αvβ3 Integrin as a Link between the Development of Fibrosis and Thyroid Hormones in Systemic Sclerosis
Abstract
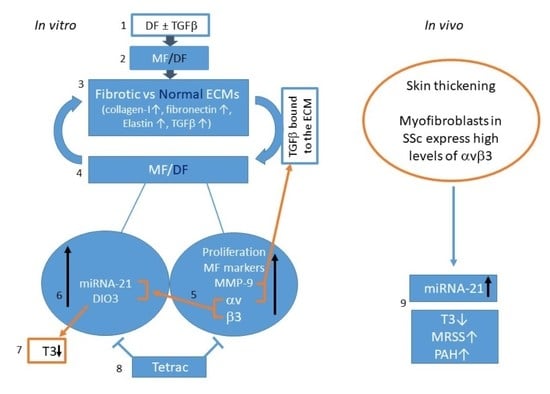
:1. Introduction
2. Results
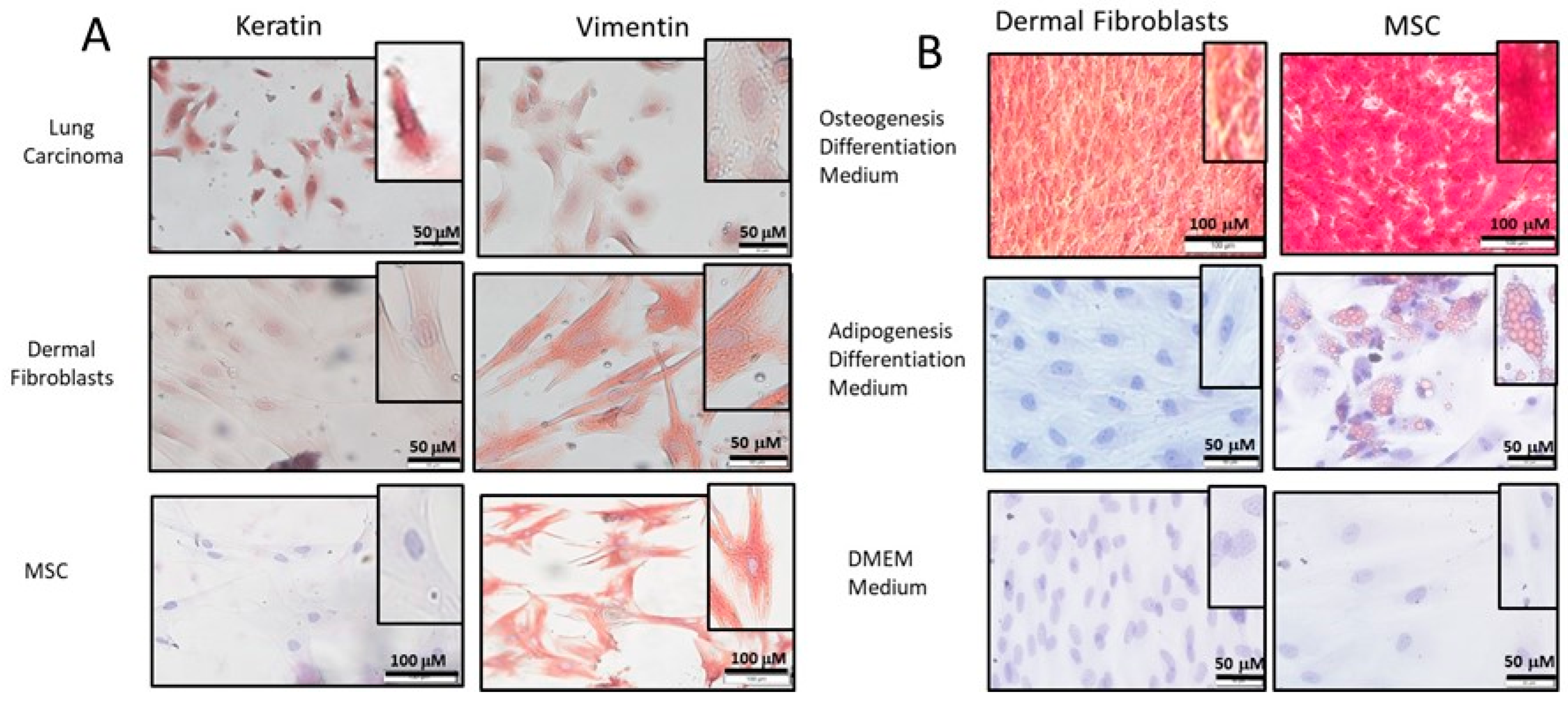
2.1. Isolation and Characterization of DF
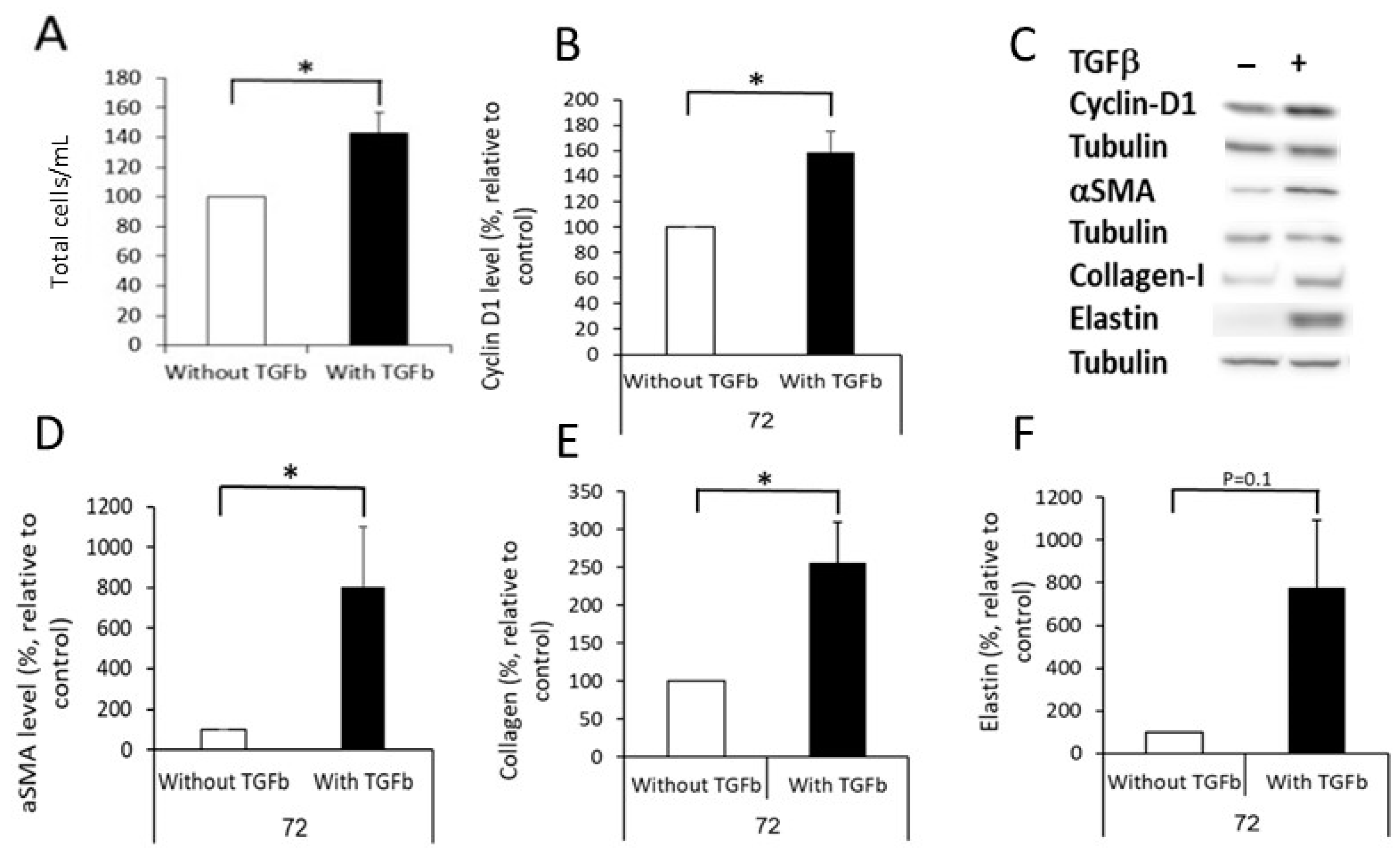
2.2. Differentiation of DF into MF
2.3. Preparation of Extracellular Matrices (ECM)
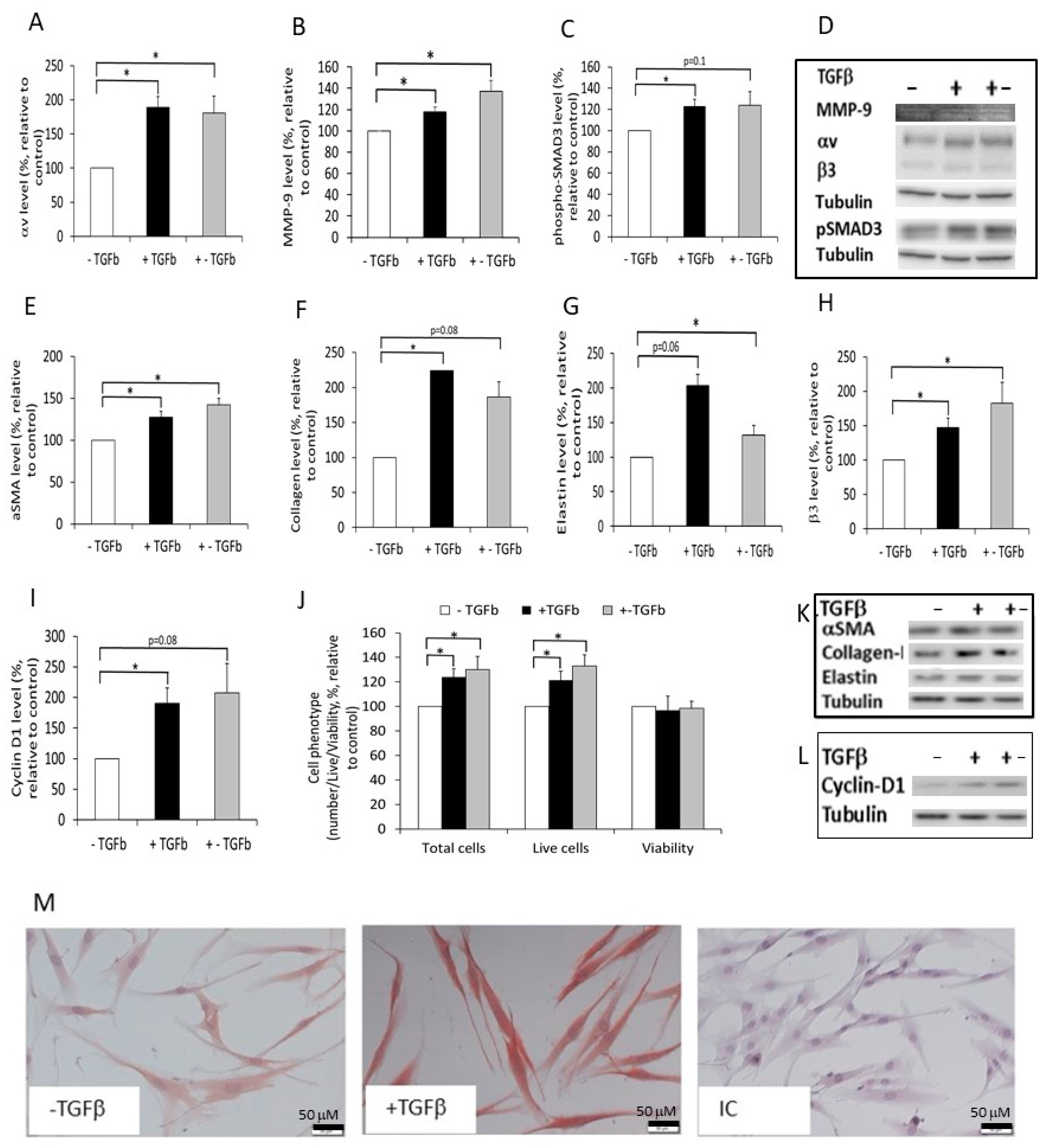
2.4. The Effect of Normal and Fibrotic-ECM on Naive DF
2.5. The Effect of Normal and Fibrotic-ECM on DF αvβ3 Expression
2.6. The Effect of Tetrac on the Response of DF to Fibrotic-ECM
2.7. The Effect of Fibrotic-ECM and Tetrac on miRNA-21 and D3 Expression
2.8. Analyzing miRNA-21 and fT3 Levels in SSc Patients’ Sera
3. Discussion
4. Materials and Methods
4.1. Isolation of Dermal Fibroblasts (DF)
4.2. Immunocytochemistry (ICC)
4.3. Adipogenesis/Osteogenesis Differentiation
4.4. Extracellular Matrix Preparation
4.5. ECM Histochemical Staining
4.6. Cell Culture
4.7. Cell Count
4.8. Protein Extraction
4.9. Western Blotting
4.10. Gelatin Zymography
4.11. RNA Extraction
4.12. RT cDNA Synthesis
4.13. Real-Time Quantitative PCR
4.14. Analyzing the Effect of TGFβ on DF Differentiation into MF, and the Cells’ Ability to Create Fibrotic ECM
4.15. Analyzing the Effect of ECM on DF
4.16. Ethical Approval
4.17. Patient Data
4.18. Biobank
4.19. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| αSMA | alpha-smooth muscle actin |
| D3 (protein) | DIO3 (gene) deiodinase-type-3 |
| DDW | double distilled water |
| DF | dermal fibroblasts |
| DMEM | Dulbecco’s Modified Eagle Medium |
| ECM | extracellular matrix |
| fT3 | free T3 |
| ICC | immunocytochemistry |
| LAP | latency-associated peptide |
| MF | myofibroblasts |
| MMP | matrix metalloproteinase |
| MRSS | modified Rodnan skin score |
| MSC | mesenchymal stem cells |
| PAH | pulmonary arterial hypertension |
| pSMAD3 | phosphorylated-Smad3 |
| RGD | arginine-glycine-aspartic |
| SSC | systemic sclerosis |
| T3 | triodothyronine |
| T4 | L-thyroxine |
| TBS | tris-buffered saline |
| Tetrac | tetraiodothyroacetic acid |
| TGFβ | transforming growth factor β |
| THs | thyroid hormones |
References
- Asano, Y. Systemic sclerosis. J. Dermatol. 2018, 45, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Knobler, R.; Moinzadeh, P.; Hunzelmann, N.; Kreuter, A.; Cozzio, A.; Mouthon, L.; Cutolo, M.; Rongioletti, F.; Denton, C.; Rudnicka, L.; et al. European Dermatology Forum S1-guideline on the diagnosis and treatment of sclerosing diseases of the skin, Part 1: Localized scleroderma, systemic sclerosis and overlap syndromes. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1401–1424. [Google Scholar] [CrossRef] [PubMed]
- Strong, A.L.; Rubin, J.P.; Kozlow, J.H.; Cederna, P.S. Fat Grafting for the Treatment of Scleroderma. Plast. Reconstr. Surg. 2019, 144, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Lin, C.J.F.; Furst, D.E.; Goldin, J.; Kim, G.; Kuwana, M.; Allanore, Y.; Matucci-Cerinic, M.; Distler, O.; Shima, Y.; et al. Tocilizumab in systemic sclerosis: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2020, 8, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Distler, O.; Cozzio, A. Systemic sclerosis and localized scleroderma—Current concepts and novel targets for therapy. Semin. Immunopathol. 2016, 38, 87–95. [Google Scholar] [CrossRef]
- Dees, C.; Chakraborty, D.; Distler, J.H.W. Cellular and molecular mechanisms in fibrosis. Exp. Dermatol. 2021, 30, 121–131. [Google Scholar] [CrossRef]
- Maldonado, H.; Hagood, J.S. Cooperative signaling between integrins and growth factor receptors in fibrosis. J. Mol. Med. 2021, 99, 213–224. [Google Scholar] [CrossRef]
- Liakouli, V.; Cipriani, P.; Di Benedetto, P.; Ruscitti, P.; Carubbi, F.; Berardicurti, O.; Panzera, N.; Giacomelli, R. The role of extracellular matrix components in angiogenesis and fibrosis: Possible implication for Systemic Sclerosis. Mod. Rheumatol. 2018, 28, 922–932. [Google Scholar] [CrossRef]
- Birembaut, P.; Cahuzac, P.; Delhomme, H.; Caron, Y.; Labat-Robert, J.; Robert, L.; Kalis, B. Distribution of fibronectin in the skin of patients with scleroderma. Ann. Dermatol. Venereol. 1982, 109, 933–937. [Google Scholar]
- van Caam, A.; Vonk, M.; van den Hoogen, F.; van Lent, P.; van der Kraan, P. Unraveling SSc Pathophysiology; The Myofibroblast. Front. Immunol. 2018, 9, 2452. [Google Scholar] [CrossRef]
- Sivakumar, P.; Kitson, C.; Jarai, G. Modeling and measuring extracellular matrix alterations in fibrosis: Challenges and perspectives for antifibrotic drug discovery. Connect. Tissue Res. 2019, 60, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Darby, I.A.; Laverdet, B.; Bonté, F.; Desmouliere, A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar] [PubMed]
- Zhu, H.; Luo, H.; Li, Y.; Zhou, Y.; Jiang, Y.; Chai, J.; Xiao, X.; You, Y.; Zuo, X. MicroRNA-21 in scleroderma fibrosis and its function in TGF-β-regulated fibrosis-related genes expression. J. Clin. Immunol. 2013, 33, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Di Girolamo, D.; Ambrosio, R.; De Stefano, M.A.; Mancino, G.; Porcelli, T.; Luongo, C.; Di Cicco, E.; Scalia, G.; Del Vecchio, L.; Colao, A.; et al. Reciprocal interplay between thyroid hormone and microRNA-21 regulates hedgehog pathway-driven skin tumorigenesis. J. Clin. Investig. 2016, 126, 2308–2320. [Google Scholar] [CrossRef] [PubMed]
- Baqui, M.; Botero, D.; Gereben, B.; Curcio, C.; Harney, J.W.; Salvatore, D.; Sorimachi, K.; Larsen, P.R.; Bianco, A.C. Human type 3 iodothyronine selenodeiodinase is located in the plasma membrane and undergoes rapid internalization to endosomes. J. Biol. Chem. 2003, 278, 1206–1211. [Google Scholar] [CrossRef]
- Alonso-Merino, E.; Orozco, R.M.; Ruíz-Llorente, L.; Martínez-Iglesias, O.A.; Velasco-Martín, J.P.; Montero-Pedrazuela, A.; Fanjul-Rodríguez, L.; Contreras-Jurado, C.; Regadera, J.; Aranda, A. Thyroid hormones inhibit TGF-β signaling and attenuate fibrotic responses. Proc. Natl. Acad. Sci. USA 2016, 113, E3451–E3460. [Google Scholar] [CrossRef]
- Shumnalieva, R.; Kachakova, D.; Shoumnalieva-Ivanova, V.; Miteva, P.; Kaneva, R.; Kolarov, Z.; Monov, S. P109 Expression levels of miR-21 and miR-29 in the serum of systemic sclerosis patients. Ann. Rheum. Dis. 2019, 78 (Suppl. S1), A48. [Google Scholar]
- Wolska-Gawron, K.; Bartosińska, J.; Rusek, M.; Kowal, M.; Raczkiewicz, D.; Krasowska, D. Circulating miRNA-181b-5p, miRNA-223-3p, miRNA-210-3p, let 7i-5p, miRNA-21-5p and miRNA-29a-3p in patients with localized scleroderma as potential biomarkers. Sci. Rep. 2020, 10, 20218. [Google Scholar] [CrossRef]
- Frangogiannis, N. Transforming growth factor-β in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef]
- Song, K.H.; Cho, S.J.; Song, J.Y. αvβ1 integrin as a novel therapeutic target for tissue fibrosis. Ann. Transl. Med. 2016, 4, 411. [Google Scholar] [CrossRef]
- Brown, N.F.; Marshall, J.F. Integrin-Mediated TGFβ Activation Modulates the Tumour Microenvironment. Cancers 2019, 11, 1221. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, F.; Chen, X. Integrin αvβ3-Targeted Cancer Therapy. Drug Dev. Res. 2008, 69, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.L. Integrin-mediated transforming growth factor-beta activation, a potential therapeutic target in fibrogenic disorders. Am. J. Pathol. 2009, 175, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Bergh, J.J.; Lin, H.Y.; Lansing, L.; Mohamed, S.N.; Davis, F.B.; Mousa, S.; Davis, P.J. Integrin αVβ3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology 2005, 146, 2864–2871. [Google Scholar] [CrossRef] [PubMed]
- Nappi, A.; Murolo, M.; Sagliocchi, S.; Miro, C.; Cicatiello, A.G.; Di Cicco, E.; Di Paola, R.; Raia, M.; D’Esposito, L.; Stornaiuolo, M.; et al. Selective Inhibition of Genomic and Non-Genomic Effects of Thyroid Hormone Regulates Muscle Cell Differentiation and Metabolic Behavior. Int. J. Mol. Sci. 2021, 22, 7175. [Google Scholar] [CrossRef]
- Liu, Y.C.; Yeh, C.T.; Lin, K.H. Molecular Functions of Thyroid Hormone Signaling in Regulation of Cancer Progression and Anti-Apoptosis. Int. J. Mol. Sci. 2019, 20, 4986, Correct in Int. J. Mol. Sci. 2020, 21, 3185. [Google Scholar] [CrossRef]
- Cohen, K.; Abadi, U.; Hercbergs, A.; Davis, P.J.; Ellis, M.; Ashur-Fabian, O. The induction of myeloma cell death and DNA damage by tetrac, a thyroid hormone derivative. Endocr. Relat. Cancer 2018, 25, 21–34. [Google Scholar] [CrossRef]
- Cody, V.; Davis, P.J.; Davis, F.B. Molecular modeling of the thyroid hormone interactions with αvβ3 integrin. Steroids 2007, 72, 165–170. [Google Scholar] [CrossRef]
- Jabbar, A.; Pingitore, A.; Pearce, S.H.S.; Zaman, A.; Iervasi, G.; Razvi, S. Thyroid hormones and cardiovascular disease. Nat. Rev. Cardiol. 2017, 14, 39–55. [Google Scholar] [CrossRef]
- Fallahi, P.; Ruffilli, I.; Giuggioli, D.; Colaci, M.; Ferrari, S.M.; Antonelli, A.; Ferri, C. Associations between Systemic Sclerosis and Thyroid Diseases. Front. Endocrinol. 2017, 8, 266. [Google Scholar] [CrossRef]
- Zhou, J.; Tripathi, M.; Ho, J.P.; Widjaja, A.A.; Shekeran, S.G.; Camat, M.D.; James, A.; Wu, Y.; Ching, J.; Kovalik, J.-P.; et al. Thyroid Hormone Decreases Hepatic Steatosis, Inflammation, and Fibrosis in a Dietary Mouse Model of Nonalcoholic Steatohepatitis. Thyroid 2022, 32, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Tzouvelekis, A.; Wang, R.; Herazo-Maya, J.D.; Ibarra, G.H.; Srivastava, A.; Werneck-De-Castro, J.P.; DeIuliis, G.; Ahangari, F.; Woolard, T.; et al. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat. Med. 2018, 24, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.C.; Shyu, W.C.; Lin, S.Z. Mesenchymal stem cells. Cell Transpl. 2011, 20, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Tobi, D.; Krashin, E.; Davis, P.J.; Cody, V.; Ellis, M.; Ashur-Fabian, O. Three-Dimensional Modeling of Thyroid Hormone Metabolites Binding to the Cancer-Relevant αvβ3 Integrin: In-Silico Based Study. Front. Endocrinol. 2022, 13, 895240. [Google Scholar] [CrossRef]
- Khanna, D.; Furst, D.E.; Clements, P.J.; Allanore, Y.; Baron, M.; Czirjak, L.; Distler, O.; Foeldvari, I.; Kuwana, M.; Matucci-Cerinic, M.; et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J. Scleroderma Relat. Disord. 2017, 2, 11–18. [Google Scholar] [CrossRef]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef]
- Duca, L.; Blaise, S.; Romier, B.; Laffargue, M.; Gayral, S.; El Btaouri, H.; Kawecki, C.; Guillot, A.; Martiny, L.; Debelle, L.; et al. Matrix ageing and vascular impacts: Focus on elastin fragmentation. Cardiovasc. Res. 2016, 110, 298–308. [Google Scholar] [CrossRef]
- Deng, B.; Zhao, Z.; Kong, W.; Han, C.; Shen, X.; Zhou, C. Biological role of matrix stiffness in tumor growth and treatment. J. Transl. Med. 2022, 20, 540. [Google Scholar] [CrossRef]
- Santos, A.; Lagares, D. Matrix Stiffness: The Conductor of Organ Fibrosis. Curr. Rheumatol. Rep. 2018, 20, 2. [Google Scholar] [CrossRef]
- Blaauboer, M.E.; Boeijen, F.R.; Emson, C.L.; Turner, S.M.; Zandieh-Doulabi, B.; Hanemaaijer, R.; Smit, T.H.; Stoop, R.; Everts, V. Extracellular matrix proteins: A positive feedback loop in lung fibrosis? Matrix Biol. 2014, 34, 170–178. [Google Scholar] [CrossRef]
- Rapisarda, V.; Borghesan, M.; Miguela, V.; Encheva, V.; Snijders, A.P.; Lujambio, A.; O’loghlen, A. Integrin Beta 3 Regulates Cellular Senescence by Activating the TGF-β Pathway. Cell Rep. 2017, 18, 2480–2493. [Google Scholar] [CrossRef] [PubMed]
- Leong, E.; Bezuhly, M.; Marshall, J.S. Distinct Metalloproteinase Expression and Functions in Systemic Sclerosis and Fibrosis: What We Know and the Potential for Intervention. Front. Physiol. 2021, 12, 727451. [Google Scholar] [CrossRef] [PubMed]
- Conroy, K.P.; Kitto, L.J.; Henderson, N.C. αv integrins: Key regulators of tissue fibrosis. Cell Tissue Res. 2016, 365, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y.; Ihn, H.; Yamane, K.; Jinnin, M.; Mimura, Y.; Tamaki, K. Increased expression of integrin αvβ3 contributes to the establishment of autocrine TGF-β signaling in scleroderma fibroblasts. J. Immunol. 2005, 175, 7708–7718. [Google Scholar] [CrossRef]
- Cobb, D.A.; de Rossi, J.; Liu, L.; An, E.; Lee, D.W. Targeting of the αvβ3 integrin complex by CAR-T cells leads to rapid regression of diffuse intrinsic pontine glioma and glioblastoma. J. Immunother. Cancer 2022, 10, e003816. [Google Scholar] [CrossRef]
- Schnittert, J.; Bansal, R.; Storm, G.; Prakash, J. Integrins in wound healing, fibrosis and tumor stroma: High potential targets for therapeutics and drug delivery. Adv. Drug Deliv. Rev. 2018, 129, 37–53. [Google Scholar] [CrossRef]
- Yokosaki, Y.; Nishimichi, N. New Therapeutic Targets for Hepatic Fibrosis in the Integrin Family, α8β1 and α11β1, Induced Specifically on Activated Stellate Cells. Int. J. Mol. Sci. 2021, 22, 12794. [Google Scholar] [CrossRef]
- Schmohl, K.A.; Nelson, P.J.; Spitzweg, C. Tetrac as an anti-angiogenic agent in cancer. Endocr. Relat. Cancer 2019, 26, R287–R304. [Google Scholar] [CrossRef]
- Gionfra, F.; De Vito, P.; Pallottini, V.; Lin, H.-Y.; Davis, P.J.; Pedersen, J.Z.; Incerpi, S. The Role of Thyroid Hormones in Hepatocyte Proliferation and Liver Cancer. Front. Endocrinol. 2019, 10, 532. [Google Scholar] [CrossRef]
- Abadi, U.; Weisz, A.; Kidron, D.; Katzav, A.; Hercbergs, A.; Davis, P.J.; Ellis, M.H.; Ashur-Fabian, O. αvβ3 Integrin Expression and Mitogenic Effects by Thyroid Hormones in Chronic Lymphocytic Leukemia. J. Clin. Med. 2021, 10, 1766. [Google Scholar] [CrossRef]
- Godugu, K.; Sudha, T.; Davis, P.J.; Mousa, S.A. Nano Diaminopropane tetrac and integrin αvβ3 expression in different cancer types: Anti-cancer efficacy and Safety. Cancer Treat. Res. Commun. 2021, 28, 100395. [Google Scholar] [CrossRef] [PubMed]
- Shinderman-Maman, E.; Cohen, K.; Weingarten, C.; Nabriski, D.; Twito, O.; Baraf, L.; Hercbergs, A.; Davis, P.J.; Werner, H.; Ellis, M.; et al. The thyroid hormone-αvβ3 integrin axis in ovarian cancer: Regulation of gene transcription and MAPK-dependent proliferation. Oncogene 2016, 35, 1977–1987. [Google Scholar] [CrossRef]
- Davis, P.J.; Glinsky, G.V.; Lin, H.Y.; Leith, J.T.; Hercbergs, A.; Tang, H.Y.; Ashur-Fabian, O.; Incerpi, S.; Mousa, S.A. Cancer Cell Gene Expression Modulated from Plasma Membrane Integrin αvβ3 by Thyroid Hormone and Nanoparticulate Tetrac. Front. Endocrinol. 2014, 5, 240. [Google Scholar]
- Asano, Y. The Pathogenesis of Systemic Sclerosis: An Understanding Based on a Common Pathologic Cascade across Multiple Organs and Additional Organ-Specific Pathologies. J. Clin. Med. 2020, 9, 2687. [Google Scholar] [CrossRef]
- Ghamra, Z.W.; Dweik, R.A.; Arroliga, A.C. Hypothyroidism and pulmonary arterial hypertension. Am. J. Med. 2004, 116, 354–355. [Google Scholar] [CrossRef] [PubMed]
- Wuttge, D.M.; Carlsen, A.L.; Teku, G.; Wildt, M.; Rådegran, G.; Vihinen, M.; Heegaard, N.H.H.; Hesselstrand, R. Circulating plasma microRNAs in systemic sclerosis-associated pulmonary arterial hypertension. Rheumatology 2021, 61, 309–318. [Google Scholar] [CrossRef]
- Bachmann, M.; Kukkurainen, S. Cell Adhesion by Integrins. Physiol. Rev. 2019, 99, 1655–1699. [Google Scholar] [CrossRef]
- Sarrazy, V.; Koehler, A.; Chow, M.L.; Zimina, E.; Li, C.X.; Kato, H.; Caldarone, C.A.; Hinz, B. Integrins αvβ5 and αvβ3 promote latent TGF-β1 activation by human cardiac fibroblast contraction. Cardiovasc. Res. 2014, 102, 407–417. [Google Scholar] [CrossRef]
- Huang, C.-H.; Huang, T.-Y.; Chang, W.-J.; Pan, Y.-S.; Chu, H.-R.; Li, Z.-L.; Unson, S.; Chin, Y.-T.; Lin, C.-Y.; Huang, H.-M.; et al. Combined Treatment of Heteronemin and Tetrac Induces Antiproliferation in Oral Cancer Cells. Mar. Drugs 2020, 18, 348. [Google Scholar] [CrossRef]
- Köhrle, J.; Frädrich, C. Deiodinases control local cellular and systemic thyroid hormone availability. Free Radic. Biol. Med. 2022, 193 Pt 1, 59–79. [Google Scholar] [CrossRef]


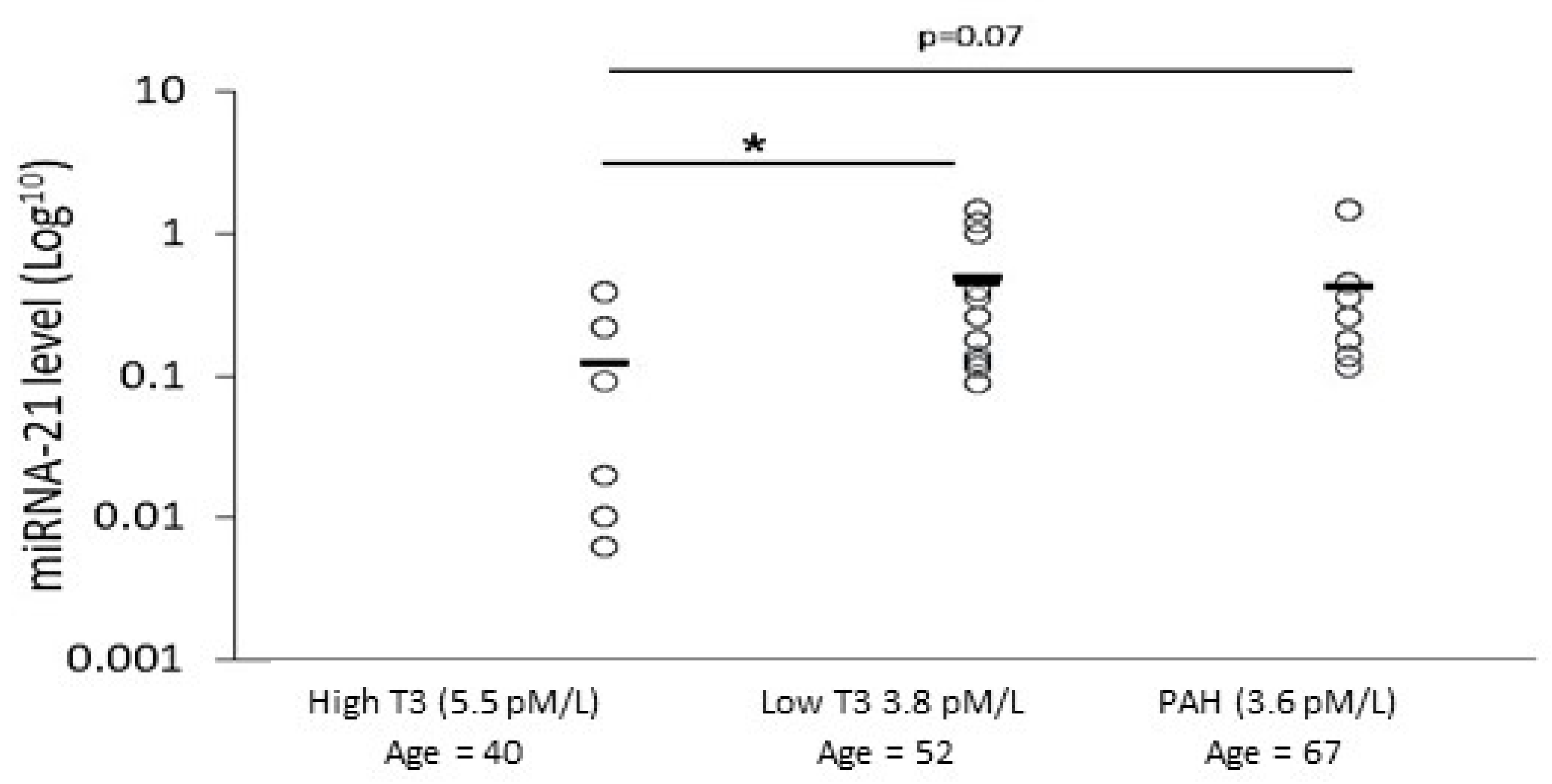
| Characteristics | Low T3 PAH | All Low T3 SSc + PAH | High T3 SSc |
|---|---|---|---|
| n = 7 | n = 12 | n = 6 | |
| Demographics | |||
| Age (years) | 67 ± 9 * | 60 * | 40 ± 16 |
| Female (% (number of males)) | 86 (1) | 91.7 (1) | 100 |
| Clinical | |||
| Diffuse vs. local SSc (%) | 57 | 66 | 83 |
| Echo-pulmonary arterial pressure (mPAP, mmHg) | 68 ± 19 * | 54.6 ± 22 * | 27.3 ± 3.6 |
| Average FT3 level (pmol/L) (normal range 3.1–6.8 pmol/L) | 3.6 ± 0.6 * | 3.7 ± 0.5 * | 5.6 ± 1.4 |
| miRNA-21 | 0.56 ± 0.46 * | 0.47 + 0.46 * | 0.12 + 0.15 |
| Variables | r(s)XY | p-Value | n | |
|---|---|---|---|---|
| X | Y | |||
| fT3 | miRNA-21 | −0.391 | 0.0505 | 18 |
| fT3 | MRSS | −0.311 | 0.104 | 18 |
| fT3 | PAH | −0.538 | 0.010 | 18 |
| fT3 | Echo | −0.701 | 0.001 | 18 |
| fT3 | Age | −0.424 | 0.036 | 18 |
| miRNA-21 | Age | 0.243 | 0.329 | 18 |
| Target | Source | Isotype | Company | Catalog No. | Dilution | Hybridization Conditions |
|---|---|---|---|---|---|---|
| Vimentin | Rabbit | mAb | Cell Signaling Technology (Danvers, MA, USA) | M0725 | 1:100 | BSA 3% |
| Pan Cytokeratin | Mouse | mAb | BIOCARE (Pacheco, CA, USA) | CM 011 A, B, C | 1:1 | As is |
| Collagen-I | Rabbit | Polyclonal Ab | Abcam (Cambridge, UK) | ab34710 | 1:1000 | BSA 5% |
| Elastin | Rabbit | Polyclonal Ab | Proteintech | 15257-1 | 1:2500 | BSA 5% |
| Fibronectin | Rabbit | mAb | Abcam (Cambridge, UK) | AB32419 | 1:1000 | Milk 5% |
| αSMA | Mouse | mAb IgG2a | Abcam (Cambridge, UK) | ab7817 | 1:2000 | BSA 5% |
| Cyclin-D1 | Rabbit | Polyclonal Ab | Cell Signaling Technology (Danvers, MA, USA) | #2922 | 1:1000 | BSA 5% |
| Integrin αv | Rabbit | Polyclonal Ab | Cell Signaling Technology (Danvers, MA, USA) | #4711 | 1:1000 | BSA 5% |
| Integrin β3 | Rabbit | Polyclonal Ab | Santa Cruz Biotechnology | SC14009 | 1:200 | BSA 5% |
| Integrin αvβ3 | Rabbit | Polyclonal Ab | ABBIOTEC (Rosemont, IL, USA) | 251672 | 1:200 | BSA 3% |
| Phospho-SMAD3 | Rabbit | mAb IgG | Cell Signaling Technology (Danvers, MA, USA) | #9520 | 1:1000 | BSA 5% |
| D3 | Rabbit | Polyclonal Ab | Novus (Centennial, CO, USA) | NBP1-05767 | 1:2000 | BSA 5% |
| TGFβ-I | Rabbit | Polyclonal Ab | Sigma Aldrich (Rehovot, Israel) | HPA008612 | 1:1000 | BSA 5% |
| α-Tubulin | Mouse | mAb IgG1 | Sigma Aldrich Rehovot, Israel) | T5168 | 1:4000 | Milk 5% |
| Peroxidase conjugated anti-mouse | Goat | Polyclonal Ab | Jackson Immuno-Research (West Grove, PA, USA) | 115035003 | 1:20,000 | Milk 1% |
| Peroxidase conjugated anti-Rabbit | Goat | Polyclonal Ab | Jackson Immuno-Research (West Grove, PA, USA) | 111035003 | 1:10,000 | Milk 1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohon, M.Y.; Zaaroor Levy, M.; Hornik-Lurie, T.; Shalom, A.; Berl, A.; Drucker, L.; Levy, Y.; Tartakover Matalon, S. αvβ3 Integrin as a Link between the Development of Fibrosis and Thyroid Hormones in Systemic Sclerosis. Int. J. Mol. Sci. 2023, 24, 8927. https://doi.org/10.3390/ijms24108927
Kohon MY, Zaaroor Levy M, Hornik-Lurie T, Shalom A, Berl A, Drucker L, Levy Y, Tartakover Matalon S. αvβ3 Integrin as a Link between the Development of Fibrosis and Thyroid Hormones in Systemic Sclerosis. International Journal of Molecular Sciences. 2023; 24(10):8927. https://doi.org/10.3390/ijms24108927
Chicago/Turabian StyleKohon, Maia Yamila, Mor Zaaroor Levy, Tzipi Hornik-Lurie, Avshalom Shalom, Ariel Berl, Liat Drucker, Yair Levy, and Shelly Tartakover Matalon. 2023. "αvβ3 Integrin as a Link between the Development of Fibrosis and Thyroid Hormones in Systemic Sclerosis" International Journal of Molecular Sciences 24, no. 10: 8927. https://doi.org/10.3390/ijms24108927
APA StyleKohon, M. Y., Zaaroor Levy, M., Hornik-Lurie, T., Shalom, A., Berl, A., Drucker, L., Levy, Y., & Tartakover Matalon, S. (2023). αvβ3 Integrin as a Link between the Development of Fibrosis and Thyroid Hormones in Systemic Sclerosis. International Journal of Molecular Sciences, 24(10), 8927. https://doi.org/10.3390/ijms24108927