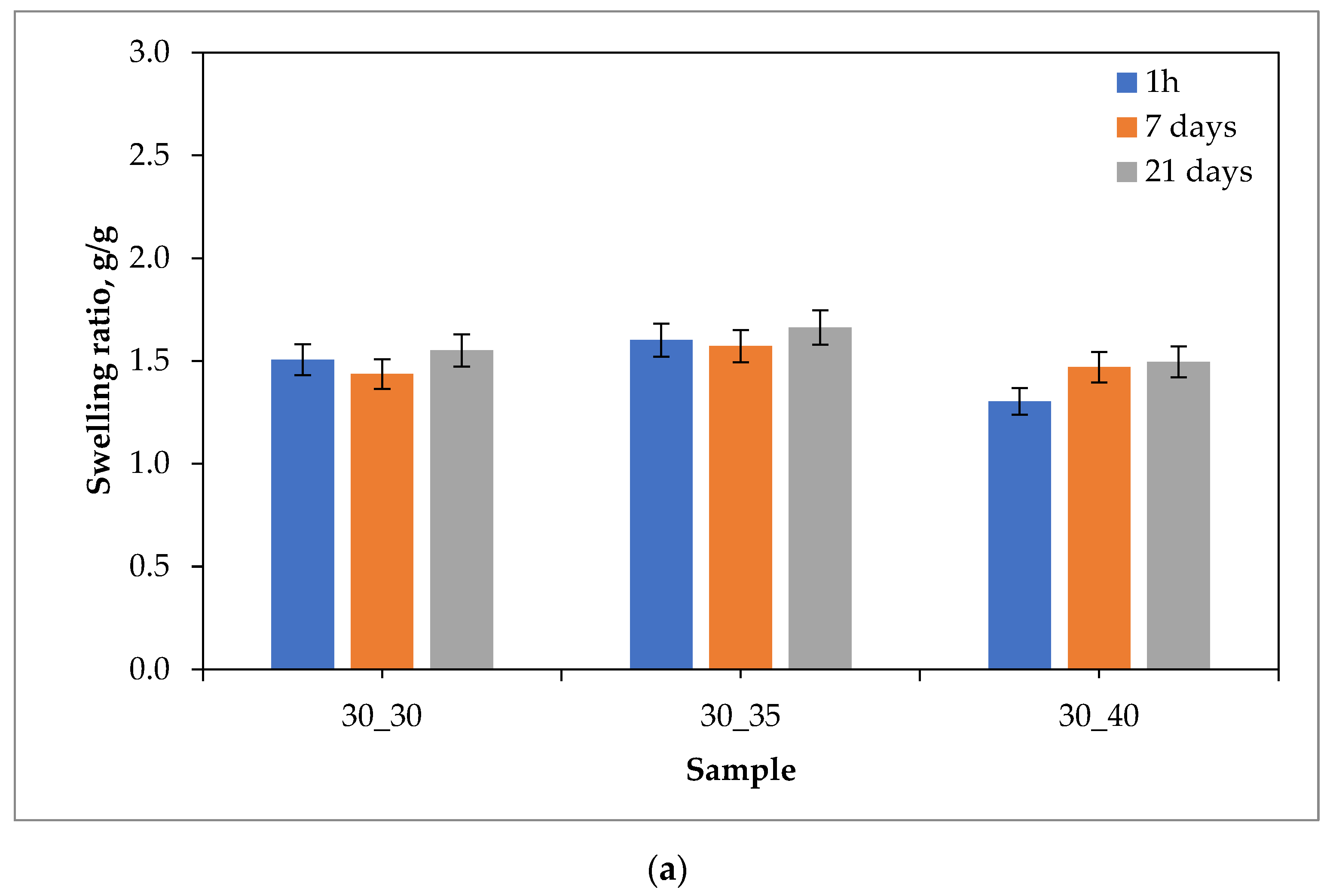




| Sample | Contact Angle, ° | Surface Free Energy | |||
|---|---|---|---|---|---|
| Distilled Water | Diiodomethane | Polar, mJ/m2 | Dispersive, mJ/m2 | Total Free Energy, mJ/m2 | |
| 30_30 | 113 ± 2.0% | 48 ± 0.8% | 1.72 | 45.73 | 47.44 |
| 30_40 | 122 ± 2.4% | 35 ± 1.2% | 6.75 | 58.44 | 65.19 |
| 60_30 | 94 ± 2.8% | 56 ± 1.7% | 0.90 | 33.69 | 34.59 |
| 60_40 | 105 ± 1.8% | 51 ± 1.1% | 0.16 | 40.87 | 41.03 |
| Wave Number, cm−1 | Functional Group | Substrate |
|---|---|---|
| 2930 | -C-H | PVP |
| 1650 | -C=O | |
| 1280 | -C-N | |
| 2925 | -C-H | Crosslinking agent |
| 1735 | -C=O | |
| 1640 | -C=C | |
| 3000 | -C-H | Photoinitiator |
| 1715 | -C=O | |
| 1600 | -C=C | |
| 3000–2800 | -CH and -CH2 | Royal yelly |
| 1700–1600 | -C=O | |
| 1500–1300 | -OH and -CH2-CH2- | |
| 1200–900 | -C-O and -C-C- |
| Sample | 15% PVP Solution, mL | Royal Jelly, wt.% * | Photoinitiator, mL | Crosslinking Agent, v/v % ** |
|---|---|---|---|---|
| 30_30 | 7 | 30 | 0.05 | 30 |
| 30_35 | 35 | |||
| 30_40 | 40 | |||
| 60_30 | 60 | 30 | ||
| 60_35 | 35 | |||
| 60_40 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudłacik-Kramarczyk, S.; Krzan, M.; Jamroży, M.; Przybyłowicz, A.; Drabczyk, A. Exploring the Potential of Royal-Jelly-Incorporated Hydrogel Dressings as Innovative Wound Care Materials. Int. J. Mol. Sci. 2023, 24, 8738. https://doi.org/10.3390/ijms24108738
Kudłacik-Kramarczyk S, Krzan M, Jamroży M, Przybyłowicz A, Drabczyk A. Exploring the Potential of Royal-Jelly-Incorporated Hydrogel Dressings as Innovative Wound Care Materials. International Journal of Molecular Sciences. 2023; 24(10):8738. https://doi.org/10.3390/ijms24108738
Chicago/Turabian StyleKudłacik-Kramarczyk, Sonia, Marcel Krzan, Mateusz Jamroży, Alicja Przybyłowicz, and Anna Drabczyk. 2023. "Exploring the Potential of Royal-Jelly-Incorporated Hydrogel Dressings as Innovative Wound Care Materials" International Journal of Molecular Sciences 24, no. 10: 8738. https://doi.org/10.3390/ijms24108738
APA StyleKudłacik-Kramarczyk, S., Krzan, M., Jamroży, M., Przybyłowicz, A., & Drabczyk, A. (2023). Exploring the Potential of Royal-Jelly-Incorporated Hydrogel Dressings as Innovative Wound Care Materials. International Journal of Molecular Sciences, 24(10), 8738. https://doi.org/10.3390/ijms24108738










