Morphine Withdrawal-Induced Hyperalgesia in Models of Acute and Extended Withdrawal Is Attenuated by l-Tetrahydropalmatine
Abstract
:1. Introduction
2. Results
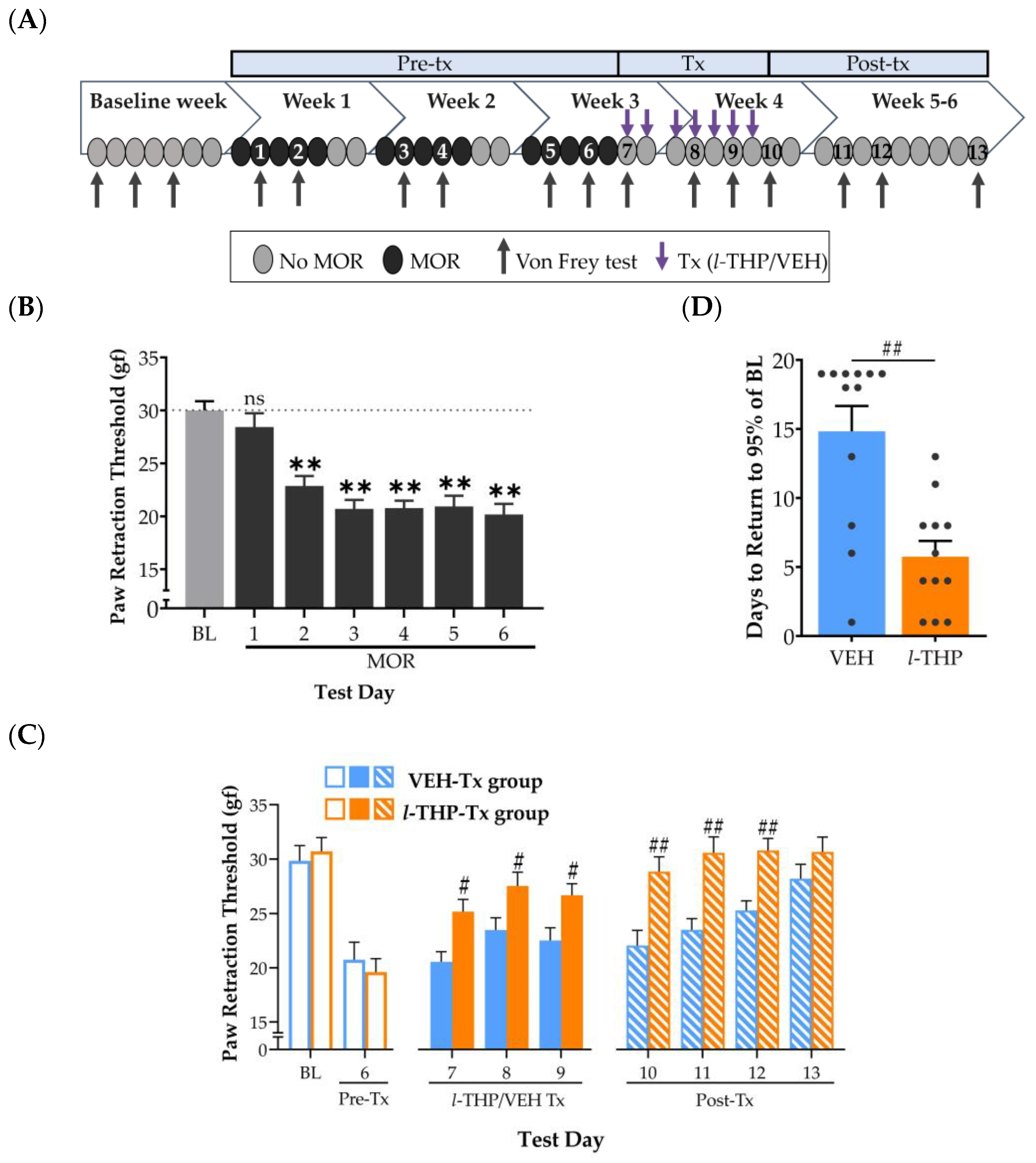
2.1. Experiment 1: Hyperalgesia during Acute Withdrawal from Morphine Is Attenuated by l-THP
2.2. Experiment 2: Repeated l-THP Facilitates Recovery of Hyperalgesia during Extended Withdrawal from Morphine
2.3. Experiment 3: Effect of l-THP in Morphine-Naïve Rats
2.4. Experiment 4: Effect of Withdrawal on Locomotor Activity in Morphine-Dependent Rats
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drug Preparation and Administration
4.3. Assessment of Hyperalgesia with Von Frey Device
4.4. Assessment of Locomotor Activity in Open Field Test
4.5. Experiment 1
4.6. Experiment 2
4.7. Experiment 3
4.8. Experiment 4
4.9. Data Presentation and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Dasgupta, N.; Beletsky, L.; Ciccarone, D. Opioid Crisis: No Easy Fix to Its Social and Economic Determinants. Am. J. Public Health 2018, 108, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Alexander, G.C.; Stoller, K.B.; Haffajee, R.L.; Saloner, B. An Epidemic in the Midst of a Pandemic: Opioid Use Disorder and COVID-19. Ann. Intern. Med. 2020, 173, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Kosten, T.R.; Baxter, L.E. Review Article: Effective Management of Opioid Withdrawal Symptoms: A Gateway to Opioid Dependence Treatment. Am. J. Addict. 2019, 28, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F. Neurobiology of Opioid Addiction: Opponent Process, Hyperkatifeia, and Negative Reinforcement. Biol. Psychiatry 2020, 87, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S.; Arcuri, E.; Santoni, A. Opioid-Induced Tolerance and Hyperalgesia. CNS Drugs 2019, 33, 943–955. [Google Scholar] [CrossRef]
- Higgins, C.; Smith, B.H.; Matthews, K. Evidence of Opioid-Induced Hyperalgesia in Clinical Populations after Chronic Opioid Exposure: A Systematic Review and Meta-Analysis. Br. J. Anaesth. 2019, 122, e114–e126. [Google Scholar] [CrossRef]
- Tompkins, D.A.; Campbell, C.M. Opioid-Induced Hyperalgesia: Clinically Relevant or Extraneous Research Phenomenon? Curr. Pain Headache Rep. 2011, 15, 129–136. [Google Scholar] [CrossRef]
- Angst, M.S.; Clark, J.D. Opioid-Induced Hyperalgesia: A Qualitative Systematic Review. Anesthesiology 2006, 104, 570–587. [Google Scholar] [CrossRef]
- Ren, Z.-Y.; Shi, J.; Epstein, D.H.; Wang, J.; Lu, L. Abnormal Pain Response in Pain-Sensitive Opiate Addicts after Prolonged Abstinence Predicts Increased Drug Craving. Psychopharmacology 2009, 204, 423–429. [Google Scholar] [CrossRef]
- Carcoba, L.M.; Contreras, A.E.; Cepeda-Benito, A.; Meagher, M.W. Negative Affect Heightens Opiate Withdrawal-Induced Hyperalgesia in Heroin Dependent Individuals. J. Addict. Dis. 2011, 30, 258–270. [Google Scholar] [CrossRef]
- Dunbar, S.A.; Karamian, I.; Zhang, J. Ketorolac Prevents Recurrent Withdrawal Induced Hyperalgesia but Does Not Inhibit Tolerance to Spinal Morphine in the Rat. Eur. J. Pain 2007, 11, 1–6. [Google Scholar] [CrossRef]
- Alvarez-Bagnarol, Y.; Marchette, R.C.N.; Francis, C.; Morales, M.; Vendruscolo, L.F. Neuronal Correlates of Hyperalgesia and Somatic Signs of Heroin Withdrawal in Male and Female Mice. eNeuro 2022, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Marchette, R.C.N.; Gregory-Flores, A.; Tunstall, B.J.; Carlson, E.R.; Jackson, S.N.; Sulima, A.; Rice, K.C.; Koob, G.F.; Vendruscolo, L.F. κ-Opioid Receptor Antagonism Reverses Heroin Withdrawal-Induced Hyperalgesia in Male and Female Rats. Neurobiol. Stress 2021, 14, 100325. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, D.S.; McKendrick, G.; Graziane, N.M. Anterior Cingulate Cortex Is Necessary for Spontaneous Opioid Withdrawal and Withdrawal-Induced Hyperalgesia in Male Mice. Neuropsychopharmacology 2021, 46, 1990–1999. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, V.; Tanga, F.Y.; DeLeo, J.A. Attenuation of Morphine Tolerance, Withdrawal-Induced Hyperalgesia, and Associated Spinal Inflammatory Immune Responses by Propentofylline in Rats. Neuropsychopharmacology 2004, 29, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F. Drug Addiction: Hyperkatifeia/Negative Reinforcement as a Framework for Medications Development. Pharmacol. Rev. 2021, 73, 163–201. [Google Scholar] [CrossRef] [PubMed]
- McHugh, R.K.; Chase, A.; Trinh, C.D.; Weiss, R.D. Are Pain and Negative Affect Coping Distinct Motives for Opioid Misuse? Subst. Use Misuse 2022, 57, 848–852. [Google Scholar] [CrossRef]
- Laroche, F.; Rostaing, S.; Aubrun, F.; Perrot, S. Pain Management in Heroin and Cocaine Users. Joint Bone Spine 2012, 79, 446–450. [Google Scholar] [CrossRef]
- Jaffal, S.; Abazid, H. Medicinal Plants and Addiction Treatment. In Handbook of Substance Misuse and Addictions: From Biology to Public Health; Patel, V.B., Preedy, V.R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–26. ISBN 978-3-030-67928-6. [Google Scholar]
- Wang, L.; Zhang, Y.; Wang, Z.; Gong, N.; Kweon, T.D.; Vo, B.; Wang, C.; Zhang, X.; Chung, J.Y.; Alachkar, A.; et al. The Antinociceptive Properties of the Corydalis Yanhusuo Extract. PLoS ONE 2016, 11, e0162875. [Google Scholar] [CrossRef]
- Min, X.; Lee, D.T.S.; Jinhua, X.; Wenjun, D.; Li, C.; Bin, D.; Pingxiang, D.; Wingho, L.; Xiaoyin, T.; Xiaohui, Z. A Database on Treating Drug Addiction with Traditional Chinese Medicine. Addiction 2007, 102, 282–288. [Google Scholar] [CrossRef]
- Sun, M.; Liu, J.; Lin, C.; Miao, L.; Lin, L. Alkaloid Profiling of the Traditional Chinese Medicine Rhizoma corydalis Using High Performance Liquid Chromatography-Tandem Quadrupole Time-of-Flight Mass Spectrometry. Acta Pharm. Sin. B 2014, 4, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Nesbit, M.O.; Phillips, A.G. Tetrahydroprotoberberines: A Novel Source of Pharmacotherapies for Substance Use Disorders? Trends Pharmacol. Sci. 2020, 41, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Desgrouas, C.; Taudon, N.; Bun, S.-S.; Baghdikian, B.; Bory, S.; Parzy, D.; Ollivier, E. Ethnobotany, Phytochemistry and Pharmacology of Stephania Rotunda Lour. J. Ethnopharmacol. 2014, 154, 537–563. [Google Scholar] [CrossRef] [PubMed]
- Semwal, D.K.; Semwal, R.B. Efficacy and Safety of Stephania Glabra: An Alkaloid-Rich Traditional Medicinal Plant. Nat. Prod. Res. 2015, 29, 396–410. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Song, N.; Lu, T.; Pan, Y.; Song, J.; Chen, G.; Sun, L.; Li, N. Rapid Characterization of TCM Qianjinteng by UPLC-QTOF-MS and Its Application in the Evaluation of Three Species of Stephania. J. Pharm. Biomed. Anal. 2018, 156, 284–296. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, M.; Liu, H.; Liu, S. A Critical Review: Traditional Uses, Phytochemistry, Pharmacology and Toxicology of Stephania tetrandra S. Moore (Fen Fang Ji). Phytochem. Rev. 2020, 19, 449–489. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Y.; Huang, Y.; Lu, L. Chinese Herbal Medicine for the Treatment of Drug Addiction. In International Review of Neurobiology; Zeng, B.-Y., Zhao, K., Eds.; Neurobiology of Chinese Herb Medicine; Academic Press: Cambridge, MA, USA, 2017; Volume 135, pp. 279–295. [Google Scholar]
- Tian, B.; Tian, M.; Huang, S.-M. Advances in Phytochemical and Modern Pharmacological Research of Rhizoma corydalis. Pharm. Biol. 2020, 58, 265–275. [Google Scholar] [CrossRef]
- Liu, J.; Dai, R.; Damiescu, R.; Efferth, T.; Lee, D.Y.W. Role of Levo-Tetrahydropalmatine and Its Metabolites for Management of Chronic Pain and Opioid Use Disorders. Phytomedicine 2021, 90, 153594. [Google Scholar] [CrossRef]
- Du, Q.; Meng, X.; Wang, S. A Comprehensive Review on the Chemical Properties, Plant Sources, Pharmacological Activities, Pharmacokinetic and Toxicological Characteristics of Tetrahydropalmatine. Front. Pharmacol. 2022, 13, 890078. [Google Scholar] [CrossRef]
- Mantsch, J.R.; Li, S.-J.; Risinger, R.; Awad, S.; Katz, E.; Baker, D.A.; Yang, Z. Levo-Tetrahydropalmatine Attenuates Cocaine Self-Administration and Cocaine-Induced Reinstatement in Rats. Psychopharmacology 2007, 192, 581–591. [Google Scholar] [CrossRef]
- Figueroa-Guzman, Y.; Mueller, C.; Vranjkovic, O.; Wisniewski, S.; Yang, Z.; Li, S.-J.; Bohr, C.; Graf, E.N.; Baker, D.A.; Mantsch, J.R. Oral Administration of Levo-Tetrahydropalmatine Attenuates Reinstatement of Extinguished Cocaine Seeking by Cocaine, Stress or Drug-Associated Cues in Rats. Drug Alcohol Depend. 2011, 116, 72–79. [Google Scholar] [CrossRef]
- Sushchyk, S.; Xi, Z.-X.; Wang, J.B. Combination of Levo-Tetrahydropalmatine and Low Dose Naltrexone: A Promising Treatment for Prevention of Cocaine Relapse. J. Pharmacol. Exp. Ther. 2016, 357, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Yue, K.; Ma, B.; Xing, J.; Gan, Y.; Wang, D.; Jin, G.; Li, C. Levo-Tetrahydropalmatine, a Natural, Mixed Dopamine Receptor Antagonist, Inhibits Methamphetamine Self-Administration and Methamphetamine-Induced Reinstatement. Pharmacol. Biochem. Behav. 2016, 144, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Li, R.; Wu, J. Effects of Yuanhu-Zhitong Tablets on Alcohol-Induced Conditioned Place Preference in Mice. Biomed. Pharmacother. 2021, 133, 110962. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Ma, B.; Ru, Q.; Chen, L.; Gan, Y.; Wang, D.; Jin, G.; Li, C. The Dopamine Receptor Antagonist Levo-Tetrahydropalmatine Attenuates Heroin Self-Administration and Heroin-Induced Reinstatement in Rats. Pharmacol. Biochem. Behav. 2012, 102, 1–5. [Google Scholar] [CrossRef]
- Jiang, W.-N.; Jing, X.; Li, M.; Deng, H.; Jiang, T.; Xiong, K.-Z.; Chen, Y.; Wang, X.-F.; Wang, Q.-J. Corydaline and L-Tetrahydropalmatine Attenuate Morphine-Induced Conditioned Place Preference and the Changes in Dopamine D2 and GluA1 AMPA Receptor Expression in Rats. Eur. J. Pharmacol. 2020, 884, 173397. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, J.; Yan, L.; Su, R.; Wu, C.; Gong, Z. Effects of L-Tetrahydropalmatine on Locomotor Sensitization to Oxycodone in Mice. Acta Pharmacol. Sin. 2005, 26, 533–538. [Google Scholar] [CrossRef]
- Du, K.; Wang, Z.; Zhang, H.; Zhang, Y.; Su, H.; Wei, Z.; Zhang, C.; Yun, K.; Cong, B. Levo-Tetrahydropalmatine Attenuates the Acquisition of Fentanyl-Induced Conditioned Place Preference and the Changes in ERK and CREB Phosphorylation Expression in Mice. Neurosci. Lett. 2021, 756, 135984. [Google Scholar] [CrossRef]
- Alhassen, L.; Nuseir, K.; Ha, A.; Phan, W.; Marmouzi, I.; Shah, S.; Civelli, O. The Extract of Corydalis Yanhusuo Prevents Morphine Tolerance and Dependence. Pharmaceuticals 2021, 14, 1034. [Google Scholar] [CrossRef]
- Yang, Z.; Shao, Y.; Li, S.; Qi, J.; Zhang, M.; Hao, W.; Jin, G. Medication of L-Tetrahydropalmatine Significantly Ameliorates Opiate Craving and Increases the Abstinence Rate in Heroin Users: A Pilot Study1. Acta Pharmacol. Sin. 2008, 29, 781–788. [Google Scholar] [CrossRef]
- Hassan, H.E.; Kelly, D.; Honick, M.; Shukla, S.; Ibrahim, A.; Gorelick, D.A.; Glassman, M.; McMahon, R.P.; Wehring, H.J.; Kearns, A.M.; et al. Pharmacokinetics and Safety Assessment of L-Tetrahydropalmatine in Cocaine Users: A Randomized, Double-Blind, Placebo-Controlled Study. J. Clin. Pharmacol. 2017, 57, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, S.; Qing, G.; Zhang, X. Determination of L-Tetrahydropalmatine in Human Plasma by HPLC and Pharmacokinetics of Its Disintegrating Tablets in Healthy Chinese. Eur. J. Drug Metab. Pharmacokinet. 2011, 36, 257–262. [Google Scholar] [CrossRef]
- Kang, D.-W.; Moon, J.-Y.; Choi, J.-G.; Kang, S.-Y.; Ryu, Y.; Park, J.B.; Lee, J.-H.; Kim, H.-W. Antinociceptive Profile of Levo-Tetrahydropalmatine in Acute and Chronic Pain Mice Models: Role of Spinal Sigma-1 Receptor. Sci. Rep. 2016, 6, 37850. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Wang, T.-X.; Zhou, J.-C.; Qu, W.-M.; Huang, Z.-L. Dopamine D1 and D2 Receptors Mediate Analgesic and Hypnotic Effects of L-Tetrahydropalmatine in a Mouse Neuropathic Pain Model. Psychopharmacology 2019, 236, 3169–3182. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-H.; Wu, D.-L.; Gao, L.-Y.; Fang, Y.; Ge, W.-H. L-Tetrahydropalmatine Alleviates Mechanical Hyperalgesia in Models of Chronic Inflammatory and Neuropathic Pain in Mice. NeuroReport 2016, 27, 476. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.Y.; Jin, G.Z. Supraspinal D2 Receptor Involved in Antinociception Induced by L-Tetrahydropalmatine. Zhongguo Yao Li Xue Bao 1999, 20, 715–719. [Google Scholar]
- Chu, H.; Jin, G.; Friedman, E.; Zhen, X. Recent Development in Studies of Tetrahydroprotoberberines: Mechanism in Antinociception and Drug Addiction. Cell. Mol. Neurobiol. 2008, 28, 491–499. [Google Scholar] [CrossRef]
- Ingram, S.L. Pain: Novel Analgesics from Traditional Chinese Medicines. Curr. Biol. CB 2014, 24, R114–R116. [Google Scholar] [CrossRef]
- Ahn, S.; Nesbit, M.O.; Zou, H.; Vacca, G.; Axerio-Cilies, P.; Van Sung, T.; Phillips, A.G. Neural Bases for Attenuation of Morphine Withdrawal by Heantos-4: Role of l -Tetrahydropalmatine. Sci. Rep. 2020, 10, 21275. [Google Scholar] [CrossRef]
- Jin, G.-Z. (−)-Tetrahydropalmatine and Its Analogues as New Dopamine Receptor Antagonists. Trends Pharmacol. Sci. 1987, 8, 81–82. [Google Scholar] [CrossRef]
- Wang, J.B.; Mantsch, J.R. L-Tetrahydropalamatine: A Potential New Medication for the Treatment of Cocaine Addiction. Future Med. Chem. 2012, 4, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Faison, S.L.; Schindler, C.W.; Goldberg, S.R.; Wang, J.B. L-Tetrahydropalmatine Reduces Nicotine Self-Administration and Reinstatement in Rats. BMC Pharmacol. Toxicol. 2016, 17, 49. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Hinton, D.J.; Johng, S.; Wang, J.B.; Choi, D.-S. Levo-Tetrahydropalmatine Decreases Ethanol Drinking and Antagonizes Dopamine D2 Receptor-Mediated Signaling in the Mouse Dorsal Striatum. Behav. Brain Res. 2013, 244, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Leung, W.C.; Zheng, H.; Huen, M.; Law, S.L.; Xue, H. Anxiolytic-like Action of Orally Administered Dl-Tetrahydropalmatine in Elevated plus-Maze. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 775–779. [Google Scholar] [CrossRef]
- Mantsch, J.R.; Wisniewski, S.; Vranjkovic, O.; Peters, C.; Becker, A.; Valentine, A.; Li, S.-J.; Baker, D.A.; Yang, Z. Levo-Tetrahydropalmatine Attenuates Cocaine Self-Administration under a Progressive-Ratio Schedule and Cocaine Discrimination in Rats. Pharmacol. Biochem. Behav. 2010, 97, 310–316. [Google Scholar] [CrossRef]
- Wang, W.; Liu, J.; Zhao, X.; Peng, Y.; Wang, N.; Lee, D.Y.W.; Dai, R. Simultaneous Determination of L-Tetrahydropalmatine and Its Active Metabolites in Rat Plasma by a Sensitive Ultra-High-Performance Liquid Chromatography with Tandem Mass Spectrometry Method and Its Application in a Pharmacokinetic Study. Biomed. Chromatogr. BMC 2017, 31, e3903. [Google Scholar] [CrossRef]
- Alsalem, M.; Haddad, M.; Altarifi, A.; Aldossary, S.A.; Kalbouneh, H.; Abojaradeh, A.M.; El-Salem, K. Impairment in Locomotor Activity as an Objective Measure of Pain and Analgesia in a Rat Model of Osteoarthritis. Exp. Ther. Med. 2020, 20, 165. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Bannon, A.W.; Joshi, S.K. Complete Freund’s Adjuvant-Induced Reduction of Exploratory Activity in a Novel Environment as an Objective Nociceptive Endpoint for Sub-Acute Inflammatory Pain Model in Rats. Eur. J. Pain 2015, 19, 1527–1536. [Google Scholar] [CrossRef]
- Yun, J. L-Tetrahydropalmatine Inhibits Methamphetamine-Induced Locomotor Activity via Regulation of 5-HT Neuronal Activity and Dopamine D3 Receptor Expression. Phytomedicine Int. J. Phytother. Phytopharm. 2014, 21, 1287–1291. [Google Scholar] [CrossRef]
- Dias, C.; Ahn, S. Behavioural and Neurochemical Assessment of Heantos 4 on Preclinical Models of Morphine-Dependence. J. Addict. Res. Ther. 2016, 7, 292–303. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, Z.; Gambeta, E.; Xu, S.C.; Thomas, C.; Godfrey, N.; Chen, L.; M’Dahoma, S.; Borgland, S.L.; Zamponi, G.W. Dopamine Inputs from the Ventral Tegmental Area into the Medial Prefrontal Cortex Modulate Neuropathic Pain-Associated Behaviors in Mice. Cell Rep. 2020, 31, 107812. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.B.; Koob, G.F. Sex Differences in Animal Models: Focus on Addiction. Pharmacol. Rev. 2016, 68, 242–263. [Google Scholar] [CrossRef] [PubMed]
- Kokane, S.S.; Perrotti, L.I. Sex Differences and the Role of Estradiol in Mesolimbic Reward Circuits and Vulnerability to Cocaine and Opiate Addiction. Front. Behav. Neurosci. 2020, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Bakhti-Suroosh, A.; Towers, E.B.; Lynch, W.J. A Buprenorphine-Validated Rat Model of Opioid Use Disorder Optimized to Study Sex Differences in Vulnerability to Relapse. Psychopharmacology 2021, 238, 1029–1046. [Google Scholar] [CrossRef] [PubMed]
- Gipson, C.D.; Dunn, K.E.; Bull, A.; Ulangkaya, H.; Hossain, A. Establishing Preclinical Withdrawal Syndrome Symptomatology Following Heroin Self-Administration in Male and Female Rats. Exp. Clin. Psychopharmacol. 2021, 29, 636–649. [Google Scholar] [CrossRef]
- Bobzean, S.A.M.; Kokane, S.S.; Butler, B.D.; Perrotti, L.I. Sex Differences in the Expression of Morphine Withdrawal Symptoms and Associated Activity in the Tail of the Ventral Tegmental Area. Neurosci. Lett. 2019, 705, 124–130. [Google Scholar] [CrossRef]
- Doyle, H.H.; Murphy, A.Z. Sex-Dependent Influences of Morphine and Its Metabolites on Pain Sensitivity in the Rat. Physiol. Behav. 2018, 187, 32–41. [Google Scholar] [CrossRef]
- Lefevre, E.M.; Pisansky, M.T.; Toddes, C.; Baruffaldi, F.; Pravetoni, M.; Tian, L.; Kono, T.J.Y.; Rothwell, P.E. Interruption of Continuous Opioid Exposure Exacerbates Drug-Evoked Adaptations in the Mesolimbic Dopamine System. Neuropsychopharmacology 2020, 45, 1781–1792. [Google Scholar] [CrossRef]
- Harris, G.C.; Aston-Jones, G. Involvement of D2 Dopamine Receptors in the Nucleus Accumbens in the Opiate Withdrawal Syndrome. Nature 1994, 371, 155–157. [Google Scholar] [CrossRef]
- Brewer, A.L.; Lewis, C.C.; Eggerman, L.; Blokker, A.; Burkland, J.A.; Johnsen, M.; Quock, R.M. Modeling Spontaneous Opioid Withdrawal in Male and Female Outbred Mice Using Traditional Endpoints and Hyperalgesia. Behav. Pharmacol. 2023, 34, 112–122. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oleinichenko, D.; Ahn, S.; Song, R.; Snutch, T.P.; Phillips, A.G. Morphine Withdrawal-Induced Hyperalgesia in Models of Acute and Extended Withdrawal Is Attenuated by l-Tetrahydropalmatine. Int. J. Mol. Sci. 2023, 24, 8872. https://doi.org/10.3390/ijms24108872
Oleinichenko D, Ahn S, Song R, Snutch TP, Phillips AG. Morphine Withdrawal-Induced Hyperalgesia in Models of Acute and Extended Withdrawal Is Attenuated by l-Tetrahydropalmatine. International Journal of Molecular Sciences. 2023; 24(10):8872. https://doi.org/10.3390/ijms24108872
Chicago/Turabian StyleOleinichenko, Daria, Soyon Ahn, Ru Song, Terrance P. Snutch, and Anthony G. Phillips. 2023. "Morphine Withdrawal-Induced Hyperalgesia in Models of Acute and Extended Withdrawal Is Attenuated by l-Tetrahydropalmatine" International Journal of Molecular Sciences 24, no. 10: 8872. https://doi.org/10.3390/ijms24108872




