Antimicrobial Peptides: Challenging Journey to the Pharmaceutical, Biomedical, and Cosmeceutical Use
Abstract
1. Introduction
2. Classification
3. Mechanism of Antimicrobial Peptide Action
3.1. Direct Killing
3.2. Immune Modulation
3.3. Mechanism of Action against Cancer Cells
4. Possible Applications in Pharmaceutical, Biomedical and Cosmeceutical Fields
4.1. Pharmaceutical Applications
4.1.1. Antibacterial Activity
4.1.2. Antiviral Activity
4.1.3. Anticancer Activity
4.1.4. Broad Spectrum of Antimicrobial Activities
4.2. Biomedical Applications
4.2.1. Implantable Devices
4.2.2. Biomedical Devices
4.2.3. Multifunctional Coatings
4.2.4. Water Purification Membranes
4.2.5. Detection Biosensors
4.3. Cosmeceutical Applications
5. Methods of AMP Production
6. Market
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S.; Gigante, V.; Sati, H.; Paulin, S.; Al-Sulaiman, L.; Rex, J.H.; Fernandes, P.; Arias, C.A.; Paul, M.; Thwaites, G.E.; et al. Analysis of the Clinical Pipeline of Treatments for Drug-Resistant Bacterial Infections: Despite Progress, More Action Is Needed. Antimicrob. Agents Chemother. 2022, 66, e0199121. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Improved methods for classification, prediction, and design of antimicrobial peptides. Methods Mol. Biol. 2015, 1268, 43–66. [Google Scholar] [CrossRef] [PubMed]
- Vizioli, J.; Salzet, M. Antimicrobial peptides from animals: Focus on invertebrates. Trends Pharmacol. Sci. 2002, 23, 494–496. [Google Scholar] [CrossRef]
- Van Epps, H.L. René Dubos: Unearthing antibiotics. J. Exp. Med. 2006, 20, 203–259. [Google Scholar] [CrossRef]
- Boparai, J.K.; Sharma, P.K. Mini Review on Antimicrobial Peptides, Sources, Mechanism and Recent Applications. Protein Pept. Lett. 2020, 27, 4–16. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Brandenburg, L.-O.; Merres, J.; Albrecht, L.-J.; Varoga, D.; Pufe, T. Antimicrobial Peptides: Multifunctional Drugs for Different Applications. Polymers 2012, 4, 539–560. [Google Scholar] [CrossRef]
- Brown, K.L.; Hancock, R.E. Cationic host defence (antimicrobial) peptides. Curr Opin Immunol. 2006, 18, 24–30. [Google Scholar] [CrossRef]
- Haney, E.F.; Hancock, R.E. Peptide design for antimicrobial and immunomodulatory applications. Biopolymers 2013, 100, 572–583. [Google Scholar] [CrossRef]
- Harder, J.; Schröder, J.M. Antimicrobial Peptides. Role in Human Health and Disease, 1st ed.; Springer International Publishing Switzerland: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Lalani, R.; Misra, A.; Amrutiya, J.; Patel, H.; Bhatt, P.; Patel, V. Challenges in Dermal Delivery of Therapeutic Antimicrobial Protein and Peptides. Curr. Drug. Metab. 2017, 18, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Lou, T.; Leung, M.; Wang, X.; Chang, J.Y.F.; Tsao, C.T.; Sham, J.G.C.; Edmondson, D.; Zhang, M. Bi-layer scaffold of chitosan/PCL-nanofibrous mat and PLLA-microporous disc for skin tissue engineering. J. Biomed. Nanotechnol. 2014, 10, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Gallo, R.L. Antimicrobial Peptides: Old Molecules with New Ideas. J. Investig. Dermatol. 2012, 132, 887–895. [Google Scholar] [CrossRef]
- Seo, M.-D.; Won, H.-S.; Kim, J.-H.; Mishig-Ochir, T.; Lee, B.-J. Antimicrobial Peptides for Therapeutic Applications: A Review. Molecules 2012, 17, 12276–12286. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Wei, R.-B.; Luo, H.-Y.; Wang, D.-F. Isolation and Characterization of an Antibacterial Peptide Fraction from the Pepsin Hydrolysate of Half-Fin Anchovy (Setipinna taty). Molecules 2012, 17, 2980–2991. [Google Scholar] [CrossRef]
- Pasupuleti, M.; Schmidtchen, A.; Malmsten, M. Antimicrobial peptides: Key components of the innate immune system. Crit. Rev. Biotechnol. 2012, 32, 143–171. [Google Scholar] [CrossRef]
- Chen, J.; Tian, S.; Han, X.; Chu, Y.; Wang, Q.; Zhou, B.; Shang, H. Is the superbug fungus really so scary? A systematic review and meta-analysis of global epidemiology and mortality of Candida auris. BMC Infect. Dis. 2020, 20, 827. [Google Scholar] [CrossRef]
- McClure, N.S.; Day, T. A theoretical examination of the relative importance of evolution management and drug development for managing resistance. Proc. Biol. Sci. 2022, 281, 20141861. [Google Scholar] [CrossRef]
- Luong, T.; Salabarria, A.C.; Edwards, R.A.; Roach, D.R. Standardized bacteriophage purification for personalized phage therapy. Nat. Protoc. 2020, 15, 2867–2890. [Google Scholar] [CrossRef]
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Front. Cell. Infect. Microbiol. 2021, 11, 668632. [Google Scholar] [CrossRef]
- Kurpe, S.R.; Grishin, S.Y.; Surin, A.K.; Panfilov, A.V.; Slizen, M.V.; Chowdhury, S.D.; Galzitskaya, O.V. Antimicrobial and Amyloidogenic Activity of Peptides. Can Antimicrobial Peptides Be Used against SARS-CoV-2? Int. J. Mol. Sci. 2020, 21, 9552. [Google Scholar] [CrossRef] [PubMed]
- Pushpanathan, M.; Gunasekaran, P.; Rajendhran, J. Antimicrobial peptides: Versatile biological properties. Int. J. Pept. 2013, 2013, 675391. [Google Scholar] [CrossRef] [PubMed]
- Gelband, H.; Miller-Petrie, M.; Pant, S.; Gandra, S.; Levinson, J.; Barter, D.; White, A.; Laxminarayan, R. State of the World’s Antibiotics; Center for Disease Dynamics, Economics & Policy: Washington, DC, USA, 2015. [Google Scholar]
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The future of peptide-based drugs. Chem. Biol. Drug. Des. 2013, 81, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef]
- Alencar-Silva, T.; Braga, M.C.; Santana, G.O.S.; Saldanha-Araujo, F.; Pogue, R.; Dias, S.C.; Franco, O.L.; Carvalho, J.L. Breaking the frontiers of cosmetology with antimicrobial peptides. Biotechnol. Adv. 2018, 36, 2019–2031. [Google Scholar] [CrossRef]
- Lee, T.H.N.; Hall, K.N.; Aguilar, M.I. Antimicrobial peptide structure and mechanism of action: A focus on the role of membrane structure. Curr. Top. Med. Chem. 2016, 16, 25–39. [Google Scholar] [CrossRef]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef]
- Rozek, T.; Bowie, J.H.; Wallace, J.C.; Tyler, M.J. The antibiotic and anticancer active aurein peptides from the Australian Bell Frogs Litoria aurea and Litoria raniformis. Part 2. Sequence determination using electrospray mass spectrometry. Rapid Commun. Mass. Spectrom. 2000, 14, 2002–2011. [Google Scholar] [CrossRef]
- Rozek, T.; Wegener, K.L.; Bowie, J.H.; Olver, I.N.; Carver, J.A.; Wallace, J.C.; Tyler, M.J. The antibiotic and anticancer active aurein peptides from the Australian Bell Frogs Litoria aurea and Litoria raniformis. Eur. J. Biochem. 2000, 267, 5330–5341. [Google Scholar] [CrossRef]
- Hancock, R.E.; Haney, E.F.; Gill, E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Publ. Gr. 2016, 16, 321–334. [Google Scholar] [CrossRef]
- Da Mata, É.C.G.; Mourão, C.B.F.; Rangel, M.; Schwartz, E.F. Antiviral activity of animal venom peptides and related compounds. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 3. [Google Scholar] [CrossRef] [PubMed]
- Niidome, T.; Kobayashi, K.; Arakawa, H.; Hatakeyama, T.; Aoyagi, H. Structure–activity relationship of an antibacterial peptide, maculatin 1.1, from the skin glands of the tree frog, Litoria genimaculata. J. Pept. Sci. 2004, 10, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, E.; Greber, K.; Rodziewicz-Motowidło, S.; Szultka, Ł.; Łukasiak, J.; Kamysz, W. Synthesis and antimicrobial activity of truncated fragments and analogs of citropin 1.1: The solution structure of the SDS micelle-bound citropin-like peptides. J. Struct. Biol. 2009, 168, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Park, C.B.; Kim, H.S.; Kim, S.C. Mechanism of Action of the Antimicrobial Peptide Buforin II: Buforin II Kills Microorganisms by Penetrating the Cell Membrane and Inhibiting Cellular Functions. Biochem. Biophys. Res. Commun. 1998, 244, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Kościuczuk, E.M.; Lisowski, P.; Jarczak, J.; Strzałkowska, N.; Jóźwik, A.; Horbańczuk, J.; Krzyżewski, J.; Zwierzchowski, L.; Bagnicka, E. Cathelicidins: Family of antimicrobial peptides. A review. Mol. Biol. Rep. 2012, 39, 10957–10970. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Defensins: Antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003, 3, 710–720. [Google Scholar] [CrossRef]
- Ulm, H.; Wilmes, M.; Shai, Y.; Sahl, H.-G. Antimicrobial Host Defensins—Specific Antibiotic Activities and Innate Defense Modulation. Front. Immunol. 2012, 3, 249. [Google Scholar] [CrossRef]
- Lehrer, R.I.; Barton, A.; Daher, K.A.; Harwig, S.S.; Ganz, T.; Selsted, M.E. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J. Clin. Investig. 1989, 84, 553–561. [Google Scholar] [CrossRef]
- Tincu, J.A.; Taylor, S.W. Antimicrobial peptides from marine invertebrates. Antimicrob. Agents Chemother. 2004, 48, 3645–3654. [Google Scholar] [CrossRef]
- Takahashi, D.; Shukla, S.K.; Prakash, O.; Zhang, G. Structural determinants of host defense peptides for antimicrobial activity and target cell selectivity. Biochimie 2010, 92, 1236–1241. [Google Scholar] [CrossRef]
- University of Nebraska Medical Center. Antimicrobial Peptide Database. Available online: https://aps.unmc.edu/ (accessed on 24 March 2023).
- Nawrot, R.; Barylski, J.; Nowicki, G.; Broniarczyk, J.; Buchwald, W.; Goździcka-Józefiak, A. Plant antimicrobial peptides. Folia microbiol 2014, 59, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Mak, A.S.; Jones, B.L. Separation and characterisation of chymotryptic peptides from alpha- and beta-purothionins of wheat. J. Sci. Food Agric. 1976, 27, 205–213. [Google Scholar] [CrossRef]
- Hughes, P.; Dennis, E.; Whitecross, M.; Llewellyn, D.; Gage, P. The cytotoxic plant protein, beta-purothionin, forms ion channels in lipid membranes. J. Biol. Chem. 2000, 275, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Stec, B. Plant thionins—The structural perspective. Cell. Mol. Life Sci. 2006, 63, 1370–1385. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.P.; Wang, S.; Wong, K.H.; Tan, W.L. Antimicrobial Peptides from Plants. Pharmaceuticals 2015, 8, 711–757. [Google Scholar] [CrossRef]
- Milbradt, A.G.; Kerek, F.; Moroder, L.; Renner, C. Structural characterization of hellethionins from Helleborus purpurascens. Biochemistry 2003, 42, 2404–2411. [Google Scholar] [CrossRef]
- Rao, U.; Stec, B.; Teeter, M.M. Refinement of purothionins reveals solute particles important for lattice formation and toxicity. Part 1: Alpha1-purothionin revisited. Acta Crystallogr. Section D Biol. Crystallogr. 1995, 51, 904–913. [Google Scholar] [CrossRef]
- Rao, U.; Stec, B.; Teeter, M.M. Refinement of purothionins reveals solute particles important for lattice formation and toxicity. Part 2: Structure of beta-purothionin at 1.7 Å resolution. Acta Cryst. D Biol. Cryst. 1995, 51, 914–924. [Google Scholar] [CrossRef]
- Majewski, J.; Stec, B. X-ray scattering studies of model lipid membrane interacting with purothionins provide support for a previously proposed mechanism of membrane lysis. Eur. Biophys. J. 2001, 39, 1155–1165. [Google Scholar] [CrossRef]
- Fernandez de Caleya, R.; Gonzalez-Pascual, B.; García-Olmedo, F.; Carbonero, P. Susceptibility of phytopathogenic bacteria to wheat purothionins In Vitro. Appl. Microbiol. 1972, 23, 998–1000. [Google Scholar] [CrossRef]
- Oard, S.; Rush, M.C.; Oard, J.H. Characterization of antimicrobial peptides against a US strain of the rice pathogen Rhizoctonia solani. J. Appl. Microbiol. 2004, 97, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Giudici, M.; Poveda, J.A.; Molina, M.L.; de la Canal, L.; González-Ros, J.M.; Pfüller, K.; Pfüller, U.; Villalaín, J. Antifungal effects and mechanism of action of viscotoxin A3. FEBS J. 2004, 273, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef]
- Graham, M.A.; Silverstein, K.A.; VandenBosch, K.A. Defensin-like genes: Genomic perspectives on a diverse superfamily in plants. Crop. Sci. 2008, 48, S-3–S-11. [Google Scholar] [CrossRef]
- Bruix, M.; Jiménez, M.A.; Santoro, J.; González, C.; Colilla, F.J.; Méndez, E.; Rico, M. Solution structure of gamma 1-H and gamma 1-P thionins from barley and wheat endosperm determined by 1H-NMR: A structural motif common to toxic arthropod proteins. Biochemistry 1993, 32, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, W.F.; Terras, F.R.; Cammue, B.P.; Osborn, R.W. Plant defensins: Novel antimicrobial peptides as components of the host defense system. Plant Physiol. 1995, 108, 1353–1358. [Google Scholar] [CrossRef]
- Mendez, E.; Moreno, A.; Colilla, F.; Pelaez, F.; Limas, G.G.; Mendez, R.; Soriano, F.; Salinas, M.; de Haro, C. Primary structure and inhibition of protein synthesis in eukaryotic cell-free system of a novel thionin, gamma-hordothionin, from barley endosperm. Eur. J. Chem. 1990, 194, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Pelegrini, P.B.; Franco, O.L. Plant gamma-thionins: Novel insights on the mechanism of action of a multi-functional class of defense proteins. Int. J. Biochem. Cell. Biol. 2005, 37, 2239–2253. [Google Scholar] [CrossRef]
- Carvalho, A.d.O.; Gomes, V.M. Plant defensins and defensin-like peptides—Biological activities and biotechnological applications. Curr. Pharm. Des. 2011, 17, 4270–4293. [Google Scholar] [CrossRef]
- Fujimura, M.; Minami, Y.; Watanabe, K.; Tadera, K. Purification, characterization, and sequencing of a novel type of antimicrobial peptides, Fa-AMP1 and Fa-AMP2, from seeds of buckwheat (Fagopyrum esculentum Moench.). Biosci. Biotechnol. Biochem. 2003, 67, 1636–1642. [Google Scholar] [CrossRef]
- Gao, A.G.; Hakimi, S.M.; Mittanck, C.A.; Wu, Y.; Woerner, B.M.; Stark, D.M.; Shah, D.M.; Liang, J.; Rommens, C.M. Fungal pathogen protection in potato by expression of a plant defensin peptide. Nat. Biotechnol. 2000, 18, 1307–1310. [Google Scholar] [CrossRef] [PubMed]
- Sitaram, N. Antimicrobial peptides with unusual amino acid compositions and unusual structures. Curr. Med. Chem 2006, 13, 679–696. [Google Scholar] [CrossRef] [PubMed]
- Terras, F.R.; Eggermont, K.; Kovaleva, V.; Raikhel, N.V.; Osborn, R.W.; Kester, A.; Rees, S.B.; Torrekens, S.; Van Leuven, F.; Vanderleyden, J. Small cysteine-rich antifungal proteins from radish: Their role in host defense. Plant Cell. 1995, 7, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, R.; Neumann, G.M.; Condron, R.; Hughes, A.B.; Polya, G.M. Defense proteins from seed of Cassia fistula include a lipid transfer protein homologue and a protease inhibitory plant defensin. Int. J. Plant Sci. 2000, 159, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Segura, A.; Moreno, M.; Molina, A.; García-Olmedo, F. Novel defensin subfamily from spinach (Spinacia oleracea). FEBS Lett. 1998, 435, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Portieles, R.; Ayra, C.; Gonzalez, E.; Gallo, A.; Rodriguez, R.; Chacón, O.; López, Y.; Rodriguez, M.; Castillo, J.; Pujol, M.; et al. NmDef02, a novel antimicrobial gene isolated from Nicotiana megalosiphon confers high-level pathogen resistance under greenhouse and field conditions. Plant Biotechnol. J. 2010, 8, 678–690. [Google Scholar] [CrossRef]
- van der Weerden, N.L.; Hancock, R.E.; Anderson, M.A. Permeabilization of fungal hyphae by the plant defensin NaD1 occurs through a cell wall-dependent process. J. Biol. Chem. 2010, 285, 37513–37520. [Google Scholar] [CrossRef]
- Ngai, P.H.; Ng, T.B. Phaseococcin, an antifungal protein with antiproliferative and anti-HIV-1 reverse transcriptase activities from small scarlet runner beans. Biochem. Cell. Biol. 2005, 83, 212–220. [Google Scholar] [CrossRef]
- Wong, J.H.; Ng, T.B. Sesquin, a potent defensin-like antimicrobial peptide from ground beans with inhibitory activities toward tumor cells and HIV-1 reverse transcriptase. Peptides 2005, 26, 1120–1126. [Google Scholar] [CrossRef]
- Falco, A.; Mas, V.; Tafalla, C.; Perez, L.; Coll, J.M.; Estepa, A. Dual antiviral activity of human alpha-defensin-1 against viral haemorrhagic septicaemia rhabdovirus (VHSV): Inactivation of virus particles and induction of a type I interferon-related response. Antivir. Res. 2007, 76, 111–123. [Google Scholar] [CrossRef]
- Poon, I.K.; Baxter, A.A.; Lay, F.T.; Mills, G.D.; Adda, C.G.; Payne, J.A.; Hulett, M.D. Phosphoinositide-mediated oligomerization of a defensin induces cell lysis. Struct. Biol. Mol. Bioph. 2014, 3, e01808. [Google Scholar] [CrossRef] [PubMed]
- Hoover, D.M.; Wu, Z.; Tucker, K.; Lu, W.; Lubkowski, J. Antimicrobial characterization of human β-defensin 3 derivatives. Antimicrob. Agents Chemother. 2003, 47, 2804–2809. [Google Scholar] [CrossRef] [PubMed]
- Yeats, T.H.; Rose, J.K. The biochemistry and biology of extracellular plant lipid-transfer proteins (LTPs). Protein Sci. A Publ. Protein Soc. 2008, 17, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.O.; Gomes, V.M. Role of plant lipid transfer proteins in plant cell physiology—A concise review. Peptides 2007, 28, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, X.; Lu, C.; Zeng, X.; Li, Y.; Fu, D.; Wu, G. Non-specific lipid transfer proteins in plants: Presenting new advances and an integrated functional analysis. J. Exp. Bot. 2015, 66, 5663–5681. [Google Scholar] [CrossRef] [PubMed]
- Sels, J.; Mathys, J.; De Coninck, B.M.; Cammue, B.P.; De Bolle, M.F. Plant pathogenesis-related (PR) proteins: A focus on PR peptides. Plant Physiol. Biochem. 2008, 46, 941–950. [Google Scholar] [CrossRef]
- Blein, J.P.; Coutos-Thévenot, P.; Marion, D.; Ponchet, M. From elicitins to lipid-transfer proteins: A new insight in cell signalling involved in plant defence mechanisms. Trends Plant Sci. 2002, 7, 293–296. [Google Scholar] [CrossRef]
- Bogdanov, I.V.; Shenkarev, Z.O.; Finkina, E.I.; Melnikova, D.N.; Rumynskiy, E.I.; Arseniev, A.S.; Ovchinnikova, T.V. A novel lipid transfer protein from the pea Pisum sativum: Isolation, recombinant expression, solution structure, antifungal activity, lipid binding, and allergenic properties. BMC Plant Biol. 2016, 16, 107. [Google Scholar] [CrossRef]
- Bogdanov, I.V.; Finkina, E.I.; Balandin, S.V.; Melnikova, D.N.; Stukacheva, E.A.; Ovchinnikova, T.V. Structural and functional characterization of recombinant isoforms of the lentil lipid transfer protein. Acta Nat. 2015, 7, 65–73. [Google Scholar] [CrossRef]
- Souza, A.A.; Costa, A.S.; Campos, D.C.O.; Batista, A.H.M.; Sales, G.W.P.; Nogueira, N.A.P.; Alves, K.M.M.; Coelho-de-Souza, A.N.; Oliveira, H.D. Lipid transfer protein isolated from noni seeds displays antibacterial activity in vitro and improves survival in lethal sepsis induced by CLP in mice. Biochimie 2018, 149, 9–17. [Google Scholar] [CrossRef]
- Kirubakaran, S.I.; Begum, S.M.; Ulaganathan, K.; Sakthivel, N. Characterization of a new antifungal lipid transfer protein from wheat. Plant Physiol. Biochem. 2008, 46, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Diz, M.S.; Carvalho, A.O.; Ribeiro, S.F.; Da Cunha, M.; Beltramini, L.; Rodrigues, R.; Nascimento, V.V.; Machado, O.L.; Gomes, V.M. Characterisation, immunolocalisation and antifungal activity of a lipid transfer protein from chili pepper (Capsicum annuum) seeds with novel α-amylase inhibitory properties. Physiol. Plant 2011, 142, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Chouabe, C.; Eyraud, V.; Da Silva, P.; Rahioui, I.; Royer, C.; Soulage, C.; Bonvallet, R.; Huss, M.; Gressent, F. New mode of action for a knottin protein bioinsecticide: Pea albumin 1 subunit b (PA1b) is the first peptidic inhibitor of V-ATPase. J. Biol. Chem. 2011, 286, 36291–36296. [Google Scholar] [CrossRef]
- Archer, B.L. The proteins of Hevea brasiliensis Latex. 4. Isolation and characterization of crystalline hevein. Biochem. J. 1960, 75, 236–240. [Google Scholar] [CrossRef]
- Van Parijs, J.; Broekaert, W.F.; Goldstein, I.J.; Peumans, W.J. Hevein: An antifungal protein from rubber-tree (Hevea brasiliensis) latex. Planta 1991, 183, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Beintema, J.J. Structural features of plant chitinases and chitin-binding proteins. FEBS Lett. 1994, 350, 159–163. [Google Scholar] [CrossRef]
- Jiménez-Barbero, J.; Cañada, F.J.; Asensio, J.L.; Aboitiz, N.; Vidal, P.; Canales, A.; Siebert, H.C. Hevein domains: An attractive model to study carbohydrate–protein interactions at atomic resolution. Adv. Carbohydr. Chem. Biochem. 2006, 60, 303–354. [Google Scholar] [CrossRef]
- Kini, S.G.; Nguyen, P.Q.; Weissbach, S.; Mallagaray, A.; Shin, J.; Yoon, H.S.; Tam, J.P. Studies on the chitin binding property of novel cysteine-rich peptides from Alternanthera sessilis. Biochemistry 2015, 54, 6639–6649. [Google Scholar] [CrossRef]
- Porto, W.F.; Pires, Á.S.; Franco, O.L. CS-AMPPred: An Updated SVM Model for Antimicrobial Activity Prediction in Cysteine-Stabilized Peptides. PLoS ONE 2012, 7, e51444. [Google Scholar] [CrossRef]
- Koo, J.C.; Lee, S.Y.; Chun, H.J.; Cheong, Y.H.; Choi, J.S.; Kawabata, S.; Miyagi, M.; Tsunasawa, S.; Ha, K.S.; Bae, D.W.; et al. Two hevein homologs isolated from the seed of Pharbitis nil L. exhibit potent antifungal activity. Biochim. Biophys. Acta 1998, 1382, 80–90. [Google Scholar] [CrossRef]
- Kiba, A.; Saitoh, H.; Nishihara, M.; Omiya, K.; Yamamura, S. C-terminal domain of a hevein-like protein from Wasabia japonica has potent antimicrobial activity. Plant Cell. Physiol. 2003, 44, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.H.; Xiang, Y.; Tu, G.Z.; Zhang, Y.; Wang, D.C. Solution structure of Eucommia antifungal peptide: A novel structural model distinct with a five-disulfide motif. Biochemistry 2004, 43, 6005–6012. [Google Scholar] [CrossRef] [PubMed]
- Saether, O.; Craik, D.J.; Campbell, I.D.; Sletten, K.; Juul, J.; Norman, D.G. Elucidation of the primary and three-dimensional structure of the uterotonic polypeptide kalata B1. Biochemistry 1995, 34, 4147–4158. [Google Scholar] [CrossRef] [PubMed]
- Pelegrini, P.B.; Quirino, B.F.; Franco, O.L. Plant Cyclotides: An Unusual Class of Defense Compounds. Peptides 2007, 28, 1475–1481. [Google Scholar] [CrossRef]
- Craik, D.J.; Mylne, J.S.; Daly, N.L. Cyclotides: Macrocyclic peptides with applications in drug design and agriculture. Cell. Mol. Life Sci. 2010, 67, 9–16. [Google Scholar] [CrossRef]
- Harris, F.; Dennison, S.R.; Phoenix, D.A. Anionic antimicrobial peptides from eukaryotic organisms. Curr. Protein Pept. Sci. 2009, 10, 585–606. [Google Scholar] [CrossRef]
- Prabhu, S.; Dennison, S.R.; Mura, M.; Lea, R.W.; Snape, T.J.; Harris, F. Cn-AMP2 from green coconut water is an anionic anticancer peptide. J. Pept. Sci. 2014, 20, 909–915. [Google Scholar] [CrossRef]
- Pränting, M.; Lööv, C.; Burman, R.; Göransson, U.; Andersson, D.I. The cyclotide cycloviolacin O2 from Viola odorata has potent bactericidal activity against Gram-negative bacteria. J. Antimicrob. Chemother. 2010, 65, 1964–1971. [Google Scholar] [CrossRef]
- Ireland, D.C.; Clark, R.J.; Daly, N.L.; Craik, D.J. Isolation, sequencing, and structure-activity relationships of cyclotides. J. Nat. Prod. 2010, 73, 1610–1622. [Google Scholar] [CrossRef]
- Mehta, L.; Dhankhar, R.; Gulati, P.; Kapoor, R.K.; Mohanty, A.; Kumar, S. Natural and grafted cyclotides in cancer therapy: An insight. J. Pept. Sci. 2020, 26, e3246. [Google Scholar] [CrossRef]
- Lindholm, P.; Göransson, U.; Johansson, S.; Claeson, P.; Gullbo, J.; Larsson, R.; Bohlin, L.; Backlund, A. Cyclotides: A novel type of cytotoxic agents. Mol. Cancer Ther. 2002, 1, 365–369. [Google Scholar] [PubMed]
- Sangphukieo, A.; Nawae, W.; Laomettachit, T.; Supasitthimethee, U.; Ruengjitchatchawalya, M. Computational Design of Hypothetical New Peptides Based on a Cyclotide Scaffold as HIV gp120 Inhibitor. PLoS ONE 2015, 10, e0139562. [Google Scholar] [CrossRef] [PubMed]
- Colgrave, M.L.; Kotze, A.C.; Huang, Y.H.; O’Grady, J.; Simonsen, S.M.; Craik, D.J. Cyclotides: Natural, circular plant peptides that possess significant activity against gastrointestinal nematode parasites of sheep. Biochemistry 2008, 47, 5581–5589. [Google Scholar] [CrossRef]
- Jennings, C.; West, J.; Waine, C.; Craik, D.; Anderson, M. Biosynthesis and insecticidal properties of plant cyclotides: The cyclic knotted proteins from Oldenlandia affinis. Proc. Natl. Acad. Sci. USA 2001, 98, 10614–10619. [Google Scholar] [CrossRef]
- Ireland, D.C.; Wang, C.K.; Wilson, J.A.; Gustafson, K.R.; Craik, D.J. Cyclotides as natural anti-HIV agents. Biopolymers 2008, 90, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Plan, M.R.; Saska, I.; Cagauan, A.G.; Craik, D.J. Backbone cyclised peptides from plants show molluscicidal activity against the rice pest Pomacea canaliculata (golden apple snail). J. Agric. Food Chem. 2008, 56, 5237–5241. [Google Scholar] [CrossRef] [PubMed]
- Daly, N.L.; Gustafson, K.R.; Craik, D.J. The role of the cyclic peptide backbone in the anti-HIV activity of the cyclotide kalata B1. FEBS Lett. 2004, 574, 69–72. [Google Scholar] [CrossRef]
- Ovesen, R.G.; Brandt, K.K.; Göransson, U.; Nielsen, J.; Hansen, H.C.; Cedergreen, N. Biomedicine in the environment: Cyclotides constitute potent natural toxins in plants and soil bacteria. Environ. Toxicol. Chem. 2011, 30, 1190–1196. [Google Scholar] [CrossRef]
- Craik, D.J.; Henriques, S.T.; Mylne, J.S.; Wang, C.K. Cyclotide isolation and characterization. Methods. Enzym. 2012, 516, 37–62. [Google Scholar] [CrossRef]
- Gustafson, K.R.; Sowder, R.C., II; Henderson, L.E.; Parsons, I.C.; Kashman, Y.; Cardellina, J.H., II; McMahon, J.B.; Buckheit, R.W., Jr.; Pannell, L.K.; Boyd, M.R. Circulins A and B. Novel human immunodeficiency virus (HIV)-inhibitory macrocyclic peptides from the tropical tree Chassalia parvifolia. J. Am. Chem. Soc. 1994, 116, 9337–9338. [Google Scholar] [CrossRef]
- Thongyoo, P.; Bonomelli, C.; Leatherbarrow, R.J.; Tate, E.W. Potent inhibitors of beta-tryptase and human leukocyte elastase based on the MCoTI-II scaffold. J. Med. Chem. 2009, 52, 6197–6200. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Singh, V.; Tellis, M.B.; Joshi, R.S.; Singh, S. Repurposing the McoTI-II Rigid Molecular Scaffold in to Inhibitor of ’Papain Superfamily’ Cysteine Proteases. Pharmaceuticals 2020, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Cheng, C.S.; Lai, S.M.; Hsu, M.P.; Chen, C.S.; Lyu, P.C. Solution structure of the plant defensin VrD1 from mung bean and its possible role in insecticidal activity against bruchids. Proteins 2006, 63, 777–786. [Google Scholar] [CrossRef]
- Guzmán-Rodríguez, J.J.; López-Gómez, R.; Suárez-Rodríguez, L.M.; Salgado-Garciglia, R.; Rodríguez-Zapata, L.C.; Ochoa-Zarzosa, A.; López-Meza, J.E. Antibacterial activity of defensin PaDef from avocado fruit (Persea americana var. drymifolia) expressed in endothelial cells against Escherichia coli and Staphylococcus aureus. Biomed. Res. Int. 2013, 2013, 986273. [Google Scholar] [CrossRef] [PubMed]
- Taveira, G.B.; Mathias, L.S.; da Motta, O.V.; Machado, O.L.; Rodrigues, R.; Carvalho, A.O.; Teixeira-Ferreira, A.; Perales, J.; Vasconcelos, I.M.; Gomes, V.M. Thionin-like peptides from Capsicum annuum fruits with high activity against human pathogenic bacteria and yeasts. Biopolymers 2014, 102, 30–39. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.C.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q.Y. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar]
- Rima, M.; Rima, M.; Fajloun, Z.; Sabatier, J.M.; Bechinger, B.; Naas, T. Antimicrobial Peptides: A Potent Alternative to Antibiotics. Antibiotics 2021, 10, 1095. [Google Scholar] [CrossRef]
- Sinha, S.; Zheng, L.; Mu, Y.; Ng, W.J.; Bhattacharjya, S. Structure and Interactions of A Host Defense Antimicrobial Peptide Thanatin in Lipopolysaccharide Micelles Reveal Mechanism of Bacterial Cell Agglutination. Sci. Rep. 2017, 7, 17795. [Google Scholar] [CrossRef]
- Hollmann, A.; Martinez, M.; Maturana, P.; Semorile, L.C.; Maffia, P.C. Antimicrobial Peptides: Interaction with Model and Biological Membranes and Synergism with Chemical Antibiotics. Front. Chem. 2018, 6, 204. [Google Scholar] [CrossRef]
- Juhász, T.; Quemé-Peña, M.; Kővágó, B.; Mihály, J.; Ricci, M.; Horváti, K.; Bősze, S.; Zsila, F.; Beke-Somfai, T. Interplay between membrane active host defense peptides and heme modulates their assemblies and in vitro activity. Sci. Rep. 2021, 11, 18328. [Google Scholar] [CrossRef]
- Meroueh, S.O.; Bencze, K.Z.; Hesek, D.; Lee, M.; Fisher, J.F.; Stemmler, T.L.; Mobashery, S. Three-dimensional structure of the bacterial cell wall peptidoglycan. Proc. Natl. Acad. Sci. USA 2006, 103, 4404–4409. [Google Scholar] [CrossRef]
- Ruiz, N.; Kahne, D.; Silhavy, T.J. Advances in understanding bacterial outer-membrane biogenesis. Nat. Rev. Microbiol. 2006, 4, 57–66. [Google Scholar] [CrossRef]
- Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef]
- Li, J.; Koh, J.J.; Liu, S.; Lakshminarayanan, R.; Verma, C.S.; Beuerman, R.W. Membrane active antimicrobial peptides: Translating mechanistic insights to design. Front. Neurosci. 2017, 11, 73. [Google Scholar] [CrossRef]
- Tally, F.P.; DeBruin, M.F. Development of daptomycin for Gram-positive infections. J. Antimicrob. Chemother. 2000, 46, 523–526. [Google Scholar] [CrossRef]
- Hilchie, A.L.; Wuerth, K.; Hancock, R.E.W. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat. Chem. Biol. 2013, 9, 761–768. [Google Scholar] [CrossRef]
- Afacan, N.J.; Yeung, A.T.Y.; Pena, O.M.; Hancock, R.E.W. Therapeutic potential of host defense peptides in antibiotic-resistant infections. Curr. Pharm. Des. 2012, 18, 807–819. [Google Scholar] [CrossRef]
- Mader, J.S.; Hoskin, D.W. Cationic antimicrobial peptides as novel cytotoxic agents for cancer treatment. Expert Opin. Investig. Drugs 2006, 15, 933–946. [Google Scholar] [CrossRef]
- Nijnik, A.; Hancock, R. Host defence peptides: Antimicrobial and immunomodulatory activity and potential applications for tackling antibiotic-resistant infections. Emerg. Health Threats. J. 2009, 2, e1. [Google Scholar] [CrossRef]
- Lai, Y.; Gallo, R.L. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Nijnik, A.; Philpott, D.J. Modulating immunity as a therapy for bacterial infections. Nat. Rev. Microbiol. 2012, 10, 243–254. [Google Scholar] [CrossRef]
- Jenssen, H.; Hamill, P.; Hancock, R.E. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef]
- Smolarczyk, R.; Cichoń, T.; Szala, S. Peptides: A new class of anticancer drugs. Pos. Hig. Med. Dosw. 2009, 63, 360–368. [Google Scholar]
- Ellerby, H.M.; Arap, W.; Ellerby, L.M.; Kain, R.; Andrusiak, R.; Del Rio, G.; Krajewski, S.; Lombardo, C.R.; Rao, R.; Ruoslahti, E.; et al. Anti-cancer activity of targeted pro-apoptotic peptides. Nat. Med. 1999, 5, 1032–1038. [Google Scholar] [CrossRef]
- Hoskin, D.W.; Ramamoorthy, A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta 2008, 1778, 357–375. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Felício, M.R.; Silva, O.N.; Gonçalves, S.; Santos, N.C.; Franco, O.L. Peptides with dual antimicrobial and anticancer activities. Front. Chem. 2017, 5, 5. [Google Scholar] [CrossRef]
- Divyashree, M.; Mani, M.K.; Reddy, D.; Kumavath, R.; Ghosh, P.; Azevedo, V.; Barh, D. Clinical Applications of Antimicrobial Peptides (AMPs): Where do we Stand Now? Protein Pept. Lett. 2020, 27, 120–134. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D.; Kubicek-Sutherland, J.Z. Mechanisms and consequences of bacterial resistance to antimicrobial peptides. Drug. Resist. Updat. 2016, 26, 43–57. [Google Scholar] [CrossRef]
- Vaara, M. New approaches in peptide antibiotics. Curr. Opin. Pharmacol. 2009, 9, 571–576. [Google Scholar] [CrossRef]
- Dong, P.; Zhou, Y.; He, W.; Hua, D. A strategy for enhanced anti-bacterial activity against Staphylococcus aureus by the assembly of alamethicin with a thermo-sensitive polymeric carrier. Chem. Commun. 2016, 52, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Sahariah, P.; Sørensen, K.K.; Hjálmarsdóttir, M.A.; Sigurjónsson, Ó.E.; Jensen, K.J.; Másson, M.; Thygesen, M.B. Antimicrobial peptide shows enhanced activity and reduced toxicity upon grafting to chitosan polymers. Chem. Commun. 2015, 51, 11611–11614. [Google Scholar] [CrossRef] [PubMed]
- Bayer, I.S. Hyaluronic Acid and Controlled Release: A Review. Molecules 2020, 25, 2649. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, A.; Neundorf, I. Design and application of antimicrobial peptide conjugates. Int. J. Mol. Sci. 2016, 17, 701. [Google Scholar] [CrossRef]
- Veronese, F.M.; Mero, A. The impact of PEGylation on biological therapies. Bio. Drugs 2008, 22, 315–329. [Google Scholar] [CrossRef]
- Kumar, P.; Shenoi, R.A.; Lai, B.F.L.; Nguyen, M.; Kizhakkedathu, J.N.; Straus, S.K. Conjugation of Aurein 2.2 to HPG Yields an Antimicrobial with Better Properties. Biomacromolecules 2015, 16, 913–923. [Google Scholar] [CrossRef]
- Taylor, T.M.; Gaysinsky, S.; Davidson, P.M.; Bruce, B.D.; Weiss, J. Characterization of antimicrobial-bearing liposomes by ζ—Potential, vesicle size, and encapsulation efficiency. Food Biophys. 2007, 2, 1–9. [Google Scholar] [CrossRef]
- Syryamina, V.N.; Samoilova, R.I.; Tsvetkov, Y.D.; Ischenko, A.V.; De Zotti, M.; Gobbo, M.; Dzuba, S.A. Peptides on the surface: Spin-label EPR and PELDOR study of adsorption of the antimicro-bial peptides Trichogin GA IV and Ampullosporin A on the silica nanoparticles. Appl. Magn. Reson. 2016, 47, 309–320. [Google Scholar] [CrossRef]
- Galdiero, E.; Siciliano, A.; Maselli, V.; Gesuele, R.; Guida, M.; Fulgione, D.; Galdiero, S.; Lombardi, L.; Falanga, A. An integrated study on antimicrobial activity and ecotoxicity of quantum dots and quantum dots coated with the antimicrobial peptide indolicidin. Int. J. Nanomed. 2016, 11, 4199–4211. [Google Scholar] [CrossRef]
- Chen, W.Y.; Chang, H.Y.; Lu, J.K.; Huang, Y.C.; Harroun, S.G.; Tseng, Y.T.; Chang, H.T. Self-assembly of antimicrobial peptides on gold nanodots: Against multidrug-resistant bacteria and wound-healing application. Adv. Funct. Mater. 2015, 25, 7189–7199. [Google Scholar] [CrossRef]
- Chaudhari, A.A.; Ashmore, D.; Nath, S.D.; Kate, K.; Dennis, V.; Singh, S.R.; Owen, D.R.; Palazzo, C.; Arnold, R.D.; Miller, M.E.; et al. A novel covalent approach to bio-conjugate silver coated single walled carbon nanotubes with antimicrobial peptide. J. Nanobiotechnol. 2016, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Gallardo, M.; Mas-Moruno, C.; Yu, K.; Manero, J.M.; Gil, F.J.; Kizhakkedathu, J.N.; Rodriguez, D. Antibacterial properties of hLf1-11 peptide onto titanium surfaces: A comparison study be-tween silanization and surface initiated polymerization. Biomacromolecules 2015, 16, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Kanchanapally, R.; Viraka Nellore, B.P.; Sinha, S.S.; Pedraza, F.; Jones, S.J.; Pramanik, A.; Chavva, S.R.; Tchounwou, C.; Shi, Y.; Vangara, A.; et al. Antimicrobial peptide-conjugated graphene oxide membrane for efficient removal and effective killing of multiple drug resistant bacteria. RSC Adv. 2015, 5, 18881–18887. [Google Scholar] [CrossRef] [PubMed]
- Dostalova, S.; Moulick, A.; Milosavljevic, V.; Guran, R.; Kominkova, M.; Cihalova, K.; Vaculovicova, M. Antiviral activity of fullerene C60 nanocrystals modified with derivatives of anionic antimicrobial peptide maximin H5. Mon. Für Chem. Chemie. Mon. 2016, 147, 905–918. [Google Scholar] [CrossRef]
- Sultana, A.; Luo, H.; Ramakrishna, S. Antimicrobial Peptides and Their Applications in Biomedical Sector. Antibiotics 2021, 10, 1094. [Google Scholar] [CrossRef]
- Shoemaker, D.M.; Simou, J.; Roland, W.E. A review of daptomycin for injection (Cubicin) in the treatment of complicated skin and skin structure infections. Ther. Clin. Risk Manag. 2006, 2, 169–174. [Google Scholar] [CrossRef]
- Velkov, T.; Roberts, K.D.; Nation, R.L.; Thompson, P.E.; Li, J. Pharmacology of polymyxins: New insights into an ‘old’ class of antibiotics. Future Microbiol. 2013, 8, 711–724. [Google Scholar] [CrossRef]
- Xellia Pharmaceuticals, Polymyxin B Vials. Available online: https://www.xellia.com/products/Polymyxin%20B%20vials/ (accessed on 2 April 2023).
- Mühlberg, E.; Umstätter, F.; Kleist, C.; Domhan, C.; Mier, W.; Uhl, P. Renaissance of vancomycin: Approaches for breaking antibiotic resistance in multidrug-resistant bacteria. Can. J. Microbiol. 2019, 66, 11–16. [Google Scholar] [CrossRef]
- Dowling, P.M. Peptide Antibiotics: Polymyxins, Glycopeptides, Bacitracin, and Fosfomycin. In Antimicrobial Therapy in Veterinary Medicine; Giguère, S., Prescott, J.F., Dowling, P.M., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 189–198. ISBN 978-0-470-96302-9. [Google Scholar]
- Benner, K.W.; Worthington, M.A.; Kimberlin, D.W.; Hill, K.; Buckley, K.; Tofil, N.M. Correlation of vancomycin dosing to serum concentrations in pediatric patients: A retrospective database review. J. Pediatr. Pharmacol. Ther. JPPT 2009, 14, 86–93. [Google Scholar] [CrossRef]
- Cammarota, G.; Masucci, L.; Ianiro, G.; Bibbo, S.; Dinoi, G.; Costamagna, G.; Gasbarrini, A. Randomised clinical trial: Faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment. Pharmacol. Ther. 2015, 41, 835–843. [Google Scholar] [CrossRef]
- Huvelle, S.; Godet, M.; Hecq, J.-D.; Gillet, P.; Jamart, J.; Galanti, L.M. Long-term Stability of Vancomycin Hydrochloride in Oral Solution: The Brand Name Versus a Generic Product. Int. J. Pharm. Compd. 2016, 20, 347–350. [Google Scholar] [PubMed]
- Stone, K.J.; Strominger, J.L. Mechanism of action of bacitracin: Complexation with metal ion and C 55 -isoprenyl pyrophosphate. Proc. Natl. Acad. Sci. USA 1971, 68, 3223–3227. [Google Scholar] [CrossRef] [PubMed]
- Spann, C.T.; Taylor, S.C.; Weinberg, J.M. Topical antimicrobial agents in dermatology. Dis. A Month. 2004, 50, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Ramdeen, S.; Boucher, H.W. Dalbavancin for the treatment of acute bacterial skin and skin structure infections. Expert Opin. Pharmacother. 2015, 16, 2073–2081. [Google Scholar] [CrossRef]
- Buzón-Martín, L.; Zollner-Schwetz, I.; Tobudic, S.; Cercenado, E.; Lora-Tamayo, J. Dalbavancin for the Treatment of Prosthetic Joint Infections: A Narrative Review. Antibiotics 2021, 10, 656. [Google Scholar] [CrossRef]
- Bouza, E.; Valerio, M.; Soriano, A.; Morata, L.; Carus, E.G.; Rodríguez-González, C.; Hidalgo-Tenorio, M.C.; Plata, A.; Muñoz, P.; Vena, A.; et al. Dalbavancin in the treatment of different gram-positive infections: A real-life experience. Int. J. Antimicrob. Agents 2018, 51, 571–577. [Google Scholar] [CrossRef]
- U.S. Securities and Exchange Commission. Amendment No. 1 to Form S-1 Registration Statement Under the Securities Act of 1933. Available online: https://www.sec.gov/Archives/edgar/data/1659323/000119312518152964/d522368ds1a.htm (accessed on 3 April 2023).
- Stogios, P.J.; Savchenko, A. Molecular mechanisms of vancomycin resistance. Protein Sci. 2020, 29, 654–669. [Google Scholar] [CrossRef]
- Lampejo, T. Dalbavancin and telavancin in the treatment of infective endocarditis: A literature review. Int. J. Antimicrob. Agents 2020, 56, 106072. [Google Scholar] [CrossRef]
- Medscape, Vibativ (Telavancin) Dosing, Indications, Interactions, Adverse Effects, and More. Available online: https://reference.medscape.com/drug/vibativ-telavancin-345210 (accessed on 3 April 2023).
- Zhang, H.; Zhou, W.; Wang, J.; Cai, Y. Efficacy and safety of oritavancin for the treatment of acute bacterial skin and skin-structure infections: A systematic review and meta-analysis. J. Glob. Antimicrob. Resist. 2021, 25, 380–389. [Google Scholar] [CrossRef]
- Arbeit, R.D.; Maki, D.; Tally, F.P.; Campanaro, E.; Eisenstein, B.I. Daptomycin 98-01 and 99-01 Investigators; The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin. Infect. Dis. 2004, 38, 1673–1681. [Google Scholar] [CrossRef]
- David, J.M.; Rajasekaran, A.K. Gramicidin A: A New Mission for an Old Antibiotic. J. Kidney Cancer VHL 2015, 2, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Mousavi Maleki, M.S.; Sardari, S.; Ghandehari Alavijeh, A.; Madanchi, H. Recent Patents and FDA-Approved Drugs Based on Antiviral Peptides and Other Peptide-Related Antivirals. Int. J. Pept. Res. Ther. 2023, 29, 5. [Google Scholar] [CrossRef]
- Kapić, E.; Becić, F.; Zvizdić, S. Enfuvirtid, mehanizam djelovanja i farmakoloske osobine [Enfuvirtide, mechanism of action and pharmacological properties]. Med. Arh. 2005, 59, 313–316. [Google Scholar] [PubMed]
- Pan, Q.; Peppelenbosch, M.P.; Janssen, H.L.; de Knegt, R.J. Telaprevir/boceprevir era: From bench to bed and back. World J. Gastroenterol. 2012, 18, 6183–6188. [Google Scholar] [CrossRef]
- Liscano, Y.; Oñate-Garzón, J.; Ocampo-Ibáñez, I.D. In Silico Discovery of Antimicrobial Peptides as an Alternative to Control SARS-CoV-2. Molecules 2020, 25, 5535. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Y.; Gao, S.; Cui, Y.; He, D.; Wang, L.; Chen, Y. Comparison on effect of hydrophobicity on the antibacterial and antifungal activities of α-helical antimicrobial peptides. Sci. China Chem. 2013, 56, 1307–1314. [Google Scholar] [CrossRef]
- Hu, C.; Chen, X.; Huang, Y.; Chen, Y. Co-administration of iRGD with peptide HPRP-A1 to improve anticancer activity and membrane penetrability. Sci. Rep. 2018, 2, 2274. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.E.F.; Najas, J.Z.G.; Magalhães, L.G.; Bobey, A.F.; Mendonça, J.N.; Lopes, N.P.; Leme, F.; Teixeira, S.P.; Trovó, M.; Andricopulo, A.D.; et al. Inhibition of Breast Cancer Cell Migration by Cyclotides Isolated from Pombalia calceolaria. J. Nat. Prod. 2018, 81, 1203–1208. [Google Scholar] [CrossRef]
- Rai, D.; Qian, S. Interaction of the Antimicrobial Peptide Aurein 1.2 and Charged Lipid Bilayer. Sci. Rep. 2017, 7, 3719. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Li, Y.; Lan, X.; Leung, P.; Li, J.; Li, G.; Xie, M.; Han, Y.; Lin, X. Composite Membranes of Recombinant Silkworm Antimicrobial Peptide and Poly (l-lactic Acid) (PLLA) for biomedical application. Sci. Rep. 2016, 6, 31149. [Google Scholar] [CrossRef]
- Baindara, P.; Gautam, A.; Raghava, G.; Korpole, S. Anticancer properties of a defensin like class IId bacteriocin Laterosporulin10. Sci. Rep. 2017, 7, srep46541. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Hou, X.; Wang, L.; Gao, Y.; Wu, D.; Xi, X.; Zhou, M.; Kwok, H.F.; Duan, J.; Chen, T.; et al. Two Novel Dermaseptin-Like Antimicrobial Peptides with Anticancer Activities from the Skin Secretion of Pachymedusa dacnicolor. Toxins 2016, 8, 144. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, I.-W.; Kim, S.-H.; Kim, M.-A.; Yun, E.-Y.; Nam, S.-H.; Ahn, M.-Y.; Kang, D.; Hwang, J.S. Anticancer Activity of the Antimicrobial Peptide Scolopendrasin VII Derived from the Centipede, Scolopendra subspinipes mutilans. J. Microbiol. Biotechnol. 2015, 25, 1275–1280. [Google Scholar] [CrossRef]
- Li, C.; Liu, H.; Yang, Y.; Xu, X.; Lv, T.; Zhang, H.; Liu, K.; Zhang, S.; Chen, Y. N-myristoylation of Antimicrobial Peptide CM4 Enhances Its Anticancer Activity by Interacting with Cell Membrane and Targeting Mitochondria in Breast Cancer Cells. Front. Pharmacol. 2018, 9, 1297. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.-L.; Yip, B.-S.; Chen, K.-H.; Yu, H.-Y.; Chih, Y.-H.; Cheng, H.-T.; Chou, Y.-T.; Cheng, J.-W. Novel Antimicrobial Peptides with High Anticancer Activity and Selectivity. PLoS ONE 2015, 10, e0126390. [Google Scholar] [CrossRef]
- Gaglione, R.; Pirone, L.; Farina, B.; Fusco, S.; Smaldone, G.; Aulitto, M.; Dell’Olmo, E.; Roscetto, E.; Del Gatto, A.; Fattorusso, R.; et al. Insights into the anticancer properties of the first antimicrobial peptide from Archaea. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 2155–2164. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, I.-W.; Kim, S.-H.; Yun, E.-Y.; Nam, S.-H.; Ahn, M.-Y.; Kang, D.-C.; Hwang, J.S. Anticancer activity of CopA3 dimer peptide in human gastric cancer cells. BMB Rep. 2015, 48, 324–329. [Google Scholar] [CrossRef]
- Han, Y.; Cui, Z.; Li, Y.-H.; Hsu, W.-H.; Lee, B.-H. In Vitro and in Vivo Anticancer Activity of Pardaxin against Proliferation and Growth of Oral Squamous Cell Carcinoma. Mar. Drugs 2015, 14, 2. [Google Scholar] [CrossRef]
- Ridyard, K.E.; Overhage, J. The Potential of Human Peptide LL-37 as an Antimicrobial and Anti-Biofilm Agent. Antibiotics 2021, 10, 650. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Sidorowicz, A.; Mikosinski, J.; Krzyżanowski, M.; Orleanski, J.; Twardowska-Saucha, K.; Nykaza, A.; Dyaczynski, M.; Belz-Lagoda, B.; Dziwiszek, G.; et al. Evaluation of LL-37 in healing of hard-to-heal venous leg ulcers: A multicentric prospective randomized placebo-controlled clinical trial. Wound Repair Regen. 2021, 29, 938–950. [Google Scholar] [CrossRef]
- Perez-Favila, A.; Martinez-Fierro, M.L.; Rodriguez-Lazalde, J.G.; Cid-Baez, M.A.; Zamudio-Osuna, J.; Martinez-Blanco, R.; Mollinedo-Montaño, F.E.; Rodriguez-Sanchez, I.P.; Castañeda-Miranda, R.; Garza-Veloz, I. Current Therapeutic Strategies in Diabetic Foot Ulcers. Medicina 2019, 55, 714. [Google Scholar] [CrossRef]
- Puar, N.; Chovatiya, R.; Paller, A.S. New treatments in atopic dermatitis. Ann. Allergy Asthma Immunol. 2021, 126, 21–31. [Google Scholar] [CrossRef]
- Niemeyer-van der Kolk, T.; van der Wall, H.; Hogendoorn, G.K.; Rijneveld, R.; Luijten, S.; van Alewijk, D.C.J.G.; van den Munckhof, E.H.A.; de Kam, M.L.; Feiss, G.L.; Prens, E.P.; et al. Pharmacodynamic Effects of Topical Omiganan in Patients With Mild to Moderate Atopic Dermatitis in a Randomized, Placebo-Controlled, Phase II Trial. Clin. Transl. Sci. 2020, 13, 994–1003. [Google Scholar] [CrossRef]
- Mitra, D.; Yadav, A.; Prithyani, S.; John, L.E.; Rodrigues, S.; Shah, R. The antiplaque efficacy of lantibiotic Nisin extract mouthrinse. Indian Soc. Periodontol. 2018, 23, 31–34. [Google Scholar] [CrossRef]
- Santos, R.; Ruza, D.; Cunha, E.; Tavares, L.; Oliveira, M. Diabetic foot infections: Application of a nisin-biogel to complement the activity of conventional antibiotics and antiseptics against Staphylococcus aureus biofilms. PLoS ONE 2019, 14, e0220000. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Li, M.; Fan, S. The clinical effect of Chitosan nanoparticles against Helicobacter pylori. Mater. Express 2020, 10, 1116–1121. [Google Scholar] [CrossRef]
- Lee, G.; Bae, H. Anti-Inflammatory Applications of Melittin, a Major Component of Bee Venom: Detailed Mechanism of Action and Adverse Effects. Molecules 2016, 21, 616. [Google Scholar] [CrossRef]
- Kwon, N.Y.; Sung, S.H.; Sung, H.K.; Park, J.K. Anticancer Activity of Bee Venom Components against Breast Cancer. Toxins 2022, 14, 460. [Google Scholar] [CrossRef]
- Khurshid, Z.; Najeeb, S.; Mali, M.; Moin, S.F.; Raza, S.Q.; Zohaib, S.; Sefat, F.; Zafar, M.S. Histatin peptides: Pharmacological functions and their applications in dentistry. Saudi Pharm. J. 2017, 25, 25–31. [Google Scholar] [CrossRef]
- Paquette, D.W.; Simpson, D.M.; Friden, P.; Braman, V.; Williams, R.C. Safety and clinical effects of topical histatin gels in humans with experimental gingivitis. J. Clin. Periodontol. 2002, 29, 1051–1058. [Google Scholar] [CrossRef]
- Wantstats Research and Media Private Limited, Global Obesity Treatment Market Overview. Available online: https://www.marketresearchfuture.com/reports/obesity-treatment-market-587 (accessed on 3 April 2023).
- Wilkins, L.J.; Monga, M.; Miller, A.W. Defining dysbiosis for a cluster of chronic diseases. Sci. Rep. 2019, 9, 12918. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Bell, C.M.; Matelski, J.J.; Detsky, A.S.; Cram, P. Payments by US pharmaceutical and medical device manufacturers to US medical journal editors: Retrospective observational study. BMJ Clin. Res. 2017, 359, j4619. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Neuzil, P.; Dukkipati, S.R.; Reddy, V.Y. Leadless cardiac pacemakers: Back to the future. J. Am. Coll. Cardiol. 2015, 66, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Ruben, R.B.; Fernandes, P.R.; Folgado, J. On the optimal shape of hip implants. J. Biomech. 2012, 45, 239–246. [Google Scholar] [CrossRef]
- Franco, P.; de Marco, I. Contact lenses as ophthalmic drug delivery systems: A review. Polymers 2021, 13, 1102. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Kanamori, H.; Gergen, M.F.; Knelson, L.P.; Sickbert-Bennett, E.E.; Chen, L.F.; Anderson, D.J.; Sexton, D.J.; Weber, D.J.; CDC Prevention Epicenters Program. Enhanced disinfection leads to reduction of microbial contamination and a decrease in patient colonization and infection. Infect. Control. Hosp. Epidemiol. 2018, 39, 1118–1121. [Google Scholar] [CrossRef]
- Caldara, M.; Belgiovine, C.; Secchi, E.; Rusconi, R. Environmental, Microbiological, and Immunological Features of Bacterial Biofilms Associated with Implanted Medical Devices. Clin. Microbiol. Rev. 2022, 35, e00221-20. [Google Scholar] [CrossRef]
- Muñoz, M.; Acheson, A.G.; Bisbe, E.; Butcher, A.; Gómez-Ramírez, S.; Khalafallah, A.; Kehlet, H.; Kietaibl, S.; Liumbruno, G.M.; Meybohm, P.; et al. An international consensus statement on the management of postoperative anaemia after major surgical procedures. Anaesthesia 2018, 73, 1418–1431. [Google Scholar] [CrossRef]
- Leahy, M.F.; Hofmann, A.; Towler, S.; Trentino, K.M.; Burrows, S.A.; Swain, S.G.; Hamdorf, J.; Gallagher, T.; Koay, A.; Geelhoed, G.C.; et al. Improved outcomes and reduced costs associated with a health-system–wide patient blood management program: A retrospective observational study in four major adult tertiary-care hospitals. Transfusion 2017, 57, 1347–1358. [Google Scholar] [CrossRef]
- Frieri, K.K.M.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef]
- Namivandi-Zangeneh, R.; Wong, E.H.; Boyer, C. Synthetic antimicrobial polymers in combination therapy: Tackling antibiotic resistance. ACS Infect. Dis. 2021, 7, 215–253. [Google Scholar] [CrossRef] [PubMed]
- Ong, G.; Kasi, R.; Subramaniam, R. A review on plant extracts as natural additives in coating applications. Prog. Org. Coat. 2021, 151, 106091. [Google Scholar] [CrossRef]
- Vaz, J.M.; Pezzoli, D.; Chevallier, P.; Campelo, C.S.; Candiani, G.; Mantovani, D. Antibacterial coatings based on chitosan for pharmaceutical and biomedical applications. Curr. Pharm. Des. 2018, 24, 866–885. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Tian, L.; Bing, W.; Zhao, J.; Ren, L. Bioinspired marine antifouling coatings: Status, prospects, and future. Prog. Mater. Sci. 2022, 124, 100889. [Google Scholar] [CrossRef]
- Nguyen, T.K.N.; Dierre, B.; Grasset, F.; Dumait, N.; Cordier, S.; Lemoine, P.; Renaud, A.; Fudouzi, H.; Ohashi, N.; Uchikoshi, T. Electrophoretic coating of octahedral molybdenum metal clusters for UV/NIR light screening. Coatings 2017, 7, 114. [Google Scholar] [CrossRef]
- Floroian, L.; Samoila, C.; Badea, M.; Munteanu, D.; Ristoscu, C.; Sima, F.; Negut, I.; Chifiriuc, M.C.; Mihailescu, I.N. Stainless steel surface biofunctionalization with PMMA-bioglass coatings: Compositional, electrochemical corrosion studies and microbiological assay. J. Mater. Sci Mater. Med. 2015, 26, 195. [Google Scholar] [CrossRef] [PubMed]
- Ramburrun, P.; Pringle, N.A.; Dube, A.; Adam, R.Z.; D’Souza, S.; Aucamp, M. Recent Advances in the Development of Antimicrobial and Antifouling Biocompatible Materials for Dental Applications. Materials 2021, 14, 3167. [Google Scholar] [CrossRef] [PubMed]
- Mateescu, M.; Baixe, S.; Garnier, T.; Jierry, L.; Ball, V.; Haikel, Y.; Metz-Boutigue, M.H.; Nardin, M.; Schaaf, P.; Etienne, O.; et al. Antibacterial Peptide-Based Gel for Prevention of Medical Implanted-Device Infection. PLoS ONE 2015, 10, e0145143. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Yue, K.; Kazemzadeh-Narbat, M.; Liu, Y.; Khalilpour, A.; Li, B.; Zhang, Y.S.; Annabi, N.; Khademhosseini, A. Mussel-Inspired Multifunctional Hydrogel Coating for Prevention of Infections and Enhanced Osteogenesis. ACS Appl. Mater. Interfaces 2017, 9, 11428–11439. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Sun, J.-L.; Huang, F.; Wang, J.; Wang, P.; Zheng, Y.-F.; Wang, F.; Ma, C.-F. Antibacterial Peptide HHC-36 Sustained-Release Coating Promotes Antibacterial Property of Percutaneous Implant. Front. Bioeng. Biotechnol. 2021, 9, 735889. [Google Scholar] [CrossRef]
- Li, T.; Wang, N.; Chen, S.; Lu, R.; Li, H.; Zhang, Z. Antibacterial activity and cytocompatibility of an implant coating consisting of TiO2 nanotubes combined with a GL13K antimicrobial peptide. Int. J. Nanomed. 2017, 12, 2995–3007. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, J.; Xue, Y.; Ding, T.; Zhu, S.; Mao, M.; Zhang, L.; Han, Y. Polymer brush grafted antimicrobial peptide on hydroxyapatite nanorods for highly effective antibacterial performance. Chem. Eng. J. 2021, 423, 130133. [Google Scholar] [CrossRef]
- Divakarla, S.K.; Das, T.; Chatterjee, C.; Ionescu, M.; Pastuovic, Z.; Jang, J.-H.; Al-Khoury, H.; Loppnow, H.; Yamaguchi, S.; Groth, T.; et al. Antimicrobial and Anti-inflammatory Gallium–Defensin Surface Coatings for Implantable Devices. ACS Appl. Mater. Interfaces 2022, 14, 9685–9696. [Google Scholar] [CrossRef] [PubMed]
- Zarghami, V.; Ghorbani, M.; Bagheri, K.P.; Shokrgozar, M.A. Melittin antimicrobial peptide thin layer on bone implant chitosan-antibiotic coatings and their bactericidal properties. Mater. Chem. Phys. 2021, 263, 124432. [Google Scholar] [CrossRef]
- He, Y.; Li, Y.; Zuo, E.; Chai, S.; Ren, X.; Fei, T.; Ma, G.; Wang, X.; Liu, H. A Novel Antibacterial Titanium Modification with a Sustained Release of Pac-525. Nanomaterials 2021, 11, 3306. [Google Scholar] [CrossRef]
- Mitra, A.K.; Cholkar, K.; Mandal, A. Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; William, Andrew: Norwich, NY, USA, 2017. [Google Scholar]
- Levien, M.; Farka, Z.; Pastucha, M.; Skládal, P.; Nasri, Z.; Weltmann, K.-D.; Fricke, K. Functional plasma-polymerized hydrogel coatings for electrochemical biosensing. Appl. Surf. Sci. 2022, 584, 152511. [Google Scholar] [CrossRef]
- Gherasim, O.; Grumezescu, A.; Grumezescu, V.; Negut, I.; Dumitrescu, M.; Stan, M.; Nica, I.; Holban, A.; Socol, G.; Andronescu, E. Bioactive Coatings Based on Hydroxyapatite, Kanamycin, and Growth Factor for Biofilm Modulation. Antibiotics 2021, 10, 160. [Google Scholar] [CrossRef]
- Gherasim, O.; Grumezescu, A.M.; Grumezescu, V.; Andronescu, E.; Negut, I.; Bîrcă, A.C.; Gălățeanu, B.; Hudiță, A. Bioactive Coatings Loaded with Osteogenic Protein for Metallic Implants. Polymers 2021, 13, 4303. [Google Scholar] [CrossRef]
- Chen, L.; Song, X.; Xing, F.; Wang, Y.; Wang, Y.; He, Z.; Sun, L. A Review on Antimicrobial Coatings for Biomaterial Implants and Medical Devices. J. Biomed. Nanotechnol. 2020, 16, 789–809. [Google Scholar] [CrossRef]
- Atriwal, T.; Azeem, K.; Husain, F.M.; Hussain, A.; Khan, M.N.; Alajmi, M.F.; Abid, M. Mechanistic Understanding of Candida albicans Biofilm Formation and Approaches for Its Inhibition. Front. Microbiol. 2021, 12, 932. [Google Scholar] [CrossRef]
- DeFlorio, W.; Liu, S.; White, A.R.; Taylor, T.M.; Cisneros-Zevallos, L.; Min, Y.; Scholar, E.M.A. Recent developments in antimicrobial and antifouling coatings to reduce or prevent contamination and cross-contamination of food contact surfaces by bacteria. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3093–3134. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Bita, B.; Groza, A. Polymeric Coatings and Antimicrobial Peptides as Efficient Systems for Treating Implantable Medical Devices Associated-Infections. Polymers 2022, 14, 1611. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.R.; Avelino, K.Y.; Ribeiro, K.L.; Franco, O.L.; Oliveira, M.D.; Andrade, C.A. Chemical immobilization of antimicrobial peptides on biomaterial surfaces. Front Biosci. 2016, 8, 129–142. [Google Scholar] [CrossRef]
- Costa, F.; Carvalho, I.F.; Montelaro, R.C.; Gomes, P.; Martins, M.C.L. Covalent immobilization of antimicrobial peptides (AMPs) onto biomaterial surfaces. Acta Biomater. 2011, 7, 1431–1440. [Google Scholar] [CrossRef]
- Cleophas, R.T.C.; Riool, M.; van Ufford, H.C.Q.; Zaat, S.A.J.; Kruijtzer, J.A.W.; Liskamp, R.M.J. Convenient Preparation of Bactericidal Hydrogels by Covalent Attachment of Stabilized Antimicrobial Peptides Using Thiolene Click Chemistry. ACS Macro Lett. 2014, 3, 477–480. [Google Scholar] [CrossRef]
- Gao, G.; Lange, D.; Hilpert, K.; Kindrachuk, J.; Zou, Y.; Cheng, J.T.J.; Kazemzadeh-Narbat, M.; Yu, K.; Wang, R.; Straus, S.K.; et al. The biocompatibility and biofilm resistance of implant coatings based on hydrophilic polymer brushes conjugated with antimicrobial peptides. Biomaterials 2011, 32, 3899–3909. [Google Scholar] [CrossRef]
- Gao, G.; Yu, K.; Kindrachuk, J.; Brooks, D.E.; Hancock, R.E.W.; Kizhakkedathu, J.N. Antibacterial Surfaces Based on Polymer Brushes: Investigation on the Influence of Brush Properties on Antimicrobial Peptide Immobilization and Antimicrobial Activity. Biomacromolecules 2011, 12, 3715–3727. [Google Scholar] [CrossRef]
- Lu, Q.; Ganesan, K.; Simionescu, D.T.; Vyavahare, N.R. Novel porous aortic elastin and collagen scaffolds for tissue engineering. Biomaterials 2004, 25, 5227–5237. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, X.C.; Liu, S.; Wu, R.F.; Aparicio, C.; Wu, J.Y. In vivo osseointegration of dental implants with an antimicrobial peptide coating. J. Mater. Sci. Mater. Med. 2017, 28, 76. [Google Scholar] [CrossRef]
- Hoyos-Nogués, M.; Velasco, F.; Ginebra, M.-P.; Manero, J.M.; Gil, F.J.; Mas-Moruno, C. Regenerating Bone via Multifunctional Coatings: The Blending of Cell Integration and Bacterial Inhibition Properties on the Surface of Biomaterials. ACS Appl. Mater. Interfaces 2017, 9, 21618–21630. [Google Scholar] [CrossRef]
- Nilebäck, L.; Hedin, J.; Widhe, M.; Floderus, L.S.; Krona, A.; Bysell, H.; Hedhammar, M. Self-Assembly of Recombinant Silk as a Strategy for Chemical-Free Formation of Bioactive Coatings: A Real-Time Study. Biomacromolecules 2017, 18, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Yuan, C.; Xiao, J.; He, X.; Bai, X. A biofilm resistance surface yielded by grafting of antimicrobial peptides on stainless steel surface. Surf. Interface Anal. 2018, 50, 516–521. [Google Scholar] [CrossRef]
- Yu, K.; Lo, J.C.Y.; Mei, Y.; Haney, E.F.; Siren, E.; Kalathottukaren, M.T.; Hancock, R.E.; Lange, D.; Kizhakkedathu, J.N. Toward Infection-Resistant Surfaces: Achieving High Antimicrobial Peptide Potency by Modulating the Functionality of Polymer Brush and Peptide. ACS Appl. Mater. Interfaces 2015, 7, 28591–28605. [Google Scholar] [CrossRef] [PubMed]
- Muszanska, A.K.; Rochford, E.T.J.; Gruszka, A.; Bastian, A.A.; Busscher, H.J.; Norde, W.; Van Der Mei, H.C.; Herrmann, A. Antiadhesive Polymer Brush Coating Functionalized with Antimicrobial and RGD Peptides to Reduce Biofilm Formation and Enhance Tissue Integration. Biomacromolecules 2014, 15, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.; Costa, F.; Pirttilä, A.M.; Tejesvi, M.V.; Martins, M.C.L. Prevention of urinary catheter-associated infections by coating antimicrobial peptides from crowberry endophytes. Sci. Rep. 2019, 9, 10753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Zhao, Y.-Q.; Zhang, Y.; Wang, A.; Ding, X.; Li, Y.; Duan, S.; Ding, X.; Xu, F.-J. Antimicrobial Peptide-Conjugated Hierarchical Antifouling Polymer Brushes for Functionalized Catheter Surfaces. Biomacromolecules 2019, 20, 4171–4179. [Google Scholar] [CrossRef]
- Acosta, S.; Ibañez-Fonseca, A.; Aparicio, C.; Rodríguez-Cabello, J.C. Antibiofilm coatings based on protein-engineered polymers and antimicrobial peptides for preventing implant-associated infections. Biomater. Sci. 2020, 8, 2866–2877. [Google Scholar] [CrossRef]
- Bhalani, D.V.; Bera, A.; Chandel, A.K.S.; Kumar, S.B.; Jewrajka, S.K. Multifunctionalization of Poly(vinylidene fluoride)/Reactive Copolymer Blend Membranes for Broad Spectrum Applications. ACS Appl. Mater. Interfaces 2017, 9, 3102–3112. [Google Scholar] [CrossRef]
- Pal, S.; Mondal, R.; Chandel, A.K.S.; Chatterjee, U. Composite Anion Exchange Membranes with Antibacterial Properties for Desalination and Fluoride Ion Removal. ACS EST Water 2021, 1, 2206–2216. [Google Scholar] [CrossRef]
- Salimi, A.; Noorbakhsh, A.; Mamkhezri, H.; Ghavami, R. Electrocatalytic Reduction of H2O2 and Oxygen on the Surface of Thionin Incorporated onto MWCNTs Modified Glassy Carbon Electrode: Application to Glucose Detection. Electroanalysis 2007, 19, 1100–1108. [Google Scholar] [CrossRef]
- Zhang, D.; Li, W.; Wang, H.; Ma, Z. A novel immunoprobe composed of reduced graphene oxide-hemin-thionin-Au nanohybrid for ultrasensitive detection of tumor marker. Sens. Actuators B Chem. 2018, 258, 141–147. [Google Scholar] [CrossRef]
- Schagen, S.K. Topical Peptide Treatments with Effective Anti-Aging Results. Cosmetics 2017, 4, 16. [Google Scholar] [CrossRef]
- Pai, V.V.; Bhandari, P.; Shukla, P. Topical peptides as cosmeceuticals. Indian J. Derm. Venereol. Leprol. 2017, 83, 9. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Anil, S.; Kim, S.K.; Shim, M.S. Marine Fish Proteins and Peptides for Cosmeceuticals: A Review. Mar. Drugs 2017, 15, 143. [Google Scholar] [CrossRef]
- Yoo, J.H.; Ho, S.; Tran, D.H.; Cheng, M.; Bakirtzi, K.; Kukota, Y.; Ichikawa, R.; Su, B.; Tran, D.H.; Hing, T.C.; et al. Anti-fibrogenic effects of the anti-microbial peptide cathelicidin in murine colitis-associated fibrosis. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 55–74. [Google Scholar] [CrossRef]
- Park, H.J.; Cho, D.H.; Kim, H.J.; Lee, J.Y.; Cho, B.K.; Bang, S.I.; Song, S.Y.; Yamasaki, K.; Di Nardo, A.; Gallo, R.L. Collagen synthesis is suppressed in dermal fibroblasts by the human antimicrobial peptide LL-37. J. Investig. Dermatol. 2009, 129, 843–850. [Google Scholar] [CrossRef]
- Sun, C.; Zhu, M.; Yang, Z.; Pan, X.; Zhang, Y.; Wang, Q.; Xiao, W. LL-37 secreted by epithelium promotes fibroblast collagen production: A potential mechanism of small airway remodeling in chronic obstructive pulmonary disease. Lab. Investig. 2014, 94, 991–1002. [Google Scholar] [CrossRef]
- Steinstraesser, L.; Hirsch, T.; Schulte, M.; Kueckelhaus, M.; Jacobsen, F.; Mersch, E.A.; Stricker, I.; Afacan, N.; Jenssen, H.; Hancock, R.E.; et al. Innate defense regulator peptide 1018 in wound healing and wound infection. PLoS ONE 2012, 7, e39373. [Google Scholar] [CrossRef]
- Kim, S.K. Marine cosmeceuticals. J. Cosmet. Dermatol. 2014, 13, 56–67. [Google Scholar] [CrossRef]
- Poindexter, B.J.; Bhat, S.; Buja, L.M.; Bick, R.J.; Milner, S.M. Localization of antimicrobial peptides in normal and burned skin. Burns 2006, 32, 402–407. [Google Scholar] [CrossRef]
- Supp, D.M.; Karpinski, A.C.; Boyce, S.T. Expression of human beta-defensins HBD-1, HBD-2, and HBD-3 in cultured keratinocytes and skin substitutes. Burns 2004, 30, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Oono, T.; Shirafuji, Y.; Huh, W.K.; Akiyama, H.; Iwatsuki, K. Effects of human neutrophil peptide-1 on the expression of interstitial collagenase and type I collagen in human dermal fibroblasts. Arch. Dermatol. Res. 2002, 294, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.M.C.; Dean, S.N.; Propst, C.N.; Bishop, B.M.; van Hoek, M.L. Komodo dragon-inspired synthetic peptide DRGN-1 promotes wound-healing of a mixed-biofilm infected wound. NPJ Biofilms Microbiomes 2017, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Guha, D.; Murmu, K.C.; Sur, A.; Ray, P.; Das, D.; Aich, P. Comparative efficacy analysis of anti-microbial peptides, LL-37 and indolicidin upon conjugation with CNT, in human monocytes. J Nanobiotechnology 2017, 15, 44. [Google Scholar] [CrossRef]
- Nan, Y.H.; Bang, J.K.; Shin, S.Y. Design of novel indolicidin-derived antimicrobial peptides with enhanced cell specificity and potent anti-inflammatory activity. Peptides 2009, 30, 832–838. [Google Scholar] [CrossRef]
- Dong, W.; Mao, X.; Guan, Y.; Kang, Y.; Shanget, D. Antimicrobial and anti-inflammatory activities of three chensinin-1 peptides containing mutation of glycine and histidine residues. Sci. Rep. 2017, 7, 40228. [Google Scholar] [CrossRef]
- Wu, J.; Mu, L.; Zhuang, L.; Han, Y.; Liu, T.; Li, J.; Yang, Y.; Yang, H.; Wei, L. A cecropin-like antimicrobial peptide with anti-inflammatory activity from the black fly salivary glands. Parasites Vectors 2015, 8, 561. [Google Scholar] [CrossRef]
- Brunetti, J.; Roscia, G.; Lampronti, I.; Gambari, R.; Quercini, L.; Falciani, C.; Bracci, L.; Pini, A. Immunomodulatory and Anti-inflammatory Activity in Vitro and in Vivo of a Novel Antimicrobial Candidate. J. Biol. Chem. 2016, 291, 25742–25748. [Google Scholar] [CrossRef]
- Rajasekaran, G.; Kamalakannan, R.; Shin, S.Y. Enhancement of the anti-inflammatory activity of temporin-1Tl-derived antimicrobial peptides by tryptophan, arginine and lysine substitutions. J. Pept. Sci. 2015, 21, 779–785. [Google Scholar] [CrossRef]
- Dhople, V.; Krukemeyer, A.; Ramamoorthy, A. The human beta-defensin-3, an antibacterial peptide with multiple biological functions. Biochim. Biophys. Acta 2006, 1758, 1499–1512. [Google Scholar] [CrossRef]
- Bayer, A.; Lammel, J.; Tohidnezhad, M.; Lippross, S.; Behrendt, P.; Klüter, T.; Pufe, T.; Cremer, J.; Jahr, H.; Rademacher, F.; et al. The Antimicrobial Peptide Human Beta-Defensin-3 Is Induced by Platelet-Released Growth Factors in Primary Keratinocytes. Mediat. Inflamm. 2017, 2017, 6157491. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Suh, J.S.; Kim, J.M.; Kim, J.H.; Park, H.J.; Park, Y.J.; Chung, C.P. Identification of a cell-penetrating peptide domain from human beta-defensin 3 and characterization of its anti-inflammatory activity. Int. J. Nanomed. 2015, 10, 5423–5434. [Google Scholar] [CrossRef]
- Jönsson, D.; Nilsson, B.O. The antimicrobial peptide LL-37 is anti-inflammatory and proapoptotic in human periodontal ligament cells. J. Periodontal Res. 2012, 47, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Kahlenberg, J.M.; Kaplan, M.J. Little peptide, big effects: The role of LL-37 in inflammation and autoimmune disease. J. Immunol. 2013, 191, 4895–4901. [Google Scholar] [CrossRef] [PubMed]
- Ostorhazi, E.; Voros, E.; Nemes-Nikodem, E.; Pinter, D.; Sillo, P.; Mayer, B.; Wade, J.D.; Otvos, L., Jr. Rapid systemic and local treatments with the antibacterial peptide dimer A3-APO and its monomeric metabolite eliminate bacteria and reduce inflammation in intradermal lesions infected with Propionibacterium acnes and meticillin-resistant Staphylococcus aureus. Int. J. Antimicrob. Agents. 2013, 42, 537–543. [Google Scholar] [CrossRef]
- Popovic, S.; Urbán, E.; Lukic, M.; Conlon, J.M. Peptides with antimicrobial and anti-inflammatory activities that have therapeutic potential for treatment of acne vulgaris. Peptides 2012, 34, 275–282. [Google Scholar] [CrossRef]
- Koczulla, R.; Bals, R. Cathelicidin antimicrobial peptides modulate angiogenesis. Ther. Neovascularization Quo Vadis 2003, 63, 191–196. [Google Scholar] [CrossRef]
- Pachón-Ibáñez, M.E.; Smani, Y.; Pachon, J.; Sanchez-Cespedes, J. Perspectives for clinical use of engineered human host defense antimicrobial peptides. FEMS Microbiol. Rev. 2017, 41, 323–342. [Google Scholar] [CrossRef]
- Mansour, S.C.; de la Fuente-Núñez, C.; Hancock, R.E. Peptide IDR-1018: Modulating the immune system and targeting bacterial biofilms to treat antibiotic-resistant bacterial infections. J. Pept. Sci. 2015, 21, 323–329. [Google Scholar] [CrossRef]
- Müller, C.A.; Markovic-Lipkovski, J.; Klatt, T.; Gamper, J.; Schwarz, G.; Beck, H.; Deeg, M.; Kalbacher, H.; Widmann, S.; Wessels, J.T.; et al. Human alpha-defensins HNPs-1, -2, and -3 in renal cell carcinoma: Influences on tumor cell proliferation. Am. J. Pathol. 2002, 160, 1311–1324. [Google Scholar] [CrossRef]
- Sørensen, O.E. Antimicrobial peptides in cutaneous wound healing. Antimicrob. Pept. Role Hum. Health Dis. 2016, 1–15. [Google Scholar] [CrossRef]
- Mangoni, M.L.; McDermott, A.M.; Zasloff, M. Antimicrobial peptides and wound healing: Biological and therapeutic considerations. Exp. Dermatol. 2016, 25, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Niyonsaba, F.; Ushio, H.; Nakano, N.; Ng, W.; Sayama, K.; Hashimoto, K.; Nagaoka, I.; Okumura, K.; Ogawa, H. Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J. Investig. Dermatol. 2007, 127, 594–604. [Google Scholar] [CrossRef]
- Kosikowska, P.; Pikula, M.; Langa, P.; Trzonkowski, P.; Obuchowski, M.; Lesner, A. Synthesis and Evaluation of Biological Activity of Antimicrobial--Pro-Proliferative Peptide Conjugates. PLoS ONE 2015, 10, e0140377. [Google Scholar] [CrossRef] [PubMed]
- Rakers, S.; Niklasson, L.; Steinhagen, D.; Kruse, C.; Schauber, J.; Sundell, K.; Paus, R. Antimicrobial peptides (AMPs) from fish epidermis: Perspectives for investigative dermatology. J. Investig. Dermatol. 2013, 133, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Gauglitz, G.G.; Bureik, D.; Zwicker, S.; Ruzicka, T.; Wolf, R. The antimicrobial peptides psoriasin (S100A7) and koebnerisin (S100A15) suppress extracellular matrix production and proliferation of human fibroblasts. Ski. Pharmacol. Physiol. 2015, 28, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Huggenberger, R.; Detmar, M. The cutaneous vascular system in chronic skin inflammation. J. Investig. Dermatology. Symp. Proc. 2011, 15, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, K.; Okumura, K.; Ogawa, H.; Niyonsaba, F. An antimicrobial peptide with angiogenic properties, AG-30/5C, activates human mast cells through the MAPK and NF-κB pathways. Immunol. Res. 2016, 64, 594–603. [Google Scholar] [CrossRef]
- Marcinkiewicz, M.; Majewski, S. The role of antimicrobial peptides in chronic inflammatory skin diseases. Postep. Dermatol. I Alergol. 2016, 33, 6–12. [Google Scholar] [CrossRef]
- Li, J.; Post, M.; Volk, R.; Gao, Y.; Li, M.; Metais, C.; Sato, K.; Tsai, J.; Aird, W.; Rosenberg, R.D.; et al. PR39, a peptide regulator of angiogenesis. Nat. Med. 2000, 6, 49–55. [Google Scholar] [CrossRef]
- Kurosaka, K.; Chen, Q.; Yarovinsky, F.; Oppenheim, J.J.; Yang, D. Mouse cathelin-related antimicrobial peptide chemoattracts leukocytes using formyl peptide receptor-like 1/mouse formyl peptide receptor-like 2 as the receptor and acts as an immune adjuvant. J. Immunol. 2005, 174, 6257–6265. [Google Scholar] [CrossRef] [PubMed]
- Marin-Luevano, P.; Trujillo, V.; Rodriguez-Carlos, A.; González-Curiel, I.; Enciso-Moreno, J.A.; Hancock, R.E.W.; Rivas-Santiago, B. Induction by innate defence regulator peptide 1018 of pro-angiogenic molecules and endothelial cell migration in a high glucose environment. Peptides 2018, 101, 135–144. [Google Scholar] [CrossRef]
- Huang, H.C.; Lin, H.; Huang, M.C. The lactoferricin B-derived peptide, LfB17-34, induces melanogenesis in B16F10 cells. Int. J. Mol. Med. 2017, 39, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L.; Weisberg, E.; Percival, S.L. Skin aging and microbiology. In Microbiology and Aging: Clinical Manifestations; Janaway, R.C., Percival, S.L., Wilson, A.S., Eds.; Humana Press: Totowa, NJ, USA, 2009; pp. 57–94. [Google Scholar] [CrossRef]
- Chandika, P.; Ko, S.C.; Jung, W.K. Marine-derived biological macromolecule-based biomaterials for wound healing and skin tissue regeneration. Int. J. Biol. Macromol. 2015, 77, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H. Marine Peptides: Bioactivities and Applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.; Me, L.C.; Aleman, A.; López de Lacey, A.; Giménez, B.; Montero, P. Antioxidant and antimicrobial peptide fractions from squid and tuna skin gelatin. In Sea By-Products as Real Material: New Ways of Application; Le Bihan, E., Ed.; Transworld Research Network: Trivandrum, India, 2010; pp. 89–115. [Google Scholar]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive peptides from marine processing waste and shellfish: A review. J. Funct. Foods 2012, 4, 6–24. [Google Scholar] [CrossRef]
- Najafian, L.; Babji, A.S. A review of fish-derived antioxidant and antimicrobial peptides: Their production, assessment, and applications. Peptides 2012, 33, 178–185. [Google Scholar] [CrossRef]
- Borquaye, L.S.; Darko, G.; Ocansey, E.; Ankomah, E. Antimicrobial and antioxidant properties of the crude peptide extracts of Galatea paradoxa and Patella rustica. Springerplus 2015, 4, 500. [Google Scholar] [CrossRef]
- Guo, C.; Hu, Y.; Li, J.; Liu, Y.; Li, S.; Yan, K.; Wang, X.; Liu, J.; Wang, H. Identification of multiple peptides with antioxidant and antimicrobial activities from skin and its secretions of Hylarana taipehensis, Amolops lifanensis, and Amolops granulosus. Biochimie 2014, 105, 192–201. [Google Scholar] [CrossRef]
- Alexander, T.C.; Ghosh, R.; Majumdar, A.; Mathapathi, M.S.; Thimmaiah, S.; Waskar, M. Hydroxystearic Acid for Inducing Generation of Antimicrobial Peptides. WO/2022/117404, 9 June 2022. [Google Scholar]
- Picherau, C.; Allary, C. Therapeutic peptides under the spotlight. Eur. Biopharm. Rev. 2005, 5, 88–91. [Google Scholar]
- Kim, D.S.; Kim, S.W.; Song, J.M.; Kim, S.Y.; Kwon, K.C. A new prokaryotic expression vector for the expression of antimicrobial peptide abaecin using SUMO fusion tag. BMC Biotechnol. 2019, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Zhang, S.Q.; Li, B.C.; Qiu, W.; Jiao, B.; Zhang, J.; Diao, Z.Y. Expression of a cytotoxic cationic antibacterial peptide in Escherichia coli using two fusion partners. Protein Expr. Purif. 2008, 57, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.H.; Pei, C.; Yeh, J.Y.; Shih, C.H.; Chung, Y.C.; Hung, L.T.; Ou, B.R. Production of bioactive human alpha-defensin 5 in Pichia pastoris. J. Gen. Appl. Microbiol. 2009, 55, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Carrier proteins for fusion expression of antimicrobial peptides in Escherichia coli. Biotechnol. Appl. Biochem. 2009, 54, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Piers, K.L.; Brown, M.H.; Hancock, R.E. Recombinant DNA procedures for producing small antimicrobial cationic peptides in bacteria. Gene 1993, 134, 7–13. [Google Scholar] [CrossRef]
- Zhang, L.; Falla, T.; Wu, M.; Fidai, S.; Burian, J.; Kay, W.; Hancock, R.E.W. Determinants of recombinant production of antimicrobial cationic peptides and creation of peptide variants in bacteria. Biochem. Biophys. Res. Commun. 1998, 247, 674–680. [Google Scholar] [CrossRef]
- Luiz, D.P.; Almeida, J.F.; Goulart, L.R.; Nicolau-Junior, N.; Ueira-Vieira, C. Heterologous expression of abaecin peptide from Apis mellifera in Pichia pastoris. Microb. Cell. Factories 2017, 16, 76. [Google Scholar] [CrossRef]
- Li, L.; Mu, L.; Wang, X.; Yu, J.; Hu, R.; Li, Z. A novel expression vector for the secretion of abaecin in Bacillus subtilis. Braz. J. Microbiol. 2017, 48, 809–814. [Google Scholar] [CrossRef]
- Ingham, A.B.; Moore, R.J. Recombinant production of antimicrobial peptides in heterologous microbial systems. Biotechnol. Appl. Biochem. 2007, 47, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z. RAPD: A database of recombinantly-produced antimicrobial peptides. FEMS Microbiol. Lett. 2008, 289, 126–129. [Google Scholar] [CrossRef]
- Zhou, Y.; Cao, W.; Wang, J.; Ma, Y.; Wei, D. Comparison of expression of monomeric and multimeric adenoregulin genes in Escherichia coli and Pichia pastorias. Protein Pept. Lett. 2005, 12, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Hong, I.P.; Lee, S.J.; Kim, Y.S.; Choi, S.G. Recombinant expression of human cathelicidin (hCAP18/LL-37) in Pichia pastoris. Biotechnol. Lett. 2007, 29, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Li, B.; Jin, S.; Daniell, H. Expression and characterization of antimicrobial peptides Retrocyclin-101 and Protegrin-1 in chloroplasts to control viral and bacterial infections. Plant Biotechnol. J. 2011, 9, 100–115. [Google Scholar] [CrossRef]
- Liu, Y.; Kamesh, A.C.; Xiao, Y.; Sun, V.; Hayes, M.; Daniell, H.; Koo, H. Topical delivery of low-cost protein drug candidates made in chloroplasts for biofilm disruption and uptake by oral epithelial cells. Biomaterials 2016, 105, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Kotian, A.; Subramanian, H.; Daniell, H.; Ali, H. Activation of human mast cells by retrocyclin and protegrin highlight their immunomodulatory and antimicrobial properties. Oncotarget 2015, 6, 28573–28587. [Google Scholar] [CrossRef]
- Kwon, K.C.; Daniel, H. Oral Delivery of Protein Drugs Bioencapsulated in Plant Cells. Mol. Ther. 2016, 24, 1342–1350. [Google Scholar] [CrossRef]
- Xiao, Y.; Kwon, K.C.; Hoffman, B.E.; Kamesh, A.; Jones, N.T.; Herzog, R.W.; Daniell, H. Low cost delivery of proteins bioencapsulated in plant cells to human non-immune or immune modulatory cells. Biomaterials 2016, 80, 68–79. [Google Scholar] [CrossRef]
- Jin, S.; Daniell, H. The Engineered Chloroplast Genome Just Got Smarter. Trends Plant Sci. 2015, 20, 622–640. [Google Scholar] [CrossRef]
- Kwon, K.C.; Daniell, H. Low-cost oral delivery of protein drugs bioencapsulated in plant cells. Plant Biotechnol. J. 2015, 13, 1017–1022. [Google Scholar] [CrossRef]
- Verma, D.; Daniell, H. Chloroplast vector systems for biotechnology applications. Plant Physiol. 2007, 145, 1129–1143. [Google Scholar] [CrossRef]
- Dhingra, A.; Daniell, H. Chloroplast genetic engineering via organogenesis or somatic embryogenesis. Methods Mol. Biol. 2006, 323, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research, Peptide Antibiotics Market Report. Available online: https://www.grandviewresearch.com/industry-analysis/peptide-antibiotics-market-report (accessed on 5 April 2023).
- GlobeNewsWire, Antimicrobial Peptides Market Worth USD 539.32 million by 2027 Growth, Size, Shares, Revenue, Types, Applications, Key Players, Top Countries, Growing Factors, Key Dynamics. Available online: https://www.globenewswire.com/news-release/2022/08/18/2500859/0/en/Antimicrobial-Peptides-Market-Worth-USD-539-32-million-by-2027-Growth-Size-Shares-Revenue-Types-Applications-Key-Players-Top-Countries-Growing-Factors-Key-Dynamics.html (accessed on 5 April 2023).
- Data Bridge Market Research, Global Antimicrobial Peptides Market. Available online: https://www.databridgemarketresearch.com/reports/global-antimicrobial-peptides-market (accessed on 5 April 2023).
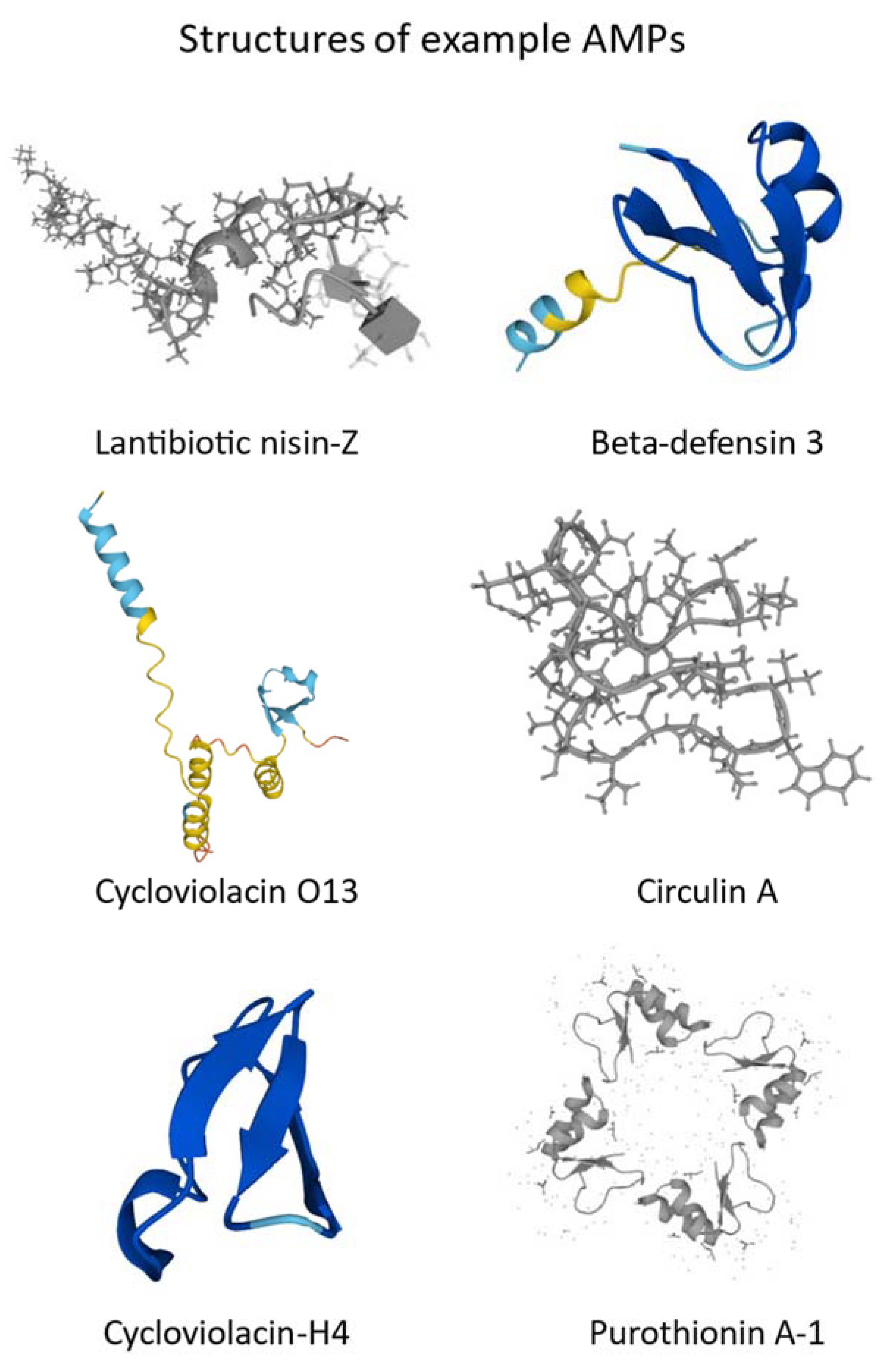
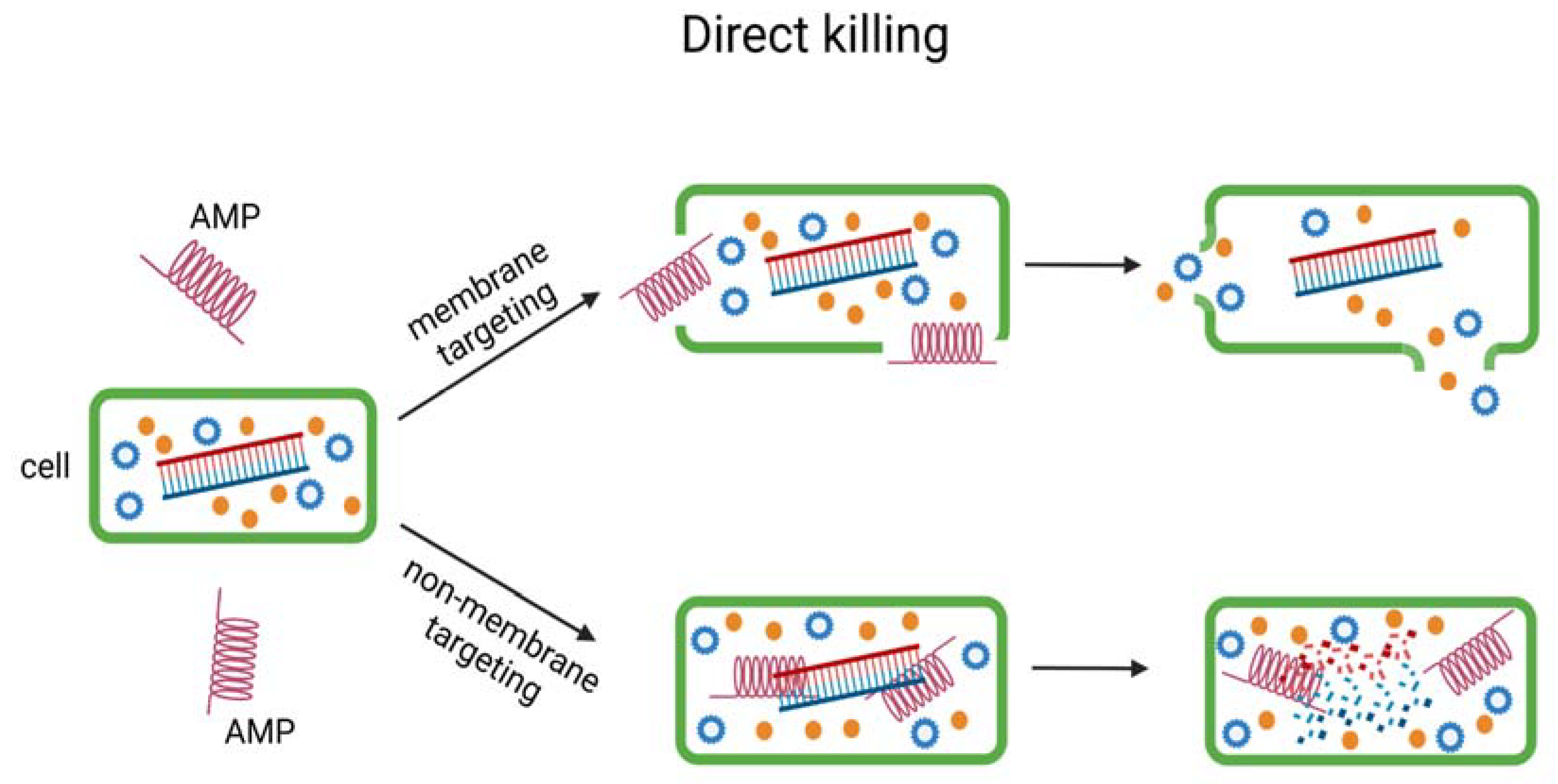
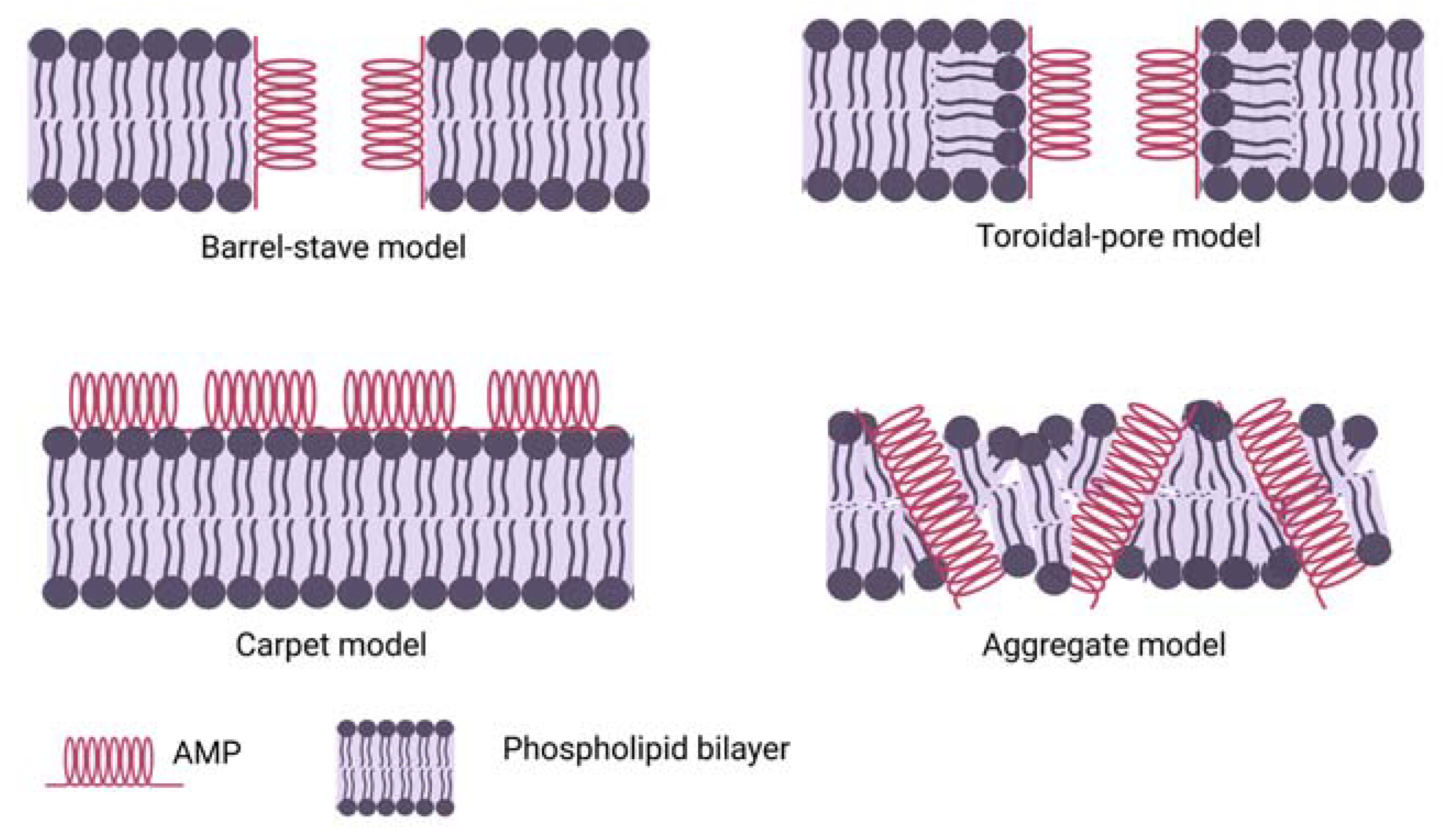
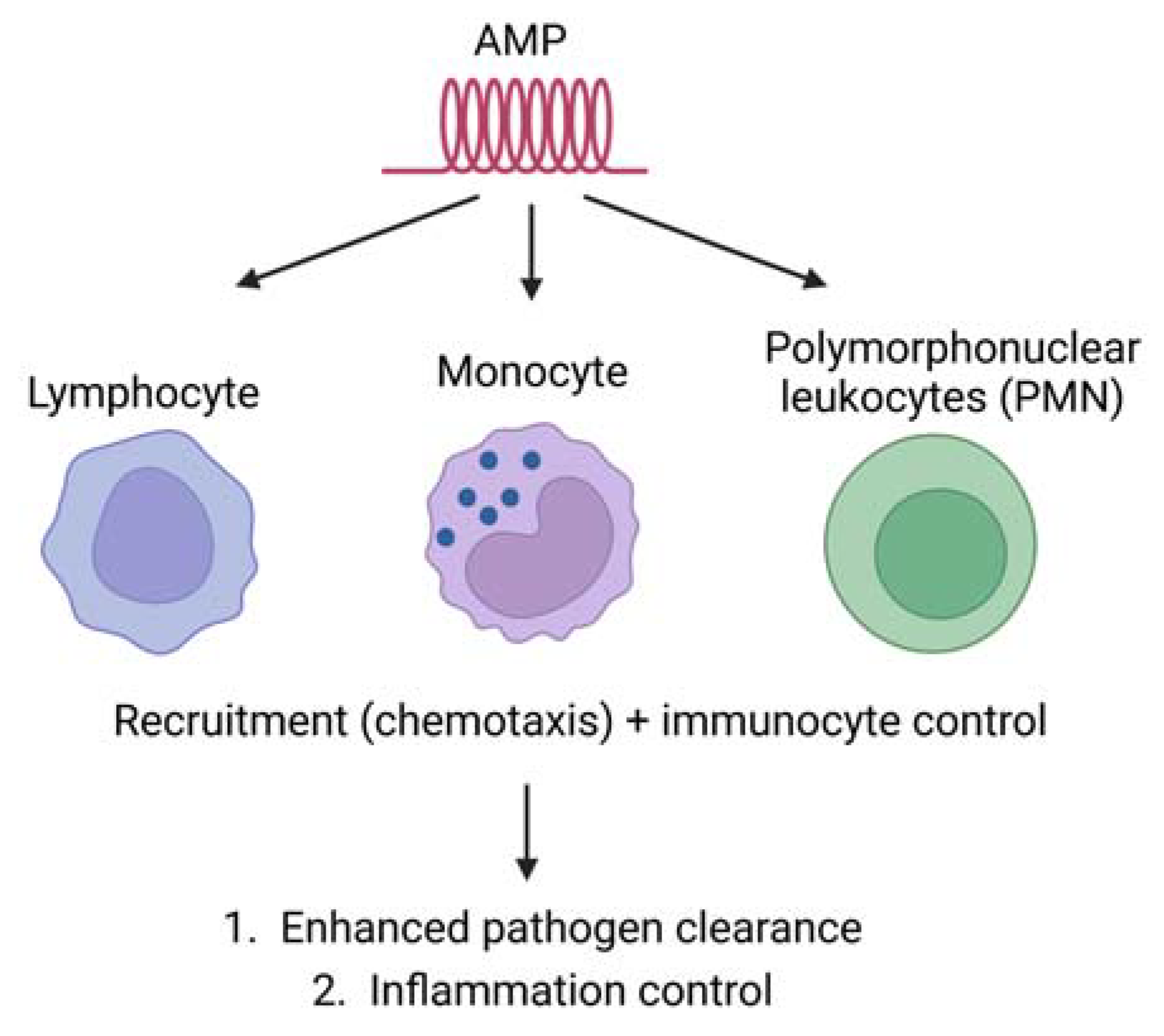

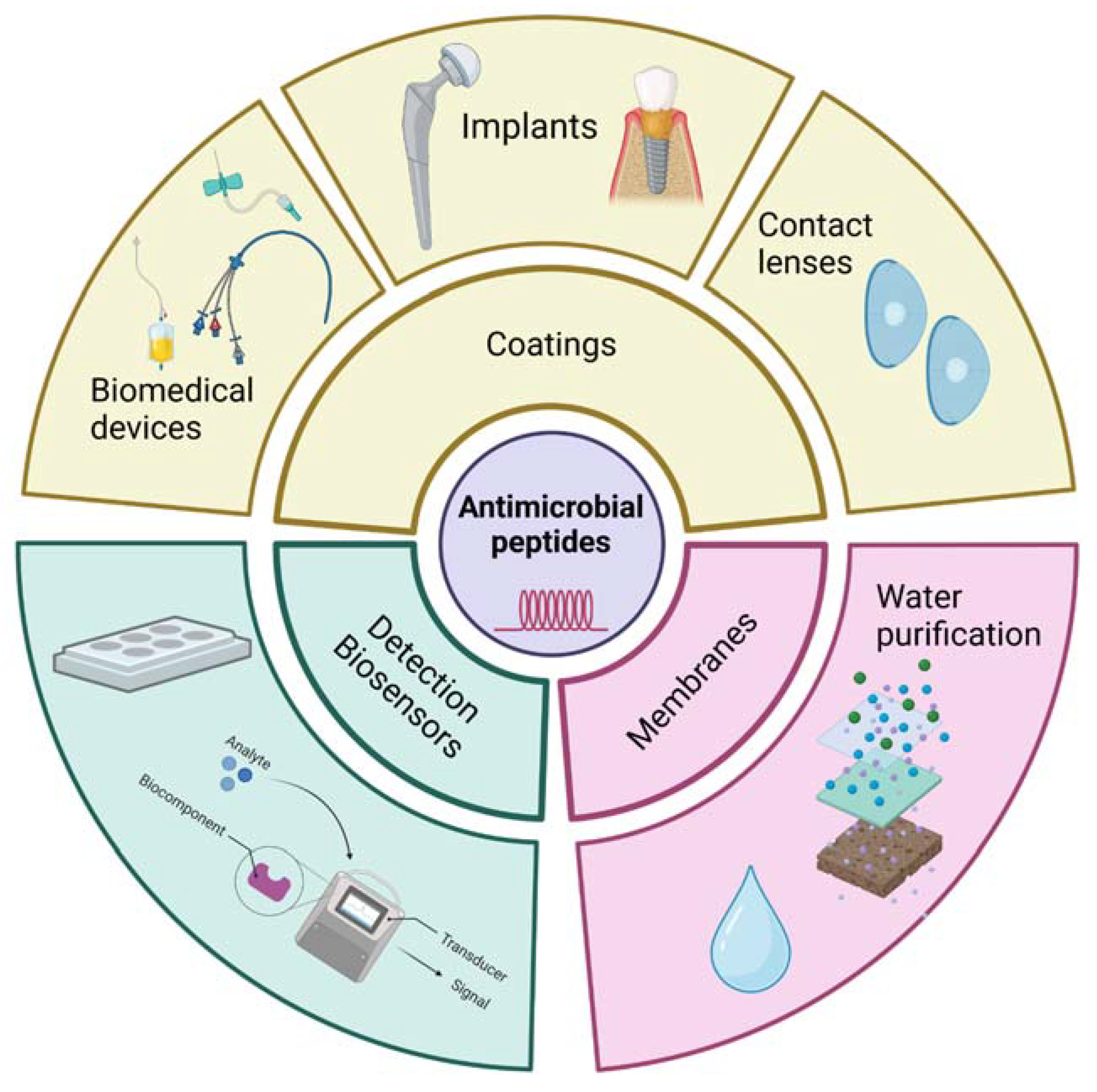
| Cat. | AMP | Structure | Source | Ref. |
|---|---|---|---|---|
| α-helical peptides | Aurerin 1–2 | Amidated C-terminus | Frogs | [30,31] |
| Melittin | Amidated C-terminus | Bees | [32] | |
| Brevinin 1 | - | Frogs | [33] | |
| Maculatins | Amidated C-terminus | Frogs | [34] | |
| Citropin | Amidated C-terminus | Frogs | [35] | |
| Buforin II | - | Toad | [36] | |
| Cathelicidins | [37] | |||
| (LL-37 | Amidated C-terminus | Human | ||
| BMAP-27,28,34 | - | Bovine | ||
| Magainins | - | Frogs | ||
| Cecropin) | Amidated C-terminus | Insect | ||
| β-sheet peptides | Cathelicidins (Protegrins Bactenecin) Defensins | Cysteine-rich Disulphide forming loop | Pigs Bovine Mammals | [37] |
| Tachyplesins and Polyphemusin | Three disulphide bonds Cysteine/arginine-rich and amidated C-terminus | Horse Crab | [32,38,39,40] | |
| Horse Crab | [41] | |||
| Extended structure | Cathelicidins (PR-39 Tritrpticin Indolicidin Crotalicidin 15–34) | Proline and arginine-rich Tryptophan and arginine-rich Tryptophan and amidated C-terminus Lysine rich | Pigs Pigs Bovine Snakes Humans | [37] |
| Histatins | Histidine-rich and amidated C-terminus | [42] |
| AMPs | Source | Significance | Ref. |
|---|---|---|---|
| Poca A, Poca B and CyO4 | Pombalia calceolaria | Reduced the breast cancer cell up to 80% | [185] |
| Aurein 1.2 | Frog Litoria aurea | Among 54 cancer cells, 52 are inhibited in NCI testing method | [186] |
| Bmattacin2 | Bombyx mori | Disrupted A375 and HCT116 cancer cells | [187] |
| Laterosporulin10 | Brevibacillus sp. | MCF-7, H1299, HEK293T, HT1080, and HeLa cancer cells were disrupted | [188] |
| Dermaseptin-PD-1 and dermaseptin-PD-2 | Phyllomedusine leaf frogs | Growth of H157, PC-3, and U251 MG cancer cell was inhibited | [189] |
| Scolopendrasin VII | Centipede | Reduction in the viability of leukaemia cells | [190] |
| Myristoyl-CM4 | Synthetic | Activates caspase 9, caspase 3, and cleavage of Poly(ADP-ribose) polymerase (PARP) in breast cancer cells | [191] |
| K4R2-Nal2-S1 | Synthetic | Binds with lung cancer cells and results in apoptosis | [192] |
| VLL-28 | Sulfolobus islandicus | Inhibits murine and human tumour cells | [193] |
| CopA3 | Copris tripartitus | Reduction in cell viability of gastric cancer cells | [194] |
| Pardaxin | Pardachirus armoratus | Improved the activation of caspase-3 | [195] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazurkiewicz-Pisarek, A.; Baran, J.; Ciach, T. Antimicrobial Peptides: Challenging Journey to the Pharmaceutical, Biomedical, and Cosmeceutical Use. Int. J. Mol. Sci. 2023, 24, 9031. https://doi.org/10.3390/ijms24109031
Mazurkiewicz-Pisarek A, Baran J, Ciach T. Antimicrobial Peptides: Challenging Journey to the Pharmaceutical, Biomedical, and Cosmeceutical Use. International Journal of Molecular Sciences. 2023; 24(10):9031. https://doi.org/10.3390/ijms24109031
Chicago/Turabian StyleMazurkiewicz-Pisarek, Anna, Joanna Baran, and Tomasz Ciach. 2023. "Antimicrobial Peptides: Challenging Journey to the Pharmaceutical, Biomedical, and Cosmeceutical Use" International Journal of Molecular Sciences 24, no. 10: 9031. https://doi.org/10.3390/ijms24109031
APA StyleMazurkiewicz-Pisarek, A., Baran, J., & Ciach, T. (2023). Antimicrobial Peptides: Challenging Journey to the Pharmaceutical, Biomedical, and Cosmeceutical Use. International Journal of Molecular Sciences, 24(10), 9031. https://doi.org/10.3390/ijms24109031





