Therapeutic Potential of Targeting Complement C5a Receptors in Diabetic Kidney Disease
Abstract
:1. Introduction
2. Diabetic Kidney Disease
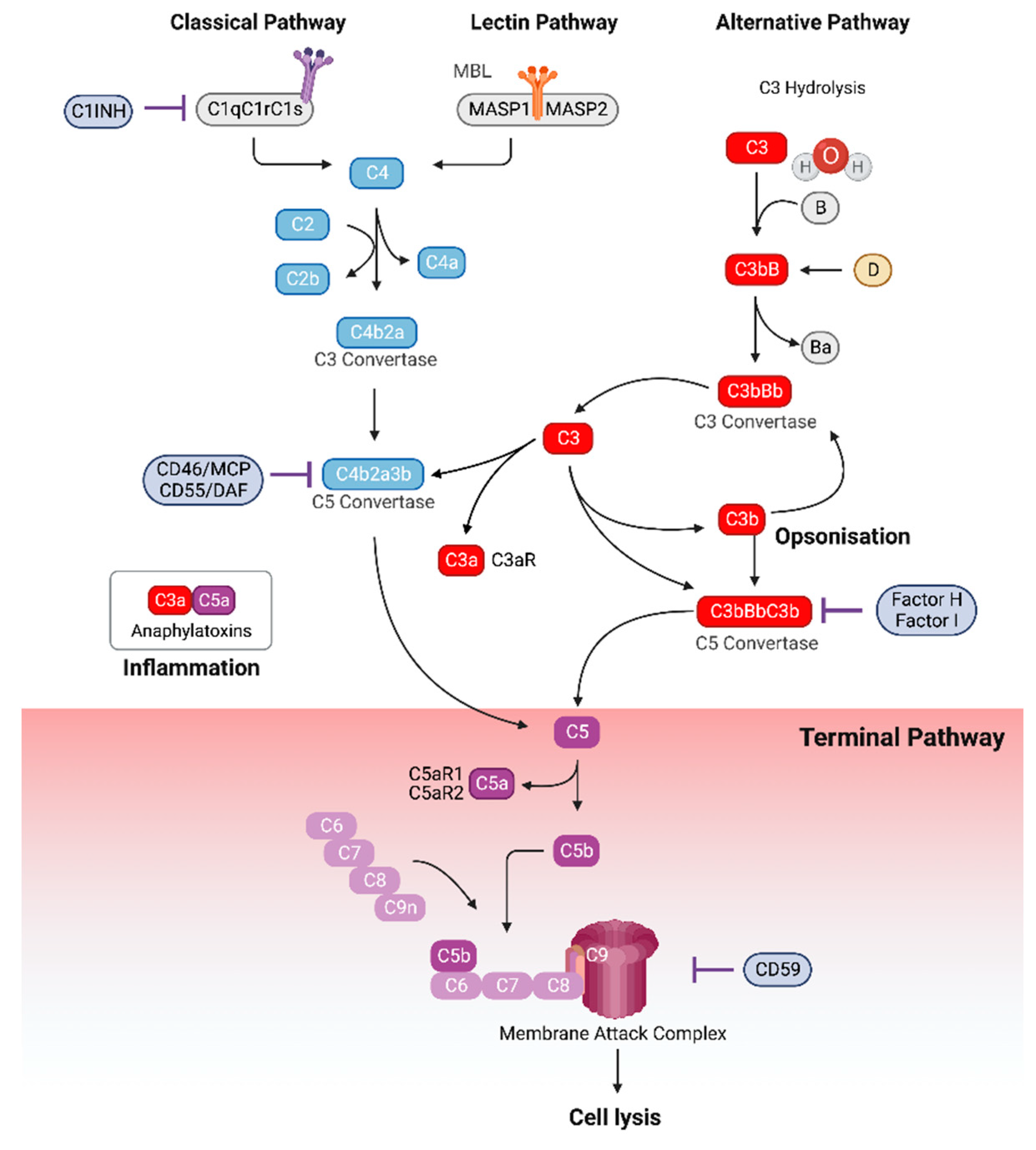
3. The Complement System
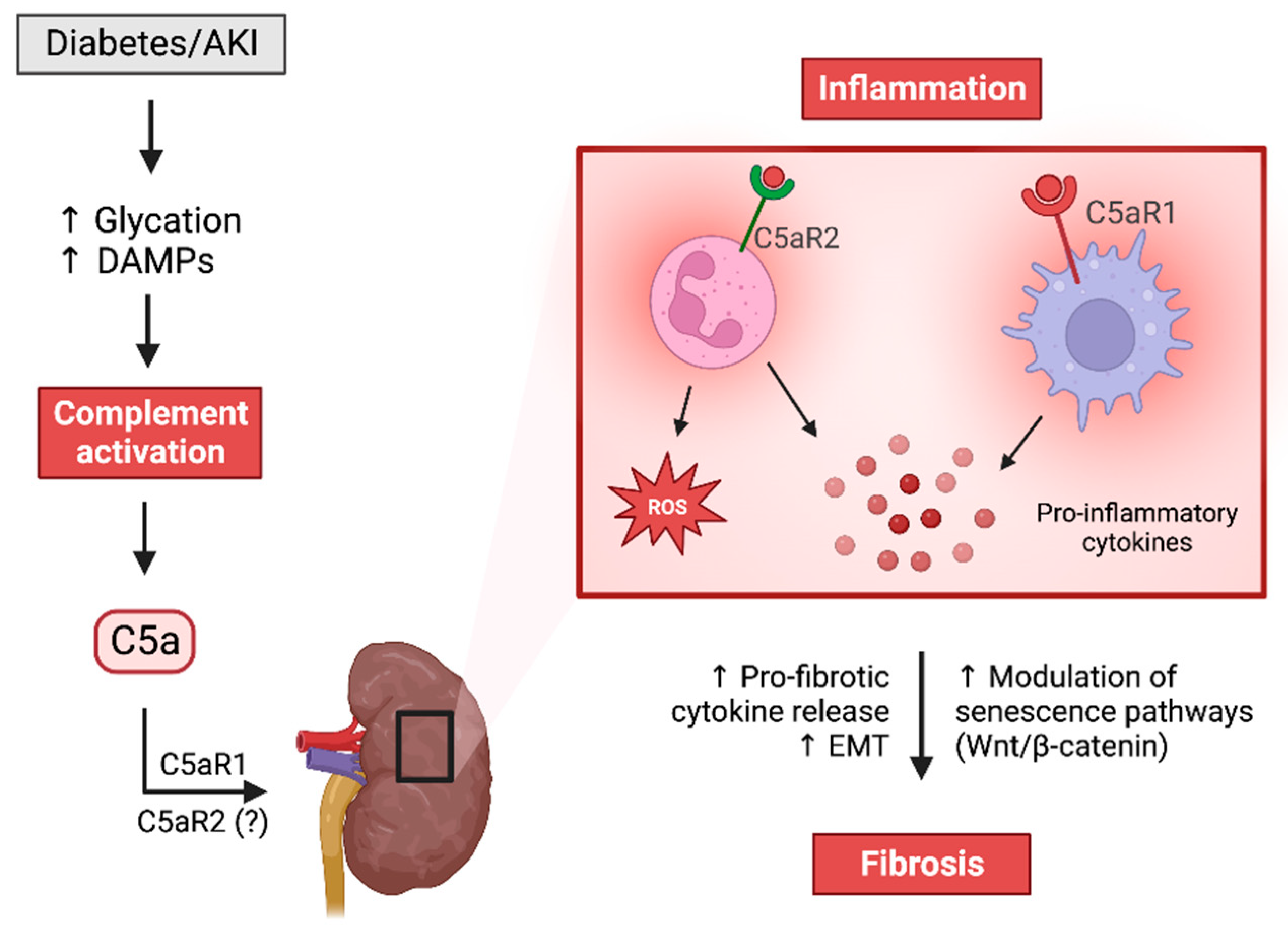
4. The Hyperglycemic Milieu Promotes Complement Activation
| References | Biospecimen Type | Complement Protein | Key Finding/s |
|---|---|---|---|
| [46,54] | Renal tissue | C4d | C4d deposition associated with diabetic nephropathy and correlated with disease severity |
| [46,54] | Renal tissue | C1q | C1q deposition associated with renal dysfunction and disease progression |
| [46] | Renal tissue | C5b-9 | MAC deposits associated with diabetes and correlated with severity of disease |
| [21,26,55] | Renal tissue | C3 | Increased C3 deposition or expression associated with kidney function decline (including albuminuria) and/or progression to ESRD |
| [43] | Renal tissue | C5 | C5 deposition increased in diabetes vs. non-diabetic controls |
| [43,44] | Plasma | C3a | Plasma C3a is significantly elevated in diabetic disease and DKD, and is associated with albuminuria |
| [43,44] | Plasma | C5a | Plasma C5a is significantly elevated in the diabetic milieu and is associated with reduced eGFR |
| [43] | Plasma | sC5b-9 | Circulating C5b-9 is upregulated in DKD versus diabetic patients without kidney involvement |
| [47,48,49,50,51,52,58] | Serum | MBL | MBL levels are associated with the progression and/or progression of albuminuria in patients with T1DM and T2DM |
| [53] | Serum | MAp19 | MAp19 concentration associated with increased risk of progression of albuminuria in T1DM patients |
| [59] | Serum | C7 | Serum C7 levels are increased in patients with early diabetic nephropathy in comparison to controls |
| [54,56] | Urine | C3 | Urinary C3 abundance is negatively correlated with progressive decline in eGFR; urinary C3 is elevated in DKD versus diabetes alone |
| [54] | Urine | C9 | Urinary C9 abundance is negatively correlated with progressive decline in eGFR |
| [56] | Urine | C3b | Urinary C3b significantly increased in DKD versus diabetes alone |
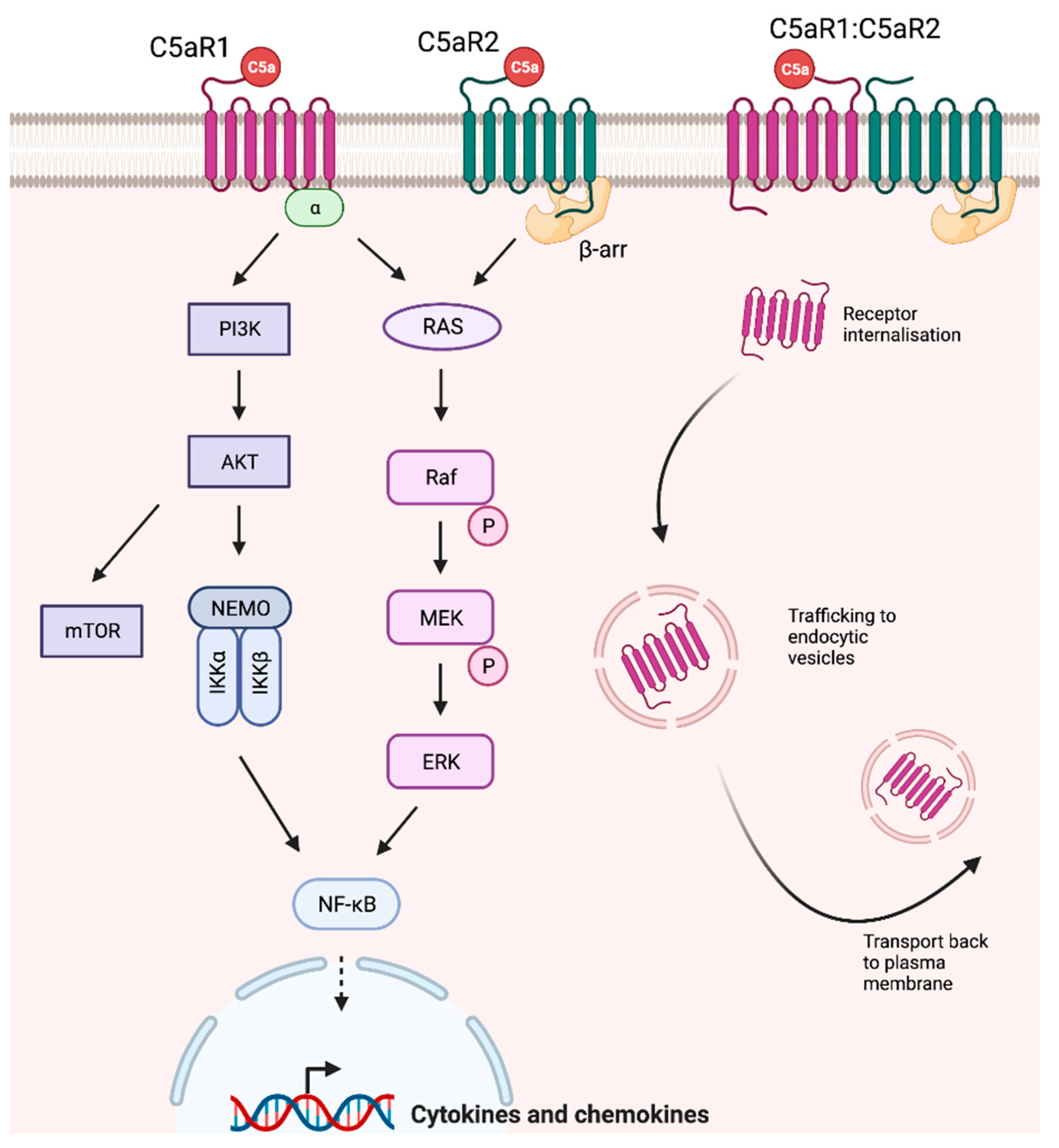
5. C5a and C5a Receptors
6. C5a-C5aR1 Signaling
7. C5aR1 Promotes Inflammation and Tissue Damage in Diverse Models of Acute Kidney Injury
8. C5aR1 Mediates Pathogenesis in Diabetic Kidney Disease
9. C5a-C5aR2 Signaling
10. C5aR2 Promotes Inflammation in AKI
11. Dysregulated C5aR2 Is Associated with Impaired Immunometabolism
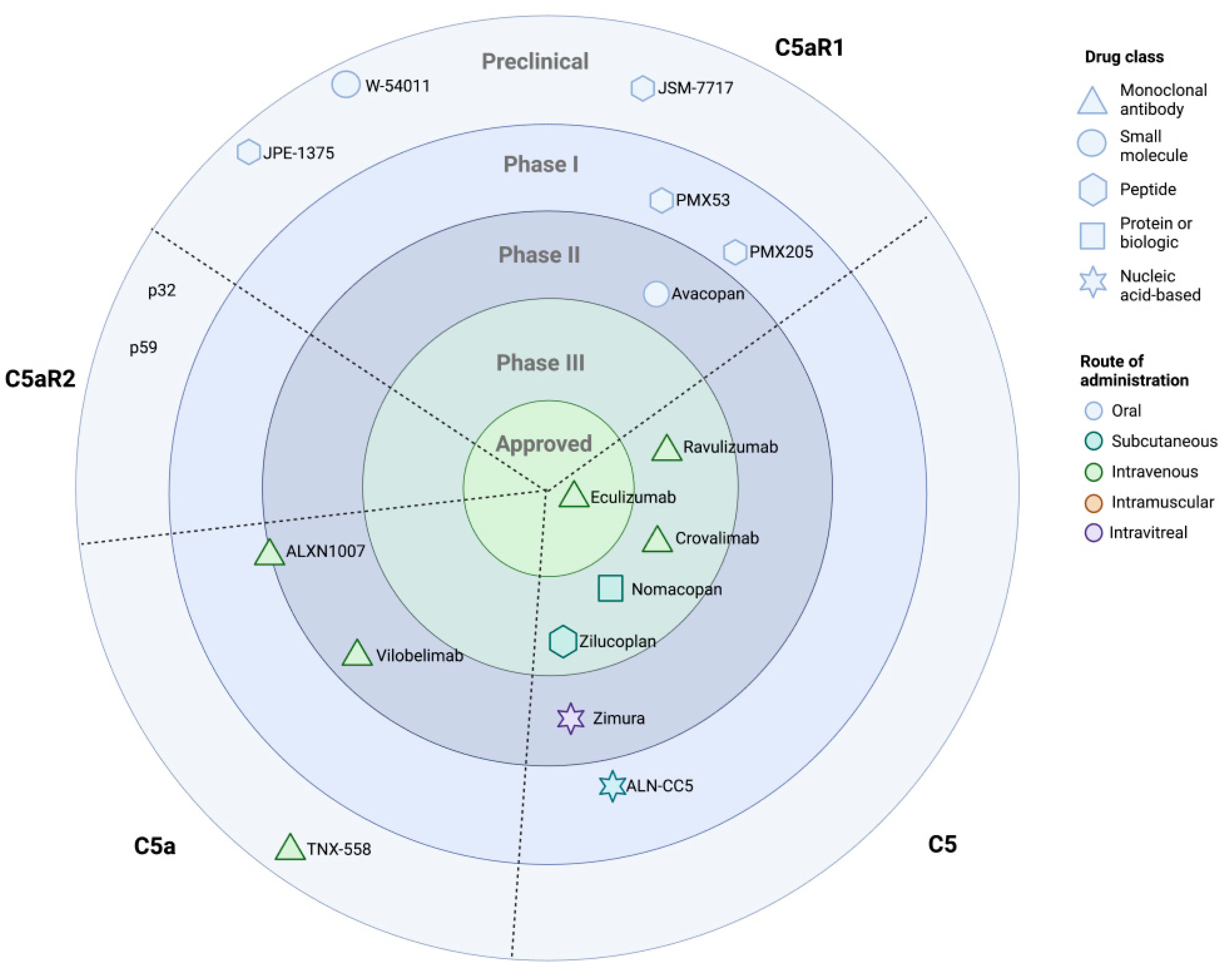
12. Therapeutic Targeting of C5 and the C5a-Signalling Axis
12.1. Eculizumab and C5-Targeted Therapeutics
12.2. Inhibition of C5aRs in Preclinical Models
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.Z.; Magliano, D.J.; Herman, W.H.; Shaw, J.E. Diabetes: A 21st century challenge. Lancet Diabetes Endocrinol. 2014, 2, 56–64. [Google Scholar] [CrossRef]
- Macisaac, R.J.; Ekinci, E.I.; Jerums, G. Markers of and risk factors for the development and progression of diabetic kidney disease. Am. J. Kidney Dis. 2014, 63, S39–S62. [Google Scholar] [CrossRef]
- Flyvbjerg, A. The role of the complement system in diabetic nephropathy. Nat. Rev. Nephrol. 2017, 13, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Krolewski, A.S.; Warram, J.H.; Christlieb, A.R.; Busick, E.J.; Kahn, C.R. The changing natural history of nephropathy in type I Diabetes. Am. J. Med. 1985, 78, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.I.; Stevens, R.J.; Manley, S.E.; Bilous, R.W.; Cull, C.A.; Holman, R.R.; Group, U. Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 2003, 63, 225–232. [Google Scholar] [CrossRef]
- Retnakaran, R.; Cull, C.A.; Thorne, K.I.; Adler, A.I.; Holman, R.R.; Group, f.t.U.S. Risk Factors for Renal Dysfunction in Type 2 Diabetes: U.K. Prospective Diabetes Study 74. Diabetes 2006, 55, 1832–1839. [Google Scholar] [CrossRef]
- de Boer, I.H.; Afkarian, M.; Rue, T.C.; Cleary, P.A.; Lachin, J.M.; Molitch, M.E.; Steffes, M.W.; Sun, W.; Zinman, B. Renal Outcomes in Patients with Type 1 Diabetes and Macroalbuminuria. J. Am. Soc. Nephrol. 2014, 25, 2342–2350. [Google Scholar] [CrossRef]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef]
- Anders, H.J.; Huber, T.B.; Isermann, B.; Schiffer, M. CKD in diabetes: Diabetic kidney disease versus nondiabetic kidney disease. Nat. Rev. Nephrol. 2018, 14, 361–377. [Google Scholar] [CrossRef]
- Ruggenenti, P.; Cravedi, P.; Remuzzi, G. The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nat. Rev. Nephrol. 2010, 6, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Helal, I.; Fick-Brosnahan, G.M.; Reed-Gitomer, B.; Schrier, R.W. Glomerular hyperfiltration: Definitions, mechanisms and clinical implications. Nat. Rev. Nephrol. 2012, 8, 293–300. [Google Scholar] [CrossRef]
- Ghosh, P.; Sahoo, R.; Vaidya, A.; Chorev, M.; Halperin, J.A. Role of complement and complement regulatory proteins in the complications of diabetes. Endocr. Rev. 2015, 36, 272–288. [Google Scholar] [CrossRef]
- Wei, P.Z.; Szeto, C.C. Mitochondrial dysfunction in diabetic kidney disease. Clin. Chim. Acta 2019, 496, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2011, 7, 684–696. [Google Scholar] [CrossRef]
- Meng, X.-M.; Nikolic-Paterson, D.J.; Lan, H.Y. Inflammatory processes in renal fibrosis. Nat. Rev. Nephrol. 2014, 10, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Reidy, K.; Kang, H.M.; Hostetter, T.; Susztak, K. Molecular mechanisms of diabetic kidney disease. J. Clin. Invest. 2014, 124, 2333–2340. [Google Scholar] [CrossRef]
- Budge, K.; Dellepiane, S.; Yu, S.M.; Cravedi, P. Complement, a Therapeutic Target in Diabetic Kidney Disease. Front. Med. 2020, 7, 599236. [Google Scholar] [CrossRef] [PubMed]
- Hung, P.H.; Hsu, Y.C.; Chen, T.H.; Lin, C.L. Recent Advances in Diabetic Kidney Diseases: From Kidney Injury to Kidney Fibrosis. Int. J. Mol. Sci. 2021, 22, 11857. [Google Scholar] [CrossRef]
- Woroniecka, K.I.; Park, A.S.; Mohtat, D.; Thomas, D.B.; Pullman, J.M.; Susztak, K. Transcriptome analysis of human diabetic kidney disease. Diabetes 2011, 60, 2354–2369. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Huang, S.; Park, J.; Park, Y.; Ko, Y.A.; Seasock, M.J.; Bryer, J.S.; Xu, X.X.; Song, W.C.; Palmer, M.; et al. Renal compartment-specific genetic variation analyses identify new pathways in chronic kidney disease. Nat. Med. 2018, 24, 1721–1731. [Google Scholar] [CrossRef] [PubMed]
- Niewczas, M.A.; Pavkov, M.E.; Skupien, J.; Smiles, A.; Md Dom, Z.I.; Wilson, J.M.; Park, J.; Nair, V.; Schlafly, A.; Saulnier, P.J.; et al. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat. Med. 2019, 25, 805–813. [Google Scholar] [CrossRef]
- Tang, S.C.W.; Yiu, W.H. Innate immunity in diabetic kidney disease. Nat. Rev. Nephrol. 2020, 16, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, M.; Zhang, Y. Identification of fibronectin 1 (FN1) and complement component 3 (C3) as immune infiltration-related biomarkers for diabetic nephropathy using integrated bioinformatic analysis. Bioengineered 2021, 12, 5386–5401. [Google Scholar] [CrossRef]
- Jiao, Y.; Jiang, S.; Wang, Y.; Yu, T.; Zou, G.; Zhuo, L.; Li, W. Activation of complement C1q and C3 in glomeruli might accelerate the progression of diabetic nephropathy: Evidence from transcriptomic data and renal histopathology. J. Diabetes Investig. 2022, 13, 839–849. [Google Scholar] [CrossRef]
- Pan, Z.; Yang, Y.; Cao, R.; Qiu, Y.; Li, S.; Zhao, Y.; Chang, S.; Chen, S.; Chen, Z.; Zhang, W.; et al. Identification and Verification of Potential Biomarkers in Renal Ischemia-Reperfusion Injury by Integrated Bioinformatic Analysis. BioMed Res. Int. 2023, 2023, 7629782. [Google Scholar] [CrossRef]
- Wada, T.; Nangaku, M. Novel roles of complement in renal diseases and their therapeutic consequences. Kidney Int. 2013, 84, 441–450. [Google Scholar] [CrossRef]
- Danobeitia, J.S.; Djamali, A.; Fernandez, L.A. The role of complement in the pathogenesis of renal ischemia-reperfusion injury and fibrosis. Fibrogenesis Tissue Repair. 2014, 7, 16. [Google Scholar] [CrossRef]
- Ricklin, D.; Reis, E.S.; Lambris, J.D. Complement in disease: A defence system turning offensive. Nat. Rev. Nephrol. 2016, 12, 383–401. [Google Scholar] [CrossRef]
- Thurman, J.M. Complement and the Kidney: An Overview. Adv. Chronic Kidney Dis. 2020, 27, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Atanes, P.; Ruz-Maldonado, I.; Pingitore, A.; Hawkes, R.; Liu, B.; Zhao, M.; Huang, G.C.; Persaud, S.J.; Amisten, S. C3aR and C5aR1 act as key regulators of human and mouse beta-cell function. Cell. Mol. Life Sci. 2018, 75, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Cui, Z.; Zhao, M.H. The Complement C3a and C3a Receptor Pathway in Kidney Diseases. Front. Immunol. 2020, 11, 1875. [Google Scholar] [CrossRef] [PubMed]
- El-Lati, S.G.; Church, M.K.; Dahinden, C.A. Complement Peptides C3a- and C5a-Induced Mediator Release from Dissociated Human Skin Mast Cells. J. Investig. Dermatol. 1994, 102, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zhou, W.; Sheerin, N.S.; Vaughan, R.W.; Sacks, S.H. Contribution of Renal Secreted Complement C3 to the Circulating Pool in Humans. J. Immunol. 1999, 162, 4336–4341. [Google Scholar] [CrossRef]
- Farrar, C.A.; Zhou, W.; Lin, T.; Sacks, S.H. Local extravascular pool of C3 is a determinant of postischemic acute renal failure. Faseb J. 2006, 20, 217–226. [Google Scholar] [CrossRef]
- Abbate, M.; Zoja, C.; Corna, D.; Rottoli, D.; Zanchi, C.; Azzollini, N.; Tomasoni, S.; Berlingeri, S.; Noris, M.; Morigi, M.; et al. Complement-mediated dysfunction of glomerular filtration barrier accelerates progressive renal injury. J. Am. Soc. Nephrol. 2008, 19, 1158–1167. [Google Scholar] [CrossRef]
- Sheerin, N.S.; Risley, P.; Abe, K.; Tang, Z.; Wong, W.; Lin, T.; Sacks, S.H. Synthesis of complement protein C3 in the kidney is an important mediator of local tissue injury. Faseb J. 2008, 22, 1065–1072. [Google Scholar] [CrossRef]
- Tampe, D.; Hakroush, S.; Tampe, B. Molecular signatures of intrarenal complement receptors C3AR1 and C5AR1 correlate with renal outcome in human lupus nephritis. Lupus Sci. Med. 2022, 9, e000831. [Google Scholar] [CrossRef]
- Østergaard, J.A.; Ruseva, M.M.; Malik, T.H.; Hoffmann-Petersen, I.T.; Pickering, M.C.; Thiel, S.; Hansen, T.K. Increased Autoreactivity of the Complement-Activating Molecule Mannan-Binding Lectin in a Type 1 Diabetes Model. J. Diabetes Res. 2016, 2016, 1825738. [Google Scholar] [CrossRef]
- Axelgaard, E.; Østergaard, J.A.; Thiel, S.; Hansen, T.K. Diabetes Is Associated with Increased Autoreactivity of Mannan-Binding Lectin. J. Diabetes Res. 2017, 2017, 6368780. [Google Scholar] [CrossRef]
- Zheng, J.M.; Ren, X.G.; Jiang, Z.H.; Chen, D.J.; Zhao, W.J.; Li, L.J. Lectin-induced renal local complement activation is involved in tubular interstitial injury in diabetic nephropathy. Clin. Chim. Acta 2018, 482, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Q.; Chang, D.Y.; Chen, M.; Zhao, M.H. Complement activation in patients with diabetic nephropathy. Diabetes Metab. 2019, 45, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.M.; Ziemann, M.; Thallas-Bonke, V.; Snelson, M.; Kumar, V.; Laskowski, A.; Nguyen, T.V.; Huynh, K.; Clarke, M.V.; Libianto, R.; et al. Complement C5a Induces Renal Injury in Diabetic Kidney Disease by Disrupting Mitochondrial Metabolic Agility. Diabetes 2020, 69, 83–98. [Google Scholar] [CrossRef]
- Qin, X.; Goldfine, A.; Krumrei, N.; Grubissich, L.; Acosta, J.; Chorev, M.; Hays, A.P.; Halperin, J.A. Glycation Inactivation of the Complement Regulatory Protein CD59. Diabetes 2004, 53, 8. [Google Scholar] [CrossRef]
- Bus, P.; Chua, J.S.; Klessens, C.Q.F.; Zandbergen, M.; Wolterbeek, R.; van Kooten, C.; Trouw, L.A.; Bruijn, J.A.; Baelde, H.J. Complement Activation in Patients with Diabetic Nephropathy. Kidney Int. Rep. 2018, 3, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.K.; Thiel, S.; Knudsen, S.T.; Gravholt, C.H.; Christiansen, J.S.; Mogensen, C.E.; Poulsen, P.L. Elevated Levels of Mannan-Binding Lectin in Patients with Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2003, 88, 4857–4861. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, L.H.; Eerligh, P.; Terpstra, O.T.; Daha, M.R.; de Knijff, P.; Ballieux, B.E.P.B.; Bruining, G.J.; van der Slik, A.R.; Roos, A.; Roep, B.O. Elevated Levels of Mannose-Binding Lectin at Clinical Manifestation of Type 1 Diabetes in Juveniles. Diabetes 2005, 54, 3002–3006. [Google Scholar] [CrossRef]
- Saraheimo, M.; Forsblom, C.; Hansen, T.K.; Teppo, A.M.; Fagerudd, J.; Pettersson-Fernholm, K.; Thiel, S.; Tarnow, L.; Ebeling, P.; Flyvbjerg, A.; et al. Increased levels of mannan-binding lectin in type 1 diabetic patients with incipient and overt nephropathy. Diabetologia 2005, 48, 198–202. [Google Scholar] [CrossRef]
- Kaunisto, M.A.; Sjolind, L.; Sallinen, R.; Pettersson-Fernholm, K.; Saraheimo, M.; Frojdo, S.; Forsblom, C.; Fagerudd, J.; Hansen, T.K.; Flyvbjerg, A.; et al. Elevated MBL concentrations are not an indication of association between the MBL2 gene and type 1 diabetes or diabetic nephropathy. Diabetes 2009, 58, 1710–1714. [Google Scholar] [CrossRef]
- Guan, L.-Z.; Tong, Q.; Xu, J. Elevated Serum Levels of Mannose-Binding Lectin and Diabetic Nephropathy in Type 2 Diabetes. PLoS ONE 2015, 10, e0119699. [Google Scholar] [CrossRef]
- Østergaard, J.A.; Thiel, S.; Lajer, M.; Steffensen, R.; Parving, H.-H.; Flyvbjerg, A.; Rossing, P.; Tarnow, L.; Hansen, T.K. Increased All-Cause Mortality in Patients with Type 1 Diabetes and High-Expression Mannan-Binding Lectin Genotypes: A 12-Year Follow-up Study. Diabetes Care 2015, 38, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, J.A.; Thiel, S.; Hoffmann-Petersen, I.T.; Hovind, P.; Parving, H.H.; Tarnow, L.; Rossing, P.; Hansen, T.K. Incident microalbuminuria and complement factor mannan-binding lectin-associated protein 19 in people with newly diagnosed type 1 diabetes. Diabetes Metab. Res. Rev. 2017, 33. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Liu, F.; Yang, H.; Zhong, Y.; Wang, Y.; Li, S.; Su, Q.; Tang, L.; Bai, L.; et al. Urinary complement proteins and risk of end-stage renal disease: Quantitative urinary proteomics in patients with type 2 diabetes and biopsy-proven diabetic nephropathy. J. Endocrinol. Invest. 2021, 44, 2709–2723. [Google Scholar] [CrossRef]
- Li, M.R.; Sun, Z.J.; Chang, D.Y.; Yu, X.J.; Wang, S.X.; Chen, M.; Zhao, M.H. C3c deposition predicts worse renal outcomes in patients with biopsy-proven diabetic kidney disease in type 2 diabetes mellitus. J. Diabetes 2022, 14, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Wendt, R.; Siwy, J.; He, T.; Latosinska, A.; Wiech, T.; Zipfel, P.F.; Tserga, A.; Vlahou, A.; Rupprecht, H.; Catanese, L.; et al. Molecular Mapping of Urinary Complement Peptides in Kidney Diseases. Proteomes 2021, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Qamar, A.; Liu, H. The complement system testing in clinical laboratory. Clin. Chim. Acta 2023, 541, 117238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhuang, M.; Ma, A.; Wang, G.; Cheng, P.; Yang, Y.; Wang, X.; Zhang, J.; Chen, X.; Lu, M. Association of levels of mannose-binding lectin and the MBL2 gene with type 2 diabetes and diabetic nephropathy. PLoS ONE 2013, 8, e83059. [Google Scholar] [CrossRef]
- Sircar, M.; Rosales, I.A.; Selig, M.K.; Xu, D.; Zsengeller, Z.K.; Stillman, I.E.; Libermann, T.A.; Karumanchi, S.A.; Thadhani, R.I. Complement 7 Is Up-Regulated in Human Early Diabetic Kidney Disease. Am. J. Pathol. 2018, 188, 2147–2154. [Google Scholar] [CrossRef]
- Jiang, S.; Di, D.; Jiao, Y.; Zou, G.; Gao, H.; Li, W. Complement Deposition Predicts Worsening Kidney Function and Underlines the Clinical Significance of the 2010 Renal Pathology Society Classification of Diabetic Nephropathy. Front. Immunol. 2022, 13, 868127. [Google Scholar] [CrossRef]
- Biancone, L.; David, S.; Pietra, V.D.; Montrucchio, G.; Cambi, V.; Camussi, G. Alternative pathway activation of complement by cultured human proximal tubular epithelial cells. Kidney Int. 1994, 45, 451–460. [Google Scholar] [CrossRef]
- Ichida, S.; Yuzawa, Y.; Okada, H.; Yoshioka, K.; Matsuo, S. Localization of the complement regulatory proteins in the normal human kidney. Kidney Int. 1994, 46, 89–96. [Google Scholar] [CrossRef]
- Laursen, N.S.; Magnani, F.; Gottfredsen, R.H.; Petersen, S.V.; Andersen, G.R. Structure, Function and Control of Complement C5 and its Proteolytic Fragments. Curr. Mol. Med. 2012, 12, 1083–1097. [Google Scholar] [CrossRef]
- Li, X.X.; Lee, J.D.; Kemper, C.; Woodruff, T.M. The Complement Receptor C5aR2: A Powerful Modulator of Innate and Adaptive Immunity. J. Immunol. 2019, 202, 3339–3348. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; George, S.R.; Cheng, R.; Nguyen, T.; Liu, Y.; Brown, M.; Lynch, K.R.; O’Dowd, B.F. Identification of four novel human G protein-coupled receptors expressed in the brain. Brain Res. Mol. Brain Res. 2001, 86, 13–22. [Google Scholar] [CrossRef]
- Mery, L.; Boulay, F. Evidence that the extracellular N-terminal domain of C5aR contains amino-acid residues crucial for C5a binding. Eur. J. Haematol. 1993, 51, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Okinaga, S.; Slattery, D.; Humbles, A.; Zsengeller, Z.; Morteau, O.; Kinrade, M.B.; Brodbeck, R.M.; Krause, J.E.; Choe, H.; Gerard, N.P.; et al. C5L2, a Nonsignaling C5A Binding Protein. Biochemistry 2003, 42, 9406–9415. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Neff, T.A.; Guo, R.; Speyer, C.L.; Sarma, J.V.; Tomlins, S.; Man, Y.; Riedemann, N.C.; Hoesel, L.M.; Younkin, E.; et al. Evidence for a functional role of the second C5a receptor C5L2. FASEB J. 2005, 19, 1003-–1005. [Google Scholar] [CrossRef]
- Bamberg, C.E.; Mackay, C.R.; Lee, H.; Zahra, D.; Jackson, J.; Lim, Y.S.; Whitfeld, P.L.; Craig, S.; Corsini, E.; Lu, B.; et al. The C5a receptor (C5aR) C5L2 is a modulator of C5aR-mediated signal transduction. J. Biol. Chem. 2010, 285, 7633–7644. [Google Scholar] [CrossRef]
- Zhang, T.; Garstka, M.A.; Li, K. The Controversial C5a Receptor C5aR2: Its Role in Health and Disease. J. Immunol. Res. 2017, 2017, 8193932. [Google Scholar] [CrossRef]
- Gerard, N.P.; Lu, B.; Liu, P.; Craig, S.; Fujiwara, Y.; Okinaga, S.; Gerard, C. An anti-inflammatory function for the complement anaphylatoxin C5a-binding protein, C5L2. J. Biol. Chem. 2005, 280, 39677–39680. [Google Scholar] [CrossRef]
- Rabiet, M.J.; Huet, E.; Boulay, F. Complement component 5a receptor oligomerization and homologous receptor down-regulation. J. Biol. Chem. 2008, 283, 31038–31046. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.K.; Xavier, S.; Chauss, D.; Wang, L.; Chew, C.; Taylor, R.; Stallcup, W.B.; Ma, J.Z.; Kazemian, M.; Afzali, B.; et al. Folic acid-mediated fibrosis is driven by C5a receptor 1-mediated activation of kidney myeloid cells. Am. J. Physiol. Ren. Physiol. 2022, 322, F597–F610. [Google Scholar] [CrossRef]
- Abe, K.; Miyazaki, M.; Koji, T.; Furusu, A.; Nakamura-Kurashige, T.; Nishino, T.; Ozono, Y.; Harada, T.; Sakai, H.; Kohno, S. Enhanced expression of complement C5a receptor mRNA in human diseased kidney assessed by in situ hybridization. Kidney Int. 2001, 60, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Arias-Cabrales, C.; Rodriguez-Garcia, E.; Gimeno, J.; Benito, D.; Pérez-Sáez, M.J.; Redondo-Pachón, D.; Buxeda, A.; Burballa, C.; Crespo, M.; Riera, M.; et al. Role of C5aR1 and C5L2 Receptors in Ischemia-Reperfusion Injury. J. Clin. Med. 2021, 10, 974. [Google Scholar] [CrossRef]
- van Werkhoven, M.B.; Damman, J.; Daha, M.R.; Krikke, C.; van Goor, H.; van Son, W.J.; Hillebrands, J.L.; van Dijk, M.C.; Seelen, M.A. Novel insights in localization and expression levels of C5aR and C5L2 under native and post-transplant conditions in the kidney. Mol. Immunol. 2013, 53, 237–245. [Google Scholar] [CrossRef]
- Weiss, S.; Rosendahl, A.; Czesla, D.; Meyer-Schwesinger, C.; Stahl, R.A.; Ehmke, H.; Kurts, C.; Zipfel, P.F.; Kohl, J.; Wenzel, U.O. The complement receptor C5aR1 contributes to renal damage but protects the heart in angiotensin II-induced hypertension. Am. J. Physiol. Ren. Physiol. 2016, 310, F1356–F1365. [Google Scholar] [CrossRef]
- de Vries, B.; Köhl, J.; Leclercq, W.K.; Wolfs, T.G.; van Bijnen, A.A.; Heeringa, P.; Buurman, W.A. Complement factor C5a mediates renal ischemia-reperfusion injury independent from neutrophils. J. Immunol. 2003, 170, 3883–3889. [Google Scholar] [CrossRef]
- Peng, Q.; Wu, W.; Wu, K.Y.; Cao, B.; Qiang, C.; Li, K.; Sacks, S.H.; Zhou, W. The C5a/C5aR1 axis promotes progression of renal tubulointerstitial fibrosis in a mouse model of renal ischemia/reperfusion injury. Kidney Int. 2019, 96, 117–128. [Google Scholar] [CrossRef]
- Peng, Q.; Li, K.; Smyth, L.A.; Xing, G.; Wang, N.; Meader, L.; Lu, B.; Sacks, S.H.; Zhou, W. C3a and C5a promote renal ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2012, 23, 1474–1485. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wu, K.Y.; Wu, W.; Wang, N.; Zhang, T.; Choudhry, N.; Song, Y.; Farrar, C.A.; Ma, L.; Wei, L.L.; et al. C5aR1 promotes acute pyelonephritis induced by uropathogenic E. coli. JCI Insight 2017, 2, e97626. [Google Scholar] [CrossRef]
- Choudhry, N.; Li, K.; Zhang, T.; Wu, K.Y.; Song, Y.; Farrar, C.A.; Wang, N.; Liu, C.F.; Peng, Q.; Wu, W.; et al. The complement factor 5a receptor 1 has a pathogenic role in chronic inflammation and renal fibrosis in a murine model of chronic pyelonephritis. Kidney Int. 2016, 90, 540–554. [Google Scholar] [CrossRef] [PubMed]
- Phieler, J.; Chung, K.J.; Chatzigeorgiou, A.; Klotzsche-von Ameln, A.; Garcia-Martin, R.; Sprott, D.; Moisidou, M.; Tzanavari, T.; Ludwig, B.; Baraban, E.; et al. The complement anaphylatoxin C5a receptor contributes to obese adipose tissue inflammation and insulin resistance. J. Immunol. 2013, 191, 4367–4374. [Google Scholar] [CrossRef]
- Tsai, I.J.; Lin, W.C.; Yang, Y.H.; Tseng, Y.L.; Lin, Y.H.; Chou, C.H.; Tsau, Y.K. High Concentration of C5a-Induced Mitochondria-Dependent Apoptosis in Murine Kidney Endothelial Cells. Int. J. Mol. Sci. 2019, 20, 4465. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lv, D.; Jiang, S.; Hou, Q.; Zhang, L.; Li, S.; Zhu, X.; Xu, X.; Wen, J.; Zeng, C.; et al. Complement induces podocyte pyroptosis in membranous nephropathy by mediating mitochondrial dysfunction. Cell. Death Dis. 2022, 13, 281. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wei, T.; Liu, S.; Wang, C.; Zhao, M.; Feng, Y.; Ma, L.; Lu, Y.; Fu, P.; Liu, J. Complement C5 activation promotes type 2 diabetic kidney disease via activating STAT3 pathway and disrupting the gut-kidney axis. J. Cell. Mol. Med. 2021, 25, 960–974. [Google Scholar] [CrossRef]
- Yiu, W.H.; Li, R.X.; Wong, D.W.L.; Wu, H.J.; Chan, K.W.; Chan, L.Y.Y.; Leung, J.C.K.; Lai, K.N.; Sacks, S.H.; Zhou, W.; et al. Complement C5a inhibition moderates lipid metabolism and reduces tubulointerstitial fibrosis in diabetic nephropathy. Nephrol. Dial. Transplant. 2017, 33, 1323–1332. [Google Scholar] [CrossRef]
- Castellano, G.; Franzin, R.; Sallustio, F.; Stasi, A.; Banelli, B.; Romani, M.; De Palma, G.; Lucarelli, G.; Divella, C.; Battaglia, M.; et al. Complement component C5a induces aberrant epigenetic modifications in renal tubular epithelial cells accelerating senescence by Wnt4/βcatenin signaling after ischemia/reperfusion injury. Aging 2019, 11, 4382–4406. [Google Scholar] [CrossRef]
- Coughlan, M.T.; Ziemann, M.; Laskowski, A.; Woodruff, T.M.; Tan, S.M. Valproic acid attenuates cellular senescence in diabetic kidney disease through the inhibition of complement C5a receptors. Nat. Sci. Rep. 2022, 12, 20278. [Google Scholar] [CrossRef]
- Xiao, L.; Zhou, D.; Tan, R.J.; Fu, H.; Zhou, L.; Hou, F.F.; Liu, Y. Sustained Activation of Wnt/β-Catenin Signaling Drives AKI to CKD Progression. J. Am. Soc. Nephrol. 2016, 27, 1727–1740. [Google Scholar]
- Schunk, S.J.; Floege, J.; Fliser, D.; Speer, T. WNT–β-catenin signalling—A versatile player in kidney injury and repair. Nat. Rev. Nephrol. 2021, 17, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Hirata, T.; Enomoto, M.; Araki, T.; Ishimaru, H.; Takahashi, T.A. A putative chemoattractant receptor, C5L2, is expressed in granulocyte and immature dendritic cells, but not in mature dendritic cells. Mol. Immunol. 2000, 37, 407–412. [Google Scholar] [CrossRef]
- Scola, A.M.; Johswich, K.O.; Morgan, B.P.; Klos, A.; Monk, P.N. The human complement fragment receptor, C5L2, is a recycling decoy receptor. Mol. Immunol. 2009, 46, 1149–1162. [Google Scholar] [CrossRef]
- Zhang, X.; Schmudde, I.; Laumonnier, Y.; Pandey, M.K.; Clark, J.R.; Konig, P.; Gerard, N.P.; Gerard, C.; Wills-Karp, M.; Kohl, J. A critical role for C5L2 in the pathogenesis of experimental allergic asthma. J. Immunol. 2010, 185, 6741–6752. [Google Scholar] [CrossRef]
- Wang, R.; Lu, B.; Gerard, C.; Gerard, N.P. Disruption of the complement anaphylatoxin receptor C5L2 exacerbates inflammation in allergic contact dermatitis. J. Immunol. 2013, 191, 4001–4009. [Google Scholar] [CrossRef]
- Wang, R.; Lu, B.; Gerard, C.; Gerard, N.P. C5L2, the Second C5a Anaphylatoxin Receptor, Suppresses LPS-Induced Acute Lung Injury. Am. J. Respir. Cell. Mol. Biol. 2016, 55, 657–666. [Google Scholar] [CrossRef]
- Kovtun, A.; Bergdolt, S.; Hagele, Y.; Matthes, R.; Lambris, J.D.; Huber-Lang, M.; Ignatius, A. Complement receptors C5aR1 and C5aR2 act differentially during the early immune response after bone fracture but are similarly involved in bone repair. Sci. Rep. 2017, 7, 14061. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.C.L.; Lee, J.D.; Ruitenberg, M.J.; Woodruff, T.M. Absence of the C5a Receptor C5aR2 Worsens Ischemic Tissue Injury by Increasing C5aR1-Mediated Neutrophil Infiltration. J. Immunol. 2020, 205, 2834–2839. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.J.; Mirtsos, C.; Suh, D.; Lu, Y.C.; Lin, W.J.; McKerlie, C.; Lee, T.; Baribault, H.; Tian, H.; Yeh, W.C. C5L2 is critical for the biological activities of the anaphylatoxins C5a and C3a. Nature 2007, 446, 203–207. [Google Scholar] [CrossRef]
- Horst, S.A.; Itzek, A.; Klos, A.; Beineke, A.; Medina, E. Differential Contributions of the Complement Anaphylotoxin Receptors C5aR1 and C5aR2 to the Early Innate Immune Response against Staphylococcus aureus Infection. Pathogens 2015, 4, 722–738. [Google Scholar] [CrossRef] [PubMed]
- Pundir, P.; MacDonald, C.A.; Kulka, M. The Novel Receptor C5aR2 Is Required for C5a-Mediated Human Mast Cell Adhesion, Migration, and Proinflammatory Mediator Production. J. Immunol. 2015, 195, 2774–2787. [Google Scholar] [CrossRef]
- Poppelaars, F.; van Werkhoven, M.B.; Kotimaa, J.; Veldhuis, Z.J.; Ausema, A.; Broeren, S.G.M.; Damman, J.; Hempel, J.C.; Leuvenink, H.G.D.; Daha, M.R.; et al. Critical role for complement receptor C5aR2 in the pathogenesis of renal ischemia-reperfusion injury. FASEB J. 2017, 31, 3193–3204. [Google Scholar] [CrossRef] [PubMed]
- Thorenz, A.; Derlin, K.; Schroder, C.; Dressler, L.; Vijayan, V.; Pradhan, P.; Immenschuh, S.; Jorns, A.; Echtermeyer, F.; Herzog, C.; et al. Enhanced activation of interleukin-10, heme oxygenase-1, and AKT in C5aR2-deficient mice is associated with protection from ischemia reperfusion injury-induced inflammation and fibrosis. Kidney Int. 2018, 94, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, D.; Huang, L.; Zhang, Y.; Luo, R.; Adah, D.; Tang, Y.; Zhao, K.; Lu, B. The complement receptor C5aR2 promotes protein kinase R expression and contributes to NLRP3 inflammasome activation and HMGB1 release from macrophages. J. Biol. Chem. 2019, 294, 8384–8394. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Coulthard, L.G.; Wu, M.C.; Taylor, S.M.; Woodruff, T.M. C5L2: A controversial receptor of complement anaphylatoxin, C5a. FASEB J. 2013, 27, 855–864. [Google Scholar] [CrossRef]
- Coulthard, L.G.; Hawksworth, O.A.; Woodruff, T.M. C5aR2. In The Complement FactsBook, 2nd ed.; Barnum, S., Schein, T., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 407–414. [Google Scholar]
- Lee, H.; Whitfeld, P.L.; Mackay, C.R. Receptors for complement C5a. The importance of C5aR and the enigmatic role of C5L2. Immunol. Cell. Biol. 2008, 86, 153–160. [Google Scholar] [CrossRef]
- Audet, M.; Bouvier, M. Restructuring G-Protein- Coupled Receptor Activation. Cell 2012, 151, 14–23. [Google Scholar] [CrossRef]
- Schönegge, A.-M.; Gallion, J.; Picard, L.-P.; Wilkins, A.D.; Le Gouill, C.; Audet, M.; Stallaert, W.; Lohse, M.J.; Kimmel, M.; Lichtarge, O.; et al. Evolutionary action and structural basis of the allosteric switch controlling β2AR functional selectivity. Nat. Commun. 2017, 8, 2169. [Google Scholar] [CrossRef]
- Graham, G.J. D6 and the atypical chemokine receptor family: Novel regulators of immune and inflammatory processes. Eur. J. Immunol. 2009, 39, 342–351. [Google Scholar] [CrossRef]
- Torphy, R.J.; Yee, E.J.; Schulick, R.D.; Zhu, Y. Atypical chemokine receptors: Emerging therapeutic targets in cancer. Trends Pharmacol. Sci. 2022, 43, 1085–1097. [Google Scholar] [CrossRef]
- Kalant, D.; MacLaren, R.; Cui, W.; Samanta, R.; Monk, P.N.; Laporte, S.A.; Cianflone, K. C5L2 is a functional receptor for acylation-stimulating protein. J. Biol. Chem. 2005, 280, 23936–23944. [Google Scholar] [CrossRef] [PubMed]
- Croker, D.E.; Halai, R.; Kaeslin, G.; Wende, E.; Fehlhaber, B.; Klos, A.; Monk, P.N.; Cooper, M.A. C5a2 can modulate ERK1/2 signaling in macrophages via heteromer formation with C5a1 and beta-arrestin recruitment. Immunol. Cell. Biol. 2014, 92, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.C.; Yang, F.C.; Lin, C.H.; Hsieh, S.L.; Chen, N.J. C5L2 is required for C5a-triggered receptor internalization and ERK signaling. Cell. Signal. 2014, 26, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Wang, C.; Yuan, J.; Chen, M.; Zhao, M.H. A pro-inflammatory role of C5L2 in C5a-primed neutrophils for ANCA-induced activation. PLoS ONE 2013, 8, e66305. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Clark, R.J.; Woodruff, T.M. C5aR2 Activation Broadly Modulates the Signaling and Function of Primary Human Macrophages. J. Immunol. 2020, 205, 1102–1112. [Google Scholar] [CrossRef]
- Croker, D.E.; Halai, R.; Fairlie, D.P.; Cooper, M.A. C5a, but not C5a-des Arg, induces upregulation of heteromer formation between complement C5a receptors C5aR and C5L2. Immunol. Cell. Biol. 2013, 91, 625–633. [Google Scholar] [CrossRef]
- Poursharifi, P.; Lapointe, M.; Petrin, D.; Devost, D.; Gauvreau, D.; Hebert, T.E.; Cianflone, K. C5L2 and C5aR interaction in adipocytes and macrophages: Insights into adipoimmunology. Cell. Signal. 2013, 25, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Poursharifi, P.; Lapointe, M.; Fisette, A.; Lu, H.; Roy, C.; Munkonda, M.N.; Fairlie, D.P.; Cianflone, K. C5aR and C5L2 act in concert to balance immunometabolism in adipose tissue. Mol. Cell. Endocrinol. 2014, 382, 325–333. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, K.Y.; Ma, N.; Wei, L.L.; Garstka, M.; Zhou, W.; Li, K. The C5a/C5aR2 axis promotes renal inflammation and tissue damage. JCI Insight 2020, 5, e134081. [Google Scholar] [CrossRef]
- Arumugam, T.V.; Magnus, T.; Woodruff, T.M.; Proctor, L.M.; Shiels, I.A.; Taylor, S.M. Complement mediators in ischemia-reperfusion injury. Clin. Chim. Acta 2006, 374, 33–45. [Google Scholar] [CrossRef]
- Gorsuch, W.B.; Chrysanthou, E.; Schwaeble, W.J.; Stahl, G.L. The complement system in ischemia-reperfusion injuries. Immunobiology 2012, 217, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Castellano, G.; Franzin, R.; Stasi, A.; Divella, C.; Sallustio, F.; Pontrelli, P.; Lucarelli, G.; Battaglia, M.; Staffieri, F.; Crovace, A.; et al. Complement Activation During Ischemia/Reperfusion Injury Induces Pericyte-to-Myofibroblast Transdifferentiation Regulating Peritubular Capillary Lumen Reduction Through pERK Signaling. Front. Immunol. 2018, 9, 1002. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wei, Q.; Liu, J.; Yi, M.; Liu, Y.; Liu, H.; Sun, L.; Peng, Y.; Liu, F.; Venkatachalam, M.A.; et al. AKI on CKD: Heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int. 2017, 92, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Infante, B.; Conserva, F.; Pontrelli, P.; Leo, S.; Stasi, A.; Fiorentino, M.; Troise, D.; dello Strologo, A.; Alfieri, C.; Gesualdo, L. Recent advances in molecular mechanisms of acute kidney injury in patients with diabetes mellitus. Front. Endocrinol. 2022, 13, 903970. [Google Scholar] [CrossRef] [PubMed]
- Marcil, M.; Vu, H.; Cui, W.; Dastani, Z.; Engert, J.C.; Gaudet, D.; Castro-Cabezas, M.; Sniderman, A.D.; Genest, J., Jr.; Cianflone, K. Identification of a novel C5L2 variant (S323I) in a French Canadian family with familial combined hyperlipemia. Arter. Arterioscler.Thromb. Vasc. Biol. 2006, 26, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Simaan, M.; Laporte, S.; Lodge, R.; Cianflone, K. C5a- and ASP-mediated C5L2 activation, endocytosis and recycling are lost in S323I-C5L2 mutation. Mol. Immunol. 2009, 46, 3086–3098. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Y.; Xie, X.; Ma, Y.T.; Yang, Y.N.; Fu, Z.Y.; Li, X.M.; Ma, X.; Chen, B.D.; Liu, F. Relationship between a novel polymorphism of the C5L2 gene and coronary artery disease. PLoS ONE 2011, 6, e20984. [Google Scholar] [CrossRef]
- Zheng, Y.-Y.; Xie, X.; Ma, Y.-T.; Fu, Z.-Y.; Ma, X.; Yang, Y.-N.; Li, X.-M.; Pan, S.; Adi, D.; Chen, B.-D.; et al. Association of C5L2 genetic polymorphisms with coronary artery disease in a Han population in Xinjiang, China. Oncotarget 2017, 8, 8590–8596. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Xie, X.; Ma, Y.T.; Yang, Y.N.; Fu, Z.Y.; Li, X.M.; Ma, X.; Chen, B.D.; Liu, F. Relationship between type 2 diabetes mellitus and a novel polymorphism C698T in C5L2 in the Chinese Han population. Endocrine 2012, 41, 296–301. [Google Scholar] [CrossRef]
- Alharbi, K.K.; Khan, I.A.; Syed, R. Circulating C5L2 gene polymorphism is associated with type 2 diabetes mellitus in Saudi population. Mol. Biol. Rep. 2013, 40, 6323–6327. [Google Scholar] [CrossRef]
- Vijayan, S.; Asare, Y.; Grommes, J.; Soehnlein, O.; Lutgens, E.; Shagdarsuren, G.; Togtokh, A.; Jacobs, M.J.; Fischer, J.W.; Bernhagen, J.; et al. High expression of C5L2 correlates with high proinflammatory cytokine expression in advanced human atherosclerotic plaques. Am. J. Pathol. 2014, 184, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.O.; Ritter, R.; Abel, K.J.; Manning, A.; Panhuysen, C.; Farrer, L.A. Complement Factor H Polymorphism and Age-Related Macular Degeneration. Science 2005, 308, 421–424. [Google Scholar] [CrossRef]
- Zareparsi, S.; Branham, K.E.H.; Li, M.; Shah, S.; Klein, R.J.; Ott, J.; Hoh, J.; Abecasis, G.R.; Swaroop, A. Strong Association of the Y402H Variant in Complement Factor H at 1q32 with Susceptibility to Age-Related Macular Degeneration. Am. J. Hum. Human. Genet. 2005, 77, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.L.; Pouw, R.B.; Kavanagh, D.; Sun, R.; Ricklin, D. Developments in anti-complement therapy; from disease to clinical trial. Mol. Immunol. 2018, 102, 89–119. [Google Scholar] [CrossRef]
- Hillmen, P.; Young, N.S.; Schubert, J.; Brodsky, R.A.; Socié, G.; Muus, P.; Röth, A.; Szer, J.; Elebute, M.O.; Nakamura, R.; et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N. Engl. J. Med. 2006, 355, 1233–1243. [Google Scholar] [CrossRef]
- Rother, R.P.; Rollins, S.A.; Mojcik, C.F.; Brodsky, R.A.; Bell, L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat. Biotechnol. 2007, 25, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Ricklin, D.; Mastellos, D.C.; Reis, E.S.; Lambris, J.D. The renaissance of complement therapeutics. Nat. Rev. Nephrol. 2018, 14, 26–47. [Google Scholar] [CrossRef] [PubMed]
- Le Quintrec, M.; Lionet, A.; Kandel, C.; Bourdon, F.; Gnemmi, V.; Colombat, M.; Goujon, J.-M.; Frémeaux-Bacchi, V.; Fakhouri, F. Eculizumab for Treatment of Rapidly Progressive C3 Glomerulopathy. Am. J. Kidney Dis. 2015, 65, 484–489. [Google Scholar] [CrossRef]
- Zipfel, P.F.; Wiech, T.; Rudnick, R.; Afonso, S.; Person, F.; Skerka, C. Complement Inhibitors in Clinical Trials for Glomerular Diseases. Front. Immunol. 2019, 10, 2166. [Google Scholar] [CrossRef]
- Rosenblad, T.; Rebetz, J.; Johansson, M.; Békássy, Z.; Sartz, L.; Karpman, D. Eculizumab treatment for rescue of renal function in IgA nephropathy. Pediatr. Nephrol. 2014, 29, 2225–2228. [Google Scholar] [CrossRef]
- Lonze, B.E.; Zachary, A.A.; Magro, C.M.; Desai, N.M.; Orandi, B.J.; Dagher, N.N.; Singer, A.L.; Carter-Monroe, N.; Nazarian, S.M.; Segev, D.L.; et al. Eculizumab Prevents Recurrent Antiphospholipid Antibody Syndrome and Enables Successful Renal Transplantation. Am. J. Transplant. 2014, 14, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Siedlecki, A.M.; Isbel, N.; Vande Walle, J.; James Eggleston, J.; Cohen, D.J.; Licht, C.; Frémeaux-Bacchi, V.; Ariceta, G.; Ardissino, G.; Fakhouri, F.; et al. Eculizumab Use for Kidney Transplantation in Patients with a Diagnosis of Atypical Hemolytic Uremic Syndrome. Kidney Int. Rep. 2019, 4, 434–446. [Google Scholar] [CrossRef]
- Tan, E.K.; Bentall, A.; Dean, P.G.; Shaheen, M.F.; Stegall, M.D.; Schinstock, C.A. Use of Eculizumab for Active Antibody-mediated Rejection That Occurs Early Post-kidney Transplantation: A Consecutive Series of 15 Cases. Transplantation 2019, 103, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Baldwin, W.M., 3rd; Jordan, S.C. Potential Roles for C1 Inhibitor in Transplantation. Transplantation 2016, 100, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Cernoch, M.; Viklicky, O. Complement in Kidney Transplantation. Front. Med. 2017, 4, 66. [Google Scholar] [CrossRef]
- Gonzalez Suarez, M.L.; Thongprayoon, C.; Mao, M.A.; Leeaphorn, N.; Bathini, T.; Cheungpasitporn, W. Outcomes of Kidney Transplant Patients with Atypical Hemolytic Uremic Syndrome Treated with Eculizumab: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 919. [Google Scholar] [CrossRef]
- Qi, R.; Qin, W. Role of Complement System in Kidney Transplantation: Stepping from Animal Models to Clinical Application. Front. Immunol. 2022, 13, 811696. [Google Scholar] [CrossRef]
- Grossman, T.R.; Hettrick, L.A.; Johnson, R.B.; Hung, G.; Peralta, R.; Watt, A.; Henry, S.P.; Adamson, P.; Monia, B.P.; McCaleb, M.L. Inhibition of the alternative complement pathway by antisense oligonucleotides targeting complement factor B improves lupus nephritis in mice. Immunobiology 2016, 221, 701–708. [Google Scholar] [CrossRef]
- Shi, Y.; Yao, W.; Sun, L.; Li, G.; Liu, H.; Ding, P.; Hu, W.; Xu, H. The new complement inhibitor CRIg/FH ameliorates lupus nephritis in lupus-prone MRL/lpr mice. BMC Nephrol. 2019, 20, 424. [Google Scholar] [CrossRef]
- Tortajada, A.; Gutierrez, E.; Pickering, M.C.; Praga Terente, M.; Medjeral-Thomas, N. The role of complement in IgA nephropathy. Mol. Immunol. 2019, 114, 123–132. [Google Scholar] [CrossRef]
- Carrara, C.; Podestà, M.A.; Abbate, M.; Rizzo, P.; Piras, R.; Alberti, M.; Daina, E.; Ruggenenti, P.; Remuzzi, G. Morphofunctional Effects of C5 Convertase Blockade in Immune Complex-Mediated Membranoproliferative Glomerulonephritis: Report of Two Cases with Evidence of Terminal Complement Activation. Nephron 2020, 144, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Nester, C.; Appel, G.B.; Bomback, A.S.; Bouman, K.P.; Cook, H.T.; Daina, E.; Dixon, B.P.; Rice, K.; Najafian, N.; Hui, J.; et al. Clinical Outcomes of Patients with C3G or IC-MPGN Treated with the Factor D Inhibitor Danicopan: Final Results from Two Phase 2 Studies. Am. J. Nephrol. 2022, 53, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shao, D.; Ricklin, D.; Hilkin, B.M.; Nester, C.M.; Lambris, J.D.; Smith, R.J.H. Compstatin analog Cp40 inhibits complement dysregulation in vitro in C3 glomerulopathy. Immunobiology 2015, 220, 993–998. [Google Scholar] [CrossRef]
- Ruseva, M.M.; Peng, T.; Lasaro, M.A.; Bouchard, K.; Liu-Chen, S.; Sun, F.; Yu, Z.-X.; Marozsan, A.; Wang, Y.; Pickering, M.C. Efficacy of Targeted Complement Inhibition in Experimental C3 Glomerulopathy. J. Am. Soc. Nephrol. JASN 2016, 27, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Ricklin, D.; Lambris, J.D. Complement-targeted therapeutics. Nat. Biotechnol. 2007, 25, 1265–1275. [Google Scholar] [CrossRef]
- Cullinan, N.; Gorman, K.M.; Riordan, M.; Waldron, M.; Goodship, T.H.; Awan, A. Case report: Benefits and challenges of long-term eculizumab in atypical hemolytic uremic syndrome. Pediatrics 2015, 135, e1506–e1509. [Google Scholar] [CrossRef]
- Socié, G.; Caby-Tosi, M.-P.; Marantz, J.L.; Cole, A.; Bedrosian, C.L.; Gasteyger, C.; Mujeebuddin, A.; Hillmen, P.; Vande Walle, J.; Haller, H. Eculizumab in paroxysmal nocturnal haemoglobinuria and atypical haemolytic uraemic syndrome: 10-year pharmacovigilance analysis. Br. J. Haematol. 2019, 185, 297–310. [Google Scholar] [CrossRef]
- Kulasekararaj, A.G.; Hill, A.; Rottinghaus, S.T.; Langemeijer, S.; Wells, R.; Gonzalez-Fernandez, F.A.; Gaya, A.; Lee, J.W.; Gutierrez, E.O.; Piatek, C.I.; et al. Ravulizumab (ALXN1210) vs eculizumab in C5-inhibitor–experienced adult patients with PNH: The 302 study. Blood 2019, 133, 540–549. [Google Scholar] [CrossRef]
- Stern, R.M.; Connell, N.T. Ravulizumab: A novel C5 inhibitor for the treatment of paroxysmal nocturnal hemoglobinuria. Ther. Adv. Hematol. 2019, 10, 2040620719874728. [Google Scholar] [CrossRef]
- Liu, H.; Xia, L.; Weng, J.; Zhang, F.; He, C.; Gao, S.; Jia, J.; Chang, A.C.; Lundberg, P.; Sima, C.S.; et al. Results from the First Phase 3 Crovalimab (C5 Inhibitor) Study (COMMODORE 3): Efficacy and Safety in Complement Inhibitor-Naive Patients with Paroxysmal Nocturnal Hemoglobinuria (PNH). Blood 2022, 140, 714–716. [Google Scholar] [CrossRef]
- Köhl, J. Igniting the flame in arthritis- C5aR2 controls endothelial transcytosis of C5a. Sci. Immunol. 2019, 4, eaax0352. [Google Scholar] [CrossRef] [PubMed]
- Vergunst, C.E.; Gerlag, D.M.; Dinant, H.; Schulz, L.; Vinkenoog, M.; Smeets, T.J.M.; Sanders, M.E.; Reedquist, K.A.; Tak, P.P. Blocking the receptor for C5a in patients with rheumatoid arthritis does not reduce synovial inflammation. Rheumatology 2007, 46, 1773–1778. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Lee, J.D.; Clark, R.J.; Woodruff, T.M. Development and validation of a LC-MS/MS assay for pharmacokinetic studies of complement C5a receptor antagonists PMX53 and PMX205 in mice. Sci. Rep. 2018, 8, 8101. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Lee, J.D.; Clark, R.J.; Noakes, P.G.; Taylor, S.M.; Woodruff, T.M. Preclinical Pharmacokinetics of Complement C5a Receptor Antagonists PMX53 and PMX205 in Mice. ACS Omega 2020, 5, 2345–2354. [Google Scholar] [CrossRef]
- Köhl, J. Drug evaluation: The C5a receptor antagonist PMX-53. Curr. Opin. Mol. Ther. 2006, 8, 529–538. [Google Scholar] [PubMed]
- Australian New Zealand Clinical Trials Registry. Trial Review ACTRN12619001639112. Available online: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=378702 (accessed on 3 April 2023).
- Xu, W.; Kumar, V.; Cui, C.S.; Li, X.X.; Whittaker, A.K.; Xu, Z.P.; Smith, M.T.; Woodruff, T.M.; Han, F.Y. Success in Navigating Hurdles to Oral Delivery of a Bioactive Peptide Complement Antagonist through Use of Nanoparticles to Increase Bioavailability and In Vivo Efficacy. Adv. Ther. 2022, 5, 2200109. [Google Scholar] [CrossRef]
- Schnatbaum, K.; Locardi, E.; Scharn, D.; Richter, U.; Hawlisch, H.; Knolle, J.; Polakowski, T. Peptidomimetic C5a receptor antagonists with hydrophobic substitutions at the C-terminus: Increased receptor specificity and in vivo activity. Bioorg Med. Chem. Lett. 2006, 16, 5088–5092. [Google Scholar] [CrossRef]
- Boor, P.; Konieczny, A.; Villa, L.; Schult, A.L.; Bucher, E.; Rong, S.; Kunter, U.; van Roeyen, C.R.; Polakowski, T.; Hawlisch, H.; et al. Complement C5 mediates experimental tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 2007, 18, 1508–1515. [Google Scholar] [CrossRef]
- Pennington, M.W.; Zell, B.; Bai, C.J. Commercial manufacturing of current good manufacturing practice peptides spanning the gamut from neoantigen to commercial large-scale products. Med. Drug. Discov. 2021, 9, 100071. [Google Scholar] [CrossRef]
- Bruchfeld, A.; Magin, H.; Nachman, P.; Parikh, S.; Lafayette, R.; Potarca, A.; Miao, S.; Bekker, P. C5a receptor inhibitor avacopan in immunoglobulin A nephropathy—An open-label pilot study. Clin. Kidney J. 2022, 15, 922–928. [Google Scholar] [CrossRef]
- Croker, D.E.; Monk, P.N.; Halai, R.; Kaeslin, G.; Schofield, Z.; Wu, M.C.; Clark, R.J.; Blaskovich, M.A.; Morikis, D.; Floudas, C.A.; et al. Discovery of functionally selective C5aR2 ligands: Novel modulators of C5a signalling. Immunol. Cell. Biol. 2016, 94, 787–795. [Google Scholar] [CrossRef] [PubMed]
| C5aR1 | C5aR2 | |
|---|---|---|
| Topology | Seven transmembrane receptor | Seven transmembrane receptor |
| G protein coupling | Coupling to G proteins of Gα family | No known coupling to Gα family proteins |
| β-arrestin recruitment | Recruitment of β-arrestins 1 and 2 | Recruitment of β-arrestins 1 and 2 |
| Expression | Immune cells: myeloid lineage, some lymphocytes Non-immune cells: epithelial cells | Immune cells of myeloid lineage with particularly strong expression on granulocytes Non-immune cells: neurons, fibroblasts, adipose cells, hepatocytes |
| Cellular localisation | Predominantly on cellular surface | Predominantly intracellular |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trambas, I.A.; Coughlan, M.T.; Tan, S.M. Therapeutic Potential of Targeting Complement C5a Receptors in Diabetic Kidney Disease. Int. J. Mol. Sci. 2023, 24, 8758. https://doi.org/10.3390/ijms24108758
Trambas IA, Coughlan MT, Tan SM. Therapeutic Potential of Targeting Complement C5a Receptors in Diabetic Kidney Disease. International Journal of Molecular Sciences. 2023; 24(10):8758. https://doi.org/10.3390/ijms24108758
Chicago/Turabian StyleTrambas, Inez A., Melinda T. Coughlan, and Sih Min Tan. 2023. "Therapeutic Potential of Targeting Complement C5a Receptors in Diabetic Kidney Disease" International Journal of Molecular Sciences 24, no. 10: 8758. https://doi.org/10.3390/ijms24108758
APA StyleTrambas, I. A., Coughlan, M. T., & Tan, S. M. (2023). Therapeutic Potential of Targeting Complement C5a Receptors in Diabetic Kidney Disease. International Journal of Molecular Sciences, 24(10), 8758. https://doi.org/10.3390/ijms24108758







