Quality and Agronomic Trait Analyses of Pyramids Composed of Wheat Genes NGli-D2, Sec-1s and 1Dx5+1Dy10
Abstract
1. Introduction
2. Results
2.1. Identification and Analysis of Wheat Gene Pyramids
2.2. Effects of Gene Pyramiding on Sedimentation Value and Gluten in Wheat Flour
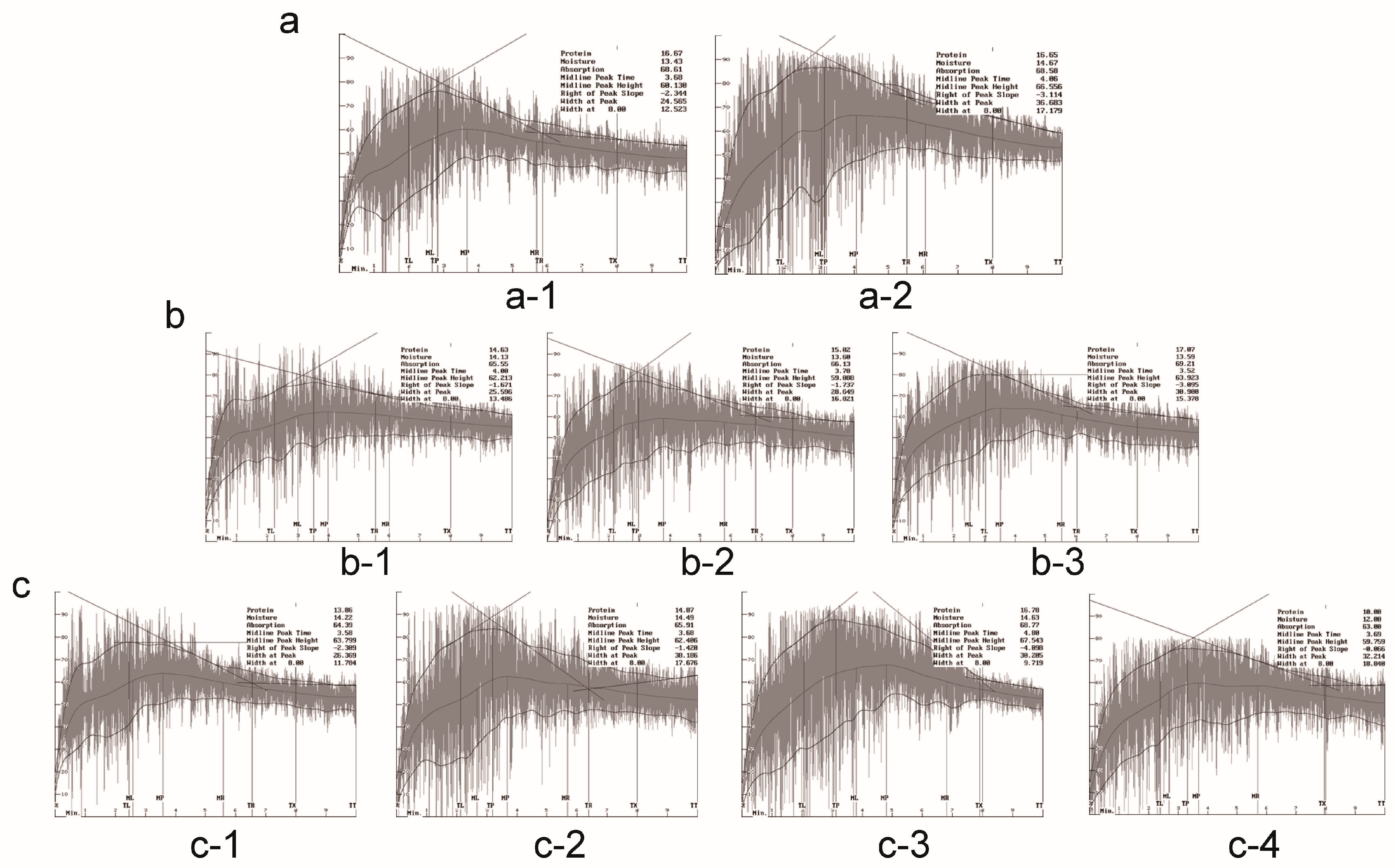
2.3. Effects of Gene Pyramiding on Flour Mixograph Parameters
2.4. Effects of Gene Pyramiding on Grain Protein Components
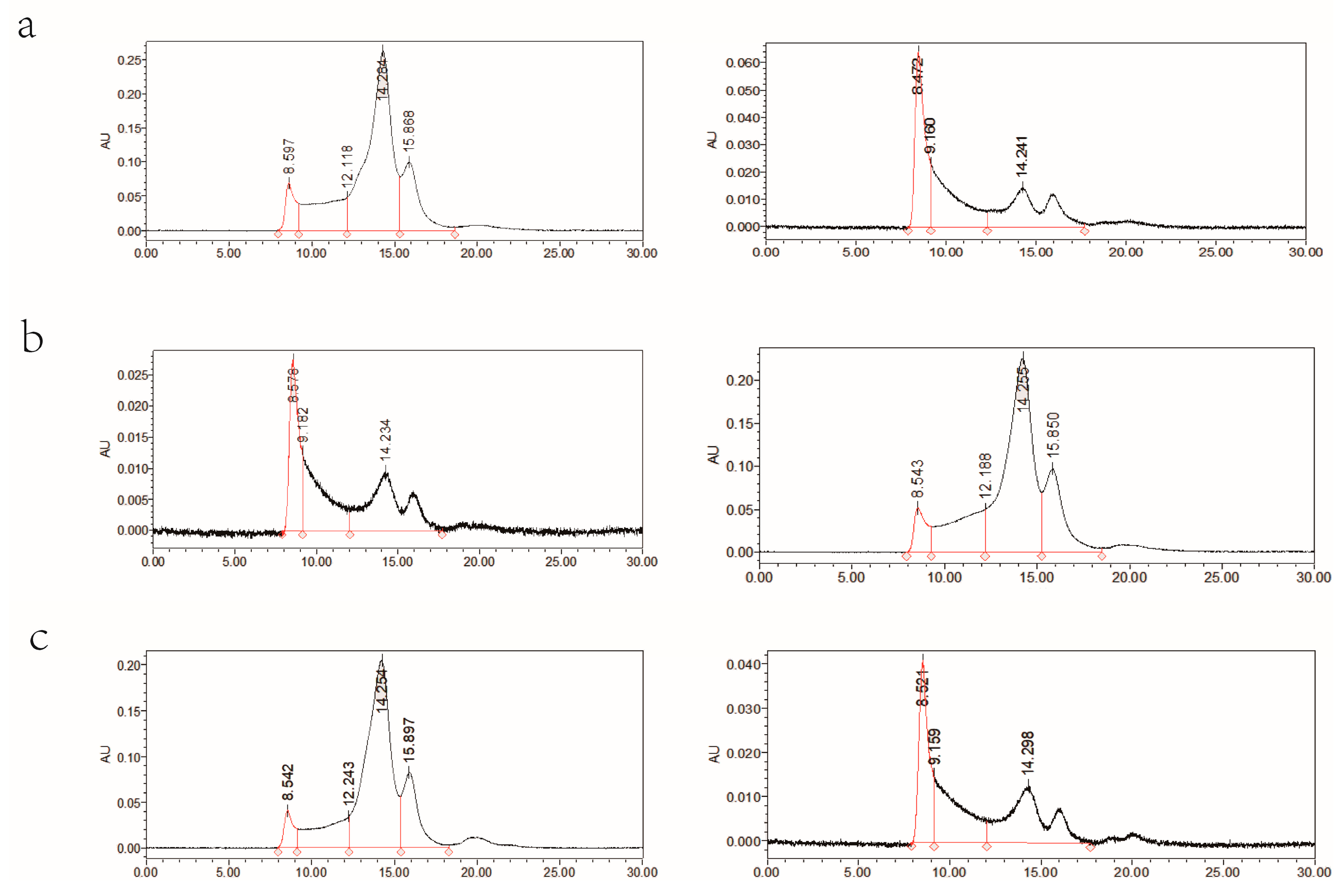
2.5. Determination and Analysis of CD Epitopes in Near-Isodeletion Lines at the NGli-D2 Locus
2.6. Effects of Gene Pyramiding on Agronomic Traits
3. Discussion
3.1. Marker-Assisted Selection Accelerating the Cultivation of High-Quality Wheat through Gene Pyramiding
3.2. Improvement in Gene Pyramiding Affected by Genetic Background and Complementarity Factors
3.3. Significance of 1Dx5+1Dy10, Sec-1s and NGli-D2 Gene Pyramiding for Wheat Processing Quality and Food Safety Improvement
3.4. 1Dx5+1Dy10, Sec-1s and NGli-D2 Gene Pyramiding Have No Negative Impact on Wheat Agronomic Traits
4. Materials and Methods
4.1. Plant Materials and Cultivation Procedure
4.2. Extraction of Wheat Leaf DNA
4.3. Agarose Gel Electrophoresis
4.4. Preparation of SDS—PAGE Solution
4.5. Protein Extraction from Wheat Grains
4.6. SDS—PAGE Agarose Gel Electrophoresis
4.7. Wheat Flour Milling and Determination of Protein Content and Moisture
4.8. Determination of Wheat Flour Sedimentation Value
4.9. Determination of Wheat Mixograph Parameters
4.10. Determination of Wheat Protein Fractions
4.11. Determination of Agronomic Traits in Wheat
4.12. Determination of CD Epitope in Wheat Flour
4.13. Data Processing
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meira, D.; Panho, M.C.; Beche, E.; Woyann, L.G.; Madella, L.A.; Milioli, A.S.; Colonelli, L.L.; Malone, G.; Brito, S.L.; Benin, G. Gene pyramiding combinations confer resistance of Asian soybean rust. Crop. Sci. 2022, 62, 792–801. [Google Scholar] [CrossRef]
- Eathington, S.R.; Crosbie, T.M.; Edwards, M.D.; Reiter, R.S.; Bull, J.K. Molecular Markers in a Commercial Breeding Program. Crop. Sci. 2007, 47, S154. [Google Scholar] [CrossRef]
- Pan, Q.M.; Yu, Z.W. Effects of nitrogen supplementation on grain quality and yield of winter wheat. J. Triticeae Crops 2002, 65–69. [Google Scholar]
- Bangur, R.; Batey, I.; McKenzie, E.; MacRitchie, F. Dependence of Extensograph Parameters on Wheat Protein Composition Measured by SE-HPLC. J. Cereal Sci. 1997, 25, 237–241. [Google Scholar] [CrossRef]
- Li, D.; Jin, H.; Zhang, K.; Wang, Z.; Wang, F.; Zhao, Y.; Huo, N.; Liu, X.; Gu, Y.Q.; Wang, D.; et al. Analysis of theGli-D2locus identifies a genetic target for simultaneously improving the breadmaking and health-related traits of common wheat. Plant J. 2018, 95, 414–426. [Google Scholar] [CrossRef]
- Fan, J.; Chen, X.; Zhang, J.; Cheng, Z.; Wang, J.; Zhang, F.; Yang, B.; Isotope Institute Co., Ltd. Composition of High Molecular Weight Glutenin Subunits and Theirs Relationship with Wheat Quality Traits. J. Triticeae Crops 2021, 41, 9. [Google Scholar]
- Wrigley, C.W. Giant proteins with flour power. Nature 1996, 381, 738–739. [Google Scholar] [CrossRef]
- Liu, S.; Gao, X.; Xia, G. Characterizing HMW-GS alleles of decaploid Agropyron elongatum in relation to evolution and wheat breeding. Theor. Appl. Genet. 2008, 116, 325–334. [Google Scholar] [CrossRef]
- Lin, J.-t.; Guo, X.-d.; Su, D.-m.; College of Food Science and Technology, Zhengzhou University of Light Industry. Study on the Relationship between Water Absorption Speed and Protein Composition Characteristics of Wheat Flour. Mod. Food Sci. Technol. 2021, 37, 108–114+97. [Google Scholar]
- Koga, S.; Böcker, U.; Wieser, H.; Koehler, P.; Uhlen, A.; Moldestad, A. Polymerisation of gluten proteins in developing wheat grain as affected by desiccation. J. Cereal Sci. 2017, 73, 122–129. [Google Scholar] [CrossRef]
- Wang, P.; Jin, Z.; Xu, X. Physicochemical alterations of wheat gluten proteins upon dough formation and frozen storage—A review from gluten, glutenin and gliadin perspectives. Trends Food Sci. Technol. 2015, 46, 189–198. [Google Scholar] [CrossRef]
- Koh, A.; Nishimura, K.; Urade, R. Relationship between Endogenous Protein Disulfide Isomerase Family Proteins and Glutenin Macropolymer. J. Agric. Food Chem. 2010, 58, 12970–12975. [Google Scholar] [CrossRef]
- Osipova, S.V.; Permyakova, M.; Permyakov, A.V. Role of Non-prolamin Proteins and Low Molecular Weight Redox Agents in Protein Folding and Polymerization in Wheat Grains and Influence on Baking Quality Parameters. J. Agric. Food Chem. 2012, 60, 12065–12073. [Google Scholar] [CrossRef]
- Simsek, S.; Ohm, J.-B.; Cariou, V.; Mergoum, M. Effect of flour polymeric proteins on dough thermal properties and breadmaking characteristics for hard red spring wheat genotypes. J. Cereal Sci. 2016, 68, 164–171. [Google Scholar] [CrossRef]
- Shewry, P.R.; Halford, N.G.; Tatham, A.S. High molecular weight subunits of wheat glutenin. J. Cereal Sci. 1992, 15, 105–120. [Google Scholar] [CrossRef]
- Zhensheng, L.; Li, L.; Meifang, W.; Jun, Y.; Pan, Y.; Yan, Z.; Zhonghu, H. Effects of HMW-GS and LMW-GS components on wheat processing quality. Acta Agron. Sin. 2009, 35, 8. [Google Scholar]
- Shewry, P.R.; Halford, N.G.; Belton, P.S.; Tatham, A.S. The structure and properties of gluten: An elastic protein from wheat grain. Philosophical Transactions of the Royal Society of London. Ser. B Biol. Sci. 2002, 357, 133–142. [Google Scholar] [CrossRef]
- Veraverbeke, W.S.; Delcour, J. Wheat Protein Composition and Properties of Wheat Glutenin in Relation to Breadmaking Functionality. Crit. Rev. Food Sci. Nutr. 2002, 42, 179–208. [Google Scholar] [CrossRef]
- Liu, Z.; Yan, Z.; Wan, Y.; Liu, K.; Zheng, Y.; Wang, D. Analysis of HMW glutenin subunits and their coding sequences in two, diploid Aegilops species. Theor. Appl. Genet. 2003, 106, 1368–1378. [Google Scholar] [CrossRef]
- Sun, X.; Hu, S.; Liu, X.; Qian, W.; Hao, S.; Zhang, A.; Wang, D. Characterization of the HMW glutenin subunits from Aegilops searsii and identification of a novel variant HMW glutenin subunit. Theor. Appl. Genet. 2006, 113, 631–641. [Google Scholar] [CrossRef]
- Yu, Z.; Peng, Y.; Islam, S.; She, M.; Lu, M.; Lafiandra, D.; Roy, N.; Juhasz, A.; Yan, G.; Ma, W. Molecular characterization and phylogenetic analysis of active y-type high molecular weight glutenin subunit genes at Glu-A1 locus in wheat. J. Cereal Sci. 2019, 86, 9–14. [Google Scholar] [CrossRef]
- Zheng, W.; Peng, Y.; Ma, J.; Appels, R.; Sun, D.; Ma, W. High frequency of abnormal high molecular weight glutenin alleles in Chinese wheat landraces of the Yangtze-River region. J. Cereal Sci. 2011, 54, 401–408. [Google Scholar] [CrossRef]
- Peng, Y.; Yu, K.; Zhang, Y.; Islam, S.; Sun, D.; Ma, W. Two Novel Y-Type High Molecular Weight Glutenin Genes in Chinese Wheat Landraces of the Yangtze-River Region. PLoS ONE 2015, 10, e0142348. [Google Scholar] [CrossRef]
- McIntosh, R.; Hart, G.; Gale, M. Catalogue of gene symbols for wheat 1991 supplement. Cereal Res. Commun. 1991, 18, 491–508. [Google Scholar]
- Junmei, C.; Xinzhong, Z.; Guiqiang, F.; Jing, L.; Xinyuan, W.; Anding, Z.; Lianzheng, L.; Shihui, N. Xinjiang Wheat Varieties: HMW-GS Expression and the Correlation Between HMW-GS Expression and Quality Characters. Chin. Agric. Sci. Bull. 2020, 36, 8–14. [Google Scholar]
- Payne, P.I.; Nightingale, M.A.; Krattiger, A.F.; Holt, L.M. The relationship between HMW glutenin subunit composition and the bread-making quality of British-grown wheat varieties. J. Sci. Food Agric. 1987, 40, 51–65. [Google Scholar] [CrossRef]
- Kolster, P.; Van Eeuwijk, F.A.; Van Gelder, W.M.J. Additive and epistatic effects of allelic variation at the high molecular weight glutenin subunit loci in determining the bread-making quality of breeding lines of wheat. Euphytica 1991, 55, 277–285. [Google Scholar] [CrossRef]
- Bushuk, W.; Zillman, R.R. Wheat cultivar identification by gliadin electrophoregrams. i. apparatus, method and nomenclature. Can. J. Plant Sci. 1979, 58, 505–515. [Google Scholar] [CrossRef]
- Redaelli, R.; Metakovsky, E.V.; Davidov, S.D.; Pogna, N.E. Two-Dimensional Mapping of Gliadins Using Biotypes and Null Mutants of Common Wheat Cultivar Saratovskaya 29. Hereditas 2004, 121, 131–137. [Google Scholar] [CrossRef]
- Barone, M.V.; Zimmer, K.P. Endocytosis and transcytosis of gliadin peptides. Mol. Cell. Pediatr. 2016, 3, 1–5. [Google Scholar] [CrossRef]
- Scherf, K.A.; Koehler, P.; Wieser, H. Gluten and wheat sensitivities—An overview. J. Cereal Sci. 2016, 67, 2–11. [Google Scholar] [CrossRef]
- Scherf, K.A.; Ciccocioppo, R.; Pohanka, M.; Rimarova, K.; Opatrilova, R.; Rodrigo, L.; Kruzliak, P. Biosensors for the Diagnosis of Celiac Disease: Current Status and Future Perspectives. Mol. Biotechnol. 2016, 58, 381–392. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biagi, F.; Fasano, A.; Green, P.H.R.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; et al. The Oslo definitions for coeliac disease and related terms. Gut 2013, 62, 43–52. [Google Scholar] [CrossRef]
- Buddrick, O.; Cornell, H.J.; Small, D.M. Reduction of toxic gliadin content of wholegrain bread by the enzyme caricain. Food Chem. 2015, 170, 343–347. [Google Scholar] [CrossRef]
- Scherf, K.A.; Wieser, H.; Koehler, P. Novel approaches for enzymatic gluten degradation to create high-quality gluten-free products. Food Res. Int. 2018, 110, 62–72. [Google Scholar] [CrossRef]
- Xue, L.; Li, Y.; Li, T.; Pan, H.; Liu, J.; Fan, M.; Qian, H.; Zhang, H.; Ying, H.; Wang, L. Phosphorylation and Enzymatic Hydrolysis with Alcalase and Papain Effectively Reduce Allergic Reactions to Gliadins in Normal Mice. J. Agric. Food Chem. 2019, 67, 6313–6323. [Google Scholar] [CrossRef]
- Abedi, E.; Pourmohammadi, K. Chemical modifications and their effects on gluten protein: An extensive review. Food Chem. 2021, 343, 128398. [Google Scholar] [CrossRef]
- Gil-Humanes, J.; Pistón, F.; Tollefsen, S.; Sollid, L.M.; Barro, F. Effective shutdown in the expression of celiac disease-related wheat gliadin T-cell epitopes by RNA interference. Proc. Natl. Acad. Sci. USA 2010, 107, 17023–17028. [Google Scholar] [CrossRef]
- Gil Humanes, J.; Pistón, F.; Barro, F.; Rosell, C.M. The Shutdown of Celiac Disease-Related Gliadin Epitopes in Bread Wheat by RNAi Provides Flours with Increased Stability and Better Tolerance to Over-Mixing. PLoS ONE 2014, 9, e91931. [Google Scholar] [CrossRef]
- Sánchez-León, S.; Gil Humanes, J.; Ozuna, C.V.; Gimenez, M.J.; Sousa, C.; Voytas, D.; Barro, F. Low-gluten, nontransgenic wheat engineered with CRISPR/Cas9. Plant Biotechnol. J. 2018, 16, 902–910. [Google Scholar] [CrossRef]
- Wang, D.W.; Li, D.; Wang, J.; Zhao, Y.; Wang, Z.; Yue, G.; Liu, X.; Qin, H.; Zhang, K.; Dong, L.; et al. Genome-wide analysis of complex wheat gliadins, the dominant carriers of celiac disease epitopes. Sci. Rep. 2017, 7, 44609. [Google Scholar] [CrossRef] [PubMed]
- Farrell, A.D.; Kettlewell, P.S.; Simmonds, J.; Flintham, J.E.; Snape, J.W.; Werner, P.; Jack, P.L. Control of late maturity alpha-amylase in wheat by the dwarfing gene Rht-D1b and genes on the 1B/1R translocation. Mol. Breed. 2013, 32, 425–436. [Google Scholar] [CrossRef]
- Xiangcun, Z.; Zhengling, L.; Yingying, C.; Haibin, D.; Chong, G.; Liupeng, H.; Rui, G.; Mingzhong, Z.; Lin, H.; Weigang, X. Effects of Sec-1 site deletion on grain quality of 1BL/1RS translocation lines. J. Triticeae Crops 2021, 41, 827–833. [Google Scholar]
- Yaqing, L.; Nan, Z.; Shichang, Z.; Mingqi, H.; Mengjun, L. Composition and quality analysis of 1BL/1RS translocation system HMW-GS. J. Triticeae Crops 2019, 39, 7. [Google Scholar]
- Graybosch, R.A. Mini Review: Uneasy Unions: Quality Effects of Rye Chromatin Transfers to Wheat. J. Cereal Sci. 2001, 33, 3–16. [Google Scholar] [CrossRef]
- Hohn, C.E.; Bektas, H. Preliminary tests indicate the absence of polymorphism among 1RS.1BL translocations in wheat for root biomass enhancement. Euphytica 2021, 217, 1–11. [Google Scholar] [CrossRef]
- Xiaole, M.; Qian, C.; Juncheng, W.; Lirong, Y.; Yaxiong, M.; Baochun, L.; Ke, Y.; Erjing, S.; Lulu, L.; Huajun, W.; et al. Determination of HMW-GS and 1BL/1RS translocation lines of wheat varieties of Gansu Province and the effects on quality. J. Gansu Agric. Univ. 2020, 55, 8. [Google Scholar]
- Li, Z.; Ren, T.; Yan, B.; Tan, F.; Yang, M.; Ren, Z. A Mutant with Expression Deletion of Gene Sec-1 in a 1RS.1BL Line and Its Effect on Production Quality of Wheat. PLoS ONE 2016, 11, e0146943. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, X.; Zhang, W.; Chen, X.; Yan, J.; Zhang, Y.; DeSen, W.; ZhongWei, W.; YueFang, L.; He, Z.; et al. Marker-assisted selection of HMW-glutenin 1Dx5+ 1Dy10 gene and 1B/1R translocation for improving industry quality in common wheat. Acta Agron. Sin. 2012, 38, 1743–1751. [Google Scholar] [CrossRef]
- Mago, R.; Spielmeyer, W.; Lawrence, G.J.; Ellis, J.G.; Pryor, A.J. Resistance genes for rye stem rust (SrR) and barley powdery mildew (Mla) are located in syntenic regions on short arm of chromosome. Genome 2004, 47, 112–121. [Google Scholar] [CrossRef]
- Xiaobo, L.; Yanbin, Z.; Qingjie, S.; Decai, Y.; Chunli, Z.; Haibin, Z. Effect of HMW-GS 5+10 on Quality Parameters of Strong-gluten Spring Wheat Cultivar Xiaobingmai 33. Acta Tritical. Crops 2004, 2, 45–48. [Google Scholar]
- He, Z.; Yang, J.; Zhang, Y.; Quail, K.; Peña, R. Pan bread and dry white Chinese noodle quality in Chinese winter wheats. Euphytica 2004, 139, 257–267. [Google Scholar] [CrossRef]
- Zhang, Y.; Nagamine, T.; He, Z.H.; Ge, X.X.; Yoshida, H.; Peña, R.J. Variation in quality traits in common wheat as related to Chinese fresh white noodle quality. Euphytica 2005, 141, 113–120. [Google Scholar] [CrossRef]
- Zhao, L.; Song, W.; Yang, X.; Song, Q.; Zhang, C.; Wenli, X.; Xiao, Z. Application of Marker-assisted Back-crossing Breeding in Quality Improvement of Strong-gluten Wheat. Chin. Agric. Sci. Bull. 2020, 36, 8–11. [Google Scholar]
- Pingping, Z.; Dongsheng, C.; Yong, Z. Gliadin Composition and Their Effects on Quality Properties in Spring Wheat. Acta Agron. Sin. 2006, 32, 1796–1801. [Google Scholar]
- Chai, J.; Wang, H.; Ma, X.; Zhang, C.; Dong, F. Effect of ω-Secalin Gene Silencing on Processing Quality of Wheat 1B/1R Translocation Line. Acta Agron. Sin. 2016, 42, 6. [Google Scholar] [CrossRef]
- He, Z.H.; Liu, L.; Xia, X.C.; Liu, J.J.; Peña, R.J. Composition of HMW and LMW Glutenin Subunits and Their Effects on Dough Properties, Pan Bread, and Noodle Quality of Chinese Bread Wheats. Cereal Chem. 2005, 82, 345–350. [Google Scholar] [CrossRef]
- Zhao, D.; Jun, Y.; Yu-Lian, H.; Xian-Chun, X.; Yan, Z.; Tian, Y.; He, Z.; Zhang, Y. Effect of 1BL/1RS Translocation on Gluten Protein Fraction Quantities and Dough Rheological Properties. Acta Agron. Sin. 2015, 41, 1648–1656. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, Y.; Xia, X.; He, Z.; Shen, X.; Zhang, W.; Chen, X.; Yan, J.; Zhang, Y.; Wang, D. Application of high molecular weight gluten 5 + 10 subunit and 1b/1R translocation molecular marker-assisted selection in wheat quality breeding. Acta Agron. Sin. 2012, 38, 9. [Google Scholar]
- Zhu, J.; Huang, S.; Khan, K.; O Brien, L. Relationship of Protein Quantity, Quality and Dough Properties with Chinese Steamed Bread Quality. J. Cereal Sci. 2001, 33, 205–212. [Google Scholar] [CrossRef]
- Aamodt, A.; Magnus, E.M.; Færgestad, E.M. Effect of Protein Quality, Protein Content, Bran Addition, DATEM, Proving Time, and Their Interaction on Hearth Bread. Cereal Chem. 2004, 81, 722–734. [Google Scholar] [CrossRef]
- van Herpen, T.W.; Goryunova, S.V.; van der Schoot, J.; Mitreva, M.; Salentijn, E.; Vorst, O.; Schenk, M.F.; van Veelen, P.A.; Koning, F.; Smulders, M.J.; et al. Alpha-gliadin genes from the A, B, and D genomes of wheat contain different sets of celiac disease epitopes. BMC Genom. 2006, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R.; Tatham, A.S. Improving wheat to remove coeliac epitopes but retain functionality. J. Cereal Sci. 2016, 67, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Rui, W.; Shuobi, L. The Relation Between More Spikelet And High Molecular Weight Subunit Compositions In Hexaploid Wheat. Acta Bot. Boreali-Occident. Sin. 1995, 15, 5. [Google Scholar]
- Yangjie, H.; Huifang, T.; Quanhao, S.; Faji, L.; Daojie, S. Genetic effects of 1BL/1RS and 7DL·7Ag translocations on major agronomic traits in wheat. J. Triticeae Crops 2012, 32, 610–615. [Google Scholar]
- Xiaoke, Z.; Yimin, W. Method and Its Effect of Rapid Introduction of HMW-GS Genes with Good Baking Properties into High-Yielding Wheat. Line Sci. Agric. Sin. 2005, 1, 208–212. [Google Scholar]
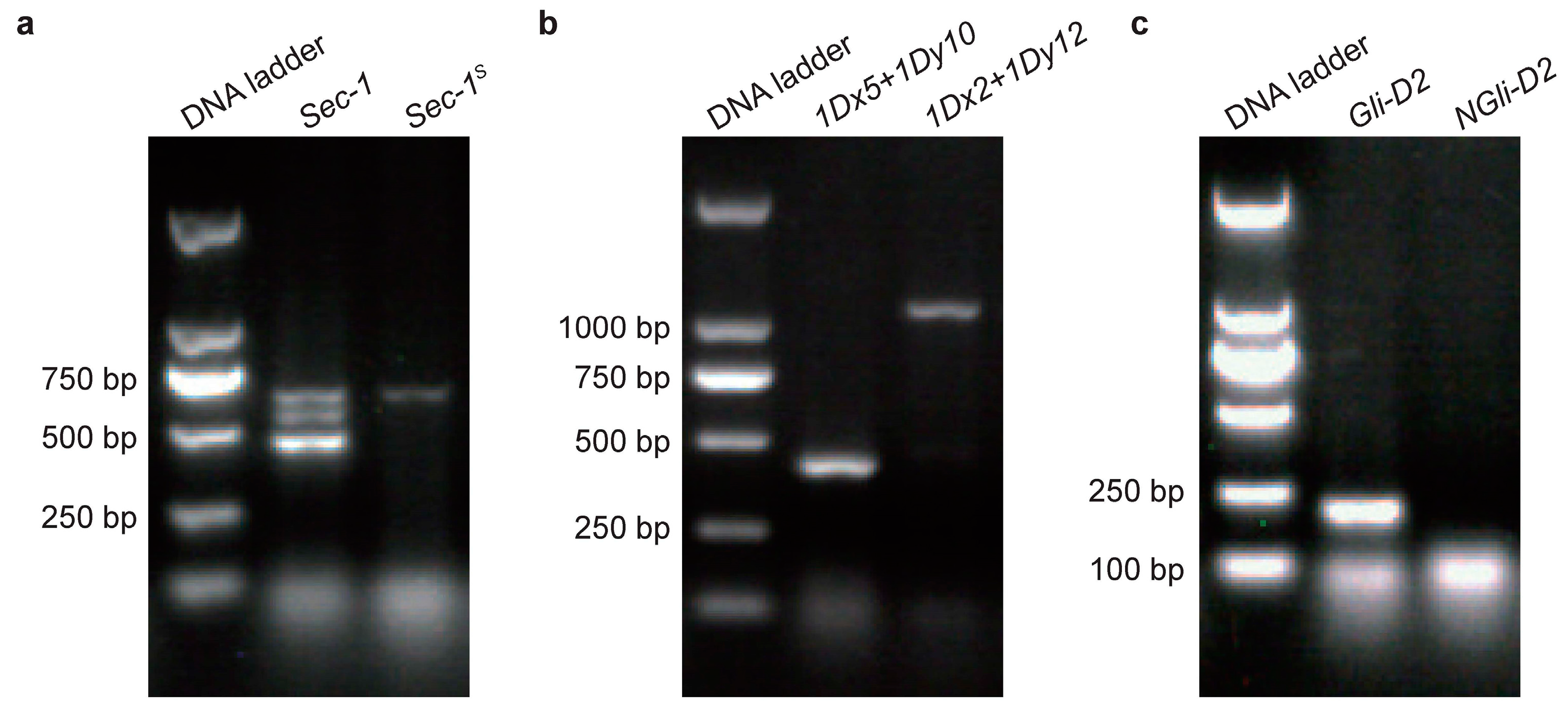

| Background | Pyramid | Sedimentation Value (mL) | Wet Gluten Content (%) | Gluten Index (%) |
|---|---|---|---|---|
| Hengguan 35 | CK | 28.2 ± 3.4b | 33.83 ± 0.81b | 63.67 ± 2.02b |
| Type I digenic lines | 41.30 ± 4.88a | 38.6 ± 2.48ab | 92.58 ± 4.15a | |
| Type II digenic lines | 48.67 ± 6.30a | 44.07 ± 7.47a | 84.03 ± 14.47a | |
| Trigenic lines | 45.82 ± 6.08a | 43.35 ± 6.48ab | 82.69 ± 3.52a | |
| Zhengmai 7698 | CK | 31 ± 1.21b | 37.47 ± 3.25b | 70.2 ± 1.11a |
| Type II digenic lines | 47.81 ± 4.85a | 46.41 ± 5.23a | 76.37 ± 10.04a | |
| Trigenic lines | 54.00 ± 2.88a | 42.67 ± 6.96ab | 88.23 ± 5.59a | |
| Zhengmai 366 | CK | 44.17 ± 0.76a | 36.87 ± 1.44a | 81.37 ± 3.95b |
| Type I digenic lines | 45.61 ± 4.22a | 38.06 ± 1.7a | 95.36 ± 1.91a |
| Background | Pyramids | MPT (Midline Peak Time) | MPV (Midline Peak Value) | MPW (Midline Peak Width) | CTV (Curve Tail Value) | CTW (Curve Tail Width) | MTxV (Time = 8 min Value) | MTxW (Time = 8 min Width) | MTxI (Integral Area) |
|---|---|---|---|---|---|---|---|---|---|
| Hengguan 35 | CK | 1.82 ± 0.02b | 59.71 ± 2.08b | 16.36 ± 1.21b | 41.12 ± 0.58b | 4.81 ± 0.58b | 43.77 ± 1.53b | 3.93 ± 0.58b | 410.54 ± 51.03b |
| Type Idigenic lines | 3.59 ± 0.72a | 66.14 ± 8.18ab | 26.70 ± 8.55a | 51.95 ± 7.19a | 9.89 ± 4.95a | 54.43 ± 7.39a | 11.24 ± 3.36a | 444.86 ± 44.61ab | |
| Type IIdigenic lines | 3.86 ± 0.37a | 66.91 ± 7.17ab | 26.44 ± 4.80a | 48.95 ± 10.47ab | 7.12 ± 3.15ab | 54.62 ± 6.51a | 9.24 ± 3.54ab | 453.34 ± 51.00ab | |
| Trigenic lines | 3.36 ± 0.74a | 69.91 ± 5.38a | 23.30 ± 3.73ab | 53.43 ± 4.32a | 10.08 ± 2.12a | 56.30 ± 4.29a | 10.07 ± 3.51a | 474.12 ± 33.74a | |
| Zhengmai 7698 | CK | 1.88 ± 0.15b | 56.93 ± 1.15b | 21.32 ± 1.00b | 41.59 ± 1.53b | 3.91 ± 0.25b | 40.07 ± 1.15b | 4.41 ± 0.26b | 387.07 ± 10.00b |
| Type IIdigenic lines | 3.19 ± 0.70a | 66.48 ± 6.42a | 20.44 ± 5.04b | 48.16 ± 2.98ab | 5.66 ± 0.85b | 50.39 ± 3.23a | 6.33 ± 1.41b | 441.25 ± 26.65a | |
| Trigenic lines | 4.23 ± 1.08a | 65.24 ± 6.36ab | 32.27 ± 7.68a | 54.05 ± 7.89a | 13.25 ± 3.99a | 57.16 ± 8.27a | 16.10 ± 4.35a | 447.84 ± 39.57a | |
| Zhengmai 366 | CK | 3.21 ± 0.09b | 58.31 ± 1.00b | 19.01 ± 2.00b | 49.95 ± 2.08a | 5.43 ± 0.58b | 47.07 ± 2.65a | 7.83 ± 0.58b | 413.30 ± 10.00a |
| Type Idigenic lines | 4.09 ± 0.57a | 66.10 ± 4.94a | 28.05 ± 5.43a | 52.32 ± 5.01a | 9.46 ± 2.12a | 55.54 ± 5.56a | 12.48 ± 2.86a | 499.78 ± 32.83a |
| Background | Pyramid | UPP% | Glu/Gli |
|---|---|---|---|
| Hengguan 35 | CK | 0.24 ± 0.01c | 0.44 ± 0.09b |
| Type I digenic lines | 0.38 ± 0.06bc | 0.60 ± 0.65a | |
| Type II digenic lines | 0.48 ± 0.06a | 0.54 ± 0.60ab | |
| Trigenic lines | 0.44 ± 0.11ab | 0.65 ± 0.13a | |
| Zhengmai 7698 | CK | 0.21 ± 0.02c | 0.44 ± 0.18b |
| Type II digenic lines | 0.44 ± 0.08a | 0.56 ± 0.07a | |
| Trigenic lines | 0.35 ± 0.05b | 0.57 ± 0.05a | |
| Zhengmai 366 | CK | 0.41 ± 0.02a | 0.51 ± 0.02b |
| Type I digenic lines | 0.40 ± 0.06a | 0.64 ± 0.05a |
| Background | Pyramid | Spike Length (cm) | Spikelet Number | Grain Number per Spike | Plant Height (cm) | Thousand-Kernel Weight (g) |
|---|---|---|---|---|---|---|
| Hengguan 35 | CK | 9.56 ± 0.50a | 16.60 ± 1.14a | 50.60 ± 4.51a | 82.20 ± 5.54a | 42.86 ± 0.67b |
| Type I digenic lines | 9.94 ± 0.56a | 17.00 ± 1.87a | 51.20 ± 5.17a | 76.40 ± 10.33ab | 45.68 ± 4.23ab | |
| Type II digenic lines | 9.72 ± 0.52a | 15.60 ± 2.30a | 54.20 ± 1.48a | 71.20 ± 3.77b | 48.36 ± 1.33a | |
| Trigenic lines | 9.82 ± 0.86a | 16.60 ± 1.87a | 53.20 ± 5.26a | 78.80 ± 4.60ab | 42.10 ± 2.60b | |
| Zhengmai 7698 | CK | 9.52 ± 0.47a | 19.20 ± 2.39a | 40.40 ± 1.67a | 77.80 ± 3.27a | 44.88 ± 0.32a |
| Type II digenic lines | 10.04 ± 0.30ab | 21.40 ± 3.21a | 45.40 ± 4.39ab | 76.60 ± 5.27a | 50.80 ± 7.96a | |
| Trigenic lines | 10.26 ± 0.48a | 21.00 ± 3.67a | 47.20 ± 4.76a | 72.60 ± 5.59a | 45.58 ± 1.54a | |
| Zhengmai 366 | CK | 9.92 ± 0.30a | 19.40 ± 2.70a | 39.60 ± 5.94a | 69.60 ± 3.05a | 42.42 ± 1.72b |
| Type I digenic lines | 10.04 ± 0.55a | 21.00 ± 1.87a | 43.60 ± 6.43a | 72.20 ± 4.32a | 46.34 ± 1.77a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bu, Z.; Fang, G.; Yu, H.; Kong, D.; Huo, Y.; Ma, X.; Chong, H.; Guan, X.; Liu, D.; Fan, K.; et al. Quality and Agronomic Trait Analyses of Pyramids Composed of Wheat Genes NGli-D2, Sec-1s and 1Dx5+1Dy10. Int. J. Mol. Sci. 2023, 24, 9253. https://doi.org/10.3390/ijms24119253
Bu Z, Fang G, Yu H, Kong D, Huo Y, Ma X, Chong H, Guan X, Liu D, Fan K, et al. Quality and Agronomic Trait Analyses of Pyramids Composed of Wheat Genes NGli-D2, Sec-1s and 1Dx5+1Dy10. International Journal of Molecular Sciences. 2023; 24(11):9253. https://doi.org/10.3390/ijms24119253
Chicago/Turabian StyleBu, Zhimu, Gongyan Fang, Haixia Yu, Dewei Kong, Yanbing Huo, Xinyu Ma, Hui Chong, Xin Guan, Daxin Liu, Kexin Fan, and et al. 2023. "Quality and Agronomic Trait Analyses of Pyramids Composed of Wheat Genes NGli-D2, Sec-1s and 1Dx5+1Dy10" International Journal of Molecular Sciences 24, no. 11: 9253. https://doi.org/10.3390/ijms24119253
APA StyleBu, Z., Fang, G., Yu, H., Kong, D., Huo, Y., Ma, X., Chong, H., Guan, X., Liu, D., Fan, K., Yan, M., Ma, W., & Chen, J. (2023). Quality and Agronomic Trait Analyses of Pyramids Composed of Wheat Genes NGli-D2, Sec-1s and 1Dx5+1Dy10. International Journal of Molecular Sciences, 24(11), 9253. https://doi.org/10.3390/ijms24119253









