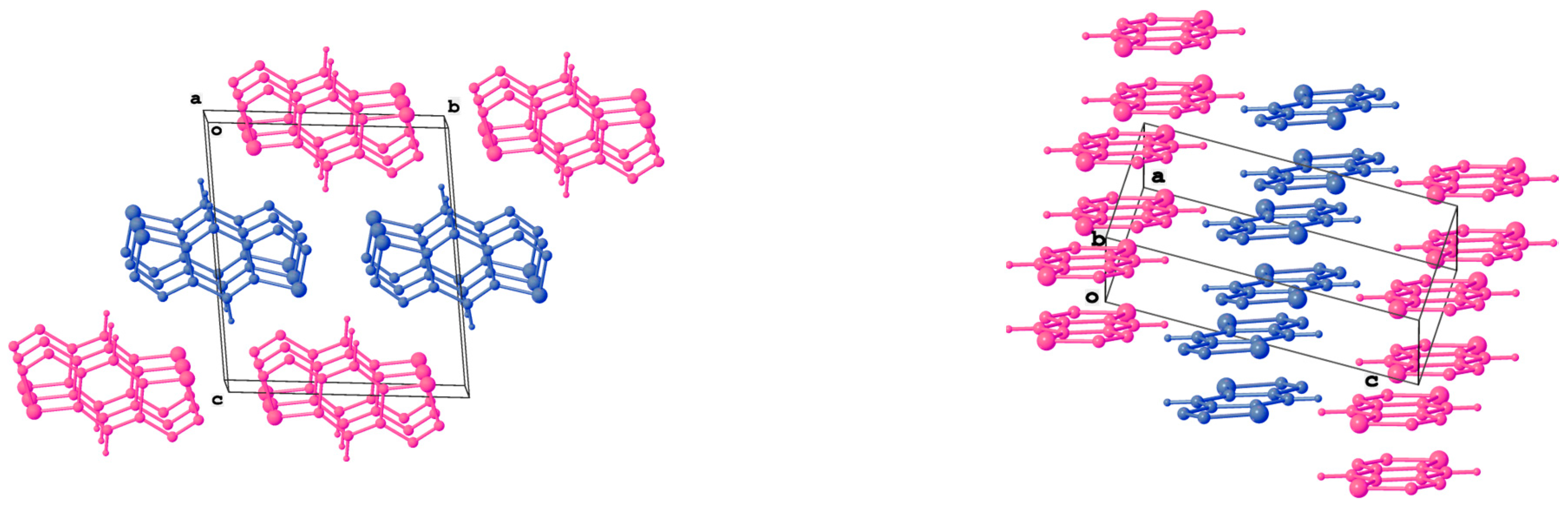
3.5. Synthesis of Compounds
3.5.1. General Procedure for the Preparation of Aminated Products 4a–c
Amine 5a–c (0.36 mmol) was added to a solution of 4-bromobenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) 2 (50 mg, 0.18 mmol) in dry MeCN (10 mL) at room temperature, and the mixture was stirred at reflux for 24 h, poured into water (20 mL), and extracted with CH2Cl2 (3 × 35 mL). The combined organic layers were washed with brine, dried over MgSO4, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography (silica gel Merck 60).
4-(benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole)-4-yl)morpholine (4a)
Orange solid, 39 mg (85%), eluent–CH2Cl2/hexane, 1:2 (v/v). Rf = 0.2 (CH2Cl2/hexane, 1:1, (ν/ν)). Mp = 195–197 °C. IR νmax (KBr, cm–1): 2961, 2918, 1851, 1563, 1537, 1441, 1388, 1339, 1290, 1262, 1243, 1108, 1067, 1025, 980, 925, 848,835, 809, 680, 611, 514. 1H NMR (300 MHz, CDCl3): δ 8.72 (s, 1H), 4.06–3.96 (m, 8H). 13C NMR (100 MHz, CDCl3): δ 160.2, 150.3, 142.3, 139.6, 129.2, 104.4, 67.4, 52.2. HRMS (ESI-TOF), m/z: calcd for C10H9N5OS2 [M]+, 279.0243; found, 279.0240. MS (EI, 70 eV), m/z (I, %): 279 ([M]+, 6), 251 (20), 165 (55), 93 (4), 69 (80), 28 (100).
4-(Piperidin-1-yl)benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) (4b)
Orange solid, 39 mg (80%), eluent–CH2Cl2/hexane, 1:1 (v/v). Rf = 0.5 (CH2Cl2/hexane, 1:1, (ν/ν)). Mp = 155–157 °C. IR νmax (KBr, cm–1): 2928, 2847, 2809, 1565, 1534, 1443, 1387, 1341, 1288, 1243, 1222, 1123, 1086, 975, 844, 810, 678, 609, 625, 609, 543. 1H NMR (300 MHz, CDCl3): δ 8.59 (s, 1H), 3.98–3.88 (m, 4H), 1.96–1.98 (m, 6H). 13C NMR (100 MHz, CDCl3): δ 160.2, 149.8, 142.4, 140.7, 129.0, 102.6, 53.4, 26.8, 24.4. HRMS (ESI-TOF), m/z: calcd for C11H11N5S2 [M]+, 277.0450; found, 277.0444. MS (EI, 70 eV), m/z (I, %): 277 ([M]+, 45), 249 (90), 220 (6), 192 (46), 165 (30), 96 (40), 69 (100), 41 (95), 27 (80).
4-(Pyrrolidin-1-yl)benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) (4c)
Orange solid, 37 mg (79%), eluent–CH2Cl2/hexane, 1:2 (v/v). Rf = 0.4 (CH2Cl2/Hexane, 1:1, (ν/ν)). Mp = 196 –198 °C. IR νmax (KBr, cm–1): 2969, 2950, 2875, 2856, 1635, 1555, 1538, 1450, 1388, 1337, 1315, 1278, 1194, 1037, 982, 844, 807, 669, 640, 528. 1H NMR (300 MHz, CDCl3): δ 8.19 (s, 1H), 4.39–4.28 (m, 4H), 2.23–2.14 (m, 4H). 13C NMR (100 MHz, CDCl3): δ 160.5, 145.4, 143.3, 138.8, 124.8, 97.5, 53.6, 26.1. HRMS (ESI-TOF), m/z: calcd for C10H9N5S2 [M]+, 263.0294; found, 263.0295. MS (EI, 70 eV), m/z (I, %): 263 ([M]+, 8), 235 (15), 206 (5), 192 (3), 178 (6), 96 (13), 69 (100), 41 (50), 27 (55), 18 (10).
N-Phenylbenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole)-4-amine (4e)
Aniline 5e (33 mg, 0.36 mmol) was added to a solution of 4-bromobenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) 2 (50 mg, 0.18 mmol) in dry DMF (10 mL), and the mixture was stirred at 130 °C for 24 h, poured into water, and extracted with CH2Cl2 (3 × 35 mL). The combined organic layers were washed with water, brine, dried over MgSO4, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography (silica gel Merck 60). Red solid, yield 20 mg (40%), eluent–CH2Cl2/hexane, 1:1 (v/v)). Rf = 0.4 (CH2Cl2/hexane, 1:1 (v/v)). Mp = 157–160 °C. IR νmax (KBr, cm–1): 3344, 1581, 1546, 1495, 1449, 1429, 1385, 1343, 1298, 1255, 1072, 854, 816, 764, 702, 677, 538. 1H NMR (300 MHz, CDCl3): δ 8.59 (s, 1H), 8.33 (br. s, 1H), 7.56–7.45 (m, 3H), 7.31 (d, J = 7.1, 2H). 13C NMR (100 MHz, CDCl3): δ 161.3, 146.7, 141.3, 137.2, 134.7, 129.8, 127.8, 126.9, 121.4, 101.7. HRMS (ESI-TOF), m/z: calcd for C12H8N5S2 [M + H]+, 286.0216; found, 286.0221. MS (EI, 70 eV), m/z (I, %): 285 ([M]+, 4), 257 (55), 228 (4), 196 (5), 185 (30), 160 (12), 153 (13), 125 (10), 93 (14), 77 (60), 69 (100), 51 (58).
3.5.2. General Procedure for the Reaction of 4-Bromobenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) 2 with Thiols
Sodium hydride (7.2 mg, 0.18 mmol) was added to a solution of thiol (0.18 mmol) in dry THF (15 mL) at 0 °C with stirring. The reaction mixture was stirred at 0 °C for 30 min, then 4-bromobenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) 2 (50 mg, 0.18 mmol) was added. The mixture was stirred for 6 h at room temperature. On completion (monitored by TLC), the mixture was poured into water (20 mL) and extracted with CH2Cl2 (3 × 5 mL). The combined organic layers were washed with brine, dried over MgSO4, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography.
4-(Phenylthio)benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) (6a)
Yellow solid, 43 mg (80%), Mp = 140–142 °C, eluent–CH2Cl2/hexane, 1:2 (v/v). Rf = 0.5 (CH2Cl2/hexane, 1:1 (v/v)). IR νmax (KBr, cm–1): 1638, 1578, 1475, 1438, 1388, 1330, 1286, 1196, 1075, 1019, 901, 866, 832, 800, 734, 686, 660, 541, 515, 470. 1H NMR (300 MHz, CDCl3): δ 9.11 (s, 1H), 7.65 (d, J = 7.4, 2H), 7.60–7.54 (m, 1H), 7.46 (t, J = 7.4, 2H). 13C NMR (75 MHz, CDCl3): δ 158.6, 155.5, 140.2, 139.0, 135.7, 130.6, 129.9, 129.2, 127.4, 111.3. HRMS (ESI-TOF), m/z: calcd for C12H6N4S3Ag [M + Ag]+, 408.8800; found, 408.8790. MS (EI, 70 eV), m/z (I, %): 304 ([M + 2]+, 2), 303 ([M + 1]+, 3), 302 ([M + 1]+, 35), 274 (70), 245 (8), 214 (14), 201 (100), 177 (90), 170 (65), 158 (85), 145 (50), 133 (53), 124 (80), 109 (90), 100 (92), 93(95), 76 (94), 70 (95), 65 (94), 52 (94), 45 (96), 39 (95), 27 (55).
4-(Hexylthio)benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) (6b)
Yellow solid, 41 mg (75%), eluent–CH2Cl2/hexane, 1:4 (v/v). Rf = 0.7 (CH2Cl2/hexane, 1:1 (v/v)). Mp = 112–115 °C. IR νmax (KBr, cm–1): 2956, 2924, 2853, 1615, 1510, 1456, 1391, 1334, 1285, 1196, 1104, 901, 868, 798, 752, 721, 658, 543. 1H NMR (300 MHz, CDCl3): δ 9.12 (s, 1H), 3.68 (t, J = 7.3, 2H), 1.67 (p, J = 7.3, 2H), 1.49–1.40 (m, 2H), 1.27–1.21 (m, 4H), 0.84 (t, J = 6.7, 3H). 13C NMR (75 MHz, CDCl3): δ 157.1, 156.4, 140.8, 126.5, 125.8, 111.5, 36.8, 31.2, 30.0, 28.2, 22.4, 13.9. HRMS (ESI-TOF), m/z: calcd for C12H14N4S3Ag [M + Ag]+, 416.9426; found, 416.9425. MS (EI, 70 eV), m/z (I, %): 311 ([M + 1]+, 10), 310 ([M]+, 66), 311 ([M − 1]+, 15), 281 (60), 225 (55), 197 (35), 183 (65), 125 (36), 93 (58), 69 (100), 41 (80), 29 (79).
4-(Dodecylthio)benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) (6c)
Green solid, 53 mg (76%), eluent–CH2Cl2/hexane, 1:2 (v/v). Rf = 0.8 (CH2Cl2/hexane, 1:1 (v/v). Mp = 31–33 °C. IR νmax (KBr, cm–1): 2954, 2920, 2849, 1653, 1469, 1390, 1287, 1195, 903, 871, 833, 798, 718, 656, 542. 1H NMR (300 MHz, CDCl3): δ 9.11 (s, 1H), 3.67 (t, J = 7.3, 2H), 1.67 (p, J = 7.3, 2H), 1.48–1.36 (m, 2H), 1.29–1.16 (m, 16H), 0.86 (t, J = 6.6, 3H). 13C NMR (75 MHz, CDCl3): δ 157.1, 156.4, 143.0, 140.8, 125.8, 111.4, 36.8, 31.9, 30.0, 29.6, 29.59, 29.55, 29.5, 29.4, 29.0, 28.5, 22.7, 14.1. HRMS (ESI-TOF), m/z: calcd for C18H26N4S3Na [M + Na]+, 417.1212; found, 417.1212. MS (EI, 70 eV), m/z (I, %): 396 ([M + 2]+, 5), 395 ([M + 1]+, 10), 394 ([M]+, 46), 355 (100), 341 (70), 295 (44), 281 (90), 221 (85), 207 (49), 69 (50), 55 (40).
3.5.3. General Procedure for the Preparation of Arylated Benzo-bis-thiadiazoles 8 under Suzuki Coupling Conditions (Procedure A)
A mixture of 4-bromobenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) 2 (50 mg, 0.18 mmol), boronic ether 16a–h (0.18 mmol), K2CO3 (24 mg, 0.18 mmol), 1 mL (H2O), and Pd(PPh3)4 (20 mg, 10% mmol) in dry toluene (8 mL) was degassed by argon and heated at 110 °C in a sealed vial. On completion (monitored by TLC), the mixture was poured into water and extracted with CH2Cl2 (3 × 35 mL). The combined organic layers were washed with brine, dried over MgSO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography.
3.5.4. General Procedure for the Preparation of Arylated Benzo-bis-thiadiazoles 8 under Stille Coupling Conditions (Procedure B)
PdCl2(PPh3)2 (12 mg, 10% mmol) and stannane 20a–h (0.21 mmol) were added to a solution of 4-bromobenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) 2 (50 mg, 0.18 mmol) in anhydrous toluene (4 mL) The resulting cloudy, yellow mixture was stirred and degassed by argon in a sealed vial. The resulting yellow mixture was then stirred at 60 °C for the desired time. On completion (monitored by TLC), the mixture was washed with water and the organic layer was extracted with CH2Cl2 (3 × 35 mL), dried over MgSO4, and then concentrated in vacuo. The crude product was purified by column chromatography.
3.5.5. General Procedure for the Synthesis of Arylated Benzo-bis-thiadiazoles 8 by Palladium-catalyzed C-H Direct Arylation Reaction of 4-Bromobenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) 2 (Procedure C)
A mixture of 4-bromobenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) 2 (50 mg, 0.18 mmol), thiophene 3a–e (0.20 mmol), Pd(OAc)2 (6 mg, 15% mmol), pivalic acid (20 mg, 0.20 mmol), and K2CO3 (27 mg, 0.20 mmol) were added to an air-free flask, which was then purged in dry toluene (8 mL), degassed by argon, and heated at 110 °C in a sealed vial. On completion (monitored by TLC), the mixture was poured into water and extracted with CH2Cl2 (3 × 35 mL). The combined organic layers were washed with brine, dried over MgSO4, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography.
4-(Thiophen-2-yl)benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) (8a)
Orange solid, 34 mg (70%, Procedure A), 34 mg (70%, Procedure B), or 18 mg (37%, Procedure C), eluent–CH2Cl2/hexane, 1:2 (v/v). Rf = 0.3 (CH2Cl2/hexane, 1:1, (ν/ν)). Mp = 173–150 °C. IR νmax (KBr, cm–1): 1636, 1532, 1437, 1432, 1393, 1328, 1286, 1258, 1142, 858, 812, 715, 666, 544. 1H NMR (300 MHz, CDCl3): δ 9.19 (s, 1H), 8.25 (d, J = 3.7, 1H), 7.75 (d, J = 5.0, 1H), 7.43–7.32 (m, 1H). 13C NMR (100 MHz, CDCl3): δ 158.4, 154.0, 140.9, 138.9, 137.6, 131.6, 130.5, 129.8, 128.6, 112.1. HRMS (ESI-TOF), m/z: calcd for C10H5N4S3 [M + H]+, 276.9671; found, 276.9663. MS (EI, 70 eV), m/z (I, %): 276 ([M]+, 6), 248 (75), 220 (10), 176 (11), 151 (100), 93 (25), 69 (95), 45 (12), 28 (5).
4-(4-Hexylthiophen-2-yl)benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) (8b)
Yellow solid, 44 mg (69%, Procedure A), 46 mg (71%, Procedure B), or 23 mg (37%, Procedure C), eluent–CH2Cl2/hexane, 1:2 (v/v). Rf = 0.4 (CH2Cl2/hexane, 1:1, (ν/ν)). Mp = 65–68 °C. IR νmax (KBr, cm–1): 2956, 2924, 2853, 1640, 1540, 1513, 1494, 1451, 1398, 13754, 1333, 1287, 1249, 1188, 1081, 967, 854, 815, 775, 725, 661, 615, 522. 1H NMR (300 MHz, CDCl3): δ 9.16 (s, 1H), 8.14 (s, 1H), 7.34 (s, 1H), 3.18–2.61 (m, 2H), 1.79–1.70 (m, 2H), 1.40–1.30 (m, 6H), 0.91 (t, J = 8.0, 3H). 13C NMR (100 MHz, CDCl3): δ 157.2, 152.8, 144.1, 138.9, 136.2, 136.1, 132.2, 124.3, 122.5, 110.6, 30.7, 29.6, 29.5, 28.0, 21.6, 13.1 HRMS (ESI-TOF), m/z: calcd for C16H17N4S3 [M + H]+, 361.0610; found, 361.0606. MS (EI, 70 eV), m/z (I, %): 362 ([M + 2]+, 3), 361 ([M + 1]+, 6), 360 ([M]+, 50), 332 (100), 248 (20), 235 (19), 220 (12), 165 (18), 120 (13), 69 (60), 43 (57), 29 (48).
4-(5′-(Trimethylsilyl)-[2,2′-bithiophen]-5-yl)benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) (8c)
Red solid, 54 mg (70%, Procedure A), 52 mg (68%, Procedure B), or 31 mg (40%, Procedure C), eluent–CH2Cl2/hexane, 1:2 (v/v). Rf = 0.3 (CH2Cl2/hexane, 1:1, (ν/ν)). Mp = 155–157 °C. IR νmax (KBr, cm–1): 2958, 2924, 2853, 1724, 1641, 1494, 1464, 1364, 1279, 1263, 1187, 1081, 968, 892, 818, 725, 486. 1H NMR (300 MHz, CDCl3): δ 9.15 (s, 1H), 8.15 (d, J = 4.0, 1H), 7.44 (d, J = 3.5, 1H), 7.40 (d, J = 4.0, 1H), 7.22 (d, J = 3.5, 1H), 0.37 (s, 9H). 13C NMR (100 MHz, CDCl3): δ 158.6, 153.6, 143.1, 142.2, 141.2, 141.05, 136.8, 135.9, 135.2, 132.6, 126.5, 124.9, 123.1, 111.7, 0.00(TMS). HRMS (ESI-TOF), m/z: calcd for C17H15N4S4Si [M + H]+, 430.9943; found, 430.9928. MS (EI, 70 eV), m/z (I, %): 432 ([M + 2]+, 1), 431 ([M + 1]+, 2), 430 ([M]+, 8), 402 (7), 305 (6), 200 (10), 175 (12), 93 (45), 69 (100), 45 (30).
4-(5-(2-Ethylhexyl)thiophen-2-yl)benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) (8d)
Orange solid, 47 mg (68%, Procedure A), 48 mg (70%, Procedure B), or 38 mg (55%, Procedure C), eluent–CH2Cl2/hexane, 1:2 (v/v). Rf = 0.4 (CH2Cl2/hexane, 1:1, (ν/ν)). Mp = 55–57 °C. IR νmax (KBr, cm–1): 2958, 2923, 2855, 1618, 1507, 1457, 1389, 1324, 1282, 1262, 1144, 1078, 1032, 881, 861, 847, 812, 786, 739, 618, 547. 1H NMR (300 MHz, CDCl3): δ 9.10 (s, 1H), 8.09 (d, J = 3.7, 1H), 7.01 (d, J = 3.7, 1H), 2.91 (d, J = 6.8, 2H), 1.78–1.68 (m, 1H), 1.48–1.29 (m, 8H), 0.98–0.89 (m, 6H). 13C NMR (100 MHz, CDCl3): δ 158.3, 151.1, 140.8, 136.7, 135.1, 131.8, 127.0, 126.5, 123.6, 111.1, 41.6, 34.4, 32.5, 28.9, 25.7, 23.0, 14.1, 10.9. HRMS (ESI-TOF), m/z: calcd for C18H21N4S3 [M + H]+, 389.0923; found, 389.0921. MS (EI, 70 eV), m/z (I, %): 390 ([M + 2]+, 3), 389 ([M + 1]+, 6), 388 ([M]+, 35), 360 (80), 332 (15), 261 (18), 248 (38), 233 (100), 69 (28), 57 (60), 41 (45), 29 (37).
4-Phenylbenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) (8e)
Yellow solid, 33 mg (68%, Procedure A), 30 mg (63%, Procedure B), eluent–CH2Cl2/hexane, 1:2 (v/v). Rf = 0.3 (CH2Cl2/hexane, 1:1, (ν/ν)). Mp = 203–205 °C. IR νmax (KBr, cm–1): 1637, 1492, 1431, 1386, 1277, 1148, 1075, 893, 862, 813, 745, 696, 673, 623, 545, 523. 1H NMR (300 MHz, CDCl3): δ 9.28 (s, 1H), 7.99 (d, J = 6.7, 2H), 7.69–7.59 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 157.9, 155.6, 140.8, 140.1, 136.9, 130.3, 129.9, 129.7, 129.3, 112.7. HRMS (ESI-TOF), m/z: calcd for C12H7N4S2 [M + H]+, 271.0107/ found, 271.0109. MS (EI, 70 eV), m/z (I, %): 270 ([M+]+, 3), 242 (58), 214 (26), 170 (23), 145 (90), 93 (20), 69 (100), 28 (40).
4-(p-Tolyl)benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) (8f)
Green solid, 32 mg (64%, Procedure A), 34 mg (68%, Procedure B), eluent–CH2Cl2/hexane, 1:2 (v/v). Rf = 0.3 (CH2Cl2/hexane, 1:1, (ν/ν)). Mp = 229–232 °C. IR νmax (KBr, cm–1): 2925, 1639, 1609, 1507, 1427, 1379, 1331, 1317, 1291, 1275, 1192, 1147, 1120, 895, 865, 828, 804, 763, 716, 670, 609, 556, 536, 488. 1H NMR (300 MHz, CDCl3): δ 9.24 (s, 1H), 7.89 (d, J = 7.9, 2H), 7.46 (d, J = 7.9, 2H), 2.51 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 157.8, 155.5, 140.6, 140.4, 139.8, 134.0, 130.0, 129.8, 129.4, 112.1, 21.3. HRMS (ESI-TOF), m/z: calcd for C13H8BrN4S2 [M + H]+, 285.0263; found, 285.0266. MS (EI, 70 eV), m/z (I, %): 284 ([M]+, 3), 256 (8), 227 (5), 159 (25), 139 (5), 93 (7), 69 (100), 63 (7), 51 (10), 39 (30), 28 (45), 18 (70).
4-(4-Methoxyphenyl)benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) (8g)
Orange solid, 37 mg (69%, Procedure A), 37 mg (70%, Procedure B), eluent–CH2Cl2/hexane, 1:2 (v/v). Rf = 0.2 (CH2Cl2/hexane, 1:1, (ν/ν)). Mp = 198–201 °C. IR νmax (KBr, cm–1): 3076, 1609, 1509, 1457, 1430, 1383, 1300, 1279, 1262, 1178, 1150, 1116, 1030, 896, 863, 835, 806, 670, 540. 1H NMR (300 MHz, CDCl3): δ 9.22 (s, 1H), 7.97 (d, J = 8.8, 2H), 7.17 (d, J = 8.8, 2H), 3.95 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 161.2, 158.0, 155.6, 140.8, 139.4, 131.2, 129.9, 129.2, 114.8, 112.0, 55.6. HRMS (ESI-TOF), m/z: calcd for C13H9N4OS2 [M + H]+, 301.0212; found, 301.0215. MS (EI, 70 eV), m/z (I, %): 302 ([M + 2]+, 3), 301 ([M + 1]+, 4), 300 ([M]+, 30), 272(50), 229 (45), 201 (25), 175 (80), 132 (65), 93 (35), 69 (100), 28 (30).
4-(benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole)-4-yl)-N,N-diphenylaniline (8h)
Orange solid, 55 mg (70%, Procedure A), 50 mg (64%, Procedure B), eluent–CH2Cl2/hexane, 1:2 (v/v). Rf = 0.25 (CH2Cl2/hexane, 1:1, (ν/ν)). Mp = 213–215 °C. IR νmax (KBr, cm–1): 1727, 1590, 1487, 1428, 1321, 1276, 1195, 1125, 1073, 894, 865, 835, 808, 748, 696, 624, 512. 1H NMR (300 MHz, CDCl3): δ 9.18 (s, 1H), 7.88 (d, J = 8.8, 2H), 7.35 (t, J = 7.8 Hz, 3H), 7.28–7.12 (m, 9H). 13C NMR (100 MHz, CDCl3): δ 158.0, 155.3, 150.0, 146.9, 140.8, 139.3, 130.6, 129.8, 129.6, 129.0, 125.7, 124.3, 121.4, 111.5. HRMS (ESI-TOF), m/z: calcd for C24H15N5S2 [M]+, 437.0763; found, 437.0757. MS (EI, 70 eV), m/z (I, %): 438 ([M + 1]+, 8), 437 ([M]+, 55), 409 (6), 381 (4), 312 (12), 168 (3), 69 (15), 18 (100).
3.5.6. General Procedure for the Preparation of Arylated Benzo-bis-thiadiazoles 14 from 4-Bromobenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) 2 (Procedure D)
A mixture of 4-bromobenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) 2 (50 mg, 0.18 mmol), bromide or iodide 13a–d,f–j (1.03 mmol), Pd(OAc)2 (17 mg, 0.076 mmol), (P(But)2Me·HBF4) (41 mg, 0.18 mmol), pivalic acid (105 mg, 1.03 mmol), and K2CO3 (142 mg, 1.03 mmol) were added to an air-free flask, which was then purged in dry toluene (8 mL), degassed by argon, and heated at 110 °C in a sealed vial. On completion (monitored by TLC), the mixture was poured into water and extracted with CH2Cl2 (3 × 35 mL). The combined organic layers were washed with brine, dried over MgSO4, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography.
3.5.7. General Procedure for the Preparation of Arylated Benzo-bis-thiadiazoles 14 under C-H Oxidative Coupling Conditions (Procedure E)
A mixture of 4-bromobenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) 2 (50 mg, 0.18 mmol), thiophene (0.36 mmol), Ag2O (83 mg, 0.36 mmol), and Pd(OAc)2 (6 mg, 0.027 mmol), were added to an air-free flask which, was then purged in dry DMSO (5 mL), degassed by argon, and heated at 90 °C in a sealed vial. On completion (monitored by TLC), the mixture was poured into water and extracted with CH2Cl2 (3 × 35 mL). The combined organic layers were washed with brine, dried over MgSO4, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography.
4-Bromo-8-(thiophen-2-yl)benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) (14a)
Yellow solid, 20 mg (32%, Procedure D), 19 mg (30%, Procedure E), eluent–CH
2Cl
2/hexane, 1:1 (
v/
v). R
f = 0.4 (CH
2Cl
2). Mp = 198–200 °C (lit. mp 198–200 °C [
18]). The data of the
1H and
13C NMR spectra correspond to the literature data [
18].
4-Bromo-8-(4-hexylthiophen-2-yl)benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) (14b)
Yellow solid, 31 mg (40%, Procedure D), 30 mg (38%, Procedure E), eluent–CH
2Cl
2/hexane, 1:2 (
v/
v). R
f = 0.6 (CH
2Cl
2/hexane, 1:1 (
v/
v)). Mp = 67–69 °C (lit. mp 67–69 °C [
18]). The data of the
1H and
13C NMR spectra correspond to the literature data [
18].
4-([2,20-Bithiophen]-5-yl)-8-bromobenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) (14c)
Red solid, 39 mg (50%, Procedure D), 37 mg (48%, Procedure E), eluent–CH
2Cl
2/hexane, 1:1 (
v/
v). R
f = 0.4 (CH
2Cl
2/hexane, 1:1 (
v/
v)). Mp = 130–132 °C (lit. mp 130–132 °C [
18]). The data of the
1H and
13C NMR spectra correspond to the literature data [
18].
4-Bromo-8-(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) (14d)
Yellow solid, 54 mg (65%, Procedure D), 57 mg (68%, Procedure E), eluent–CH
2Cl
2/hexane, 1:1 (
v/
v). R
f = 0.6 (CH
2Cl
2). Mp = 57–60 °C (lit. mp 57–60 °C [
18]). The data of the
1H and
13C NMR spectra correspond to the literature data [
18].
4-Bromo-8-phenylbenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) (14e)
Yellow solid, 32 mg (52%, procedure D), eluent–CH
2Cl
2/hexane, 1:1 (
v/
v). R
f = 0.4 (CH
2Cl
2). Mp = 163–165 °C (lit. mp 163–165 °C [
18]). The data of the
1H and
13C NMR spectra correspond to the literature data [
18].
4-Bromo-8-(p-tolyl)benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) (14f)
Yellow solid, 44 mg (68%, procedure D), eluent–CH
2Cl
2/hexane, 1:1 (
v/
v). R
f = 0.5 (CH
2Cl
2). Mp = 135–137 °C (lit. mp 135–137 °C [
18]). The data of the
1H and
13C NMR spectra correspond to the literature data [
18].
4-Bromo-8-(4-methoxyphenyl)benzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole) (14g)
Yellow solid, 41 mg (60%, procedure D), eluent–CH
2Cl
2/hexane, 1:1 (
v/
v). R
f = 0.2 (CH
2Cl
2). Mp = 125–127 °C (lit. mp 125–127 °C [
18]). The data of the
1H and
13C NMR spectra correspond to the literature data [
18].
4-(8-Bromobenzo[1,2-d:4,5-d′]bis([1,2,3]thiadiazole)-4-yl)-N,N-diphenylaniline (14h)
Red solid, 53 mg (58%, procedure D), eluent–CH
2Cl
2/hexane, 1:2 (
v/
v). R
f = 0.5 (CH
2Cl
2/hexane, 1:1 (
v/
v)). Mp = 165–168 °C. (lit. mp 165–168 °C [
18]). The data of the
1H and
13C NMR spectra correspond to the literature data [
18].