Novel GPR18 Ligands in Rodent Pharmacological Tests: Effects on Mood, Pain, and Eating Disorders
Abstract
1. Introduction
2. Results
2.1. Locomotor Activity
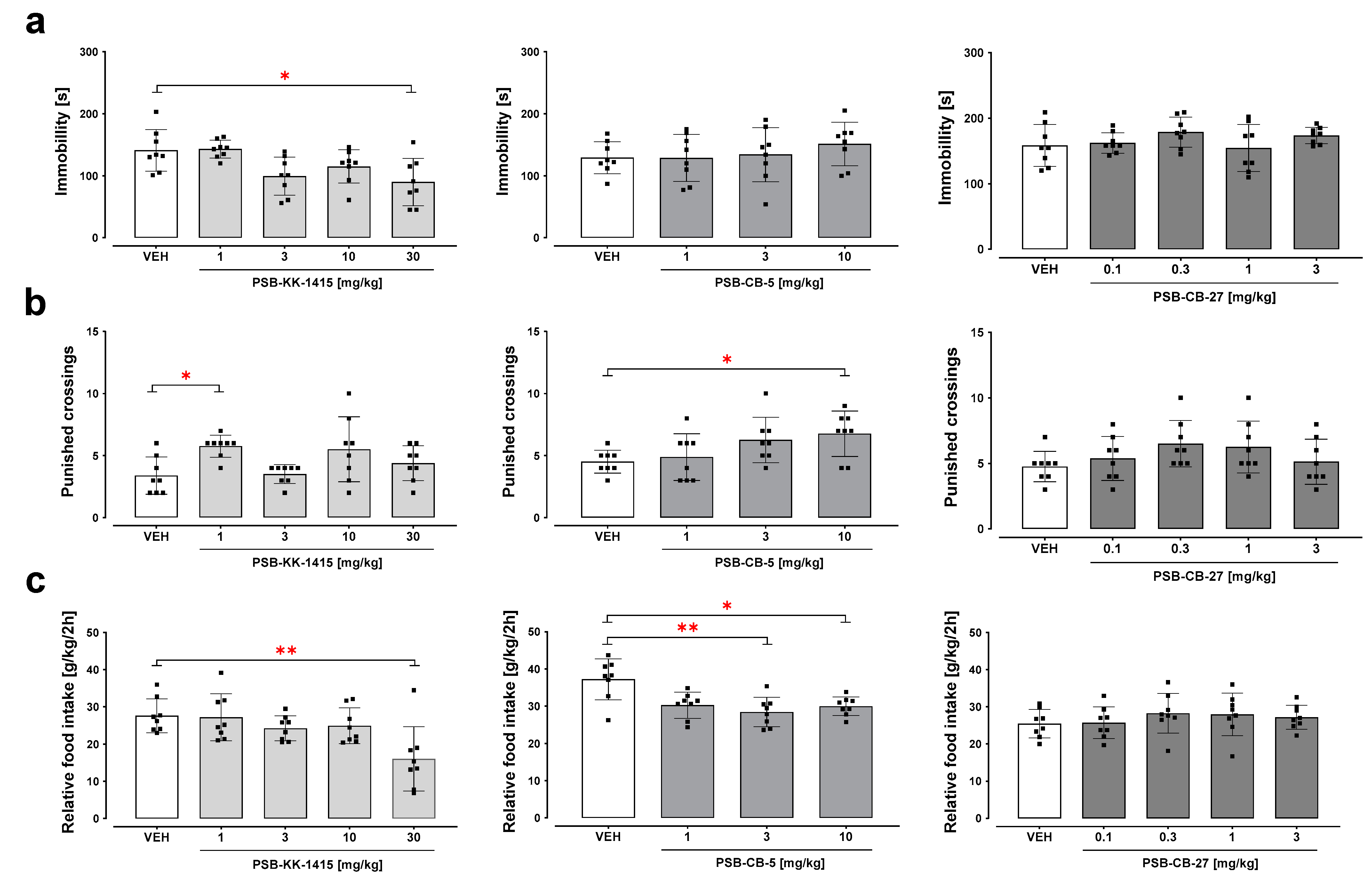
2.2. Forced Swim Test
2.3. Four-Plate Test
2.4. Food Intake
2.5. Hot Plate Test (Acute Pain)
2.6. Oxaliplatin-Induced Neuropathic Pain (Chronic, Neuropathic Pain Model)
2.6.1. Effect on the Mechanical Nociceptive Threshold in Oxaliplatin-Treated Mice
2.6.2. Effect on the Thermal (Cold) Nociceptive Threshold in Oxaliplatin-Treated Mice
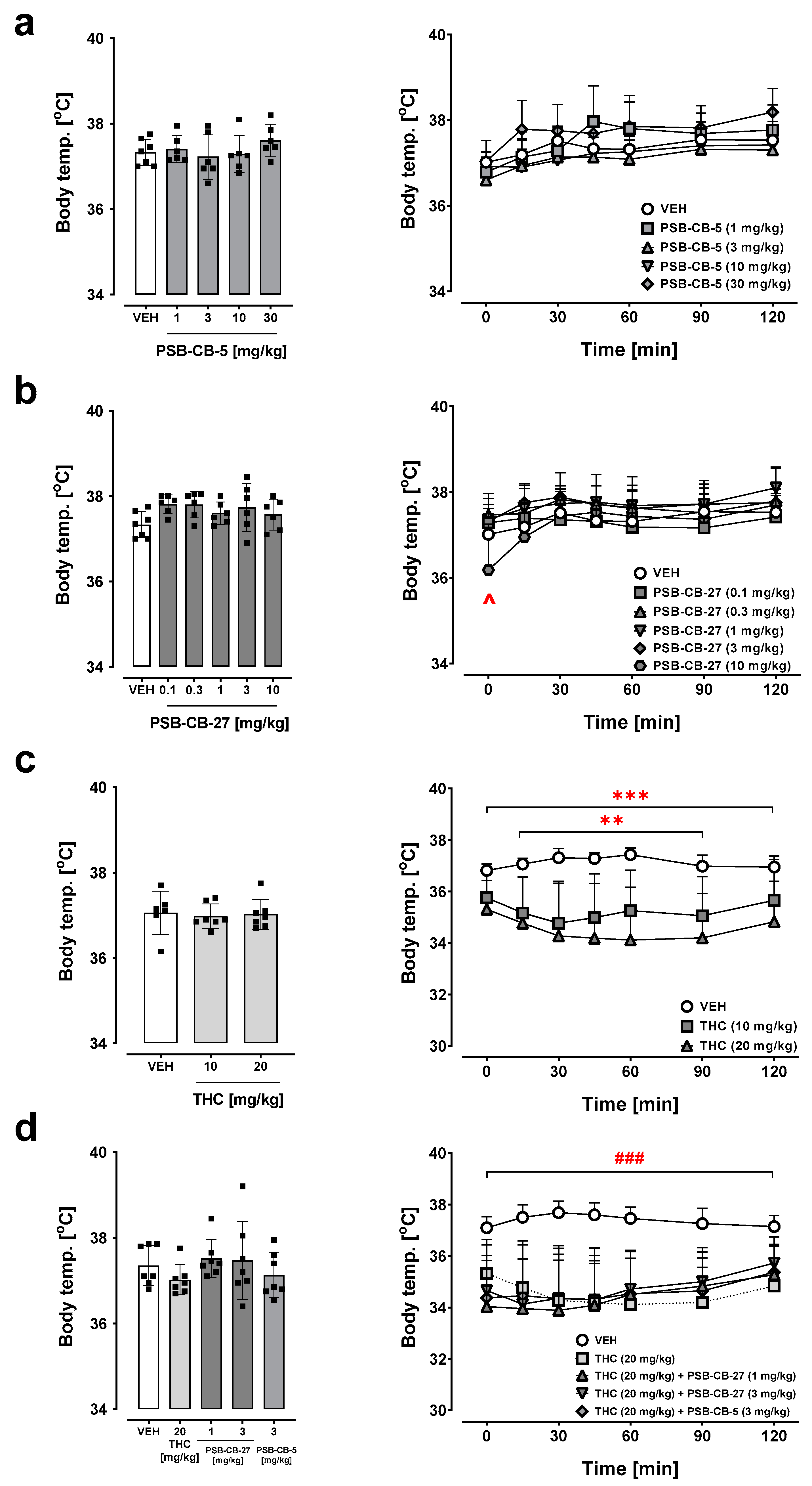
2.7. Body Temperature
2.7.1. Effects of PSB-CB-5 and PSB-CB-27
2.7.2. Combination Studies of PSB-CB-5 or PSB-CB-27 and THC
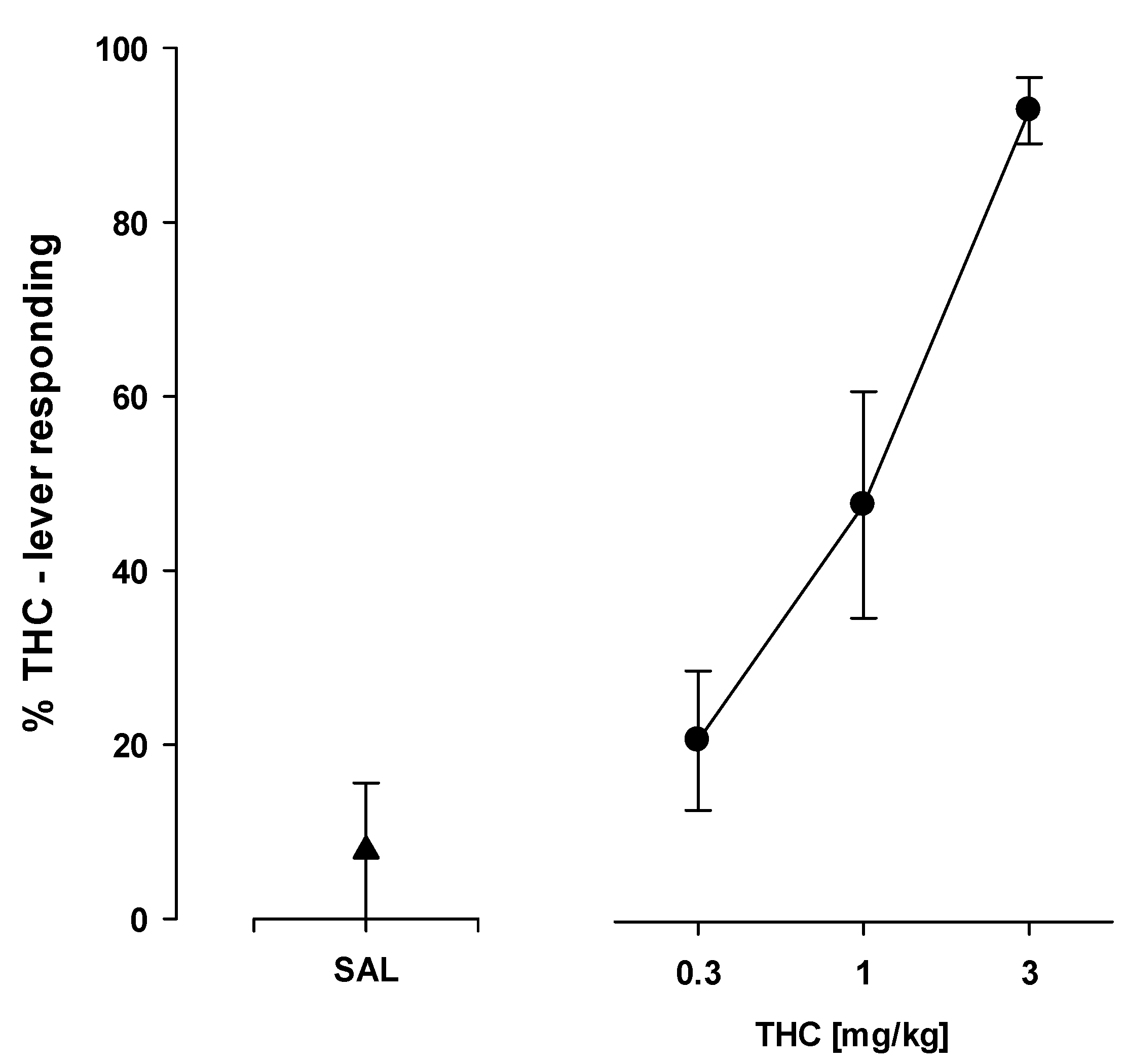
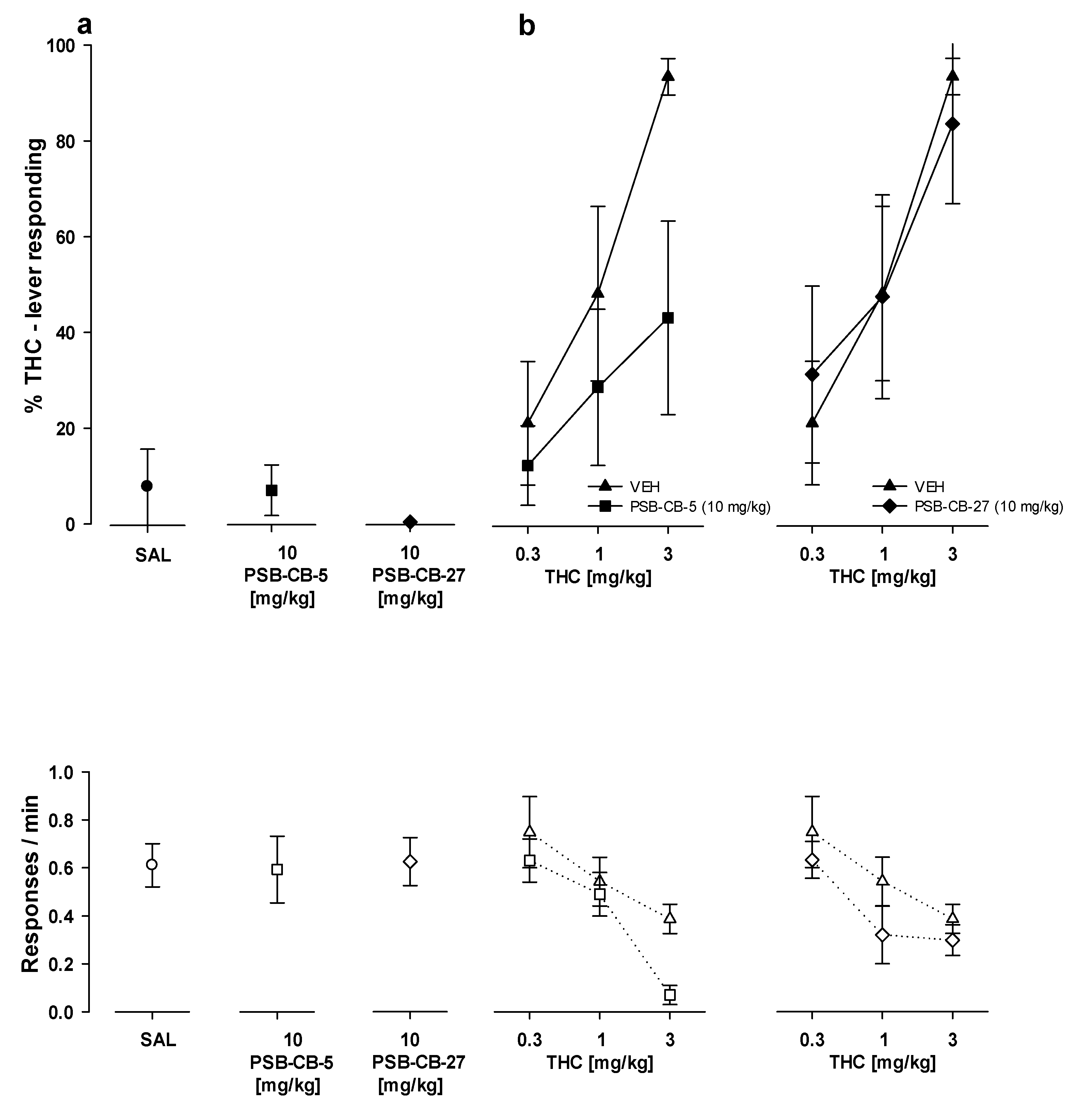
2.8. Drug Discrimination
2.8.1. THC—Saline Discrimination
2.8.2. Substitution Studies
2.8.3. Combination Studies
3. Discussion
4. Materials and Methods
4.1. Drugs, Chemical Reagents, and Other Materials
4.2. Animals
4.3. Locomotor Activity
4.4. Forced Swim Test
4.5. Four-Plate Test
4.6. Hot Plate Test
4.7. Food Intake
4.8. Oxaliplatin-Induced Neuropathic Pain
4.8.1. Induction of Oxaliplatin-Induced Peripheral Neuropathy
4.8.2. Assessment of Mechanical Nociceptive Threshold—Von Frey Test
4.8.3. Assessment of Thermal (Cold) Nociceptive Threshold—Cold Plate Test
4.9. Body Temperature
4.10. Drug Discrimination
4.11. Data Analysis and Statistical Procedures
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Latek, D.; Modzelewska, A.; Trzaskowski, B.; Palczewski, K.; Filipek, S. G protein-coupled receptors—Recent advances. Acta Biochim. Pol. 2012, 59, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnan, A.J.; Deupi, X.; Lebon, G.; Tate, C.G.; Schertler, G.F.; Babu, M.M. Molecular signatures of G-protein-coupled receptors. Nature 2013, 494, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Lago-Fernandez, A.; Hurst, D.P.; Sotudeh, N.; Brailoiu, E.; Reggio, P.H.; Abood, M.E.; Jagerovic, N. Therapeutic exploitation of GPR18: Beyond the cannabinoids? J. Med. Chem. 2020, 63, 14216–14227. [Google Scholar] [CrossRef] [PubMed]
- Finlay, D.B.; Joseph, W.R.; Grimsey, N.L.; Glass, M. GPR18 undergoes a high degree of constitutive trafficking but is unresponsive to N-Arachidonoyl Glycine. Peer J. 2016, 4, e1835. [Google Scholar] [CrossRef] [PubMed]
- Schoeder, C.T.; Mahardhika, A.B.; Drabczyńska, A.; Kieć-Kononowicz, K.; Müller, C.E. Discovery of Tricyclic Xanthines as Agonists of the Cannabinoid-Activated Orphan G-Protein-Coupled Receptor GPR18. ACS Med. Chem. Lett. 2020, 11, 2024–2031. [Google Scholar] [CrossRef]
- McHugh, D. GPR18 in microglia: Implications for the CNS and endocannabinoid system signalling. Br. J. Pharmacol. 2012, 167, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Page, J.; Dunn, E.; Bradshaw, H.B. Δ9-Tetrahydrocannabinol and N-arachidonyl glycine are full agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells. Br. J. Pharmacol. 2012, 165, 2414–2424. [Google Scholar] [CrossRef] [PubMed]
- Flegel, C.; Vogel, F.; Hofreuter, A.; Wojcik, S.; Schoeder, C.; Kieć-Kononowicz, K.; Brockmeyer, N.H.; Müller, C.E.; Becker, C.; Altmüller, J.; et al. Characterization of non-olfactory GPCRs in human sperm with a focus on GPR18. Sci. Rep. 2016, 6, 32255. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Cao, Z.; Wang, W.; Zhou, N. New Insights in Cannabinoid Receptor Structure and Signaling. Curr. Mol. Pharmacol. 2019, 12, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P. So what do we call GPR18 now? Br. J. Pharmacol. 2012, 165, 2411–2413. [Google Scholar] [CrossRef]
- Console-Bram, L.; Marcu, J.; Abood, M.E. Cannabinoid receptors: Nomenclature and pharmacological principles. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 38, 4–15. [Google Scholar] [CrossRef]
- Chiang, N.; Dalli, J.; Colas, R.A.; Serhan, C.N. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J. Exp. Med. 2015, 212, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Resina, I.; Navarro, G.; Aguinaga, D.; Canela, E.I.; Schoeder, C.T.; Załuski, M.; Kieć-Kononowicz, K.; Saura, C.A.; Müller, C.E.; Franco, R. Molecular and functional interaction between GPR18 and cannabinoid CB2 G-protein-coupled receptors. Relevance in neurodegenerative diseases. Biochem. Pharmacol. 2018, 157, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Rempel, V.; Atzler, K.; Behrenswerth, A.; Karcz, T.; Schoeder, C.; Hinz, S.; Kaleta, M.; Thimm, D.; Kieć-Kononowicz, K.; Mueller, C.E. Bicyclic imidazole-4-one derivatives: A new class of antagonists for the orphan G protein-coupled receptors GPR18 and GPR55. Med. Chem. Comm. 2014, 5, 632–649. [Google Scholar] [CrossRef]
- Schoeder, C.T.; Kaleta, M.; Mahardhika, A.B.; Olejarz-Maciej, A.; Łażewska, D.; Kieć-Kononowicz, K.; Müller, C.E. Structure-activity relationships of imidazothiazinones and analogs as antagonists of the cannabinoid-activated orphan G protein-coupled receptor GPR18. Eur. J. Med. Chem. 2018, 155, 381–397. [Google Scholar] [CrossRef]
- Malek, N.; Popiolek-Barczyk, K.; Mika, J.; Przewlocka, B.; Starowicz, K. Anandamide, Acting via CB2 Receptors, Alleviates LPS-Induced Neuroinflammation in Rat Primary Microglial Cultures. Neural Plast. 2015, 2015, 130639. [Google Scholar] [CrossRef] [PubMed]
- Simcocks, A.C.; Jenkin, K.A.; O’Keefe, L.; Samuel, C.S.; Mathai, M.L.; McAinch, A.J.; Hryciw, D.H. Atypical cannabinoid ligands O-1602 and O-1918 administered chronically in diet-induced obesity. Endocr. Connect. 2019, 8, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.H.; Prus, A.J.; Overton, D.A. Drug Discrimination: Historical Origins, Important Concepts, and Principles. Curr. Top. Behav. Neurosci. 2018, 39, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.J., III; Mittelstadt, S.W. Can locomotor screening be utilized as a first-tiered approach for pre-clinical CNS/neurobehavioral safety testing? J. Pharmacol. Toxicol. Met. 2009, 60, 232. [Google Scholar] [CrossRef]
- Lynch, J.J.; Castagné, V.; Moser, P.C.; Mittelstadt, S.W. Comparison of methods for the assessment of locomotor activity in rodent safety pharmacology studies. J. Pharmacol. Toxicol. Methods 2011, 64, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1997, 229, 327–336. [Google Scholar]
- Porsolt, R.D. Animal models of depression: Utility for transgenic research. Rev. Neurosci. 2000, 11, 53–58. [Google Scholar] [CrossRef] [PubMed]
- El-Alfy, A.T.; Ivey, K.; Robinson, K.; Ahmed, S.; Radwan, M.; Slade, D.; Khan, I.; ElSohly, M.; Ross, S. Antidepressant-like effect of delta9-tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L. Pharmacol. Biochem. Behav. 2010, 9, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Aron, C.; Simon, P.; Larousse, C.; Boissier, J.R. Evaluation of a rapid technique for detecting minor tranquilizers. Neuropharmacology 1971, 10, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Viveros, M.P.; Marco, E.M.; Feli, S.E. Endocannabinoid system and stress and anxiety responses. Pharmacol. Biochem. Behav. 2005, 81, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Gaetani, S.; Dipasquale, P.; Romano, A.; Righetti, L.; Cassano, T.; Piomelli, D.; Cuomo, V. Chapter 5 The Endocannabinoid System as A Target for Novel Anxiolytic and Antidepressant Drugs. Inter. Rev. Neurobiol. 2009, 85, 57–72. [Google Scholar] [CrossRef]
- Guerrero-Alba, R.; Barragán-Iglesias, P.; González-Hernández, A.; Valdez-Moráles, E.E.; Granados-Soto, V.; Condés-Lara, M.; Rodríguez, M.G.; Marichal-Cancino, B.A. Some Prospective Alternatives for Treating Pain: The Endocannabinoid System and Its Putative Receptors GPR18 and GPR55. Front. Pharmacol. 2019, 9, 1496. [Google Scholar] [CrossRef]
- Ripoll, N.; Hascoet, M.; Bourin, M. The four-plates test: Anxiolytic or analgesic paradigm? Prog. Neuro-Psychopharm. Biol. Psych. 2006, 30, 873–880. [Google Scholar] [CrossRef]
- Xiao, W.H.; Zheng, H.; Bennett, G.J. Characterization of oxaliplatin-induced chronic painful peripheral neuropathy in the rat and comparison with the neuropathy induced by paclitaxel. Neuroscience 2012, 203, 194–206. [Google Scholar] [CrossRef]
- Ewertz, M.; Qvortrup, C.; Eckhoff, L. Chemotherapy-induced peripheral neuropathy in patients treated with taxanes and platinum derivatives. Acta Oncol. 2015, 54, 587–591. [Google Scholar] [CrossRef]
- Sałat, K. Chemotherapy-induced peripheral neuropathy: Part 1-current state of knowledge and perspectives for pharmacotherapy. Pharmacol. Rep. 2020, 72, 486–507. [Google Scholar] [CrossRef] [PubMed]
- Sałat, K. Chemotherapy-induced peripheral neuropathy-part 2: Focus on the prevention of oxaliplatin-induced neurotoxicity. Pharmacol. Rep. 2020, 72, 508–527. [Google Scholar] [CrossRef]
- Xu, D.; Zhao, H.; Gao, H.; Zhao, H.; Liu, D.; Li, J. Participation of pro-inflammatory cytokines in neuropathic pain evoked by chemotherapeutic oxaliplatin via central GABAergic pathway. Mol. Pain. 2018, 14, 1–10. [Google Scholar] [CrossRef]
- Mulpuri, Y.; Marty, V.N.; Munier, J.J.; Mackie, K.; Schmidt, B.L.; Seltzman, H.H.; Spigelman, I. Synthetic peripherally-restricted cannabinoid suppresses chemotherapy-induced peripheral neuropathy pain symptoms by CB1 receptor activation. Neuropharmacology 2018, 139, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Blanton, H.L.; Brelsfoard, J.; DeTurk, N.; Pruitt, K.; Narasimhan, M.; Morgan, D.J.; Guindon, J. Cannabinoids: Current and Future Options to Treat Chronic and Chemotherapy-Induced Neuropathic Pain. Drugs 2019, 79, 969–995. [Google Scholar] [CrossRef]
- MacDonald, D.I.; Wood, J.N.; Emery, E.C. Molecular mechanisms of cold pain. Neurobiol. Pain 2020, 7, 100044. [Google Scholar] [CrossRef] [PubMed]
- Rawls, S.M.; Benamar, K. Effects of opioids, cannabinoids, and vanilloids on body temperature. Front. Biosci. 2011, 3, 822–845. [Google Scholar]
- Rawls, S.M.; Cabassa, J.; Geller, E.B.; Adler, M.W. CB1 receptors in the preoptic anterior hypothalamus regulate WIN 55212-2 [(4,5-dihydro-2-methyl-4(4-morpholinylmethyl)-1-(1-naphthalenyl-carbonyl)-6H-pyrrolo[3,2,1ij]quinolin-6-one]-induced hypothermia. J. Pharmacol. Exp. Ther. 2002, 301, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Nava, F.; Carta, G.; Gessa, G.L. Permissive role of dopamine D2 receptors in the hypothermia induced by Δ9-tetrahydrocannabinol in rats. Pharmacol. Biochem. Behav. 2000, 66, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Kotańska, M.; Mika, K.; Szafarz, M.; Kubacka, M.; Müller, C.E.; Sapa, J.; Kieć-Kononowicz, K. Effects of GPR18 Ligands on Body Weight and Metabolic Parameters in a Female Rat Model of Excessive Eating. Pharmaceuticals 2021, 14, 270. [Google Scholar] [CrossRef] [PubMed]
- Bellocchio, L.; Lafenêtre, P.; Cannich, A.; Cota, D.; Puente, N.; Grandes, P.; Chaouloff, F.; Piazza, P.V.; Marsicano, G. Bimodal control of stimulated food intake by the endocannabinoid system. Nat. Neurosci. 2010, 13, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Wiley, J.L.; Burston, J.J.; Leggett, D.C.; Alekseeva, O.O.; Razdan, R.K.; Mahadevan, A.; Martin, B.R. CB1 cannabinoid receptor- mediated modulation of food intake in mice. Br. J. Pharmacol. 2005, 145, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Järbe, T.U.; Johansson, J.O.; Henriksson, B.G. Characteristics of tetrahydrocannabinol (THC)-produced discrimination in rats. Psychopharmacology 1976, 48, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Rowton, S.S.; Rocha, B.A.; Porter, I.E. Δ9-tetrahydrocannabinol: Drug discrimination abuse liability testing in female Lister Hooded rats: Trials, tribulations and triumphs. J. Pharmacol. Toxicol. Methods 2020, 106, 106937. [Google Scholar] [CrossRef]
- Wiley, J.L. Cannabis: Discrimination of “internal bliss”? Pharmacol. Biochem. Behav. 1999, 64, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Bourin, M.; Masse, F.; Dailly, E.; Hascoët, M. Anxiolytic-like effect of milnacipran in the four-plate test in mice: Mechanism of action. Pharmacol. Biochem. Behav. 2005, 81, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Sałat, K.; Podkowa, A.; Kowalczyk, P.; Kulig, K.; Dziubina, A.; Filipek, B.; Librowski, T. Anticonvulsant active inhibitor of GABA transporter subtype 1, tiagabine, with activity in mouse models of anxiety, pain and depression. Pharmacol. Rep. 2015, 67, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Sałat, K.; Cios, A.; Wyska, E.; Sałat, R.; Mogilski, S.; Filipek, B.; Więckowski, K.; Malawska, B. Antiallodynic and antihyperalgesic activity of 3-[4-(3-trifluoromethyl-phenyl)-piperazin-1-yl]-dihydrofuran-2-one compared to pregabalin in chemotherapy-induced neuropathic pain in mice. Pharmacol. Biochem. Behav. 2014, 122, 173–181. [Google Scholar] [CrossRef]
- Furgała, A.; Fijałkowski, Ł.; Nowaczyk, A.; Sałat, R.; Sałat, K. Time-shifted co-administration of sub-analgesic doses of ambroxol and pregabalin attenuates oxaliplatin-induced cold allodynia in mice. Biomed. Pharmacother. 2018, 106, 930–940. [Google Scholar] [CrossRef]
- Mokrosz, J.L.; Paluchowska, M.H.; Chojnacka-Wójcik, E.; Filip, M.; Charakchieva-Minol, S.; Dereń-Wesołek, A.; Mokrosz, M.J. Structure-activity relationship studies of central nervous system agents. 13.4-[3-(Benzotriazol-1-yl)propyl]-1-(2-methoxyphenyl)piperazine, a new putative 5-HT1A receptor antagonist, and its analogs. J. Med. Chem. 1994, 37, 2754–2760. [Google Scholar] [CrossRef]
- Tallarida, R.J.; Murray, R.B. Manual of Pharmacological Calculations with Computer Programs, 2nd ed.; Springer: New York, NY, USA, 1987. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frankowska, M.; Wydra, K.; Suder, A.; Zaniewska, M.; Gawliński, D.; Miszkiel, J.; Furgała-Wojas, A.; Sałat, K.; Filip, M.; Müller, C.E.; et al. Novel GPR18 Ligands in Rodent Pharmacological Tests: Effects on Mood, Pain, and Eating Disorders. Int. J. Mol. Sci. 2023, 24, 9046. https://doi.org/10.3390/ijms24109046
Frankowska M, Wydra K, Suder A, Zaniewska M, Gawliński D, Miszkiel J, Furgała-Wojas A, Sałat K, Filip M, Müller CE, et al. Novel GPR18 Ligands in Rodent Pharmacological Tests: Effects on Mood, Pain, and Eating Disorders. International Journal of Molecular Sciences. 2023; 24(10):9046. https://doi.org/10.3390/ijms24109046
Chicago/Turabian StyleFrankowska, Małgorzata, Karolina Wydra, Agata Suder, Magdalena Zaniewska, Dawid Gawliński, Joanna Miszkiel, Anna Furgała-Wojas, Kinga Sałat, Małgorzata Filip, Christa E. Müller, and et al. 2023. "Novel GPR18 Ligands in Rodent Pharmacological Tests: Effects on Mood, Pain, and Eating Disorders" International Journal of Molecular Sciences 24, no. 10: 9046. https://doi.org/10.3390/ijms24109046
APA StyleFrankowska, M., Wydra, K., Suder, A., Zaniewska, M., Gawliński, D., Miszkiel, J., Furgała-Wojas, A., Sałat, K., Filip, M., Müller, C. E., Kieć-Kononowicz, K., & Kotańska, M. (2023). Novel GPR18 Ligands in Rodent Pharmacological Tests: Effects on Mood, Pain, and Eating Disorders. International Journal of Molecular Sciences, 24(10), 9046. https://doi.org/10.3390/ijms24109046







