Investigating Mammalian Formins with SMIFH2 Fifteen Years in: Novel Targets and Unexpected Biology
Abstract
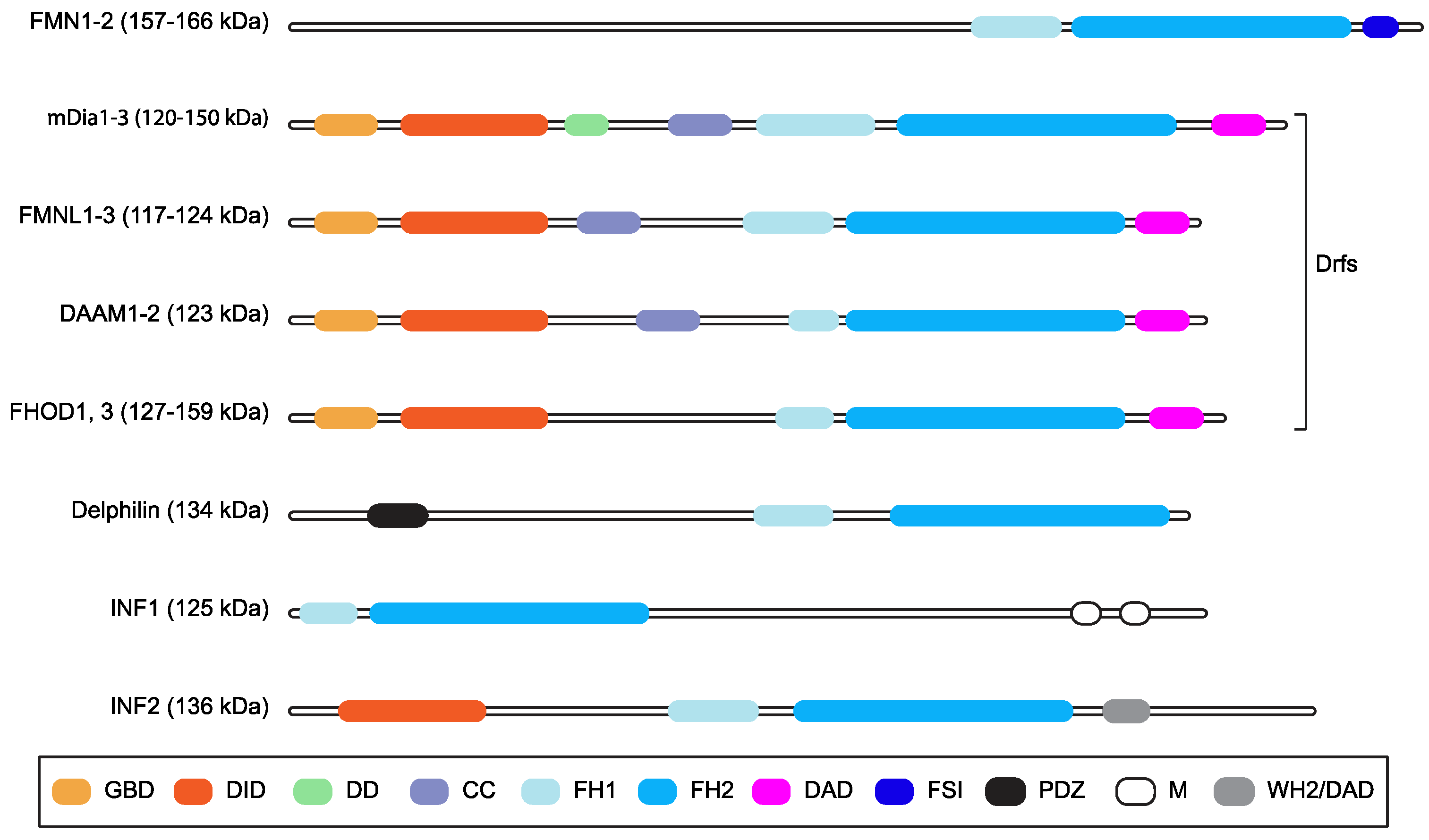
:1. Introduction
2. SMIFH2 Discovery and Characterization In Vitro and in Cells
3. Identification of Mammalian Myosins and Interferons as SMIFH2 Targets
3.1. Myosins
3.2. Interferons
4. High-Throughput Bioactivity Assays Reveal Novel SMIFH2 Targets
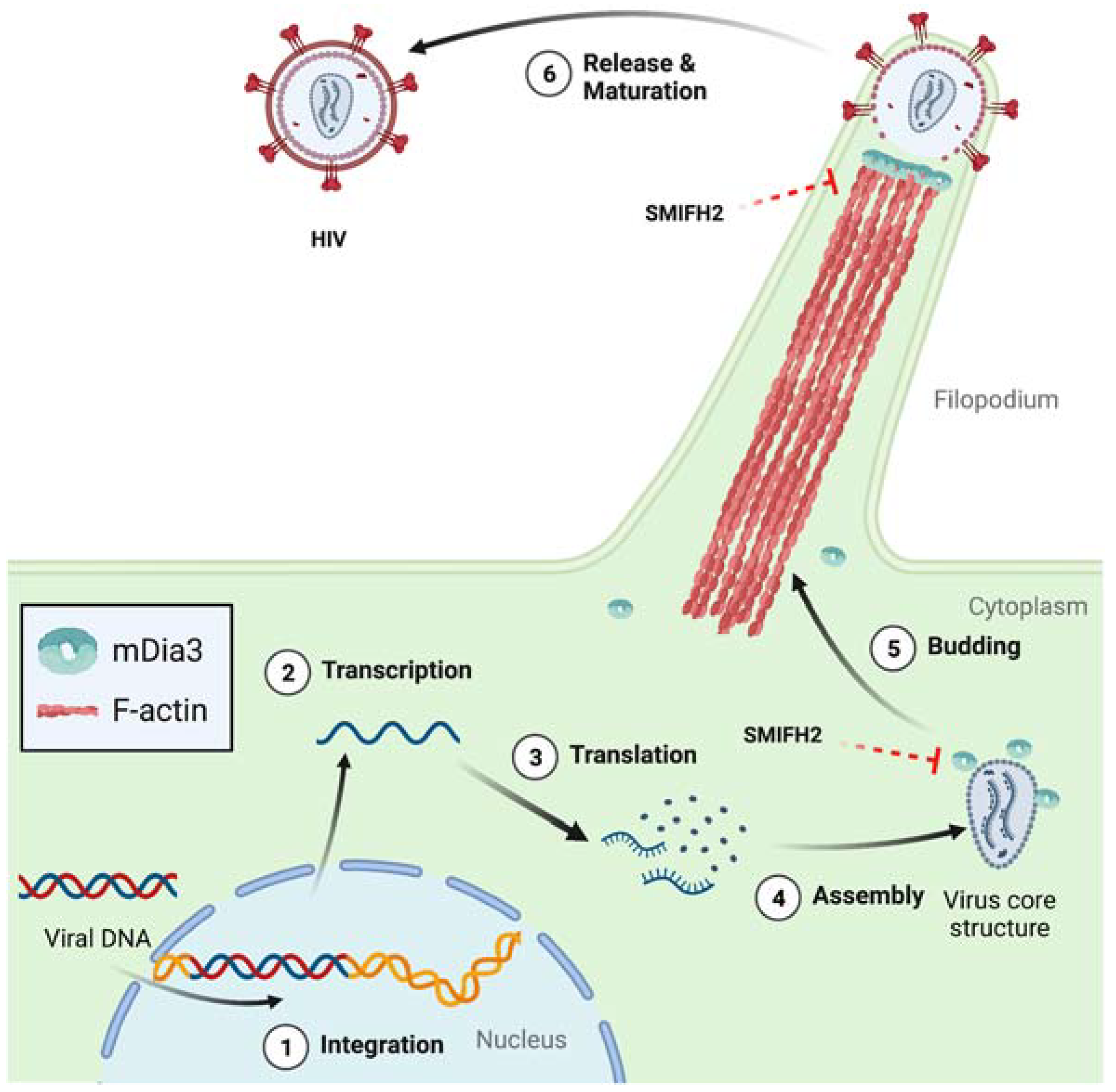
4.1. Ribonuclease H (RNase H) Activity of the HIV-1 Reverse Transcriptase
4.2. Dual-Specificity Phosphatase 3 and Dual-Specificity Phosphatase 6
4.3. Tyrosine-Protein Phosphatase Non-Receptor Type 7 Isoform 2
4.4. Heat Shock 70kDa Protein 1A
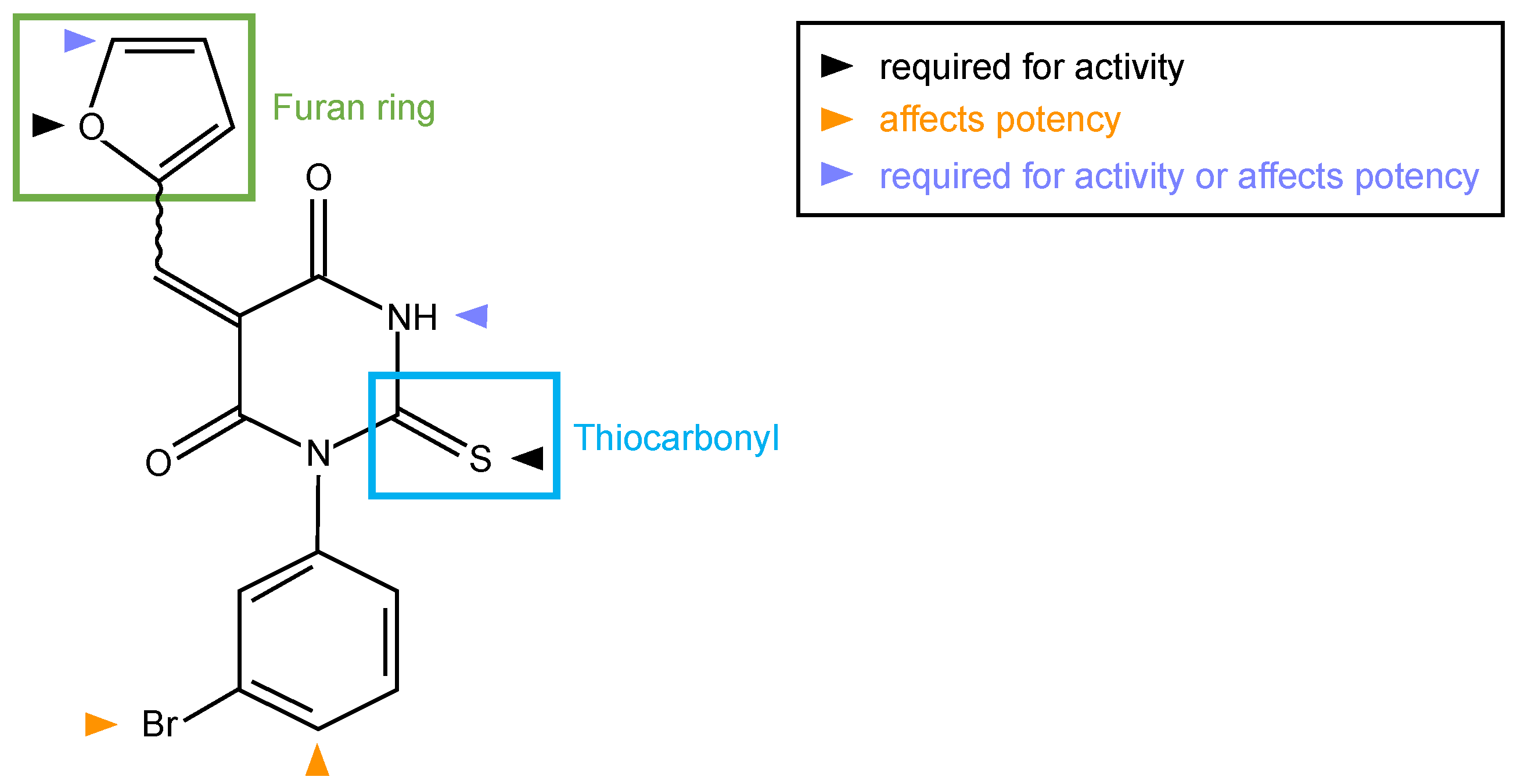
5. Possible Mechanism(s) for Formin Inhibition by SMIFH2
5.1. Non-Covalent Reversible Inhibition of the FH2 Domain
5.2. Covalent Inhibition of Formins
6. Can SMIFH2 Be Modified to Obtain an Isoform-Specific Formin Inhibitor?
7. Conclusions and Future Directions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chesarone, M.A.; DuPage, A.G.; Goode, B.L. Unleashing Formins to Remodel the Actin and Microtubule Cytoskeletons. Nat. Rev. Mol. Cell Biol. 2010, 11, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Isogai, T.; Innocenti, M. New Nuclear and Perinuclear Functions of Formins. Biochem. Soc. Trans. 2016, 44, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Valencia, D.A.; Quinlan, M.E. Formins. Curr. Biol. 2021, 31, R517–R522. [Google Scholar] [CrossRef]
- Schonichen, A.; Geyer, M. Fifteen Formins for an Actin Filament: A Molecular View on the Regulation of Human Formins. Biochim. Biophys. Acta 2010, 1803, 152–163. [Google Scholar] [CrossRef]
- Peng, J.; Wallar, B.J.; Flanders, A.; Swiatek, P.J.; Alberts, A.S. Disruption of the Diaphanous-Related Formin Drf1 Gene Encoding MDia1 Reveals a Role for Drf3 as an Effector for Cdc42. Curr. Biol. 2003, 13, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Wallar, B.J.; Stropich, B.N.; Schoenherr, J.A.; Holman, H.A.; Kitchen, S.M.; Alberts, A.S. The Basic Region of the Diaphanous-Autoregulatory Domain (DAD) Is Required for Autoregulatory Interactions with the Diaphanous-Related Formin Inhibitory Domain. J. Biol. Chem. 2006, 281, 4300–4307. [Google Scholar] [CrossRef]
- Alberts, A.S. Identification of a Carboxyl-Terminal Diaphanous-Related Formin Homology Protein Autoregulatory Domain. J. Biol. Chem. 2001, 276, 2824–2830. [Google Scholar] [CrossRef] [PubMed]
- Gould, C.J.; Maiti, S.; Michelot, A.; Graziano, B.R.; Blanchoin, L.; Goode, B.L. The Formin DAD Domain Plays Dual Roles in Autoinhibition and Actin Nucleation. Curr. Biol. 2011, 21, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Thurston, S.F.; Kulacz, W.A.; Shaikh, S.; Lee, J.M.; Copeland, J.W. The Ability to Induce Microtubule Acetylation Is a General Feature of Formin Proteins. PLoS ONE 2012, 7, e48041. [Google Scholar] [CrossRef]
- Bartolini, F.; Moseley, J.B.; Schmoranzer, J.; Cassimeris, L.; Goode, B.L.; Gundersen, G.G. The Formin MDia2 Stabilizes Microtubules Independently of Its Actin Nucleation Activity. J. Cell Biol. 2008, 181, 523–536. [Google Scholar] [CrossRef]
- Gaillard, J.; Ramabhadran, V.; Neumanne, E.; Gurel, P.; Blanchoin, L.; Vantard, M.; Higgs, H.N. Differential Interactions of the Formins INF2, MDia1, and MDia2 with Microtubules. Mol. Biol. Cell 2011, 22, 4575–4587. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Eng, C.H.; Schmoranzer, J.; Cabrera-Poch, N.; Morris, E.J.S.; Chen, M.; Wallar, B.J.; Alberts, A.S.; Gundersen, G.G. EB1 and APC Bind to MDia to Stabilize Microtubules Downstream of Rho and Promote Cell Migration. Nat. Cell Biol. 2004, 6, 820–830. [Google Scholar] [CrossRef]
- Manor, U.; Bartholomew, S.; Golani, G.; Christenson, E.; Kozlov, M.; Higgs, H.; Spudich, J.; Lippincott-Schwartz, J. A Mitochondria-Anchored Isoform of the Actin-Nucleating Spire Protein Regulates Mitochondrial Division. eLife 2015, 4, e08828. [Google Scholar] [CrossRef] [PubMed]
- Cangkrama, M.; Liu, H.; Whipman, J.; Zubair, M.; Matsushita, M.; Di Filippo, M.; Kopf, M.; Innocenti, M.; Werner, S. A Pro-Tumorigenic MDia2-MIRO1 Axis Controls Mitochondrial Positioning and Function in Cancer-Associated Fibroblasts. Cancer Res 2022, 82, 3701–3717. [Google Scholar] [CrossRef] [PubMed]
- Wallar, B.J.; Deward, A.D.; Resau, J.H.; Alberts, A.S. RhoB and the Mammalian Diaphanous-Related Formin MDia2 in Endosome Trafficking. Exp. Cell Res. 2007, 313, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Copeland, J. Actin-Based Regulation of Ciliogenesis–The Long and the Short of It. Semin. Cell Dev. Biol. 2020, 102, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Beli, P.; Mascheroni, D.; Xu, D.; Innocenti, M. WAVE and Arp2/3 Jointly Inhibit Filopodium Formation by Entering into a Complex with MDia2. Nat. Cell Biol. 2008, 10, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Isogai, T.; van der Kammen, R.; Leyton-Puig, D.; Kedziora, K.M.; Jalink, K.; Innocenti, M. Initiation of Lamellipodia and Ruffles Involves Cooperation between MDia1 and the Arp2/3 Complex. J. Cell Sci. 2015, 128, 3796–3810. [Google Scholar] [CrossRef]
- Kedziora, K.M.; Isogai, T.; Jalink, K.; Innocenti, M. Invadosomes-Shaping Actin Networks to Follow Mechanical Cues. Front. Biosci. (Landmark Ed) 2016, 21, 1092–1117. [Google Scholar]
- Dhanda, A.S.; Vogl, A.W.; Ness, F.; Innocenti, M.; Guttman, J.A. MDia1 Assembles a Linear F-Actin Coat at Membrane Invaginations To Drive Listeria Monocytogenes Cell-to-Cell Spreading. mBio 2021, 12, e0293921. [Google Scholar] [CrossRef]
- Colucci-Guyon, E.; Niedergang, F.; Wallar, B.J.; Peng, J.; Alberts, A.S.; Chavrier, P. A Role for Mammalian Diaphanous-Related Formins in Complement Receptor (CR3)-Mediated Phagocytosis in Macrophages. Curr. Biol. 2005, 15, 2007–2012. [Google Scholar] [CrossRef] [PubMed]
- Damiani, D.; Goffinet, A.M.; Alberts, A.; Tissir, F. Lack of Diaph3 Relaxes the Spindle Checkpoint Causing the Loss of Neural Progenitors. Nat. Commun. 2016, 7, 13509. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.O.-C.; Damiani, D.; Chehade, G.; Ruiz-Reig, N.; Saade, R.; Jossin, Y.; Aittaleb, M.; Schakman, O.; Tajeddine, N.; Gailly, P.; et al. DIAPH3 Deficiency Links Microtubules to Mitotic Errors, Defective Neurogenesis, and Brain Dysfunction. eLife 2021, 10, e61974. [Google Scholar] [CrossRef]
- Olson, E.N.; Nordheim, A. Linking Actin Dynamics and Gene Transcription to Drive Cellular Motile Functions. Nat. Rev. Mol. Cell Biol. 2010, 11, 353–365. [Google Scholar] [CrossRef]
- Hyrskyluoto, A.; Vartiainen, M.K. Regulation of Nuclear Actin Dynamics in Development and Disease. Curr. Opin. Cell Biol. 2020, 64, 18–24. [Google Scholar] [CrossRef]
- Labat-de-Hoz, L.; Alonso, M.A. Formins in Human Disease. Cells 2021, 10, 2554. [Google Scholar] [CrossRef] [PubMed]
- Chiereghin, C.; Robusto, M.; Massa, V.; Castorina, P.; Ambrosetti, U.; Asselta, R.; Soldà, G. Role of Cytoskeletal Diaphanous-Related Formins in Hearing Loss. Cells 2022, 11, 1726. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.A.; Neidt, E.M.; Cui, J.; Feiger, Z.; Skau, C.T.; Gardel, M.L.; Kozmin, S.A.; Kovar, D.R. Identification and Characterization of a Small Molecule Inhibitor of Formin-Mediated Actin Assembly. Chem. Biol. 2009, 16, 1158–1168. [Google Scholar] [CrossRef]
- Orman, M.; Landis, M.; Oza, A.; Nambiar, D.; Gjeci, J.; Song, K.; Huang, V.; Klestzick, A.; Hachicho, C.; Liu, S.Q.; et al. Alterations to the Broad-Spectrum Formin Inhibitor SMIFH2 Modulate Potency but Not Specificity. Sci. Rep. 2022, 12, 13520. [Google Scholar] [CrossRef]
- Nishimura, Y.; Shi, S.; Zhang, F.; Liu, R.; Takagi, Y.; Bershadsky, A.D.; Viasnoff, V.; Sellers, J.R. The Formin Inhibitor SMIFH2 Inhibits Members of the Myosin Superfamily. J. Cell Sci. 2021, 134, jcs253708. [Google Scholar] [CrossRef]
- Isogai, T.; van der Kammen, R.; Innocenti, M. SMIFH2 Has Effects on Formins and P53 That Perturb the Cell Cytoskeleton. Sci. Rep. 2015, 5, 9802. [Google Scholar] [CrossRef]
- Thoidingjam, L.K.; Blouin, C.M.; Gaillet, C.; Brion, A.; Solier, S.; Niyomchon, S.; El Marjou, A.; Mouasni, S.; Sepulveda, F.E.; de Saint Basile, G.; et al. Small Molecule Inhibitors of Interferon-Induced JAK-STAT Signalling. Angew. Chem. Int. Ed. Engl. 2022, 61, e202205231. [Google Scholar] [CrossRef]
- Houdusse, A.; Titus, M.A. The Many Roles of Myosins in Filopodia, Microvilli and Stereocilia. Curr. Biol. 2021, 31, R586–R602. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, M. New Insights into the Formation and the Function of Lamellipodia and Ruffles in Mesenchymal Cell Migration. Cell Adhes. Migr. 2018, 12, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Limouze, J.; Straight, A.F.; Mitchison, T.; Sellers, J.R. Specificity of Blebbistatin, an Inhibitor of Myosin II. J. Muscle Res. Cell Motil. 2004, 25, 337–341. [Google Scholar] [CrossRef]
- Tee, Y.H.; Shemesh, T.; Thiagarajan, V.; Hariadi, R.F.; Anderson, K.L.; Page, C.; Volkmann, N.; Hanein, D.; Sivaramakrishnan, S.; Kozlov, M.M.; et al. Cellular Chirality Arising from the Self-Organization of the Actin Cytoskeleton. Nat. Cell Biol. 2015, 17, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Alieva, N.O.; Efremov, A.K.; Hu, S.; Oh, D.; Chen, Z.; Natarajan, M.; Ong, H.T.; Jégou, A.; Romet-Lemonne, G.; Groves, J.T.; et al. Myosin IIA and Formin Dependent Mechanosensitivity of Filopodia Adhesion. Nat. Commun. 2019, 10, 3593. [Google Scholar] [CrossRef] [PubMed]
- Jacquemet, G.; Hamidi, H.; Ivaska, J. Filopodia in Cell Adhesion, 3D Migration and Cancer Cell Invasion. Curr. Opin. Cell Biol. 2015, 36, 23–31. [Google Scholar] [CrossRef]
- Benci, J.L.; Xu, B.; Qiu, Y.; Wu, T.J.; Dada, H.; Twyman-Saint Victor, C.; Cucolo, L.; Lee, D.S.M.; Pauken, K.E.; Huang, A.C.; et al. Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell 2016, 167, 1540–1554 e12. [Google Scholar] [CrossRef]
- Macarron, R.; Banks, M.N.; Bojanic, D.; Burns, D.J.; Cirovic, D.A.; Garyantes, T.; Green, D.V.; Hertzberg, R.P.; Janzen, W.P.; Paslay, J.W.; et al. Impact of High-Throughput Screening in Biomedical Research. Nat. Rev. Drug Discov. 2011, 10, 188–195. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug Repurposing: Progress, Challenges and Recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Madia, V.N.; Messore, A.; De Leo, A.; Tudino, V.; Pindinello, I.; Saccoliti, F.; De Vita, D.; Scipione, L.; Costi, R.; Di Santo, R. Small-Molecule Inhibitors of HIV-1 Reverse Transcriptase-Associated Ribonuclease H Function: Challenges and Recent Developments. Curr. Med. Chem. 2021, 28, 6146–6178. [Google Scholar] [CrossRef] [PubMed]
- Parniak, M.A.; Min, K.L.; Budihas, S.R.; Le Grice, S.F.; Beutler, J.A. A Fluorescence-Based High-Throughput Screening Assay for Inhibitors of Human Immunodeficiency Virus-1 Reverse Transcriptase-Associated Ribonuclease H Activity. Anal. Biochem. 2003, 322, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Rawle, D.J.; Harrich, D. Toward the “Unravelling” of HIV: Host Cell Factors Involved in HIV-1 Core Uncoating. PLoS Pathog. 2018, 14, e1007270. [Google Scholar] [CrossRef]
- Naghavi, M.H. HIV-1 Capsid Exploitation of the Host Microtubule Cytoskeleton during Early Infection. Retrovirology 2021, 18, 19. [Google Scholar] [CrossRef]
- Sabo, Y.; Walsh, D.; Barry, D.S.; Tinaztepe, S.; de Los Santos, K.; Goff, S.P.; Gundersen, G.G.; Naghavi, M.H. HIV-1 Induces the Formation of Stable Microtubules to Enhance Early Infection. Cell Host Microbe 2013, 14, 535–546. [Google Scholar] [CrossRef]
- Delaney, M.K.; Malikov, V.; Chai, Q.; Zhao, G.; Naghavi, M.H. Distinct Functions of Diaphanous-Related Formins Regulate HIV-1 Uncoating and Transport. Proc. Natl. Acad. Sci. USA 2017, 114, E6932–E6941. [Google Scholar] [CrossRef]
- Naranatt, P.P.; Krishnan, H.H.; Smith, M.S.; Chandran, B. Kaposi’s Sarcoma-Associated Herpesvirus Modulates Microtubule Dynamics via RhoA-GTP-Diaphanous 2 Signaling and Utilizes the Dynein Motors to Deliver Its DNA to the Nucleus. J. Virol. 2005, 79, 1191–1206. [Google Scholar] [CrossRef]
- del Real, G.; Jimenez-Baranda, S.; Mira, E.; Lacalle, R.A.; Lucas, P.; Gomez-Mouton, C.; Alegret, M.; Pena, J.M.; Rodriguez-Zapata, M.; Alvarez-Mon, M.; et al. Statins Inhibit HIV-1 Infection by down-Regulating Rho Activity. J. Exp. Med. 2004, 200, 541–547. [Google Scholar] [CrossRef]
- Lucera, M.B.; Fleissner, Z.; Tabler, C.O.; Schlatzer, D.M.; Troyer, Z.; Tilton, J.C. HIV Signaling through CD4 and CCR5 Activates Rho Family GTPases That Are Required for Optimal Infection of Primary CD4+ T Cells. Retrovirology 2017, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, F.; Ramalingam, N.; Gundersen, G.G. Actin-Capping Protein Promotes Microtubule Stability by Antagonizing the Actin Activity of MDia1. Mol. Biol. Cell 2012, 23, 4032–4040. [Google Scholar] [CrossRef]
- Destaing, O.; Saltel, F.; Gilquin, B.; Chabadel, A.; Khochbin, S.; Ory, S.; Jurdic, P. A Novel Rho-MDia2-HDAC6 Pathway Controls Podosome Patterning through Microtubule Acetylation in Osteoclasts. J. Cell Sci. 2005, 118, 2901–2911. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.; Iemma, T.L.; Shih, I.; Newsome, T.P.; McAllery, S.; Cunningham, A.L.; Turville, S.G. Mobilization of HIV Spread by Diaphanous 2 Dependent Filopodia in Infected Dendritic Cells. PLoS Pathog. 2012, 8, e1002762. [Google Scholar] [CrossRef] [PubMed]
- Sowinski, S.; Jolly, C.; Berninghausen, O.; Purbhoo, M.A.; Chauveau, A.; Kohler, K.; Oddos, S.; Eissmann, P.; Brodsky, F.M.; Hopkins, C.; et al. Membrane Nanotubes Physically Connect T Cells over Long Distances Presenting a Novel Route for HIV-1 Transmission. Nat. Cell Biol. 2008, 10, 211–219. [Google Scholar] [CrossRef]
- Zurzolo, C. Tunneling Nanotubes: Reshaping Connectivity. Curr. Opin. Cell Biol. 2021, 71, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Argenzio, E.; Innocenti, M. The Chloride Intracellular Channel Protein CLIC4 Inhibits Filopodium Formation Induced by Constitutively Active Mutants of Formin MDia2. FEBS Lett. 2020, 594, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Argenzio, E.; Klarenbeek, J.; Kedziora, K.M.; Nahidiazar, L.; Isogai, T.; Perrakis, A.; Jalink, K.; Moolenaar, W.H.; Innocenti, M. Profilin Binding Couples Chloride Intracellular Channel Protein CLIC4 to RhoA-MDia2 Signaling and Filopodium Formation. J. Biol. Chem. 2018, 293, 19161–19176. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.K.; Lappalainen, P. Filopodia: Molecular Architecture and Cellular Functions. Nat. Rev. Mol. Cell Biol. 2008, 9, 446–454. [Google Scholar] [CrossRef]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small Molecules in Targeted Cancer Therapy: Advances, Challenges, and Future Perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef]
- Lang, R.; Raffi, F.A.M. Dual-Specificity Phosphatases in Immunity and Infection: An Update. Int. J. Mol. Sci. 2019, 20, 2710. [Google Scholar] [CrossRef]
- Pavic, K.; Duan, G.; Kohn, M. VHR/DUSP3 Phosphatase: Structure, Function and Regulation. FEBS J. 2015, 282, 1871–1890. [Google Scholar] [CrossRef]
- Amand, M.; Erpicum, C.; Bajou, K.; Cerignoli, F.; Blacher, S.; Martin, M.; Dequiedt, F.; Drion, P.; Singh, P.; Zurashvili, T.; et al. DUSP3/VHR Is a pro-Angiogenic Atypical Dual-Specificity Phosphatase. Mol. Cancer 2014, 13, 108. [Google Scholar] [CrossRef] [PubMed]
- Maillet, M.; Purcell, N.H.; Sargent, M.A.; York, A.J.; Bueno, O.F.; Molkentin, J.D. DUSP6 (MKP3) Null Mice Show Enhanced ERK1/2 Phosphorylation at Baseline and Increased Myocyte Proliferation in the Heart Affecting Disease Susceptibility. J. Biol. Chem. 2008, 283, 31246–31255. [Google Scholar] [CrossRef]
- Francis, D.M.; Rozycki, B.; Koveal, D.; Hummer, G.; Page, R.; Peti, W. Structural Basis of P38alpha Regulation by Hematopoietic Tyrosine Phosphatase. Nat. Chem. Biol. 2011, 7, 916–924. [Google Scholar] [CrossRef]
- Saxena, M.; Williams, S.; Tasken, K.; Mustelin, T. Crosstalk between CAMP-Dependent Kinase and MAP Kinase through a Protein Tyrosine Phosphatase. Nat. Cell Biol. 1999, 1, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Sergienko, E.; Xu, J.; Liu, W.H.; Dahl, R.; Critton, D.A.; Su, Y.; Brown, B.T.; Chan, X.; Yang, L.; Bobkova, E.V.; et al. Inhibition of Hematopoietic Protein Tyrosine Phosphatase Augments and Prolongs ERK1/2 and P38 Activation. ACS Chem. Biol. 2012, 7, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Thumkeo, D.; Katsura, Y.; Nishimura, Y.; Kanchanawong, P.; Tohyama, K.; Ishizaki, T.; Kitajima, S.; Takahashi, C.; Hirata, T.; Watanabe, N.; et al. MDia1/3-Dependent Actin Polymerization Spatiotemporally Controls LAT Phosphorylation by Zap70 at the Immune Synapse. Sci. Adv. 2020, 6, eaay2432. [Google Scholar] [CrossRef]
- Gomez, T.S.; Kumar, K.; Medeiros, R.B.; Shimizu, Y.; Leibson, P.J.; Billadeau, D.D. Formins Regulate the Actin-Related Protein 2/3 Complex-Independent Polarization of the Centrosome to the Immunological Synapse. Immunity 2007, 26, 177–190. [Google Scholar] [CrossRef]
- Murugesan, S.; Hong, J.; Yi, J.; Li, D.; Beach, J.R.; Shao, L.; Meinhardt, J.; Madison, G.; Wu, X.; Betzig, E.; et al. Formin-Generated Actomyosin Arcs Propel T Cell Receptor Microcluster Movement at the Immune Synapse. J. Cell Biol. 2016, 215, 383–399. [Google Scholar] [CrossRef]
- Vostakolaei, M.A.; Hatami-Baroogh, L.; Babaei, G.; Molavi, O.; Kordi, S.; Abdolalizadeh, J. Hsp70 in Cancer: A Double Agent in the Battle between Survival and Death. J. Cell Physiol. 2021, 236, 3420–3444. [Google Scholar] [CrossRef]
- Isogai, T.; van der Kammen, R.; Goerdayal, S.S.; Heck, A.J.; Altelaar, A.F.; Innocenti, M. Proteomic Analyses Uncover a New Function and Mode of Action for Mouse Homolog of Diaphanous 2 (MDia2). Mol. Cell Proteom. 2015, 14, 1064–1078. [Google Scholar] [CrossRef] [PubMed]
- Cangkrama, M.; Wietecha, M.; Mathis, N.; Okumura, R.; Ferrarese, L.; Al-Nuaimi, D.; Antsiferova, M.; Dummer, R.; Innocenti, M.; Werner, S. A Paracrine Activin A-MDia2 Axis Promotes Squamous Carcinogenesis via Fibroblast Reprogramming. EMBO Mol. Med. 2020, 12, e11466. [Google Scholar] [CrossRef] [PubMed]
- Isogai, T.; van der Kammen, R.; Bleijerveld, O.B.; Goerdayal, S.S.; Argenzio, E.; Altelaar, A.F.; Innocenti, M. Quantitative Proteomics Illuminates a Functional Interaction between MDia2 and the Proteasome. J. Proteome Res. 2016, 15, 4624–4637. [Google Scholar] [CrossRef] [PubMed]
- Boysen, M.; Kityk, R.; Mayer, M.P. Hsp70- and Hsp90-Mediated Regulation of the Conformation of P53 DNA Binding Domain and P53 Cancer Variants. Mol. Cell 2019, 74, 831–843 e4. [Google Scholar] [CrossRef]
- Nishikawa, S.; Kaida, A.; Parrales, A.; Ranjan, A.; Alalem, M.; Ren, H.; Schoenen, F.J.; Johnson, D.K.; Iwakuma, T. DNAJA1- and Conformational Mutant P53-Dependent Inhibition of Cancer Cell Migration by a Novel Compound Identified through a Virtual Screen. Cell Death Discov. 2022, 8, 437. [Google Scholar] [CrossRef]
- Baell, J.B. Observations on Screening-Based Research and Some Concerning Trends in the Literature. Future Med. Chem. 2010, 2, 1529–1546. [Google Scholar] [CrossRef]
- Gauvin, T.J.; Fukui, J.; Peterson, J.R.; Higgs, H.N. Isoform-Selective Chemical Inhibition of MDia-Mediated Actin Assembly. Biochemistry 2009, 48, 9327–9329. [Google Scholar] [CrossRef]

| Target Name (Organism) | AID | Source |
|---|---|---|
| Chain A, RIBONUCLEASE H (HIV-1) | 372 | Molecular Targets Development Program |
| Dusp6 (Rattus norvegicus) | 425 | Burnham Center for Chemical Genomics |
| PTPN7 (Homo sapiens) | 521 | Burnham Center for Chemical Genomics |
| HSPA1A (Homo sapiens) | 583 | Burnham Center for Chemical Genomics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Innocenti, M. Investigating Mammalian Formins with SMIFH2 Fifteen Years in: Novel Targets and Unexpected Biology. Int. J. Mol. Sci. 2023, 24, 9058. https://doi.org/10.3390/ijms24109058
Innocenti M. Investigating Mammalian Formins with SMIFH2 Fifteen Years in: Novel Targets and Unexpected Biology. International Journal of Molecular Sciences. 2023; 24(10):9058. https://doi.org/10.3390/ijms24109058
Chicago/Turabian StyleInnocenti, Metello. 2023. "Investigating Mammalian Formins with SMIFH2 Fifteen Years in: Novel Targets and Unexpected Biology" International Journal of Molecular Sciences 24, no. 10: 9058. https://doi.org/10.3390/ijms24109058
APA StyleInnocenti, M. (2023). Investigating Mammalian Formins with SMIFH2 Fifteen Years in: Novel Targets and Unexpected Biology. International Journal of Molecular Sciences, 24(10), 9058. https://doi.org/10.3390/ijms24109058






