Pharmacoinformatic Investigation of Silymarin as a Potential Inhibitor against Nemopilema nomurai Jellyfish Metalloproteinase Toxin-like Protein
Abstract
1. Introduction
2. Results
2.1. The Sequencing and De Novo Transcriptome Assembly for Nemopilema nomurai Tentacle
2.2. The Putative Toxin-like Metalloprotein Gene Profile of Nemopilema nomurai Tentacle
2.3. Selection of Target Metalloproteinase
2.4. The Structure Prediction of NnV-Mlp by Alpha Fold2
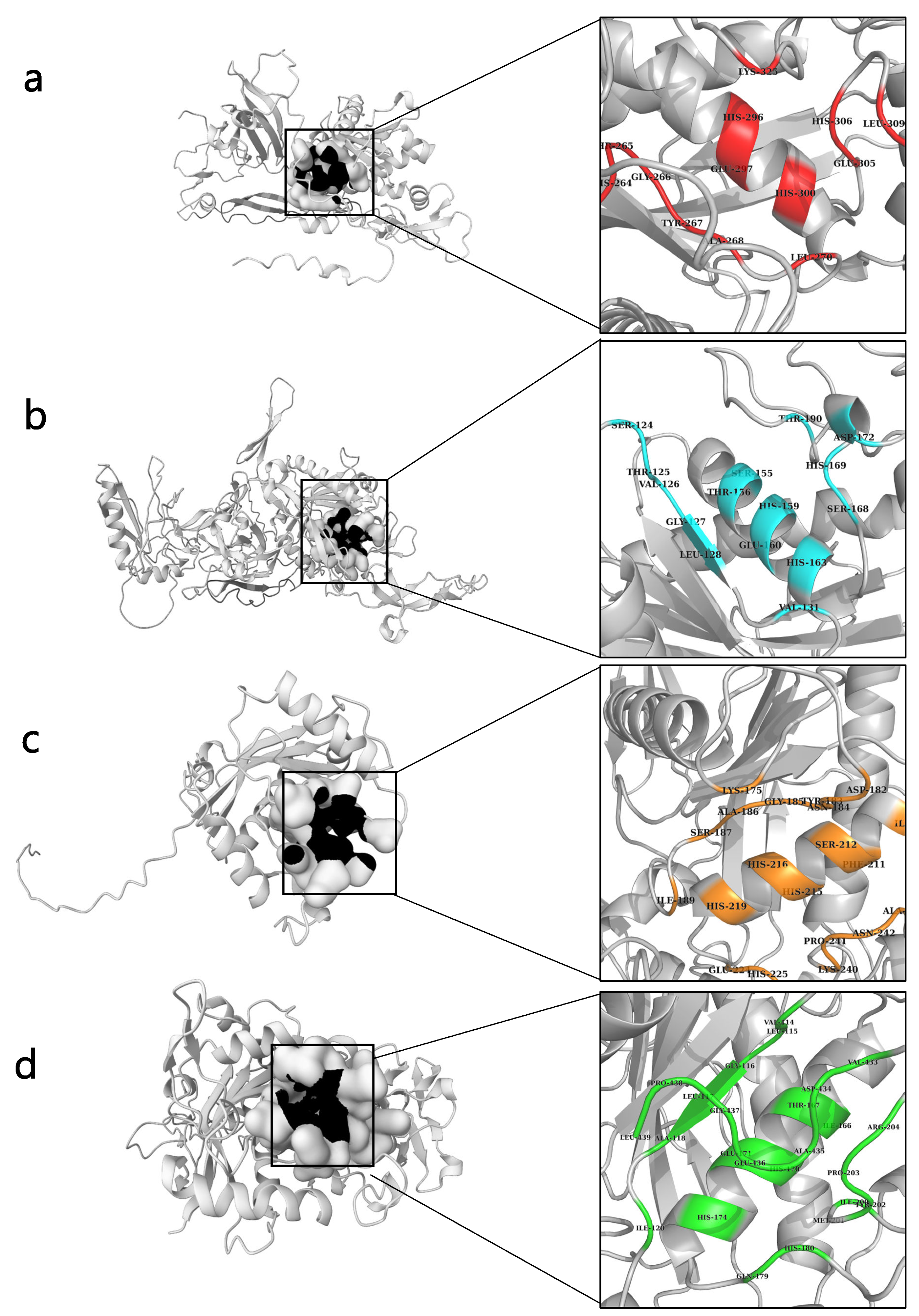
2.5. Active Side Prediction
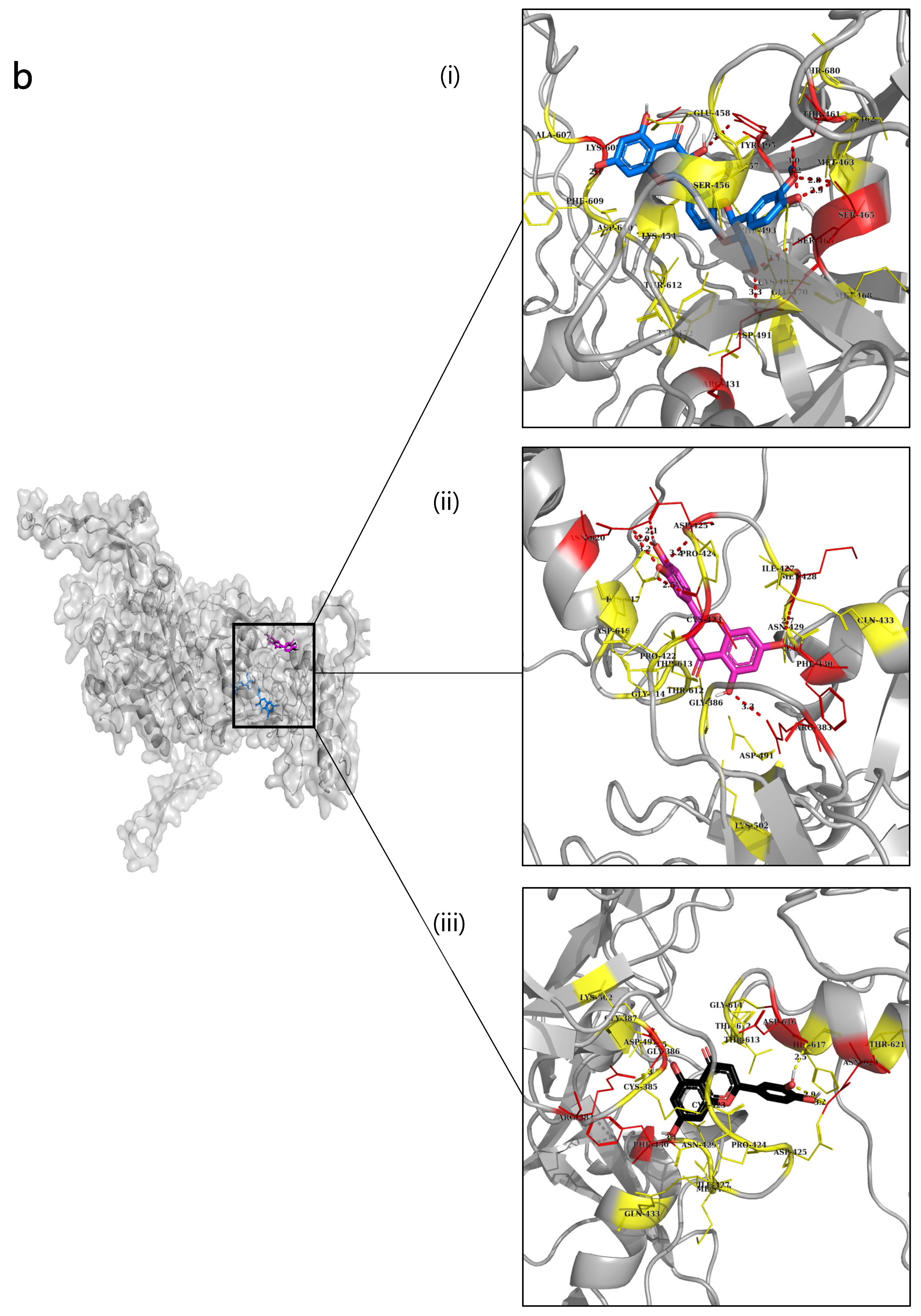
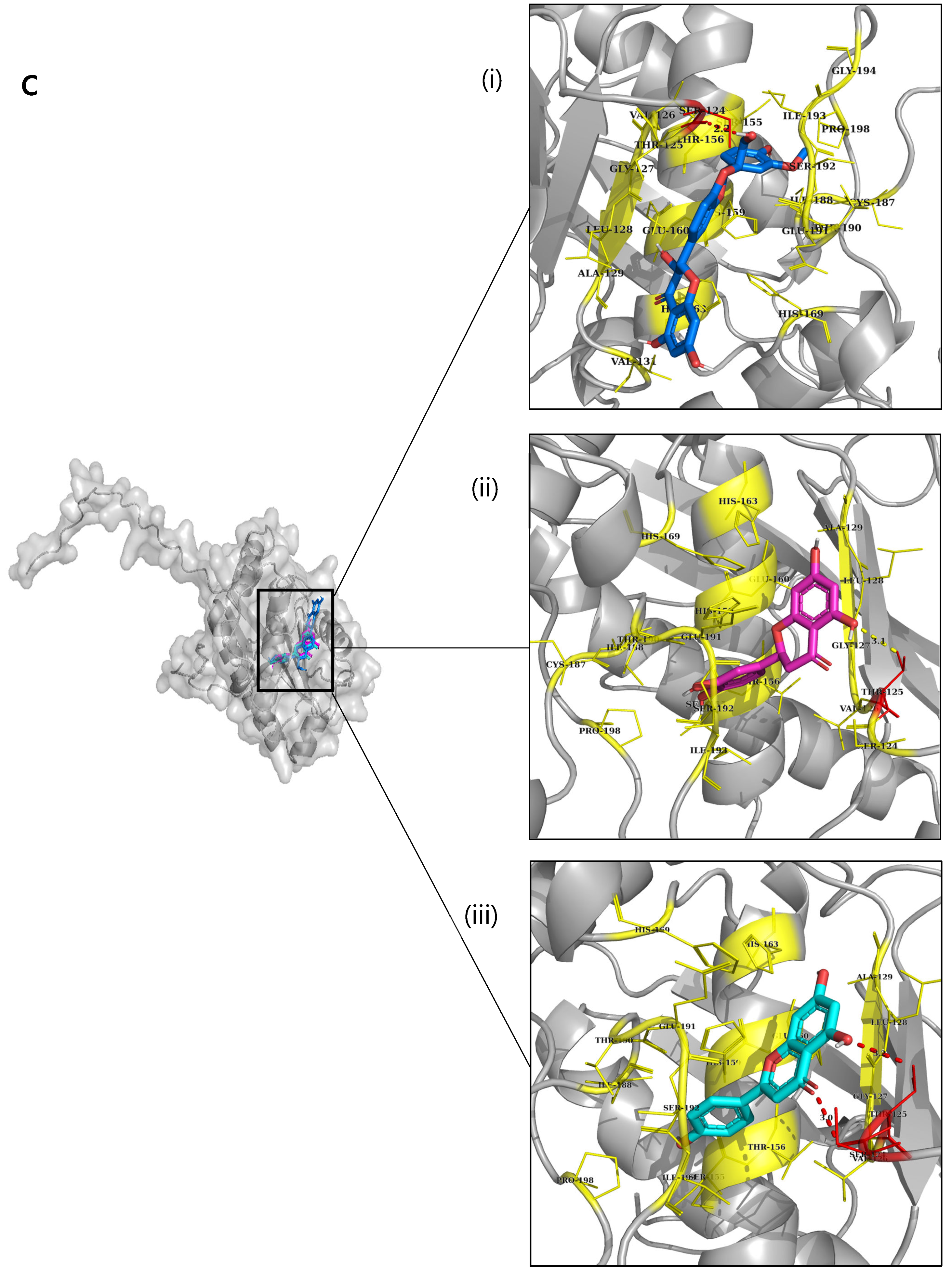
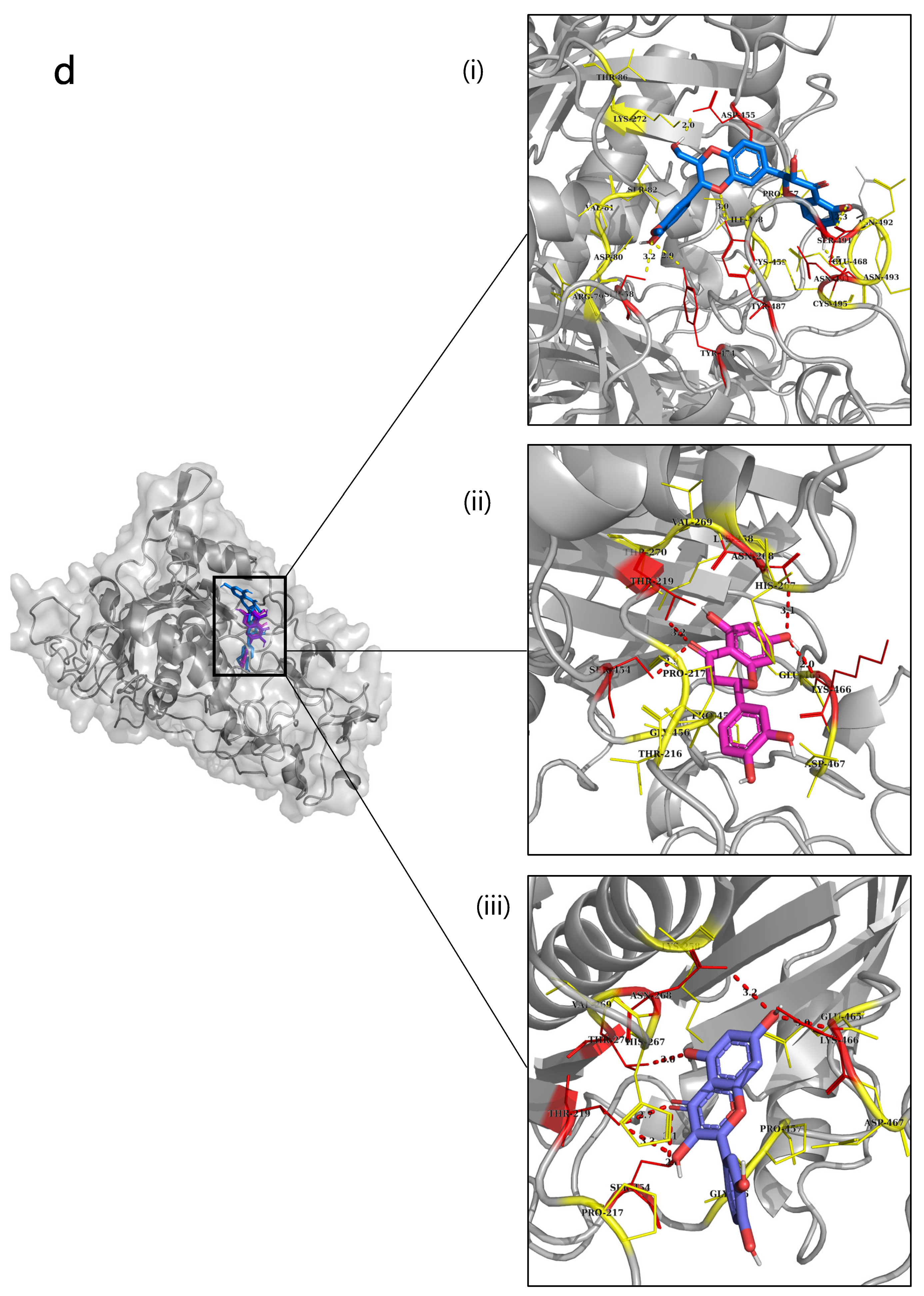
2.6. Molecular Docking
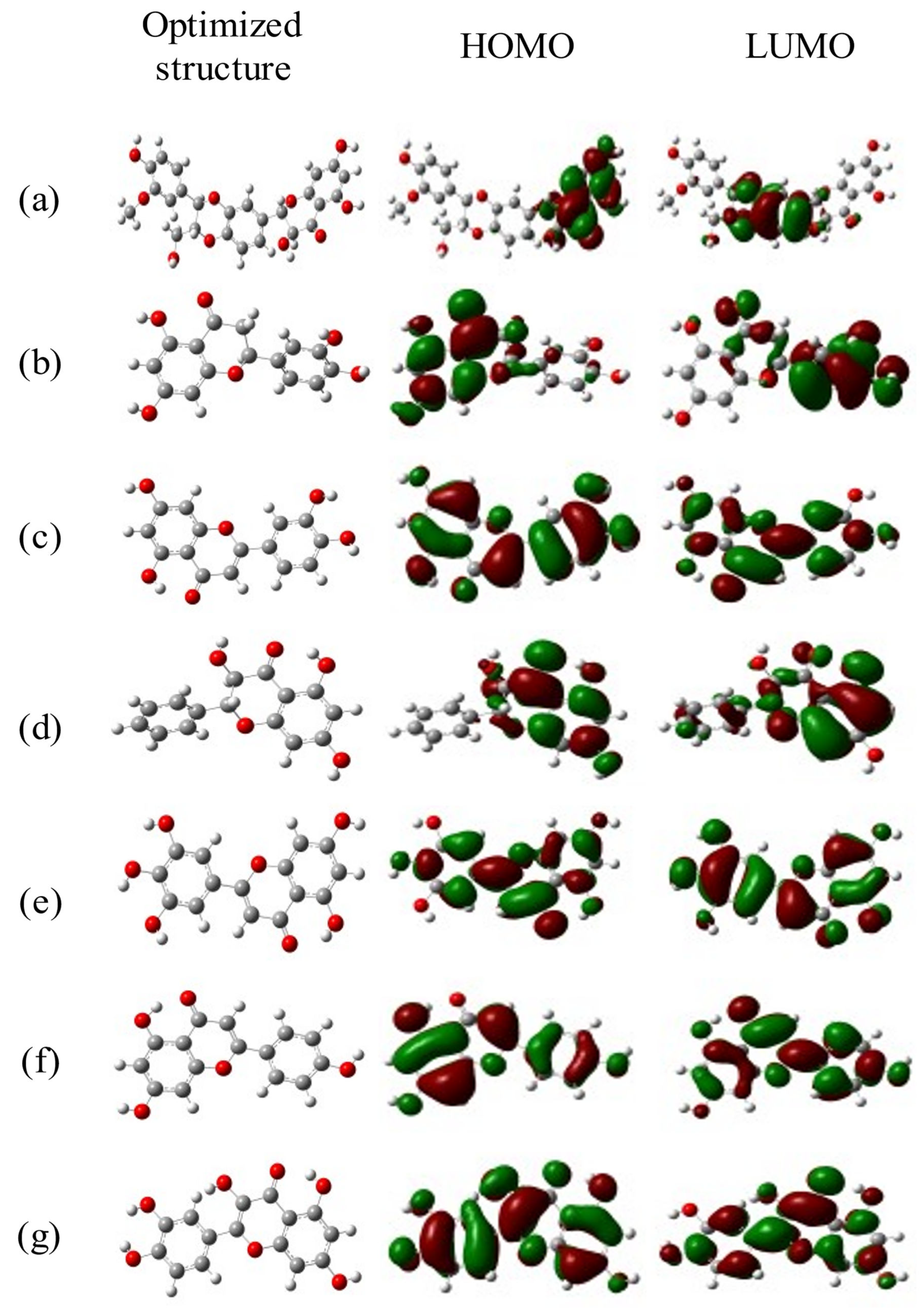
2.7. DFT Calculations
2.8. ADMET Prediction
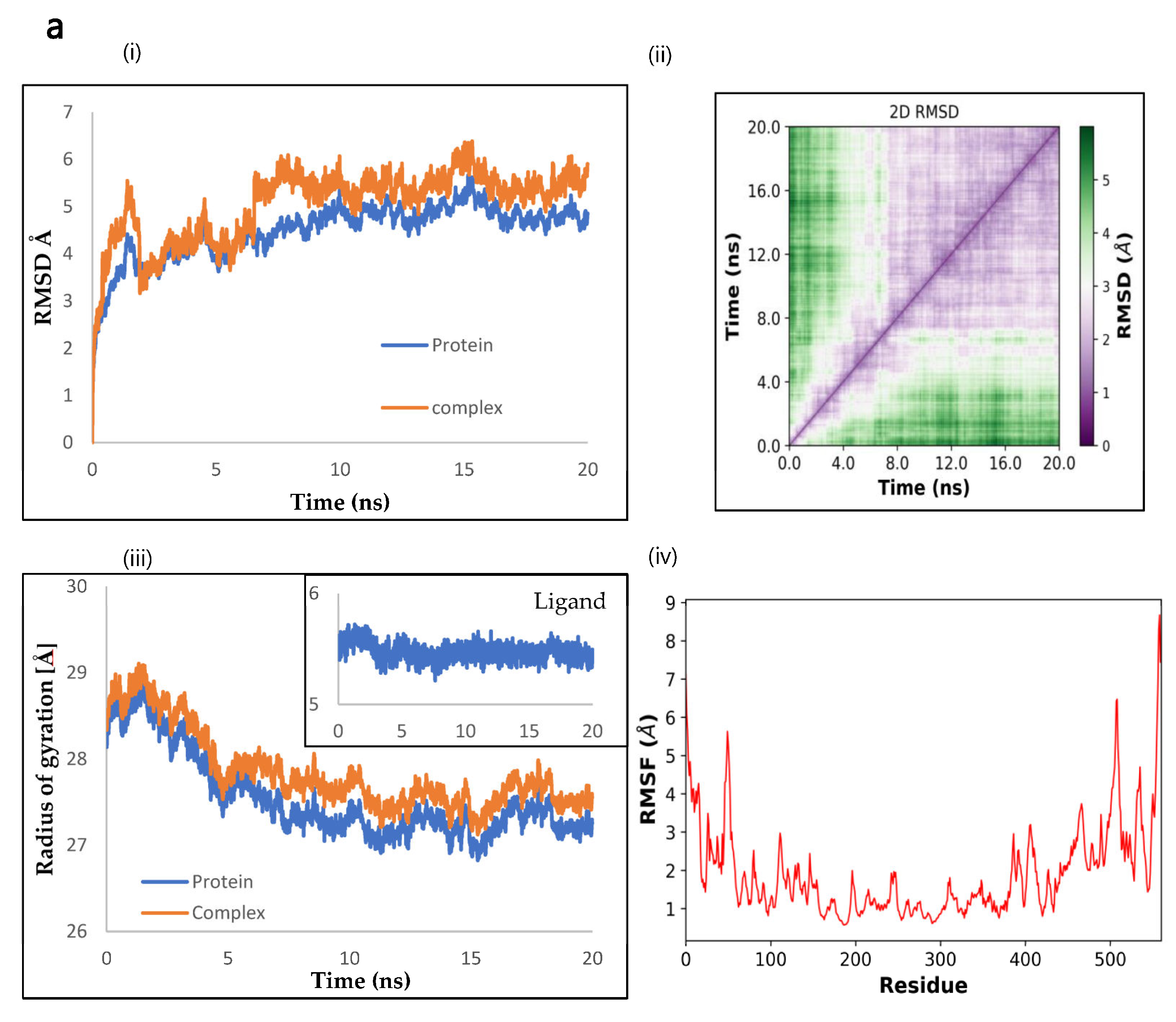
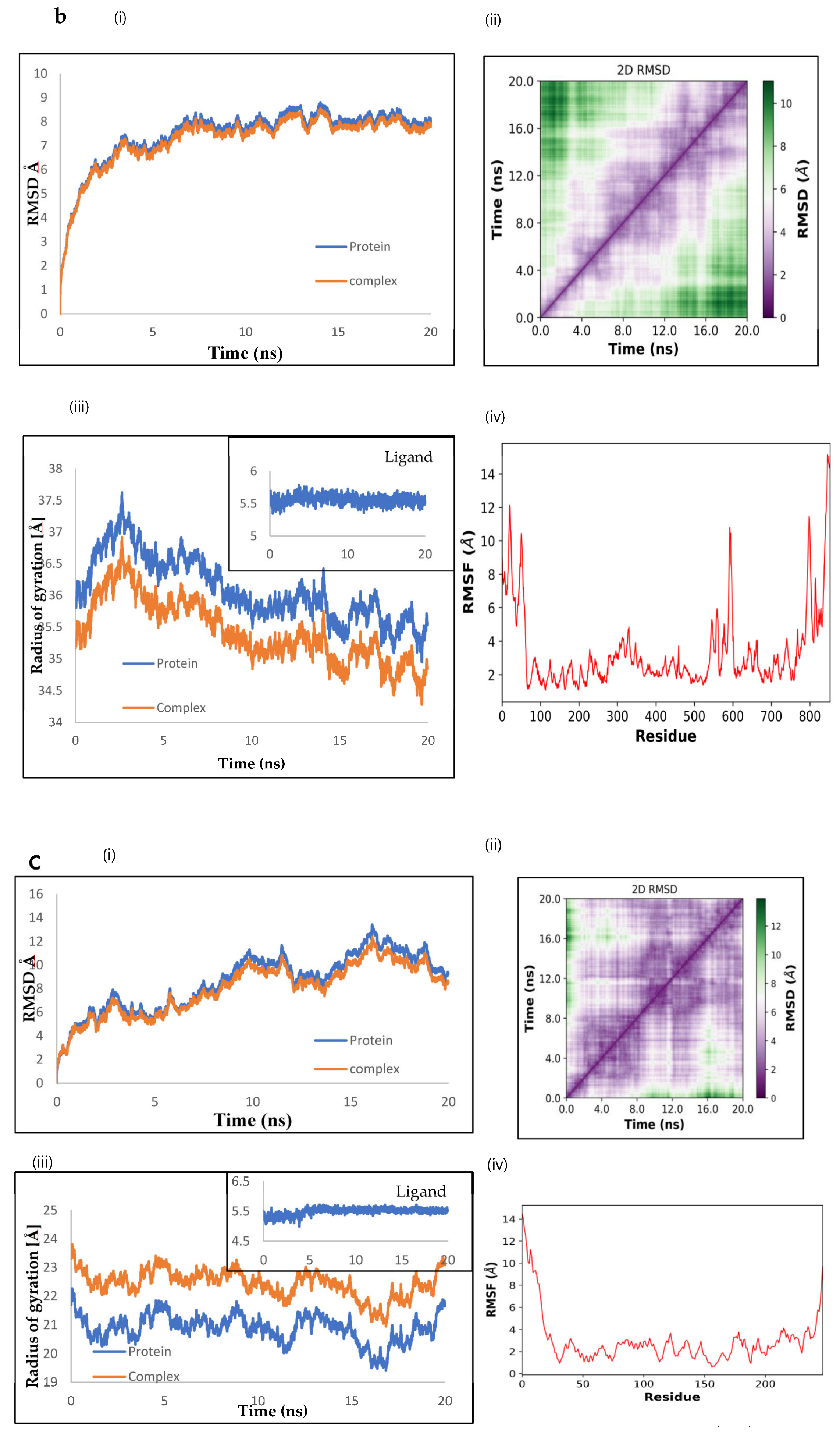
2.9. Molecular Dynamics by openMM
2.10. The Binding Energy between NnV-Mlp Types and Silymarin Using the MMPBSA Method
3. Discussion
4. Materials and Methods
4.1. Bioinformatics Analysis
4.2. Selection of Metalloproteinase
4.3. Preparation of Proteins
4.4. Selection of Lligands
4.5. Preparation of Ligands
4.6. Active site Prediction
4.7. Molecular Docking
4.8. Density Functional Theory
4.9. ADMET Properties
4.10. Molecular Dynamics Simulation
4.11. The Prediction of Relative Binding Energy between NnV-Mlp Types and Silymarin Using MMPBSA Method
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Halstead, B. Poisonous and Venomous Marine Animals of the World. Darwin Press and Princeton: Pennington, NJ, USA, 1988. [Google Scholar]
- Kang, C.; Munawir, A.; Cha, M.; Sohn, E.T.; Lee, H.; Kim, J.S.; Yoon, W.D.; Lim, D.; Kim, E. Cytotoxicity and hemolytic activity of jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) venom. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 150, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sun, H. Arthropoda Crustacea. Brachyura. Marine Primitive Crabs; Fauna Sinica: Invertebrata; Science Press: Beijing, China, 2002; Volume 30, pp. 1–597. [Google Scholar]
- Kawahara, M.; Uye, S.I.; Ohtsu, K.; Iizumi, H. Unusual Population Explosion of the Giant Jellyfish Nemopilema Nomurai (Scyphozoa: Rhizostomeae) in East Asian Waters. Mar. Ecol. Prog. Ser. 2006, 307, 161–173. [Google Scholar] [CrossRef]
- Torres, M.; Aguilar, M.B.; Falcón, A.; Sánchez, L.; Radwan, F.F.; Burnett, J.W.; Heimer-de la Cotera, E.P.; Arellano, R.O. Electrophysiological and Hemolytic Activity Elicited by the Venom of the Jellyfish Cassiopea Xamachana. Toxicon 2001, 39, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.; Isbister, G.K.; Seymour, J.E.; Hodgson, W.C. Pharmacologically Distinct Cardiovascular Effects of Box Jellyfish (Chironex Fleckeri) Venom and a Tentacle-Only Extract in Rats. Toxicol. Lett. 2005, 155, 219–226. [Google Scholar] [CrossRef]
- Ramasamy, S.; Isbister, G.K.; Seymour, J.E.; Hodgson, W.C. The in Vivo Cardiovascular Effects of Box Jellyfish Chironex Fleckeri Venom in Rats: Efficacy of Pre-Treatment with Antivenom, Verapamil and Magnesium Sulphate. Toxicon 2004, 43, 685–690. [Google Scholar] [CrossRef]
- Yu, H.; Liu, X.; Xing, R.; Liu, S.; Li, C.; Li, P. Radical Scavenging Activity of Protein from Tentacles of Jellyfish Rhopilema Esculentum. Bioorg. Med. Chem. Lett. 2005, 15, 2659–2664. [Google Scholar] [CrossRef]
- Helmholz, H.; Ruhnau, C.; Schütt, C.; Prange, A. Comparative Study on the Cell Toxicity and Enzymatic Activity of Two Northern Scyphozoan Species Cyanea Capillata (L.) and Cyanea Lamarckii (Péron & Léslieur). Toxicon 2007, 50, 53–64. [Google Scholar]
- Carli, A.; Bussotti, S.; Mariottini, G.; Robbiano, L. Toxicity of jellyfish and sea-anemone venoms on cultured V79 cells. Toxicon 1996, 34, 496–500. [Google Scholar] [CrossRef]
- Li, R.; Yu, H.; Xue, W.; Yue, Y.; Liu, S.; Xing, R.; Li, P. Jellyfish Venomics and Venom Gland Transcriptomics Analysis of Stomolophus Meleagris to Reveal the Toxins Associated with Sting. J. Proteom. 2014, 106, 17–29. [Google Scholar] [CrossRef]
- Tamkun, M.M.; Hessinger, D.A. Isolation and Partial Characterization of a Hemolytic and Toxic Protein from the Nematocyst Venom of the Portuguese Man-of-War, Physalia Physalis. Biochim. Et Biophys. Acta (BBA)-Protein Struct. 1981, 667, 87–98. [Google Scholar] [CrossRef]
- Hessinger, D.A.; Lenhoff, H.H. Binding of Active and Inactive Hemolytic Factor of Sea Anemone Nematocyst Venom to Red Blood Cells. Biochem. Biophys. Res. Commun. 1973, 53, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Hessinger, D.A.; Lenhoff, H.M. Undefined Mechanism of Hemolysis Induced by Nematocyst Venom: Roles of Phospholipase A and Direct Lytic Factor. Arch. Biochem. Biophys. 1976, 173, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Endean, R.; Monks, S.A.; Cameron, A.M. Toxins from the Box-Jellyfish Chironex Fleckeri. Toxicon 1993, 31, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jung, E.-S.; Kang, C.; Yoon, W.D.; Kim, J.-S.; Kim, E. Scyphozoan jellyfish venom metalloproteinases and their role in the cytotoxicity. Toxicon 2011, 58, 277–284. [Google Scholar] [CrossRef]
- Li, A.; Yu, H.; Li, R.; Liu, S.; Xing, R.; Li, P. Inhibitory Effect of Metalloproteinase Inhibitors on Skin Cell Inflammation Induced by Jellyfish Nemopilema nomurai Nematocyst Venom. Toxins 2019, 11, 156. [Google Scholar] [CrossRef]
- Kim, H.-M.; Weber, J.A.; Lee, N.; Park, S.G.; Cho, Y.S.; Bhak, Y.; Jeon, Y.; Jeon, S.; Luria, V.; Karger, A.; et al. The genome of the giant Nomura’s jellyfish sheds light on the early evolution of active predation. BMC Biol. 2019, 17, 28. [Google Scholar] [CrossRef]
- Li, A.; Yu, H.; Li, R.; Yue, Y.; Yu, C.; Geng, H.; Liu, S.; Xing, R.; Li, P. Jellyfish Nemopilema nomurai causes myotoxicity through the metalloprotease component of venom. Biomed. Pharmacother. 2022, 151, 113192. [Google Scholar] [CrossRef]
- Cegolon, L.; Heymann, W.C.; Lange, J.H.; Mastrangelo, G. Jellyfish Stings and Their Management: A Review. Mar. Drugs 2013, 11, 523–550. [Google Scholar] [CrossRef]
- Bais, D.S.; Che, W.; Jiang, G.; Xu, Z.; Xiao, L. Jellyfish Envenomation with Skin and Cardiovascular Manifestations and Treatments. Toxicol. Open Access 2017, 3, 132. [Google Scholar] [CrossRef]
- Hossain, M.K.; Dayem, A.A.; Han, J.; Yin, Y.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. Molecular Mechanisms of the Anti-Obesity and Anti-Diabetic Properties of Flavonoids. Int. J. Mol. Sci. 2016, 17, 569. [Google Scholar] [CrossRef]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomas-Barberan, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Vale, L.H.F.; Mendes, M.M.; Fernandes, R.S.; Costa, T.R.; Hage-Melim, L.I.S.; Sousa, M.A.; Hamaguchi, A.; Homsi-Brandeburgo, M.I.; Franca, S.C.; Silva, C.H.; et al. Protective Effect of Schizolobium parahyba Flavonoids Against Snake Venoms and Isolated Toxins. Curr. Top. Med. Chem. 2011, 11, 2566–2577. [Google Scholar] [CrossRef]
- Gómez-Betancur, I.; Benjumea, D.; Patiño, A.; Jiménez, N.; Osorio, E. Inhibition of the toxic effects of Bothrops asper venom by pinostrobin, a flavanone isolated from Renealmia alpinia (Rottb.) MAAS. J. Ethnopharmacol. 2014, 155, 1609–1615. [Google Scholar] [CrossRef]
- Gopi, K.; Anbarasu, K.; Renu, K.; Jayanthi, S.; Vishwanath, B.; Jayaraman, G. Quercetin-3-O-rhamnoside from Euphorbia hirta protects against snake Venom induced toxicity. Biochim. et Biophys. Acta (BBA)-Gen. Subj. 2016, 1860, 1528–1540. [Google Scholar] [CrossRef]
- Esmeraldino, L.; Souza, A.; Sampaio, S. Evaluation of the effect of aqueous extract of Croton urucurana Baillon (Euphorbiaceae) on the hemorrhagic activity induced by the venom of Bothrops jararaca, using new techniques to quantify hemorrhagic activity in rat skin. Phytomedicine 2005, 12, 570–576. [Google Scholar] [CrossRef]
- Hwang, D.H.; Lee, H.; Choudhary, I.; Kang, C.; Chae, J.; Kim, E. Protective effect of epigallocatechin-3-gallate (EGCG) on toxic metalloproteinases-mediated skin damage induced by Scyphozoan jellyfish envenomation. Sci. Rep. 2020, 10, 18644. [Google Scholar] [CrossRef]
- Andersen, O.; Markham, K. Flavonoids: Chemistry, Biochemistry and Applications; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Jakubec, D.; Skoda, P.; Krivak, R.; Novotny, M.; Hoksza, D. PrankWeb 3: Accelerated ligand-binding site predictions for experimental and modelled protein structures. Nucleic Acids Res. 2022, 50, W593–W597. [Google Scholar] [CrossRef]
- Krivák, R.; Hoksza, D. Improving protein-ligand binding site prediction accuracy by classification of inner pocket points using local features. J. Cheminform. 2015, 7, 12. [Google Scholar] [CrossRef]
- Abbaz, T.; Bendjeddou, A.; Villemin, D. Molecular Orbital Studies (Hardness, Chemical Potential, Electro Negativity and Electrophilicity) of TTFs Conjugated between 1, 3-Dithiole. Int. J. Adv. Res. Sci. Eng. Technol. 2018, 5, 5150–5161. [Google Scholar]
- Raftani, M.; Abram, T.; Azaid, A.; Kacimi, R.; Bennani, M.; Bouachrine, M. Theoretical design of new organic compounds based on diketopyrrolopyrrole and phenyl for organic bulk heterojunction solar cell applications: DFT and TD-DFT study. Mater. Today Proc. 2021, 45, 7334–7343. [Google Scholar] [CrossRef]
- Nomura, J.T.; Sato, R.L.; Ahern, R.M.; Snow, J.L.; Kuwaye, T.T.; Yamamoto, L.G. A randomized paired comparison trial of cutaneous treatments for acute jellyfish (Carybdea alata) stings. Am. J. Emerg. Med. 2002, 20, 624–626. [Google Scholar] [CrossRef] [PubMed]
- Lippmann, J.M.; Fenner, P.J.; Winkel, K.; Gershwin, L. Fatal and Severe Box Jellyfish Stings, Including Irukandji Stings, in Malaysia, 2000–2010. J. Travel Med. 2011, 18, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M.; Koh, C.Y. Metalloproteases Affecting Blood Coagulation, Fibrinolysis and Platelet Aggregation from Snake Venoms: Definition and Nomenclature of Interaction Sites. Toxins 2016, 8, 284. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Christen, P.; Cuendet, M. Plants as a Source of Therapeutic and Health Products. Chimia 2012, 66, 320–323. [Google Scholar] [CrossRef]
- Da Silva, M.L.; Marcussi, S.; Fernandes, R.S.; Pereira, P.S.; Januário, A.H.; França, S.C.; Da Silva, S.L.; Soares, A.M.; Lourenço, M.V. Anti-snake venom activities of extracts and fractions from callus cultures of Sapindus saponaria. Pharm. Biol. 2012, 50, 366–375. [Google Scholar] [CrossRef]
- Ribeiro, D.; Freitas, M.; Tomé, S.M.; Silva, A.M.S.; Laufer, S.; Lima, J.L.F.C.; Fernandes, E. Flavonoids Inhibit COX-1 and COX-2 Enzymes and Cytokine/Chemokine Production in Human Whole Blood. Inflammation 2015, 38, 858–870. [Google Scholar] [CrossRef]
- Sartor, L.; Pezzato, E.; Dell’aica, I.; Caniato, R.; Biggin, S.; Garbisa, S. Inhibition of matrix-proteases by polyphenols: Chemical insights for anti-inflammatory and anti-invasion drug design. Biochem. Pharmacol. 2002, 64, 229–237. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, G.; Liao, Y.; Pan, J.; Gong, D. Dietary Flavonoids as Xanthine Oxidase Inhibitors: Structure–Affinity and Structure–Activity Relationships. J. Agric. Food Chem. 2015, 63, 7784–7794. [Google Scholar] [CrossRef]
- Zhou, Z.-G.; Yao, Q.-Z.; Lei, D.; Zhang, Q.-Q.; Zhang, J. Investigations on the mechanisms of interactions between matrix metalloproteinase 9 and its flavonoid inhibitors using a combination of molecular docking, hybrid quantum mechanical/molecular mechanical calculations, and molecular dynamics simulations. Can. J. Chem. 2014, 92, 821–830. [Google Scholar] [CrossRef]
- Hou, D.-X.; Kumamoto, T. Flavonoids as Protein Kinase Inhibitors for Cancer Chemoprevention: Direct Binding and Molecular Modeling. Antioxidants Redox Signal. 2010, 13, 691–719. [Google Scholar] [CrossRef]
- Köksal, E.; Gülçin, I.; Beyza, S.; Sarikaya, Ö.; Bursal, E. In vitro antioxidant activity of silymarin. J. Enzym. Inhib. Med. Chem. 2009, 24, 395–405. [Google Scholar] [CrossRef]
- Han, H.; Yılmaz, H.; Gülçin, I. Antioxidant Activity of Flaxseed (Linum usitatissimum L.) shell and Analysis of Its Polyphenol Contents by LC-MS/MS. Rec. Nat. Prod. 2018, 12, 397–402. [Google Scholar] [CrossRef]
- Ghosh, N.; Chakraborty, T.; Mallick, S.; Mana, S.; Singha, D.; Ghosh, B.; Roy, S. Synthesis, characterization and study of antioxidant activity of quercetin–magnesium complex. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 151, 807–813. [Google Scholar] [CrossRef]
- Leinonen, R.; Sugawara, H.; Shumway, M.; on behalf of the International Nucleotide Sequence Database Collaboration. The Sequence Read Archive. Nucleic Acids Res. 2011, 39, D19–D21. [Google Scholar] [CrossRef]
- Babraham Bioinformatics—FastQC a Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 27 October 2022).
- Bushmanova, E.; Antipov, D.; Lapidus, A.; Prjibelski, A.D. rnaSPAdes: A de novo transcriptome assembler and its application to RNA-Seq data. Gigascience 2019, 8, giz100. [Google Scholar] [CrossRef]
- Seppey, M.; Manni, M.; Zdobnov, E.M. BUSCO: Assessing Genome Assembly and Annotation Completeness. Methods Mol. Biol. 2019, 1962, 227–245. [Google Scholar] [CrossRef]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Jungo, F.; Bougueleret, L.; Xenarios, I.; Poux, S. The UniProtKB/Swiss-Prot Tox-Prot program: A central hub of integrated venom protein data. Toxicon 2012, 60, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- AlphaFold2.Ipynb—Colaboratory. Available online: https://colab.research.google.com/github/sokrypton/ColabFold/blob/main/AlphaFold2.ipynb (accessed on 14 March 2023).
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 7 January 2021).
- PrankWeb. Available online: https://prankweb.cz/ (accessed on 1 February 2023).
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Vieira, T.F.; Sousa, S.F. Comparing AutoDock and Vina in Ligand/Decoy Discrimination for Virtual Screening. Appl. Sci. 2019, 9, 4538. [Google Scholar] [CrossRef]
- Dallakyan, S.; Olson, A.J. Small-molecule library screening by docking with pyrx. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar]
- Welcome to the PyRx Website. Available online: https://pyrx.sourceforge.io/ (accessed on 1 February 2023).
- Kulkarni, S.A.; Nagarajan, S.K.; Ramesh, V.; Palaniyandi, V.; Selvam, S.P.; Madhavan, T. Computational evaluation of major components from plant essential oils as potent inhibitors of SARS-CoV-2 spike protein. J. Mol. Struct. 2020, 1221, 128823. [Google Scholar] [CrossRef]
- Leach, A.R.; Shoichet, B.K.; Peishoff, C.E. Prediction of Protein−Ligand Interactions. Docking and Scoring: Successes and Gaps. J. Med. Chem. 2006, 49, 5851–5855. [Google Scholar] [CrossRef]
- Deghady, A.M.; Hussein, R.K.; Alhamzani, A.G.; Mera, A. Density Functional Theory and Molecular Docking Investigations of the Chemical and Antibacterial Activities for 1-(4-Hydroxyphenyl)-3-phenylprop-2-en-1-one. Molecules 2021, 26, 3631. [Google Scholar] [CrossRef]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P.W.; Tang, Y. admetSAR: A Comprehensive Source and Free Tool for Assessment of Chemical ADMET Properties. J. Chem. Inf. Model. 2012, 52, 3099–3105. [Google Scholar] [CrossRef]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef]
- Protein_ligand.Ipynb—Colaboratory. Available online: https://colab.research.google.com/github/pablo-arantes/making-it-rain/blob/main/Protein_ligand.ipynb (accessed on 1 February 2023).
- Arantes, P.R.; Polêto, M.D.; Pedebos, C.; Ligabue-Braun, R. Making it Rain: Cloud-Based Molecular Simulations for Everyone. J. Chem. Inf. Model. 2021, 61, 4852–4856. [Google Scholar] [CrossRef]
- Eastman, P.; Swails, J.; Chodera, J.D.; McGibbon, R.T.; Zhao, Y.; Beauchamp, K.A.; Wang, L.-P.; Simmonett, A.C.; Harrigan, M.P.; Stern, C.D.; et al. OpenMM 7: Rapid development of high performance algorithms for molecular dynamics. PLoS Comput. Biol. 2017, 13, e1005659. [Google Scholar] [CrossRef]
- Izadi, S.; Anandakrishnan, R.; Onufriev, A.V. Building Water Models: A Different Approach. J. Phys. Chem. Lett. 2014, 5, 3863–3871. [Google Scholar] [CrossRef]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef]
- Case, D.A.; Ben-Shalom, I.Y.; Brozell, S.R.; Cerutti, D.S.; Cheatham, T.E., III; Cruzeiro, V.W.D.; Darden, T.A.; Duke, R.E.; Ghoreishi, D.; Giambasu, G.; et al. Amber 2019; University of California: San Francisco, CA, USA, 2019. [Google Scholar]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Chow, K.-H.; Ferguson, D.M. Isothermal-isobaric molecular dynamics simulations with Monte Carlo volume sampling. Comput. Phys. Commun. 1995, 91, 283–289. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Jakalian, A.; Jack, D.B.; Bayly, C.I. Fast, efficient generation of high-quality atomic charges. AM1-BCC model: II. Parameterization and validation. J. Comput. Chem. 2002, 23, 1623–1641. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph. Model. 2006, 25, 247–260. [Google Scholar] [CrossRef] [PubMed]
- McGibbon, R.T.; Beauchamp, K.A.; Harrigan, M.P.; Klein, C.; Swails, J.M.; Hernández, C.X.; Schwantes, C.R.; Wang, L.-P.; Lane, T.J.; Pande, V.S. MDTraj: A Modern Open Library for the Analysis of Molecular Dynamics Trajectories. Biophys. J. 2015, 109, 1528–1532. [Google Scholar] [CrossRef] [PubMed]
- Roe, D.R.; Cheatham, T.E. PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0 Contributors. SciPy 1.0 Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef]
- Silva, J.; Vargas, J.; Cabrera, D.; García, P.; Pineda, O.B.; Gonzalez, F.M.; Mercado, C.V. Algorithm for Detecting Opinion Polarity in Laptop and Restaurant Domains. Procedia Comput. Sci. 2020, 170, 977–982. [Google Scholar] [CrossRef]
- Miller, B.R.; McGee, T.D.; Swails, J.M.; Homeyer, N.; Gohlke, H.; Roitberg, A.E. MMPBSA.py: An Efficient Program for End-State Free Energy Calculations. J. Chem. Theory Comput. 2012, 8, 3314–3321. [Google Scholar] [CrossRef]
- Hou, T.; Wang, J.; Li, Y.; Wang, W. Assessing the Performance of the MM/PBSA and MM/GBSA Methods. 1. The Accuracy of Binding Free Energy Calculations Based on Molecular Dynamics Simulations. J. Chem. Inf. Model. 2011, 51, 69–82. [Google Scholar] [CrossRef]
- Kollman, P.A.; Massova, I.; Reyes, C.; Kuhn, B.; Huo, S.; Chong, L.; Lee, M.; Lee, T.; Duan, Y.; Wang, W.; et al. Calculating Structures and Free Energies of Complex Molecules: Combining Molecular Mechanics and Continuum Models. Accounts Chem. Res. 2000, 33, 889–897. [Google Scholar] [CrossRef]




| Parts | Reads | Transcripts | Genes | N50 | BUSCO% | Diamond Tool |
|---|---|---|---|---|---|---|
| Tentacle | 19,627,288 | 62,127 | 52,072 | 2538 | 92.9 | 11,293 |
| Tox-Prot_Top_Hit | Molecular Function | Class | Length |
|---|---|---|---|
| *Snake venom metalloproteinase adamalysin-2 | Metalloendopeptidase activity | P-I | 561 |
| *Snake venom metalloproteinase HT-2 | Hemostatic and hemorrhagic | P-I | 852 |
| Snake venom metalloproteinase | Hemostasis imparing toxin | P-I | 863 |
| Snake venom metalloproteinase acutolysin-A | Hemostatic and hemorrhagic | P-I | 1569 |
| Snake venom metalloproteinase acutolysin-C | Hemostatic and hemorrhagic | P-I | 1543 |
| Snake venom metalloproteinase atrolysin-B | Hemostatic and hemorrhagic | P-I | 1482 |
| Snake venom metalloproteinase BITM02A | Hemostatic and hemorrhagic | P-I | 1145 |
| *Zinc metalloproteinase-disintegrin bilitoxin-1 | Fibrinogenolytic toxin | P-II | 240 |
| Zinc metalloproteinase-disintegrin BlatH1 | Hemostatic and hemorrhagic | P-II | 520 |
| Zinc metalloproteinase-disintegrin BA-5A | Hemostatic and hemorrhagic | P-II | 756 |
| Zinc metalloproteinase/disintegrin | Hemostatic and hemorrhagic | P-II | 627 |
| Zinc metalloproteinase/disintegrin | Hemostatic and hemorrhagic | P-II | 1869 |
| *Zinc metalloproteinase-disintegrin-like 2a | Hemostasis imparing toxin | P-III | 439 |
| Zinc metalloproteinase-disintegrin-like 8 | Hemostasis imparing toxin | P-III | 1130 |
| Zinc metalloproteinase-disintegrin-like ACLD | Toxin activity | P-IIIa | 192 |
| Zinc metalloproteinase-disintegrin-like BfMP | Toxin activity | P-IIIa | 1131 |
| Zinc metalloproteinase-disintegrin-like BjussuMP-1 | Fibrinogenolytic toxin | P-IIIa | 1209 |
| Zinc metalloproteinase-disintegrin-like Eoc1 | Hemostatic and hemorrhagic | P-IIIc | 240 |
| Zinc metalloproteinase-disintegrin-like daborhagin-K | Protease | - | 96 |
| Astacin-like metalloprotease toxin 1 | Auxillary | - | 744 |
| Astacin-like metalloprotease toxin 2 | Auxillary | - | 548 |
| Astacin-like metalloprotease toxin 3 | Auxillary | - | 459 |
| Astacin-like metalloprotease toxin 4 | Auxillary | - | 317 |
| Modeled Metalloproteinase | Compound Name | Autodock Score (kcal/mol) | Hydrogen Bond Interactions | Hydrophobic Interactions |
|---|---|---|---|---|
| Type 1 NnV-Mlp | Silymarin | −9.5 | 36-GLN, 96-ARG, 97-GLN 296-HIS, | 39-LEU, 40-GLU, 64-LEU, 88-LYS, 95-LYS, 226-GLN, 228-ARG, 230-MET, 231-GLY, 267-TYR, 270-LEU, 278-HIS, 300-HIS, 305-GLU, 306-HIS, 309-LEU, 310-TYR |
| Pinobanksin | −8 | 296-HIS | 264-HIS, 265-THR, 266-GLY,267-TYR,268-ALA,269-PRO,292-HIS,293-VAL,297-GLU,300-HIS,306-HIS | |
| Tricetin | −8 | 39-LEU, 96-ARG, 267-TYR | 36-GLN,37-SER,38-GLN,40-GLU, 97-GLN, 98-LEU,99-THR, 227-ARG,230-MET, 231-GLY,234-CYS,278-HIS | |
| Type 2 NnV-Mlp | Silymarin | −9.6 | 461-THR, 465-SER | 431-ARG, 454-LYS, 456-SER, 457-GLY, 462-SER,463-MET, 466-SER, 493-PHE, 495-TYR, 608-LYS, 610-ASP, 612-THR |
| Eriodictyol | −8.9 | 381ARG, 423CYS, 425-ASP, 428MET, 430-PHE, 620-ASN | 386-GLY,422-PRO,424-PRO, ILE-427, ASN-429, GLN-433, ASP-491,502-LYS,612-THR,613-THR,614-GLY,616-ASP,617-ASP | |
| Luteolin | −8.9 | 383ARG, 386GLY, 430-PHE, 616-ASP, 620-ASN | 385-CYS,387-GLY,423-CYS,424-PRO,425-ASP,427-ILE,428-MET,429-ASN,433-GLN,491-ASP,502-LYS,612-THR,613-THR,614-GLY,617-HIS,621-THR | |
| Type 3 NnV-Mlp | Eriodictyol | −8.8 | 125-THR, | 124-SER, 126-VAL, GLY-127,128-LEU,129-ALA,155-SER,156-THR,159-HIS,160-GLU,163-HIS,169-HIS,187-CYS, ILE-188,190-THR,191-GLU,192-SER,193-ILE,198-PRO |
| Apigenin | −8.8 | 124-SER, 125-THR | 124-SER, 126-VAL, GLY-127,128-LEU,129-ALA,155-SER,156-THR,159-HIS,160-GLU,163-HIS,169-HIS,187-CYS, ILE-188,190-THR,191-GLU,192-SER,193-ILE,198-PRO | |
| Silymarin | −8.8 | 124-SER | 125-THR, 126-Val, 129-LEU, 131-VAL, 155-SER, 156-THR, 159-HIE, 163-HIE, 169-HIE,190-THR, 191-GLU,192-SER, 193-ILE,198-PRO | |
| Type 4 NnV-Mlp | Eriodictyol | −9.3 | 170-HIS, 200-ILE | 113-GLY, 114-VAL, 115-LEU, 117-LEU, 118-ALA, 163-VAL, 166- ILE, 167-THR, 170-HIS, 171-GLU, 174-HIS, 200-ILE, 201-MET, 202-TYR, 203-PRO, 204-ARG, 206-THR, 423-TYR, 433-VAL, 434-ASP, 437-GLY, 438-PRO |
| Silymarin | −9 | 115-LEU, 120-ILE | 114-Val, 117-LEU, 118-ALA, 163-VAL, 166-ILE, 167-THR, 170-HIE, 174-HIE, 180-HIE, 202-TRY, 203-PRO, 204-ARG, 206-THR, 436-GLU, 437-GLY, 438-PRO, 439-LEU. | |
| Quercetin | −8.8 | 167-THR, 203-PRO, 434-ASP, 435-ALA, 437-GLY | 115-LEU,116-GLY, 117-LEU,118-ALA, 163-VAL, 166-ILE, 170-HIS, 171-GLU,174-HIS,180-HIS, 200-ILE, 202-TYR, 204-ARG, 205-ALA, 206-THR, 423-TYR, 433-VAL, 438-PRO |
| Compound | Dipole (eV) | HOMO (eV) | LUMO (eV) | Energy Gap (eV) | Chemical Hardness (eV) | Chemical Potential (eV) | Electro-Negativity (eV) |
|---|---|---|---|---|---|---|---|
| Silymarin | 3.991373 | −0.05581 | −0.21953 | 0.16372 | 0.096935 | −0.098335 | −2061.506039 |
| Eriodictyol | 4.295892 | −0.05173 | −0.22653 | 0.1748 | 0.10579 | −0.10379 | −1031.355218 |
| Luteolin | 3.9532421 | −0.22785 | −0.07296 | 0.30081 | 0.08467 | −0.12126 | −1601.415599 |
| Pinobanksin | 3.003841 | −0.06836 | −0.23964 | 0.17128 | 0.09728 | −0.10047 | −2061.505616 |
| Tricetin | 2.455043 | −0.0744 | −0.22859 | 0.15419 | 0.12351 | −0.09515 | −1248.589238 |
| Apigenin | 3.8518676 | −0.21666 | −0.0638 | 0.28046 | 0.10482 | −0.09754 | −1106.568277 |
| Quercetin | 4.1933281 | −0.22615 | −0.07566 | 0.30181 | 0.07932 | −0.14842 | −1138.910421 |
| Properties | Silymarin | Eriodictyol | Luteolin | Pinobanksin | Tricetin | Apigenin | Quercetin |
|---|---|---|---|---|---|---|---|
| Absorption Properties | |||||||
| Caco-2 Permeability (Optimal: higher than −5.15 Log unit or −4.70 or −4.80) | −6.255 | −5.189 | −5.028 | −5.193 | −5.255 | −4.847 | −5.204 |
| Human Intestinal Absorption (HIA) ≥ 30%: HIA+; (HIA) <30%: HIA− | - | --- | --- | --- | -- | --- | --- |
| P-glycoprotein Substrate | --- | --- | -- | --- | -- | ++ | --- |
| P-glycoprotein Inhibitor | - | --- | --- | --- | --- | --- | --- |
| Distribution Properties | |||||||
| PPB (Plasma Protein Binding): optimal- < 90% | 96.66% | 93.32% | 95.44% | 82.08% | 92.23% | 97.26% | 95.50% |
| VD (Volume Distribution) 0.04–20 L/kg | 0.649 | 0.561 | 0.533 | 1.686 | 0.603 | 0.51 | 0.579 |
| Blood–Brain Barrier (BBB) BB ratio ≥ 0.1: BBB+; BB ratio < 0.1:BBB− | --- | --- | --- | --- | --- | --- | --- |
| Metabolism | |||||||
| CYP1A2 inhibitor | -- | ++ | +++ | -- | +++ | +++ | +++ |
| CYP1A2 substrate | -- | -- | -- | -- | -- | -- | -- |
| CYP2C19 inhibitor | -- | - | -- | --- | --- | + | --- |
| CYP2C19 substrate | --- | --- | --- | - | --- | --- | --- |
| CYP2C9 inhibitor | + | ++ | + | - | + | + | + |
| CYP2C9 substrate | ++ | ++ | ++ | +++ | + | +++ | + |
| CYP2D6 inhibitor | - | + | + | - | -- | ++ | - |
| CYP2D6 substrate | - | - | + | - | -- | ++ | -- |
| CYP3A4 inhibitor | ++ | + | + | --- | -- | ++ | - |
| CYP3A4 substrate | - | -- | --- | - | --- | -- | --- |
| Excretion | |||||||
| T 1/2 (Half Lifetime) (long half-life 3 h, short half-life <3 h) | 0.274 | 0.856 | 0.898 | 0.68 | 0.922 | 0.856 | 0.929 |
| Toxicity Properties | |||||||
| hERG (hERG Blockers) | --- | --- | --- | --- | -- | --- | --- |
| AMES (Ames Mutagenicity) | - | + | + | -- | - | - | + |
| DILI (Drug Induced Liver Injury) | +++ | +++ | +++ | +++ | +++ | ++ | +++ |
| Physicochemical properties | |||||||
| LogS (Solubility) | −4.792 | −3.827 | −3.629 | −2.709 | −3.59 | −3.606 | −3.671 |
| LogD (Distribution Coefficient D) | 2.524 | 2.307 | 2.361 | 0.688 | 1.501 | 2.704 | 1.767 |
| LogP (Distribution Coefficient P) | 2.015 | 2.118 | 2.902 | 1.535 | 2.52 | 3.307 | 2.155 |
| Energy Component (kcal/mol) | NnV-Mlp Type 1 | NnV-Mlp Type 2 | NnV-Mlp Type 3 | NnV-Mlp Type 4 |
|---|---|---|---|---|
| ΔE (VDWAALS) | −43.7761 ± 1.3006 | −48.3463 ± 1.6737 | −31.9381 ± 2.4216 | −45.4069 ± 1.7482 |
| ΔE (EEL) | −29.3170 ± 2.6631 | −18.8026 ± 1.9482 | −23.3120 ± 3.3445 | −16.3162 ± 2.1864 |
| ΔE (EGB) | 51.3345 ± 2.4503 | 44.1952 ± 1.5961 | 40.2324 ± 3.6828 | 37.6677 ± 2.1287 |
| ΔE (ESURF) | −6.7133 ± 0.4014 | −7.1048 ± 0.1227 | −4.7373 ± 0.2719 | −5.9816 ± 0.2048 |
| ΔG (Gas) | −73.0931 ± 2.4925 | −67.1489 ± 2.6451 | −55.2500 ± 5.1796 | −61.7231 ± 2.8195 |
| ΔG (Solvation) | 83.7113 ± 2.5896 | 37.0903 ± 1.5079 | 35.4952 ± 3.4593 | 31.6860 ± 2.0141 |
| ΔG (Interaction) | −28.4719 ± 1.7784 | −30.0585 ± 1.3585 | −19.7549 ± 1.8149 | −30.0370 ± 1.2377 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asirvatham, R.D.; Hwang, D.H.; Prakash, R.L.M.; Kang, C.; Kim, E. Pharmacoinformatic Investigation of Silymarin as a Potential Inhibitor against Nemopilema nomurai Jellyfish Metalloproteinase Toxin-like Protein. Int. J. Mol. Sci. 2023, 24, 8972. https://doi.org/10.3390/ijms24108972
Asirvatham RD, Hwang DH, Prakash RLM, Kang C, Kim E. Pharmacoinformatic Investigation of Silymarin as a Potential Inhibitor against Nemopilema nomurai Jellyfish Metalloproteinase Toxin-like Protein. International Journal of Molecular Sciences. 2023; 24(10):8972. https://doi.org/10.3390/ijms24108972
Chicago/Turabian StyleAsirvatham, Ravi Deva, Du Hyeon Hwang, Ramachandran Loganathan Mohan Prakash, Changkeun Kang, and Euikyung Kim. 2023. "Pharmacoinformatic Investigation of Silymarin as a Potential Inhibitor against Nemopilema nomurai Jellyfish Metalloproteinase Toxin-like Protein" International Journal of Molecular Sciences 24, no. 10: 8972. https://doi.org/10.3390/ijms24108972
APA StyleAsirvatham, R. D., Hwang, D. H., Prakash, R. L. M., Kang, C., & Kim, E. (2023). Pharmacoinformatic Investigation of Silymarin as a Potential Inhibitor against Nemopilema nomurai Jellyfish Metalloproteinase Toxin-like Protein. International Journal of Molecular Sciences, 24(10), 8972. https://doi.org/10.3390/ijms24108972






