The Regulation of the Growth and Pathogenicity of Valsa mali by the Carbon Metabolism Repressor CreA
Abstract
1. Introduction
2. Results
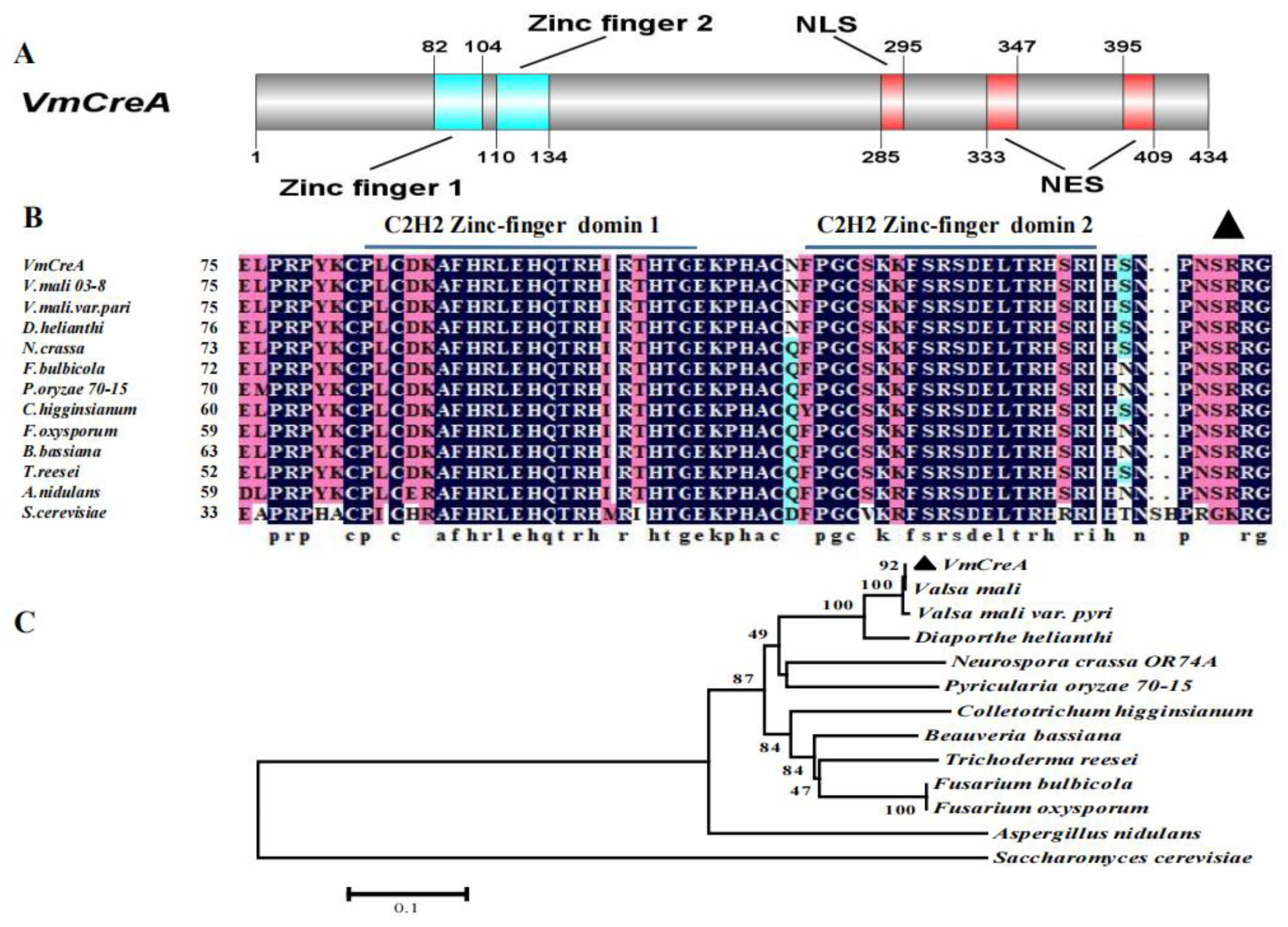
2.1. Identification of the V. mali CreA
2.2. Construction of the VmCreA Deletion and Complementation of the Mutant Strains
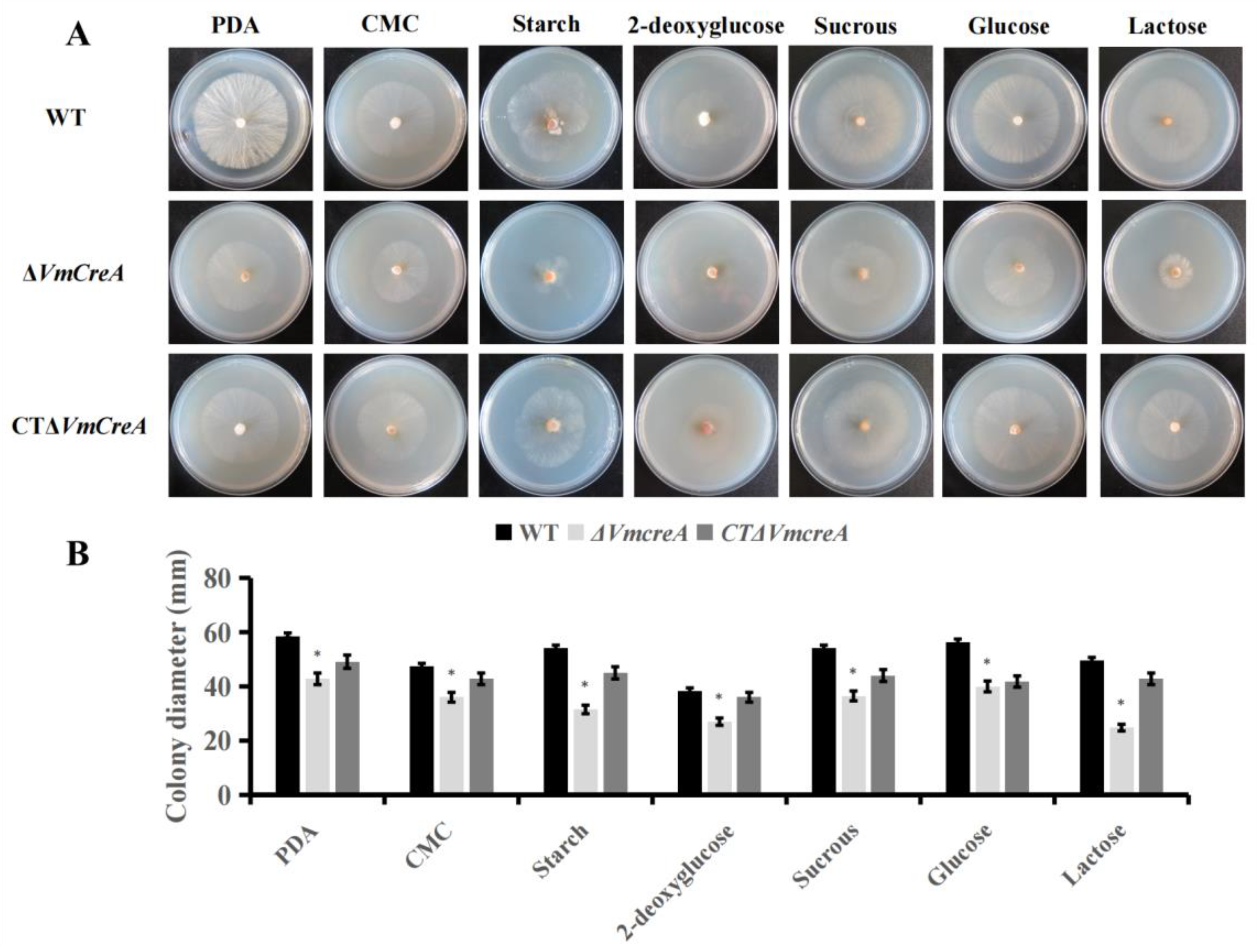
2.3. Influencing Effects of VmCreA on the Vegetative Growth and Development of V. mali
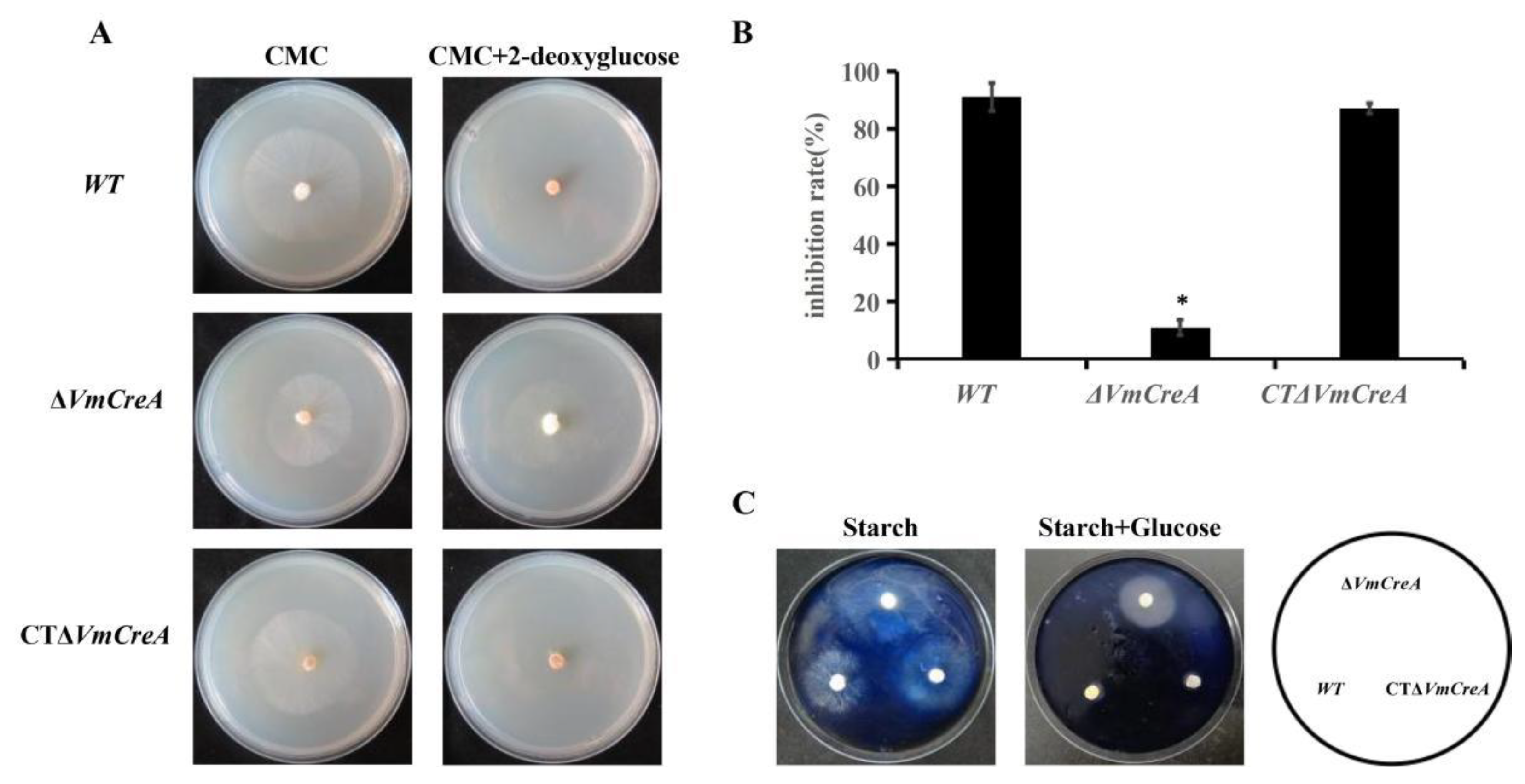
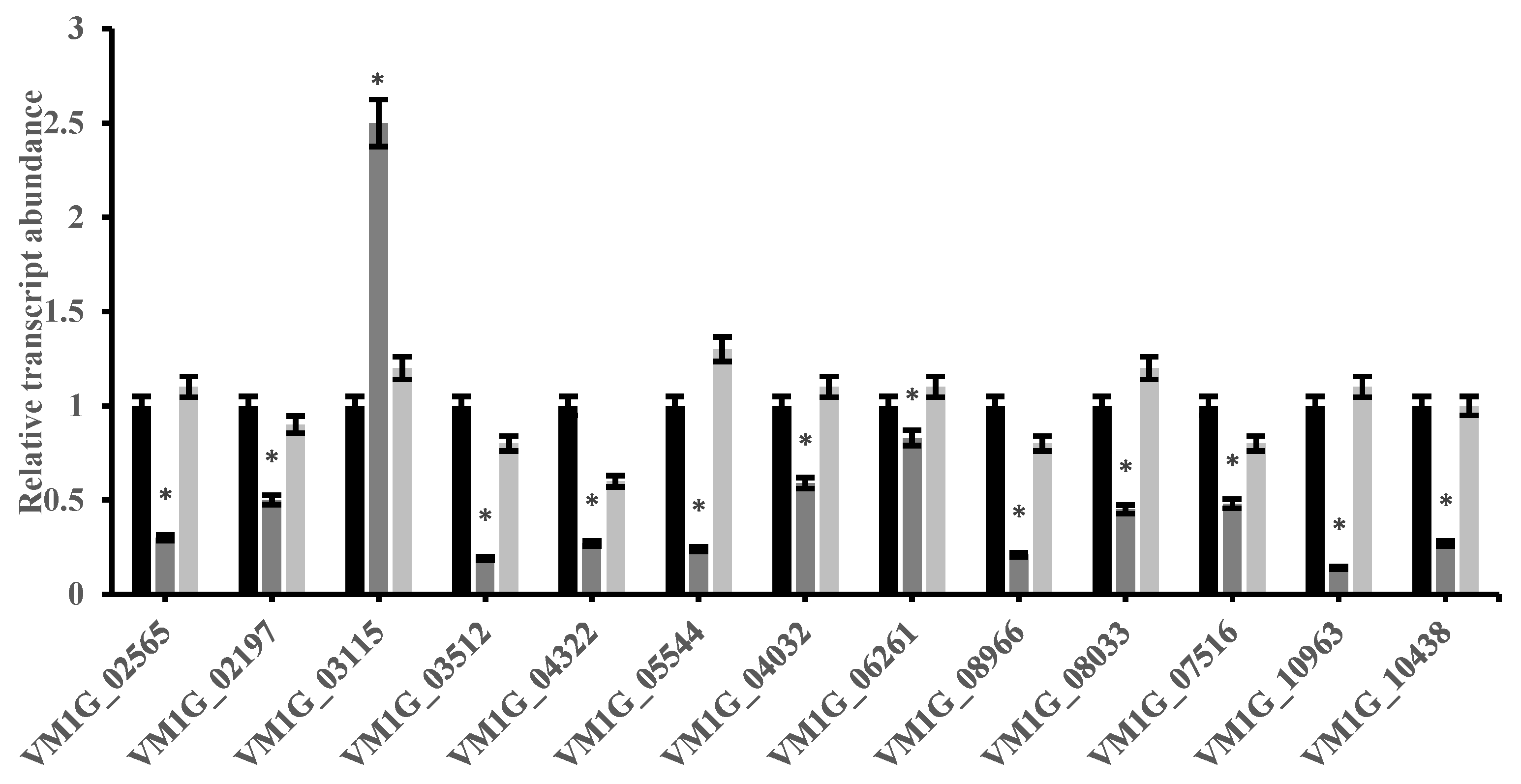
2.4. VmCreA Participates in the CCR of V. mali
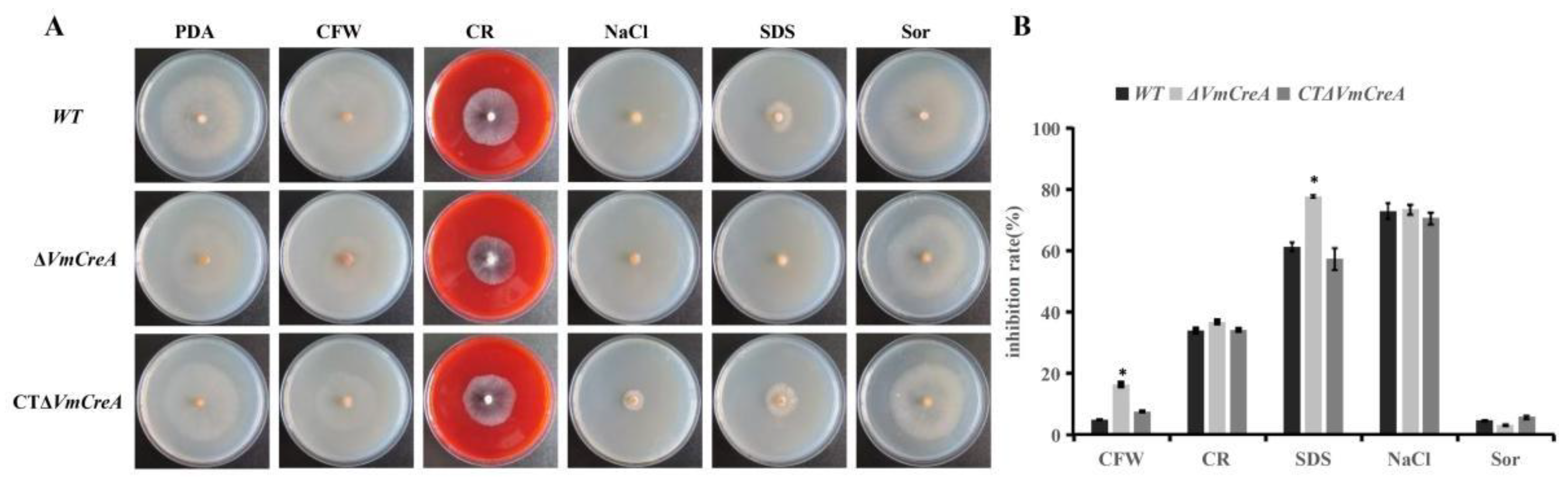
2.5. Stress Responses of the V. mali Are Influenced by VmCreA
2.6. VmCreA Is Required for V. mali Pathogenicity
2.7. VmCreA Regulates Hydrolases Production
3. Discussion
4. Materials and Methods
4.1. Fungal Strains and Culture Manipulations
4.2. Cloning and Sequencing Analysis of the VmCreA
4.3. Targeted Deletion and Complementation of the CreA Gene
4.4. Quantitative Reverse Transcription Polymerase Chain Reactions
4.5. Observations of the Growth Rates, Conidia, and Morphological Processes
4.6. Cell-Wall Integrity Inhibitor Stress Assay
4.7. Pathogenic Determination Assays
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Zang, R.; Yin, Z.; Kang, Z.; Huang, L. Delimiting cryptic pathogen species causing apple Valsa canker with multilocus data. Ecol. Evol. 2014, 4, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Liu, X. Review of researches on the Valsa canker of apple trees. J. Hebei For. Coll. 1995, 10, 87–91. [Google Scholar]
- Aldwinckle, H.; Jones, A.L. Compendium of Apple and Pear Diseases; APS Press: Algiers, Algeria, 1990. [Google Scholar]
- Hong, Y.; Cai, R.; Guo, J.; Zhong, Z.; Bao, J.; Wang, Z.; Chen, X.; Zhou, J.; Lu, G.-D. Carbon catabolite repressor MoCreA is required for the asexual development and pathogenicity of the rice blast fungus. Fungal Genet. Biol. 2021, 146, 103496. [Google Scholar] [CrossRef]
- Gancedo, J.M. Yeast carbon catabolite repression. Microbiol. Mol. Biol. Rev. 1998, 62, 334–361. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Zheng, W.; Islam, W.; Arif, M.; Abubakar, Y.S.; Wang, Z.; Lu, G. Carbon catabolite repression in filamentous fungi. Int. J. Mol. Sci. 2017, 19, 48. [Google Scholar] [CrossRef] [PubMed]
- Dowzer, C.E.; Kelly, J.M. Cloning of the cre A gene from Aspergillus nidulans: A gene involved in carbon catabolite repression. Curr. Genet. 1989, 15, 457–459. [Google Scholar] [CrossRef]
- Dowzer, C.; Kelly, J.M. Analysis of the creA gene, a regulator of carbon catabolite repression in Aspergillus nidulans. Mol. Cell. Biol. 1991, 11, 5701–5709. [Google Scholar]
- Ebbole, D.J. Carbon catabolite repression of gene expression and conidiation in Neurospora crassa. Fungal Genet. Biol. 1998, 25, 15–21. [Google Scholar] [CrossRef]
- Jin, K.; Luo, Z.; Jiang, X.; Zhang, Y.; Zhou, Y.; Pei, Y. Carbon catabolite repressor gene BbCre1 influences carbon source uptake but does not have a big impact on virulence in Beauveria bassiana. J. Invertebr. Pathol. 2011, 106, 400–406. [Google Scholar] [CrossRef]
- Nehlin, J.O.; Ronne, H. Yeast MIG1 repressor is related to the mammalian early growth response and Wilms’ tumour finger proteins. EMBO J. 1990, 9, 2891–2898. [Google Scholar] [CrossRef]
- Fasoyin, O.E.; Wang, B.; Qiu, M.; Han, X.; Chung, K.-R.; Wang, S. Carbon catabolite repression gene creA regulates morphology, aflatoxin biosynthesis and virulence in Aspergillus flavus. Fungal Genet. Biol. 2018, 115, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Diao, Y.; Yu, C.; Xiong, X.; Zhao, T.; He, B.; Liu, H. Transcriptome Analysis during Apple Stem Infected by Valsa mali. J. Shandong Agric. Univ. Nat. Sci. Ed. 2021, 52, 187–193. [Google Scholar]
- Kosugi, S.; Hasebe, M.; Tomita, M.; Yanagawa, H. Systematic identification of cell cycle-dependent yeast nucleocytoplasmic shuttling proteins by prediction of composite motifs. Proc. Natl. Acad. Sci. USA 2009, 106, 10171–10176. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wen, L.; Gao, X.; Jin, C.; Xue, Y.; Yao, X. DOG 1.0: Illustrator of protein domain structures. Cell Res. 2009, 19, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Szilágyi, M.; Miskei, M.; Karányi, Z.; Lenkey, B.; Pócsi, I.; Emri, T. Transcriptome changes initiated by carbon starvation in Aspergillus nidulans. Microbiology 2013, 159 Pt 1, 176–190. [Google Scholar] [CrossRef]
- Cziferszky, A.; Seiboth, B.; Kubicek, C.P. The Snf1 kinase of the filamentous fungus Hypocrea jecorina phosphorylates regulation-relevant serine residues in the yeast carbon catabolite repressor Mig1 but not in the filamentous fungal counterpart Cre1. Fungal Genet. Biol. 2003, 40, 166–175. [Google Scholar] [CrossRef]
- Vautard, G.; Cotton, P.; Fèvre, M. The glucose repressor CRE1 from Sclerotinia sclerotiorum is functionally related to CREA from Aspergillus nidulans but not to the Mig proteins from Saccharomyces cerevisiae. FEBS Lett. 1999, 453, 54–58. [Google Scholar] [CrossRef]
- Kulmburg, P.; Mathieu, M.; Dowzer, C.; Kelly, J.; Felenbok, B. Specific binding sites in the alcR and alcA promoters of the ethanol regulon for the CREA repressor mediating carbon cataboiite repression in Aspergillus nidulans. Mol. Microbiol. 1993, 7, 847–857. [Google Scholar] [CrossRef]
- Portnoy, T.; Margeot, A.; Linke, R.B.; Atanasova, L.; Fekete, E.; Sándor, E.; Hartl, L.; Karaffa, L.; Druzhinina, I.S.; Seiboth, B.; et al. The CRE1 carbon catabolite repressor of the fungus Trichoderma reesei: A master regulator of carbon assimilation. BMC Genom. 2011, 12, 269. [Google Scholar] [CrossRef]
- Duran, R.; Cary, J.W.; Calvo, A.M. Role of the osmotic stress regulatory pathway in morphogenesis and secondary metabolism in filamentous fungi. Toxins 2010, 2, 367–381. [Google Scholar] [CrossRef]
- Mellon, J.E.; Cotty, P.J.; Dowd, M.K. Aspergillus flavus hydrolases: Their roles in pathogenesis and substrate utilization. Appl. Microbiol. Biotechnol. 2007, 77, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Yin, Z.; Song, N.; Dai, Q.; Voegele, R.T.; Liu, Y.; Wang, H.; Gao, X.; Kang, Z.; Huang, L. Transcriptome profiling to identify genes involved in pathogenicity of Valsa mali on apple tree. Fungal Genet. Biol. 2014, 68, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-H.; Hamari, Z.; Han, K.-H.; Seo, J.-A.; Reyes-Domínguez, Y.; Scazzocchio, C. Double-joint PCR: A PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet. Biol. 2004, 41, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Ke, X.; Huang, D.; Gao, X.; Voegele, R.T.; Kang, Z.; Huang, L. Validation of reference genes for gene expression analysis in Valsa mali var. mali using real-time quantitative PCR. World J. Microbiol. Biotechnol. 2013, 29, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wu, Y.; Yin, Z.; Xu, L.; Feng, H.; Huang, L. VmPacC is required for acidification and virulence in Valsa mali. Front. Microbiol. 2018, 9, 1981. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, J.; Diao, Y.; Xiong, X.; Yu, C.; Tian, Y.; Li, C.; Liu, H. The Regulation of the Growth and Pathogenicity of Valsa mali by the Carbon Metabolism Repressor CreA. Int. J. Mol. Sci. 2023, 24, 9252. https://doi.org/10.3390/ijms24119252
Jin J, Diao Y, Xiong X, Yu C, Tian Y, Li C, Liu H. The Regulation of the Growth and Pathogenicity of Valsa mali by the Carbon Metabolism Repressor CreA. International Journal of Molecular Sciences. 2023; 24(11):9252. https://doi.org/10.3390/ijms24119252
Chicago/Turabian StyleJin, Jiyang, Yufei Diao, Xiong Xiong, Chengming Yu, Yehan Tian, Chuanrong Li, and Huixiang Liu. 2023. "The Regulation of the Growth and Pathogenicity of Valsa mali by the Carbon Metabolism Repressor CreA" International Journal of Molecular Sciences 24, no. 11: 9252. https://doi.org/10.3390/ijms24119252
APA StyleJin, J., Diao, Y., Xiong, X., Yu, C., Tian, Y., Li, C., & Liu, H. (2023). The Regulation of the Growth and Pathogenicity of Valsa mali by the Carbon Metabolism Repressor CreA. International Journal of Molecular Sciences, 24(11), 9252. https://doi.org/10.3390/ijms24119252





