Synthetic Potential of Regio- and Stereoselective Ring Expansion Reactions of Six-Membered Carbo- and Heterocyclic Ring Systems: A Review
Abstract
1. Introduction
2. Literature Review
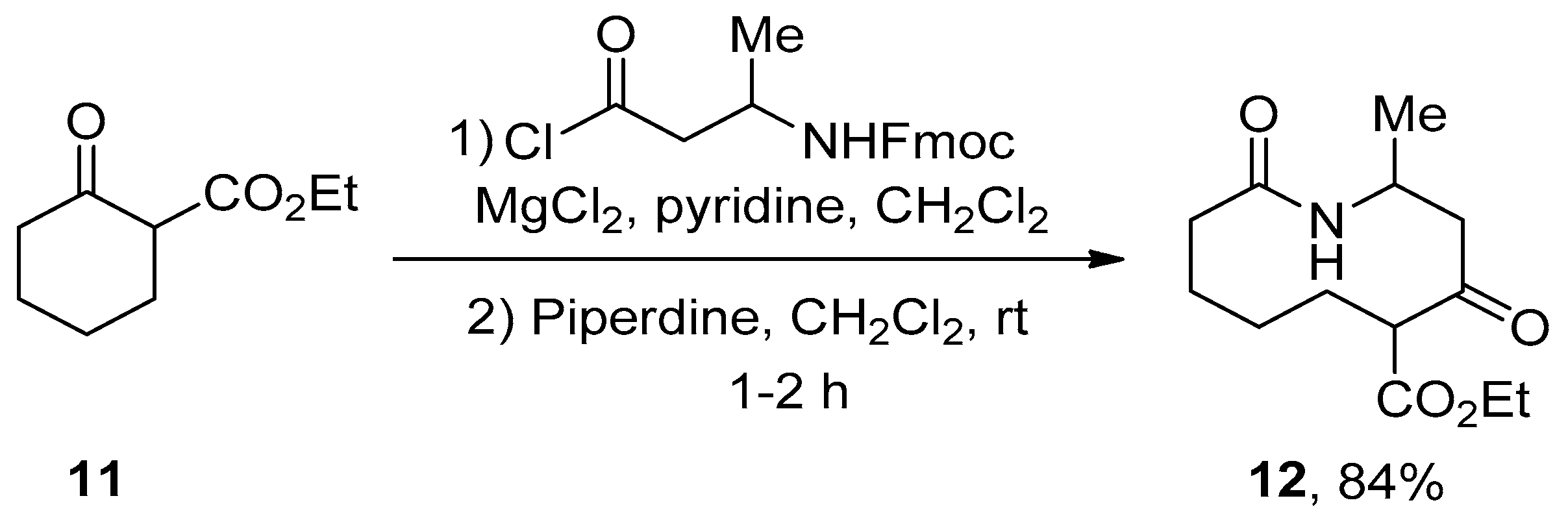
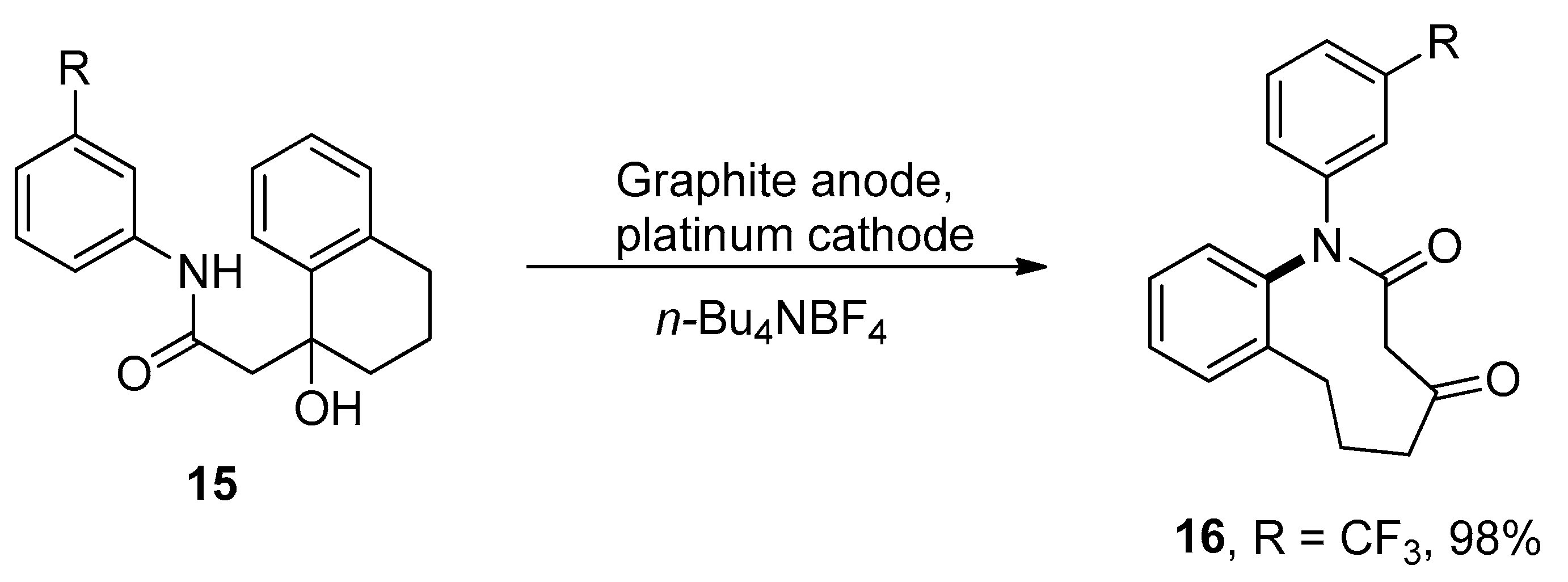
2.1. Ring Expansion Reactions for the Synthesis of Lactams
2.2. Ring Expansion Reactions for the Synthesis of Lactones
2.3. Ring Expansion Reactions for the Formation of Azulenes Derivatives
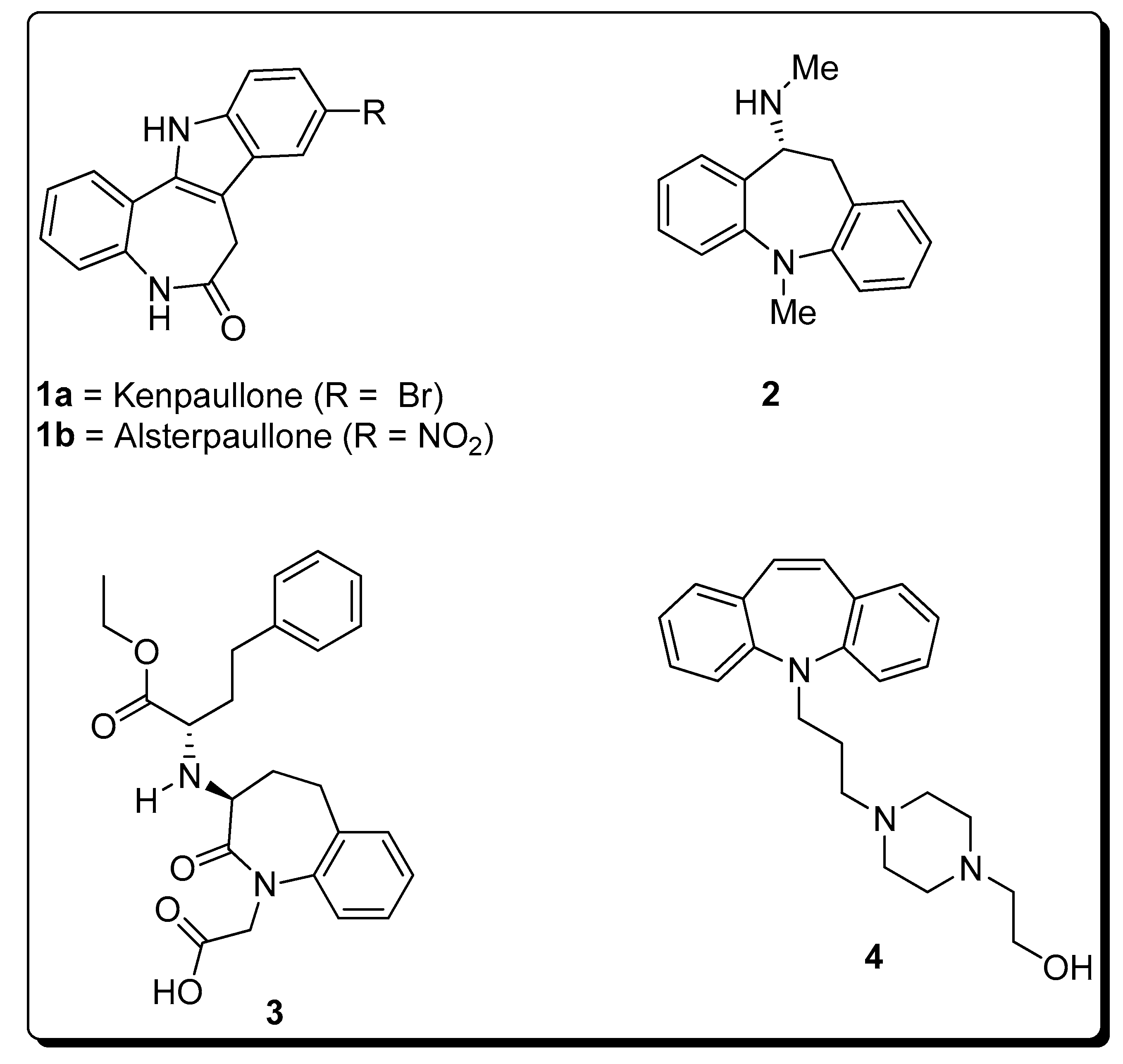
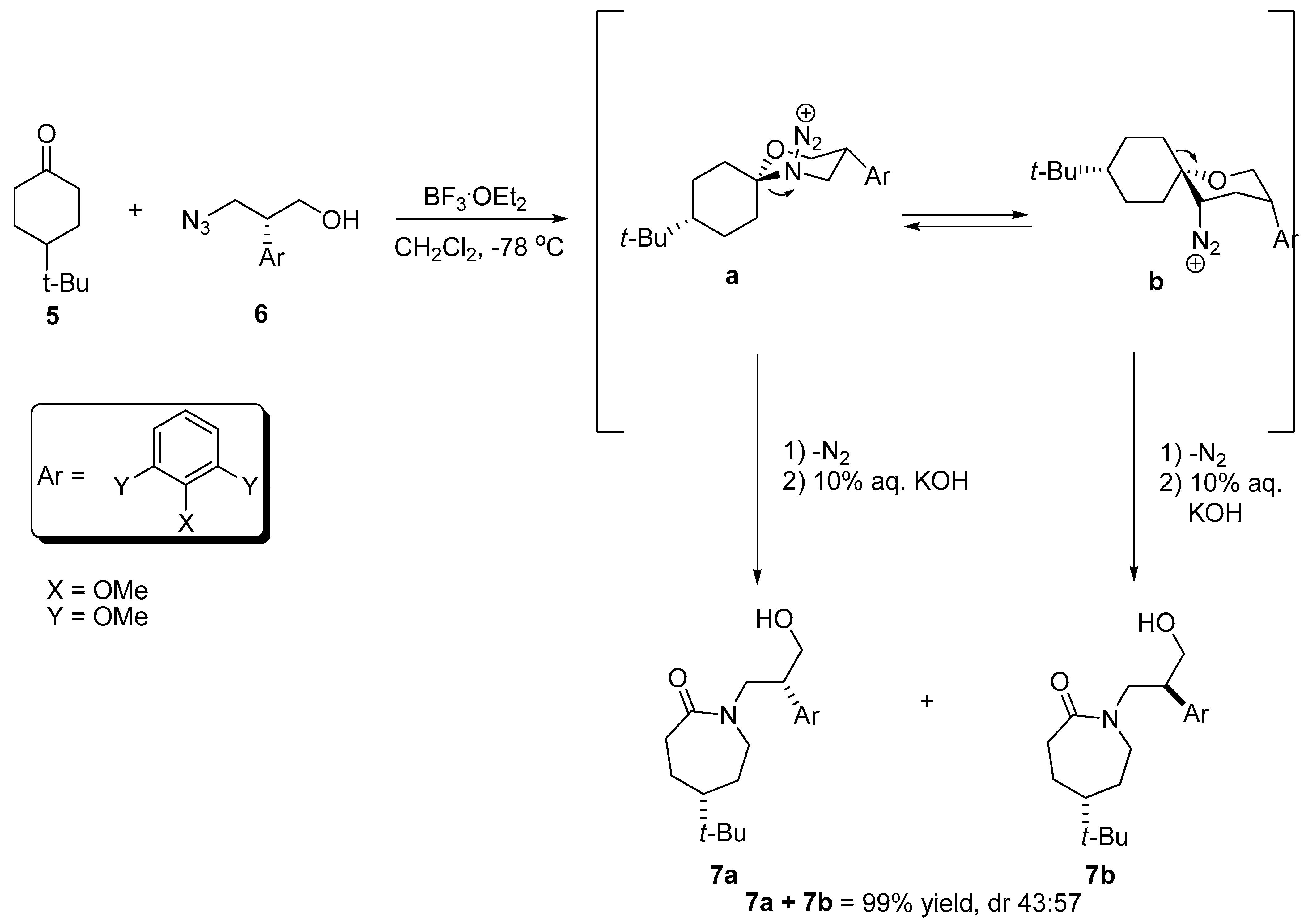
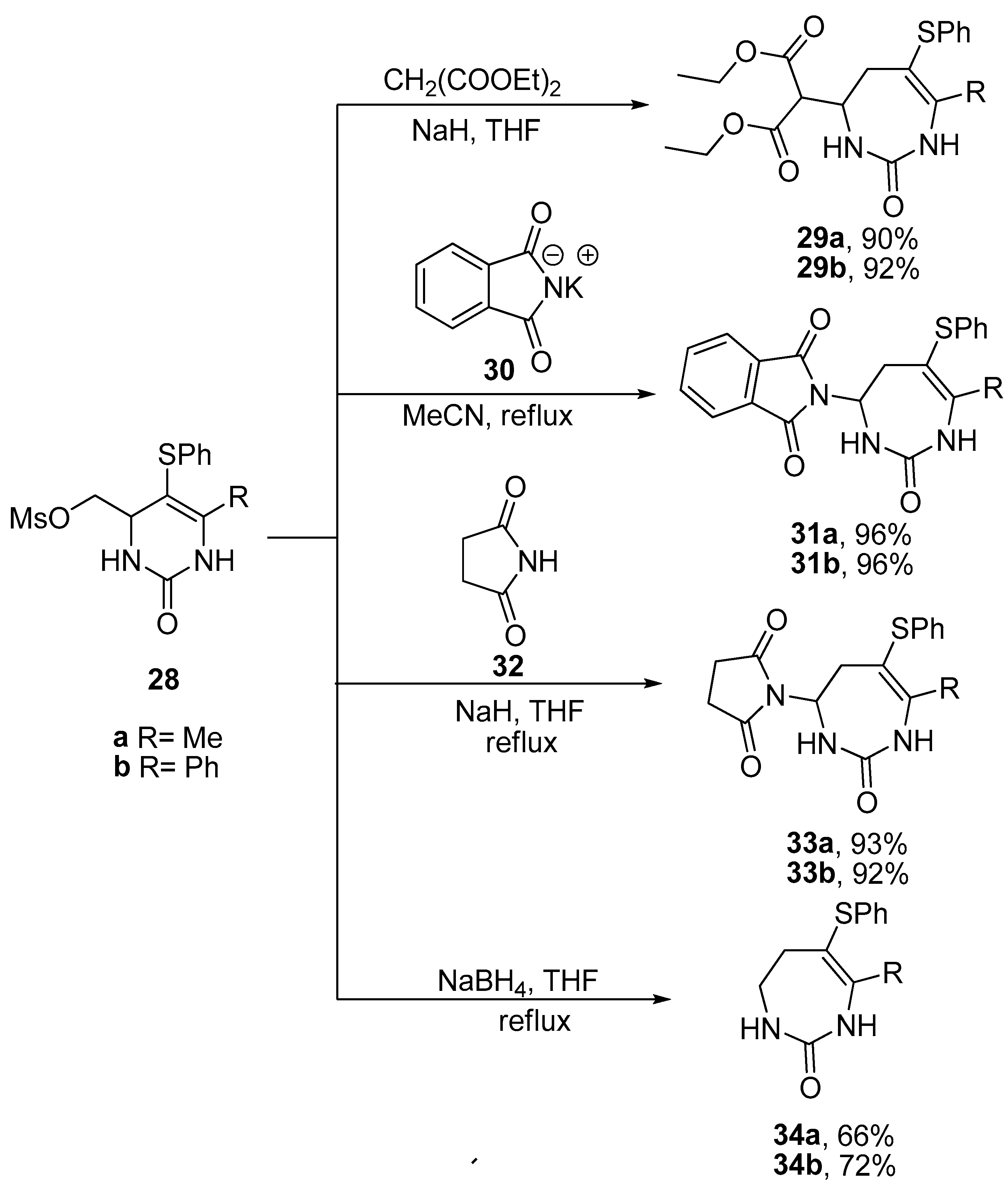
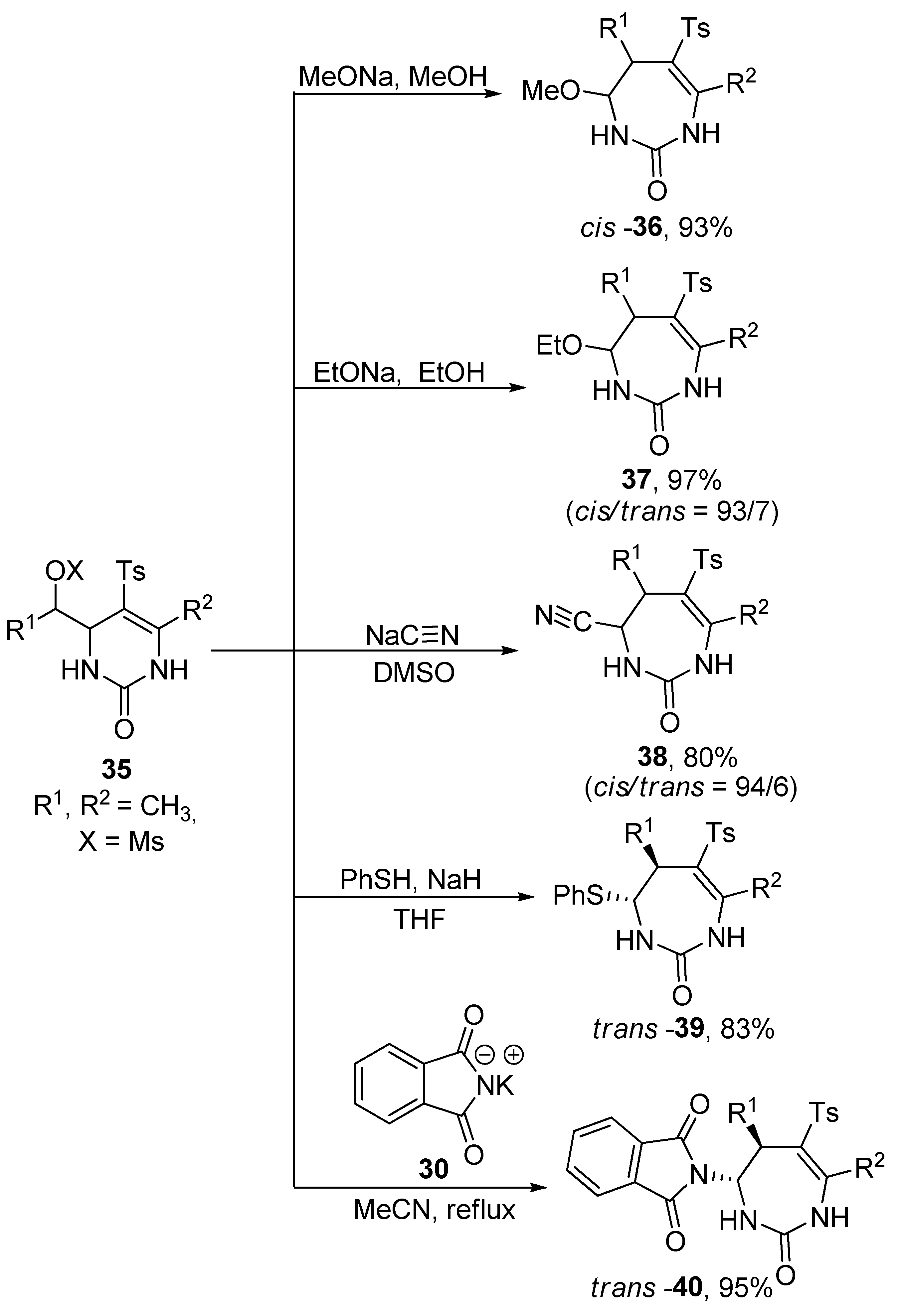
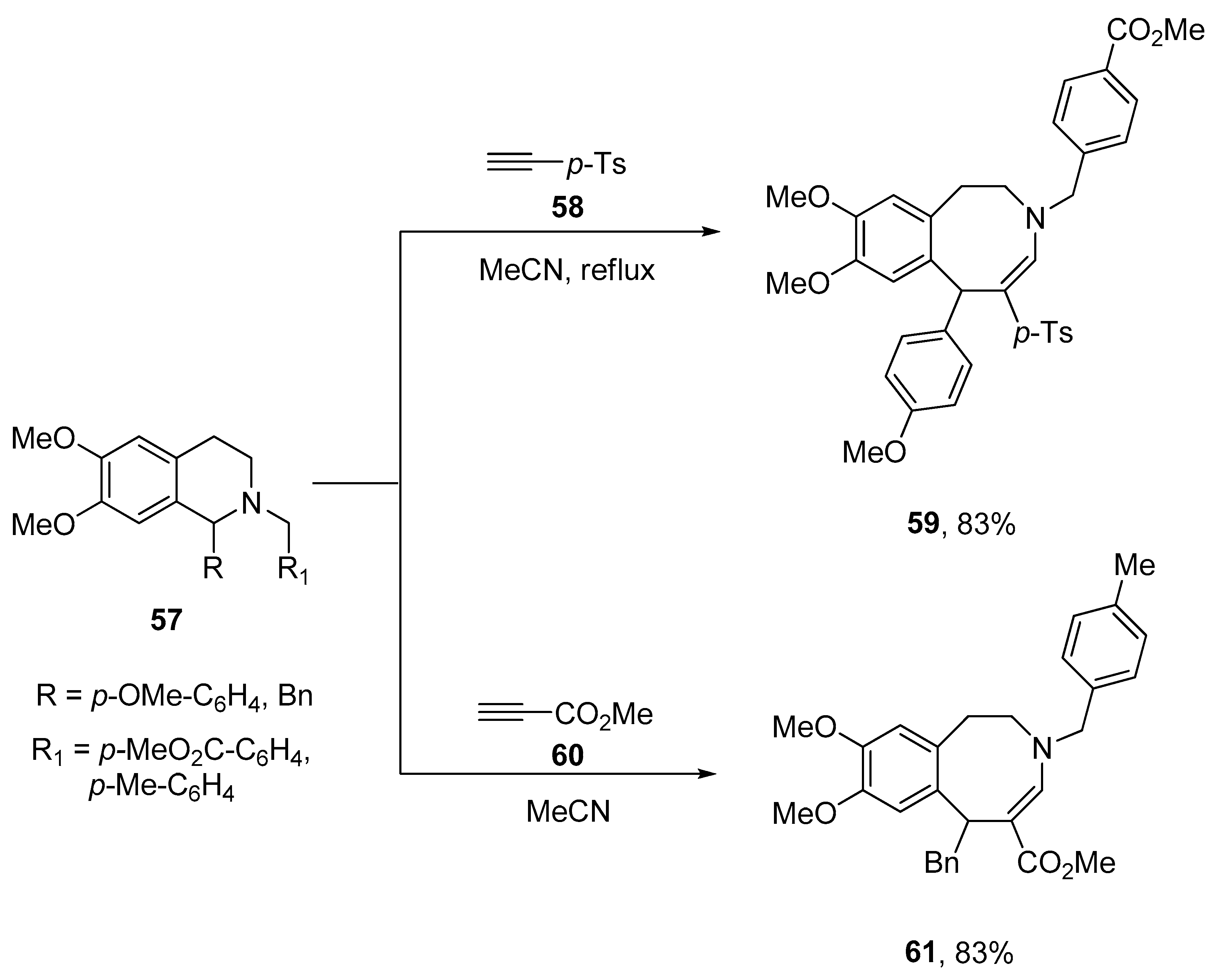
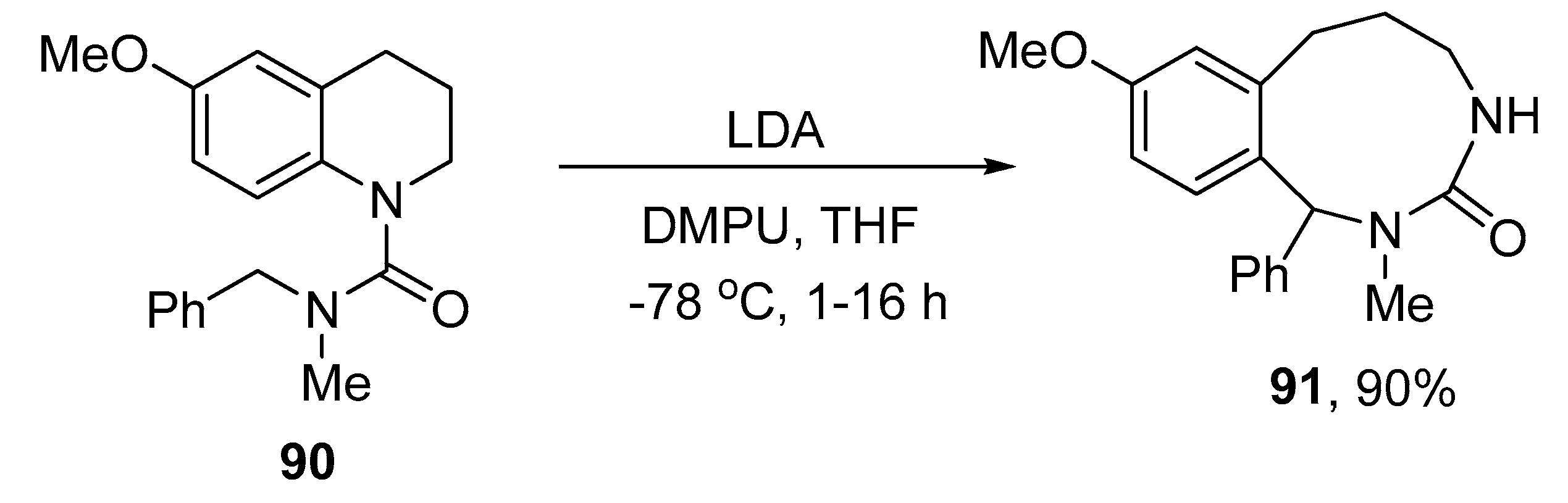
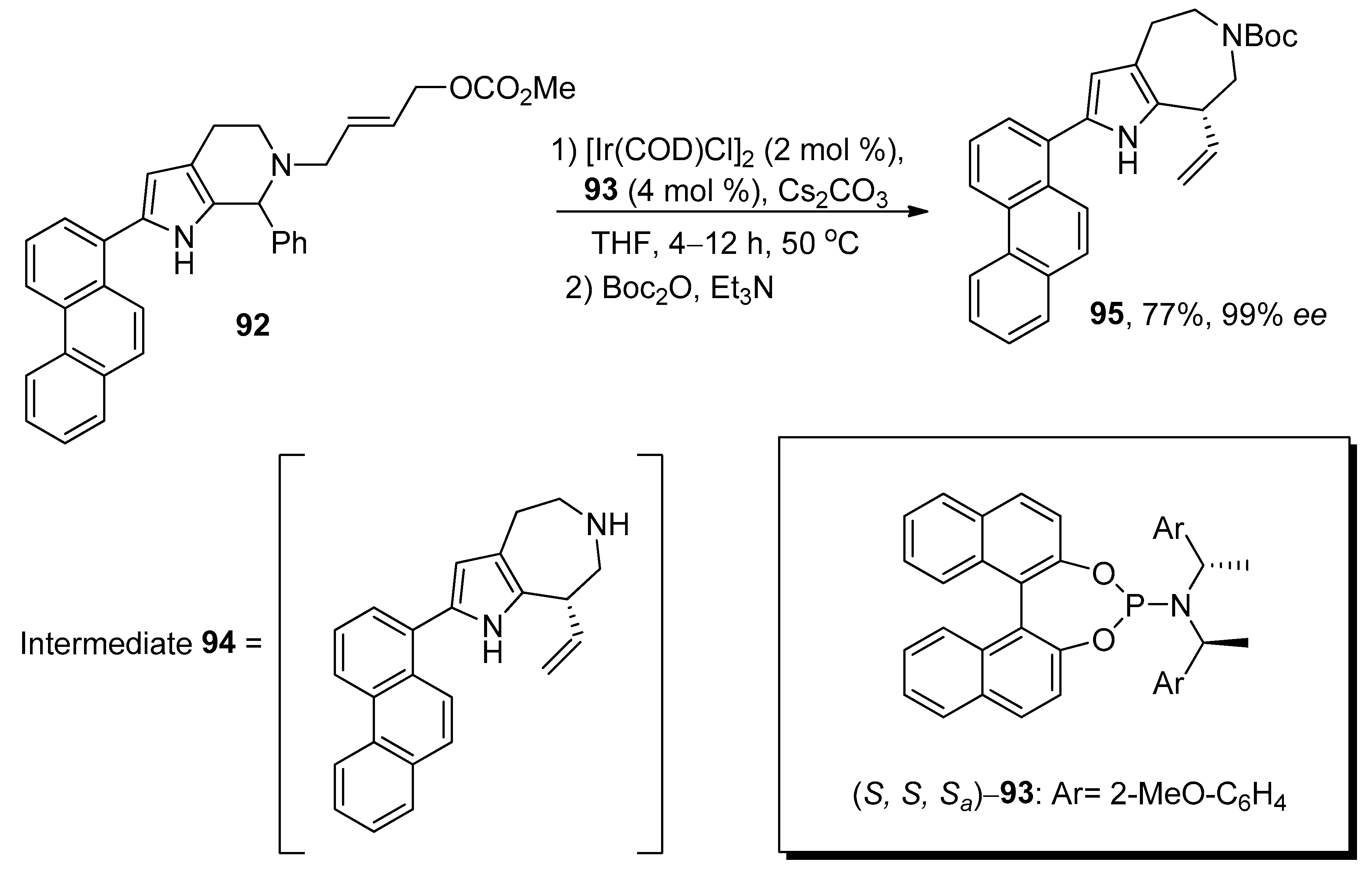
2.4. Ring Expansion Reactions for the Synthesis of Azepine Derivatives
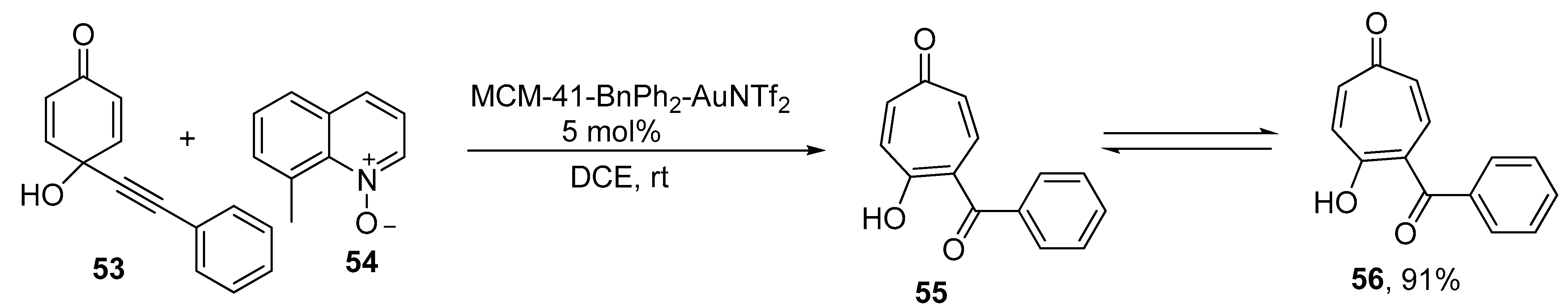
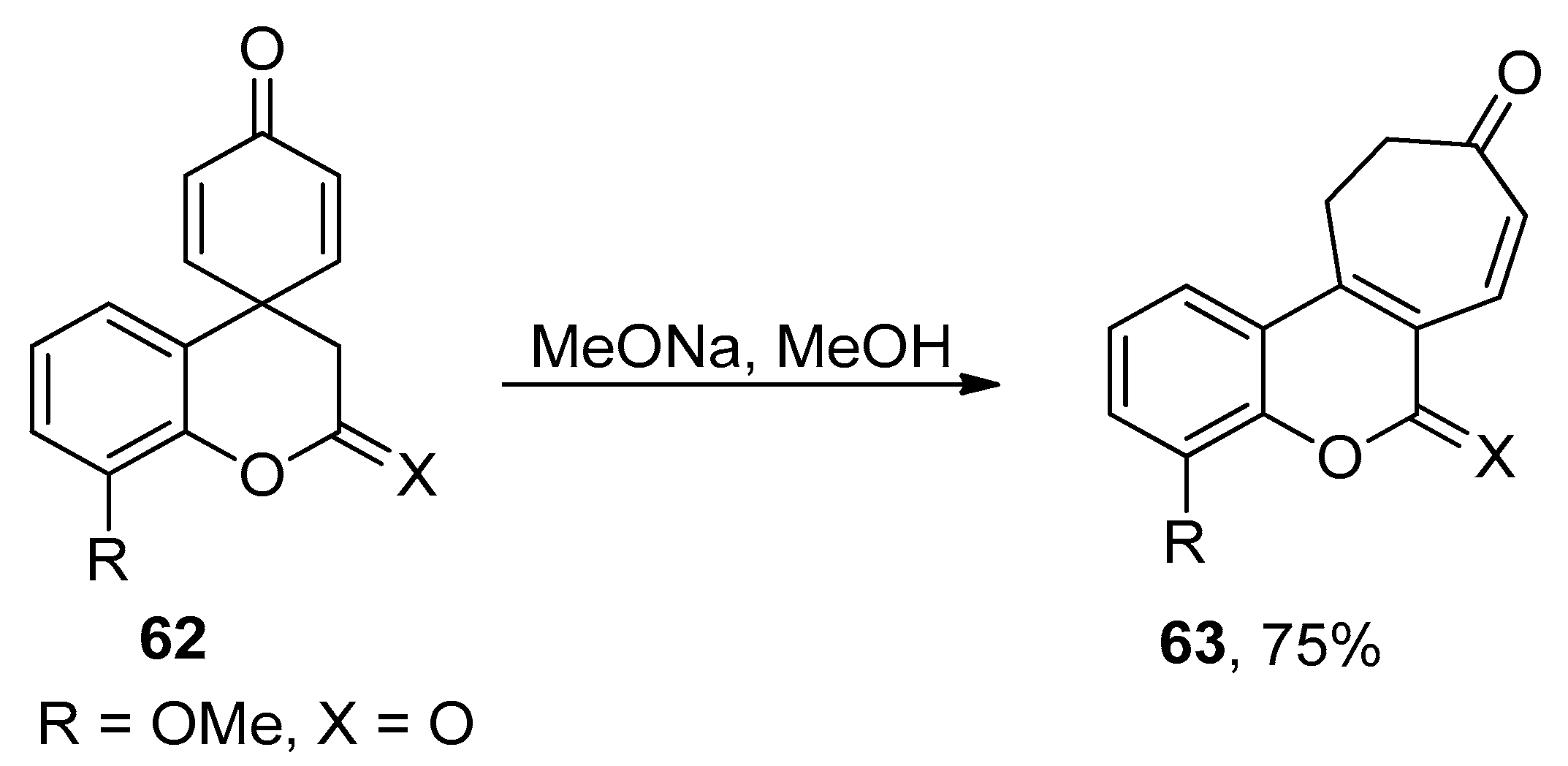
2.5. Ring Expansion Reaction for the Synthesis of Tropone Derivatives
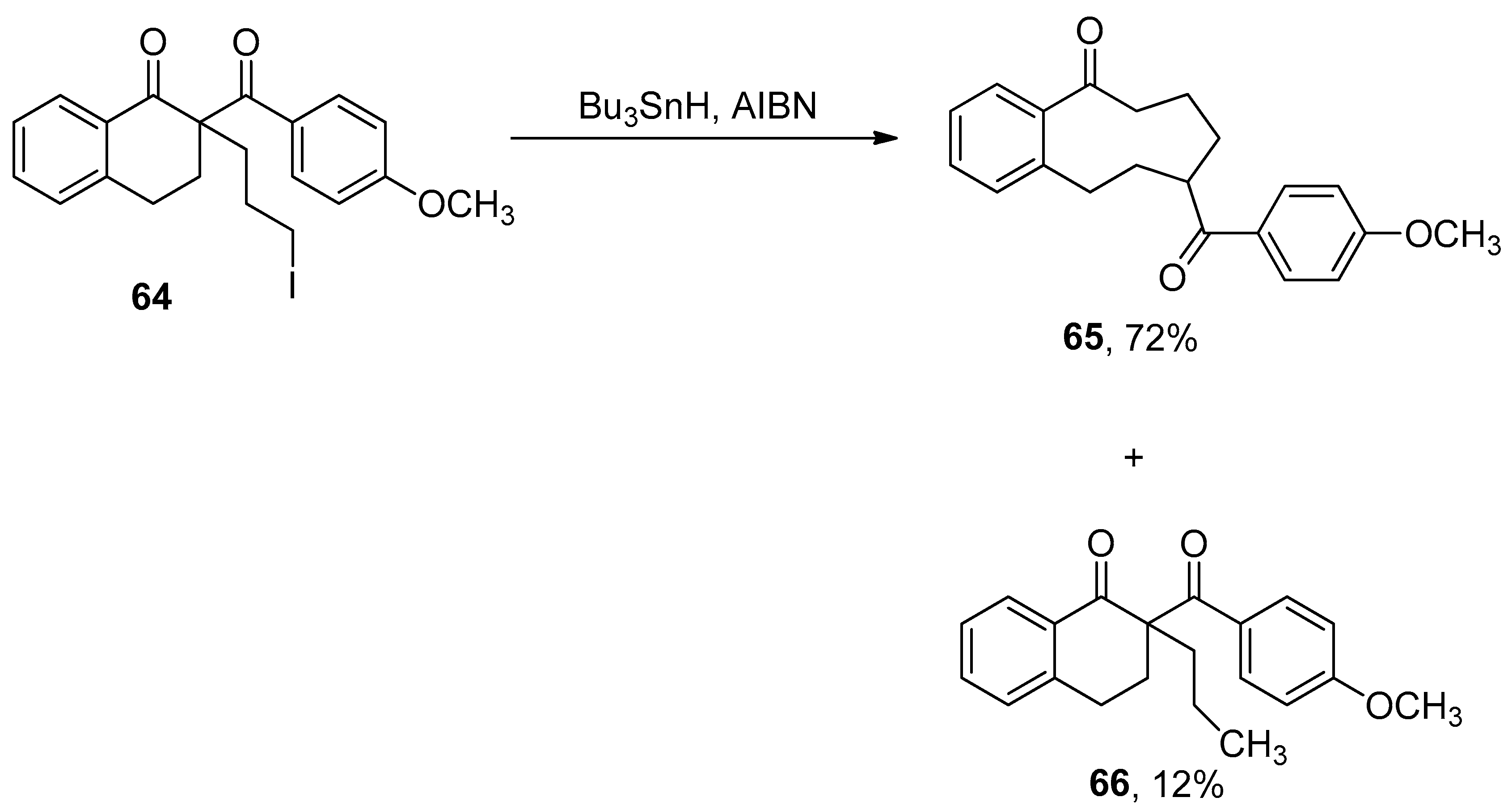
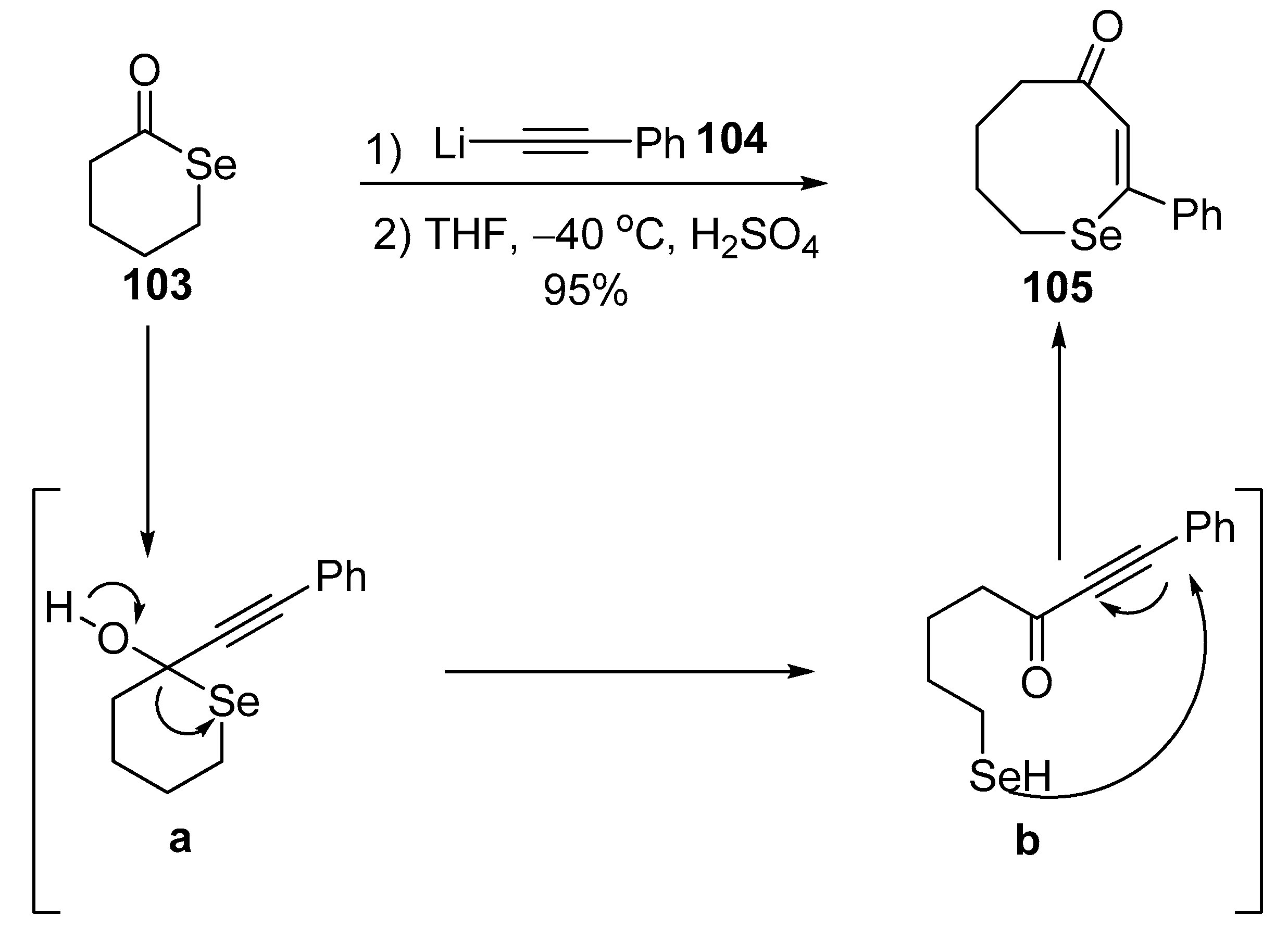
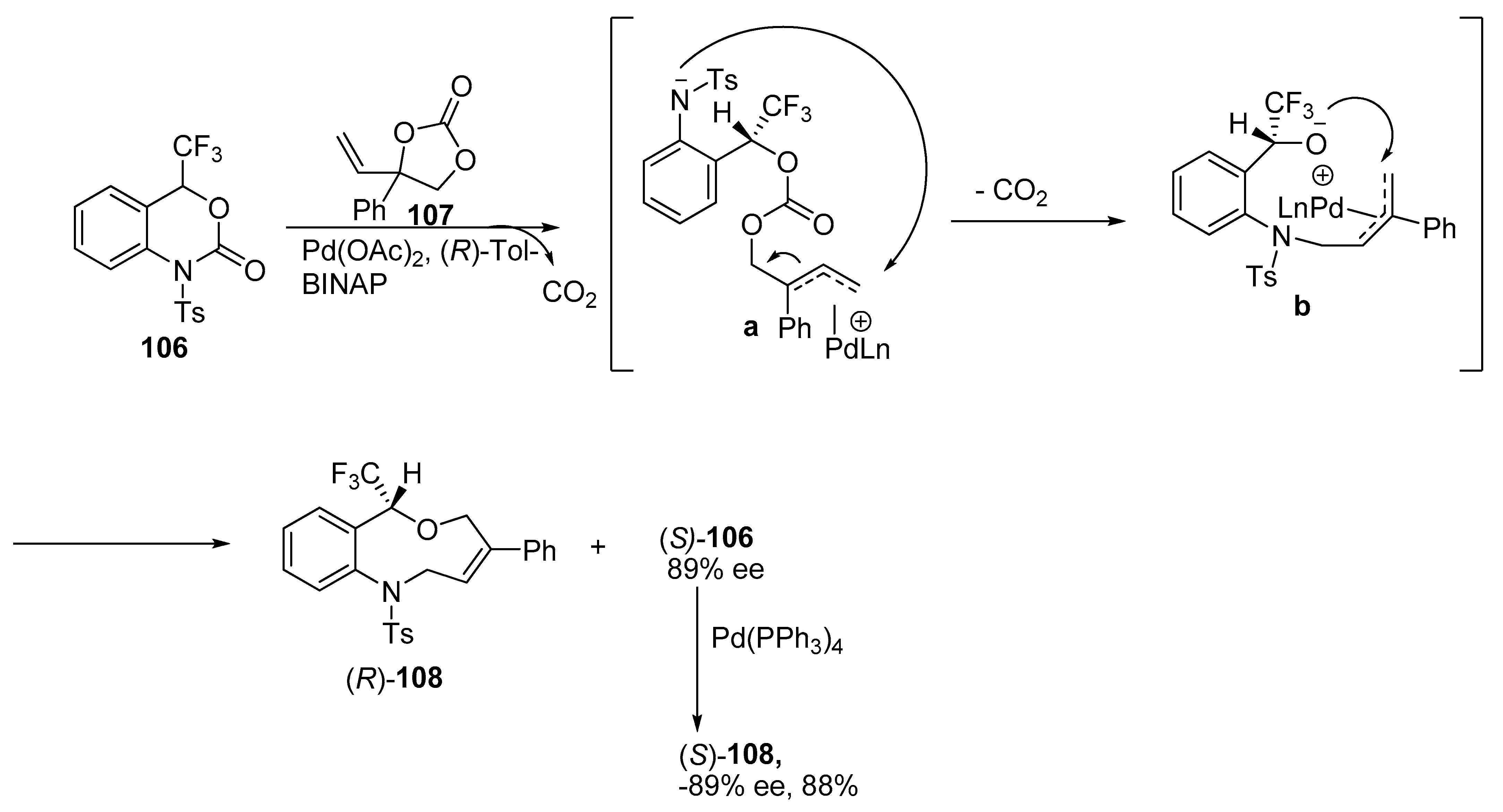
2.6. Miscellaneous Ring Expansion Reactions
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noor, R.; Zahoor, A.F.; Naqvi, S.A.R.; Haq, A.; Akhtar, R. Synthetic potential of ring expansions of 5-membered carbo-& heterocycles: A review. Synth. Commun. 2022, 52, 949–973. [Google Scholar] [CrossRef]
- Kaiser, G.; Ackermann, R.; Sioufi, A. Pharmacokinetics of a new angiotensin-converting enzyme inhibitor, benazepril hydrochloride, in special populations. Am. Heart J. 1989, 117, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Balfour, J.A.; Goa, K.L. Benazepril. Drugs 1991, 42, 511–539. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.; Link, A.; Leost, M.; Zaharevitz, D.W.; Gussio, R.; Sausville, E.A.; Kunick, C. Paullones, a series of cyclin-dependent kinase inhibitors: Synthesis, evaluation of CDK1/cyclin B inhibition, and in vitro antitumor activity. J. Med. Chem. 1999, 42, 2909–2919. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Villa, F.X.; Ávila-Zárraga, G.; Armenta-Salinas, C. Synthesis of new fused dipyrroloazepinones via a two-step tandem reaction: Comparison of the Schmidt and Beckmann pathways. Tetrahedron Lett. 2020, 61, 151751–151757. [Google Scholar] [CrossRef]
- Toogood, P.L.; Harvey, P.J.; Repine, J.T.; Sheehan, D.J.; VanderWel, S.N.; Zhou, H.; Fry, D.W. Discovery of a potent and selective inhibitor of cyclin-dependent kinase 4/6. J. Med. Chem. 2005, 48, 2388–2406. [Google Scholar] [CrossRef] [PubMed]
- Zaharevitz, D.W.; Gussio, R.; Leost, M.; Senderowicz, A.M.; Lahusen, T.; Kunick, C.; Meijer, L.; Sausville, E.A. Discovery and initial characterisation of the paullones, a novel class of small-molecule inhibitors of cyclin-dependent kinases. Cancer Res. 1999, 59, 2566–2569. [Google Scholar]
- Stopka, T.; Marzo, L.; Zurro, M.; Janich, S.; Wuerthwein, E.U.; Daniliuc, C.G.; Mancheno, O.G. Oxidative C—H Bond Functionalization and Ring Expansion with TMSCHN2: A Copper (I)-Catalyzed Approach to Dibenzoxepines and Dibenzoazepines. Angew. Chem. Int. 2015, 54, 5049–5053. [Google Scholar] [CrossRef]
- Müller, W.E.; Siebert, B.; Holoubek, G.; Gentsch, C. Neuropharmacology of the anxiolytic drug opripramol, a sigma site ligand. Pharmacopsychiatry 2004, 37, 189–197. [Google Scholar] [CrossRef]
- Kambe, M.; Arai, E.; Suzuki, M.; Tokuyama, H.; Fukuyama, T. Intramolecular 1, 3-dipolar cycloaddition strategy for enantioselective synthesis of FR-900482 analogues. Org. Lett. 2001, 3, 2575–2578. [Google Scholar] [CrossRef]
- Seto, M.; Aikawa, K.; Miyamoto, N.; Aramaki, Y.; Kanzaki, N.; Takashima, K.; Shiraishi, M. Highly potent and orally active CCR5 antagonists as anti-HIV-1 agents: Synthesis and biological activities of 1-benzazocine derivatives containing a sulfoxide moiety. J. Med. Chem. 2006, 49, 2037–2048. [Google Scholar] [CrossRef]
- Wentland, M.; VanAlstine, M.; Kucejko, R.; Lou, R.; Cohen, D.J.; Parkhill, A.L.; Bidlack, J.M. Redefining the Structure− Activity Relationships of 2, 6-Methano-3-benzazocines. 4. Opioid Receptor Binding Properties of 8-[N-(4 ‘-phenyl)-phenethyl) carboxamido] Analogues of Cyclazocine and Ethyl ketocycalzocine. J. Med. Chem. 2006, 49, 5635–5639. [Google Scholar] [CrossRef]
- Kalis, M.M.; Huff, N.A. Oxcarbazepine, an antiepileptic agent. Clin. Ther. 2001, 23, 680–700. [Google Scholar] [CrossRef] [PubMed]
- Worayuthakarn, R.; Thasana, N.; Ruchirawat, S. Three Distinct Reactions of 3, 4-Dihydroisoquinolines with Azlactones: Novel Synthesis of Imidazoloisoquinolin-3-ones, Benzo [a] quinolizin-4-ones, and Benzo [d] azocin-4-ones. Org. Lett. 2006, 8, 5845–5848. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, T.; Sasaki, O.; Itô, S. Aza-Claisen rearrangement of amide enolates. Stereoselective synthesis of 2, 3-disubstituted carboxamides. Tetrahedron Lett. 1990, 31, 727–730. [Google Scholar] [CrossRef]
- Hadimani, M.B.; Mukherjee, R.; Banerjee, R.; Shoman, M.E.; Aly, O.M.; King, S.B. Ring expansions of acyloxy nitroso compounds. Tetrahedron Lett. 2015, 6, 5870–5873. [Google Scholar] [CrossRef]
- Hashimoto, T.; Naganawa, Y.; Maruoka, K. Stereoselective Construction of Seven-Membered Rings with an All-Carbon Quaternary Center by Direct Tiffeneau-Demjanov-type Ring Expansion. J. Am. Chem. 2009, 131, 6614–6617. [Google Scholar] [CrossRef]
- Donslund, B.S.; Jessen, N.I.; Bertuzzi, G.; Giardinetti, M.; Palazzo, T.A.; Christensen, M.L.; Jørgensen, K.A. Catalytic Enantioselective [10+4]-Cycloadditions. Angew. Chem. Int. 2018, 57, 13182–13186. [Google Scholar] [CrossRef] [PubMed]
- Uno, H.; Imai, T.; Harada, K.; Shibata, N. Synthesis of highly functionalized 12-membered trifluoromethyl heterocycles via a nondecarboxylative Pd-catalyzed [6+ 6] annulation. ACS Catal. 2020, 10, 1454–1459. [Google Scholar] [CrossRef]
- Katz, C.E.; Ribelin, T.; Withrow, D.; Basseri, Y.; Manukyan, A.K.; Bermudez, A.; Aubé, J. Nonbonded, Attractive Cation−π Interactions in Azide-Mediated Asymmetric Ring Expansion Reactions. J. Org. Chem. 2008, 73, 3318–3327. [Google Scholar] [CrossRef]
- Driggers, E.M.; Hale, S.P.; Lee, J.; Terrett, N.K. The exploration of macrocycles for drug discovery—An underexploited structural class. Nat. Rev. Drug Discov. 2008, 7, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Baud, L.G.; Manning, M.A.; Arkless, H.L.; Stephens, T.C.; Unsworth, W.P. Ring expansion approach to medium-sized lactams and analysis of their medicinal lead-like properties. Chem. Eur. J. 2017, 23, 2225–2230. [Google Scholar] [CrossRef]
- Guney, T.; Wenderski, T.A.; Boudreau, M.W.; Tan, D.S. Synthesis of Benzannulated Medium-ring Lactams via a Tandem Oxidative Dearomatization–Ring Expansion Reaction. Chem. Eur. J. 2018, 24, 13150–13157. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Huang, Z.; Li, Y.; Kuniyil, R.; Zhang, C.; Ackermann, L.; Ruan, Z. Catalyst-free, direct electrochemical synthesis of annulated medium-sized lactams through C–C bond cleavage. Green Chem. 2020, 22, 1099–1104. [Google Scholar] [CrossRef]
- Agag, T.; Takeichi, T. Synthesis and Characterization of Novel Benzoxazine Monomers Containing Allyl Groups and Their High Performance Thermosets. Macromolecules 2003, 36, 6010–6017. [Google Scholar] [CrossRef]
- Ishida, H.; Allen, D.J. Physical and mechanical characterization of near-zero shrinkagepolybenzoxazines. J. Polym. Sci. B Polym. Phys. 1996, 34, 1019–1030. [Google Scholar] [CrossRef]
- Kudoh, R.; Sudo, A.; Endo, T. Synthesis of eight-membered lactone having tertiary amine moiety by ring-expansion reaction of 1, 3-benzoxazine and its anionic ring-opening polymerization behavior. Macromolecules 2009, 42, 2327–2329. [Google Scholar] [CrossRef]
- Shintani, R.; Ikehata, K.; Hayashi, T. Synthesis of nine-membered azlactones by palladium catalyzed ring-expansion of γ-methylidene-δ-valerolactones with aziridines. J. Org. Chem. 2011, 76, 4776–4780. [Google Scholar] [CrossRef]
- Cowper, P.; Pockett, A.; Kociok-Köhn, G.; Cameron, P.J.; Lewis, S.E. Azulene–Thiophene–Cyanoacrylic acid dyes with donor-π-acceptor structures. Synthesis, characterisation and evaluation in dye-sensitized solar cells. Tetrahedron 2018, 74, 2775–2786. [Google Scholar] [CrossRef]
- López-Alled, C.M.; Sanchez-Fernandez, A.; Edler, K.J.; Sedgwick, A.C.; Bull, S.D.; McMullin, C.L.; Lewis, S.E. Azulene–boronate esters: Colorimetric indicators for fluoride in drinking water. Chem. Commun. 2017, 53, 12580–12583. [Google Scholar] [CrossRef] [PubMed]
- Cristalli, G.; Volpini, R.; Vittori, S.; Camaioni, E.; Rafaiani, G.; Potenza, S.; Vita, A. Diazepinone Nucleosides as Inhibitors of Cytidine Deaminase. Nucleosides Nucleotides Nucleic Acids 1996, 15, 1567–1580. [Google Scholar] [CrossRef]
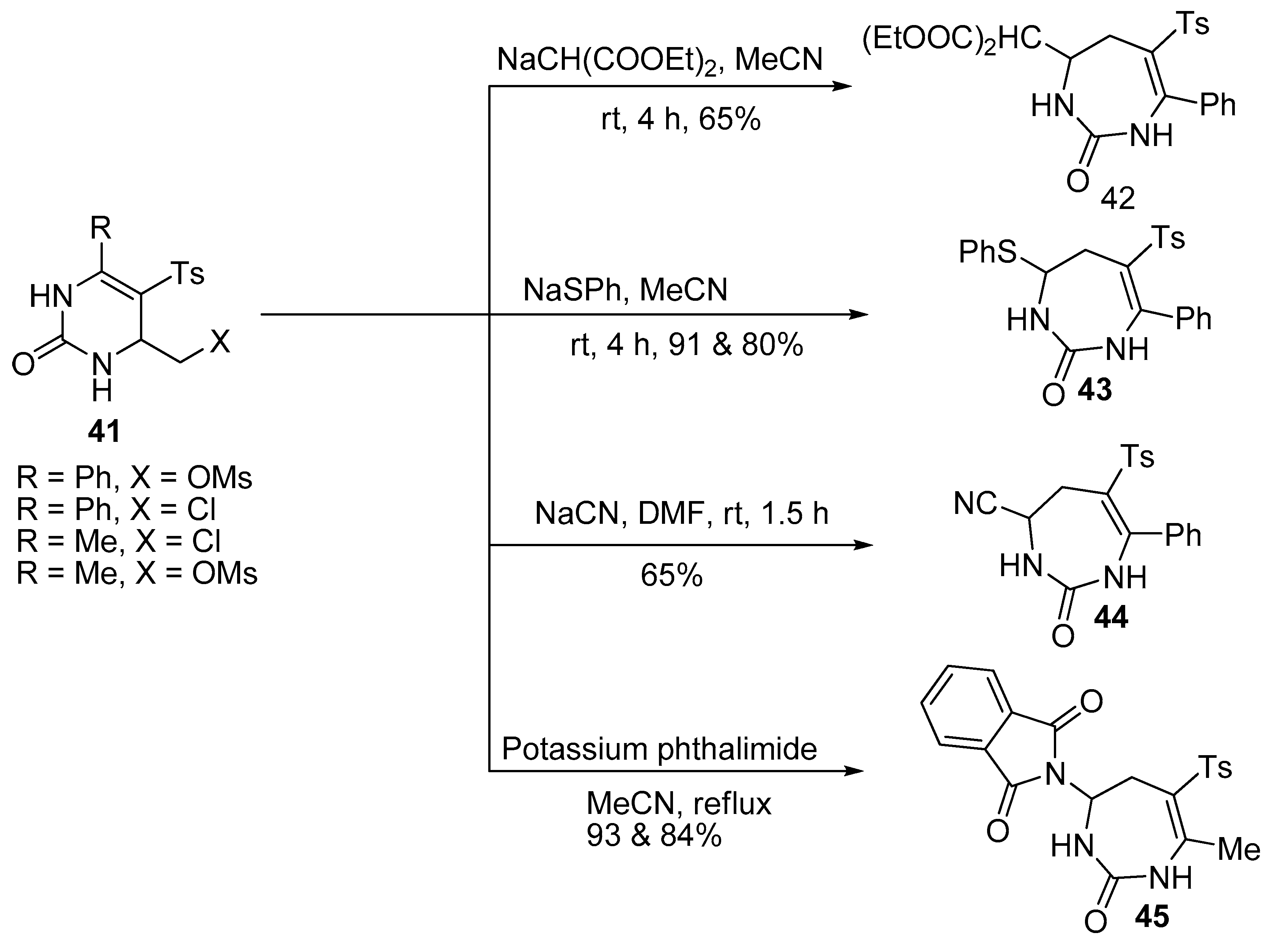
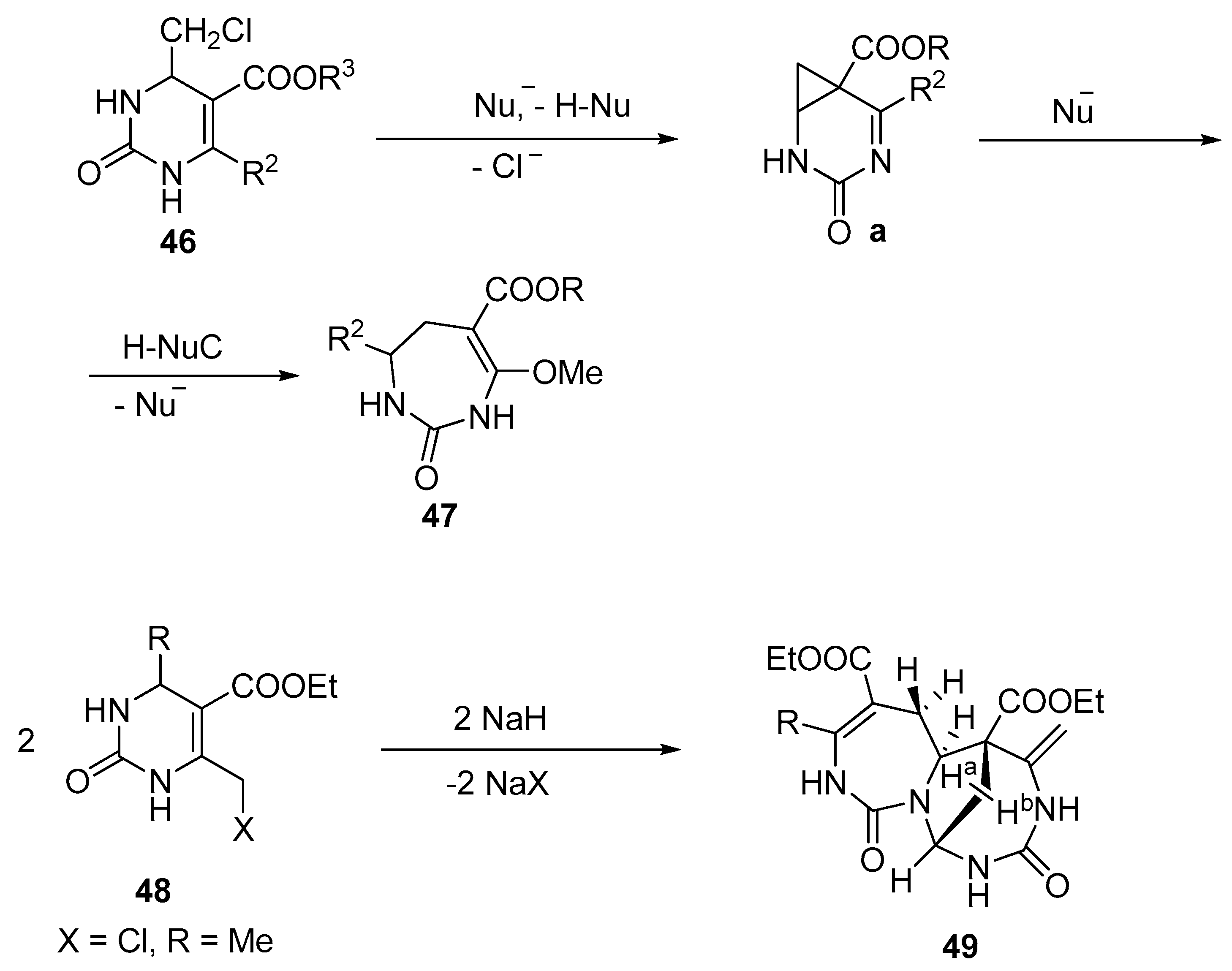
- Fesenko, A.A.; Trafimova, L.A.; Shutalev, A.D. Synthesis of functionalized tetrahydro-1, 3-diazepin-2-ones and 1-carbamoyl-1 H-pyrroles via ring expansion and ring expansion/ring contraction of tetrahydropyrimidines. Org. Biomol. Chem. 2012, 10, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Fesenko, A.A.; Trafimova, L.A.; Albov, D.V.; Shutalev, A.D. Nucleophile-dependent diastereoselectivity in the ring expansion of pyrimidines to give 1, 3-diazepines. Tetrahedron Lett. 2015, 56, 1317–1321. [Google Scholar] [CrossRef]
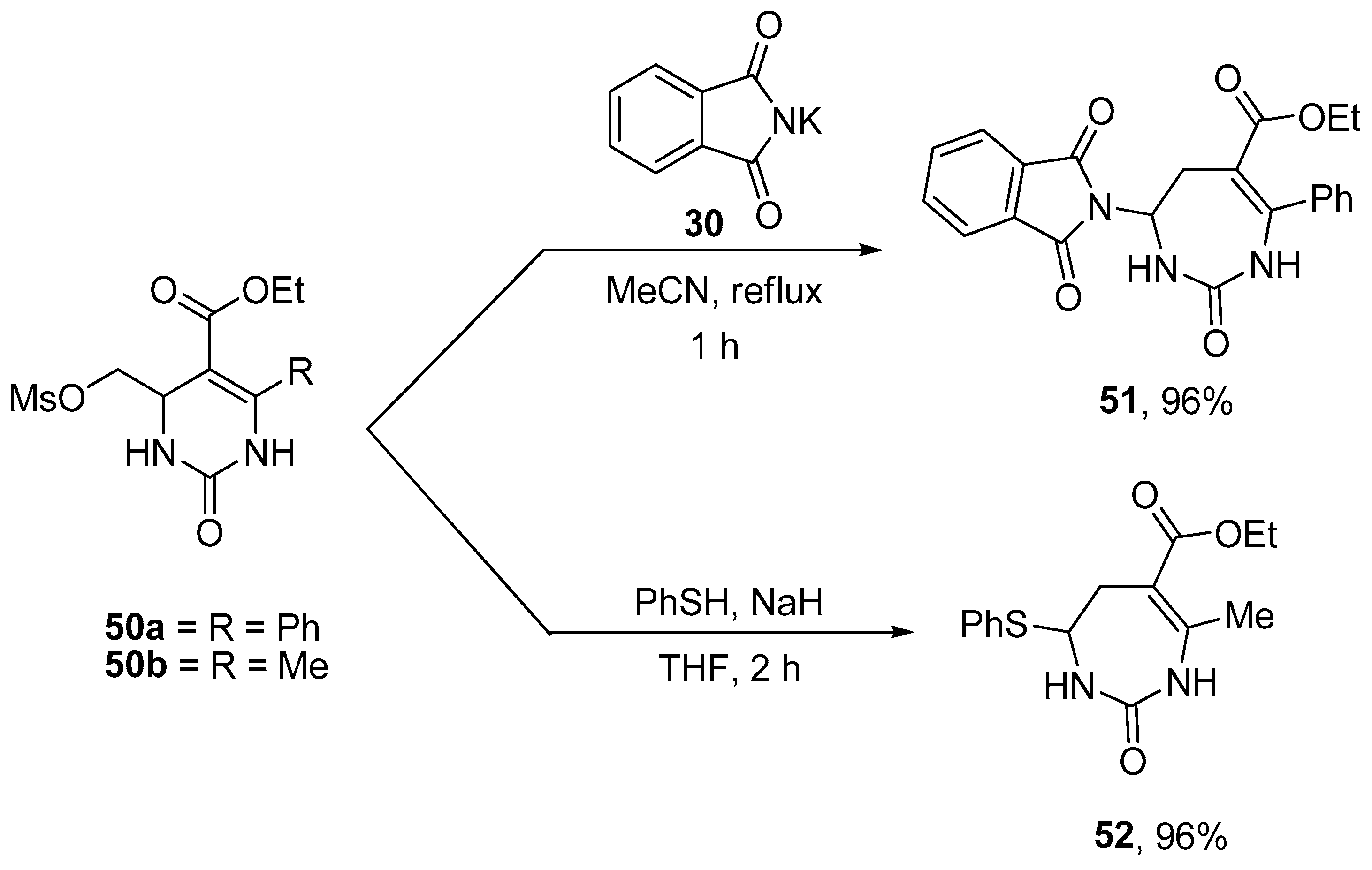
- Fesenko, A.A.; Shutalev, A.D. Nucleophile-mediated ring expansion of 4-chloromethyl- and 4-mesyloxymethyl5-tosyl-1,2,3,4-tetrahydropyrimidin-2-ones to 6-tosyl-2,3,4,5-tetrahydro-1H-1,3-diazepin-2-ones: Effect of the leaving group and the substituent at C6. Tetrahedron 2011, 36, 6876–6882. [Google Scholar] [CrossRef]
- Claremon, D.A.; Rosenthal, S.A. Preparation of 7-Alkyl-2,3,6,7-tetrahydro-4-alkyl-2-oxo- 1H-1,3-diazepine-5-carboxylates by Ring Expansion-Nucleophilic Addition. Synthesis 1986, 8, 664–665. [Google Scholar] [CrossRef]
- Bullock, E.; Carter, R.A.; Cochrane, R.M.; Gregory, B.; Shields, D.C. The synthesis of 1,3-diazepine derivatives by the ring expansion of alkyl 4-chloromethyl-1,2,3,4-tetrahydro-6-methyl-2- oxopyrimidine-5-carboxylates. Can. J. Chem. 1977, 55, 895–905. [Google Scholar] [CrossRef]
- Shutalev, A.D.; Fesenko, A.A.; Cheshkov, D.A.; Goliguzov, D.V. Unprecedented base-promoted cascade transformation of a pyrimidinone derivative into a novel tricyclic bis-diazepinone. Tetrahedron Lett. 2008, 49, 4099–4101. [Google Scholar] [CrossRef]
- Fesenko, A.A.; Grigoriev, M.S.; Shutalev, A.D. Nucleophile-Mediated Ring Expansion of 5-Acyl-substituted 4- Mesyloxymethyl-1,2,3,4-tetrahydropyrimidin-2-ones in the Synthesis of 7- Membered Analogues of Biginelli Compounds and Related Heterocycles. J. Org. Chem. 2017, 82, 8085–8110. [Google Scholar] [CrossRef]
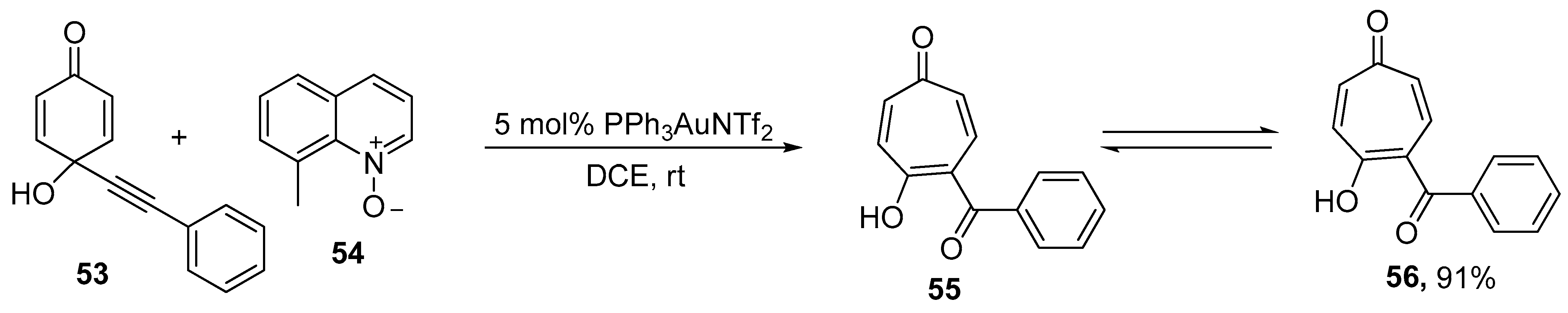
- Zhao, J.; Liu, J.; Xie, X.; Li, S.; Liu, Y. Gold-Catalyzed Synthesis of Tropone and Its Analogues via Oxidative Ring Expansion of Alkynyl Quinols. Org. Lett. 2015, 17, 5926–5929. [Google Scholar] [CrossRef]
- Du, Y.; Huang, B.; Zeng, J.; Cai, M. Recyclable heterogeneous gold(I)-catalyzed oxidative ring expansion of alkynyl quinols: A practical access to tropone and its analogues. Dalton Trans. 2021, 50, 6488–6499. [Google Scholar] [CrossRef]
- Ishihara, Y.; Hirai, K.; Miyamoto, M.; Goto, G. Central cholinergic agents. 6. Synthesis and evaluation of 3-[1-(phenylmethyl)-4-piperidinyl]-1-(2, 3, 4, 5-tetrahydro-1H-1-benzazepin-8-yl)-1-propanones and their analogs as central selective acetylcholinesterase inhibitors. J. Med. Chem. 1994, 37, 2292–2299. [Google Scholar] [CrossRef] [PubMed]
- Voskressensky, L.G.; Listratova, A.V.; Borisova, T.N.; Alexandrov, G.G.; Varlamov, A.V. Synthesis of Benzoazocines from Substituted Tetrahydroisoquinolines and Activated Alkynes in a Tetrahydropyridine Ring Expansion. Eur. J. Org. Chem. 2007, 2007, 6106–6117. [Google Scholar] [CrossRef]
- Søhoel, H.; Liljefors, T.; Ley, S.V.; Oliver, S.F.; Antonello, A.; Smith, M.D.; Christensen, S.B. Total synthesis of two novel subpicomolar sarco/endoplasmatic reticulum Ca2+-ATPase inhibitors designed by an analysis of the binding site of thapsigargin. J. Med. Chem. 2005, 48, 7005–7011. [Google Scholar] [CrossRef]
- Graening, T.; Schmalz, H.G. Total syntheses of colchicine in comparison: A journey through 50 years of synthetic organic chemistry. Angew. Chem. Int. 2004, 43, 3230–3256. [Google Scholar] [CrossRef]
- Miyashita, M.; Hara, S.; Yoshikoshi, A. Regiospecific synthesis of .beta.-thujaplicin (hinokitiol) from 2-isopropylphenol. J. Org. Chem. 1987, 52, 2602–2604. [Google Scholar] [CrossRef]
- Lallemand, A.; Varin, M.; Chiaroni, J.Y.; Iorga, B.; Guillou, C. A new access to dihydrotropones through ring expansion of spirocyclohexadienones: Synthesis and mechanism. J. Org. Chem. 2007, 72, 6421–6426. [Google Scholar] [CrossRef]
- Xu, W.; Zou, J.P.; Mu, X.J.; Zhang, W. Free radical ring expansion and spirocyclization of 1, 3-diketone derivatives. Tetrahedron Lett. 2008, 49, 7311–7314. [Google Scholar] [CrossRef]
- Ballesteros-Garrido, R.; Rix, D.; Besnard, C.; Lacour, J. Medium-Sized Rings versus Macrocycles through Rhodium-Catalyzed Ring-Expansion Reactions of Cyclic Acetals. Chem. Eur. J. 2012, 18, 6626–6631. [Google Scholar] [CrossRef]
- Silva, S.B.L.; Torre, A.D.; De Carvalho, J.E.; Ruiz, A.L.T.G.; Silva, L.F. Seven-membered rings through metal-free rearrangement mediated by hypervalent iodine. Molecules 2015, 20, 1475–1494. [Google Scholar] [CrossRef]
- Sarfraz, I.; Rasul, A.; Hussain, G.; Shah, M.A.; Zahoor, A.F.; Asrar, M.; Selamoglu, Z.; Ji, X.-Y.; Adem, S.; Sarker, S.D. 6-Phosphogluconate dehydrogenase fuels multiple aspects of cancer cells: From cancer initiation to metastasis and chemoresistance. BioFactors 2020, 46, 550. [Google Scholar] [CrossRef]
- Yang, C.S.; Lambert, J.D.; Ju, J.; Lu, G.; Sang, S. Tea and cancer prevention: Molecular mechanisms and human relevance. Toxicol. Appl. Pharmacol. 2007, 224, 265–273. [Google Scholar] [CrossRef]
- Baba, M.; Nishimura, O.; Kanzaki, N.; Okamoto, M.; Sawada, H.; Iizawa, Y.; Shiraishi, M.; Aramaki, Y.; Okonogi, K.; Ogawa, Y.; et al. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA 1999, 96, 5698–5703. [Google Scholar] [CrossRef]
- Silva, L.F.; Vasconcelos, R.S.; Nogueira, M.A. Iodine(III)-Promoted Ring Expansion of 1-Vinylcycloalkanol Derivatives: A Metal-Free Ap-proach toward Seven-Membered Rings. Org. Lett. 2008, 10, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Silva, L.F.; Rabnawaz, M. Iodine(III)-Promoted Ring Expansion Reactions: A Metal-Free Approach toward Seven-Membered Heterocyclic Rings. Asian J. Org. Chem. 2021, 10, 2549–2552. [Google Scholar] [CrossRef]
- Ganguly, A.K.; Alluri, S.S.; Caroccia, D.; Biswas, D.; Wang, C.H.; Kang, E.; Munshi, V.; Orth, P.; Strickland, C. Design, synthesis, and X-ray crystallographic analysis of a novel class of HIV-1 protease inhibitors. J. Med. Chem. 2011, 54, 7176–7183. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, L.; Gorojankina, T.; Dauban, P.; Faure, H.; Ruat, M.; Dodd, R.H. Design and synthesis of cyclic sulfonamides and sulfamates as new calcium sensing receptor agonists. Bioorg. Med. Chem. Lett. 2010, 20, 7483–7487. [Google Scholar] [CrossRef]
- Tollefson, M.B.; Kolodziej, S.A.; Fletcher, T.R.; Vernier, W.F.; Beaudry, J.A.; Keller, B.T.; Reitz, D.B. A novel class of apical sodium co-dependent bile acid transporter inhibitors: The 1, 2-benzothiazepines. Bioorg. Med. Chem. Lett. 2003, 13, 3727–3730. [Google Scholar] [CrossRef]
- Xia, A.J.; Kang, T.R.; He, L.; Chen, L.M.; Li, W.T.; Yang, J.L.; Liu, Q.Z. Metal-Free Ring-Expansion Reaction of Six-membered Sulfonylimines with Diazomethanes: An Approach toward Seven-Membered Enesulfonamides. Angew. Chem. Int. 2016, 55, 1441–1444. [Google Scholar] [CrossRef]
- Michael-Titus, A.; Costentin, J. Analgesic effects of metapramine and evidence against the involvement of endogenous enkephalins in the analgesia induced by tricyclic antidepressants. Pain 1987, 31, 391–400. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, X.; Yao, Q.; Zhao, Y.; Li, Y. Insertion of Isolated Alkynes into Carbon–Carbon σ-Bonds of Unstrained Cyclic β-Ketoesters via Transition-Metal-Free Tandem Reactions: Synthesis of Medium-Sized Ring Compounds. Chem. Eur. J. 2016, 22, 17936–17939. [Google Scholar] [CrossRef]
- Hall, J.E.; Matlock, J.V.; Ward, J.W.; Gray, K.V.; Clayden, J. Medium-Ring Nitrogen Heterocycles through Migratory Ring Expansion of Metalated Ureas. Angew. Chem. Int. Ed. 2016, 55, 11153–11157. [Google Scholar] [CrossRef] [PubMed]
- Mehlmann, J.F.; Crawley, M.L.; Lundquist IV, J.T.; Unwalla, R.J.; Harnish, D.C.; Evans, M.J.; Mahaney, P.E. Pyrrole [2, 3-d] azepino compounds as agonists of the farnesoid X receptor (FXR). Bioorg. Med. Chem. Lett. 2009, 19, 5289–5292. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Cai, Y.; Zheng, C.; Dai, L.X.; You, S.L. Iridium-Catalyzed Enantioselective Synthesis of Pyrrole-Annulated Medium-Sized-Ring Compounds. Angew. Chem. Int. Ed. 2017, 129, 10681–10684. [Google Scholar] [CrossRef]
- Ahmad, S.; Yousaf, M.; Mansha, A.; Rasool, N.; Zahoor, A.F.; Hafeez, F.; Rizvi, S.M.A. Ring-opening reactions of oxetanes: A review of methodology development and synthetic applications. Syn. Commun. 2016, 46, 1397–1416. [Google Scholar] [CrossRef]
- Grant, T.N.; Benson, C.L.; West, F.G. Ring Expansion of Lactones and Lactams via Propiolate 1-Carbon Intercalation. Org. Lett. 2008, 10, 3985–3988. [Google Scholar] [CrossRef]
- Saeed, S.; Zahoor, A.F.; Ahmad, M.; Anjum, M.N.; Akhtar, R.; Shahzadi, I. Synthetic methodologies for the construction of selenium-containing heterocycles: A review. Phosphorus Sulfur Silicon Relat. Elem. 2022, 197, 1096–1122. [Google Scholar] [CrossRef]
- Sashida, H.; Nakayama, A.; Kaname, M. A New One-Pot Synthetic Method for Selenium-Containing Medium-Sized a,b-Unsaturated Cyclic Ketones. Synthesis 2008, 20, 3229–3236. [Google Scholar] [CrossRef]
- Liu, J.; Li, Z.; Mei, H.; Soloshonok, V.A.; Han, J. Detrifluoroacetylative in Situ Generated Cyclic Fluorinated Enolates for the Preparation of Compounds Featuring a C–F Stereogenic Center. ACS Omega 2019, 4, 19505–19512. [Google Scholar] [CrossRef]
- Uno, H.; Punna, N.; Tokunaga, E.; Shiro, M.; Shibata, N. Synthesis of Both Enantiomers of Nine-Membered CF3-Substituted Heterocycles Using a Single Chiral Ligand by Palladium-Catalyzed Decarboxylative Ring Expansion with Kinetic Resolution. Angew. Chem. Int. Ed. 2020, 59, 8187–8194. [Google Scholar] [CrossRef]


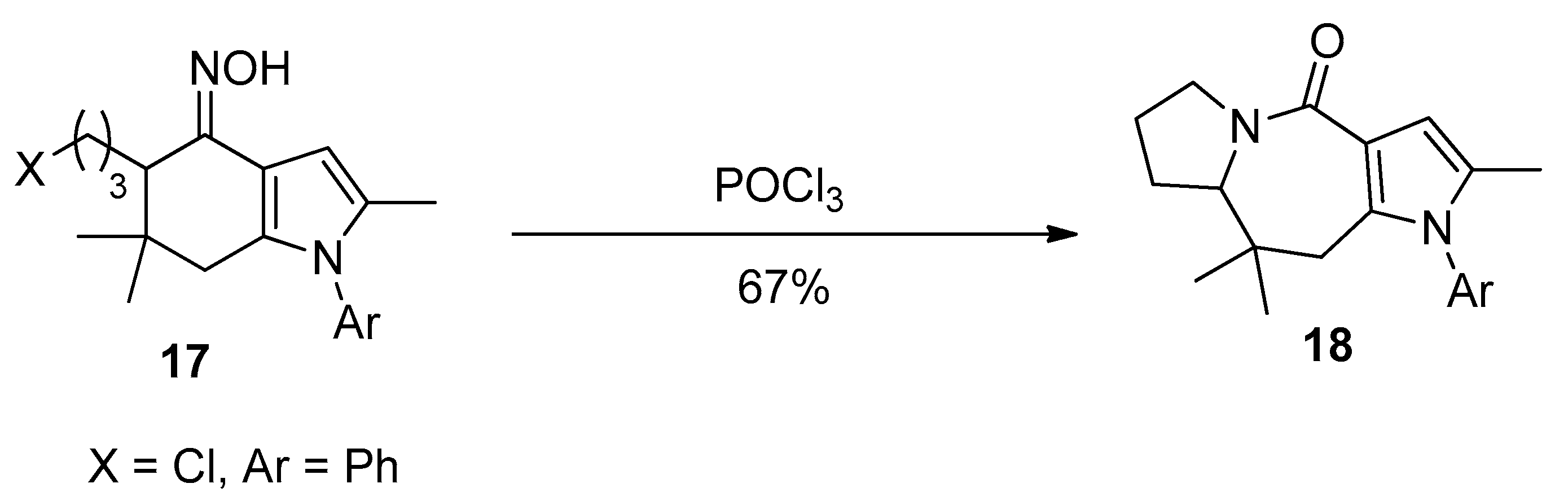

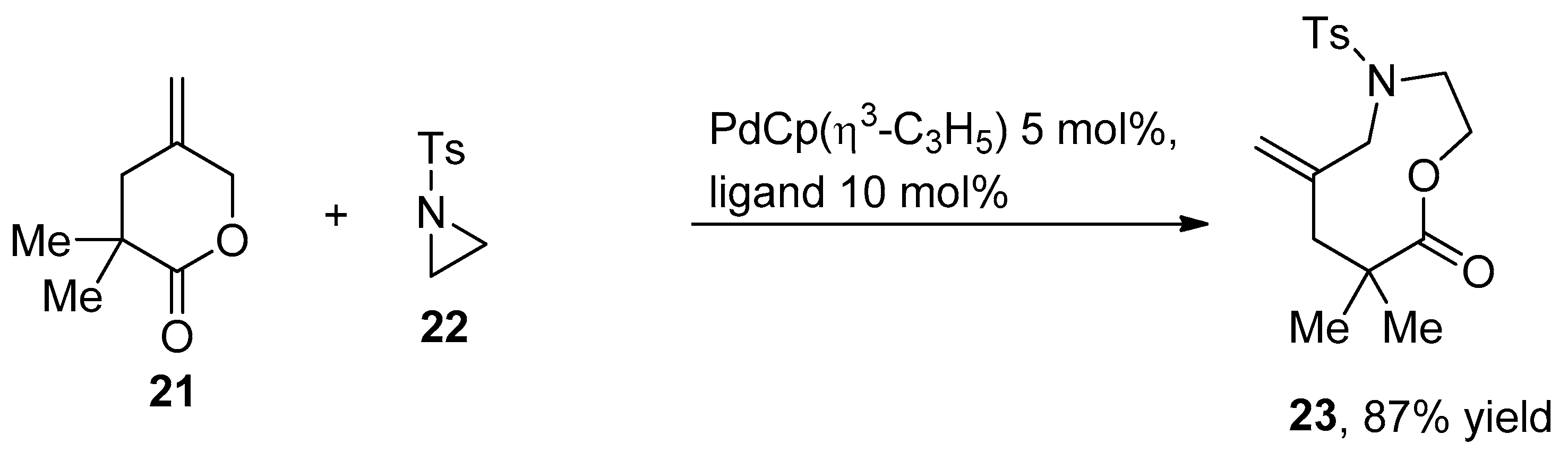

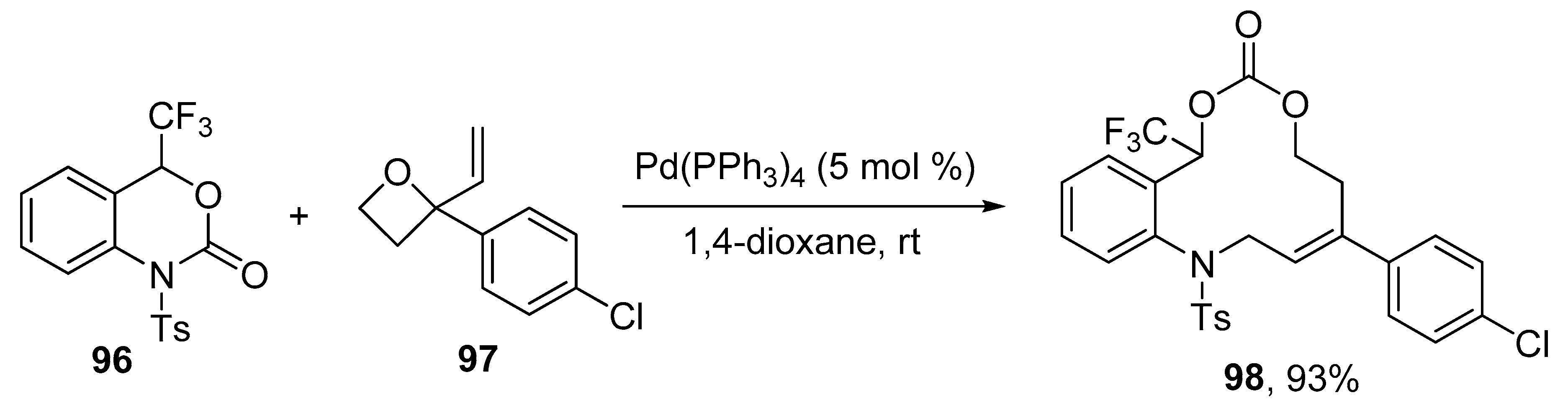

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noor, R.; Zahoor, A.F.; Mansha, A.; Khan, S.G.; Haq, A.U.; Ahmad, S.; Al-Hussain, S.A.; Irfan, A.; Zaki, M.E.A. Synthetic Potential of Regio- and Stereoselective Ring Expansion Reactions of Six-Membered Carbo- and Heterocyclic Ring Systems: A Review. Int. J. Mol. Sci. 2023, 24, 6692. https://doi.org/10.3390/ijms24076692
Noor R, Zahoor AF, Mansha A, Khan SG, Haq AU, Ahmad S, Al-Hussain SA, Irfan A, Zaki MEA. Synthetic Potential of Regio- and Stereoselective Ring Expansion Reactions of Six-Membered Carbo- and Heterocyclic Ring Systems: A Review. International Journal of Molecular Sciences. 2023; 24(7):6692. https://doi.org/10.3390/ijms24076692
Chicago/Turabian StyleNoor, Rida, Ameer Fawad Zahoor, Asim Mansha, Samreen Gul Khan, Atta Ul Haq, Sajjad Ahmad, Sami A. Al-Hussain, Ali Irfan, and Magdi E. A. Zaki. 2023. "Synthetic Potential of Regio- and Stereoselective Ring Expansion Reactions of Six-Membered Carbo- and Heterocyclic Ring Systems: A Review" International Journal of Molecular Sciences 24, no. 7: 6692. https://doi.org/10.3390/ijms24076692
APA StyleNoor, R., Zahoor, A. F., Mansha, A., Khan, S. G., Haq, A. U., Ahmad, S., Al-Hussain, S. A., Irfan, A., & Zaki, M. E. A. (2023). Synthetic Potential of Regio- and Stereoselective Ring Expansion Reactions of Six-Membered Carbo- and Heterocyclic Ring Systems: A Review. International Journal of Molecular Sciences, 24(7), 6692. https://doi.org/10.3390/ijms24076692






