SARS-CoV-2 Positive Serology and Islet Autoantibodies in Newly Diagnosed Pediatric Cases of Type 1 Diabetes Mellitus: A Single-Center Cohort Study
Abstract
1. Introduction
2. Results
2.1. Demographic and General Characteristics
2.2. Laboratory Findings
2.3. Differences between the Patients with Positive and Negative SARS-CoV-2 Antibodies
2.4. Differences between the Patients with and without DKA at T1DM Onset
2.5. Differences between the Groups of Patients Diagnosed in April 2021–April 2022 vs. March 2020–February 2021 vs. before the Pandemic
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Variables
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giwa, A.M.; Ahmed, R.; Omidian, Z.; Majety, N.; Karakus, K.E.; Omer, S.M.; Donner, T.; Hamad, A.R.A. Current understandings of the pathogenesis of type 1 diabetes: Genetics to environment. World J. Diabetes 2020, 11, 13–25. [Google Scholar] [CrossRef]
- Buschard, K. The etiology and pathogenesis of type 1 diabetes–A personal, non-systematic review of possible causes, and interventions. Front. Endocrinol. 2022, 13, 876470. [Google Scholar] [CrossRef] [PubMed]
- Ilonen, J.; Lempainen, J.; Veijola, R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Filippi, C.M.; von Herrath, M.G. Viral Trigger for Type 1 Diabetes. Diabetes 2008, 57, 2863–2871. [Google Scholar] [CrossRef] [PubMed]
- Elgenidy, A.; Awad, A.K.; Saad, K.; Atef, M.; El-Leithy, H.H.; Obiedallah, A.A.; Hammad, E.M.; Ahmad, F.-A.; Ali, A.M.; Dailah, H.G.; et al. Incidence of diabetic ketoacidosis during COVID-19 pandemic: A meta-analysis of 124,597 children with diabetes. Pediatr. Res. 2022, 93, 1149–1160. [Google Scholar] [CrossRef]
- Rahmati, M.; Keshvari, M.; Mirnasuri, S.; Yon, D.K.; Lee, S.W.; Shin, J.I.; Smith, L. The global impact of COVID-19 pandemic on the incidence of pediatric new-onset type 1 diabetes and ketoacidosis: A systematic review and meta-analysis. J. Med. Virol. 2022, 94, 5112–5127. [Google Scholar] [CrossRef]
- Borrelli, M.; Corcione, A.; Castellano, F.; Fiori Nastro, F.; Santamaria, F. Coronavirus Disease 2019 in Children. Front. Pediatr. 2021, 9, 668484. [Google Scholar] [CrossRef]
- Esposito, S.; Principi, N. Multisystem Inflammatory Syndrome in Children Related to SARS-CoV-2. Pediatr. Drugs 2021, 23, 119–129. [Google Scholar] [CrossRef]
- Chang, R.; Chen, T.Y.-T.; Wang, S.-I.; Hung, Y.-M.; Chen, H.-Y.; Wei, C.-C.J. Risk of autoimmune diseases in patients with COVID-19: A retrospective cohort study. Eclinicalmedicine 2023, 56, 101783. [Google Scholar] [CrossRef]
- Bizjak, M.; Emeršič, N.; Avramovič, M.Z.; Barbone, F.; Ronchese, F.; Della Paolera, S.; Conversano, E.; Amoroso, S.; Vidoni, M.; Tajnšek, T.V.; et al. High incidence of multisystem inflammatory syndrome and other autoimmune diseases after SARS-CoV-2 infection compared to COVID-19 vaccination in children and adolescents in south central Europe. Clin. Exp. Rheumatol. 2022, 41, 1183–1191. [Google Scholar] [CrossRef]
- Votto, M.; Castagnoli, R.; Marseglia, G.L.; Licari, A.; Brambilla, I. COVID-19 and autoimmune diseases: Is there a connection? Curr. Opin. Allergy Clin. Immunol. 2023, 23, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.; Thomas, P.G.; Randolph, A.G. Immunology of SARS-CoV-2 infection in children. Nat. Immunol. 2022, 23, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, N.; Rezaei, N. Autoimmune complications of COVID-19. J. Med. Virol. 2021, 94, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Vlad, A.; Serban, V.; Timar, R.; Sima, A.; Botea, V.; Albai, O.; Timar, B.; Vlad, M. Increased Incidence of Type 1 Diabetes during the COVID-19 Pandemic in Romanian Children. Medicina 2021, 57, 973. [Google Scholar] [CrossRef] [PubMed]
- Kamrath, C.; Mönkemöller, K.; Biester, T.; Rohrer, T.R.; Warncke, K.; Hammersen, J.; Holl, R.W. Ketoacidosis in Children and Adolescents With Newly Diagnosed Type 1 Diabetes During the COVID-19 Pandemic in Germany. JAMA 2020, 324, 801. [Google Scholar] [CrossRef]
- Lawrence, C.; Seckold, R.; Smart, C.; King, B.R.; Howley, P.; Feltrin, R.; Smith, T.A.; Roy, R.; Lopez, P. Increased paediatric presentations of severe diabetic ketoacidosis in an Australian tertiary centre during the COVID-19 pandemic. Diabet. Med. 2020, 38, e14417. [Google Scholar] [CrossRef] [PubMed]
- Unsworth, R.; Wallace, S.; Oliver, N.S.; Yeung, S.; Kshirsagar, A.; Naidu, H.; Kwong, R.M.W.; Kumar, P.; Logan, K.M. New-Onset Type 1 Diabetes in Children During COVID-19: Multicenter Regional Findings in the U.K. Diabetes Care 2020, 43, e170–e171. [Google Scholar] [CrossRef]
- Rusak, E.; Seget, S.; Macherski, M.; Furgał, N.; Dyś, P.; Jarosz-Chobot, P. Has the COVID-19 Pandemic Affected the Prevalence of Diabetic Ketoacidosis in Polish Children with Newly Diagnosed Type 1 Diabetes? An Example of the Largest Polish Pediatric Diabetes Center (Upper Silesia—Katowice, Poland). Healthcare 2022, 10, 348. [Google Scholar] [CrossRef]
- Boboc, A.A.; Novac, C.N.; Ilie, M.T.; Ieșanu, M.I.; Galoș, F.; Bălgrădean, M.; Berghea, E.C.; Ionescu, M.D. The Impact of SARS-CoV-2 Pandemic on the New Cases of T1DM in Children. A Single-Centre Cohort Study. J. Pers. Med. 2021, 11, 551. [Google Scholar] [CrossRef]
- Sørgjerd, E.P. Type 1 Diabetes-related Autoantibodies in Different Forms of Diabetes. Curr. Diabetes Rev. 2019, 15, 199–204. [Google Scholar] [CrossRef]
- Zanone, M.; Catalfamo, E.; Pietropaolo, S.; Rabbone, I.; Sacchetti, C.; Cerutti, F.; Trucco, M.; Cavallo-Perin, P. Glutamic acid decarboxylase and ICA512/IA-2 autoantibodies as disease markers and relationship to residual β-cell function and glycemic control in young type 1 diabetic patients. Metabolism 2003, 52, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Taplin, C.E.; Barker, J.M. Autoantibodies in type 1 diabetes. Autoimmunity 2008, 41, 11–18. [Google Scholar] [CrossRef] [PubMed]
- McKeigue, P.M.; Spiliopoulou, A.; McGurnaghan, S.; Colombo, M.; Blackbourn, L.; McDonald, T.J.; Onengut-Gomuscu, S.; Rich, S.S.; Palmer, C.; McKnight, J.A.; et al. Persistent C-peptide secretion in Type 1 diabetes and its relationship to the genetic architecture of diabetes. BMC Med. 2019, 17, 165. [Google Scholar] [CrossRef] [PubMed]
- Ehehalt, S.; Gauger, N.; Blumenstock, G.; Feldhahn, L.; Scheffner, T.; Schweizer, R.; Neu, A.; DIARY-Group Baden-Wuerttemberg. Hemoglobin A1c is a reliable criterion for diagnosing type 1 diabetes in childhood and adolescence. Pediatr. Diabetes 2010, 11, 446–449. [Google Scholar] [CrossRef]
- Castellanos, L.; Tuffaha, M.; Koren, D.; Levitsky, L.L. Management of Diabetic Ketoacidosis in Children and Adolescents with Type 1 Diabetes Mellitus. Pediatr. Drugs 2020, 22, 357–367. [Google Scholar] [CrossRef]
- Hirae, K.; Hoshina, T.; Koga, H. Impact of the COVID-19 pandemic on the epidemiology of other communicable diseases in Japan. Int. J. Infect. Dis. 2023, 128, 265–271. [Google Scholar] [CrossRef]
- Chow, E.J.; Uyeki, T.M.; Chu, H.Y. The effects of the COVID-19 pandemic on community respiratory virus activity. Nat. Rev. Microbiol. 2023, 21, 195–210. [Google Scholar] [CrossRef]
- Kawasaki, E. Type 1 Diabetes and Autoimmunity. Clin. Pediatr. Endocrinol. 2014, 23, 99–105. [Google Scholar] [CrossRef]
- Jia, X.; Gesualdo, P.; Rasmussen, C.G.; Alkanani, A.A.; He, L.; Dong, F.; Rewers, M.J.; Michels, A.W.; Yu, L. Prevalence of SARS-CoV-2 Antibodies in Children and Adults with Type 1 Diabetes. Diabetes Technol. Ther. 2021, 23, 517–521. [Google Scholar] [CrossRef]
- Rewers, M.; Bonifacio, E.; Ewald, D.; Rasmussen, C.G.; Jia, X.; Pyle, L.; Ziegler, A.-G.; ASK Study Group; Fr1da Study Group. SARS-CoV-2 Infections and Presymptomatic Type 1 Diabetes Autoimmunity in Children and Adolescents From Colorado, USA, and Bavaria, Germany. JAMA 2022, 328, 1252. [Google Scholar] [CrossRef]
- Decochez, K.; De Leeuw, I.; Keymeulen, B.; Mathieu, C.; Rottiers, R.; Weets, I.; Vandemeulebroucke, E.; Truyen, I.; Kaufman, L.; Schuit, F.; et al. IA-2 autoantibodies predict impending Type I diabetes in siblings of patients. Diabetologia 2002, 45, 1658–1666. [Google Scholar] [CrossRef] [PubMed]
- Vicinanza, A.; Messaaoui, A.; Tenoutasse, S.; Dorchy, H. Diabetic ketoacidosis in children newly diagnosed with type 1 diabetes mellitus: Role of demographic, clinical, and biochemical features along with genetic and immunological markers as risk factors. A 20-year experience in a tertiary Belgian center. Pediatr. Diabetes 2019, 20, 12864. [Google Scholar] [CrossRef]
- Sepa, A.; Ludvigsson, J. Psychological Stress and the Risk of Diabetes-Related Autoimmunity: A Review Article. Neuroimmunomodulation 2006, 13, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Meade, J. Mental Health Effects of the COVID-19 Pandemic on Children and Adolescents. Pediatr. Clin. N. Am. 2021, 68, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Anindya, R.; Rutter, G.A.; Meur, G. New-onset type 1 diabetes and severe acute respiratory syndrome coronavirus 2 infection. Immunol. Cell Biol. 2023, 101, 191–203. [Google Scholar] [CrossRef]
- Ehrmann, D.; Kulzer, B.; Roos, T.; Haak, T.; Al-Khatib, M.; Hermanns, N. Risk factors and prevention strategies for diabetic ketoacidosis in people with established type 1 diabetes. Lancet Diabetes Endocrinol. 2020, 8, 436–446. [Google Scholar] [CrossRef]
- Lee, H.J.; Yu, H.W.; Jung, H.W.; Lee, Y.A.; Kim, J.H.; Chung, H.R.; Yoo, J.; Kim, E.; Yu, J.; Shin, C.H.; et al. Factors Associated with the Presence and Severity of Diabetic Ketoacidosis at Diagnosis of Type 1 Diabetes in Korean Children and Adolescents. J. Korean Med. Sci. 2017, 32, 303. [Google Scholar] [CrossRef]
- Huang, A.; Chen, Q.; Yang, W.; Cui, Y.; Wang, Q.; Wei, H. Clinical characteristics of 683 children and adolescents, aged 0–18 years, newly diagnosed with type 1 diabetes mellitus in Henan Province: A single-center study. BMC Pediatr. 2023, 23, 39. [Google Scholar] [CrossRef]
- Szypowska, A.; Skórka, A. The risk factors of ketoacidosis in children with newly diagnosed type 1 diabetes mellitus. Pediatr. Diabetes 2011, 12, 302–306. [Google Scholar] [CrossRef]
| Characteristics | SARS-CoV-2 Positive Antibodies N = 37 | SARS-CoV-2 Negative Antibodies N = 121 | p-Values |
|---|---|---|---|
| Sex | <0.001 | ||
| Female | 16 (43.2%) | 67 (55.4%) | |
| Male | 21 (56.8%) | 54 (44.6%) | |
| Background | 0.40 | ||
| Rural | 15 (40.5%) | 40 (33.1%) | |
| Urban | 22 (59.5%) | 81 (66.9%) | |
| Age of onset (mean, SD) | 8.18 ± 3.58 | 8.53 ± 4.62 | 0.63 |
| pH (median, (IQR)) | 7.26 (7.11–7.40) | 7.25 (7.17–7.40) | 0.73 |
| DKA | 0.45 | ||
| Present | 26 (72.2%) | 74 (65.5%) | |
| Absent | 10 (27.8%) | 39 (34.5%) | |
| Severe DKA | 0.81 | ||
| Present | 8 (22.2%) | 23 (20.4%) | |
| Absent | 28 (77.8%) | 90 (79.6%) | |
| HbA1c (mean, SD) | 12.96 ± 1.97% | 11.88 ± 1.90% | 0.004 |
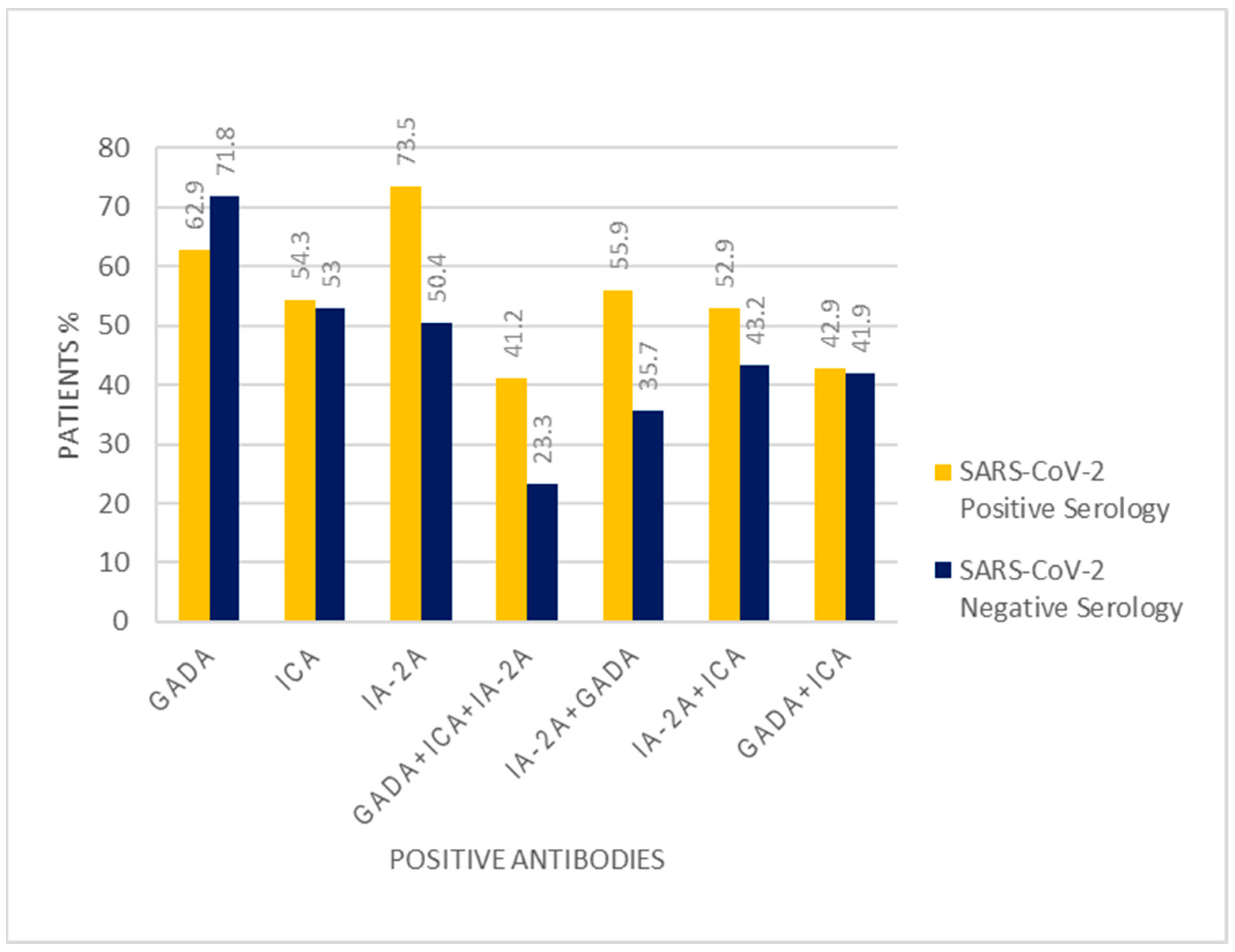
| GADA | 0.31 | ||
| Present | 22 (62.9%) | 84 (71.8%) | |
| Absent | 13 (37.1%) | 33 (28.2%) | |
| ICA | 0.89 | ||
| Present | 19 (54.3%) | 62 (53.0%) | |
| Absent | 16 (45.7%) | 55 (47.0%) | |
| IA-2A | 0.01 | ||
| Present | 25 (73.5%) | 58 (50.4%) | |
| Absent | 9 (26.5%) | 57 (49.6%) | |
| GADA + ICA + IA-2A | 0.03 | ||
| Present | 14 (41.2%) | 27 (23.3%) | |
| Absent | 20 (58.8%) | 89 (76.7%) | |
| GADA + ICA | 0.91 | ||
| Present | 15 (42.9%) | 49 (41.9%) | |
| Absent | 20 (57.1%) | 68 (58.1%) | |
| GADA + IA-2A | 0.03 | ||
| Present | 19 (55.9%) | 41 (35.7%) | |
| Absent | 15 (40.5%) | 74 (64.3%) | |
| ICA + IA-2A | 0.02 | ||
| Present | 18 (52.9%) | 37 (32.2%) | |
| Absent | 16 (43.2%) | 78 (67.8%) | |
| Vitamin D (median, IQR, ng/mL) | 23.30 (15.30–30.02) | 25.10 (15.75–30.40) | 0.75 |
| C-peptide (median, IQR, ng/mL) | 0.58 (0.42–1.02) | 0.57 (0.37–1.01) | 0.82 |
| Characteristics | DKA N = 100 | Non-DKA N = 49 | p-Values |
|---|---|---|---|
| Sex | 0.72 | ||
| Female | 50 (50.0%) | 26 (53.1%) | |
| Male | 50 (50.0%) | 23 (46.9%) | |
| Background | 0.15 | ||
| Rural | 31 (31.0%) | 21 (42.90%) | |
| Urban | 69 (69.0%) | 28 (57.10%) | |
| Age of onset (median, IQR) | 7.00 (4.00–11.00) | 11.00 (7.50–12.00) | 0.04 |
| SARS-CoV-2 antibodies | 0.45 | ||
| Present | 26 (26.0%) | 10 (20.4%) | |
| Absent | 74 (74.0%) | 39 (79.6%) | |
| HbA1c (mean, SD) | 12.34 ± 1.89% | 11.92 ± 2.13% | 0.22 |
| GADA | 0.79 | ||
| Present | 66 (68.8%) | 34 (70.8%) | |
| Absent | 30 (31.2%) | 14 (29.2%) | |
| ICA | 0.34 | ||
| Present | 54 (56.3%) | 23 (47.9%) | |
| Absent | 42 (43.8%) | 25 (52.1%) | |
| IA-2A | 0.08 | ||
| Present | 57 (61.3%) | 22 (45.8%) | |
| Absent | 36 (38.7%) | 26 (54.2%) | |
| GADA + ICA + IA-2A | 0.95 | ||
| Present | 25 (26.6%) | 13 (27.1%) | |
| Absent | 69 (73.4%) | 35 (72.9%) | |
| GADA + ICA | 1.00 | ||
| Present | 40 (41.7%) | 20 (41.7%) | |
| Absent | 56 (58.3%) | 28 (58.3%) | |
| GADA + IA-2A | 0.21 | ||
| Present | 41 (44.1%) | 16 (33.3%) | |
| Absent | 52 (55.9%) | 32 (66.7%) | |
| ICA + IA-2A | 0.53 | ||
| Present | 36 (38.7%) | 16 (66.7%) | |
| Absent | 57 (61.3%) | 32 (33.3%) | |
| Vitamin D (median, IQR, ng/mL) | 23.95 (14.37–30.37) | 25.80 (17.25–30.45) | 0.57 |
| C-peptide (median, IQR, ng/mL) | 0.49 (0.33–0.85) | 0.78 (0.51–1.16) | <0.001 |
| Characteristics | 2021 Group N = 158 | 2020 Group N = 147 | p-Values 2021 Group vs. 2020 Group | Pre-Pandemic Group N = 312 | p-Values 2021 Group vs. Pre-Pandemic Group |
|---|---|---|---|---|---|
| Gender | 0.53 | 0.15 | |||
| Female | 83 (52.5%) | 72 (48.9%) | 142 (45.5%) | ||
| Male | 75 (47.5%) | 75 (51.0%) | 170 (54.5%) | ||
| Background | 0.55 | <0.001 | |||
| Rural | 55 (34.8%) | 56 (38.1%) | 50 (16.0%) | ||
| Urban | 103 (65.2%) | 91 (61.9%) | 262 (84.0%) | ||
| Age of onset | 9.00 | 7.20 | <0.01 | 7.00 (3.00–10.00) | <0.01 |
| (median, IQR) | (5.00–12.00) | (7.07–7.30) | |||
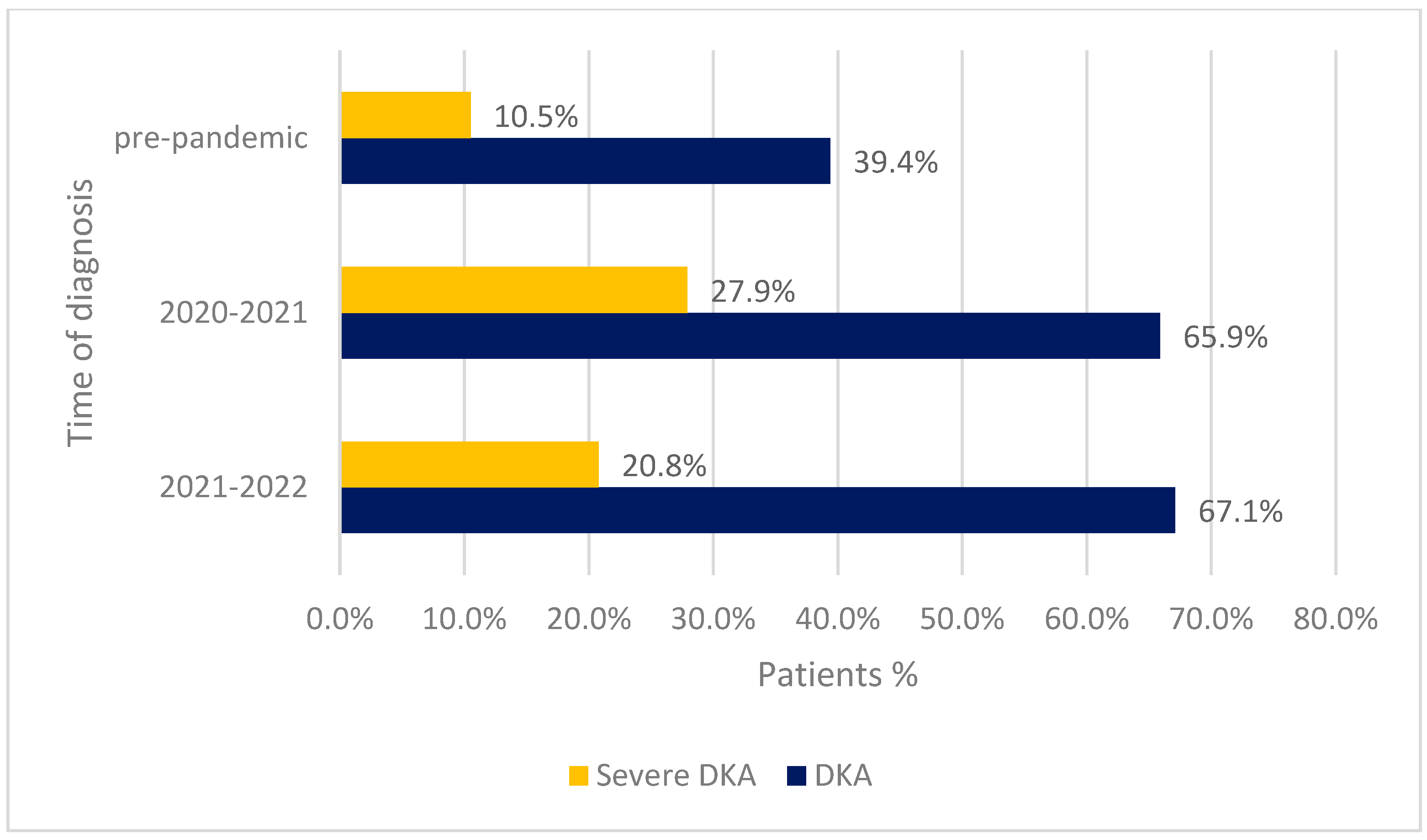
| DKA | 0.83 | <0.001 | |||
| Present | 100 (67.1%) | 97 (65.9%) | 123 (39.4%) | ||
| Absent | 49 (32.9%) | 50 (34.0%) | 189 (60.6%) | ||
| Severe DKA | 0.15 | 0.002 | |||
| Present | 31 (20.8%) | 41 (27.9%) | 33 (10.5%) | ||
| Absent | 118 (79.2%) | 106 (72.1%) | 279 (89.5%) | ||
| HbA1c | 12.14% ± 1.96% | 12.47% ± 2.19 | 0.18 | 11.32% ± 2.18% | <0.001 |
| (mean, SD) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boboc, A.A.; Novac, C.N.; Marin, A.G.; Ieșanu, M.I.; Plătică, C.; Buzescu, T.; Coșoreanu, M.T.; Galoș, F. SARS-CoV-2 Positive Serology and Islet Autoantibodies in Newly Diagnosed Pediatric Cases of Type 1 Diabetes Mellitus: A Single-Center Cohort Study. Int. J. Mol. Sci. 2023, 24, 8885. https://doi.org/10.3390/ijms24108885
Boboc AA, Novac CN, Marin AG, Ieșanu MI, Plătică C, Buzescu T, Coșoreanu MT, Galoș F. SARS-CoV-2 Positive Serology and Islet Autoantibodies in Newly Diagnosed Pediatric Cases of Type 1 Diabetes Mellitus: A Single-Center Cohort Study. International Journal of Molecular Sciences. 2023; 24(10):8885. https://doi.org/10.3390/ijms24108885
Chicago/Turabian StyleBoboc, Anca Andreea, Carmen Nicoleta Novac, Alexandra Gabriela Marin, Mara Ioana Ieșanu, Cristina Plătică, Teodora Buzescu, Maria Teodora Coșoreanu, and Felicia Galoș. 2023. "SARS-CoV-2 Positive Serology and Islet Autoantibodies in Newly Diagnosed Pediatric Cases of Type 1 Diabetes Mellitus: A Single-Center Cohort Study" International Journal of Molecular Sciences 24, no. 10: 8885. https://doi.org/10.3390/ijms24108885
APA StyleBoboc, A. A., Novac, C. N., Marin, A. G., Ieșanu, M. I., Plătică, C., Buzescu, T., Coșoreanu, M. T., & Galoș, F. (2023). SARS-CoV-2 Positive Serology and Islet Autoantibodies in Newly Diagnosed Pediatric Cases of Type 1 Diabetes Mellitus: A Single-Center Cohort Study. International Journal of Molecular Sciences, 24(10), 8885. https://doi.org/10.3390/ijms24108885







