Extraction Optimization, Characterization and Biological Activities of Polysaccharide Extracts from Nymphaea hybrid
Abstract
1. Introduction
2. Results and Discussion
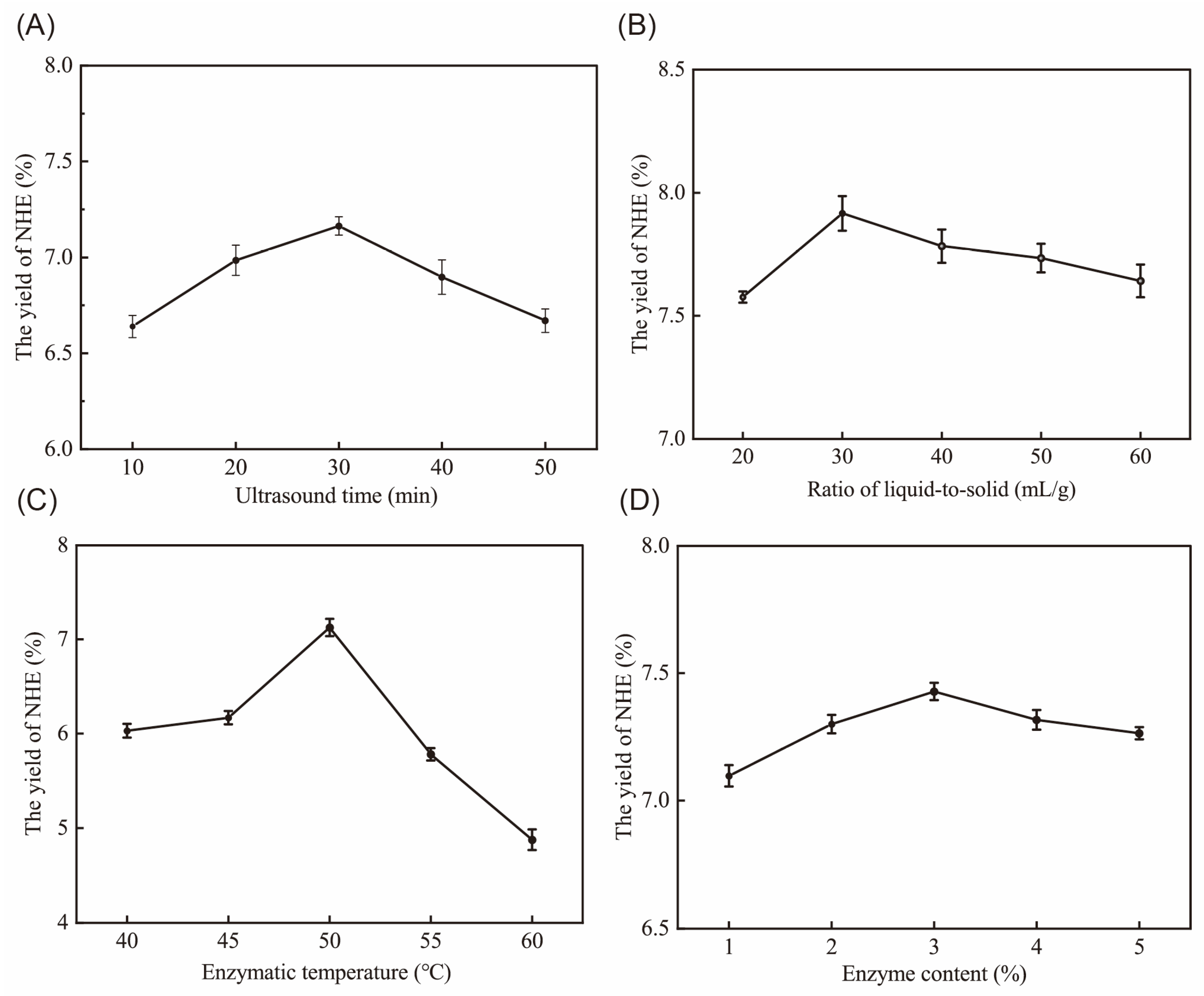
2.1. Single Factors Experiment Analysis
2.2. Optimization of Extraction Parameters
2.2.1. Optimization of NHE Yield by RSM
2.2.2. Analysis of Response Surfaces
2.2.3. Verification
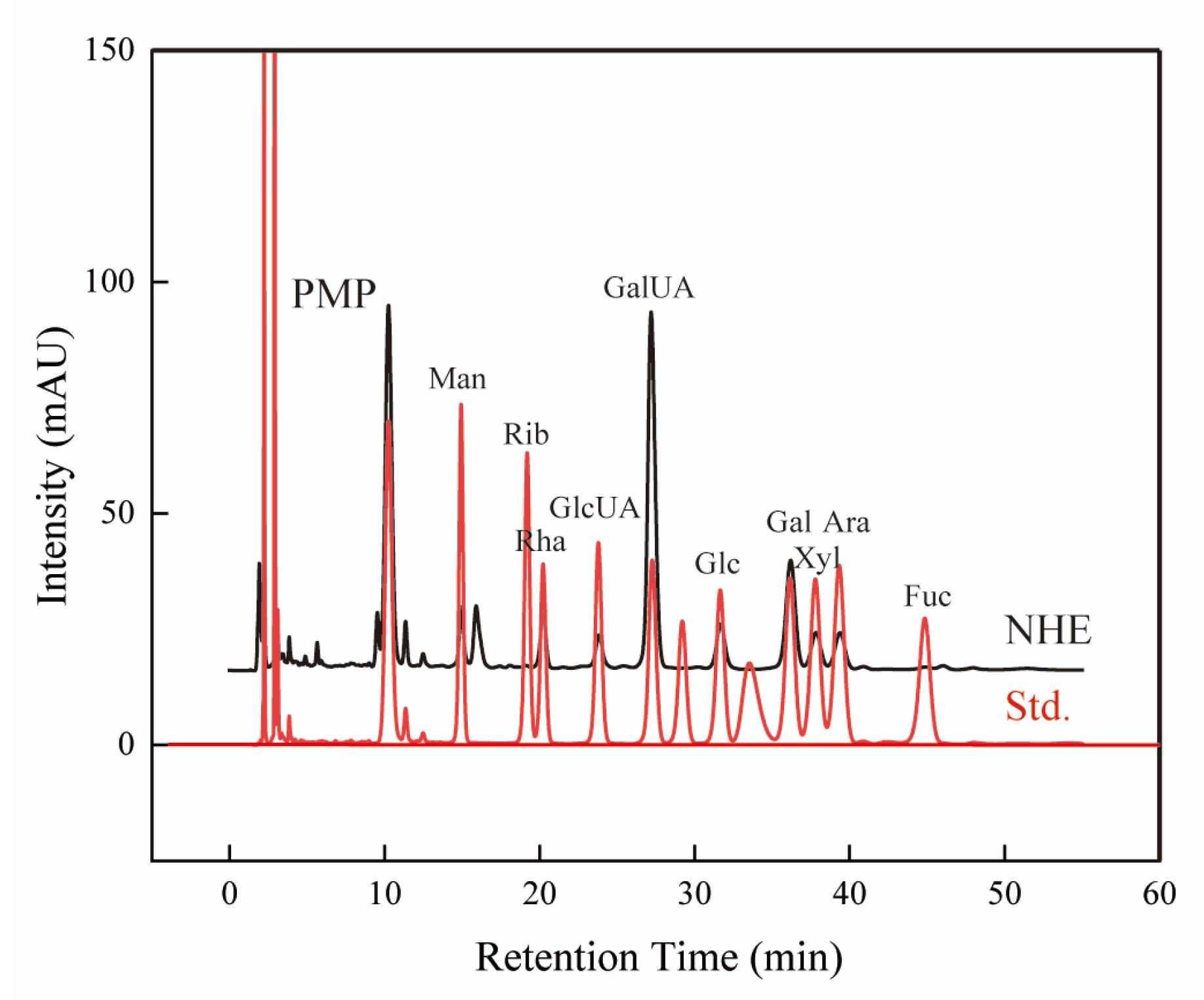
2.3. Component Analyses
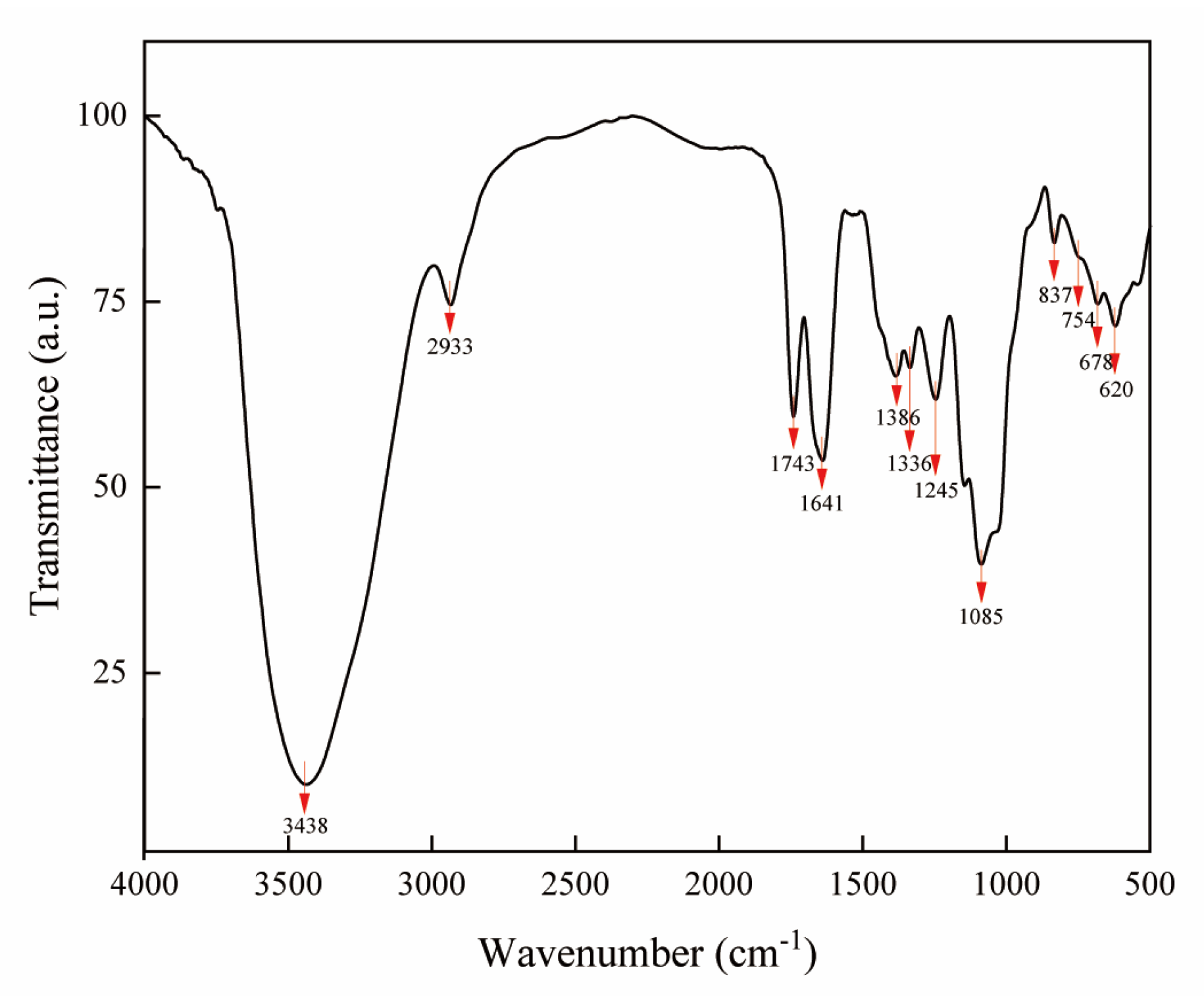
2.4. Fourier Transform Infrared Spectroscopy Analysis
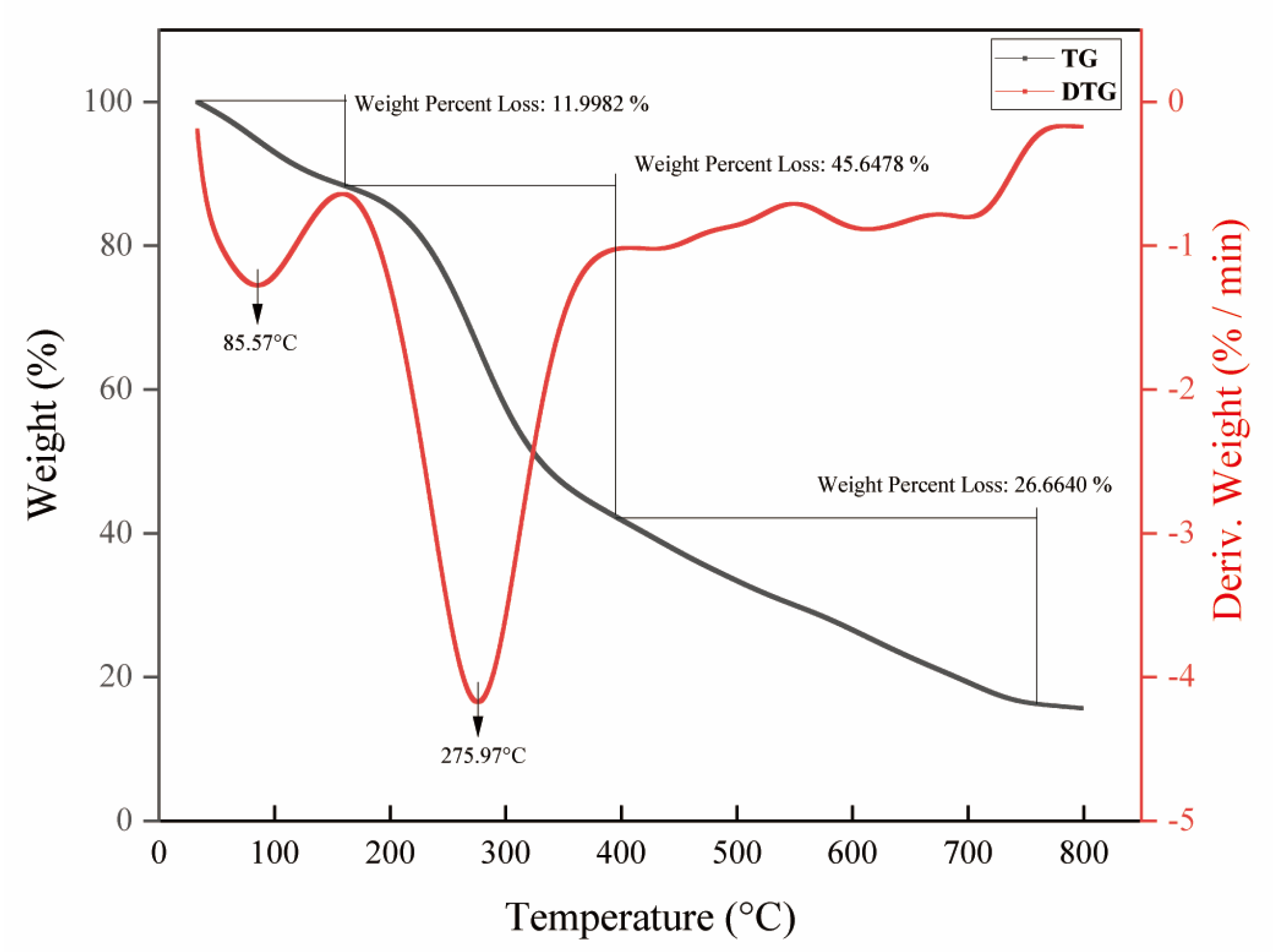
2.5. Thermal Property Analysis
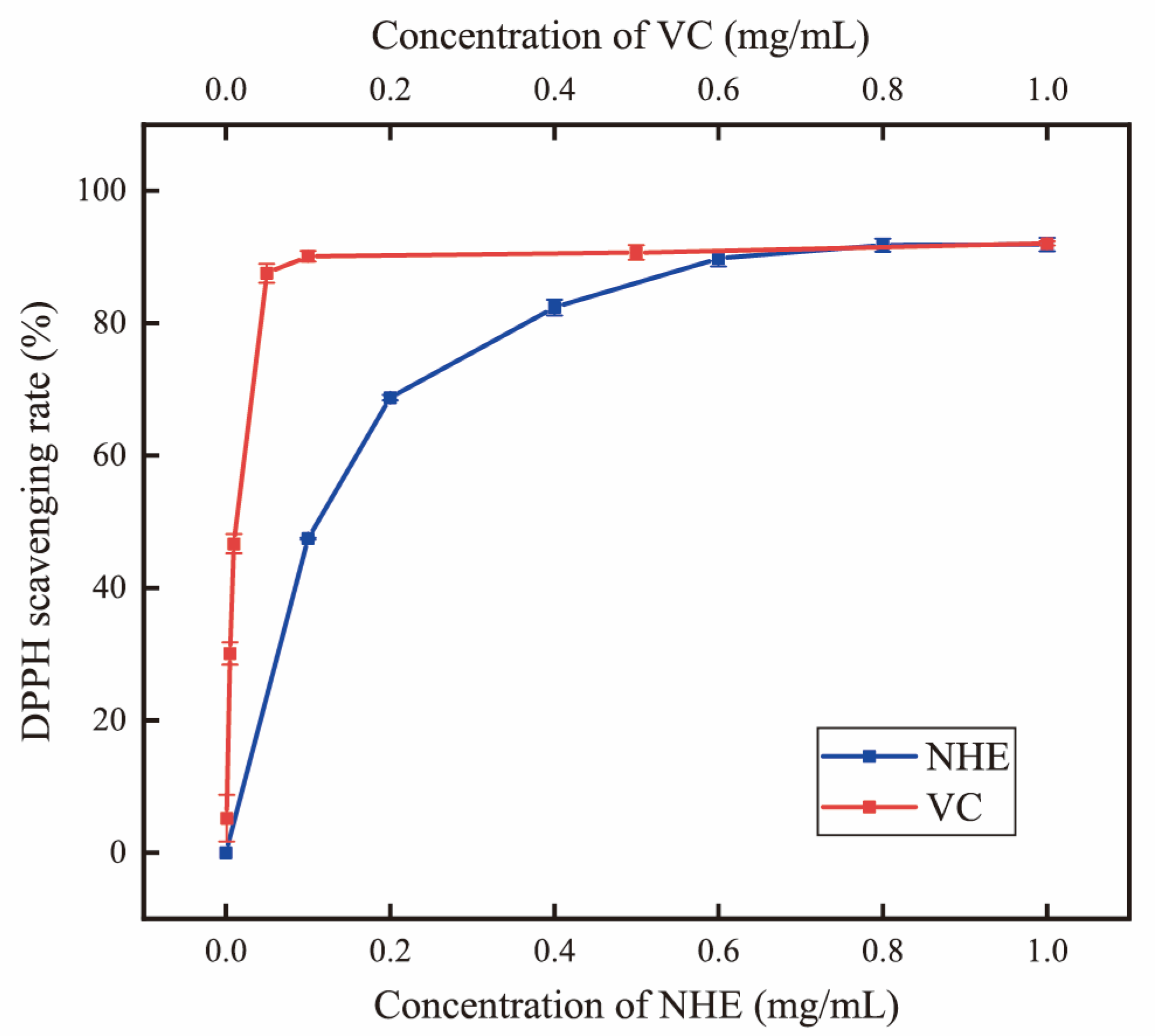
2.6. DPPH Radical Scavenging Activity
2.7. Hyaluronidase Activity Inhibition
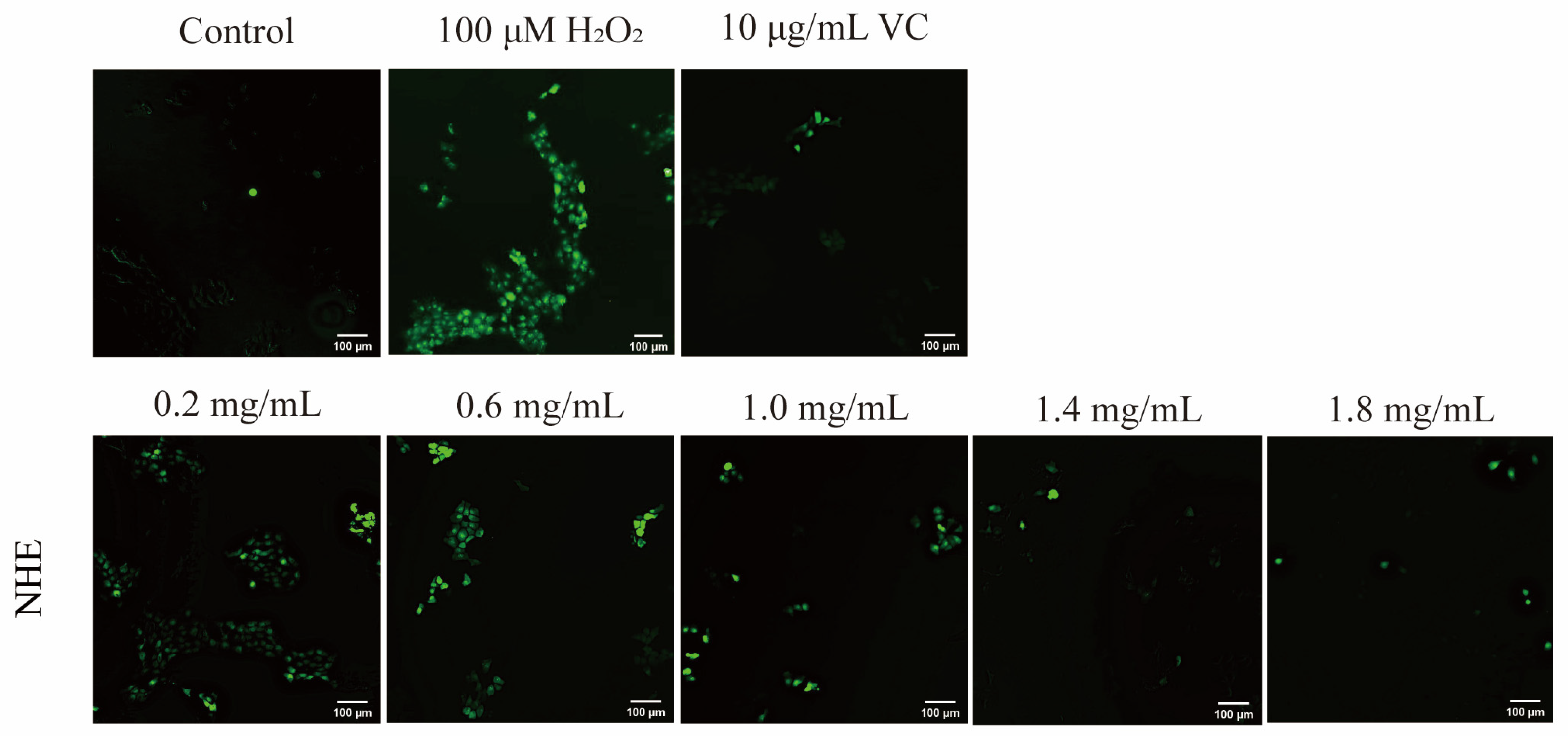
2.8. Effect of NHE on ROS Level of HaCaT Cells
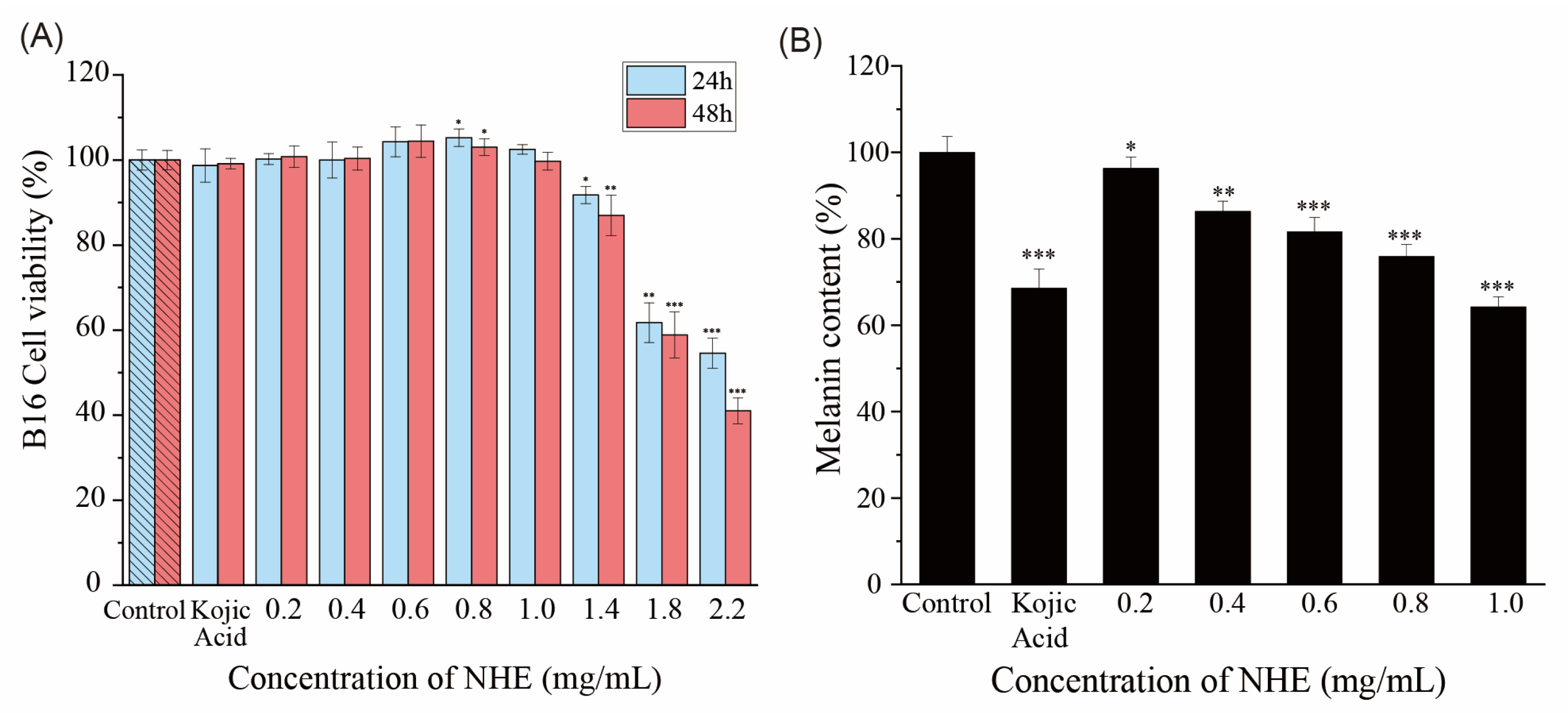
2.9. Effect of NHE on Melanin Production of B16 Cells
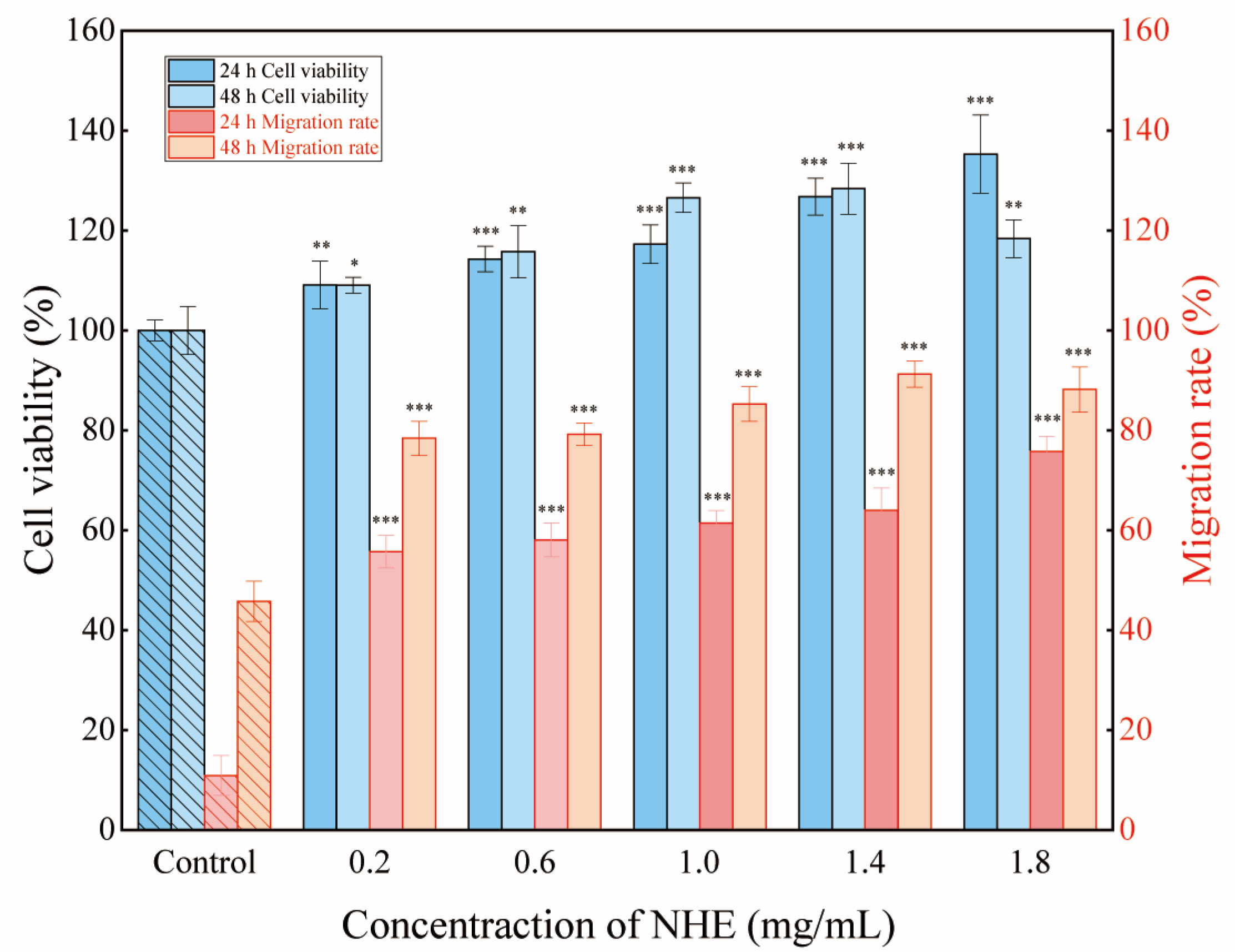
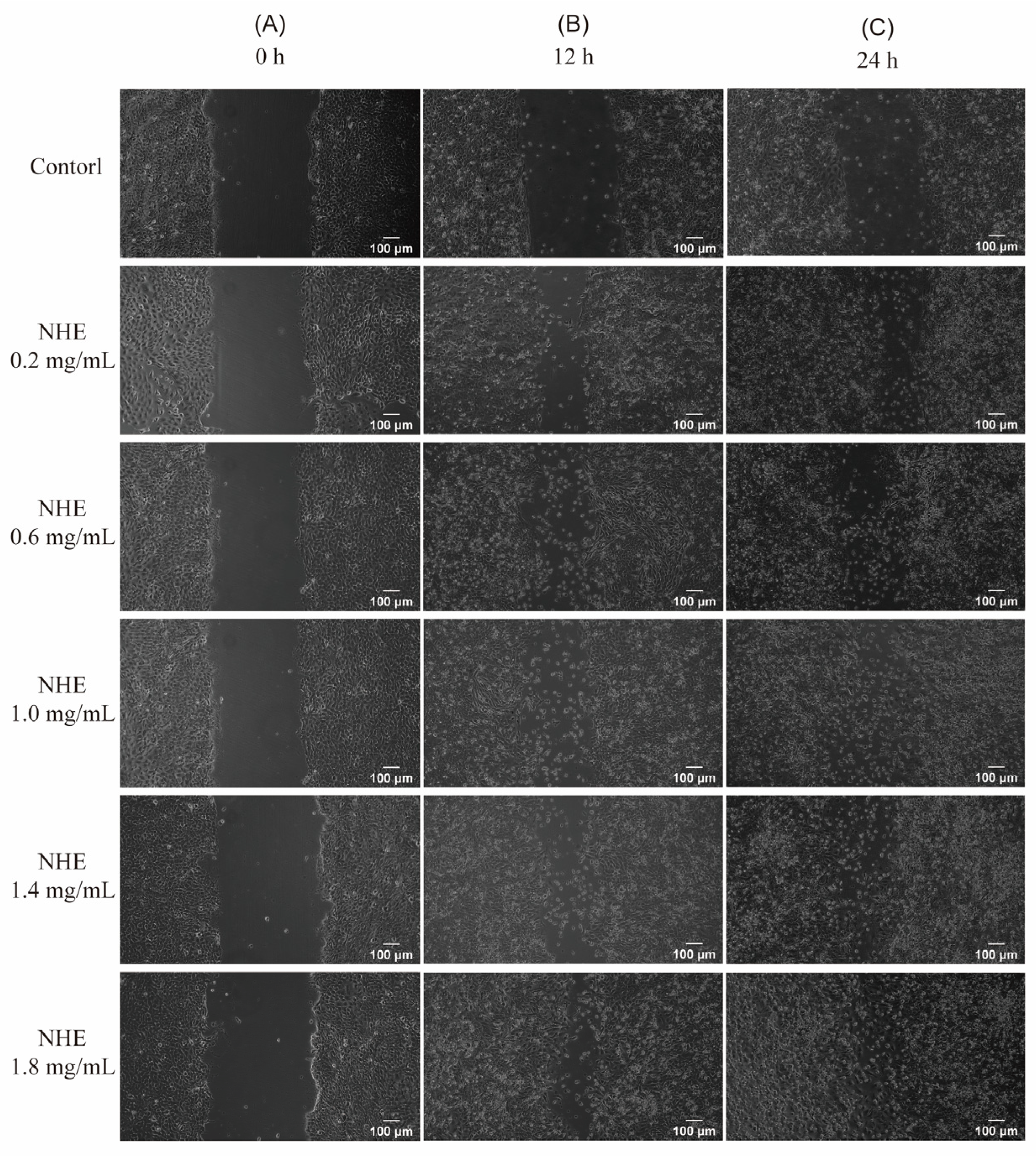
2.10. Re-Epithelialization of NHE by Scratch Assay
3. Materials and Methods
3.1. Materials and Reagent
3.2. Preparation of NHE and Single Factors Experiment
3.3. Response Surface Experiment
3.4. Determination of Component
3.5. Characterization of NHE by HPLC and FT–IR
3.6. Thermal (TG–DTG) Analysis of NHE
3.7. Antioxidant and Anti-Inflammatory Activity of NHE
3.8. Cells Culture and Cytotoxicity Assay of NHE
3.9. Measurement of ROS Production
3.10. Measurement of Melanin Content
3.11. Wound Healing Scratch Migration Assay
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sulaiman, E.S. Development of Sterilisation Procedures and In Vitro Studies of Nymphaea Lotus. Ph.D. Thesis, School of Graduate Studies, University Putra Malaysia, Selangor, Malaysia, 2004. [Google Scholar]
- Zhang, H.; Zhou, Q.; Wu, H.; Sheng, Q.; Zhu, Z. Morphology and Viability of Pollen from Three Hardy Water Lilies and Their Cross-Compatibility with Nymphaea hybrid. Diversity 2022, 14, 92. [Google Scholar] [CrossRef]
- Zhou, Q.; Shi, M.; Zhang, H.; Zhu, Z. Comparative Study of the Petal Structure and Fragrance Components of the Nymphaea hybrid, a Precious Water Lily. Molecules 2022, 27, 408. [Google Scholar] [CrossRef]
- Hang, S.; Sun, X.; He, Y.; Yuan, J. Microcapsules Preparation of Alcohol Extracts from Nymphaea Hybrid and Determination of Its Antioxidant Capability in vitro and in vivo. Food Sci. Technol. 2022, 42, e91021. [Google Scholar] [CrossRef]
- Liu, H.M.; Lei, S.N.; Tang, W.; Xun, M.H.; Zhao, Z.W.; Cheng, M.Y.; Zhang, X.D.; Wang, W. Optimization of Ultrasound-Assisted Cellulase Extraction from Nymphaea hybrid Flower and Biological Activities: Antioxidant Activity, Protective Effect against ROS Oxidative Damage in HaCaT Cells and Inhibition of Melanin Production in B16 Cells. Molecules 2022, 27, 1914. [Google Scholar] [CrossRef]
- Zhuang, Z.; Lv, T.; Li, M.; Zhang, Y.; Xue, T.; Yang, L.; Liu, H.; Zhang, W. The lifespan-extending effects of Nymphaea hybrid root extract in the nematode Caenorhabditis elegans. Plant Foods Hum. Nutr. 2014, 69, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jiang, S.; Tian, H.; Zeng, Y.; He, K.; Lin, L.; Yu, F. Ethyl acetate fraction from Nymphaea hybrida Peck modulates inflammatory responses in LPS-stimulated RAW 264.7 cells and acute inflammation murine models. J. Ethnopharmacol. 2021, 269, 113698. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xin, Y.; Xu, F.; Zheng, M.; Xi, X.; Cui, X.; Cao, H.; Guo, H.; Han, C. Maca polysaccharides: Extraction optimization, structural features and anti-fatigue activities. Int. J. Biol. Macromol. 2018, 115, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.J.; Li, J.; Chen, Y.L.; Zhang, L.J. The structures and biological functions of polysaccharides from traditional Chinese herbs. Glycans Glycosaminoglycans Clin. Biomark. Ther. 2019, 163 Pt B, 423–444. [Google Scholar]
- Dujnič, V.; Matulová, M.; Chyba, A.; Pätoprstý, V. Polysaccharides in Siraitia grosvenori flowers and herbal tea. Chem. Papers 2020, 75, 1175–1185. [Google Scholar] [CrossRef]
- Fan, Y.; Zhou, X.; Huang, G. Preparation, structure, and properties of tea polysaccharide. Chem. Biol. Drug Des. 2022, 99, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, X.Y.; Pan, L.; Su, Y.; Wang, Y. Comparing three Methods of Extraction of Auricularia Auricula Polysaccharides. Curr. Top. Nutraceutical Res. 2019, 17, 7–10. [Google Scholar]
- Qin, D.; Xi, J. Flash extraction: An ultra-rapid technique for acquiring bioactive compounds from plant materials. Trends Food Sci. Technol. 2021, 112, 581–591. [Google Scholar] [CrossRef]
- de Andrade Lima, M.; Kestekoglou, I.; Charalampopoulos, D.; Chatzifragkou, A. Supercritical Fluid Extraction of Carotenoids from Vegetable Waste Matrices. Molecules 2019, 24, 466. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, X.; Wang, Z.; Sun, Y.; Zhu, S.; Li, L.; Lv, P. Optimization of enzymatic hydrolysis for effective lipid extraction from microalgae Scenedesmus sp. Renew. Energy 2018, 125, 1049–1057. [Google Scholar] [CrossRef]
- Chen, X.; Fang, D.; Zhao, R.; Gao, J.; Kimatu, B.M.; Hu, Q.; Chen, G.; Zhao, L. Effects of ultrasound-assisted extraction on antioxidant activity and bidirectional immunomodulatory activity of Flammulina velutipes polysaccharide. Int. J. Biol. Macromol. 2019, 140, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Yolmeh, M.; Jafari, S.M. Applications of Response Surface Methodology in the Food Industry Processes. Food Bioprocess Technol. 2017, 10, 413–433. [Google Scholar] [CrossRef]
- Lin, L.; Xie, J.; Liu, S.; Shen, M.; Tang, W.; Xie, M. Polysaccharide from Mesona chinensis: Extraction optimization, physicochemical characterizations and antioxidant activities. Int. J. Biol. Macromol. 2017, 99, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zeng, G.; Pan, Y.; Chen, W.; Huang, W.; Chen, H.; Li, Y. Properties of soluble dietary fiber-polysaccharide from papaya peel obtained through alkaline or ultrasound-assisted alkaline extraction. Carbohydr. Polym. 2017, 172, 102–112. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, G.; Zhang, J.; Jia, S.; Li, F.; Wang, Y.; Wu, S. Response surface optimization of ultrasound-assisted enzymatic extraction polysaccharides from Lycium barbarum. Carbohydr. Polym. 2014, 110, 278–284. [Google Scholar] [CrossRef]
- Pandelidis, D.; Anisimov, S. Application of a statistical design for analyzing basic performance characteristics of the cross-flow Maisotsenko cycle heat exchanger. Int. J. Heat Mass Transf. 2016, 95, 45–61. [Google Scholar] [CrossRef]
- Sodeifian, G.; Sajadian, S.A.; Saadati Ardestani, N. Supercritical fluid extraction of omega-3 from Dracocephalum kotschyi seed oil: Process optimization and oil properties. J. Supercrit. Fluids 2017, 119, 139–149. [Google Scholar] [CrossRef]
- Pan, Y.; Hao, Y.; Chu, T.; Li, C.; Zhang, Z.; Zhou, Y. Ultrasonic-assisted extraction, chemical characterization of polysaccharides from Yunzhi mushroom and its effect on osteoblast cells. Carbohydr. Polym. 2010, 80, 922–926. [Google Scholar] [CrossRef]
- Prakash Maran, J.; Manikandan, S.; Thirugnanasambandham, K.; Vigna Nivetha, C.; Dinesh, R. Box-Behnken design based statistical modeling for ultrasound-assisted extraction of corn silk polysaccharide. Carbohydr. Polym. 2013, 92, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Huang, J.; Wang, S.; Yin, J.; Zhang, F. Polysaccharides from Pachyrhizus erosus roots: Extraction optimization and functional properties. Food Chem. 2022, 382, 132413. [Google Scholar] [CrossRef]
- Dinesh, S.; Vijayan, V.; Thanikaikarasan, S.; Sebastian, P.J. Productivity and Quality enhancement in Powder Mixed Electrical Discharge Machining for OHNS die steel by utilization of ANN and RSM modeling. J. New Mater. Electrochem. Syst. 2019, 22, 33–43. [Google Scholar]
- Pyun, D.G.; Choi, H.J.; Yoon, H.S.; Thambi, T.; Lee, D.S. Polyurethane foam containing rhEGF as a dressing material for healing diabetic wounds: Synthesis, characterization, in vitro and in vivo studies. Colloids Surf. B Biointerfaces 2015, 135, 699–706. [Google Scholar] [CrossRef]
- Torabi, A.; Kolahan, F. Optimizing pulsed Nd:YAG laser beam welding process parameters to attain maximum ultimate tensile strength for thin AISI316L sheet using response surface methodology and simulated annealing algorithm. Opt. Laser Technol. 2018, 103, 300–310. [Google Scholar] [CrossRef]
- Zhong, K.; Wang, Q. Optimization of ultrasonic extraction of polysaccharides from dried longan pulp using response surface methodology. Carbohydr. Polym. 2010, 80, 19–25. [Google Scholar] [CrossRef]
- Zhi, W.W.; Wei, T.C.; Jen, Y.W.; Long, W.H.; Lin, C.C.; Der, C.J.; Kuang, L.M.; Tung, L.W. Comparative study on the physicochemical and functional properties of the mucilage in the carpel of Nymphaea odorata using ultrasonic and classical heating extractions. Int. J. Biol. Macromol. 2018, 117, 1367–1373. [Google Scholar] [CrossRef]
- Ji, X.; Peng, Q.; Yuan, Y.; Shen, J.; Xie, X.; Wang, M. Isolation, structures and bioactivities of the polysaccharides from jujube fruit (Ziziphus jujuba Mill.): A review. Food Chem. 2017, 227, 349–357. [Google Scholar] [CrossRef]
- Zhao, P.; Li, X.; Wang, Y.; Yan, L.; Guo, L.; Huang, L.; Gao, W. Characterisation and saccharide mapping of polysaccharides from four common Polygonatum spp. Carbohydr. Polym. 2020, 233, 115836. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Batool, Z.; Liu, R.; Sui, G.; Sheng, B.; Zheng, X.; Xu, D. Characterization and immunological activity of polysaccharides from Potentilla chinensis. Int. J. Biol. Macromol. 2020, 165 Pt A, 683–690. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.W.; Yu, K.W.; Suh, H.J. Polysaccharides fractionated from enzyme digests of Korean red ginseng water extracts enhance the immunostimulatory activity. Int. J. Biol. Macromol. 2019, 121, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Li, C.; Wang, S.; Mei, X.; Zhang, H.; Kan, J. Characterization of physicochemical properties and antioxidant activity of polysaccharides from shoot residues of bamboo (Chimonobambusa quadrangularis): Effect of drying procedures. Food Chem. 2019, 292, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.W.; Cheng, C.; Liu, J.P.; Zhao, L.Y.; Huang, D.C.; Chen, G.T. Characterization and antitumor activities of polysaccharides obtained from ginger (Zingiber officinale) by different extraction methods. Int. J. Biol. Macromol. 2020, 152, 894–903. [Google Scholar] [CrossRef]
- Hadidi, M.; Amoli, P.I.; Jelyani, A.Z.; Hasiri, Z.; Rouhafza, A.; Ibarz, A.; Khaksar, F.B.; Tabrizi, S.T. Polysaccharides from pineapple core as a canning by-product: Extraction optimization, chemical structure, antioxidant and functional properties. Int. J. Biol. Macromol. 2020, 163, 2357–2364. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Han, X.; Wang, S.; Jiang, X. Evaluation of Structural Characteristics of Huadian Oil Shale Kerogen Using Direct Techniques (Solid-State 13C NMR, XPS, FT-IR, and XRD). Energy Fuels 2011, 25, 4006–4013. [Google Scholar] [CrossRef]
- Yang, H.; Kannappan, S.; Pandian, A.S.; Jang, J.H.; Lee, Y.S.; Lu, W. Graphene supercapacitor with both high power and energy density. Nanotechnology 2017, 28, 445401. [Google Scholar] [CrossRef]
- Sajan, D.; Ravindra, H.J.; Misra, N.; Joe, I.H. Intramolecular charge transfer and hydrogen bonding interactions of nonlinear optical material N-benzoyl glycine: Vibrational spectral study. Vib. Spectrosc. 2010, 54, 72–80. [Google Scholar] [CrossRef]
- Tchakouté, H.K.; Rüscher, C.H.; Kong, S.; Kamseu, E.; Leonelli, C. Geopolymer binders from metakaolin using sodium waterglass from waste glass and rice husk ash as alternative activators: A comparative study. Constr. Build. Mater. 2016, 114, 276–289. [Google Scholar] [CrossRef]
- Karnik, D.; Jung, J.; Hawking, S.; Wicker, L. Sugar beet pectin fractionated using isopropanol differs in galacturonic acid, protein, ferulic acid and surface hydrophobicity. Food Hydrocoll. 2016, 60, 179–185. [Google Scholar] [CrossRef]
- Upadhyay, K.K.; Kumar, A.; Upadhyay, S.; Mishra, P.C. Synthesis, characterization, structural optimization using density functional theory and superoxide ion scavenging activity of some Schiff bases. J. Mol. Struct. 2008, 873, 5–16. [Google Scholar] [CrossRef]
- Kenawi, I.M.; Barsoum, B.N.; Youssef, M.A. Drug-drug interaction between diclofenac, cetirizine and ranitidine. J. Pharm. Biomed. Anal. 2005, 37, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Huang, G.; Huang, H. The antioxidant activities of garlic polysaccharide and its derivatives. Int. J. Biol. Macromol. 2020, 145, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Abuduwaili, A.; Nuerxiati, R.; Mutailifu, P.; Gao, Y.; Lu, C.; Yili, A. Isolation, structural modification, characterization, and bioactivity of polysaccharides from Folium Isatidis. Ind. Crops Prod. 2022, 176, 114319. [Google Scholar] [CrossRef]
- Qin, Z.; Liu, H.M.; Lv, T.T.; Wang, X.D. Structure, rheological, thermal and antioxidant properties of cell wall polysaccharides from Chinese quince fruits. Int. J. Biol. Macromol. 2020, 147, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Souissi, N.; Boughriba, S.; Abdelhedi, O.; Hamdi, M.; Jridi, M.; Li, S.; Nasri, M. Extraction, structural characterization, and thermal and biomedical properties of sulfated polysaccharides from razor clam Solen marginatus. RSC Adv. 2019, 9, 11538–11551. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Netala, V.R.; Hou, T.; Zhang, Z. Optimization of complex enzyme-ultrasonic synergistic extraction of water-soluble polysaccharides from Perilla frutescens seed meal: Purification, characterization and in vitro antioxidant activity. J. Food Process. Preserv. 2022, 46, e16201. [Google Scholar] [CrossRef]
- Hou, Y.; Gong, T.; Zhang, J.; Yang, X.; Guo, Y. Structural characterization and emulsifying properties of thinned-young apples polysaccharides. Biochem. Biophys Res. Commun. 2019, 516, 1175–1182. [Google Scholar] [CrossRef]
- Hajji, M.; Hamdi, M.; Sellimi, S.; Ksouda, G.; Laouer, H.; Li, S.; Nasri, M. Structural characterization, antioxidant and antibacterial activities of a novel polysaccharide from Periploca laevigata root barks. Carbohydr. Polym. 2019, 206, 380–388. [Google Scholar] [CrossRef]
- Savi, A.; Calegari, G.C.; Santos, V.A.Q.; Pereira, E.A.; Teixeira, S.D. Chemical characterization and antioxidant of polysaccharide extracted from Dioscorea bulbifera. J. King Saud Univ. Sci. 2020, 32, 636–642. [Google Scholar] [CrossRef]
- Rozi, P.; Abuduwaili, A.; Mutailifu, P.; Gao, Y.; Rakhmanberdieva, R.; Aisa, H.A.; Yili, A. Sequential extraction, characterization and antioxidant activity of polysaccharides from Fritillaria pallidiflora Schrenk. Int. J. Biol. Macromol. 2019, 131, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.T.; O’Connell, S.; Lyons, H.; Bradley, B.; Hall, M. Antioxidant, antimicrobial, and tyrosinase inhibition activities of acetone extract of Ascophyllum nodosum. Chemical. Papers 2010, 64, 434–442. [Google Scholar] [CrossRef]
- Yao, Y.L.; Shu, C.; Feng, G.; Wang, Q.; Yan, Y.Y.; Yi, Y.; Wang, H.X.; Zhang, X.F.; Wang, L.M. Polysaccharides from Pyracantha fortuneana and its biological activity. Int. J. Biol. Macromol. 2020, 150, 1162–1174. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; He, F.; Fu, L.; Zhang, Y. Polysaccharide from rubescens: Extraction, optimization, characterization and antioxidant activities. RSC Adv. 2021, 11, 18974–18983. [Google Scholar] [CrossRef]
- Li, P.; Xue, H.; Xiao, M.; Tang, J.; Yu, H.; Su, Y.; Cai, X. Ultrasonic-Assisted Aqueous Two-Phase Extraction and Properties of Water-Soluble Polysaccharides from Malus hupehensis. Molecules 2021, 26, 2213. [Google Scholar] [CrossRef]
- Lee, H.P.; Kim, D.S.; Park, S.H.; Shin, C.Y.; Woo, J.J.; Kim, J.W.; An, R.-B.; Lee, C.; Cho, J.Y. Antioxidant Capacity of Potentilla paradoxa Nutt. and Its Beneficial Effects Related to Anti-Aging in HaCaT and B16F10 Cells. Plants 2022, 11, 873. [Google Scholar] [CrossRef]
- Abd Razak, D.L.; Jamaluddin, A.; Abd Rashid, N.Y.; Sani, N.A.; Abdul Manan, M. Assessment of Cosmeceutical Potentials of Selected Mushroom Fruitbody Extracts Through Evaluation of Antioxidant, Anti-Hyaluronidase and Anti-Tyrosinase Activity. J 2020, 3, 329–342. [Google Scholar] [CrossRef]
- Ticar, B.F.; Rohmah, Z.; Mussatto, S.I.; Lim, J.-M.; Park, S.; Choi, B.-D. Hyaluronidase-inhibitory activities of glycosaminoglycans from Liparis tessellatus eggs. Carbohydr. Polym. 2017, 161, 16–20. [Google Scholar] [CrossRef]
- Girish, K.S.; Kemparaju, K. Inhibition of Naja naja venom hyaluronidase by plant-derived bioactive components and polysaccharides. Biochemistry 2005, 70, 948–952. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Koketsu, M. Isolation and analysis of polysaccharide showing high hyaluronidase inhibitory activity in Nostochopsis lobatus MAC0804NAN. J. Biosci. Bioeng. 2016, 121, 345–348. [Google Scholar] [CrossRef]
- Olech, M.; Nowacka-Jechalke, N.; Masłyk, M.; Martyna, A.; Pietrzak, W.; Kubiński, K.; Załuski, D.; Nowak, R. Polysaccharide-rich fractions from Rosa rugosa Thunb.—Composition and chemopreventive potential. Molecules 2019, 24, 1354. [Google Scholar] [CrossRef] [PubMed]
- Skoczyńska, A.; Budzisz, E.; Trznadel-Grodzka, E.; Rotsztejn, H. Melanin and lipofuscin as hallmarks of skin aging. Adv. Dermatol. Allergol. 2017, 34, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Gillbro, J.M.; Olsson, M.J. The melanogenesis and mechanisms of skin-lightening agents-existing and new approaches. Int. J. Cosmet. Sci. 2011, 33, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Aimvijarn, P.; Rodboon, T.; Payuhakrit, W.; Suwannalert, P. Nymphaea pubescens Induces Apoptosis, Suppresses Cellular Oxidants-Related Cell Invasion in B16 Melanoma Cells. Pharm. Sci. 2018, 24, 199–206. [Google Scholar] [CrossRef]
- Teng, H.; Fan, X.; Lv, Q.; Zhang, Q.; Xiao, J.; Qian, Y.; Zheng, B.; Gao, H.; Gao, S.; Chen, L. Folium nelumbinis (Lotus leaf) volatile-rich fraction and its mechanisms of action against melanogenesis in B16 cells. Food Chem. 2020, 330, 127030. [Google Scholar] [CrossRef]
- Smeriglio, A.; D’Angelo, V.; Denaro, M.; Trombetta, D.; Raimondo, F.M.; Germanò, M.P. Polyphenol characterization, antioxidant and skin whitening properties of Alnus cordata stem bark. Chem. Biodivers. 2019, 16, e1900314. [Google Scholar] [CrossRef]
- Ko, H.-H.; Chiang, Y.-C.; Tsai, M.-H.; Liang, C.-J.; Hsu, L.-F.; Li, S.-Y.; Wang, M.-C.; Yen, F.-L.; Lee, C.-W. Eupafolin, a skin whitening flavonoid isolated from Phyla nodiflora, downregulated melanogenesis: Role of MAPK and Akt pathways. J. Ethnopharmacol. 2014, 151, 386–393. [Google Scholar] [CrossRef]
- Solano, F. Photoprotection and skin pigmentation: Melanin-related molecules and some other new agents obtained from natural sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef]
- Walter, M.N.M.; Wright, K.T.; Fuller, H.R.; MacNeil, S.; Johnson, W.E.B. Mesenchymal stem cell-conditioned medium accelerates skin wound healing: An in vitro study of fibroblast and keratinocyte scratch assays. Exp. Cell Res. 2010, 316, 1271–1281. [Google Scholar] [CrossRef]
- Choi, S.H.; Won, K.J.; Lee, R.; Cho, H.S.; Hwang, S.H.; Nah, S.Y. Wound Healing Effect of Gintonin Involves Lysophosphatidic Acid Receptor/Vascular Endothelial Growth Factor Signaling Pathway in Keratinocytes. Int. J. Mol. Sci. 2021, 22, 10155. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Xu, M.; Yuan, Y.; Zhang, X.; Tan, J.; He, J.; Tian, Y. Safety, Effect of raspberry extract on wound healing. Food Qual. Saf. 2021, 5, fyab013. [Google Scholar] [CrossRef]
- Qiu, H.; Liu, S.; Wu, K.; Zhao, R.; Cao, L.; Wang, H. Prospective application of exosomes derived from adipose-derived stem cells in skin wound healing: A review. J. Cosmet. Dermatol. 2020, 19, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; Dos Reis, P.S.; Souza, A.S. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Gu, J.; Zhang, H.; Yao, H.; Zhou, J.; Duan, Y.; Ma, H. Comparison of characterization, antioxidant and immunological activities of three polysaccharides from Sagittaria sagittifolia L. Carbohydr. Polym. 2020, 235, 115939. [Google Scholar] [CrossRef]
- Klun, K.; Šket, P.; Falnoga, I.; Faganeli, J. Variation in Colloidal Organic Matter Composition and Aggregation in Coastal Waters (Gulf of Trieste, Northern Adriatic Sea). Geomicrobiol. J. 2015, 32, 609–615. [Google Scholar] [CrossRef]
- Kunnaja, P.; Chansakaow, S.; Wittayapraparat, A.; Yusuk, P.; Sireeratawong, S. In Vitro Antioxidant Activity of Litsea martabanica Root Extract and Its Hepatoprotective Effect on Chlorpyrifos-Induced Toxicity in Rats. Molecules 2021, 26, 1906. [Google Scholar] [CrossRef]
- Shaddel, R.; Maskooki, A.; Haddad-Khodaparast, M.H.; Azadmard-Damirchi, S.; Mohamadi, M.; Fathi-Achachlouei, B. Optimization of extraction process of bioactive compounds from Bene hull using subcritical water. Food Sci. Biotechnol. 2014, 2, 1459–1468. [Google Scholar] [CrossRef]
- Mancarz, G.F.F.; Laba, L.C.; Morais Silva, T.A.; Pazzim, M.d.S.; de Souza, D.; Prado, M.R.M.; de Souza, L.M.; Nakashima, T.; Mello, R.G. Chemical composition and biological activity of Liquidambar styraciflua L. leaf essential oil. Ind. Crops Prod. 2019, 138, 111446. [Google Scholar] [CrossRef]
- Zhang, X.; Xue, H.; Zhou, P.; Liu, L.; Yu, J.; Dai, P.; Qu, M. Angelica polysaccharide alleviates oxidative response damage in HaCaT cells through up-regulation of miR-126. Exp. Mol. Pathol. 2019, 110, 104281. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Shen, T.; Ding, Q.; Lv, Y.; Li, L.; Huang, K.; Yan, L.; Song, S. Zearalenone induces ROS-mediated mitochondrial damage in porcine IPEC-J2 cells. J. Biochem. Mol. Toxicol. 2017, 31, e21944. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, W.; Li, J.; Jiao, W.H.; Liu, L.Y.; Tang, J.; Liu, L.; Sun, F.; Han, B.N.; Lin, H.W. Two sesquiterpene aminoquinones protect against oxidative injury in HaCaT keratinocytes via activation of AMPKalpha/ERK-Nrf2/ARE/HO-1 signaling. Biomed. Pharmacother. 2018, 100, 417–425. [Google Scholar] [CrossRef]
- Martinez-Liarte, J.H.; Solano, F.; García-Borrón, J.C.; Jara, J.R.; Lozano, J.A. α-MSH and Other Melanogenic Activators Mediate Opposite Effects of Tyrosinase and Dopachrome Tautomerase in B16/F10 Mouse Melanoma Cells. J. Investig. Dermatol. 1992, 99, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Karna, S. In-vitro Wound Healing Effect of 15-Hydroxyprostaglandin Dehydrogenase Inhibitor from Plant. Pharmacogn. Mag. 2017, 13 (Suppl. S1), S122–S126. [Google Scholar] [CrossRef]



| Run | A | B | C | Yield (%) |
|---|---|---|---|---|
| 1 | 40 | 20 | 50 | 6.47 ± 0.03 |
| 2 | 30 | 30 | 50 | 8.11 ± 0.08 |
| 3 | 20 | 40 | 50 | 6.55 ± 0.11 |
| 4 | 40 | 40 | 50 | 7.31 ± 0.09 |
| 5 | 30 | 20 | 55 | 6.79 ± 0.09 |
| 6 | 40 | 30 | 45 | 7.22 ± 0.13 |
| 7 | 30 | 20 | 45 | 7.14 ± 0.18 |
| 8 | 30 | 30 | 50 | 7.98 ± 0.08 |
| 9 | 30 | 30 | 50 | 8.16 ± 0.09 |
| 10 | 30 | 30 | 50 | 8.20 ± 0.21 |
| 11 | 30 | 40 | 45 | 7.75 ± 0.15 |
| 12 | 30 | 40 | 55 | 6.63 ± 0.09 |
| 13 | 30 | 30 | 50 | 7.88 ± 0.12 |
| 14 | 40 | 30 | 55 | 6.74 ± 0.13 |
| 15 | 20 | 30 | 45 | 7.35 ± 0.05 |
| 16 | 20 | 20 | 50 | 6.19 ± 0.14 |
| 17 | 20 | 30 | 55 | 6.04 ± 0.09 |
| Variables | Sum of Squares | df | Mean Square | F-Value | p-Value Prob. > F |
|---|---|---|---|---|---|
| Model | 8.01 | 9 | 0.89 | 34.47 | <0.0001 *** |
| A | 0.33 | 1 | 0.33 | 12.73 | 0.0091 ** |
| B | 0.34 | 1 | 0.34 | 13.16 | 0.0084 ** |
| C | 1.33 | 1 | 1.33 | 51.54 | 0.0002 *** |
| AB | 0.058 | 1 | 0.06 | 2.26 | 0.0176 * |
| AC | 0.17 | 1 | 0.17 | 6.70 | 0.0360 * |
| BC | 0.15 | 1 | 0.15 | 5.64 | 0.0492 * |
| A2 | 2.95 | 1 | 2.95 | 114.36 | <0.0001 *** |
| B2 | 1.51 | 1 | 1.51 | 58.55 | 0.0001 *** |
| C2 | 0.64 | 1 | 0.64 | 24.78 | 0.0016 ** |
| Residual | 0.18 | 7 | 0.035 | 2.00 | |
| Lack of fit | 0.11 | 3 | 0.036 | 34.47 | 0.2558 |
| Pure error | 0.072 | 4 | 0.034 |
| Physicochemical Property | Proportion (w/w%) |
|---|---|
| Neutral sugar | 41.31 ± 0.24 |
| Uronic acid | 41.68 ± 0.42 |
| Protein | 0.09 ± 0.01 |
| Polyphenol | 2.20 ± 0.11 |
| Yield | 8.22 ± 0.16 |
| Monosaccharide Composition | Peak Area (%) |
|---|---|
| Man | 4.555 |
| Rib | 0.139 |
| Rha | 5.655 |
| GlcUA | 3.946 |
| GalUA | 47.311 |
| Glc | 6.811 |
| Gal | 18.612 |
| Xyl | 6.323 |
| Ara | 6.382 |
| Fuc | 0.265 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.-M.; Tang, W.; Lei, S.-N.; Zhang, Y.; Cheng, M.-Y.; Liu, Q.-L.; Wang, W. Extraction Optimization, Characterization and Biological Activities of Polysaccharide Extracts from Nymphaea hybrid. Int. J. Mol. Sci. 2023, 24, 8974. https://doi.org/10.3390/ijms24108974
Liu H-M, Tang W, Lei S-N, Zhang Y, Cheng M-Y, Liu Q-L, Wang W. Extraction Optimization, Characterization and Biological Activities of Polysaccharide Extracts from Nymphaea hybrid. International Journal of Molecular Sciences. 2023; 24(10):8974. https://doi.org/10.3390/ijms24108974
Chicago/Turabian StyleLiu, Hui-Min, Wei Tang, Sheng-Nan Lei, Yun Zhang, Ming-Yan Cheng, Qing-Lei Liu, and Wei Wang. 2023. "Extraction Optimization, Characterization and Biological Activities of Polysaccharide Extracts from Nymphaea hybrid" International Journal of Molecular Sciences 24, no. 10: 8974. https://doi.org/10.3390/ijms24108974
APA StyleLiu, H.-M., Tang, W., Lei, S.-N., Zhang, Y., Cheng, M.-Y., Liu, Q.-L., & Wang, W. (2023). Extraction Optimization, Characterization and Biological Activities of Polysaccharide Extracts from Nymphaea hybrid. International Journal of Molecular Sciences, 24(10), 8974. https://doi.org/10.3390/ijms24108974





