NETosis in Parasitic Infections: A Puzzle That Remains Unsolved
Abstract
1. Introduction
2. Neutrophils and Neutrophil Extracellular Traps (NETs)
2.1. Neutrophils
2.2. NETs and NETosis
2.3. Mechanisms of NET Formation
2.4. Microbial Triggers of NETosis
3. NETosis in Parasitic Infections
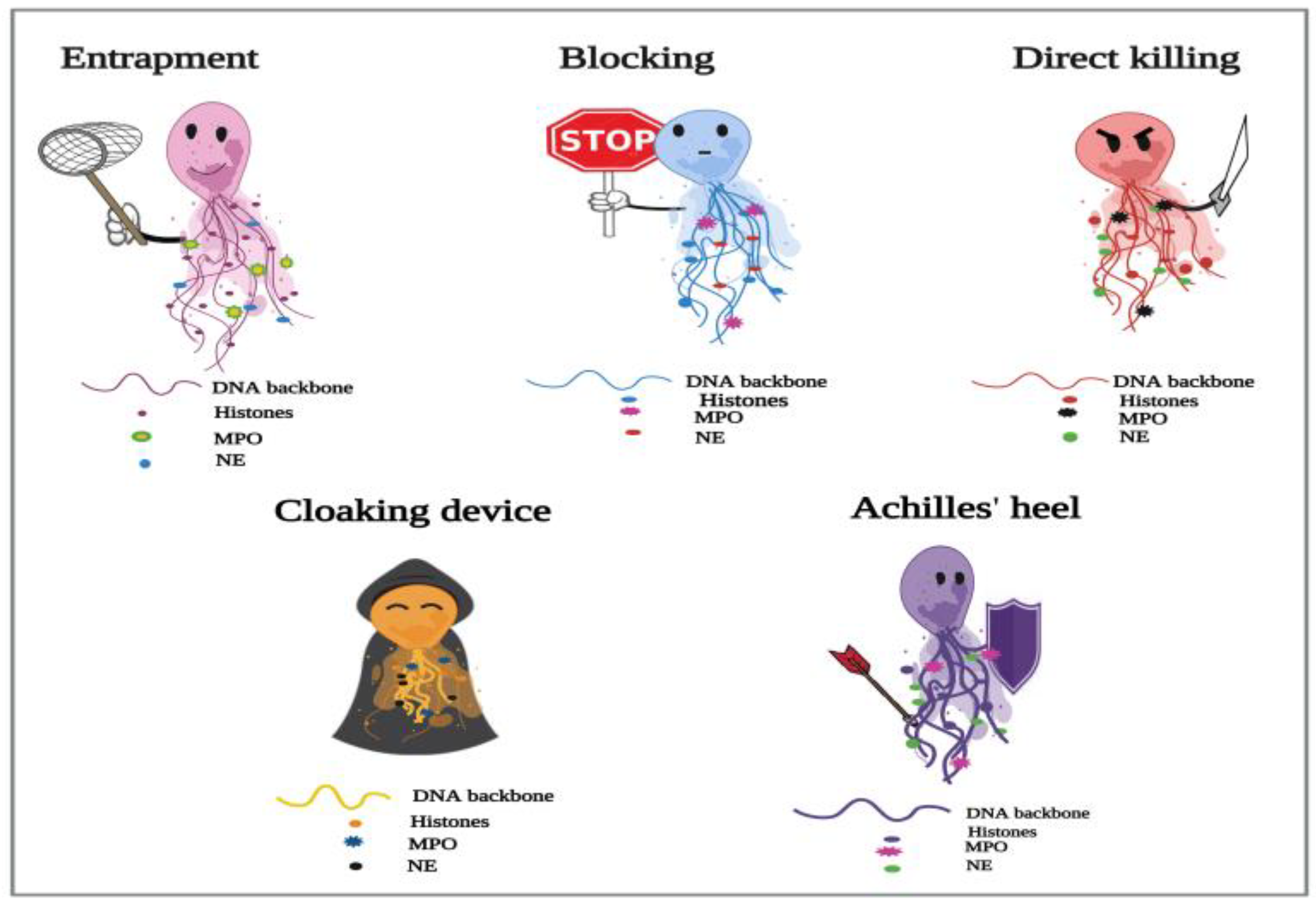
3.1. NETosis and Metazoan Infections
- Trapping without killing;
- Blocking the development;
- Direct killing (larvicidal) effects.
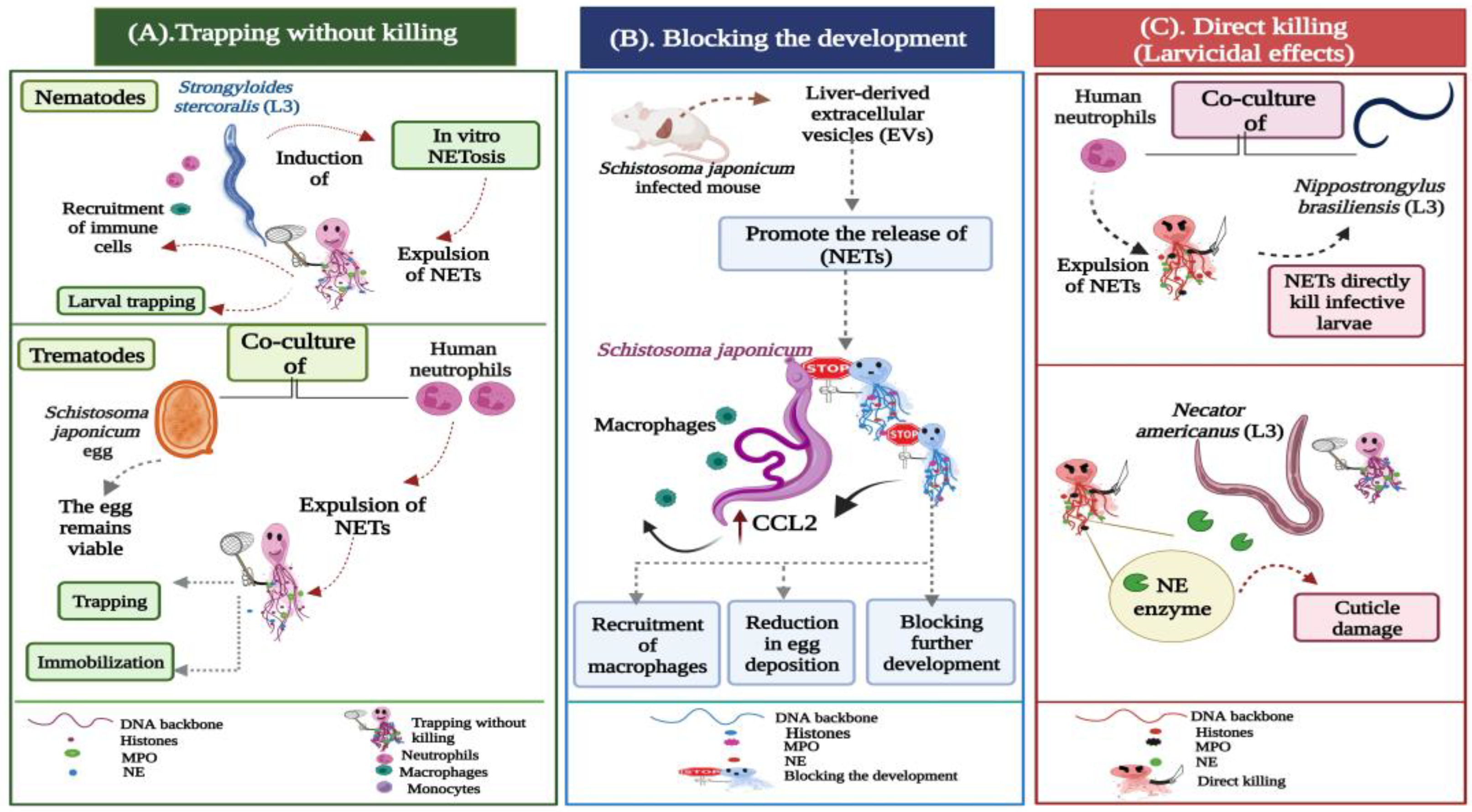
3.1.1. Trapping without Killing
3.1.2. Blocking the Development
3.1.3. Direct Killing (Larvicidal) Effects
3.1.4. Helminths Evading NET-Mediated Killing
3.1.5. NETs Acting as a Cloaking Device
3.2. NETosis and Protozoan Infections
3.2.1. The Protective Roles of NETs during Protozoan Infections
Microbicidal Effects of NETs
Prevention of Host Cell Invasion
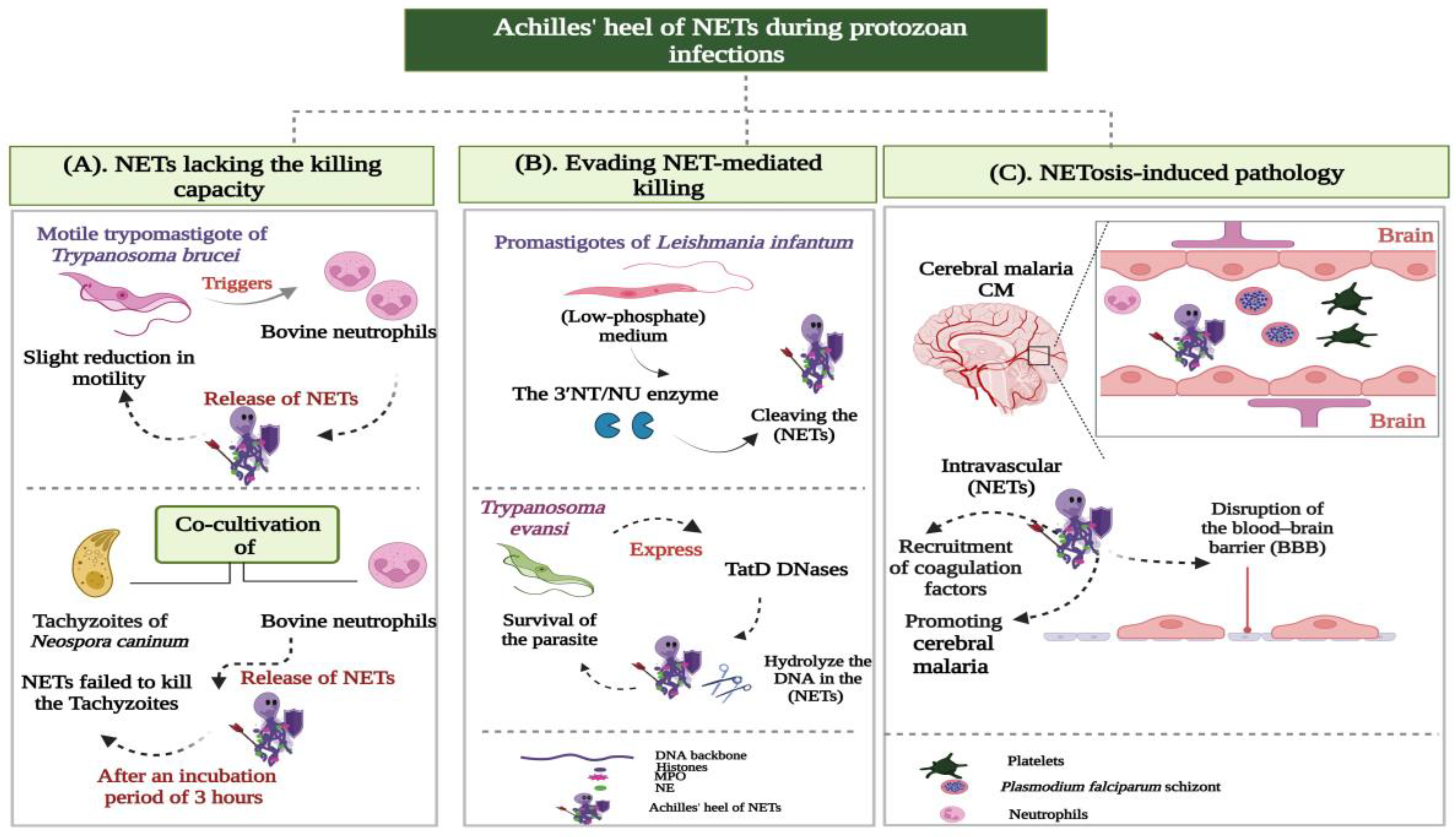
3.2.2. Achilles’ heel of NETs during Protozoan Infections
- NETs lacking the killing capacity;
- Evading NET-mediated killing;
- NETosis-induced pathology.
NETs Lacking the Killing Capacity
Evading NET-Mediated Killing
NETosis-Induced Pathology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harper, S.L.; Edge, V.L.; Schuster-Wallace, C.J.; Berke, O.; McEwen, S.A. Weather, water quality and infectious gastrointestinal illness in two inuit communities in Nunatsiavut, Canada: Potential implications for climate change. Eco Health 2011, 8, 93–108. [Google Scholar] [CrossRef]
- Chabé, M.; Lokmer, A.; Ségurel, L. Gut protozoa: Friends or foes of the human gut microbiota? Trends Parasitol. 2017, 33, 925–934. [Google Scholar] [CrossRef]
- Burrows, K.; Ngai, L.; Wong, F.; Won, D.; Mortha, A. ILC2 activation by protozoan commensal microbes. Int. J. Mol. Sci. 2019, 20, 4865. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.M.; Eichenberger, R.M.; Ruscher, R.; Giacomin, P.R.; Loukas, A. Harnessing helminth-driven immunoregulation in the search for novel therapeutic modalities. PLoS Pathog. 2020, 16, e1008508. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Godínez, C.; Carrero, J.C. The state of art of neutrophil extracellular traps in protozoan and helminthic infections. Biosci. Rep. 2019, 39, BSR20180916. [Google Scholar] [CrossRef]
- Medzhitov, R.; Janeway, C., Jr. Innate Immunity. N. Engl. J. Med. 2000, 343, 338–344. [Google Scholar] [CrossRef]
- De Veer, M.J.; Kemp, J.M.; Meeusen, E.N. The innate host defense against nematode parasites. Parasite Immunol. 2007, 29, 1–9. [Google Scholar] [CrossRef]
- Uribe, C.; Folch, H.; Enriquez, R.; Moran, G. Innate and adaptive immunity in teleost fish: A review. Vet. Med. 2011, 56, 486–503. [Google Scholar] [CrossRef]
- Rossi, M.; Young, J.W. Human dendritic cells: Potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J. Immunol. 2005, 175, 1373–1381. [Google Scholar] [CrossRef]
- Liu, K.; Nussenzweig, M.C. Origin and development of dendritic cells. Immunol. Rev. 2010, 234, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.; Simon, D.; Stojkov, D.; Karsonova, A.; Karaulov, A.; Simon, H.U. In vivo evidence for extracellular DNA trap formation. Cell Death Dis. 2020, 11, 300. [Google Scholar] [CrossRef]
- Simon, D.; Simon, H.U.; Yousefi, S. Extracellular DNA traps in allergic, infectious, and autoimmune diseases. Allergy 2013, 68, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Von Köckritz-Blickwede, M.; Nizet, V. Innate immunity turned inside-out: Antimicrobial defense by phagocyte extracellular traps. J. Mol. Med. 2009, 87, 775–783. [Google Scholar] [CrossRef]
- Borregaard, N. Neutrophils, from marrow to microbes. Immunity 2010, 33, 657–670. [Google Scholar] [CrossRef]
- Silva, L.M.; Muñoz-Caro, T.; Burgos, R.A.; Hidalgo, M.A.; Taubert, A.; Hermosilla, C. Far beyond phagocytosis: Phagocyte-derived extracellular traps act efficiently against protozoan parasites in vitro and in vivo. Mediat. Inflamm. 2016, 10, 5898074. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, P.; Becker, R.; Franzen, C.; Schell-Frederick, E.; Romer, J.; Jacobs, M.; Fätkenheuer, G.; Plum, G. Phagocytosis and killing of Mycobacterium avium complex by human neutrophils. J. Leukoc. Biol. 2001, 69, 397–404. [Google Scholar] [CrossRef]
- Lee, W.L.; Harrison, R.E.; Grinstein, S. Phagocytosis by neutrophils. Microbes Infect. 2003, 5, 1299–1306. [Google Scholar] [CrossRef]
- Wazny, T.K.; Mummaw, N.; Styrt, B. Degranulation of human neutrophils after exposure to bacterial phospholipase C. Eur. J. Clin. Microbiol. Infect. Dis. 1990, 9, 830–832. [Google Scholar] [CrossRef]
- Lacy, P. Mechanisms of degranulation in neutrophils. Allergy Asthma Clin. Immunol. 2006, 2, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goos-mann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef]
- Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps: Is immunity the second function of chromatin? J. Cell Biol. 2012, 198, 773–783. [Google Scholar] [CrossRef]
- Khan, M.A.; Palaniyar, N. Transcriptional firing helps to drive NETosis. Sci. Rep. 2017, 7, 41749. [Google Scholar] [CrossRef]
- Chapman, E.A.; Lyon, M.; Simpson, D.; Mason, D.; Beynon, R.J.; Moots, R.J.; Wright, H.L. Caught in a trap? Proteomic analysis of neutrophil extracellular traps in rheumatoid arthritis and systemic lupus erythematosus. Front. Immunol. 2019, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Petretto, A.; Bruschi, M.; Pratesi, F.; Croia, C.; Candiano, G.; Ghiggeri, G.; Migliorini, P. Neutrophil extracellular traps (NET) induced by different stimuli: A comparative proteomic analysis. PLoS ONE 2019, 14, e0218946. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Zychlinsky, A. Beneficial suicide: Why neutrophils die to make NETs. Nat. Rev. Microbiol. 2007, 8, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Takei, H.; Araki, A.; Watanabe, H.; Ichinose, A.; Sendo, F. Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J. Leukoc. Biol. 1996, 59, 229–240. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the nomenclature committee on cell death. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Guimarães-Costa, A.B.; Nascimento, M.T.; Wardini, A.B.; Pinto-da-Silva, L.H.; Saraiva, E.M. ETosis: A microbicidal mechanism beyond cell death. J. Parasitol. Res. 2012, 2012, 929743. [Google Scholar] [CrossRef]
- Wartha, F.; Henriques-Normark, B. ETosis: A novel cell death pathway. Sci. Signal. 2008, 1, pe25. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Bjornsdottir, H.; Welin, A.; Michaelsson, E.; Osla, V.; Berg, S.; Christenson, K.; Sundqvist, M.; Dahlgren, C.; Karlsson, A.; Bylund, J. Neutrophil NET formation is regulated from the inside by myeloperoxidase-processed reactive oxygen species. Free Rad. Biol. Med. 2015, 89, 1024–1035. [Google Scholar] [CrossRef] [PubMed]
- Thiam, H.R.; Wong, S.L.; Wagner, D.D.; Waterman, C.M. Cellular mechanisms of NETosis. Ann. Rev. Cell Dev. Biol. 2020, 36, 191–218. [Google Scholar] [CrossRef] [PubMed]
- Douda, D.N.; Khan, M.A.; Grasemann, H.; Palaniyar, N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc. Natl. Acad. Sci. USA 2015, 112, 2817–2822. [Google Scholar] [CrossRef]
- Villagra-Blanco, R.; Silva, L.M.R.; Gärtner, U.; Wagner, H.; Failing, K.; Wehrend, A.; Taubert, A.; Hermosilla, C. Molecular analyses on Neospora caninum-triggered NETosis in the caprine system. Dev. Comp. Immunol. 2017, 72, 119–127. [Google Scholar] [CrossRef]
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular mechanisms, role in physiology and pathology. Biochemistry 2020, 85, 1178–1190. [Google Scholar] [CrossRef]
- Yipp, B.G.; Kubes, P. NETosis: How vital is it? Blood 2013, 122, 2784–2794. [Google Scholar] [CrossRef]
- Yipp, B.G.; Petri, B.; Salina, D.; Jenne, C.N.; Scott, B.N.V.; Zbytnuik, L.D.; Pittman, K.; Asaduzzaman, M.; Wu, K.; Meijndert, H.C.; et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 2012, 18, 1386–1393. [Google Scholar] [CrossRef]
- Kayagaki, N.; Stowe, I.B.; Lee, B.L.; O’Rourke, K.; Anderson, K.; Warming, S.; Cuellar, T.; Haley, B.; Roose-Girma, M.; Phung, Q.T.; et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015, 526, 666–671. [Google Scholar] [CrossRef]
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Wardini, A.B.; Guimaraes-Costa, A.B.; Nascimento, M.T.; Nadaes, N.R.; Danelli, M.G.; Mazur, C.; Benjamim, C.F.; Saraiva, E.M.; Pinto-da-Silva, L.H. Characterization of neutrophil extracellular traps in cats naturally infected with feline leukemia virus. J. Gen. Virol. 2010, 91, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Urban, C.F.; Reichard, U.; Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006, 8, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Chang, X.; Wang, G.; Zhou, H.; Ma, Z.; Lin, H.; Fan, H. Streptococcus suis serotype 2 stimulates neutrophil extracellular traps formation via activation of p38 MAPK and ERK1/2. Front. Immunol. 2018, 9, 2854. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell. Biol. 2010, 3, 677–691. [Google Scholar] [CrossRef]
- Chavakis, T.; Wiechmann, K.; Preissner, K.T.; Herrmann, M. Staphylococcus aureus interactions with the endothelium: The role of bacterial “secretable expanded repertoire adhesive molecules” (SERAM) in disturbing host defense systems. Thromb. Haemost. 2005, 94, 278–285. [Google Scholar] [CrossRef]
- Schultz, B.M.; Acevedo, O.A.; Kalergis, A.M.; Bueno, S.M. Role of extracellular trap release during bacterial and viral infection. Front. Microbiol. 2022, 13, 798853. [Google Scholar] [CrossRef]
- Jenne, C.N.; Wong, C.H.; Zemp, F.J.; McDonald, B.; Rahman, M.M.; Forsyth, P.A.; McFadden, G.; Kubes, P. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host Microbe 2013, 13, 169–180. [Google Scholar] [CrossRef]
- Saitoh, T.; Komano, J.; Saitoh, Y.; Misawa, T.; Takahama, M.; Kozaki, T.; Uehata, T.; Iwasaki, H.; Omori, H.; Yamaoka, S.; et al. Neutrophil extracellular traps mediate a host defense response to Human Immunodeficiency Virus-1. Cell Host Microbe 2012, 12, 109–116. [Google Scholar] [CrossRef]
- Veras, F.P.; Pontelli, M.C.; Silva, C.M.; Toller-Kawahisa, J.E.; de Lima, M.; Nascimento, D.C.; Schneider, A.H.; Caetité, D.; Tavares, L.A.; Paiva, I.M.; et al. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med. 2020, 217, e20201129. [Google Scholar] [CrossRef]
- Buonacera, A.; Stancanelli, B.; Colaci, M.; Malatino, L. Neutrophil to Lymphocyte Ratio: An Emerging Marker of the Relationships between the Immune System and Diseases. Int. J. Mol. Sci. 2022, 23, 3636. [Google Scholar] [CrossRef] [PubMed]
- Regolo, M.; Vaccaro, M.; Sorce, A.; Stancanelli, B.; Colaci, M.; Natoli, G.; Russo, M.; Alessandria, I.; Motta, M.; Santangelo, N.; et al. Neutrophil-to-Lymphocyte Ratio (NLR) Is a Promising Predictor of Mortality and Admission to Intensive Care Unit of COVID-19 Patients. J. Clin. Med. 2022, 11, 2235. [Google Scholar] [CrossRef] [PubMed]
- Karkowska-Kuleta, J.; Smolarz, M.; Seweryn-Ozog, K.; Satala, D.; Zawrotniak, M.; Wronowska, E.; Bochenska, O.; Kozik, A.; Nobbs, A.H.; Gogol, M.; et al. Proteinous components of neutrophil extracellular traps are arrested by the cell wall proteins of Candida albicans during fungal infection, and can be used in the host invasion. Cells 2021, 10, 2736. [Google Scholar] [CrossRef] [PubMed]
- Branzk, N.; Lubojemska, A.; Hardison, S.E.; Wang, Q.; Gutierrez, M.G.; Brown, G.D.; Papayannopoulos, V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 2014, 15, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Warnatsch, A.; Tsourouktsoglou, T.D.; Branzk, N.; Wang, Q.; Reincke, S.; Herbst, S.; Gutierrez, M.; Papayannopoulos, V. Reactive oxygen species localization programs inflammation to clear microbes of different size. Immunity 2017, 46, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.J.; Wang, L.; Meng, X.L.; Zhang, S.H.; Sheng, Z.A.; Wei, Z.K.; Luo, X.N.; Huang, W.Y.; Zhu, X.Q.; Zhang, X.C.; et al. Newly excysted juveniles of Fasciola gigantica trigger the release of water buffalo neutrophil extracellular traps in vitro. Exp. Parasitol. 2020, 211, 107828. [Google Scholar] [CrossRef] [PubMed]
- McCoy, C.J.; Reaves, B.J.; Giguère, S.; Coates, R.; Rada, B.; Wolstenholme, A.J. Human leukocytes kill Brugia malayi microfilariae independently of DNA-based extracellular trap release. PLoS Negl. Trop. Dis. 2017, 11, e0005279. [Google Scholar] [CrossRef]
- Muñoz-Caro, T.; Conejeros, I.; Zhou, E.; Pikhovych, A.; Gärtner, U.; Hermosilla, C.; Kulke, D.; Taubert, A. Dirofilaria immitis microfilariae and third-stage larvae induce canine NETosis resulting in different types of neutrophil extracellular traps. Front. Immunol. 2018, 9, 968. [Google Scholar] [CrossRef]
- Bonne-Année, S.; Kerepesi, L.A.; Hess, J.A.; Wesolowski, J.; Paumet, F.; Lok, J.B.; Nolan, T.J.; Abraham, D. Extracellular traps are associated with human and mouse neutrophil and macrophage mediated killing of larval Strongyloides stercoralis. Microbes Infect. 2014, 6, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Caro, T.; Rubio, R.M.C.; Silva, L.M.; Magdowski, G.; Gärtner, U.; McNeilly, T.N.; Taubert, A.; Hermosilla, C. Leucocyte-derived extracellular trap formation significantly contributes to Haemonchus contortus larval entrapment. Parasite Vectors 2015, 8, 607. [Google Scholar] [CrossRef]
- Peixoto, R.; Silva, L.M.R.; López-Osório, S.; Zhou, E.; Gärtner, U.; Conejeros, I.; Taubert, A.; Hermosilla, C. Fasciola hepatica induces weak NETosis and low production of intra- and extracellular ROS in exposed bovine polymorphonuclear neutrophils. Dev. Comp. Immunol. 2021, 114, 103787. [Google Scholar] [CrossRef]
- Chuah, C.; Jones, M.K.; Burke, M.L.; Owen, H.C.; Anthony, B.J.; McManus, D.P.; Ramm, G.A.; Gobert, G.N. Spatial and temporal transcriptomics of Schistosoma japonicum-induced hepatic granuloma formation reveals novel roles for neutrophils. J. Leukoc. Biol. 2013, 94, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, Z.; Liao, Y.; Zhang, L.; Yu, Z.; Yang, R.; Wu, J.; Wu, Z.; Sun, X. Host liver-derived extracellular vesicles deliver miR-142a-3p induces neutrophil extracellular traps via targeting WASL to block the development of Schistosoma japonicum. Mol. Ther. 2022, 30, 2092–2107. [Google Scholar] [CrossRef]
- Bouchery, T.; Moyat, M.; Sotillo, J.; Silverstein, S.; Volpe, B.; Coakley, G.; Tsourouktsoglou, T.D.; Becker, L.; Shah, K.; Kulagin, M.; et al. Hookworms evade host immunity by secreting a deoxyribonuclease to degrade neutrophil extracellular traps. Cell Host Microbe 2020, 27, 277–289.e6. [Google Scholar] [CrossRef] [PubMed]
- Doolan, R.; Bouchery, T. Hookworm infections: Reappraising the evidence for a role of neutrophils in light of NETosis. Parasite Immunol. 2022, 44, e12911. [Google Scholar] [CrossRef]
- Chulanetra, M.; Chaicumpa, W. Revisiting the mechanisms of immune evasion employed by human parasites. Front. Cell Infect. Microbiol. 2021, 11, 702125. [Google Scholar] [CrossRef] [PubMed]
- El-Naccache, D.W.; Chen, F.; Chen, N.; Gause, W.C. The NET effect of neutrophils during helminth infection. Cell Host Microbe 2020, 27, 165–168. [Google Scholar] [CrossRef]
- Yeshi, K.; Ruscher, R.; Loukas, A.; Wangchuk, P. Immunomodulatory and biological properties of helminth-derived small molecules: Potential applications in diagnostics and therapeutics. Front. Parasitol. 2022, 1, 984152. [Google Scholar] [CrossRef]
- White, R.R.; Artavanis-Tsakonas, K. How helminths use excretory secretory fractions to modulate dendritic cells. Virulence 2012, 3, 668–677. [Google Scholar] [CrossRef]
- Ríos-López, A.L.; Hernández-Bello, R.; González, G.M.; Sánchez-González, A. Trichinella spiralis excretory-secretory antigens selectively inhibit the release of extracellular traps from neutrophils without affecting their additional antimicrobial functions. Cell. Immunol. 2022, 382, 104630. [Google Scholar] [CrossRef]
- Chauhan, A.; Sharma, A.; Tripathi, J.K.; Sun, Y.; Sukumran, P.; Singh, B.B.; Mishra, B.B.; Sharma, J. Helminth derived factors inhibit neutrophil extracellular trap formation and inflammation in bacterial peritonitis. Sci. Rep. 2021, 11, 12718. [Google Scholar] [CrossRef] [PubMed]
- Knox, D.P. Proteinase inhibitors and helminth parasite infection. Parasite Immunol. 2007, 29, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Milstone, A.M.; Harrison, L.M.; Bungiro, R.D.; Kuzmic, P.; Cappello, M. A broad spectrum Kunitz type serine protease inhibitor secreted by the hookworm Ancylostoma ceylanicum. J. Biol. Chem. 2000, 275, 29391–29399. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Deng, L.; Li, H.; Zhang, Z.; He, Q.; Yang, C.; Jiang, H.; Zhu, X.Q.; Peng, L. Identification and characterization of a serine protease inhibitor with two trypsin inhibitor-like domains from the human hookworm Ancylostoma Duoden. Parasitol. Res. 2011, 108, 287–295. [Google Scholar] [CrossRef]
- Tamarozzi, F.; Halliday, A.; Gentil, K.; Hoerauf, A.; Pearlman, E.; Taylor, M.J. Onchocerciasis: The role of Wolbachia bacterial endosymbionts in parasite biology, disease pathogenesis, and treatment. Clin. Microbiol. Rev. 2011, 3, 459–468. [Google Scholar] [CrossRef]
- Tamarozzi, F.; Turner, J.D.; Pionnier, N.; Midgley, A.; Guimaraes, A.F.; Johnston, K.L.; Edwards, S.W.; Taylor, M.J. Wolbachia endosymbionts induce neutrophil extracellular trap formation in human onchocerciasis. Sci. Rep. 2016, 6, 35559. [Google Scholar] [CrossRef]
- Behrendt, J.H.; Ruiz, A.; Zahner, H.; Taubert, A.; Hermosilla, C. Neutrophil extracellular trap formation as innate immune reactions against the apicomplexan parasite Eimeria Bovis. Vet. Immunol. Immunopathol. 2010, 133, 1–8. [Google Scholar] [CrossRef]
- Abi Abdallah, D.S.; Lin, C.; Ball, C.J.; King, M.R.; Duhamel, G.E.; Denkers, E.Y. Toxoplasma gondii triggers release of human and mouse neutrophil extracellular traps. Infect. Immun. 2012, 80, 768–777. [Google Scholar] [CrossRef]
- Ponte-Sucre, A. An overview of Trypanosoma brucei infections: An intense host-parasite interaction. Front. Microbiol. 2016, 7, 2126. [Google Scholar] [CrossRef]
- De Buhr, N.; Bonilla, M.C.; Jimenez-Soto, M.; Von Köckritz-Blickwede, M.; Dolz, G. Extracellular trap formation in response to Trypanosoma cruzi infection in granulocytes isolated from dogs and common opossums, natural reservoir hosts. Front. Microbiol. 2018, 9, 966. [Google Scholar] [CrossRef]
- Kho, S.; Minigo, G.; Andries, B.; Leonardo, L.; Prayoga, P.; Poespoprodjo, J.R.; Kenangalem, E.; Price, R.N.; Woodberry, T.; Anstey, N.M. Circulating neutrophil extracellular traps and neutrophil activation are increased in proportion to disease severity in human malaria. J. Infect. Dis. 2019, 219, 1994–2004. [Google Scholar] [CrossRef]
- Pereira, M.A.; Alexandre-Pires, G.; Camara, M.; Santos, M.; Martins, C.; Rodrigues, A.; Adriana, J.; Passero, L.F.D.; Pereira da Fonseca, I.; Santos-Gomes, G. Canine neutrophils cooperate with macrophages in the early stages of Leishmania infantum in vitro infection. Parasite Immunol. 2019, 41, e12617. [Google Scholar] [CrossRef]
- Niedźwiedzka-Rystwej, P.; Repka, W.; Tokarz-Deptuła, B.; Deptuła, W. “In sickness and in health” how neutrophil extracellular trap (NET) works in infections, selected diseases and pregnancy. J. Inflamm. 2019, 16, 15. [Google Scholar] [CrossRef]
- Tabrizi, Z.A.; Khosrojerdi, A.; Aslani, S.; Hemmatzadeh, M.; Babaie, F.; Bairami, A.; Shomali, N.; Hosseinzadeh, R.; Safari, R.; Mohammadi, H. Multi-facets of neutrophil extracellular trap in infectious diseases: Moving beyond immunity. Microb. Pathog. 2021, 158, 105066. [Google Scholar] [CrossRef]
- Hasheminasab, S.S.; Conejeros, I.; D Velásquez, Z.; Borggrefe, T.; Gärtner, U.; Kamena, F.; Taubert, A.; Hermosilla, C. ATP purinergic receptor P2X1-dependent suicidal NETosis induced by Cryptosporidium parvum under physioxia conditions. Biology 2022, 11, 442. [Google Scholar] [CrossRef]
- Guimarães-Costa, A.B.; Nascimento, M.T.; Froment, G.S.; Soares, R.P.; Morgado, F.N.; Conceição-Silva, F.; Saraiva, E.M. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc. Natl. Acad. Sci. USA 2009, 106, 6748–6753. [Google Scholar] [CrossRef]
- Morgado, F.N.; Nascimento, M.T.; Saraiva, E.M.; De Oliveira-Ribeiro, C.; Madeira Mde, F.; Da Costa-Santos, M.; Vasconcellos, E.C.; Pimentel, M.I.; Rosandiski Lyra, M.; Schubach Ade, O.; et al. Are neutrophil extracellular traps playing a role in the parasite control in active American tegumentary leishmaniasis lesions? PLoS ONE 2015, 10, e0133063. [Google Scholar] [CrossRef] [PubMed]
- Contis Montes de Oca, A.; Cruz Baquero, A.; Campos Rodríguez, R.; Cárdenas Jaramillo, L.M.; Aguayo Flores, J.E.; Rojas Hernández, S.; Olivos García, A.; Pacheco Yepez, J. Neutrophil extracellular traps and MPO in models of susceptibility and resistance against Entamoeba histolytica. Parasite Immunol. 2020, 42, e12714. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Ledesma, M.G.; Romero-Contreras, Y.J.; Rodríguez, M.C.; Reyes-Cortes, R.; Cuéllar-Mata, P.; Avila, E.E. Trichomonas vaginalis triggers neutrophil extracellular traps reducing parasite integrity and growth. Parasitol. Res. 2022, 121, 1355–1367. [Google Scholar] [CrossRef]
- Yildiz, K.; Gokpinar, S.; Gazyagci, A.N.; Babur, C.; Sursal, N.; Azkur, A.K. Role of NETs in the difference in host susceptibility to Toxoplasma gondii between sheep and cattle. Vet. Immunol. Immunopathol. 2017, 189, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Caro, T.; Lendner, M.; Daugschies, A.; Hermosilla, C.; Taubert, A. NADPH oxidase, MPO, NE, ERK1/2, p38 MAPK and Ca2+ influx are essential for Cryptosporidium parvum-induced NET formation. Dev. Comp. Immunol. 2015, 52, 245–254. [Google Scholar] [CrossRef]
- Silva, L.M.; Caro, T.M.; Gerstberger, R.; Vila-Viçosa, M.J.; Cortes, H.C.; Hermosilla, C.; Taubert, A. The apicomplexan parasite Eimeria arloingi induces caprine neutrophil extracellular traps. Parasitol. Res. 2014, 113, 2797–2807. [Google Scholar] [CrossRef]
- Pérez, D.; Muñoz-Caro, T.; Silva, L.M.R.; Muñoz, M.C.; Molina, J.M.; Taubert, A.; Hermosilla, C.; Ruiz, A. Eimeria ninakohlyakimovae casts NOX-independent NETosis and induces enhanced IL-12, TNF-α, IL-6, CCL2 and iNOS gene transcription in caprine PMN. Exp. Parasitol. 2021, 220, 108034. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Caro, T.; Hermosilla, C.; Silva, L.M.; Cortes, H.; Taubert, A. Neutrophil extracellular traps as innate immune reaction against the emerging apicomplexan parasite Besnoitia besnoiti. PLoS ONE 2014, 9, e91415. [Google Scholar] [CrossRef] [PubMed]
- Villagra-Blanco, R.; Silva, L.M.R.; Muñoz-Caro, T.; Yang, Z.; Li, J.; Gärtner, U.; Taubert, A.; Zhang, X.; Hermosilla, C. Bovine polymorphonuclear neutrophils cast neutrophil extracellular traps against the abortive parasite Neospora caninum. Front. Immunol. 2017, 8, 606. [Google Scholar] [CrossRef]
- Grob, D.; Conejeros, I.; Velásquez, Z.D.; Preußer, C.; Gärtner, U.; Alarcón, P.; Burgos, R.A.; Hermosilla, C.; Taubert, A. Trypanosoma brucei brucei induces polymorphonuclear neutrophil activation and neutrophil extracellular traps release. Front. Immunol. 2020, 11, 559561. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Li, X.; Wang, X.; Wang, Y.; Zhang, X.; Zhang, N.; Gong, P.; Li, J. Trypanosoma evansi triggered neutrophil extracellular traps formation dependent on myeloperoxidase, neutrophil elastase, and extracellular signal-regulated kinase 1/2 signaling pathways. Vet. Parasitol. 2021, 296, 109502. [Google Scholar] [CrossRef]
- Sousa-Rocha, D.; Thomaz-Tobias, M.; Diniz, L.F.; Souza, P.S.; Pinge-Filho, P.; Toledo, K.A. Trypanosoma cruzi and its soluble antigens induce NET release by stimulating Toll-like receptors. PLoS ONE 2015, 10, e0139569. [Google Scholar] [CrossRef]
- Hurrell, B.P.; Schuster, S.; Grün, E.; Coutaz, M.; Williams, R.A.; Held, W.; Malissen, B.; Malissen, M.; Yousefi, S.; Simon, H.U.; et al. Rapid sequestration of Leishmania mexicana by neutrophils contributes to the development of chronic lesion. PLoS Pathog. 2015, 11, e1004929. [Google Scholar] [CrossRef]
- Ávila, E.E.; Salaiza, N.; Pulido, J.; Rodríguez, M.C.; Díaz-Godínez, C.; Laclette, J.P.; Becker, I.; Carrero, J.C. Entamoeba histolytica trophozoites and lipopeptidophosphoglycan trigger human neutrophil extracellular traps. PLoS ONE 2016, 11, e0158979. [Google Scholar] [CrossRef]
- Mercer, F.; Ng, S.H.; Brown, T.M.; Boatman, G.; Johnson, P.J. Neutrophils kill the parasite Trichomonas vaginalis using trogocytosis. PLoS Biol. 2018, 16, e2003885. [Google Scholar] [CrossRef]
- Gabriel, C.; McMaster, W.R.; Girard, D.; Descoteaux, A. Leishmania donovani promastigotes evade the antimicrobial activity of neutrophil extracellular traps. J. Immunol. 2010, 185, 4319–4327. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Xin, L.; Beverley, S.M.; Carlsen, E.D.; Popov, V.; Chang, K.P.; Wang, M.; Soong, L. Differential microbicidal effects of human histone proteins H2A and H2B on Leishmania promastigotes and amastigotes. Infect. Immun. 2011, 79, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- Guimarães-Costa, A.B.; DeSouza-Vieira, T.S.; Paletta-Silva, R.; Freitas-Mesquita, A.L.; Meyer-Fernandes, J.R.; Saraiva, E.M. 3′-Nucleotidase/nuclease activity allows Leishmania parasites to escape killing by neutrophil extracellular traps. Infect. Immun. 2014, 82, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Jiang, N.; Chen, H.; Zhang, N.; Sang, X.; Feng, Y.; Chen, R.; Chen, Q. TatD DNases of African trypanosomes confer resistance to host neutrophil extracellular traps. Sci. China Life Sci. 2021, 64, 621–632. [Google Scholar] [CrossRef]
- Feintuch, C.M.; Saidi, A.; Seydel, K.; Chen, G.; Goldman-Yassen, A.; Mita-Mendoza, N.K.; Kim, R.S.; Frenette, P.S.; Taylor, T.; Daily, J.P. Activated neutrophils are associated with pediatric cerebral malaria vasculopathy in Malawian children. MBio 2016, 7, e01300-15. [Google Scholar] [CrossRef]
- Knackstedt, S.L.; Georgiadou, A.; Apel, F.; Abu-Abed, U.; Moxon, C.A.; Cunnington, A.J.; Raupach, B.; Cunningham, D.; Langhorne, J.; Krüger, R. Neutrophil extracellular traps drive inflammatory pathogenesis in malaria. Sci. Immunol. 2019, 4, eaaw0336. [Google Scholar] [CrossRef]
- Sercundes, M.K.; Ortolan, L.S.; Debone, D.; Soeiro-Pereira, P.V.; Gomes, E.; Aitken, E.H.; Condino-Neto, A.; Russo, M.; D’ Império Lima, M.R.; Alvarez, J.M.; et al. Targeting neutrophils to prevent malaria-associated acute lung injury/acute respiratory distress syndrome in mice. PLoS Pathog. 2016, 12, e1006054. [Google Scholar] [CrossRef]
- Boeltz, S.; Muñoz, L.E.; Fuchs, T.A.; Herrmann, M. Neutrophil extracellular traps open the Pandora’s box in severe malaria. Front. Immunol. 2017, 8, 874. [Google Scholar] [CrossRef]
- Gardinassi, L.G.; DeSouza-Vieira, T.S.; Da Silva, N.O.; Garcia, G.R.; Borges, V.M.; Campos, R.N.S.; De Almeida, R.P.; De Miranda Santos, I.K.F.; Saraiva, E.M. Molecular signatures of neutrophil extracellular traps in human visceral leishmaniasis. Parasite Vectors 2017, 10, 285. [Google Scholar] [CrossRef]
- Díaz-Godínez, C.; Fonseca, Z.; Néquiz, M.; Laclette, J.P.; Rosales, C.; Carrero, J.C. Entamoeba histolytica trophozoites induce a rapid non-classical NETosis mechanism independent of NOX2-derived reactive oxygen species and PAD4 activity. Front. Cell Infect. Microbiol. 2018, 8, 184. [Google Scholar] [CrossRef] [PubMed]
- Conejeros, I.; Velásquez, Z.D.; Grob, D.; Zhou, E.; Salecker, H.; Hermosilla, C.; Taubert, A. Histone H2A and bovine neutrophil extracellular traps induce damage of Besnoitia besnoiti-infected host endothelial cells but fail to affect total parasite proliferation. Biology 2019, 8, 78. [Google Scholar] [CrossRef] [PubMed]

| Helminths (Metazoa) | Evasion Strategy | Factor/Molecule Involved | PMN Origin | Effect/Result | References |
|---|---|---|---|---|---|
| Cestodes M. corti | Inhibition of ROS-induced NETs by blocking (TRPM2) channel and calcium entry; downstream AMPK and autophagy. | Parasite excretory/secretory factors named parasitic ligands (PLs) | Mouse | Improved parasite survival; reduced systemic and local bacterial load. | [71] |
| Trematodes S. japonicum | Inhibition of PMA-induced NET formation by upregulating host IL-10 expression. | S. japonicum (SWAP) and S. japonicum (SEA) | Mouse | Parasitic survival | [63] |
| F. hepatica | Induction of weak NETosis; resolving the NETs; impairment of NETosis signaling pathways and active modulation of the (PMN) response. | Excretory/secretory Bovine (ES) molecules of F. hepatica | The parasitic stages (Eggs, metacercariae, and NEJ) escape the NETs-mediated killing. | [61] | |
| Nematodes T. spiralis | Inhibition of PMA and microbe-induced release of NETs. | T. spiralis excretory/secretory (ES) antigens | Human | Establishment of the parasite inside the host; enhancing the parasitic penetration, migration, nutrition and survival. | [70] |
| Hookworms Nb | Degradation of the DNA backbone of NETs. | DNase II enzyme (Nb-DNase II) | Human; mouse | Prevention of larval damage and killing. | [64] |
| Na | Degradation of the DNA backbone of NETs. | DNase II enzyme | Human; mouse | Prevention of larval damage and killing. | [64] |
| A. ceylanicum | Inhibition of human (NE). | (AceKI-1); Soluble protein extracts and ES products | Human | Increased parasitic survival within the host intestine. | [73] |
| A. duodenale | Inhibition of human (NE). | AduTIL-1 * | Human | Increased parasitic survival within the host intestine. | [74] |
| The Protozoan | The Stages Which Trigger (NETosis) | The (Jekyll and Hyde) Effect of (NETs) * (Protective/Harmful) | References |
|---|---|---|---|
| Leishmania spp. L. amazonensis L. braziliensis L. donovani L. major L. infantum L. mexicana | Promastigote LPG Amastigotes Promastigotes Promastigotes Promastigotes Promastigotes | NETs are lethal to the protozoan: Protective NETs are lethal to the protozoan: Protective The parasite evades killing using (LPG) The parasite evades killing using (LPG) The parasite evades killing using (3′NT/NU) NETs lack the killing potential: Harmful | [86] [87] [102] [103] [104] [99] |
| Trypanosoma spp. T. cruzi (Y strain) T. brucei, T. evansi T. brucei; T. evansi | Trypomastigotes; soluble Ag Trypomastigotes Trypomastigotes (live or dead) | NETs lack the killing potential: Harmful NETs reduce motility, yet lack the killing action The parasites evade killing using (TatD DNases) | [98] [96,97] [105] |
| E. histolytica | Trophozoites Trophozoites and EhLPPG Trophozoites | NETs are lethal to the protozoan: Protective NETs lack the killing potential: Harmful NETs promote inflammation and tissue damage: Harmful | [88] [100] [111] |
| T. vaginalis | Trophozoites Trophozoites and TvLPG/LG | NETs lack the killing potential: Harmful NETs are lethal to the protozoan: Protective | [101] [89] |
| Plasmodium spp.
P. falciparum | Late-stage P. falciparum-iRBCs Haeme released from iRBCs P. falciparum ring stages | NETs contribute to pathophysiology of CM: Harmful NETs contribute to pathophysiology of CM: Harmful NETs contribute to the severity of human malaria: Harmful | [106] [107] [81] |
| P. berghei | P. berghei-iRBCs ND | NETs contribute to malaria-associated respiratory distress: Harmful | [108] |
| P. chabaudi | P. chabaudi-iRBCs ND | NETs contribute to liver damage: Harmful | [107] |
| Apicomplexan spp. Eimeria spp. E. bovis E. arloingi E.ninakohlyakimovae | Sporozoites Sporozoites and oocysts Sporozoites and oocysts | NETs prevent host cell invasion: Protective NETs lack the killing potential: Harmful NETs lack the killing potential: Harmful | [77] [92] [93] |
| C. parvum | Sporozoites and oocysts Sporozoites | NETs prevent host cell invasion: Protective NETs lack the killing potential: Harmful | [85] [91] |
| T. gondii | Tachyzoites a Tachyzoites b | NETs are lethal to the protozoan and prevent host cell invasion: Protective NETs lack the killing potential: Harmful | [78] [90] |
| N. caninum B. Besnoiti | Tachyzoites | NETs prevent host cell invasion, yet lack the killing action | [95] |
| Tachyzoites Tachyzoites | NETs prevent host cell invasion, yet lack the killing action NETs induce endothelial damage and cytotoxicity: Harmful | [94] [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omar, M.; Abdelal, H. NETosis in Parasitic Infections: A Puzzle That Remains Unsolved. Int. J. Mol. Sci. 2023, 24, 8975. https://doi.org/10.3390/ijms24108975
Omar M, Abdelal H. NETosis in Parasitic Infections: A Puzzle That Remains Unsolved. International Journal of Molecular Sciences. 2023; 24(10):8975. https://doi.org/10.3390/ijms24108975
Chicago/Turabian StyleOmar, Marwa, and Heba Abdelal. 2023. "NETosis in Parasitic Infections: A Puzzle That Remains Unsolved" International Journal of Molecular Sciences 24, no. 10: 8975. https://doi.org/10.3390/ijms24108975
APA StyleOmar, M., & Abdelal, H. (2023). NETosis in Parasitic Infections: A Puzzle That Remains Unsolved. International Journal of Molecular Sciences, 24(10), 8975. https://doi.org/10.3390/ijms24108975







