Significance of Astragaloside IV from the Roots of Astragalus mongholicus as an Acetylcholinesterase Inhibitor—From the Computational and Biomimetic Analyses to the In Vitro and In Vivo Studies of Safety
Abstract
1. Introduction
2. Results
2.1. Biomimetic Models for Lipophilicity Determination
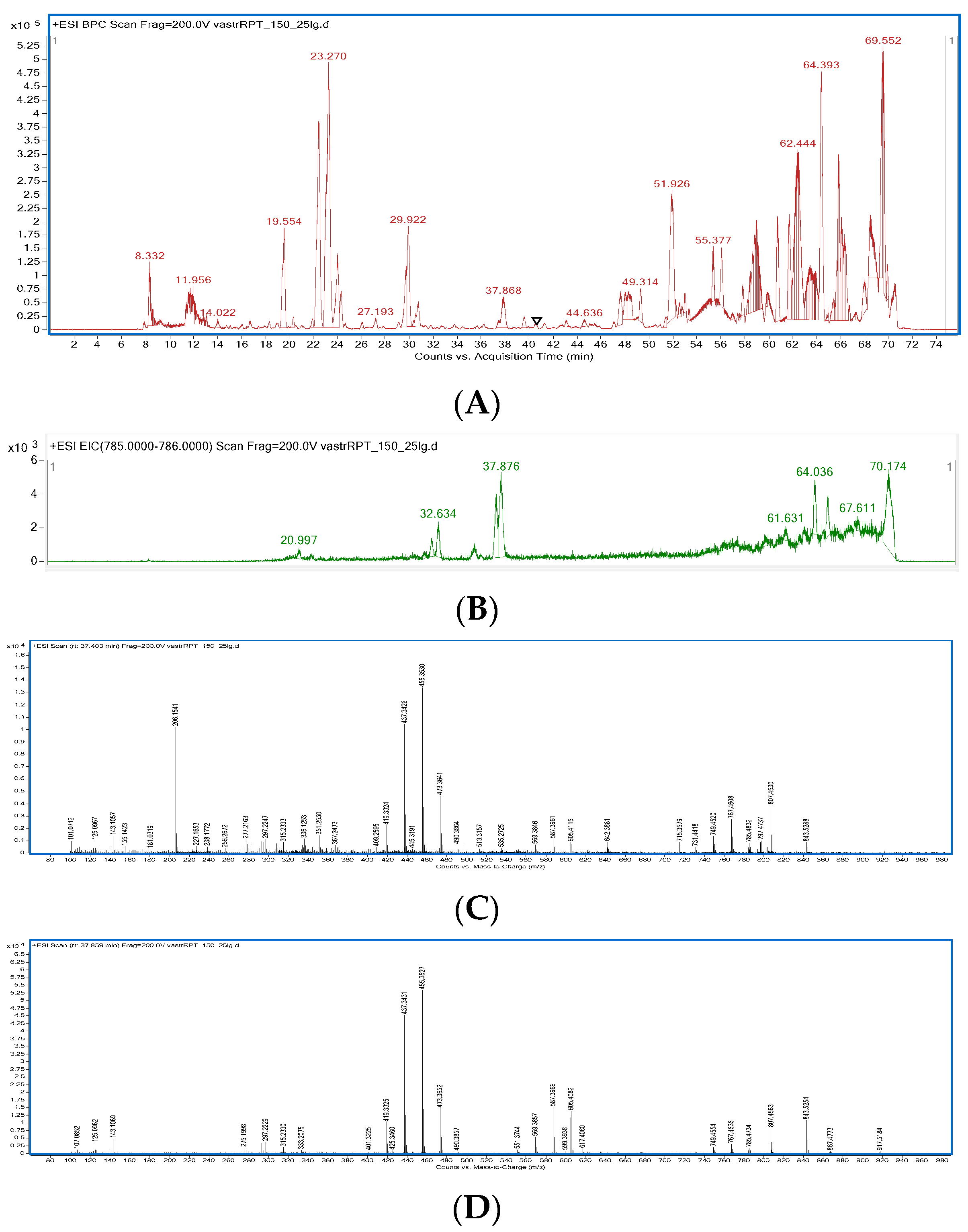
2.2. HPLC-MS Fingerprinting of Extracts
2.3. Molecular Dynamics
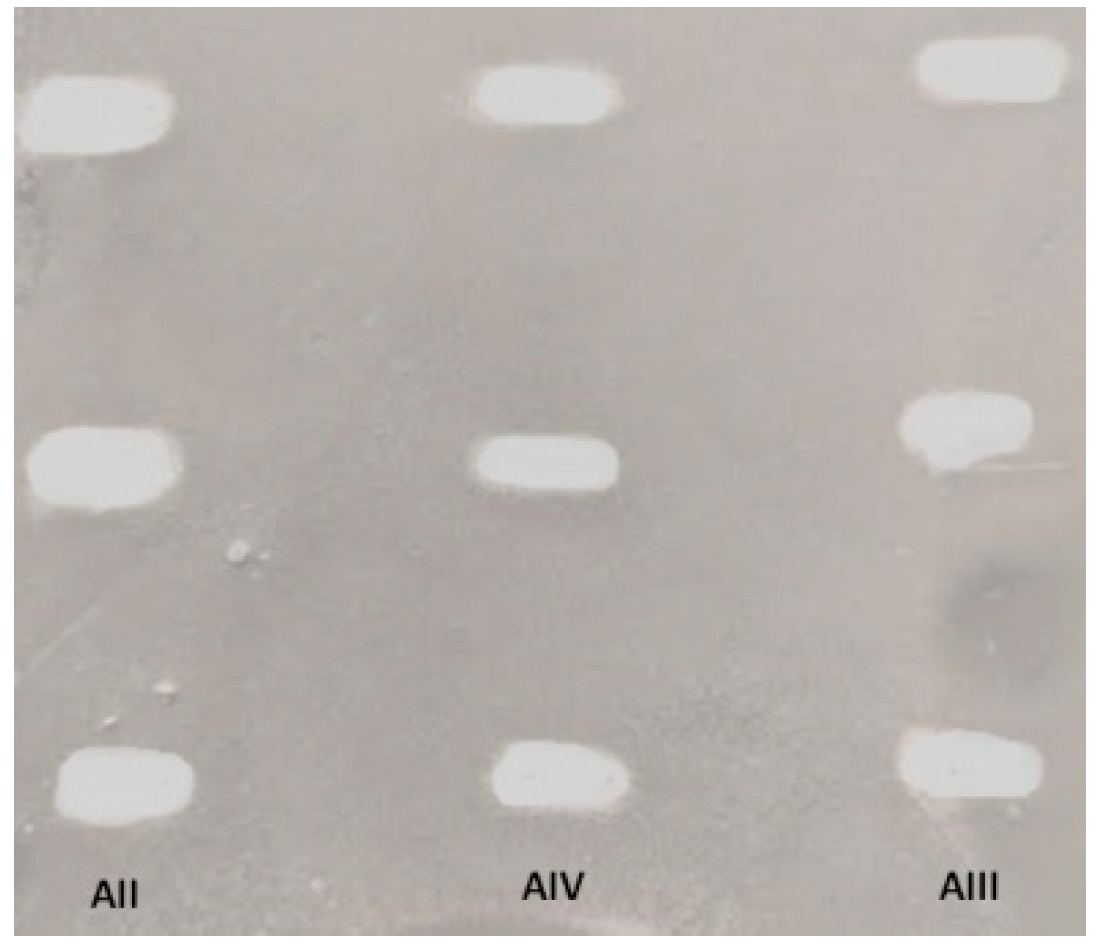
2.4. TLC Bioautography
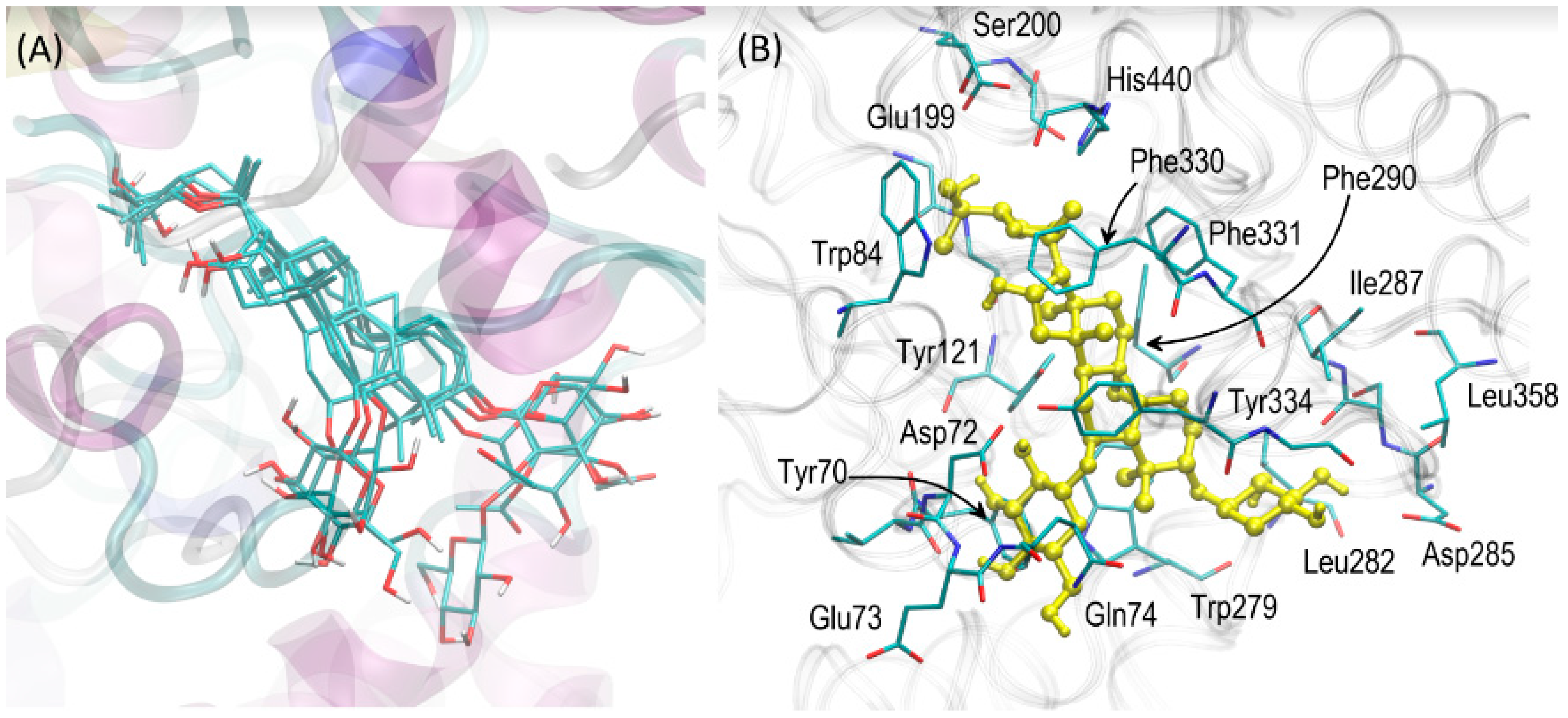
2.5. Molecular Docking
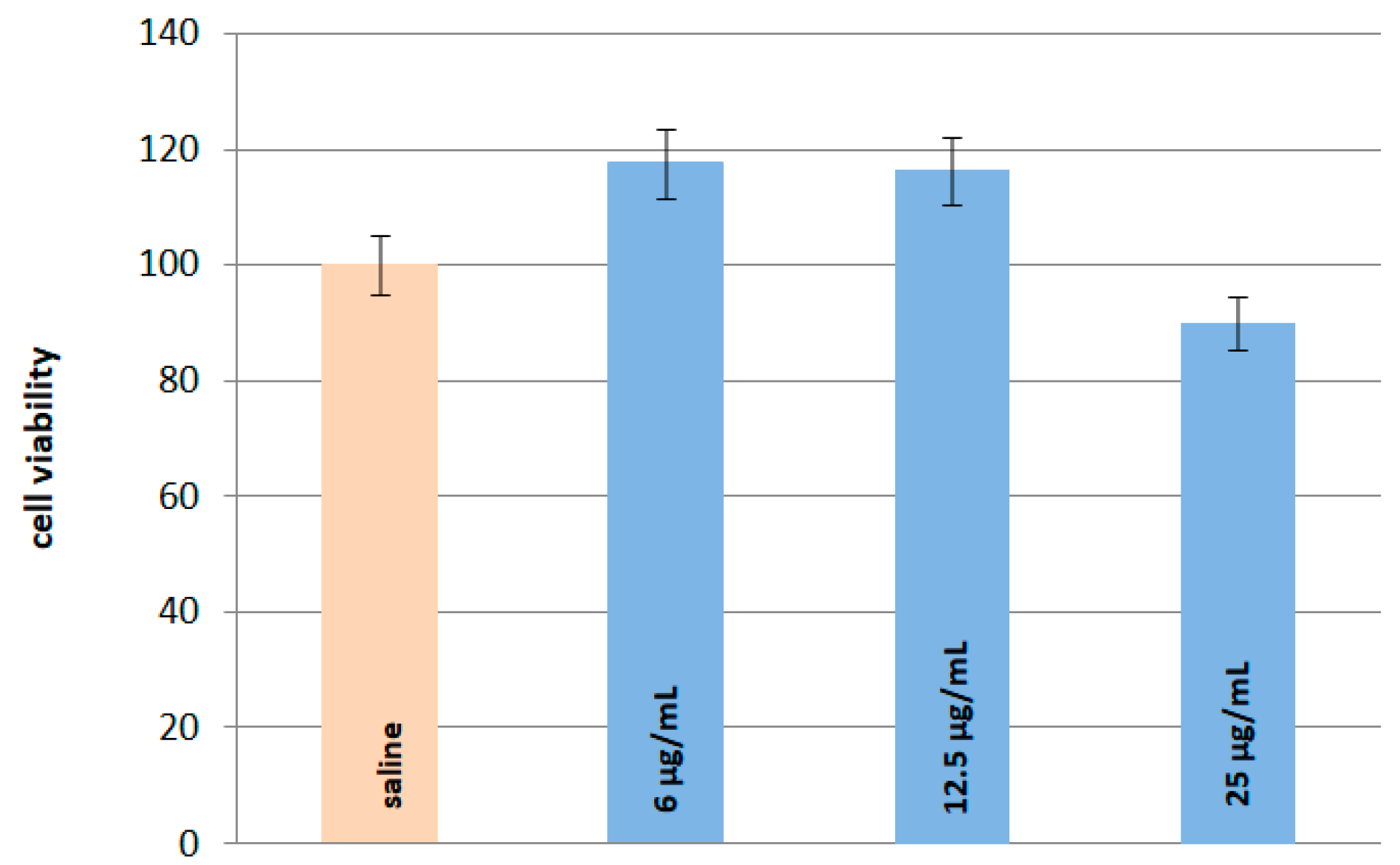
2.6. In Vitro and In Vivo Safety Studies
3. Discussion
3.1. Biomimetic Models for Lipophilicity Determination
3.2. HPLC-MS Fingerprinting of Extracts
3.3. Molecular Dynamics Simulations
3.4. Molecular Docking
3.5. In Vitro and In Vivo Safety Studies
4. Materials and Methods
4.1. The Analytes
4.2. Plant Material and Determination of Astragalosides
4.3. Chemicals
4.4. Chromatographic-Biomimetic Equipment and Conditions
4.5. HPLC-MS Based Identification of Astragalosides I–IV in Astragalus mongholicus
4.6. Molecular Dynamics
4.7. Determination of the IC50 Values of Astragalosides
4.8. Molecular Docking
4.9. In Vitro Cytotoxicity Test
4.10. The Effect of Astragaloside IV on the Zebrafish Embryos
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quinn, D.M. Acetylcholinesterase: Enzyme structure reaction dynamics and virtual transition states. Chem. Rev. 1987, 87, 955–979. [Google Scholar] [CrossRef]
- Colović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Chang, Y.; Bai, M.; Zhang, X.; Shen, S.; Hou, J.-Y.; Yao, G.-D.; Huang, X.-X.; Song, S.J. Neuroprotective and acetylcholinesterase inhibitory activities of alkaloids from Solanum lyratum Thunb.: An in vitro and in silico analyses. Phytochemistry 2023, 209, 113623. [Google Scholar] [CrossRef]
- Taylor, P.; Radic, Z. The cholinesterases: From genes to proteins. Annu. Rev. Pharmacol. 1994, 34, 281–320. [Google Scholar] [CrossRef]
- Samuel, W.; Galasko, D.; Masliah, E.; Hansen, L.A. Neocortical lewy body counts correlate with dementia in the Lewy body variant of Alzheimer’s disease. J. Neuropathol. Exp. Neurol. 1996, 55, 44–52. [Google Scholar] [CrossRef]
- Bartus, R.T.; Flicker, C.; Dean, R.L.; Pontecorvo, M.; Figueiredo, J.C.; Fisher, S.K. Selective memory loss following nucleus basalis lesions: Long term behavioral recovery despite persistent cholinergic deficiencies. Pharmacol. Biochem. Behav. 1985, 23, 125–135. [Google Scholar] [CrossRef]
- Hasselmo, M.E. The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 2006, 16, 710–715. [Google Scholar] [CrossRef]
- Okello, E.J.; Savelev, S.U.; Perry, E.K. In vitro anti-𝛽-secretase and dual anti-cholinesterase activities of Camellia sinensis L. (tea) relevant to treatment of dementia. Phytother. Res. 2004, 18, 624–627. [Google Scholar] [CrossRef]
- Douchamps, V.; Mathis, C. A second wind for the cholinergic system in Alzheimer’s therapy. Behav. Pharmacol. 2017, 28, 112–123. [Google Scholar] [CrossRef]
- Wu, T.-Y.; Chen, C.-P.; Jinn, T.-R. Traditional Chinese medicines and Alzheimer’s disease. Taiwan J. Obstet. Gynecol. 2011, 50, 131–135. [Google Scholar] [CrossRef]
- Loy, C.; Schneider, L. Galantamine for Alzheimer’s disease and mild cognitive impairment. Cochrane Database Syst. Rev. 2006, 25, CD001747. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J.; Goa, K.L. Galantamine. Drugs 2000, 60, 1095–1122. [Google Scholar] [CrossRef] [PubMed]
- Birks, J.S.; Chong, L.Y.; Grimley Evans, J. Rivastigmine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2015, 22, CD001191. [Google Scholar]
- Spencer, C.M.; Noble, S. Rivastigmine. Drugs Aging 1998, 13, 391–411. [Google Scholar] [CrossRef]
- Birks, J.S.; Harvey, R.J. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst. Rev. 2018, 18, CD001190. [Google Scholar] [CrossRef]
- Kornhuber, J.; Weller, M.; Schoppmeyer, K.; Riederer, P. Amantadine and memantine are NMDA receptor antagonists with neuroprotective properties. J. Neural. Transm. 1994, 43, 91–104. [Google Scholar]
- Burns, A. Treatment of cognitive impairment in Alzheimer’s disease. Dialogues Clin. Neurosci. 2003, 5, 35–43. [Google Scholar] [CrossRef]
- Yang, Y.F.; Qu, S.J.; Xiao, K.; Jiang, S.H.; Tan, J.J.; Tan, C.H.; Zhu, D.Y. Lycopodium alkaloids from Huperziaserrata. J. Asian Nat. Prod. Res. 2010, 12, 1005–1009. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, L.; Yan, H.; Wang, Y.; Tang, X. Effects of huperzine A on memory deficits and neurotrophic factors production after transient cerebral ischemia and reperfusion in mice. Pharmacol. Biochem. Behav. 2006, 83, 603–611. [Google Scholar] [CrossRef]
- Repantis, D.; Laisney, O.; Heuser, I. Acetylcholinesterase inhibitors and memantine for neuroenhancement in healthy individuals: A systematic review. Pharmacol. Res. 2010, 61, 473–481. [Google Scholar] [CrossRef]
- Lv, J.; Jia, H.; Jiang, Y.; Ruan, Y.; Liu, Z.; Yue, W.; Beyreuther, K.; Tu, P.; Zhang, D. Tenuifolin, an extract derived from tenuigenin, inhibits amyloid-β secretion in vitro. Acta Physiol. 2009, 196, 419–425. [Google Scholar] [CrossRef]
- Ligaa, U.; Davaasuren, B.; Ninjil, N. Medicinal Plants of Mongolia Used in Western Medicine; JCK Printing: Ulaanbaatar, Mongolia, 2005; p. 448. [Google Scholar]
- Stępnik, K.; Kukula-Koch, W. In Silico Studies on Triterpenoid Saponins Permeation through the Blood-Brain Barrier Combined with Postmortem Research on the Brain Tissues of Mice Affected by Astragaloside IV Administration. Int. J. Mol. Sci. 2020, 21, 2534. [Google Scholar] [CrossRef]
- Barai, P.; Raval, N.; Acharya, S.; Acharya, N. Neuroprotective effects of Bergeniaciliata on NMDA induced injury in SH-SY5Y cells and attenuation of cognitive deficits in scopolamine induced amnesia in rats. Biomed. Pharmacother. 2018, 108, 374–390. [Google Scholar] [CrossRef]
- Pehourcq, F.; Jarry, C.; Bannwarth, B. Potential of immobilized artificial membrane chromatography for lipophilicity determination of arylpropionic acid non-steroidal anti-inflammatory drugs. J. Pharm. Biomed. Anal. 2003, 33, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Barbato, F.; La Rotonda, M.I.; Quaglia, F. Interactions of Nonsteroidal Antiinflammatory Drugs with Phospholipids: Comparison between Octanol/Buffer Partition Coefficients and Chromatographic Indexes on Immobilized Artificial Membranes. J. Pharm. Sci. 1997, 86, 225–229. [Google Scholar] [CrossRef]
- Kaliszan, R.; Kaliszan, A.; Wainer, I.W. Deactivated hydrocarbonaceous silica and immobilized artificial membrane stationary phases in high-performance liquid chromatographic determination of hydrophobicities of organic bases: Relationship to log P and CLOGP. J. Pharm. Biomed. Anal. 1993, 11, 505–511. [Google Scholar] [CrossRef]
- Flieger, J.; Pizoń, M.; Plech, T. Chromatographic behavior of new antiepileptic active compounds on different reversed-phase materials. J. Chromatogr. A 2014, 1338, 188–196. [Google Scholar] [CrossRef]
- Sztanke, M.; Rzymowska, J.; Janicka, M.; Sztanke, K. Synthesis, structure elucidation, determination of antiproliferative activities, lipophilicity indices and pharmacokinetic properties of novel fused azaisocytosine-like congeners. Arab. J. Chem. 2019, 12, 4044–4064. [Google Scholar] [CrossRef]
- Soczewiński, E.; Wachtmeister, C.A. The relation between the composition of certain ternary two-phase solvent systems and RM values. J. Chromatogr. 1962, 7, 311–320. [Google Scholar] [CrossRef]
- Janicka, M.; Sztanke, M.; Sztanke, K. Predicting the Blood-Brain Barrier Permeability of New Drug-like Compounds via HPLC with Various Stationary Phases. Molecules 2020, 25, 487. [Google Scholar] [CrossRef]
- Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 17 February 2023).
- Xie, H.R.; Hu, L.S.; Li, G.Y. SH-SY5Y human neuroblastoma cell line: In vitro cell model of dopaminergic neurons in Parkinson’s disease. Chin. Med. J. 2010, 123, 1086–1092. [Google Scholar] [PubMed]
- Chahardehi, A.M.; Arsad, H.; Lim, V. Zebrafish as a Successful Animal Model for Screening Toxicity of Medicinal Plants. Plants 2020, 10, 1345. [Google Scholar] [CrossRef]
- Jalsrai, A.; Greckschb, G.; Becker, A. Evaluation of the effects of Astragalus mongholicus Bunge saponin extract on central nervous system functions. J. Ethnopharmacol. 2010, 131, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, C.; Gao, L.; Du, G.; Qin, X. Astragaloside IV derived from Astragalus membranaceus: A research review on the pharmacological effects. Adv. Pharmacol. 2020, 87, 89–112. [Google Scholar] [CrossRef] [PubMed]
- Hirotani, M.; Zhou, Y.; Lui, H.; Furuya, T. Astragalosides from hairy root cultures of Astragalus membranaceus. Phytochemistry 1994, 36, 665–670. [Google Scholar] [CrossRef]
- Yaxuan, Z.; Yuan, Y.; Jiawei, Z.; Yao, Z.; Yueqi, Z.; Jianliang, F. Astragaloside IV supplementation attenuates cognitive impairment by inhibiting neuroinflammation and oxidative stress in type 2 diabetic mice. Front. Aging Neurosci. 2022, 14, 1004557. [Google Scholar] [CrossRef]
- Yan, M.; Shengxue, Y.; Fang, Z.; Yu, L.; Yue, W.; Siqi, F.; Yuhong, S.; Meili, L.; Hongxin, W. Astragaloside IV Alleviates Brain Injury Induced by Hypoxia via the Calpain-1 Signaling Pathway. Neural Plast. 2022, 2022, 6509981. [Google Scholar] [CrossRef]
- Du, Q.; Deng, R.; Li, W.; Zhang, D.; Tsoi, B.; Shen, J. Baoyuan Capsule promotes neurogenesis and neurological functional recovery through improving mitochondrial function and modulating PI3K/Akt signaling pathway. Phytomedicine 2021, 93, 153795. [Google Scholar] [CrossRef]
- Li, W.; Wang, S.; Wang, H.; Wang, J.; Jin, F.; Fang, F.; Fang, C. Astragaloside IV Prevents Memory Impairment in D-galactose-induced Aging Rats Via the AGEs/RAGE/ NF-κB Axis. Arch. Med. Res. 2022, 53, 20–28. [Google Scholar] [CrossRef]
- Zu, Y.; Yan, M.; Fu, Y.; Liu, W.; Zhang, L.; Gu, C.; Efferth, T. Determination and quantification of astragalosides in Radix Astragali and its medicinal products using LC–MS. J. Sep. Sci. 2009, 32, 517–525. [Google Scholar] [CrossRef]
- Hudson, K.L.; Bartlett, G.J.; Diehl, R.C.; Agirre, J.; Gallagher, T.; Kiessling, L.L.; Woolfson, D.N. Carbohydrate–Aromatic Interactions in Proteins. J. Am. Chem. Soc. 2015, 137, 15152–15160. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, J.; Wang, S.; Qiu, J.; Yu, C. Astragaloside IV attenuates the H2O2-induced apoptosis of neuronal cells by inhibiting α-synuclein expression via the p38 MAPK pathway. Int. J. Mol. Med. 2017, 40, 1772–1780. [Google Scholar] [CrossRef] [PubMed]
- Monschein, M.; Ardjomand-Woelkart, K.; Rieder, J.; Wolf, I.; Heydel, B.; Kunert, O.; Heuberger, H.; Bauer, R. Accelerated sample preparation and formation of astragaloside IV in Astragali Radix. Pharm. Biol. 2014, 52, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Oostenbrink, C.; Villa, A.; Mark, A.E.; Van Gunsteren, W.F. A biomolecular force field based on the free enthalpy of hydration and solvation: The GROMOS force-field parameter sets 53A5 and 53A6. J. Comput. Chem. 2004, 25, 1656–1676. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; Hermans, J. Interaction Models for Water in Relation to Protein Hydration. In Intermolecular Forces: The Jerusalem Symposia on Quantum Chemistry and Biochemistry; Pullman, B., Ed.; Springer: Dordrecht, The Netherlands, 1981; Volume 14. [Google Scholar] [CrossRef]
- Kukol, A. Lipid Models for United-Atom Molecular Dynamics Simulations of Proteins. J. Chem. Theory Comput. 2009, 5, 615–626. [Google Scholar] [CrossRef]
- Stroet, M.; Caron, B.; Visscher, K.M.; Geerke, D.P.; Malde, A.K.; Mark, A.E. Automated Topology Builder Version 3.0: Prediction of Solvation Free Enthalpies in Water and Hexane. J. Chem. Theory Comput. 2018, 14, 5834–5845. [Google Scholar] [CrossRef]
- Kumar, S.; Rosenberg, J.M.; Bouzida, D.; Swendsen, R.H.; Kollman, P.A. The weighted histogram analysis method for free-energy calculations on biomolecules, I. The method. J. Comput. Chem. 1992, 13, 1011–1021. [Google Scholar] [CrossRef]
- Hub, J.S.; de Groot, B.L.; van der Spoel, D. Potentials of Mean Force and Permeabilities for Carbon Dioxide, Ammonia, and Water Flux across a Rhesus Protein Channel and Lipid Membranes. J. Chem. Theory Comput. 2010, 12, 3713–3720. [Google Scholar] [CrossRef]
- Hess, B. P-LINCS: A Parallel Linear Constraint Solver for Molecular Simulation. J. Chem. Theory Comput. 2008, 4, 116–122. [Google Scholar] [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef] [PubMed]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Available online: https://cactus.nci.nih.gov/translate/ (accessed on 17 February 2023).
- Rappé, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A., III; Skiff, W.M. A full periodic table force field for molecular mechanics molecular dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Tarabasz, D.; Szczeblewski, P.; Laskowski, T.; Płaziński, W.; Baranowska-Wójcik, E.; Szwajgier, D.; Kukula-Koch, W.; Meissner, H.O. The Distribution of Glucosinolates in Different Phenotypes of Lepidium peruvianum and Their Role as Acetyl- and Butyrylcholinesterase Inhibitors—In Silico and In Vitro Studies. Int. J. Mol. Sci. 2022, 23, 4858. [Google Scholar] [CrossRef]
- Nakonieczna, S.; Grabarska, A.; Gawel, K.; Wróblewska-Łuczka, P.; Czerwonka, A.; Stepulak, A.; Kukula-Koch, W. Isoquinoline Alkaloids from Coptischinensis Franch: Focus on Coptisine as a Potential Therapeutic Candidate against Gastric Cancer Cells. Int. J. Mol. Sci. 2022, 23, 10330. [Google Scholar] [CrossRef]
- Sztal, T.E.; Ruparelia, A.A.; Williams, C.; Bryson-Richardson, R.J. Using Touch-evoked Response and Locomotion Assays to Assess Muscle Performance and Fun ction in Zebrafish. J. Vis. Exp. 2016, 116, e54431. [Google Scholar] [CrossRef]
- Gawel, K.; Turski, W.A.; van der Ent, W.; Mathai, B.J.; Kirstein-Smardzewska, K.J.; Simonsen, A.; Esguerra, C.V. Phenotypic Characterization of Larval Zebrafish (Danio rerio) with Partial Knockdown of the cacna1a Gene. Mol. Neurobiol. 2020, 57, 1904–1916. [Google Scholar] [CrossRef]
- Ekins, S.; Waller, C.L.; Swaan, P.W.; Cruciani, G.; Wrighton, S.A.; Wikel, J.H. Progress in predicting human ADME parameters in silico. J. Pharmacol. Toxicol. Methods 2000, 44, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Smyrska-Wieleba, N.; Mroczek, T. Natural Inhibitors of Cholinesterases: Chemistry, Structure-Activity and Methods of Their Analysis. Int. J. Mol. Sci. 2023, 24, 2722. [Google Scholar] [CrossRef] [PubMed]



| Chromatographic System | Compound | logkw | s | R2 |
|---|---|---|---|---|
| IAM | A I | 2.713 | 6.040 | 0.992 |
| A II | 2.104 | 4.715 | 0.995 | |
| A III | 1.807 | 4.165 | 0.969 | |
| A IV | 1.727 | 3.965 | 0.965 | |
| CHOL | A I | 3.234 | 5.590 | 0.985 |
| A II | 3.209 | 6.570 | 0.985 | |
| A III | 2.627 | 5.681 | 0.981 | |
| A IV | 2.668 | 6.000 | 0.981 |
| No. | Name | Chemical Structure |
|---|---|---|
| 1 | Astragaloside I | 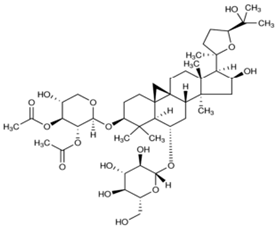 |
| 2 | Astragaloside II | 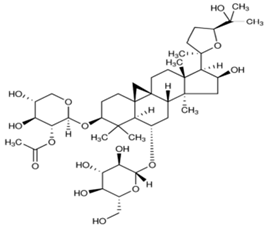 |
| 3 | Astragaloside III | 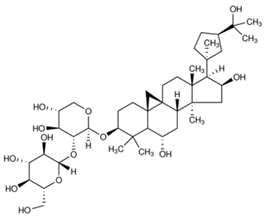 |
| 4 | Astragaloside IV | 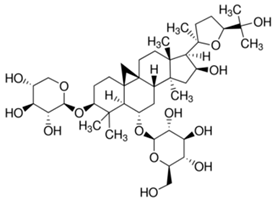 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stępnik, K.; Kukula-Koch, W.; Plazinski, W.; Gawel, K.; Gaweł-Bęben, K.; Khurelbat, D.; Boguszewska-Czubara, A. Significance of Astragaloside IV from the Roots of Astragalus mongholicus as an Acetylcholinesterase Inhibitor—From the Computational and Biomimetic Analyses to the In Vitro and In Vivo Studies of Safety. Int. J. Mol. Sci. 2023, 24, 9152. https://doi.org/10.3390/ijms24119152
Stępnik K, Kukula-Koch W, Plazinski W, Gawel K, Gaweł-Bęben K, Khurelbat D, Boguszewska-Czubara A. Significance of Astragaloside IV from the Roots of Astragalus mongholicus as an Acetylcholinesterase Inhibitor—From the Computational and Biomimetic Analyses to the In Vitro and In Vivo Studies of Safety. International Journal of Molecular Sciences. 2023; 24(11):9152. https://doi.org/10.3390/ijms24119152
Chicago/Turabian StyleStępnik, Katarzyna, Wirginia Kukula-Koch, Wojciech Plazinski, Kinga Gawel, Katarzyna Gaweł-Bęben, Daariimaa Khurelbat, and Anna Boguszewska-Czubara. 2023. "Significance of Astragaloside IV from the Roots of Astragalus mongholicus as an Acetylcholinesterase Inhibitor—From the Computational and Biomimetic Analyses to the In Vitro and In Vivo Studies of Safety" International Journal of Molecular Sciences 24, no. 11: 9152. https://doi.org/10.3390/ijms24119152
APA StyleStępnik, K., Kukula-Koch, W., Plazinski, W., Gawel, K., Gaweł-Bęben, K., Khurelbat, D., & Boguszewska-Czubara, A. (2023). Significance of Astragaloside IV from the Roots of Astragalus mongholicus as an Acetylcholinesterase Inhibitor—From the Computational and Biomimetic Analyses to the In Vitro and In Vivo Studies of Safety. International Journal of Molecular Sciences, 24(11), 9152. https://doi.org/10.3390/ijms24119152








