Phytomediated-Assisted Preparation of Cerium Oxide Nanoparticles Using Plant Extracts and Assessment of Their Structural and Optical Properties
Abstract
1. Introduction
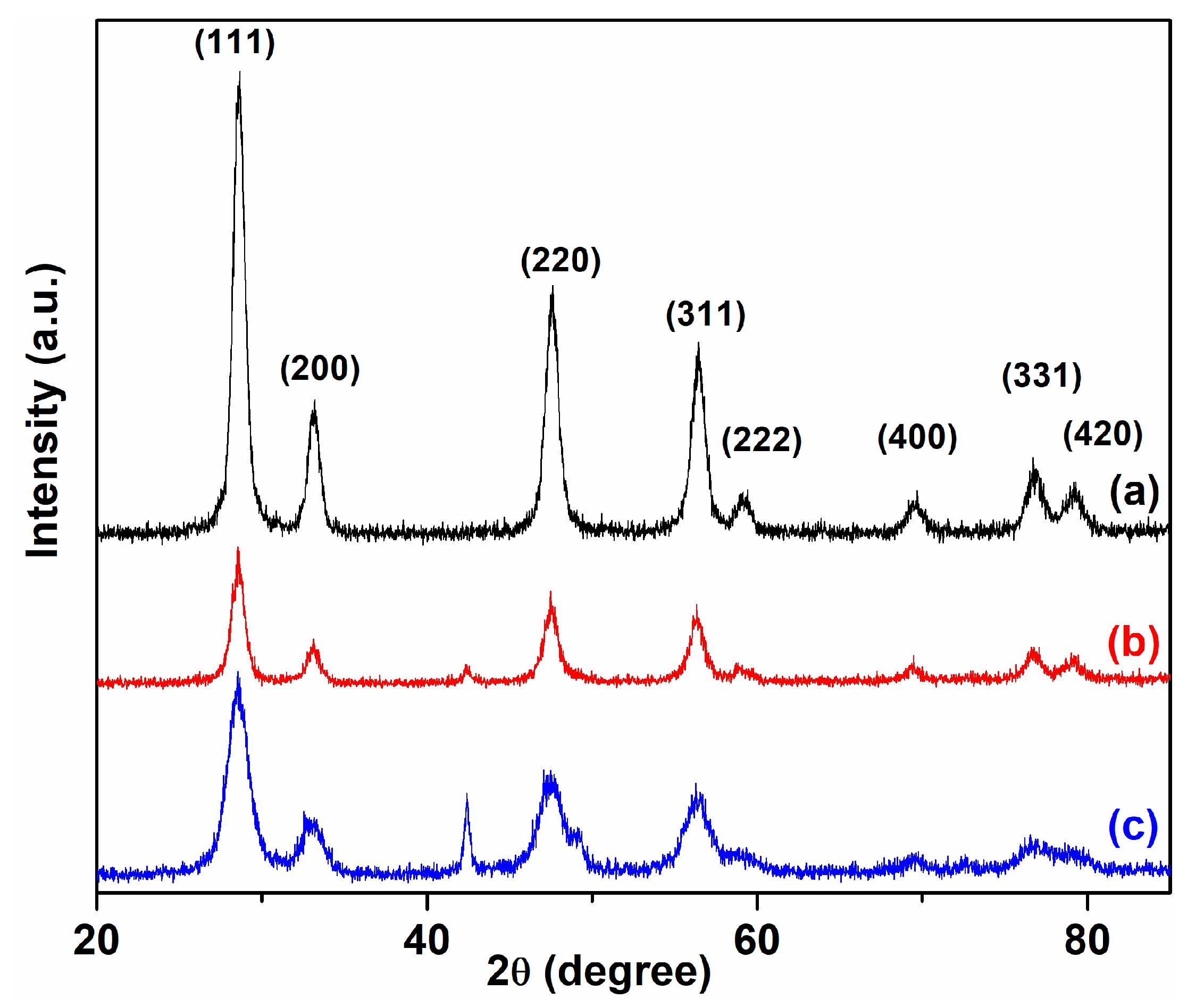
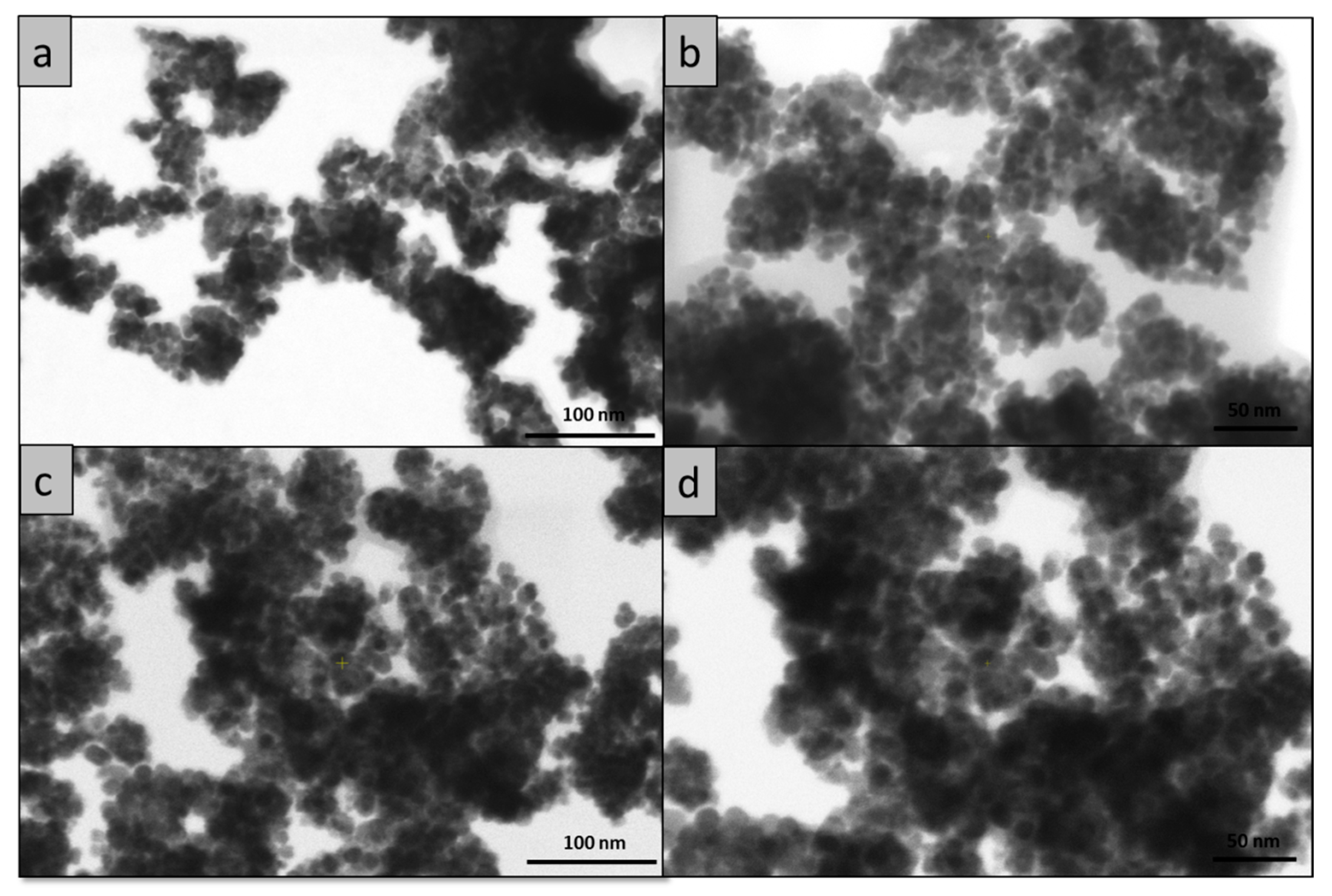
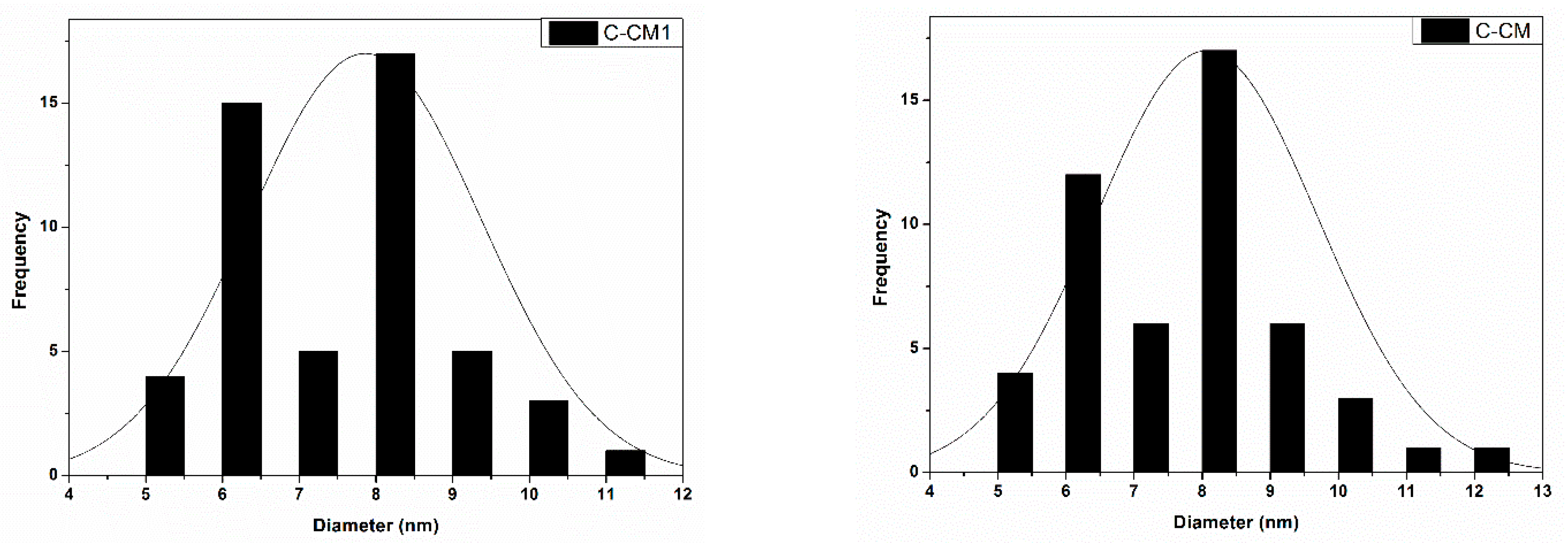
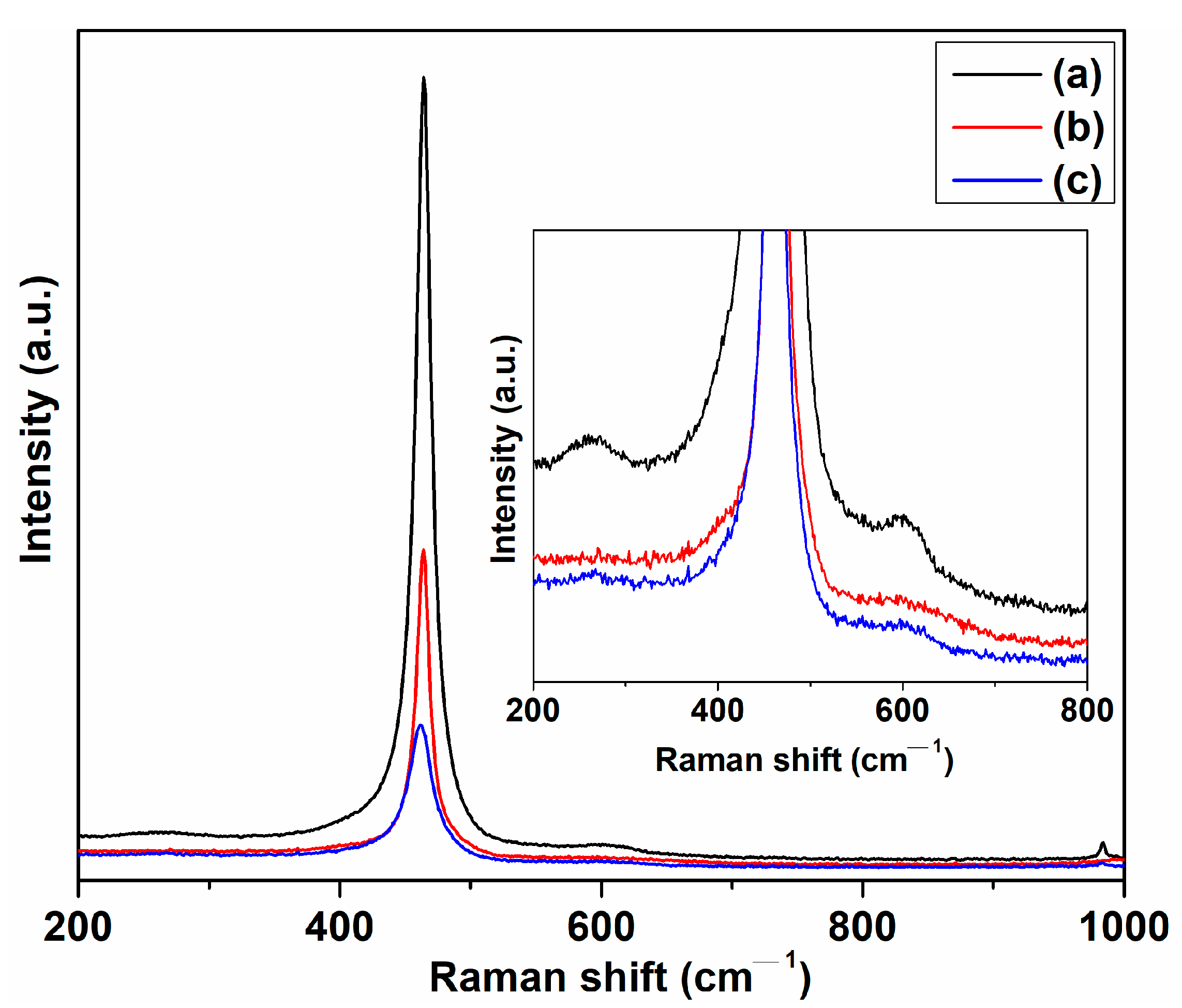
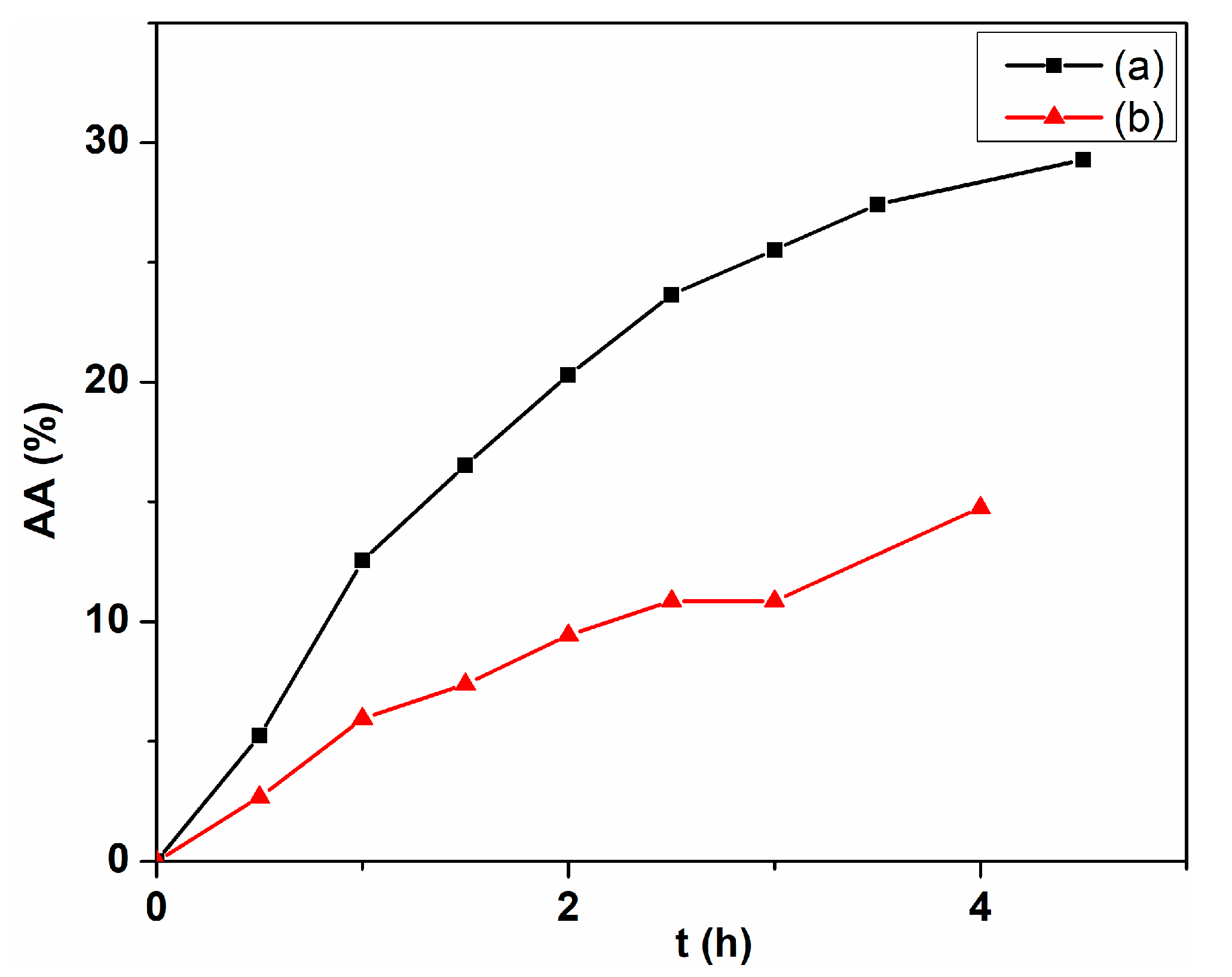
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of Chelidonium majus Plant Extract
3.3. Synthesis of Cerium Oxide Nanoparticles Using C. majus Extract
3.4. Preparation of Viscum Album Aqueous Extract
3.5. Biogenic Synthesis of Cerium Oxide Nanoparticles Using Viscum Album Extract (C-VA)
3.6. Characterization
3.6.1. X-ray Diffractometry
3.6.2. Spectral Measurements
3.6.3. SEM Investigations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, D.; Du, X.; Shi, L.; Gao, R. Shape-controlled synthesis and catalytic applications of ceria nanomaterials. Dalton Trans. 2012, 41, 14455–14475. [Google Scholar] [CrossRef] [PubMed]
- Zikalala, N.; Matshetshe, K.; Parani, S.; Oluwafemi, O.S. Biosynthesis protocols for colloidal metal oxide nanoparticles. Nano-Struct. Nano-Objects 2018, 16, 288–299. [Google Scholar] [CrossRef]
- Fauzi, A.A.; Jalil, A.A.; Hassan, N.S.; Aziz, F.F.A.; Azami, M.S.; Hussain, I.; Saravanan, R.; Vo, D.V.N. A critical review on relationship of -based photocatalyst towards mechanistic degradation of organic pollutant. Chemosphere 2022, 286, 131651. [Google Scholar] [CrossRef]
- Calvache-Munoz, J.; Prado, F.A.; Rodriguez-Paez, J.E. Cerium oxide nanoparticles: Synthesis, characterization, and tentative mechanism of particle formation. Colloids Surf. A 2017, 529, 146–159. [Google Scholar] [CrossRef]
- Liu, X.; Iocozzia, J.; Wang, Y.; Cui, X.; Chen, Y.; Zhao, S.; Li, Z.; Lin, Z. Noble metal-metal oxide naonohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Environ. Sci. 2017, 10, 402–434. [Google Scholar] [CrossRef]
- Fifere, N.; Airinei, A.; Dobromir, M.; Sacarescu, L.; Dunca, S.I. Revealing the effect of synthesis conditions on their structural, optical and antibacterial properties of cerium oxide nanoparticles. Nanomaterials 2021, 11, 2596. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Li, W.; Zheng, X.; Li, X.; Sun, Y.; Wang, Y.; Li, C.; Wang, L. Cerium and its oxidant-based materials for antibacterial applications: A state-of-the-art review. Front. Mater. 2020, 7, 213. [Google Scholar] [CrossRef]
- Malleshappa, J.; Nagabhushana, H.; Sharma, S.C.; Vidya, Y.S.; Anantharaju, K.S.; Prashantha, S.C.; Prasad, B.D.; Naika, H.R.; Lingaraju, K.; Surendra, B.S. Leucas aspera mediated multifunctional nanoparticles: Structural, photoluminescent, photocatalytic and antibacterial properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 452–462. [Google Scholar] [CrossRef]
- Vishnukumar, P.; Vivekanandhan, S.; Misra, M.; Mohanty, A.K. Recent advances and emerging opportunities in photochemical synthesis of ZnO nanostructures. Mater. Sci. Semicond. Process. 2018, 80, 143–161. [Google Scholar] [CrossRef]
- Rajan, A.R.; Vihas, V.; Rajan, A.; John, A.; Philip, D. Synthesis of nanostructured by chemical and biogenic methods: Optical properties and bioactivity. Ceram. Int. 2020, 46, 14048–14055. [Google Scholar] [CrossRef]
- Corradi, A.B.; Bondioli, F.; Ferrari, A.M.; Manfredini, T. Synthesis and characterization of nanosized ceria powders by microwave-hydrothermal method. Mater. Res. Bull. 2006, 41, 38–44. [Google Scholar] [CrossRef]
- Singh, K.R.B.; Nayak, V.; Sarkar, T.; Singh, R.P. Cerium oxide nanoparticles: Properties, biosynthesis and biomedical applications. RSC Adv. 2020, 10, 27194–27214. [Google Scholar] [CrossRef] [PubMed]
- Celardo, I.; Pedersen, J.Z.; Traversa, E.; Ghibelli, L. Pharmacological potential of cerium oxide nanoparticles. Nanoscale 2011, 3, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, L.; Hu, W.; Huang, X.; Li, Q.; Xu, Y.; Zuo, Y.; Li, G. Anchoring high-concentration oxygen vacancies at interfaces of Cu toward enhanced activity for preferential CO oxidation. ACS Appl. Mater. Interf. 2015, 7, 22999–23007. [Google Scholar] [CrossRef] [PubMed]
- Akdogan, C.S.; Gokcal, B.; Polat, M.; Haucaloglu, K.O.; Kip, C.; Tuncel, A. Porous oxygen vacancies enhanced microspheres with efficient enzyme-mimetic and photothermal properties. ACS Sustain. Chem. Eng. 2022, 10, 9492–9505. [Google Scholar] [CrossRef]
- Elahi, B.; Mirzaee, M.; Darroudi, M.; Oskuee, R.K.; Sadri, K.; Gholami, L. Role of oxygen vacancies on photocatalytic activities of green synthetized ceria nanoparticles in Cydonia oblonga miller seeds extract and evaluation of its cytoxicity effects. J. Alloys Compds. 2020, 816, 152553. [Google Scholar] [CrossRef]
- Xu, Y.; Mofarah, S.S.; Mehmood, R.; Cazorla, C.; Koshy, P.; Sorrell, C.C. Design strategies for ceria nanomaterials: Untangling key mechanistic concepts. Mater. Horiz. 2021, 8, 102–123. [Google Scholar] [CrossRef]
- Ozkan, E.; Cop, P.; Benfer, F.; Hofmann, A.; Votsmeier, M.; Guerra, J.M.; Giar, M.; Heilinger, C.; Over, H.; Smarsly, B. Rational synthesis concept for cerium oxide nanoparticles: On the impact of particle size on the oxygen storage capacity. J. Phys. Chem. C 2020, 124, 8736–8748. [Google Scholar] [CrossRef]
- de Oliveira, R.C.; Amoresi, R.A.C.; Marama, N.L.; Zaghete, M.A.; Chiquito, A.J.; Sambrano, J.R.; Longo, E.; Simones, A.Z. Influence of synthesis time on the morphology and properties of CeO2 nanoparticles. An experimental—Theoretical study. Cryst. Growth Des. 2020, 20, 5031–5042. [Google Scholar] [CrossRef]
- Pugachevskii, M.A.; Mamontov, V.A.; Syuy, A.V.; Kuzmenko, A.P. Effect of pH on oxidant properties of ablated nanoparticles on photocatalytic process. J. Ind. Eng. Chem. 2022, 106, 74–76. [Google Scholar] [CrossRef]
- Trenque, I.; Magnano, G.C.; Barta, J.; Chaput, F.; Bolzinger, M.A.; Pitault, J.; Briancon, S.; Masenelli-Varlot, K.; Bugnet, M.; Dujardin, C.; et al. Synthesis routes of nanoparticles dedicated to organophosphorus degradation: A benchmark. Cryst. Eng. Comm. 2020, 22, 1725–1737. [Google Scholar] [CrossRef]
- Yadav, T.; Srivastava, O. Synthesis of nanocrystalline cerium oxide by high energy ball milling. Ceram. Int. 2012, 38, 5782–5789. [Google Scholar] [CrossRef]
- Madler, L.; Stark, W.J.; Pratsinis, S.E. Flame-made ceria nanoparticles. J. Mater. Res. 2002, 17, 1356–1362. [Google Scholar] [CrossRef]
- Chen, H.I.; Chang, H.Y. Synthesis of nanocrystalline cerium oxide particles by the precipitation method. Ceram. Int. 2005, 31, 795–802. [Google Scholar] [CrossRef]
- Alifanti, M.; Baps, B.; Blangenois, N.; Naud, J.; Grange, P.; Delmon, B. Characterization of CeO2 mixed oxides. Comparison of the citrate and sol-gel preparation methods. Chem. Mater. 2002, 15, 395–403. [Google Scholar] [CrossRef]
- Arumugam, A.; Karthikeyan, G.; Hameed, A.S.H.; Gopinath, K.; Gowri, S.; Karthika, V. Synthesis of cerium oxide nanoparticles using Gloriosa superba L. leaf extract and their structural, optical and antibacterial properties. Mater. Sci. Eng. C 2015, 49, 408–415. [Google Scholar] [CrossRef] [PubMed]
- He, H.W.; Wu, X.Q.; Ren, W.; Shi, P.; Yao, X.; Song, Z.T. Synthesis of crystalline cerium dioxide hydrosol by a sol-gel method. Ceram. Int. 2012, 38, S501–S504. [Google Scholar] [CrossRef]
- Darroudi, M.; Hoseini, S.J.; Oskuee, R.R.; Hosseini, H.A.; Gholami, L.; Gerayli, S. Food-directed synthesis of cerium oxide nanoparticles and their neurotoxicity effects. Ceram. Int. 2014, 40, 7425–7430. [Google Scholar] [CrossRef]
- Gharagozlu, M.; Baradaran, Z.; Bayati, R. A green chemical method for synthesis of ZnO nanoparticles from solid-state decomposition of Schiff-bases derived from amino acid alanine derivatives. Ceram. Int. 2015, 41, 8382–8387. [Google Scholar] [CrossRef]
- Khan, M.; Mashwani, Z.R.; Ikram, M.; Raja, N.I.; Mohamed, A.H.; Ren, G.; Omar, A.A. Efficacy of green cerium oxide nanoparticles for potential therapeutic applications: Circumstantial insight on mechanistic aspects. Nanomaterials 2022, 12, 2117. [Google Scholar] [CrossRef]
- Dhall, A.; Self, W. Cerium oxide nanoparticles: A brief review of their synthesis methods and biomedical applications. Antioxidants 2018, 7, 97. [Google Scholar] [CrossRef]
- Navada, K.M.; Nagaraja, G.K.; D’Souza, J.N.; Kouser, S.; Nithyashree, B.R.; Manasa, D.J. Biofabrication of multifunctional nanoceria mediated from Pouteria campechiana for medical and sensing applications. J. Photochem. Photobiol. A Chem. 2022, 424, 113631. [Google Scholar] [CrossRef]
- Yulizar, Y.; Juliyanto, S.; Sudirman; Apriandanu, D.O.B.; Surya, R.M. Novel sol-gel synthesis of CeO2 nanoparticles using Morinda citrifolia L. fruit extract: Structural and optical analysis. J. Mol. Struct. 2021, 1231, 129904. [Google Scholar] [CrossRef]
- Sisubalan, N.; Ramkumar, V.S.; Pugazhendhi, A.; Karthikeyan, C.; Indira, K.; Gopinath, K.; Hameed, A.S.H.; Basha, M.H.G. ROS mediated cytotoxic activity of and nanoparticles synthetized using the Rubia cordifolia L. leaf extract on MG-63-human osteosarcoma cell lines. Environ. Sci. Pollut. Res. 2018, 25, 10482–10492. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.; Manoharadas, S.; Zeyad, M.T. Green synthesis of cerium oxide nanoparticles using Acorus calamus extract and their antibiofilm activity against bacterial pathogens. Microsc. Res. Tech. 2021, 84, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Thovhogi, N.; Diallo, A.; Gurib-Fakim, A.; Maaze, N. Nanoparticles green synthesis by Hibiscus extract: Main physical properties. J. Alloys Compds. 2015, 647, 392–396. [Google Scholar] [CrossRef]
- Dobrucka, R.; Dlugaszewska, J.; Kazmarek, M. Cytotoxic and antimicrobial effect of biosynthesized ZnO nanoparticles using Chelidonium majus extract. Biomed. Microdevices 2018, 20, 5. [Google Scholar] [CrossRef]
- Parvu, M.; Vlase, L.; Fodorpataki, L.; Parvu, O.; Rosca-Casan, O.; Bartha, C.; Barbu-Tudoran, L.; Parvu, A.E. Chemical composition of Celandine (Chelidonium majus L.) extract and its effects on Botrytis tulipae (Lib.) lind fungus and the tulip. Not. Bot. Horti Agrobot. 2013, 41, 414–426. [Google Scholar] [CrossRef]
- Hadaruga, D.I.; Hadaruga, N.G. Antioxidant activity of Chelidonium majus L. extracts from the Banat county. J. Agroalim. Process. Technol. 2009, 15, 396–402. [Google Scholar]
- Dalibarca, C.V.; Popescu, R.; Dumitrascu, V.; Cimporescu, A.; Vlad, C.S.; Vagvolgyi, C.; Krisch, J.; Dehelean, C.; Horlat, F.G. Phytocomponents identification in mistletoe (Viscum album) young leaves and branches by GC-MS and antiproliferative effect on HepG2 and MCF7 cell lines. Farmacia 2016, 64, 82–87. [Google Scholar]
- Nazaruk, J.; Orlikowski, P. Phytochemical profile and therapeutic potential of Viscum album L. Nat. Prod. Res. 2016, 30, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Alishah, H.; Seyedi, S.P.; Ebrahimipour, S.Y.; Mahani, S.E. A green approach for the synthesis of silver nanoparticles using root extract of Chelidonium majus: Characterization and antibacterial evaluation. J. Clust. Sci. 2016, 27, 421–429. [Google Scholar] [CrossRef]
- Tiseanu, C.; Parvulescu, V.I.; Boutonnet, M.; Cojocaru, B.; Primus, P.A.; Teodorescu, C.M.; Solans, C.; Sanchez Dominguez, M. Surface versus volume effects in luminescent ceria nanocrystals synthesized by an oil-in-water microemulsion method. Phys. Chem. Chem. Phys. 2011, 13, 17135–17145. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Pearson Education Ltd.: London, UK, 2014. [Google Scholar]
- Iqbal, A.; Ahmed, A.S.; Ahmad, N.; Shafi, A.; Ahamad, T.; Khan, M.Z.; Srivastava, S. Biogenic synthesis of nanoparticles and its potential application as an efficient photocatalyst for the degradation of toxic amido black dye. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100505. [Google Scholar] [CrossRef]
- Xu, H.F.; Li, H. The effect of Co-doped on the room temperature ferromagnetism of nanorods. J. Magn. Magn. Mater. 2015, 377, 272–275. [Google Scholar] [CrossRef]
- Oliveira, A.C.; da Silva, A.N.; Junior, J.A.L.; Freire, P.T.C.; Oliveria, A.C.; Filho, J.M. Structural changes in nanostructured catalytic oxides monitored by Raman spectroscopy: Effect of laser heating. J. Phys. Chem. Solids 2017, 102, 190–198. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. and oxygen vacancy mediated tuning of structural and optical properties of nanoparticles. Mater. Chem. Phys. 2012, 131, 666–671. [Google Scholar] [CrossRef]
- Askrabic, S.; Dohcevic-Mitrovic, Z.; Kremenovic, A.; Lazarevic, N.; Kahleenberg, V.; Popovic, Z.V. Oxygen vacancy-induced microstructural changes of annealed nanocrystals. J. Raman Spectrosc. 2012, 43, 76–81. [Google Scholar] [CrossRef]
- Saitzek, S.; Blach, J.F.; Villain, S.; Gavari, J.R. Nanostructured ceria: A comparative study from X-ray diffraction, Raman spectroscopy and BET specific surface measurements. Phys. Status Sol. A 2008, 205, 1534–1539. [Google Scholar] [CrossRef]
- Kosacki, A.I.; Suzuki, T.; Anderson, H.U.; Colomban, P. Raman scattering and lattice defects in nanocrystalline thin films. Solid State Ion. 2002, 149, 99–105. [Google Scholar] [CrossRef]
- Trogads, P.; Parrondo, J.; Ramani, V. surface oxygen vacancy concentration governs in situ free radical scavenging efficacy in polymer electrolytes. ACS Appl. Mater. Interf. 2012, 4, 5098–5102. [Google Scholar] [CrossRef] [PubMed]
- Burroughs, P.; Hamnett, A.; Orchard, A.F.; Thornton, G. Satellite structure in the X-ray photoelectron spectra of some binary and mixed oxides of lanthanum and cerium. J. Chem. Soc. Dalton Trans. 1976, 17, 1686–1698. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, C.; Fan, Z.; Gong, J.; Li, A.; Ding, Z.; Tang, H.; Zhang, M.; Wu, G. Hydrothermal synthesis of hexagonal nanosheets and their room temperature ferromagnetism. J. Alloys Compds. 2015, 647, 1013–1021. [Google Scholar] [CrossRef]
- Wang, L.; Meng, F. Oxygen vacancy and ion dependent magnetism of monocrystal nanopoles synthesized by a facile hydrothermal method. Mater. Res. Bull. 2013, 48, 3492–3498. [Google Scholar] [CrossRef]
- Li, H.; Meng, F.; Gong, J.; Fan, Z.; Qin, R. Structural, morphological and optical properties of shuttle-like synthesized by a facile hydrothermal method. J. Alloys Compds. 2017, 722, 489–498. [Google Scholar] [CrossRef]
- Calvache-Muñoz, J.; Prado, F.A.; Tirado, L.; Daza-Gomez, L.C.; Cuervo-Ochoa, G.; Calambas, H.L.; Rodríguez-Páez, J.E. Structural and optical properties of nanoparticles synthesized by modified polymer complex method. J. Inorg. Organomet. Polym. Mater. 2019, 29, 813–826. [Google Scholar] [CrossRef]
- Oosthuizen, D.N.; Motaung, D.E.; Swart, H.C. Gas sensors based on nanoparticles prepared by chemical precipitation method and their temperature-dependent selectivity towards and gases. Appl. Surf. Sci. 2020, 505, 144356. [Google Scholar] [CrossRef]
- Afzal, S.; Quan, X.; Lu, S. Catalytic performance and an insight into the mechanism of nanocrystals with different exposed facets in catalytic ozonation of p-nitrophenol. Appl. Catal. B Environ. 2019, 248, 526–537. [Google Scholar] [CrossRef]
- Darroudi, M.; Hakimi, M.; Sarani, M.; Oskuee, R.K.; Zak, A.K.; Gholami, L. Facile synthesis, characterization and evaluation of neurotoxicity effect of cerium oxide nanoparticles. Ceram. Int. 2013, 39, 6917–6921. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Si, R.; Liao, C.S.; Yan, C.H.; Xiao, C.X.; Kou, Y. Facile alcohothermal synthesis, size-dependent ultraviolet absorption and enhanced CO conversion activity of ceria nanocrystals. J. Phys. Chem. B. 2003, 107, 10159–10167. [Google Scholar] [CrossRef]
- Choudhury, B.; Chetri, P.; Choudhury, A. Oxygen defects and formation of affecting the photocatalytic performance of CeO2 nanoparticles. RSC Adv. 2014, 4, 4663–4671. [Google Scholar] [CrossRef]
- Jiang, L.; Tinoco, M.; Fernandez-Garcia, S.; Sun, Y.; Traviankina, M.; Nan, P.; Xue, Q.; Pan, H.; Aguinaco, A.; Gonzales-Leal, J.M.; et al. Enhanced artificial enzyme activities on the reconstructed sawtoothlike nanofacets of pure and Pr-doped ceria nanocubes. ACS Appl. Mater. Interf. 2021, 13, 38061–38073. [Google Scholar] [CrossRef]
- Escobedo Morales, A.; Sanchez Mora, E.; Pal, U. Use of diffuse reflectance spectroscopy for optical characterization of un-supported structures. Rev. Mex. Fis. 2007, S53, 18–22. [Google Scholar]
- Makula, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV-Vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Ho, C.; Yu, J.C.; Kwong, T.; Mak, A.C.; Lai, S. Morphology-controlable synthesis of mesoporous nano- and microstructures. Chem. Mater. 2005, 17, 4514–4522. [Google Scholar] [CrossRef]
- Wang, Z.; Quan, Z.; Lin, J. Remarkable changes in optical properties of nanocrystals induced by lanthanide ions doping. Inorg. Chem. 2007, 46, 5237–5242. [Google Scholar] [CrossRef]
- Wang, L.; Meng, F.; Li, K.; Lu, F. Characterizations and optical properties of pole-like nano- synthesized by a facile hydrothermal method. Appl. Surf. Sci. 2013, 286, 269–274. [Google Scholar] [CrossRef]
- Zhou, H.P.; Zhang, Y.W.; Mai, H.X.; Sun, X.; Liu, Q.; Song, W.G.; Yan, C.H. Spontaneous organization of uniform nanoflowers by 3D oriented attachment in hot surfactant solutions monitored with an in situ electrical conductance technique. Chem. Eur. J. 2008, 14, 3380–3390. [Google Scholar] [CrossRef] [PubMed]
- Murali, A.; Lan, Y.P.; Sohn, H.Y. Effect of oxygen vacancies in nonstoichiometric ceria on its photocatalytic properties. Nano-Struct. Nano Objects 2019, 18, 100257. [Google Scholar] [CrossRef]
- Urbach, F. The long-wavelength edge of photographic sensitivity and the electronic absorption of solids. Phys. Rev. 1953, 92, 1324–1333. [Google Scholar] [CrossRef]
- Boubaker, K. A physical explanation to the controversial Urbach tailing universality. Eur. Phys. J. Plus 2011, 126, 10. [Google Scholar] [CrossRef]
- Islam, M.J.; Reddy, D.A.; Choi, J.; Kim, T.K. Surface oxygen vacancy assisted electron transfer and shuttling for enhanced photocatalytic activity of Z-scheme nanocomposite. RSC Adv. 2016, 6, 19341–19350. [Google Scholar] [CrossRef]
- Wetchankun, N.; Chaiwichain, S.; Inceesungvorn, B.; Pingmuang, K.; Phanichphant, S.; Minett, A.I.; Chen, J. nanocomposites with high visible light-induced photocatalytic activity. ACS Appl. Mater. Interf. 2012, 4, 3718–3723. [Google Scholar] [CrossRef]
- Ansari, M.J.; Machek, P.; Jarosova, M.; Abed, A.M.; Khalaji, A.D. Facile coprecipitation thermal degradation synthesis of nanoparticles and their photocatalytic degradation of Rhodamine B. J. Mater. Sci. Mater. Electron. 2022, 33, 5686–5695. [Google Scholar] [CrossRef]
- Dimitrov, V.; Sakka, S. Linear and nonlinear optical properties of simple oxides. II. J. Appl. Phys. 1996, 79, 1741–1745. [Google Scholar] [CrossRef]
- Nusrath, K.; Muraleedharan, K. Synthesis, evaluation of kinetic characteristics and investigation of apoptosis of -modified ceria nano discs. J. Rare Earths 2018, 36, 1050–1059. [Google Scholar] [CrossRef]
- Maensiri, S.; Labuayai, S.; Laokul, P.; Klinkaewnarong, J.; Swatsitang, E. Structure and optical properties of nanoparticles prepared by using lemongrass plant extract solution. Jap. J. Appl. Phys. 2014, 53, 06JG14. [Google Scholar] [CrossRef]
- Babitha, K.K.; Sreedevi, A.; Priyanka, K.P.; Sabu, B.; Varghese, T. Structural characterization and optical studies of nanoparticles synthesized by chemical precipitation. Ind. J. Pure Appl. Phys. 2015, 53, 596–603. [Google Scholar]
- Poornaprakash, B.; Subramanyan, K.; Kumar, M.; Kim, Y.L. Enhanced photoluminescence characteristics and intrinsic ferromagnetism in Co-substituted nanoparticles. Mater. Sci. Semicond. Process. 2021, 123, 105566. [Google Scholar] [CrossRef]
- Tamizhdurai, P.; Saktimathan, S.; Chen, S.M.; Shanthi, K.; Sivasanker, S.; Sangeetha, P. Environmentally friendly synthesis of nanoparticles for the catalytic oxidation of benzyl alcohol to benzaldehyde and selective detection of nitrite. Sci. Rep. 2017, 7, 46372. [Google Scholar] [CrossRef]
- Fifere, N.; Airinei, A.; Asandulesa, M.; Rotaru, A.; Ursu, E.L.; Doroftei, F. Investigating the vibrational, magnetic and dielectric properties, and antioxidant activity of cerium oxide nanoparticles. Int. J. Mol. Sci. 2022, 23, 13883. [Google Scholar] [CrossRef] [PubMed]
- Shlapa, Y.; Solopan, S.; Sarnatskaya, V.; Siposova, K.; Garcarova, I.; Veltruska, K.; Timashkov, I.; Lykhova, O.; Kolesnik, D.; Musatov, A.; et al. Cerium dioxide nanoparticles synthesized via precipitation at constant pH: Synthesis, physical-chemical and antioxidant properties. Colloids Surf. B Biointerf. 2022, 220, 112960. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.; Tal, A.A.; Skallberg, A.; Brommesson, C.; Hu, Z.; Boyd, R.D.; Olovsson, W.; Fairley, N.; Abrikosov, I.A.; Zhang, X.; et al. Cerium oxide nanoparticles with antioxidant capabilities and gadolinium integration for MRI contrast enhancement. Sci. Rep. 2018, 8, 6999. [Google Scholar] [CrossRef] [PubMed]


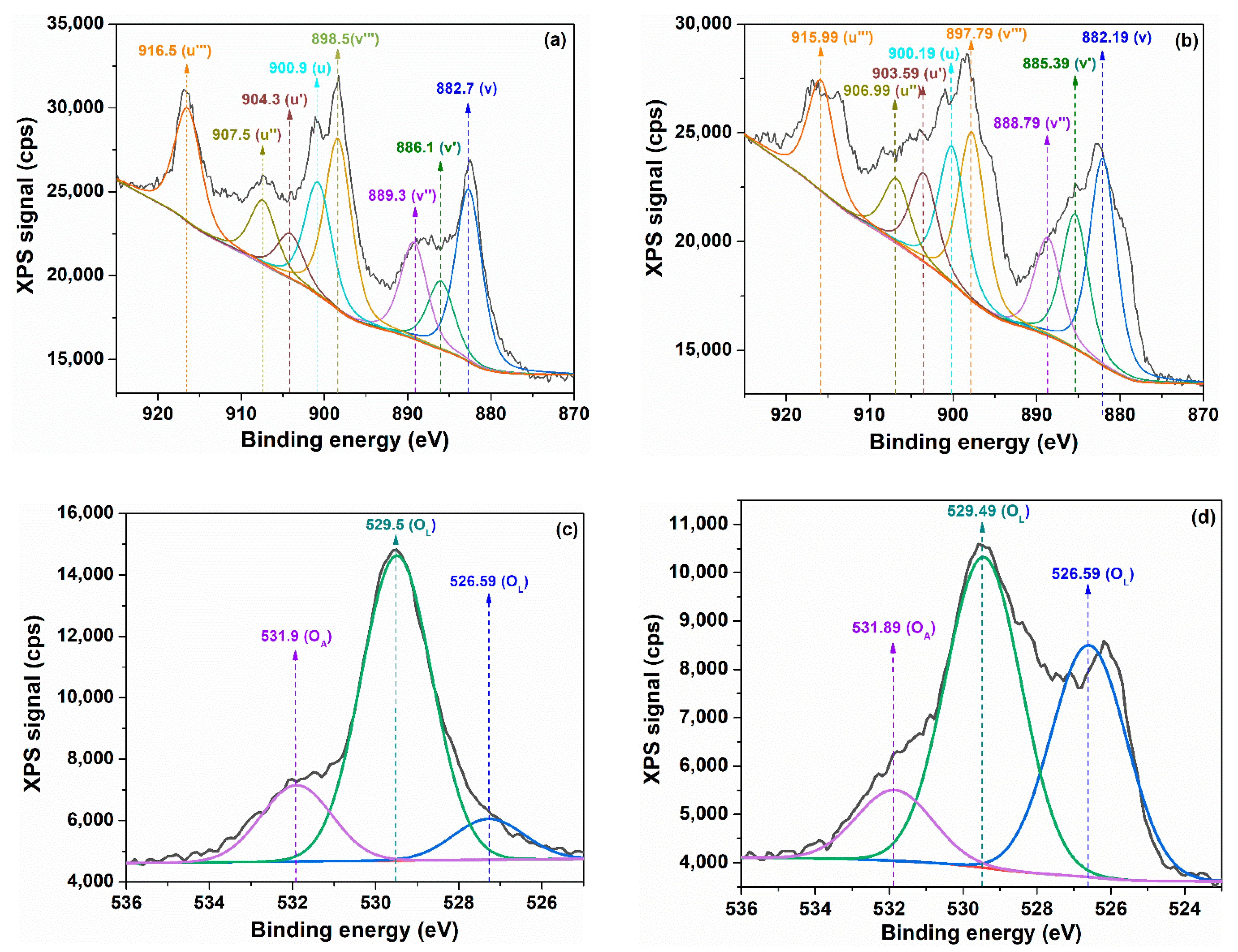
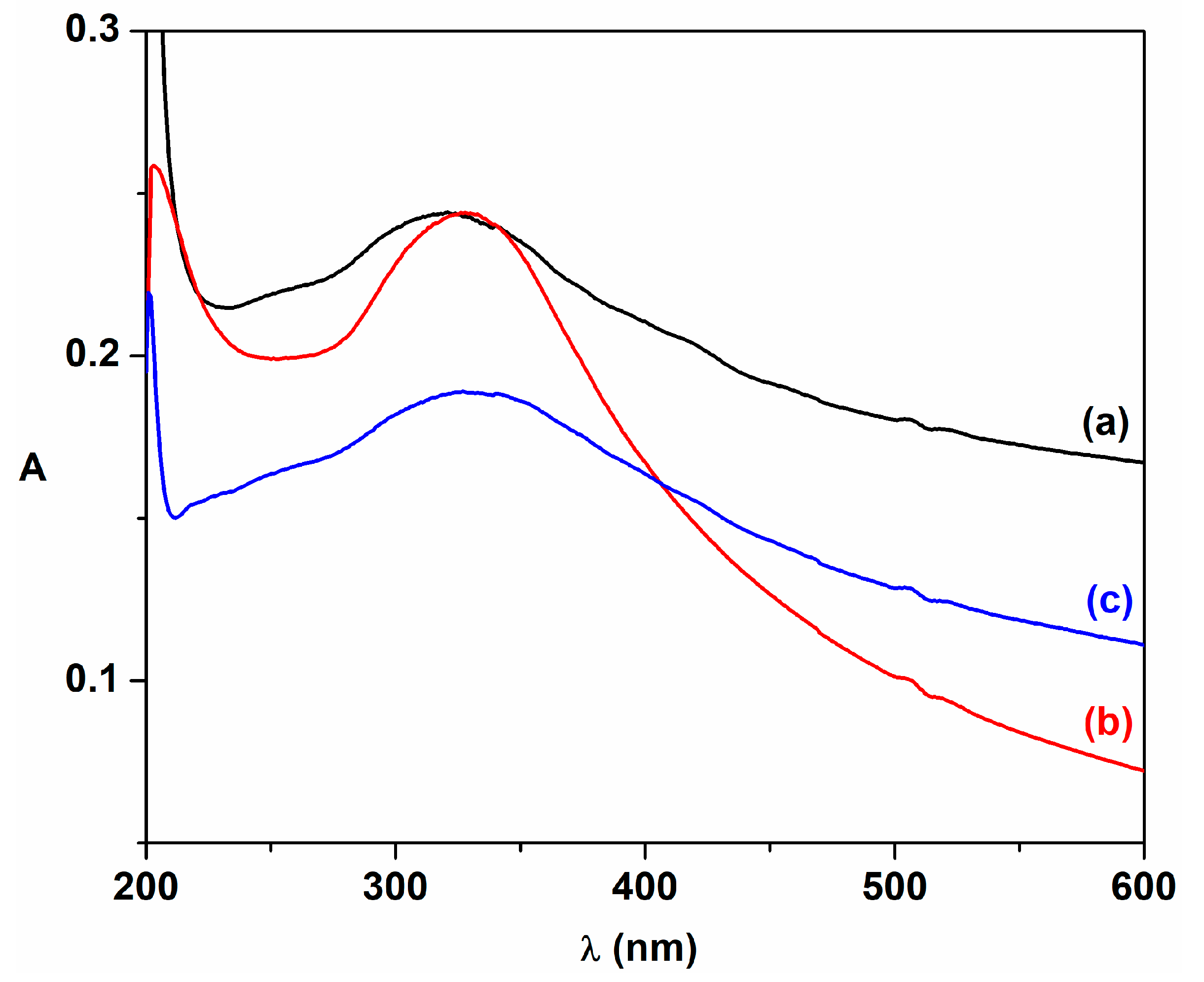
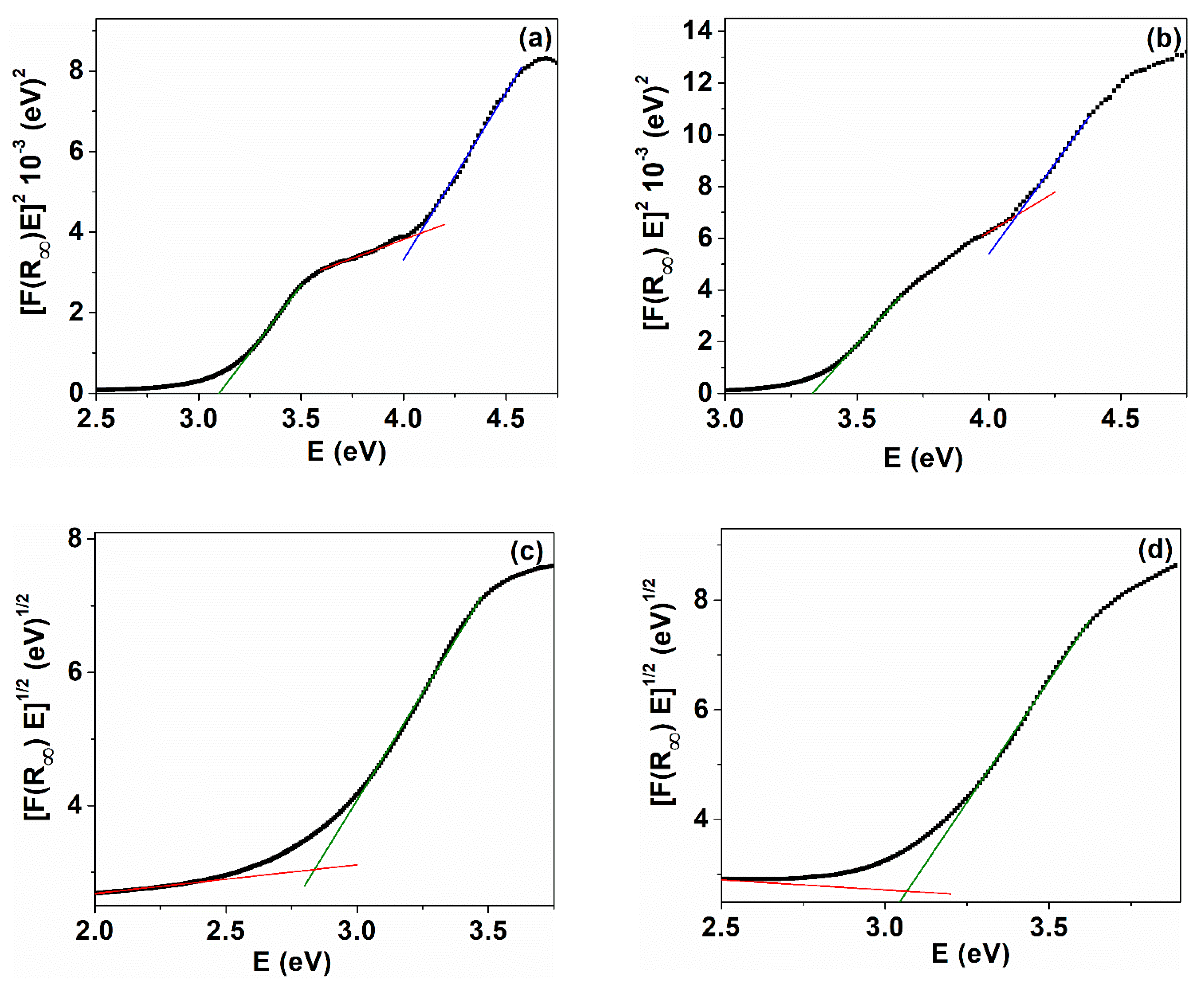

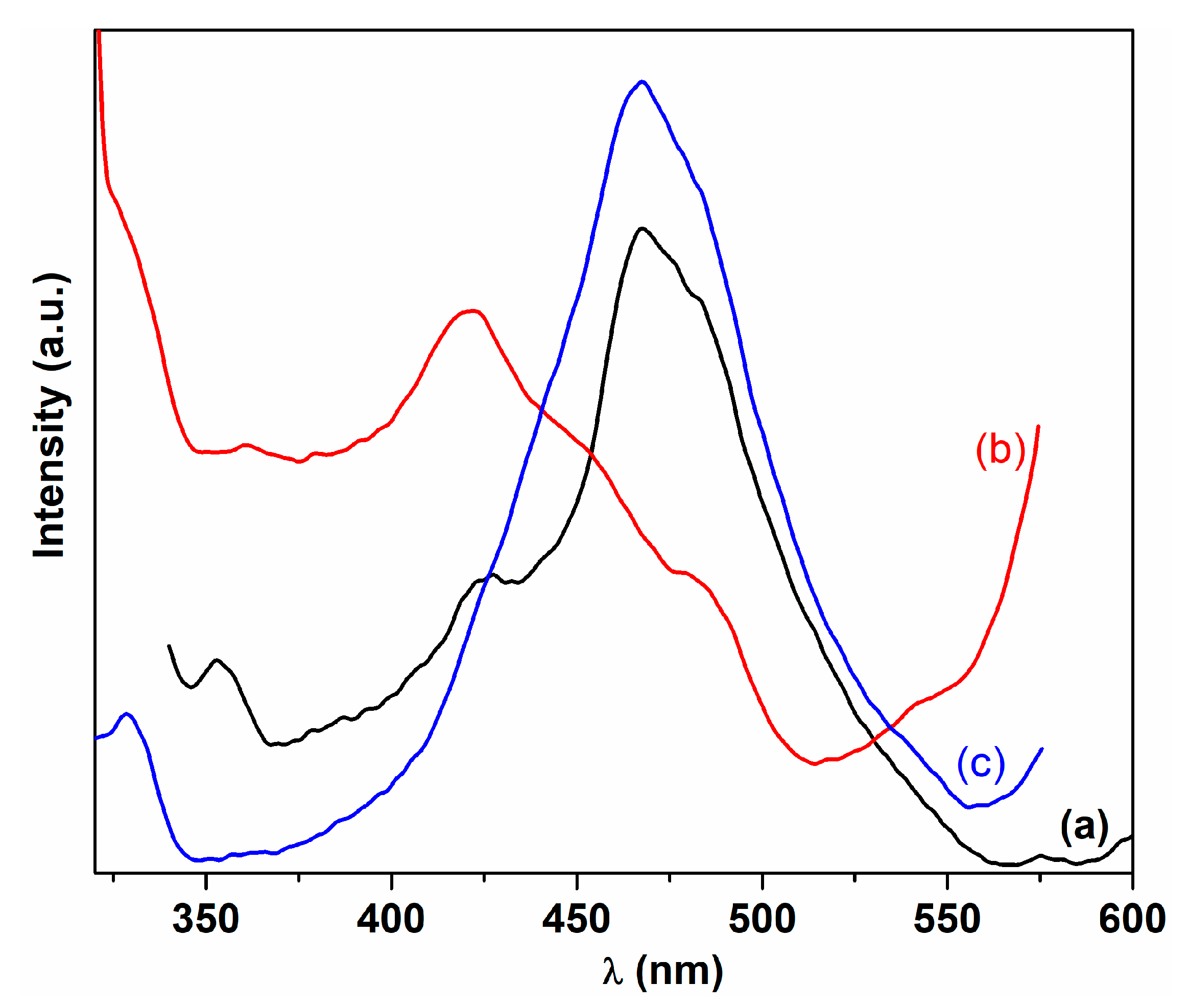
| Sample | λmax (nm) | DXRD (nm) | a (Å) | ν (cm−1) | DR (nm) | V (Å3) | N (cm−3) |
|---|---|---|---|---|---|---|---|
| C-CM | 321 | 10.14 | 5.3803 | 463.9 | 9.69 | 155.74 | 9.03 × 1020 |
| C-CM1 | 328 | 9.86 | 5.3875 | 463.6 | 12.09 | 156.37 | 7.17 × 1020 |
| C-VA | 327 | 5.97 | 5.4023 | 461.4 | 5.59 | 157.66 | 1.61 × 1021 |
| Sample | Ce 3d3/2 | Ce 3d5/2 | O 1s | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| U‴ | U″ | U′ | U | V‴ | V″ | V′ | V | OA | OL1 | OL2 | |
| C-CM1 | 24,513 | 14,310 | 19,634 | 30,158 | 37,141 | 21,682 | 29,749 | 45,694 | 3740 | 16,433 | 12,263 |
| C-CM | 32,269 | 18,315 | 12,929 | 32,362 | 48,892 | 27,750 | 19,589 | 49,033 | 5288 | 21,166 | 2832 |
| Sample |
(%) |
(%) | x | x′ | Δx | |
|---|---|---|---|---|---|---|
| C-CM1 | 22.16 | 77.84 | 0.28 | 1.89 | 1.51 | 0.37 |
| C-CM | 13.49 | 86.51 | 0.16 | 1.93 | 1.26 | 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fifere, N.; Airinei, A.; Doroftei, F.; Ardeleanu, T.S.; Dobromir, M.; Tîmpu, D.; Ursu, E.-L. Phytomediated-Assisted Preparation of Cerium Oxide Nanoparticles Using Plant Extracts and Assessment of Their Structural and Optical Properties. Int. J. Mol. Sci. 2023, 24, 8917. https://doi.org/10.3390/ijms24108917
Fifere N, Airinei A, Doroftei F, Ardeleanu TS, Dobromir M, Tîmpu D, Ursu E-L. Phytomediated-Assisted Preparation of Cerium Oxide Nanoparticles Using Plant Extracts and Assessment of Their Structural and Optical Properties. International Journal of Molecular Sciences. 2023; 24(10):8917. https://doi.org/10.3390/ijms24108917
Chicago/Turabian StyleFifere, Nicusor, Anton Airinei, Florica Doroftei, Tudor Stefan Ardeleanu, Marius Dobromir, Daniel Tîmpu, and Elena-Laura Ursu. 2023. "Phytomediated-Assisted Preparation of Cerium Oxide Nanoparticles Using Plant Extracts and Assessment of Their Structural and Optical Properties" International Journal of Molecular Sciences 24, no. 10: 8917. https://doi.org/10.3390/ijms24108917
APA StyleFifere, N., Airinei, A., Doroftei, F., Ardeleanu, T. S., Dobromir, M., Tîmpu, D., & Ursu, E.-L. (2023). Phytomediated-Assisted Preparation of Cerium Oxide Nanoparticles Using Plant Extracts and Assessment of Their Structural and Optical Properties. International Journal of Molecular Sciences, 24(10), 8917. https://doi.org/10.3390/ijms24108917







