Faujasite-Type Zeolite Obtained from Ecuadorian Clay as a Support of ZnTiO3/TiO2 NPs for Cyanide Removal in Aqueous Solutions
Abstract
1. Introduction
2. Results
2.1. Characterization
2.1.1. XRD Analysis
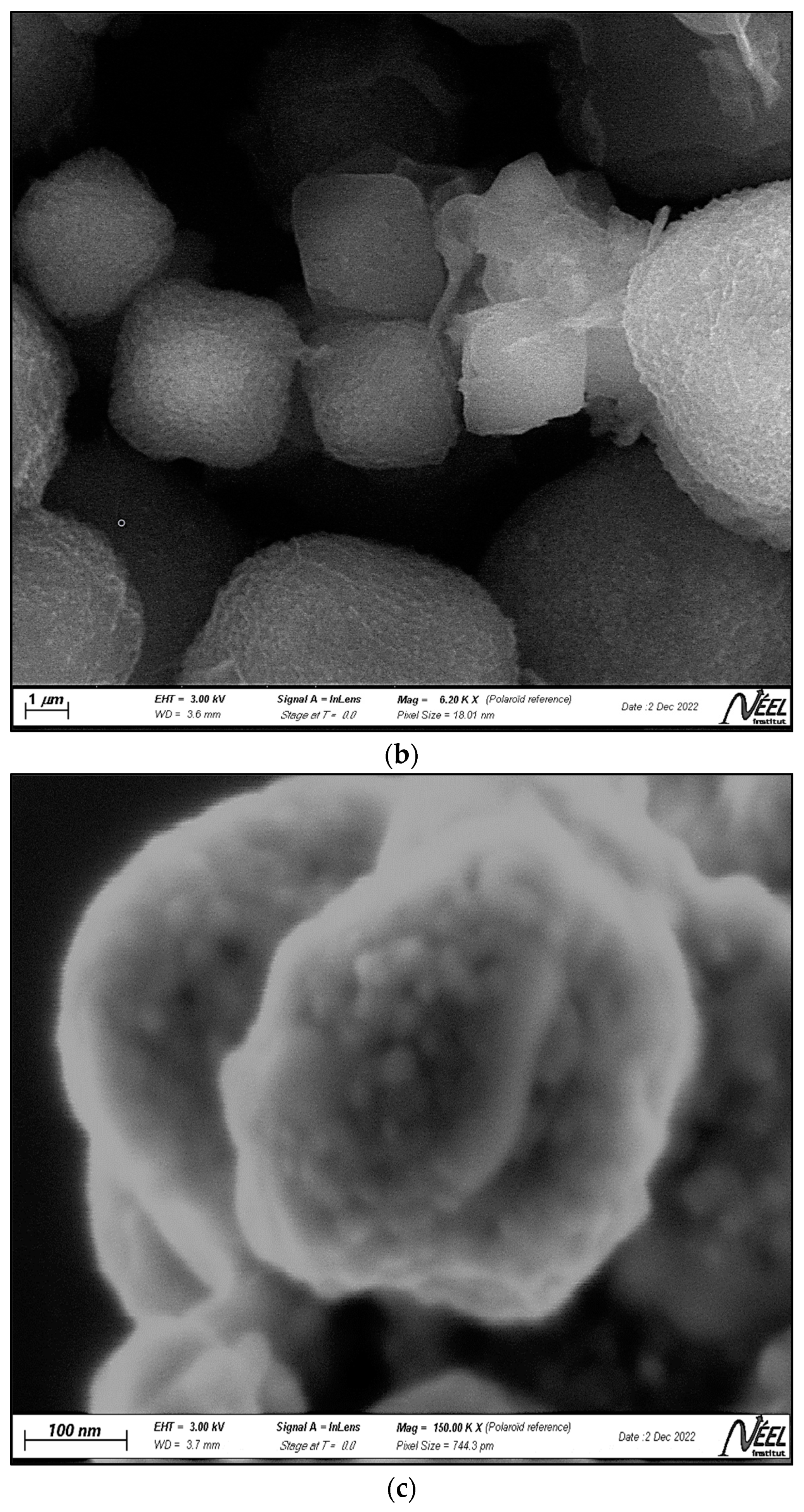
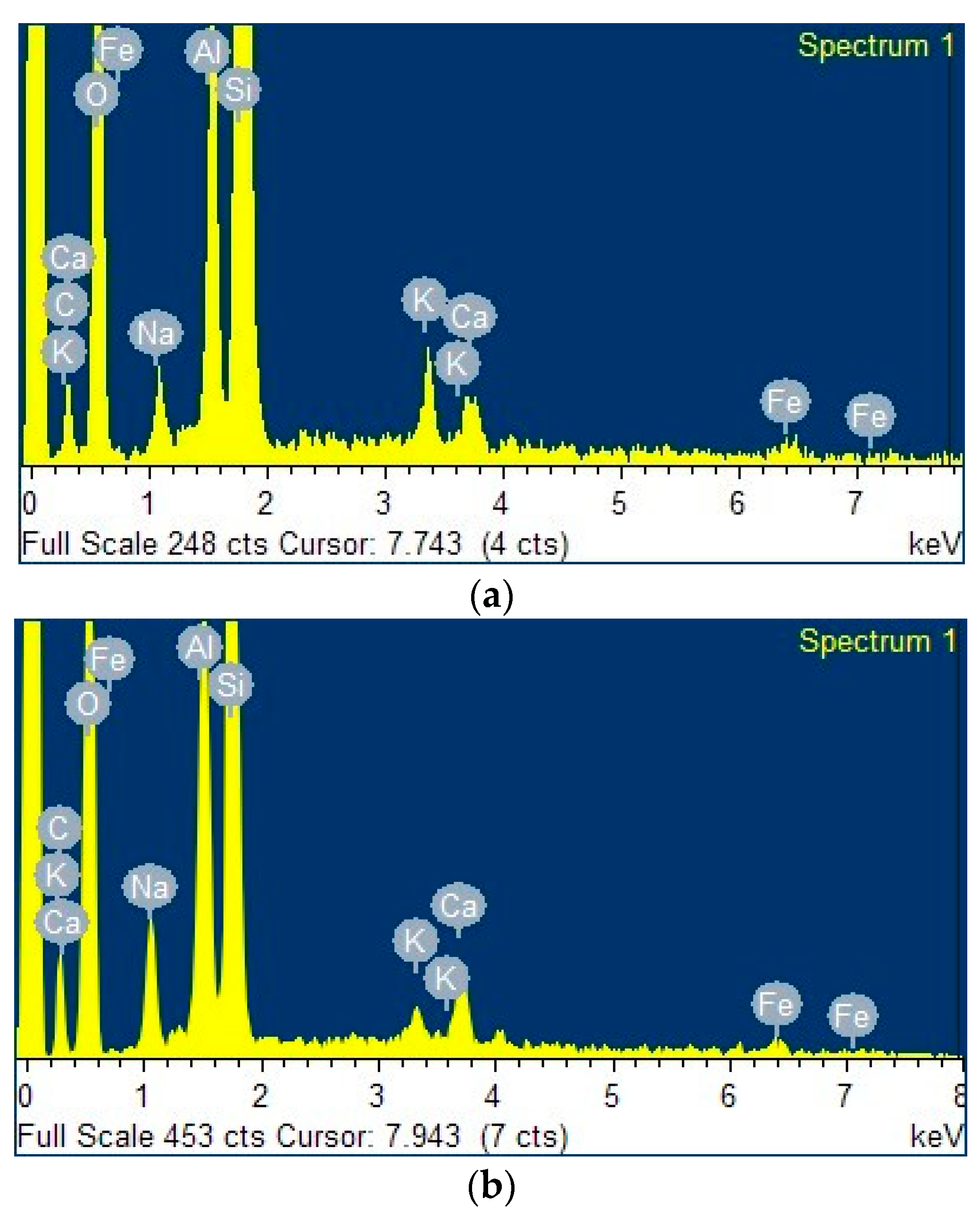
2.1.2. SEM-EDX Analysis
2.1.3. Specific Surface Area (SSA) and Point of Zero Charge (PZC) Analysis
2.2. Adsorption Studies
2.2.1. Effect of pH
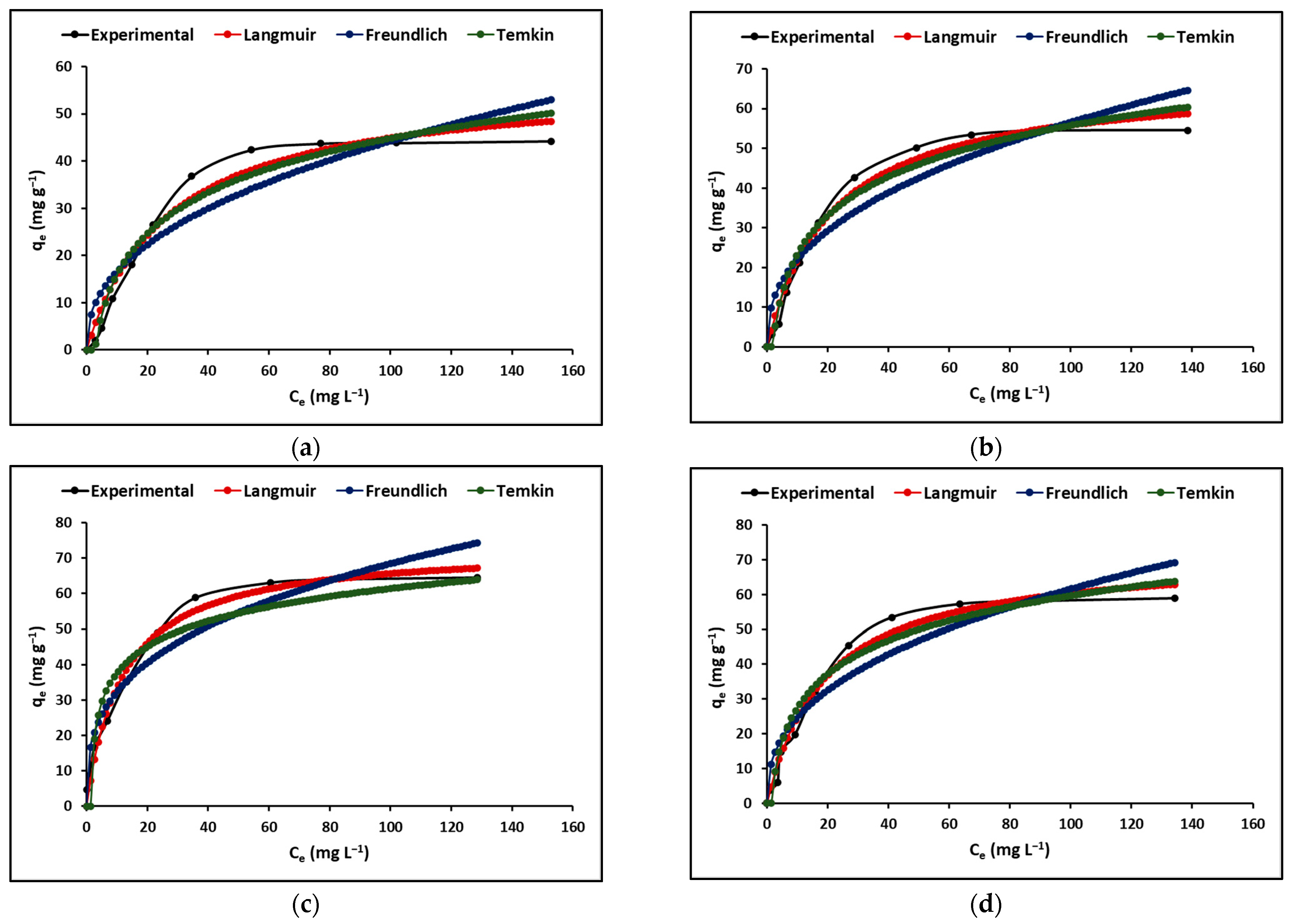
2.2.2. Adsorption Isotherm
2.2.3. Adsorption Thermodynamics
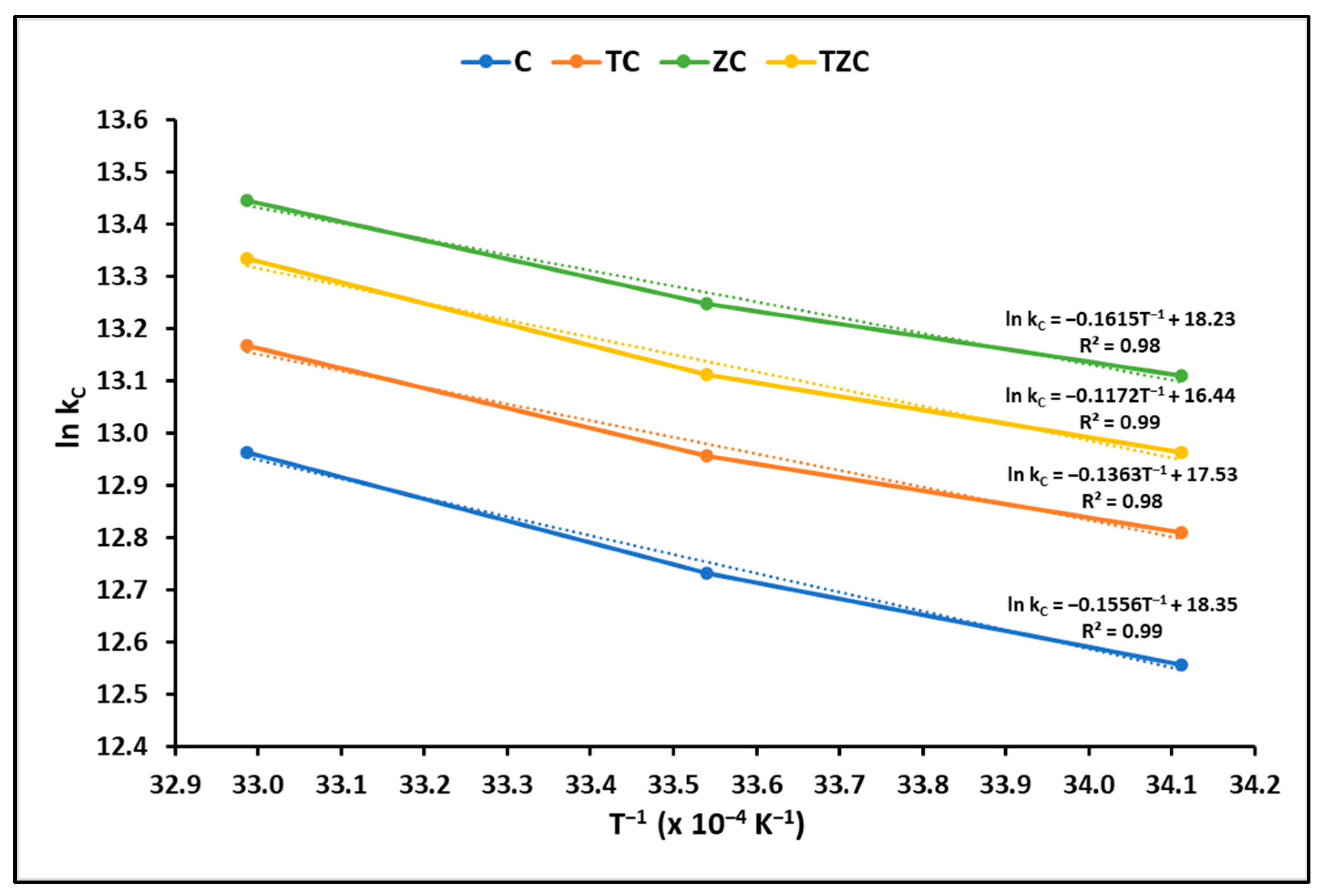
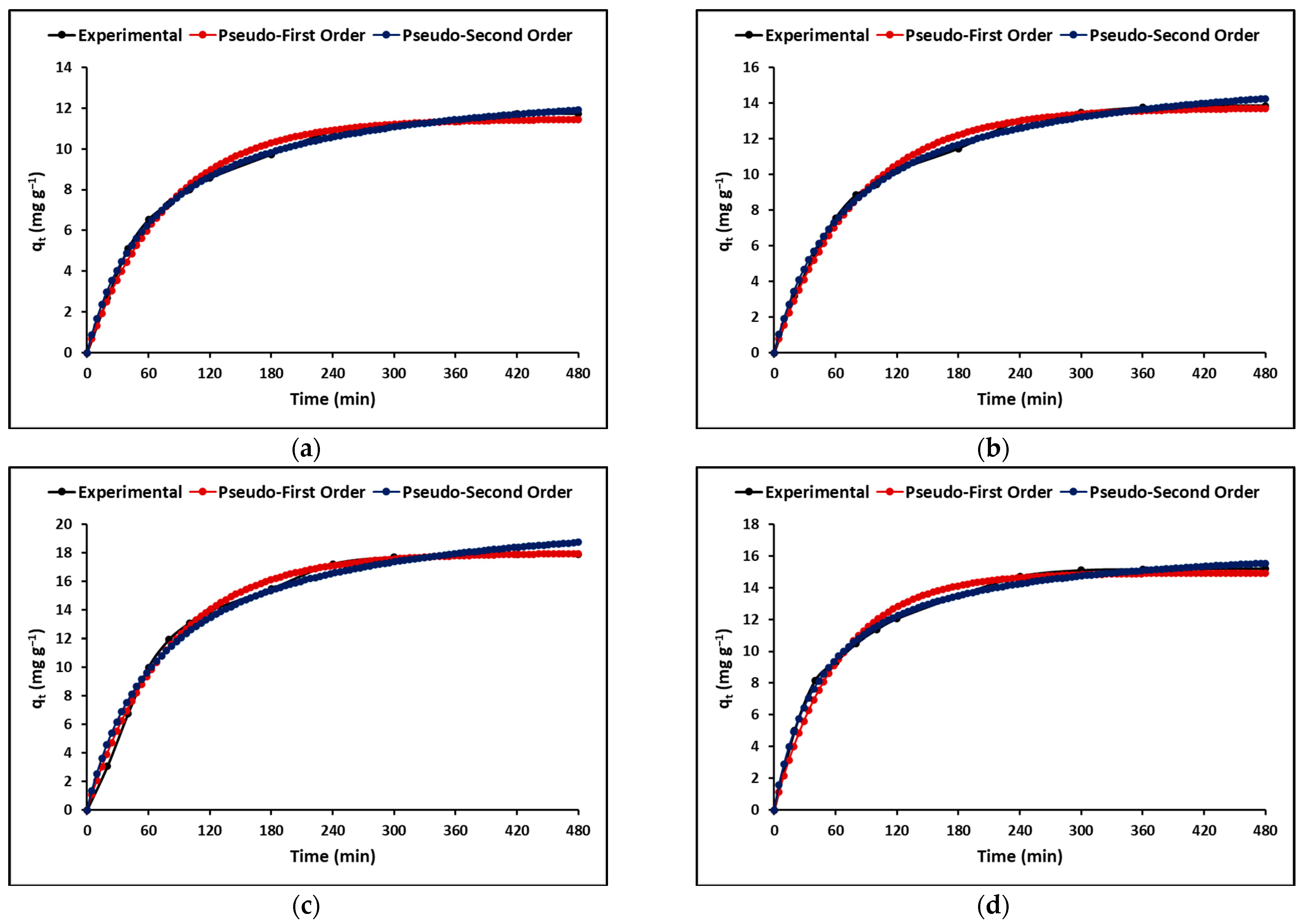
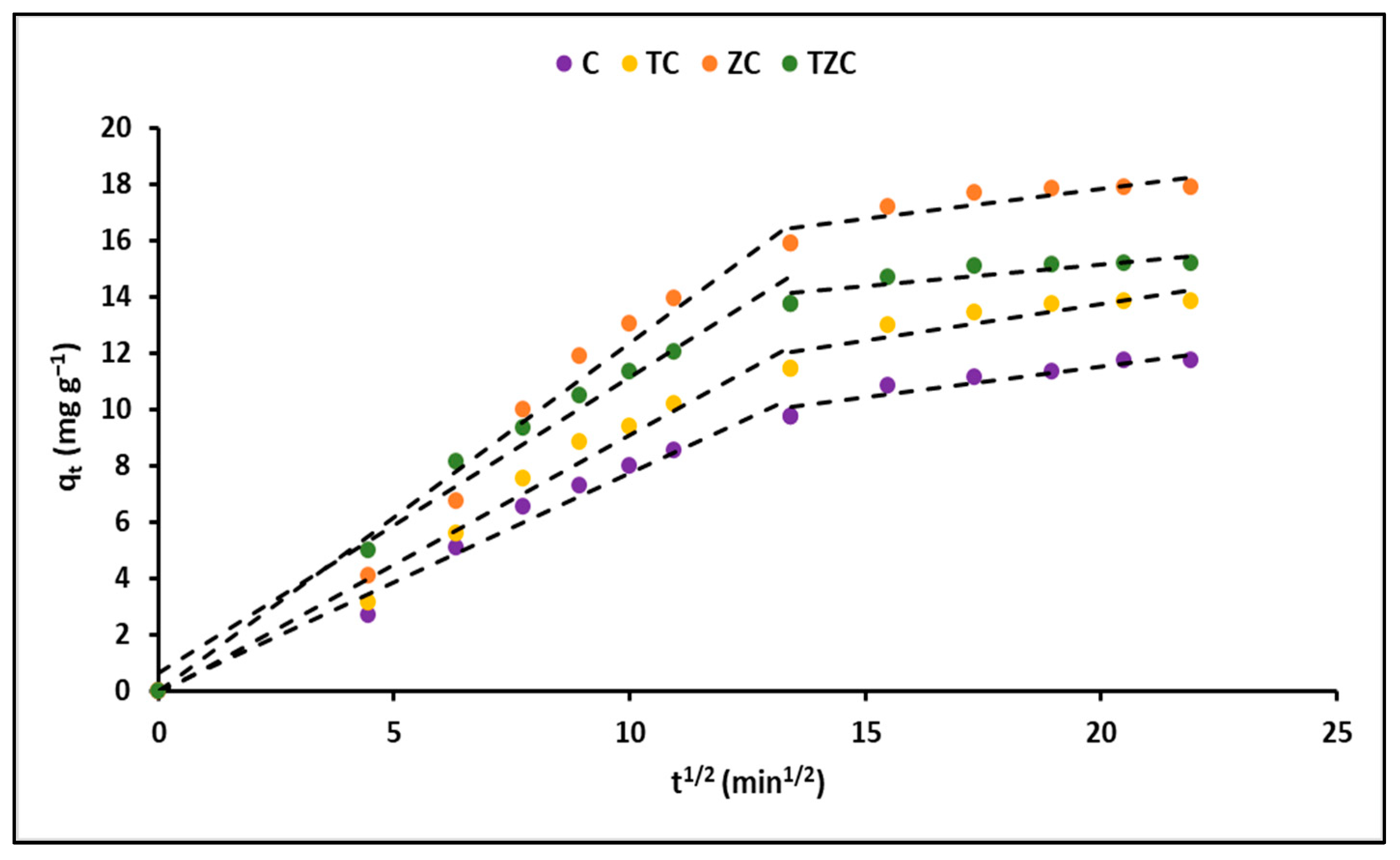
2.2.4. Adsorption Kinetics
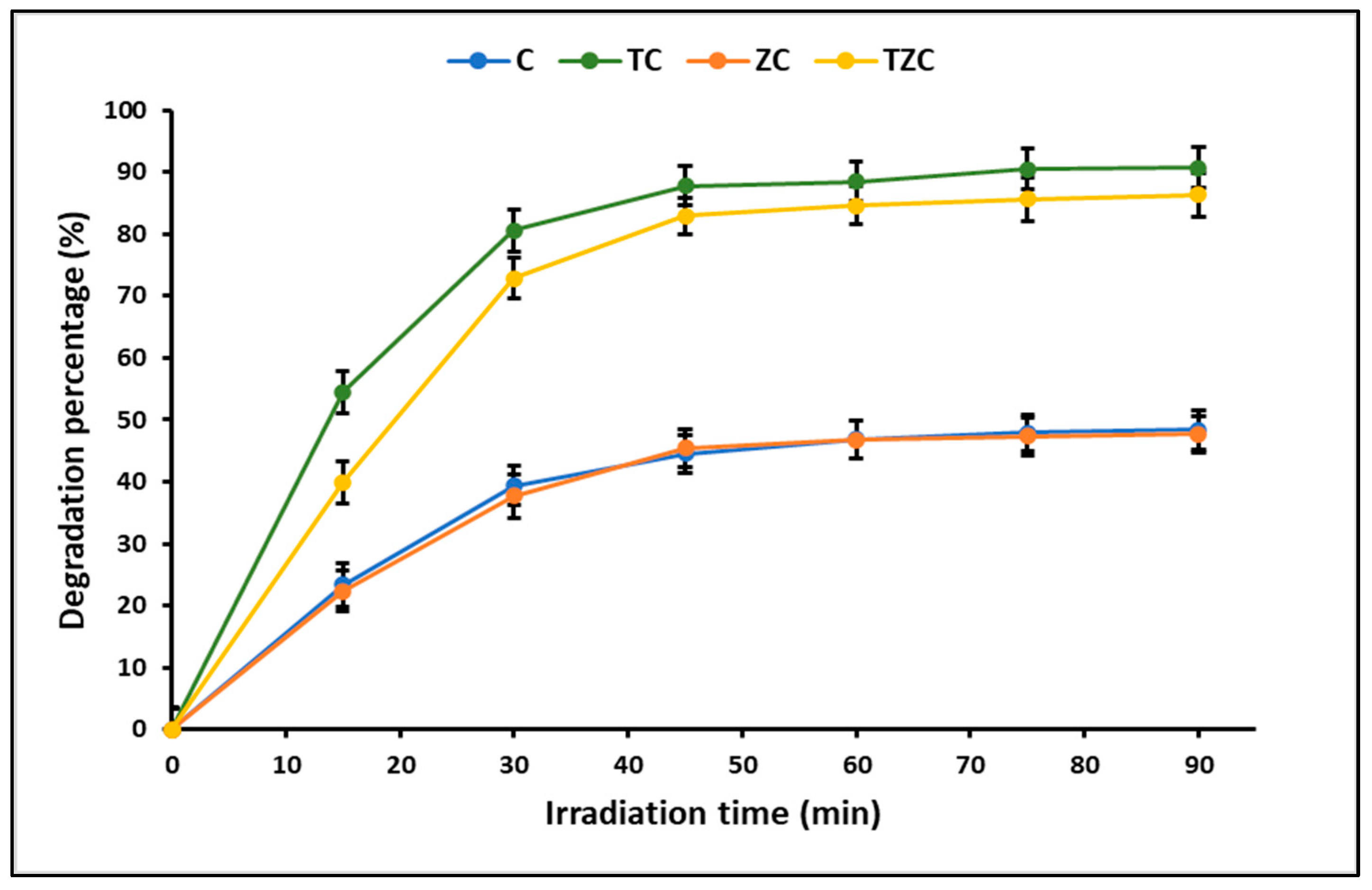
2.3. Photodegradation Studies
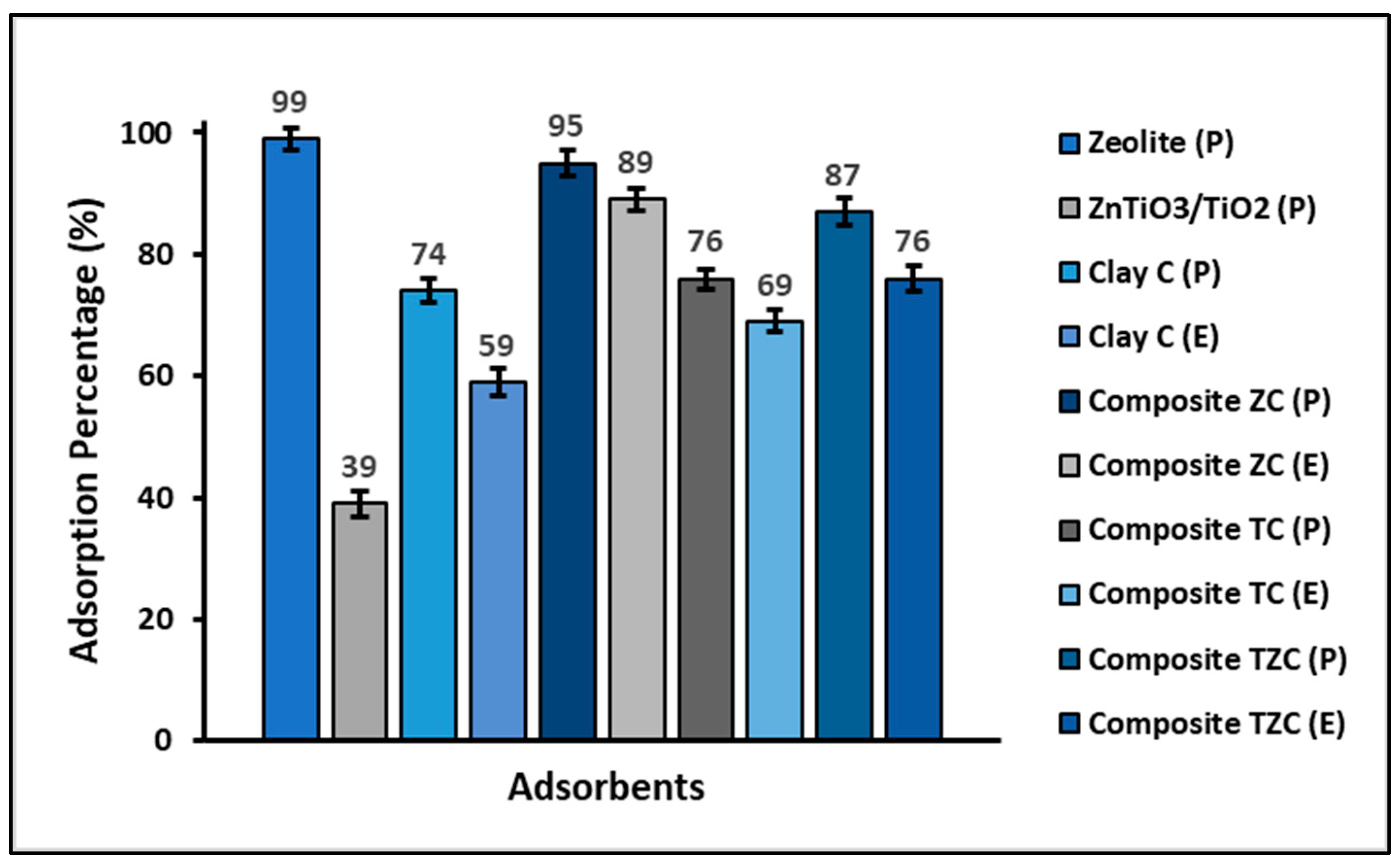
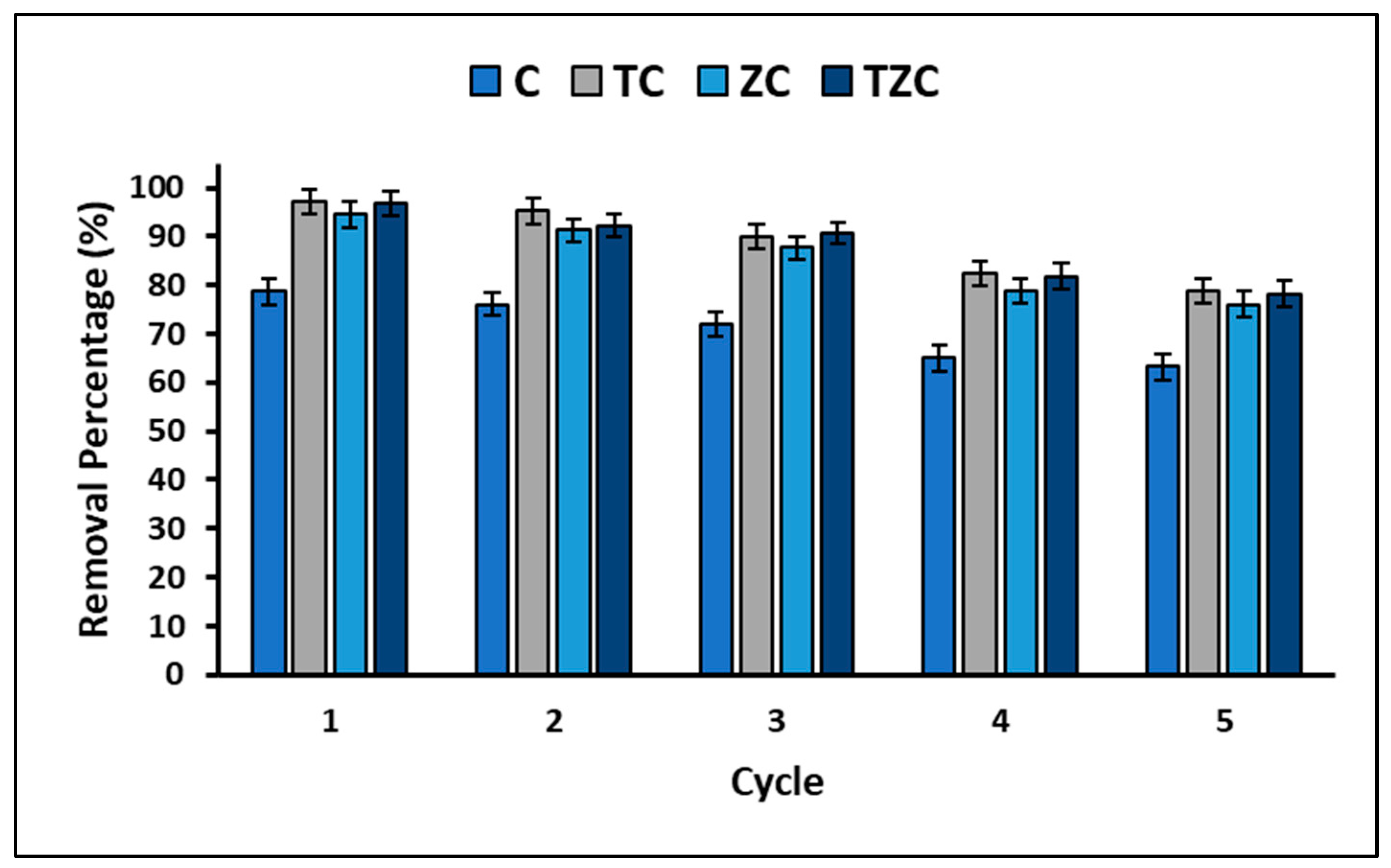
2.4. Total Efficiency and Reuse of Compounds
3. Discussion
3.1. Characterization of the Compounds
3.2. Adsorption Studies
3.2.1. Effect of pH
3.2.2. Adsorption Isotherm
3.2.3. Adsorption Thermodynamics
3.2.4. Adsorption Kinetics
3.3. Photodegradation Studies
3.4. Total Efficiency and Reuse of the Compounds
4. Materials and Methods
4.1. Materials
4.2. Clay Purification
4.3. Synthesis of ZnTiO3/TiO2 Nanoparticles
4.4. Synthesis of Zeolite from Ecuadorian Clay
4.5. Preparation of Extruded Samples
4.6. Characterization
4.7. Adsorption Studies
4.8. Photodegradation Studies
4.9. Reuse of Nanoparticles
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dwivedi, N.; Dwivedi, S.; Adetunji, C.O. Efficacy of Microorganisms in the Removal of Toxic Materials from Industrial Effluents; Springer: Berlin/Heidelberg, Germany, 2021; pp. 325–358. [Google Scholar] [CrossRef]
- Ravuru, S.S.; Jana, A.; De, S. Cyanide removal from blast furnace effluent using layered double hydroxide based mixed matrix beads: Batch and fixed bed study. J. Clean. Prod. 2022, 371, 133634. [Google Scholar] [CrossRef]
- Aranguri LLerena, G.; Reyes López, I.A. Cyanide Degradation from Mining Effluent Using Two Reagents: Sodium Metabisulphite and the Metabisulphite Mixture with Hydrogen Peroxide. Tecciencia 2019, 13, 1–9. [Google Scholar] [CrossRef]
- Aranguri-Llerena, G.; Reyes-Lázaro, W. Adsorption of cyanide contained in aqueous solution using activated carbon obtained from coffee residue: Absorption efficiency, equilibrium and kinetic model. Sci. Agropecu. 2019, 10, 315–325. [Google Scholar] [CrossRef]
- Biswas, P.; Bhunia, P.; Saha, P.; Sarkar, S.; Chandel, H.; De, S. In situ photodecyanation of steel industry wastewater in a pilot scale. Environ. Sci. Pollut. Res. 2020, 27, 33226–33233. [Google Scholar] [CrossRef] [PubMed]
- Azamat, J.; Khataee, A. Molecular dynamics simulations of removal of cyanide from aqueous solution using boron nitride nanotubes. Comput. Mater. Sci. 2017, 128, 8–14. [Google Scholar] [CrossRef]
- Mian, H.R.; Hu, G.; Hewage, K.; Rodriguez, M.J.; Sadiq, R. Prioritization of unregulated disinfection by-products in drinking water distribution systems for human health risk mitigation: A critical review. Water Res. 2018, 147, 112–131. [Google Scholar] [CrossRef]
- Rajamanikandan, R.; Sasikumar, K.; Kosame, S.; Ju, H. Optical Sensing of Toxic Cyanide Anions Using Noble Metal Nanomaterials. Nanomaterials 2023, 13, 290. [Google Scholar] [CrossRef]
- Dhongade, S.; Meher, A.K.; Mahapatra, S. Removal of Free Cyanide (CN−) from Water and Wastewater Using Activated Carbon: A Review; Springer: Singapore, 2022; pp. 355–379. [Google Scholar]
- Anning, C.; Wang, J.; Chen, P.; Batmunkh, I.; Lyu, X. Determination and detoxification of cyanide in gold mine tailings: A review. Waste Manag. Res. 2019, 37, 1117–1126. [Google Scholar] [CrossRef]
- Yang, W.; Liu, G.; Chen, Y.; Miao, D.; Wei, Q.; Li, H.; Ma, L.; Zhou, K.; Liu, L.; Yu, Z. Persulfate enhanced electrochemical oxidation of highly toxic cyanide-containing organic wastewater using boron-doped diamond anode. Chemosphere 2020, 252, 126499. [Google Scholar] [CrossRef]
- Litter, M.I. Introduction to Oxidative Technologies for Water Treatment; Springer: Cham, Switzerland, 2020; pp. 119–175. [Google Scholar]
- Dong, J.; Li, G.; Gao, J.; Zhang, H.; Bi, S.; Liu, S.; Liao, C.; Jiang, G. Catalytic degradation of brominated flame retardants in the environment: New techniques and research highlights. Sci. Total Environ. 2022, 848, 157695. [Google Scholar] [CrossRef]
- Yang, H.; Yang, B.; Chen, W.; Yang, J. Preparation and Photocatalytic Activities of TiO2-Based Composite Catalysts. Catalysts 2022, 12, 1263. [Google Scholar] [CrossRef]
- Koo, M.S.; Kim, H.; Lee, K.E.; Choi, W. Photoconversion of Cyanide to Dinitrogen Using the Durable Electrode of a TaON Overlayer-Deposited WO 3 Film and Visible Light. ACS EST Eng. 2021, 1, 228–238. [Google Scholar] [CrossRef]
- Janczarek, M.; Klapiszewski, Ł.; Jędrzejczak, P.; Klapiszewska, I.; Ślosarczyk, A.; Jesionowski, T. Progress of functionalized TiO2-based nanomaterials in the construction industry: A comprehensive review. Chem. Eng. J. 2022, 430, 132062. [Google Scholar] [CrossRef]
- Qamar, O.A.; Jamil, F.; Hussain, M.; Bae, S.; Inayat, A.; Shah, N.S.; Waris, A.; Akhter, P.; Kwon, E.E.; Park, Y.K. Advances in synthesis of TiO2 nanoparticles and their application to biodiesel production: A review. Chem. Eng. J. 2023, 460, 141734. [Google Scholar] [CrossRef]
- Yadawa, Y.; Singh, S.; Ranjan, A. Processing induced morphology change in ZnO-TiO2 multilayer thin films and its effect on their photocatalytic activity under visible light irradiation. Mater. Sci. Eng. B 2023, 288, 116164. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Gabal, M.A.; Angari, Y.M.A. Zinc titanates nanopowders: Synthesis and characterization. Mater. Res. Express 2022, 9, 025010. [Google Scholar] [CrossRef]
- Gonzales, L.L.; da Silva Hartwig, M.; Fassbender, R.U.; Moreira, E.C.; Pereira, M.B.; Jardim, P.L.G.; Raubach, C.W.; Moreira, M.L.; da Silva Cava, S. Properties of zinc titanates synthesized by microwave assisted hydrothermal method. Heliyon 2021, 7, e06521. [Google Scholar] [CrossRef]
- Souri, A.P.; Andrigiannaki, N.; Moschogiannaki, M.; Faka, V.; Kiriakidis, G.; Malankowska, A.; Zaleska-Medynska, A.; Binas, V. Metal titanate (Atio3, a: Ni, Co, Mg, Zn) nanorods for toluene photooxidation under led illumination. Appl. Sci. 2021, 11, 10850. [Google Scholar] [CrossRef]
- Saritha, M.V.; Dastagiri, S.; Lakshmaiah, M.V.; Kumar, V.R.; Supriya, K.E.; Pakardin, G. Structural and Optical Properties of ZnTiO3 Nanoparticles Synthesized by Hydrothermal Method. Int. J. Eng. Res. Technol. 2022, 11, 85–90. [Google Scholar]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Shen, Y.; Zhou, P.; Zhao, S.; Li, A.; Chen, Y.; Bai, J.; Han, C.; Wei, D.; Ao, Y. Synthesis of high-efficient TiO2/clinoptilolite photocatalyst for complete degradation of xanthate. Miner. Eng. 2020, 159, 106640. [Google Scholar] [CrossRef]
- Dutta, S. Wastewater treatment using TiO2-based photocatalysts. In Handbook of Smart Photocatalytic Materials: Fundamentals, Fabrications and Water Resources Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 303–323. ISBN 9780128190517. [Google Scholar]
- Zhang, G.; Song, A.; Duan, Y.; Zheng, S. Enhanced photocatalytic activity of TiO2/zeolite composite for abatement of pollutants. Microporous Mesoporous Mater. 2018, 255, 61–68. [Google Scholar] [CrossRef]
- Cataldo, E.; Salvi, L.; Paoli, F.; Fucile, M.; Masciandaro, G.; Manzi, D.; Masini, C.M.; Mattii, G.B. Application of zeolites in agriculture and other potential uses: A review. Agronomy 2021, 11, 1547. [Google Scholar] [CrossRef]
- Wang, H.; Pei, X.; Kalmutzki, M.J.; Yang, J.; Yaghi, O.M. Large Cages of Zeolitic Imidazolate Frameworks. Acc. Chem. Res. 2022, 55, 707–721. [Google Scholar] [CrossRef] [PubMed]
- Längauer, D.; Čablík, V.; Hredzák, S.; Zubrik, A.; Matik, M.; Danková, Z. Preparation of synthetic zeolites from coal fly ash by hydrothermal synthesis. Materials 2021, 14, 1267. [Google Scholar] [CrossRef] [PubMed]
- López Cordova, C.M.; Castillo, J.M.; Riofrio Valarezo, L.J.; García Berfon, L.V.; Jaramillo Fierro, X.V.; García López, A.L. Zeolitization of red clays from Ecuador and application in CO2 adsorption. Ing. Investig. Y Tecnol. 2020, 21, 00002. [Google Scholar] [CrossRef]
- Ramgir, N.; Bhusari, R.; Rawat, N.S.; Patil, S.J.; Debnath, A.K.; Gadkari, S.C.; Muthe, K.P. TiO2/ZnO heterostructure nanowire based NO2 sensor. Mater. Sci. Semicond. Process. 2020, 106, 104770. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; González, S.; Jaramillo, H.A.; Medina, F. Synthesis of the Zntio3 /Tio2 nanocomposite supported in ecuadorian clays for the adsorption and photocatalytic removal of methylene blue dye. Nanomaterials 2020, 10, 1891. [Google Scholar] [CrossRef]
- Ke, S.; Cheng, X.; Wang, Q.; Wang, Y.; Pan, Z. Preparation of a photocatalytic TiO2/ZnTiO3 coating on glazed ceramic tiles. Ceram. Int. 2014, 40, 8891–8895. [Google Scholar] [CrossRef]
- Irfan, H.; Racik, K.M.; Anand, S. X-ray peak profile analysis of CoAl2O4 nanoparticles by Williamson-Hall and size-strain plot methods. Mod. Electron. Mater. 2018, 4, 31–40. [Google Scholar] [CrossRef]
- Burton, A.W.; Ong, K.; Rea, T.; Chan, I.Y. On the Estimation of Average Crystallite Size of Zeolites from the Scherrer Equation: A Critical Evaluation of its Application to Zeolites with One-Dimensional Pore Systems. Microporous Mesoporous Mater. 2009, 117, 75–90. [Google Scholar] [CrossRef]
- Vinila, V.S.; Isac, J. Synthesis and structural studies of superconducting perovskite GdBa2Ca3Cu4O10.5+δ nanosystems. In Design, Fabrication, and Characterization of Multifunctional Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 319–341. ISBN 9780128205587. [Google Scholar]
- Domoroshchina, E.N.; Chernyshev, V.V.; Kuz’micheva, G.M.; Dorokhov, A.V.; Pirutko, L.V.; Kravchenko, G.V.; Chumakov, R.B. Changing the characteristics and properties of zeolite Y and nano-anatase in the formation of a nano-anatase/Y composite with improved photocatalytic and adsorption properties. Appl. Nanosci. 2018, 8, 19–31. [Google Scholar] [CrossRef]
- Eke-emezie, N.; Etuk, B.R.; Akpan, O.P.; Chinweoke, O.C. Cyanide removal from cassava wastewater onto H3PO4 activated periwinkle shell carbon. Appl. Water Sci. 2022, 12, 157. [Google Scholar] [CrossRef]
- Eletta, O.A.A.; Ajayi, O.A.; Ogunleye, O.O.; Akpan, I.C. Adsorption of cyanide from aqueous solution using calcinated eggshells: Equilibrium and optimisation studies. J. Environ. Chem. Eng. 2016, 4, 1367–1375. [Google Scholar] [CrossRef]
- Dash, R.R.; Gaur, A.; Balomajumder, C.; Roshan, R.; Gaur, A.; Balomajumder, C. Cyanide in industrial wastewaters and its removal: A review on biotreatment. J. Hazard. Mater. 2009, 163, 1–11. [Google Scholar] [CrossRef]
- Gupta, N.; Balomajumder, C.; Agarwal, V.K. Adsorption of cyanide ion on pressmud surface: A modeling approach. Chem. Eng. J. 2012, 191, 548–556. [Google Scholar] [CrossRef]
- Saxena, S.; Prasad, M.; Amritphale, S.S.; Chandra, N. Adsorption of cyanide from aqueous solutions at pyrophyllite surface. Sep. Purif. Technol. 2001, 24, 263–270. [Google Scholar] [CrossRef]
- Nassar, M.Y.; Abdelrahman, E.A. Hydrothermal tuning of the morphology and crystallite size of zeolite nanostructures for simultaneous adsorption and photocatalytic degradation of methylene blue dye. J. Mol. Liq. 2017, 242, 364–374. [Google Scholar] [CrossRef]
- Maulana, I.; Takahashi, F. Cyanide removal study by raw and iron-modified synthetic zeolites in batch adsorption experiments. J. Water Process Eng. 2018, 22, 80–86. [Google Scholar] [CrossRef]
- Eskandari, P.; Farhadian, M.; Solaimany Nazar, A.R.; Goshadrou, A. Cyanide adsorption on activated carbon impregnated with ZnO, Fe2O3, TiO2 nanometal oxides: A comparative study. Int. J. Environ. Sci. Technol. 2021, 18, 297–316. [Google Scholar] [CrossRef]
- Lin, G.; Zhuang, Q.; Cui, Q.; Wang, H.; Yao, H. Synthesis and adsorption property of zeolite FAU/LTA from lithium slag with utilization of mother liquid. Chin. J. Chem. Eng. 2015, 23, 1768–1773. [Google Scholar] [CrossRef]
- Melo, C.C.A.; Melo, B.L.S.; Angélica, R.S.; Paz, S.P.A. Gibbsite-kaolinite waste from bauxite beneficiation to obtain FAU zeolite: Synthesis optimization using a factorial design of experiments and response surface methodology. Appl. Clay Sci. 2019, 170, 125–134. [Google Scholar] [CrossRef]
- Bunmai, K.; Osakoo, N.; Deekamwong, K.; Kosri, C.; Khemthong, P.; Wittayakun, J. Fast synthesis of zeolite NaP by crystallizing the NaY gel under microwave irradiation. Mater. Lett. 2020, 272, 127845. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; Abou El-Reash, Y.G.; Youssef, H.M.; Kotp, Y.H.; Hegazey, R.M. Utilization of rice husk and waste aluminum cans for the synthesis of some nanosized zeolite, zeolite/zeolite, and geopolymer/zeolite products for the efficient removal of Co(II), Cu(II), and Zn(II) ions from aqueous media. J. Hazard. Mater. 2021, 401, 123813. [Google Scholar] [CrossRef]
- He, P.; Wang, Q.; Fu, S.; Wang, M.; Zhao, S.; Liu, X.; Jiang, Y.; Jia, D.; Zhou, Y. Hydrothermal transformation of geopolymers to bulk zeolite structures for efficient hazardous elements adsorption. Sci. Total Environ. 2021, 767, 144973. [Google Scholar] [CrossRef]
- Lee, N.K.; Khalid, H.R.; Lee, H.K. Synthesis of mesoporous geopolymers containing zeolite phases by a hydrothermal treatment. Microporous Mesoporous Mater. 2016, 229, 22–30. [Google Scholar] [CrossRef]
- Rożek, P.; Król, M.; Mozgawa, W. Geopolymer-zeolite composites: A review. J. Clean. Prod. 2019, 230, 557–579. [Google Scholar] [CrossRef]
- Parsapur, R.K.; Selvam, P. Rational design, synthesis, characterization and catalytic properties of high-quality low-silica hierarchical FAU- and LTA-type zeolites. Sci. Rep. 2018, 8, 16291. [Google Scholar] [CrossRef]
- Mańko, M.; Vittenet, J.; Rodriguez, J.; Cot, D.; Mendret, J.; Brosillon, S.; Makowski, W.; Galarneau, A. Synthesis of binderless zeolite aggregates (SOD, LTA, FAU) beads of 10, 70 μm and 1 mm by direct pseudomorphic transformation. Microporous Mesoporous Mater. 2013, 176, 145–154. [Google Scholar] [CrossRef]
- Purnomo, C.W.; Salim, C.; Hinode, H. Synthesis of pure Na-X and Na-A zeolite from bagasse fly ash. Microporous Mesoporous Mater. 2012, 162, 6–13. [Google Scholar] [CrossRef]
- Porcher, F.; Dusausoy, Y.; Souhassou, M.; Lecomte, C. Epitaxial growth of zeolite X on zeolite A and twinning in zeolite A: Structural and topological analysis. Mineral. Mag. 2000, 64, 1–8. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Javier Huertas, F.; Lettino, A.; Ragone, P.; Fiore, S. The crystallisation of zeolite (X- and A-type) from fly ash at 25 °c in artificial sea water. Microporous Mesoporous Mater. 2012, 162, 115–121. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Y.; Cui, Y.; Dai, R.; Shan, Z.; Chen, H. Fabrication of starch-based high-performance adsorptive hydrogels using a novel effective pretreatment and adsorption for cationic methylene blue dye: Behavior and mechanism. Chem. Eng. J. 2021, 405, 126953. [Google Scholar] [CrossRef]
- Shoumkova, A.; Stoyanova, V. Zeolites formation by hydrothermal alkali activation of coal fly ash from thermal power station “maritsa 3”, Bulgaria. Fuel 2013, 103, 533–541. [Google Scholar] [CrossRef]
- Murayama, N.; Yamamoto, H.; Shibata, J. Mechanism of zeolite synthesis from coal fly ash by alkali hydrothermal reaction. Int. J. Miner. Process. 2002, 64, 1–17. [Google Scholar] [CrossRef]
- Cárdenas-Ramírez, C.; Jaramillo, F.; Fernández, A.G.; Cabeza, L.F.; Gómez, M.A. Influence of thermal treatments on the absorption and thermal properties of a clay mineral support used for shape-stabilization of fatty acids. J. Energy Storage 2021, 36, 102427. [Google Scholar] [CrossRef]
- Strejcová, K.; Tišler, Z.; Svobodová, E.; Velvarská, R. Characterization of Modified Natural Minerals and Rocks for Possible Adsorption and Catalytic Use. Molecules 2020, 25, 4989. [Google Scholar] [CrossRef]
- Elgamouz, A.; Tijani, N. Dataset in the production of composite clay-zeolite membranes made from naturally occurring clay minerals. Data Br. 2018, 19, 2267–2278. [Google Scholar] [CrossRef]
- An, F.; Liu, J.; Xu, Z.; Zheng, S. Efficient removal of three dyes using porous covalent triazine frameworks: Adsorption mechanism and role of pore distribution. Water Sci. Technol. 2020, 82, 3023–3031. [Google Scholar] [CrossRef]
- Stavropoulos, G.G.; Skodras, G.S.; Papadimitriou, K.G. Effect of solution chemistry on cyanide adsorption in activated carbon. Appl. Therm. Eng. 2015, 74, 182–185. [Google Scholar] [CrossRef]
- Kosmulski, M. The pH dependent surface charging and points of zero charge. VII. Update. Adv. Colloid Interface Sci. 2018, 251, 115–138. [Google Scholar] [CrossRef] [PubMed]
- Muthirulan, P.; Nirmala Devi, C.; Meenakshi Sundaram, M. Synchronous role of coupled adsorption and photocatalytic degradation on CAC–TiO2 composite generating excellent mineralization of alizarin cyanine green dye in aqueous solution. Arab. J. Chem. 2017, 10, S1477–S1483. [Google Scholar] [CrossRef]
- Muráth, S.; Sáringer, S.; Somosi, Z.; Szilágyi, I. Effect of Ionic Compounds of Different Valences on the Stability of Titanium Oxide Colloids. Colloids Interfaces 2018, 2, 32. [Google Scholar] [CrossRef]
- Prakash, J.; Samriti; Kumar, A.; Dai, H.; Janegitz, B.C.; Krishnan, V.; Swart, H.C.; Sun, S. Novel rare earth metal–doped one-dimensional TiO2 nanostructures: Fundamentals and multifunctional applications. Mater. Today Sustain. 2021, 13, 100066. [Google Scholar] [CrossRef]
- Shu, Q.; Liu, X.; Huo, Y.; Tan, Y.; Zhang, C.; Zou, L. Construction of a Brönsted-Lewis solid acid catalyst La-PW-SiO2/SWCNTs based on electron withdrawing effect of La(III) on π bond of SWCNTs for biodiesel synthesis from esterification of oleic acid and methanol. Chin. J. Chem. Eng. 2022, 44, 351–362. [Google Scholar] [CrossRef]
- Amaro-Medina, B.M.; Martinez-Luevanos, A.; Soria-Aguilar, M.d.J.; Sanchez-Castillo, M.A.; Estrada-Flores, S.; Carrillo-Pedroza, F.R. Efficiency of Adsorption and Photodegradation of Composite TiO2/Fe2O3 and Industrial Wastes in Cyanide Removal. Water 2022, 14, 3502. [Google Scholar] [CrossRef]
- Noroozi, R.; Al-Musawi, T.J.; Kazemian, H.; Kalhori, E.M.; Zarrabi, M. Removal of cyanide using surface-modified Linde Type-A zeolite nanoparticles as an efficient and eco-friendly material. J. Water Process Eng. 2018, 21, 44–51. [Google Scholar] [CrossRef]
- Chen, M.; Ye, Q.; Jiang, S.; Shao, M.; Jin, C.; Huang, Z. Two-Step Elution Recovery of Cyanide Platinum Using Functional Metal Organic Resin. Molecules 2019, 24, 2779. [Google Scholar] [CrossRef]
- Pirmoradi, M.; Hashemian, S.; Shayesteh, M.R. Kinetics and thermodynamics of cyanide removal by ZnO@NiO nanocrystals. Trans. Nonferrous Met. Soc. China (Eng. ED.) 2017, 27, 1394–1403. [Google Scholar] [CrossRef]
- Lubis, S.; Sheilatina; Murisna. Synthesis, Characterization and Photocatalytic Activity of α-Fe2O3/Bentonite Composite Prepared by Mechanical Milling. J. Phys. Conf. Ser. 2018, 1116, 042016. [Google Scholar] [CrossRef]
- Lin, Z.; Du, C.; Yan, B.; Yang, G. Amorphous Fe2O3 for photocatalytic hydrogen evolution. Catal. Sci. Technol. 2019, 9, 5582–5592. [Google Scholar] [CrossRef]
- Mishra, M.; Chun, D.M. α-Fe2O3 as a photocatalytic material: A review. Appl. Catal. A Gen. 2015, 498, 126–141. [Google Scholar] [CrossRef]
- Hitam, C.N.C.; Jalil, A.A. A review on exploration of Fe2O3 photocatalyst towards degradation of dyes and organic contaminants. J. Environ. Manage. 2020, 258, 110050. [Google Scholar] [CrossRef] [PubMed]
- Ðorđević, V.; Milićević, B.; Dramićanin, M.D. Rare Earth-Doped Anatase TiO2 Nanoparticles. In Titanium Dioxide; Janus, M., Ed.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Chaker, H.; Ameur, N.; Saidi-Bendahou, K.; Djennas, M.; Fourmentin, S. Modeling and Box-Behnken design optimization of photocatalytic parameters for efficient removal of dye by lanthanum-doped mesoporous TiO2. J. Environ. Chem. Eng. 2021, 9, 2213–3437. [Google Scholar] [CrossRef]
- Eskandarloo, H.; Badiei, A.; Behnajady, M.A.; Tavakoli, A.; Ziarani, G.M. Ultrasonic-assisted synthesis of Ce doped cubic-hexagonal ZnTiO3 with highly efficient sonocatalytic activity. Ultrason. Sonochem. 2016, 29, 258–269. [Google Scholar] [CrossRef]
- Mediavilla, J.J.V.; Perez, B.F.; Cordoba, M.C.F.; de Espina, J.A.; Ania, C.O.; Viña Mediavilla, J.J.; Fernandez Perez, B.; Fernandez de Cordoba, M.C.; Ayala Espina, J.; Ania, C.O. Photochemical Degradation of Cyanides and Thiocyanates from an Industrial Wastewater. Molecules 2019, 24, 1373. [Google Scholar] [CrossRef]
- Betancourt-Buitrago, L.A.; Hernandez-Ramirez, A.; Colina-Marquez, J.A.; Bustillo-Lecompte, C.F.; Rehmann, L.; Machuca-Martinez, F. Recent Developments in the Photocatalytic Treatment of Cyanide Wastewater: An Approach to Remediation and Recovery of Metals. Processes 2019, 7, 225. [Google Scholar] [CrossRef]
- Dagaut, P.; Glarborg, P.; Alzueta, M.U. The oxidation of hydrogen cyanide and related chemistry. Prog. Energy Combust. Sci. 2008, 34, 1–46. [Google Scholar] [CrossRef]
- Luque-Almagro, V.M.; Cabello, P.; Sáez, L.P.; Olaya-Abril, A.; Moreno-Vivián, C.; Roldán, M.D. Exploring anaerobic environments for cyanide and cyano-derivatives microbial degradation. Appl. Microbiol. Biotechnol. 2018, 102, 1067–1074. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; León, R. Effect of Doping TiO2 NPs with Lanthanides (La, Ce and Eu) on the Adsorption and Photodegradation of Cyanide—A Comparative Study. Nanomaterials 2023, 13, 1068. [Google Scholar] [CrossRef] [PubMed]
- Martínková, L.; Bojarová, P.; Sedova, A.; Křen, V. Recent trends in the treatment of cyanide-containing effluents: Comparison of different approaches. Crit. Rev. Environ. Sci. Technol. 2023, 53, 416–434. [Google Scholar] [CrossRef]
- Yakimov, A.V.; Ravi, M.; Verel, R.; Sushkevich, V.L.; Van Bokhoven, J.A.; Copéret, C. Structure and Framework Association of Lewis Acid Sites in MOR Zeolite. J. Am. Chem. Soc. 2022, 144, 10377–10385. [Google Scholar] [CrossRef]
- Yang, X.; Lan, L.; Zhao, Z.; Zhou, S.; Kang, K.; Song, H.; Bai, S. A Review on Cyanide Gas Elimination Methods and Materials. Molecules 2022, 27, 7125. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.V.; Naresh, G.; Sudhakar, M.; Tardio, J.; Bhargava, S.K.; Venugopal, A. Role of Brønsted and Lewis acid sites on Ni/TiO2 catalyst for vapour phase hydrogenation of levulinic acid: Kinetic and mechanistic study. Appl. Catal. A Gen. 2015, 505, 217–223. [Google Scholar] [CrossRef]
- Shi, J.W.; Chen, S.H.; Wang, S.M.; Ye, Z.L.; Wu, P.; Xu, B. Favorable recycling photocatalyst TiO2/CFA: Effects of calcination temperature on the structural property and photocatalytic activity. J. Mol. Catal. A Chem. 2010, 330, 41–48. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; Cuenca, G.; Ramón, J. Comparative Study of the Effect of Doping ZnTiO3 with Rare Earths (La and Ce) on the Adsorption and Photodegradation of Cyanide in Aqueous Systems. Int. J. Mol. Sci. 2023, 24, 3780. [Google Scholar] [CrossRef] [PubMed]
- Amamou, S.; Cheniti-Belcadhi, L. Tutoring in Project-Based Learning. In Proceedings of the Procedia Computer Science; Elsevier: Amsterdam, The Netherlands, 2018; Volume 126, pp. 176–185. [Google Scholar]
- Pan, Y.; Zhang, Y.; Huang, Y.; Jia, Y.; Chen, L.; Cui, H. Functional Ag-doped coralloid titanosilicate zeolite (CTS-Ag) for efficiently catalytic and photodegradative removal of free cyanides and copper/zinc-cyanide complexes in real wastewater. J. Alloys Compd. 2022, 926, 166848. [Google Scholar] [CrossRef]
- Golbaz, S.; Jafari, A.J.; Kalantari, R.R. The study of Fenton oxidation process efficiency in the simultaneous removal of phenol, cyanide, and chromium (VI) from synthetic wastewater. Desalin. Water Treat. 2013, 51, 5761–5767. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.W.; Lee, G.M.; Lee, B.T.; Yun, S.T.; Kim, S.O. Monitoring of TiO2-catalytic UV-LED photo-oxidation of cyanide contained in mine wastewater and leachate. Chemosphere 2016, 143, 106–114. [Google Scholar] [CrossRef]
- Baeissa, E.S. Photocatalytic removal of cyanide by cobalt metal doped on TiO2–SiO2 nanoparticles by photo-assisted deposition and impregnation methods. J. Ind. Eng. Chem. 2014, 20, 3761–3766. [Google Scholar] [CrossRef]
- Faisal, M.; Jalalah, M.; Harraz, F.A.; El-Toni, A.M.; Labis, J.P.; Al-Assiri, M.S. A novel Ag/PANI/ZnTiO3 ternary nanocomposite as a highly efficient visible-light-driven photocatalyst. Sep. Purif. Technol. 2021, 256, 117847. [Google Scholar] [CrossRef]
- Karunakaran, C.; Gomathisankar, P.; Manikandan, G. Preparation and characterization of antimicrobial Ce-doped ZnO nanoparticles for photocatalytic detoxification of cyanide. Mater. Chem. Phys. 2010, 123, 585–594. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; González, S.; Montesdeoca-Mendoza, F.; Medina, F. Structuring of Zntio3 /Tio2 adsorbents for the removal of methylene blue, using zeolite precursor clays as natural additives. Nanomaterials 2021, 11, 898. [Google Scholar] [CrossRef]
- Bakatula, E.N.; Richard, D.; Neculita, C.M.; Zagury, G.J. Determination of point of zero charge of natural organic materials. Environ. Sci. Pollut. Res. 2018, 25, 7823–7833. [Google Scholar] [CrossRef] [PubMed]
- Benmessaoud, A.; Nibou, D.; Mekatel, E.H.; Amokrane, S. A Comparative Study of the Linear and Non-Linear Methods for Determination of the Optimum Equilibrium Isotherm for Adsorption of Pb2+ Ions onto Algerian Treated Clay. Iran. J. Chem. Chem. Eng. 2020, 39, 153–171. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, T.; Yao, J.; Li, J.; Jin, Y.; Cheng, J.; Shen, Z. Development of an unmanned device with picric acid strip for on-site rapid detections of sodium cyanide in marine water. IOP Conf. Ser. Earth Environ. Sci. 2021, 734, 012026. [Google Scholar] [CrossRef]
- Pramitha, A.R.; Harijono, H.; Wulan, S.N. Comparison of cyanide content in arbila beans (Phaseolus lunatus l) of East Nusa Tenggara using picrate and acid hydrolysis methods. IOP Conf. Ser. Earth Environ. Sci. 2021, 924, 012031. [Google Scholar] [CrossRef]
- Castada, H.Z.; Liu, J.; Barringer, S.A.; Huang, X. Cyanogenesis in Macadamia and Direct Analysis of Hydrogen Cyanide in Macadamia Flowers, Leaves, Husks, and Nuts Using Selected Ion Flow Tube–Mass Spectrometry. Foods 2020, 9, 174. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, X. The Unit Problem in the Thermodynamic Calculation of Adsorption Using the Langmuir Equation. Chem. Eng. Commun. 2014, 201, 1459–1467. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; González, S.; Medina, F. La-doped Zntio3 /Tio2 nanocomposite supported on ecuadorian diatomaceous earth as a highly efficient photocatalyst driven by solar light. Molecules 2021, 26, 6232. [Google Scholar] [CrossRef] [PubMed]
- Bettoni, M.; Falcinelli, S.; Rol, C.; Rosi, M.; Sebastiani, G.V. Gas-Phase TiO2 Photosensitized Mineralization of Some VOCs: Mechanistic Suggestions through a Langmuir–Hinshelwood Kinetic Approach. Catalysts 2020, 11, 20. [Google Scholar] [CrossRef]
- Daou, I.; Zegaoui, O.; Elghazouani, A. Physicochemical and photocatalytic properties of the ZnO particles synthesized by two different methods using three different precursors. Comptes Rendus Chim. 2017, 20, 47–54. [Google Scholar] [CrossRef]





| Compound | Al2O3 | SiO2 | K2O | CaO | Fe2O3 | TiO2 | ZnO |
|---|---|---|---|---|---|---|---|
| Zeolite | 19.62 (±0.79) | 41.65 (±0.68) | 0.78 (±0.02) | 1.22 (±0.01) | 0.80 (±0.01) | - | - |
| C | 21.10 (±0.85) | 45.30 (±0.63) | 1.14 (±0.02) | 3.11 (±0.02) | 2.44 (±0.01) | - | - |
| ZC | 15.70 (±0.79) | 41.80 (±0.63) | 1.34 (±0.03) | 2.96 (±0.02) | 2.38 (±0.01) | - | - |
| TC | 11.60 (±1.56) | 23.90 (±0.58) | 0.38 (±0.03) | 1.61 (±0.02) | 2.64 (±0.03) | 51.40 (±0.14) | 20.66 (±0.06) |
| TZC | 12.10 (±1.31) | 37.60 (±0.68) | 1.58 (±0.04) | 2.63 (±0.03) | 2.94 (±0.02) | 31.30 (±1.82) | 16.30 (±0.07) |
| C | O | Si | Al | Fe | Na | Ca | K | Zn | Ti | |
|---|---|---|---|---|---|---|---|---|---|---|
| Clay | 12.12 | 50.85 | 29.30 | 4.24 | 0.78 | 1.17 | 0.92 | 0.61 | - | - |
| Zeolite | 20.77 | 50.91 | 14.44 | 6.77 | 0.88 | 3.54 | 2.05 | 0.62 | - | - |
| ZnTiO3/TiO2 | 5.42 | 33.60 | - | - | - | - | - | - | 6.13 | 54.85 |
| TZC | 5.05 | 41.84 | 7.59 | 4.55 | 0.49 | 0.84 | 0.45 | 0.36 | 10.19 | 28.64 |
| Compound | Composition | SSA (m2 g−1) | pHPZC |
|---|---|---|---|
| Zeolite (P) | Zeolite | 386 | 8.4 |
| ZnTiO3/TiO2 (P) | ZnTiO3/TiO2 | 88 | 7.0 |
| Clay C (P) | Clay | 22 | 6.8 |
| Composite ZC (P) | Zeolite + Clay | 184 | 7.8 |
| Composite TC (P) | ZnTiO3/TiO2 + Clay | 45 | 6.9 |
| Composite TZC (P) | ZnTiO3/TiO2 + Zeolite + Clay | 62 | 7.3 |
| Clay C (E) | Clay | 12 | 6.8 |
| Composite ZC (E) | Zeolite + Clay | 125 | 7.8 |
| Composite TC (E) | ZnTiO3/TiO2 + Clay | 36 | 6.9 |
| Composite TZC (E) | ZnTiO3/TiO2 + Zeolite + Clay | 48 | 7.3 |
| Isotherm Parameters | 293.15 K | 298.15 K | 303.15 K | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | TC | ZC | TZC | C | TC | ZC | TZC | C | TC | ZC | TZC | ||
| Langmuir | qmax | 56.92 | 67.73 | 73.37 | 71.83 | 62.61 | 74.50 | 80.71 | 79.01 | 68.30 | 81.28 | 88.04 | 83.20 |
| (mg g−1) | (±1.41) | (±1.36) | (±1.53) | (±1.74) | (1.52) | (2.11) | (3.52) | (3.10) | (1.52) | (2.11) | (3.52) | (3.10) | |
| KL | 0.14 | 0.16 | 0.18 | 0.23 | 0.13 | 0.15 | 0.38 | 0.33 | 0.28 | 0.35 | 0.46 | 0.41 | |
| (L mg−1) | (±0.03) | (±0.02) | (±0.04) | (±0.03) | (0.03) | (0.04) | (0.05) | (0.04) | (0.03) | (0.04) | (0.05) | (0.05) | |
| RL | 0.57 | 0.52 | 0.37 | 0.49 | 0.18 | 0.15 | 0.12 | 0.13 | 0.15 | 0.13 | 0.10 | 0.11 | |
| χ2 | 1.67 | 1.90 | 1.80 | 1.41 | 2.45 | 3.97 | 2.21 | 2.89 | 2.51 | 3.97 | 2.912 | 2.89 | |
| R2 | 0.96 | 0.98 | 0.99 | 0.98 | 0.99 | 0.98 | 0.97 | 0.95 | 0.99 | 0.98 | 0.97 | 0.95 | |
| Freundlich | KF | 6.25 | 8.57 | 2.56 | 15.26 | 6.88 | 9.43 | 2.82 | 16.79 | 7.50 | 10.28 | 3.07 | 18.31 |
| (L mg−1) | (±1.20) | (±1.38) | (±2.05) | (±1.69) | (1.94) | (2.25) | (2.59) | (2.24) | (1.84) | (2.25) | (2.04) | (2.24) | |
| n | 2.35 | 2.44 | 2.54 | 2.52 | 2.58 | 2.68 | 2.79 | 2.77 | 2.82 | 2.93 | 2.59 | 3.02 | |
| (±0.43) | (±0.40) | (±0.43) | (±0.30) | (0.39) | (0.41) | (0.44) | (0.44) | (0.39) | (0.41) | (0.35) | (0.44) | ||
| 1/n | 0.42 | 0.41 | 0.39 | 0.40 | 0.41 | 0.37 | 0.36 | 0.36 | 0.39 | 0.34 | 0.44 | 0.33 | |
| χ2 | 3.34 | 5.47 | 3.67 | 5.18 | 4.70 | 3.96 | 4.15 | 3.95 | 4.97 | 3.96 | 4.15 | 3.95 | |
| R2 | 0.87 | 0.90 | 0.94 | 0.90 | 0.92 | 0.89 | 0.86 | 0.84 | 0.92 | 0.89 | 0.86 | 0.84 | |
| Temkin | qmax | 12.53 | 14.10 | 19.80 | 14.12 | 14.03 | 16.21 | 21.98 | 15.96 | 16.97 | 19.74 | 26.70 | 17.47 |
| (mg g−1) | (±0.96) | (±0.94) | (±0.19) | (±1.12) | (±1.06) | (±0.99) | (±1.19) | (±1.02) | (±1.11) | (±1.21) | (±1.23) | (±1.16) | |
| a | 0.36 | 0.52 | 0.71 | 0.53 | 0.45 | 0.60 | 0.87 | 0.65 | 0.51 | 0.67 | 0.92 | 0.69 | |
| (mol−1) | (±0.07) | (±0.09) | (±0.06) | (±0.16) | (±0.05) | (±0.05) | (±0.07) | (±0.09) | (±0.07) | (±0.09) | (±0.06) | (±0.10) | |
| χ2 | 13.9 | 16.47 | 16.75 | 12.72 | 11.7 | 12.31 | 13.14 | 10.05 | 10.8 | 11.23 | 15.13 | 12.15 | |
| R2 | 0.95 | 0.97 | 0.90 | 0.95 | 0.96 | 0.95 | 0.92 | 0.91 | 0.97 | 0.96 | 0.95 | 0.90 | |
| Samples | Temperature (K) | ln kC | ∆G° (kJ mol−1) | ∆H° (kJ mol−1) | ∆S° (kJ mol−1 K−1) |
|---|---|---|---|---|---|
| C | 293.15 | 12.56 | −30.61 | −29.93 | 0.21 |
| 298.15 | 12.73 | −31.56 | |||
| 303.15 | 12.96 | −32.67 | |||
| TC | 293.15 | 12.81 | −31.22 | −26.40 | 0.20 |
| 298.15 | 12.96 | −32.12 | |||
| 303.15 | 13.17 | −33.19 | |||
| ZC | 293.15 | 13.11 | −31.95 | −24.83 | 0.19 |
| 298.15 | 13.25 | −32.84 | |||
| 303.15 | 13.45 | −33.89 | |||
| TZC | 293.15 | 12.96 | −31.59 | −27.42 | 0.20 |
| 298.15 | 13.11 | −32.50 | |||
| 303.15 | 13.33 | −33.61 |
| Kinetic Parameters | C | TC | ZC | TZC | |
|---|---|---|---|---|---|
| Pseudo-first-order | qmax (mg g−1) | 11.47 (±2.17) | 13.73 (±1.81) | 17.98 (±2.21) | 14.93 (±2.26) |
| k1 (L mg−1) | 0.02 (±6.01 × 10−4) | 0.03 (±4.93 × 10−4) | 0.03 (±4.85 × 10−4) | 0.02 (±1.02 × 10−3) | |
| χ2 | 2.11 | 2.38 | 2.69 | 2.13 | |
| R2 | 0.99 | 0.99 | 0.99 | 0.98 | |
| Pseudo-second-order | qmax (mg g−1) | 13.63 (±1.15) | 16.42 (±1.24) | 21.55 (±1.67) | 17.10 (±2.00) |
| k2 (L mg−1) | 1.06 × 10−3 (±5.24 × 10−5) | 8.32 × 10−4 (±5.16 × 10−5) | 6.41 × 10−4 (±8.50 × 10−5) | 1.22 × 10−3 (±6.92 × 10−5) | |
| χ2 | 2.13 | 2.74 | 3.07 | 2.56 | |
| R2 | 1.00 | 1.00 | 1.00 | 1.00 | |
| Intraparticle diffusion | k3 (mg g−1 min−1/2) | 0.53 (±0.01) | 0.64 (±0.02) | 0.84 (±0.02) | 0.65 (±0.01) |
| A | 1.65 (±0.17) | 1.82 (±0.22) | 2.56 (±1.01) | 3.33 (±1.15) | |
| R2 | 0.92 | 0.91 | 0.89 | 0.87 | |
| External-film diffusion | Df (m2 min−1) | 5.97 × 10−13 | 7.43 × 10−13 | 1.15 × 10−12 | 1.02 × 10−12 |
| R2 | 0.99 | 0.98 | 0.98 | 0.99 | |
| Internal-pore diffusion | Dp (m2 min−1) | 4.00 × 10−18 | 4.20 × 10−18 | 5.30 × 10−18 | 5.70 × 10−18 |
| R2 | 0.98 | 0.96 | 0.95 | 0.97 | |
| Adsorbent | qmax (mg g−1) | Isotherm Model | Kinetic Model | Reference |
|---|---|---|---|---|
| ZnTiO3 | 57.32 | Langmuir | Pseudo-second-order | [93] |
| La/ZnTiO3 | 59.22 | Langmuir | Pseudo-second-order | [93] |
| Ce/ZnTiO3 | 42.00 | Langmuir | Pseudo-second-order | [93] |
| TiO2 | 46.48 | Langmuir | Pseudo-second-order | [87] |
| La/TiO2 | 54.96 | Langmuir | Pseudo-second-order | [87] |
| Ce/TiO2 | 51.39 | Langmuir | Pseudo-second-order | [87] |
| Eu/TiO2 | 49.25 | Langmuir | Pseudo-second-order | [87] |
| ZnO | 275.00 | Langmuir | Pseudo-second-order | [75] |
| NiO | 185.00 | Langmuir | Pseudo-first-order | [75] |
| ZnO-NiO | 320.00 | Langmuir | Pseudo-second-order | [75] |
| LTA zeolite modified with HDMTMAB | 24.09 | Langmuir | - | [73] |
| Clay-K | 253.98 | - | Pseudo-second-order | [72] |
| TiO2/Fe2O3 | 124.87 | - | Pseudo-second-order | [72] |
| Fe-MFI zeolite | 33.98 | Langmuir | Pseudo-second-order | [94] |
| C | 56.92 | Langmuir | Pseudo-second-order | This study |
| TC | 67.73 | Langmuir | Pseudo-second-order | This study |
| ZC | 73.37 | Langmuir | Pseudo-second-order | This study |
| TZC | 71.83 | Langmuir | Pseudo-second-order | This study |
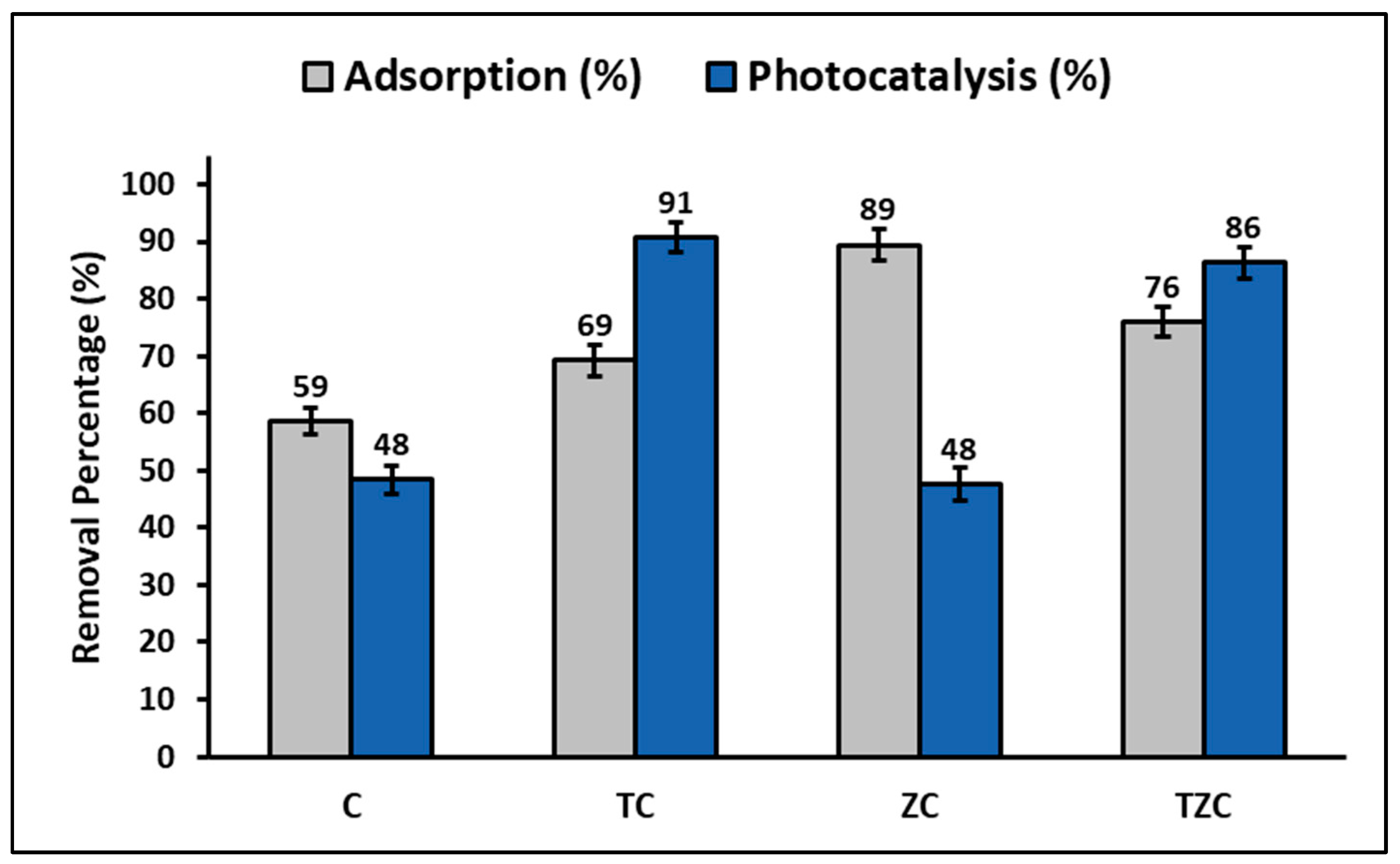
| Material | [CN] (mg L−1) | [Catalyst] (g L−1) | Time (min) | Efficiency (%) | Reference |
|---|---|---|---|---|---|
| ZnTiO3 | 20.0 | 0.2 | 90 | 90.7 | [93] |
| La/ZnTiO3 | 20.0 | 0.2 | 90 | 98.5 | [93] |
| Ce/ZnTiO3 | 20.0 | 0.2 | 90 | 95.1 | [93] |
| TiO2 | 20 | 0.2 | 90 | 84 | [87] |
| La/TiO2 | 20 | 0.2 | 90 | 97 | [87] |
| Ce/TiO2 | 20 | 0.2 | 90 | 89 | [87] |
| Eu/TiO2 | 20 | 0.2 | 90 | 86 | [87] |
| TiO2/Fe2O3/zeolite | 200 | 1.4 | 160 | 89 | [46] |
| TiO2/Fe2O3/PAC | 300 | 1.4 | 170 | 97 | [46] |
| Blast furnace sludge (BFS) | 750 | 2.0 | 120 | 97 | [72] |
| Cts-Ag | 71.6 | 2.5 | 180 | 98 | [95] |
| Fe2+ | 10 | 0.14 | 30 | 86 | [96] |
| TiO2 | 30 | 0.05 | 60 | 72 | [97] |
| Co/TiO2/SiO2 | 100 | 2.0 | 60 | 55 | [98] |
| TiO2/SiO2 | 100 | 1.7 | 180 | 93 | [99] |
| Ce/ZnO | 250 | 4.0 | 180 | 84 | [100] |
| C | 20 | 0.2 | 60 | 48 | This study |
| TC | 20 | 0.2 | 60 | 91 | This study |
| ZC | 20 | 0.2 | 60 | 48 | This study |
| TZC | 20 | 0.2 | 60 | 86 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaramillo-Fierro, X.; Alvarado, H.; Montesdeoca, F.; Valarezo, E. Faujasite-Type Zeolite Obtained from Ecuadorian Clay as a Support of ZnTiO3/TiO2 NPs for Cyanide Removal in Aqueous Solutions. Int. J. Mol. Sci. 2023, 24, 9281. https://doi.org/10.3390/ijms24119281
Jaramillo-Fierro X, Alvarado H, Montesdeoca F, Valarezo E. Faujasite-Type Zeolite Obtained from Ecuadorian Clay as a Support of ZnTiO3/TiO2 NPs for Cyanide Removal in Aqueous Solutions. International Journal of Molecular Sciences. 2023; 24(11):9281. https://doi.org/10.3390/ijms24119281
Chicago/Turabian StyleJaramillo-Fierro, Ximena, Hipatia Alvarado, Fernando Montesdeoca, and Eduardo Valarezo. 2023. "Faujasite-Type Zeolite Obtained from Ecuadorian Clay as a Support of ZnTiO3/TiO2 NPs for Cyanide Removal in Aqueous Solutions" International Journal of Molecular Sciences 24, no. 11: 9281. https://doi.org/10.3390/ijms24119281
APA StyleJaramillo-Fierro, X., Alvarado, H., Montesdeoca, F., & Valarezo, E. (2023). Faujasite-Type Zeolite Obtained from Ecuadorian Clay as a Support of ZnTiO3/TiO2 NPs for Cyanide Removal in Aqueous Solutions. International Journal of Molecular Sciences, 24(11), 9281. https://doi.org/10.3390/ijms24119281









