Substitutional Coinage Metals as Promising Defects for Adsorption and Detection of Gases on MoS2 Monolayers: A Computational Approach
Abstract
:1. Introduction
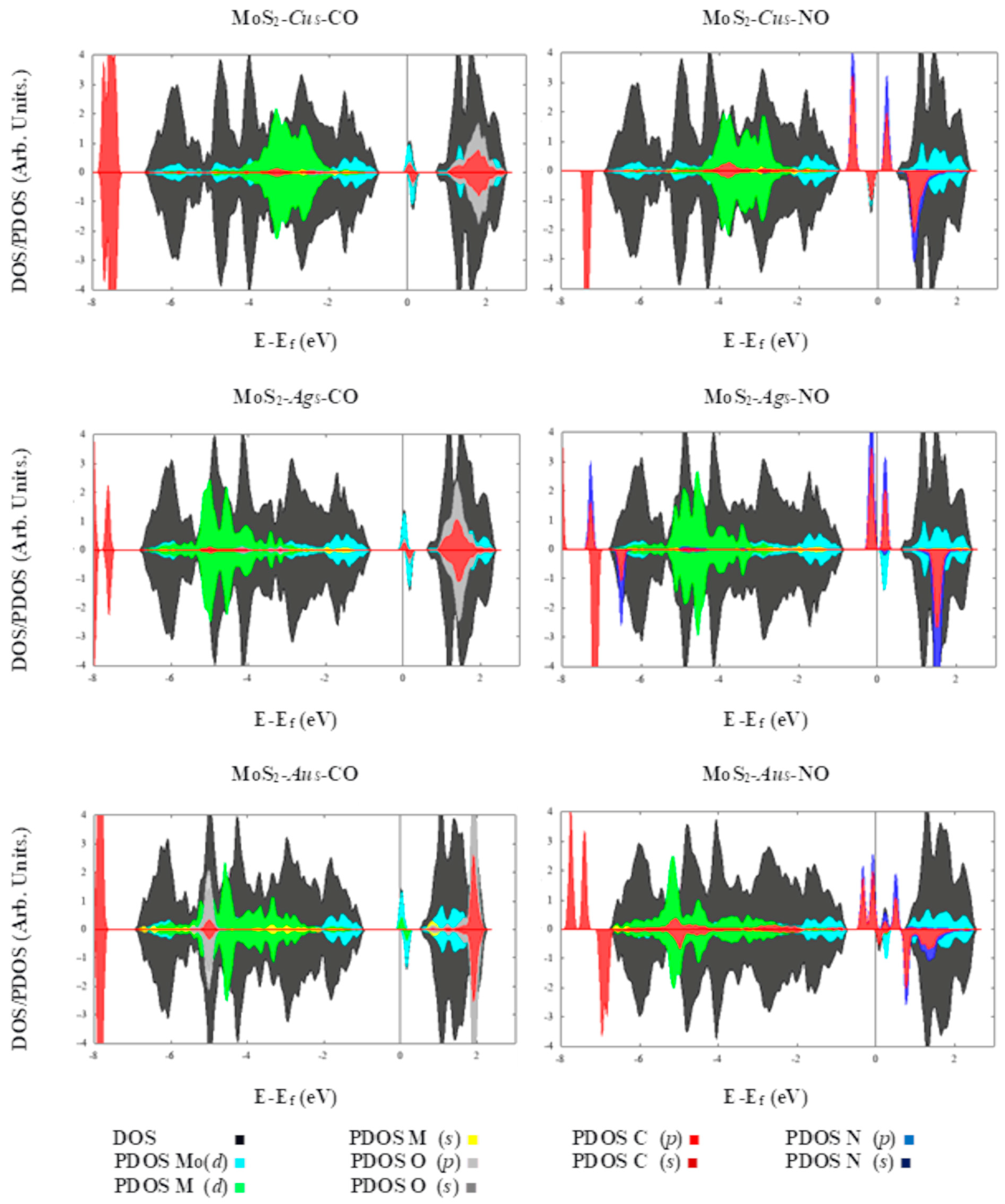
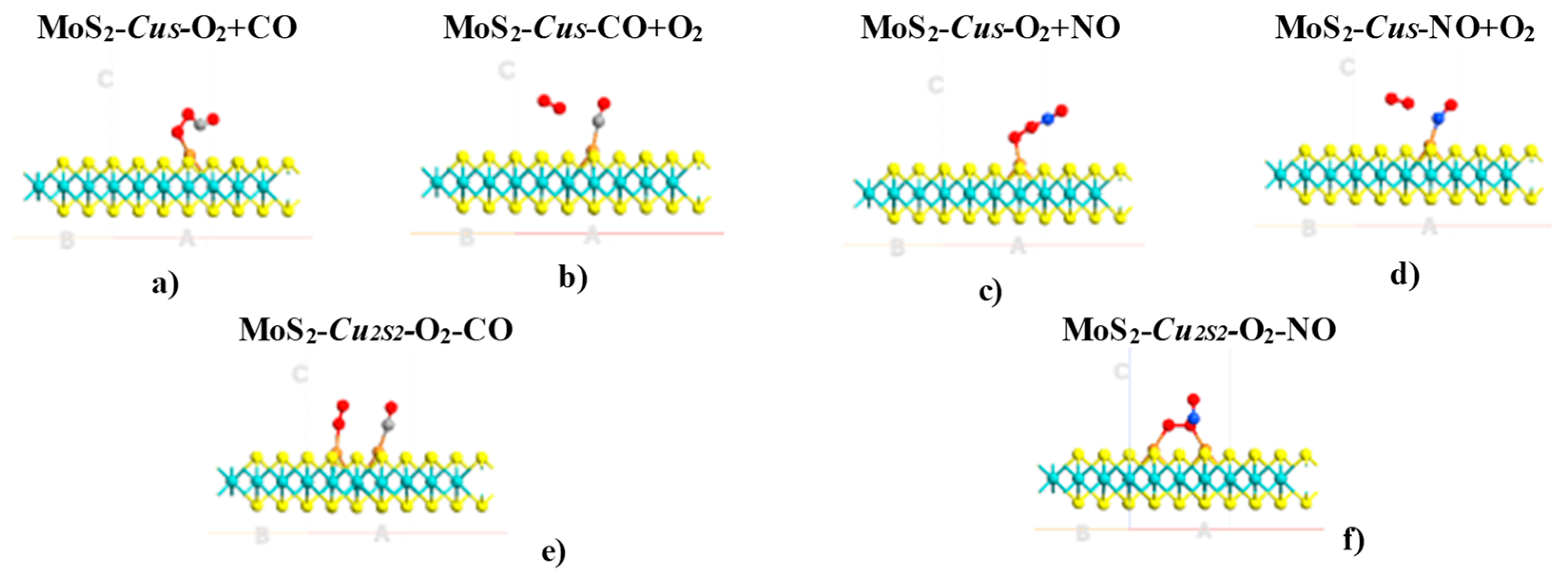
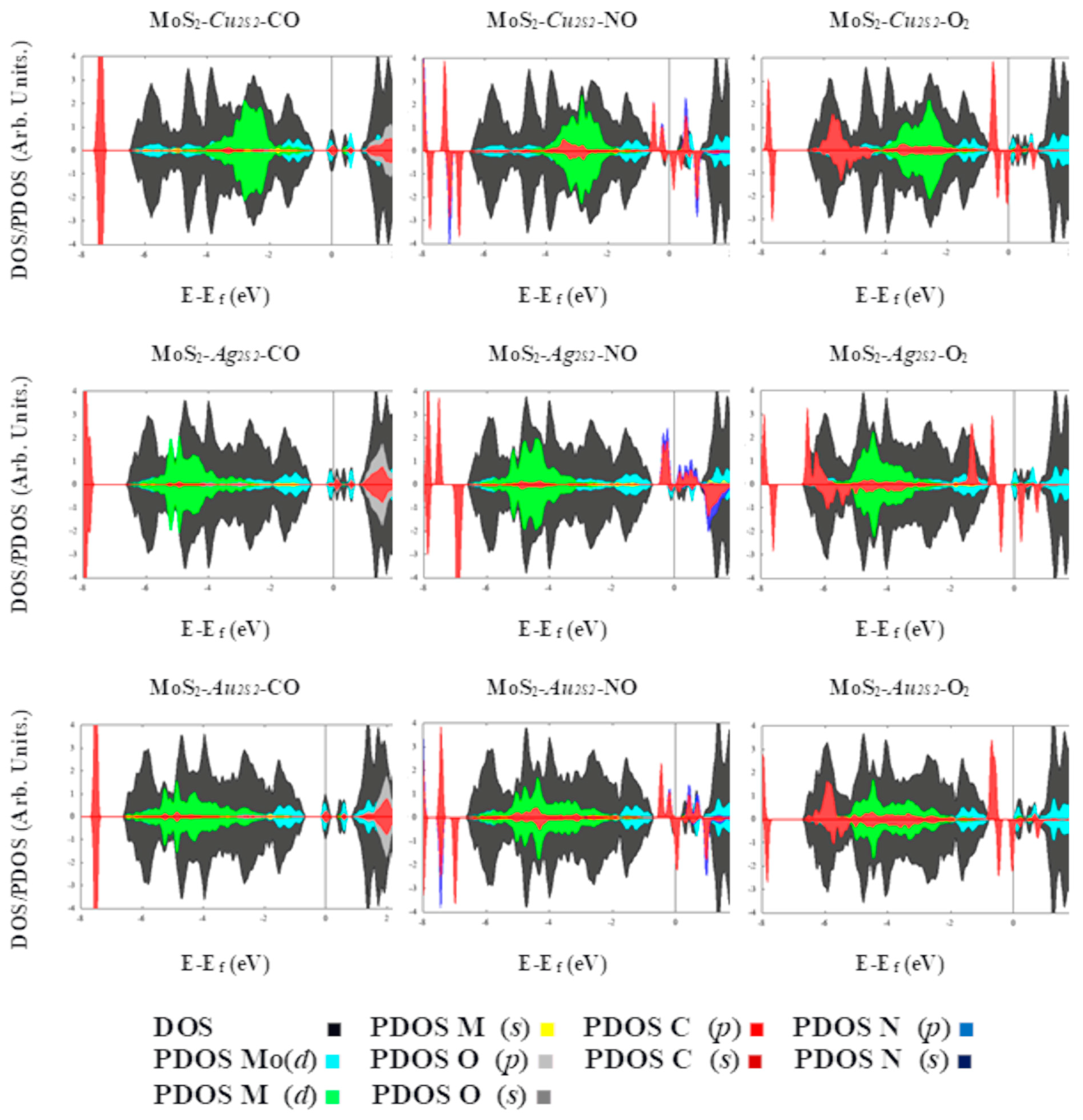
2. Results
3. Methods and Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically Thin MoS2: A New Direct-Gap Semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef] [Green Version]
- Putungan, D.B.; Lin, S.-H.; Wei, C.-M.; Kuo, J.-L. Li Adsorption, Hydrogen Storage and Dissociation Using Monolayer MoS2: An Ab Initio Random Structure Searching Approach. Phys. Chem. Chem. Phys. 2015, 17, 11367–11374. [Google Scholar] [CrossRef]
- Rangarajan, S.; Mavrikakis, M. On the Preferred Active Sites of Promoted MoS2 for Hydrodesulfurization with Minimal Organonitrogen Inhibition. ACS Catal. 2017, 7, 501–509. [Google Scholar] [CrossRef]
- Liu, B.; Chen, L.; Liu, G.; Abbas, A.N.; Fathi, M.; Zhou, C. High-Performance Chemical Sensing Using Schottky-Contacted Chemical Vapor Deposition Grown Monolayer MoS2 Transistors. ACS Nano 2014, 8, 5304–5314. [Google Scholar] [CrossRef]
- Donarelli, M.; Prezioso, S.; Perrozzi, F.; Bisti, F.; Nardone, M.; Giancaterini, L.; Cantalini, C.; Ottaviano, L. Response to NO2 and Other Gases of Resistive Chemically Exfoliated MoS2-Based Gas Sensors. Sens. Actuators B Chem. 2015, 207, 602–613. [Google Scholar] [CrossRef]
- Cho, B.; Yoon, J.; Lim, S.K.; Kim, A.R.; Kim, D.-H.; Park, S.-G.; Kwon, J.-D.; Lee, Y.-J.; Lee, K.-H.; Lee, B.H.; et al. Chemical Sensing of 2D Graphene/MoS2 Heterostructure Device. ACS Appl. Mater. Interfaces 2015, 7, 16775–16780. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.; Kim, A.R.; Park, Y.; Yoon, J.; Lee, Y.-J.; Lee, S.; Yoo, T.J.; Kang, C.G.; Lee, B.H.; Ko, H.C.; et al. Bifunctional Sensing Characteristics of Chemical Vapor Deposition Synthesized Atomic-Layered MoS2. ACS Appl. Mater. Interfaces 2015, 7, 2952–2959. [Google Scholar] [CrossRef]
- Vrubel, H.; Merki, D.; Hu, X. Hydrogen Evolution Catalyzed by MoS3 and MoS2 Particles. Energy Environ. Sci. 2012, 5, 6136. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Leuenberger, M.N. Room-Temperature Superparamagnetism Due to Giant Magnetic Anisotropy in MoS Defected Single-Layer MoS2. J. Phys. Condens. Matter 2018, 30, 155802. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Kim, M.J.; Jeong, B.G.; Kwon, C.; Cha, Y.; Choi, S.H.; Kim, K.K.; Jeong, M.S. Electrical Role of Sulfur Vacancies in MoS2: Transient Current Approach. Appl. Surf. Sci. 2023, 613, 155900. [Google Scholar] [CrossRef]
- González, C.; Biel, B.; Dappe, Y.J. Theoretical Characterisation of Point Defects on a MoS 2 Monolayer by Scanning Tunnelling Microscopy. Nanotechnology 2016, 27, 105702. [Google Scholar] [CrossRef] [PubMed]
- Le, D.; Rawal, T.B.; Rahman, T.S. Single-Layer MoS2 with Sulfur Vacancies: Structure and Catalytic Application. J. Phys. Chem. C 2014, 118, 5346–5351. [Google Scholar] [CrossRef]
- An, Y.; Fan, X.; Liu, H.; Luo, Z. Improved Catalytic Performance of Monolayer Nano-Triangles WS2 and MoS2 on HER by 3d Metals Doping. Comput. Mater. Sci. 2019, 159, 333–340. [Google Scholar] [CrossRef]
- Yin, M.Y.; Wang, X.C.; Mi, W.B.; Yang, B.H. First Principles Prediction on the Interfaces of Fe/MoS2, Co/MoS2 and Fe3O4/MoS2. Comput. Mater. Sci. 2015, 99, 326–335. [Google Scholar] [CrossRef]
- Nan, H.; Wang, Z.; Wang, W.; Liang, Z.; Lu, Y.; Chen, Q.; He, D.; Tan, P.; Miao, F.; Wang, X.; et al. Strong Photoluminescence Enhancement of MoS2 through Defect Engineering and Oxygen Bonding. ACS Nano 2014, 8, 5738–5745. [Google Scholar] [CrossRef] [Green Version]
- Miralrio, A.; Rangel Cortes, E.; Castro, M. Electronic Properties and Enhanced Reactivity of MoS2 Monolayers with Substitutional Gold Atoms Embedded into Sulfur Vacancies. Appl. Surf. Sci. 2018, 455, 758–770. [Google Scholar] [CrossRef]
- Miralrio, A.; Rangel, E.; Castro, M. Activation of MoS2 Monolayers by Substitutional Copper and Silver Atoms Embedded in Sulfur Vacancies: A Theoretical Study. Appl. Surf. Sci. 2019, 481, 611–624. [Google Scholar] [CrossRef]
- Sharma, A.; Khan, M.S.; Srivastava, A.; Khan, M.S.; Husain, M. Si-Doped MoS2 Sheet as Phosgene Gas Sensor: A First Principles Study. AIP Conf. Proc. 2019, 2115, 030438. [Google Scholar] [CrossRef]
- Zhao, P.; Zheng, J.; Guo, P.; Jiang, Z.; Cao, L.; Wan, Y. Electronic and Magnetic Properties of Re-Doped Single-Layer MoS2: A DFT Study. Comput. Mater. Sci. 2017, 128, 287–293. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, J.; Qiu, Y.; Zhu, J.; Zhang, Y.; Hu, G. A DFT Study of Transition Metal (Fe, Co, Ni, Cu, Ag, Au, Rh, Pd, Pt and Ir)-Embedded Monolayer MoS2 for Gas Adsorption. Comput. Mater. Sci. 2017, 138, 255–266. [Google Scholar] [CrossRef]
- Tong, P.; Sheng, Y.; Hou, R.; Iqbal, M.; Chen, L.; Li, J. Recent Progress on Coatings of Biomedical Magnesium Alloy. Smart Mater. Med. 2022, 3, 104–116. [Google Scholar] [CrossRef]
- Tuckett, R. Greenhouse Gases and the Emerging Climate Emergency. In Climate Change; Elsevier: Amsterdam, The Netherlands, 2021; pp. 19–45. ISBN 978-0-12-821575-3. [Google Scholar]
- Sachs, J.D. From Millennium Development Goals to Sustainable Development Goals. Lancet 2012, 379, 2206–2211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, C.Y.; Liu, L.L.; Zhou, B.; Zhang, Q.K.; Chen, Z.Q.; Tang, Z. Adsorption of Gas Molecules on Cu Impurities Embedded Monolayer MoS2: A First- Principles Study. Appl. Surf. Sci. 2016, 382, 280–287. [Google Scholar] [CrossRef]
- Wang, Z.; Mi, B. Environmental Applications of 2D Molybdenum Disulfide (MoS 2 ) Nanosheets. Environ. Sci. Technol. 2017, 51, 8229–8244. [Google Scholar] [CrossRef]
- Sharma, A.; Anu; Khan, M.S.; Husain, M.; Khan, M.S.; Srivastava, A. Sensing of CO and NO on Cu-Doped MoS2 Monolayer-Based Single Electron Transistor: A First Principles Study. IEEE Sens. J. 2018, 18, 2853–2860. [Google Scholar] [CrossRef]
- Wu, S.; Liu, H.; Qu, M.; Du, A.; Fan, J.; Sun, Q. B/C-Doped MoS2 as Promising Materials for Nitrogen Removal from Natural Gas: A First-Principles Computational Study. Comput. Mater. Sci. 2022, 214, 111735. [Google Scholar] [CrossRef]
- Sharma, A.; Srivastava, A.; Husain, M.; Khan, M.S. Computational Investigations of Cu-Embedded MoS2 Sheet for CO Oxidation Catalysis. J. Mater. Sci. 2018, 53, 9578–9588. [Google Scholar] [CrossRef]
- Reddy, B.K.S.; Borse, P.H. Review—Recent Material Advances and Their Mechanistic Approaches for Room Temperature Chemiresistive Gas Sensors. J. Electrochem. Soc. 2021, 168, 057521. [Google Scholar] [CrossRef]
- Ullah, F.; Ibrahim, K.; Mistry, K.; Samad, A.; Shahin, A.; Sanderson, J.; Musselman, K. WS2 and WS2-ZnO Chemiresistive Gas Sensors: The Role of Analyte Charge Asymmetry and Molecular Size. ACS Sens. 2023, 8, 1630–1638. [Google Scholar] [CrossRef]
- Stanislaus, A.; Marafi, A.; Rana, M.S. Recent Advances in the Science and Technology of Ultra Low Sulfur Diesel (ULSD) Production. Catal. Today 2010, 153, 1–68. [Google Scholar] [CrossRef]
- Rana, M.S.; Sámano, V.; Ancheyta, J.; Diaz, J.A.I. A Review of Recent Advances on Process Technologies for Upgrading of Heavy Oils and Residua. Fuel 2007, 86, 1216–1231. [Google Scholar] [CrossRef]
- Walton, A.S.; Lauritsen, J.V.; Topsøe, H.; Besenbacher, F. MoS2 Nanoparticle Morphologies in Hydrodesulfurization Catalysis Studied by Scanning Tunneling Microscopy. J. Catal. 2013, 308, 306–318. [Google Scholar] [CrossRef]
- Albiter, M.A.; Huirache-Acuña, R.; Paraguay-Delgado, F.; Rico, J.L.; Alonso-Nuñez, G. Synthesis of MoS2 Nanorods and Their Catalytic Test in the HDS of Dibenzothiophene. Nanotechnology 2006, 17, 3473–3481. [Google Scholar] [CrossRef] [PubMed]
- Muralikrishna, S.; Manjunath, K.; Samrat, D.; Reddy, V.; Ramakrishnappa, T.; Nagaraju, D.H. Hydrothermal Synthesis of 2D MoS2 Nanosheets for Electrocatalytic Hydrogen Evolution Reaction. RSC Adv. 2015, 5, 89389–89396. [Google Scholar] [CrossRef]
- Samy, O.; Zeng, S.; Birowosuto, M.D.; El Moutaouakil, A. A Review on MoS2 Properties, Synthesis, Sensing Applications and Challenges. Crystals 2021, 11, 355. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Tang, J.; Cui, H.; Li, Y. Noble Metal (Pt or Au)-Doped Monolayer MoS2 as a Promising Adsorbent and Gas-Sensing Material to SO2, SOF2 and SO2F2: A DFT Study. Appl. Phys. A 2018, 124, 194. [Google Scholar] [CrossRef]
- Halwidl, D.; Mayr-Schmölzer, W.; Setvin, M.; Fobes, D.; Peng, J.; Mao, Z.; Schmid, M.; Mittendorfer, F.; Redinger, J.; Diebold, U. A Full Monolayer of Superoxide: Oxygen Activation on the Unmodified Ca3Ru2O7 (001) Surface. J. Mater. Chem. A 2018, 6, 5703–5713. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.W.; Yan, J.M.; Zheng, W.T.; Jiang, Q. Cu4 Cluster Doped Monolayer MoS2 for CO Oxidation. Sci. Rep. 2015, 5, 11230. [Google Scholar] [CrossRef] [Green Version]
- Gadzhiev, O.B.; Ignatov, S.K.; Gangopadhyay, S.; Masunov, A.E.; Petrov, A.I. Mechanism of Nitric Oxide Oxidation Reaction (2NO + O2 → 2NO2) Revisited. J. Chem. Theory Comput. 2011, 7, 2021–2024. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Dou, X.; Zhang, Q.; Shah, S.N.A.; Khan, M.; Uchiyama, K.; Lin, J.-M. MoS2-Quantum Dot Triggered Reactive Oxygen Species Generation and Depletion: Responsible for Enhanced Chemiluminescence. Chem. Sci. 2019, 10, 497–500. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Hu, B.; Yang, Q.; Cai, X.; Nie, M.; Jin, Y.; Zhou, L.; Xu, Y.; Pan, Q.; Fang, L. Interaction Mechanism between Multi-Layered MoS2 and H2O2 for Self-Generation of Reactive Oxygen Species. Environ. Res. 2020, 191, 110227. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A Modular and Open-Source Software Project for Quantum Simulations of Materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Petkov, V.; Billinge, S.J.L.; Larson, P.; Mahanti, S.D.; Vogt, T.; Rangan, K.K.; Kanatzidis, M.G. Structure of Nanocrystalline Materials Using Atomic Pair Distribution Function Analysis: Study of LiMoS2. Phys. Rev. B 2002, 65, 092105. [Google Scholar] [CrossRef] [Green Version]
- Koós, A.A.; Vancsó, P.; Magda, G.Z.; Osváth, Z.; Kertész, K.; Dobrik, G.; Hwang, C.; Tapasztó, L.; Biró, L.P. STM Study of the MoS2 Flakes Grown on Graphite: A Model System for Atomically Clean 2D Heterostructure Interfaces. Carbon 2016, 105, 408–415. [Google Scholar] [CrossRef]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A Fast and Robust Algorithm for Bader Decomposition of Charge Density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]


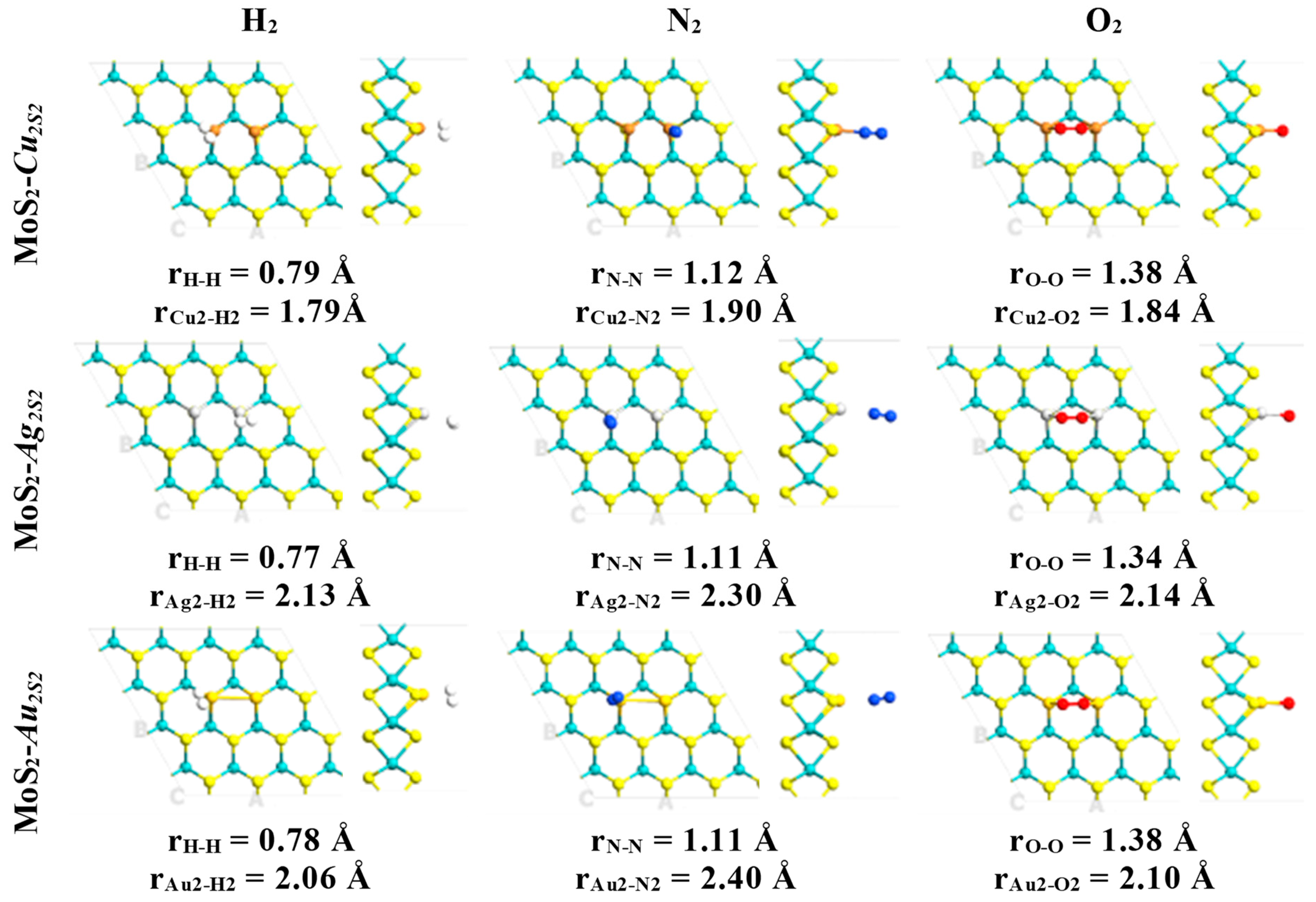
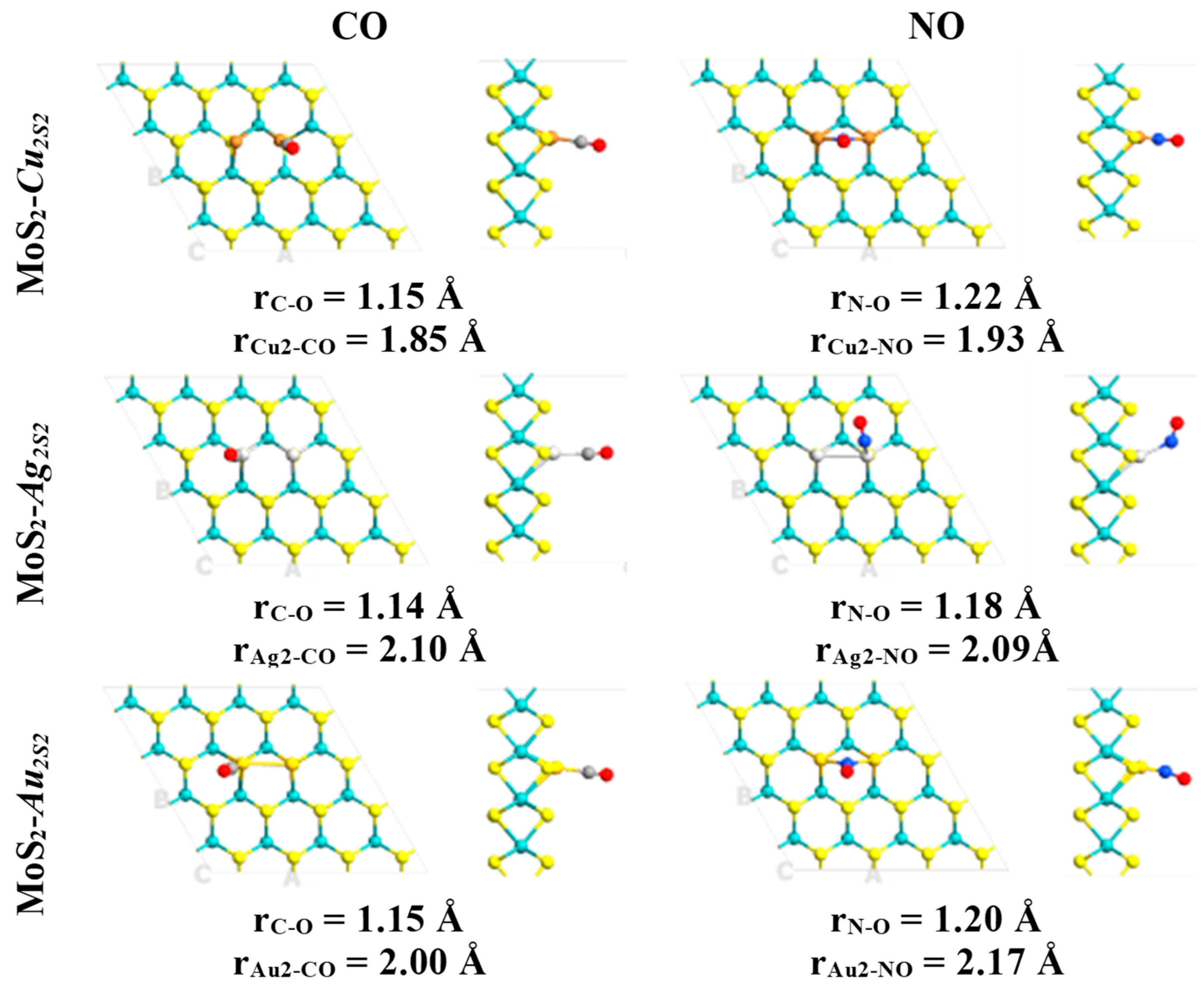
| System | Eads (kcal·mol−1)/eV | Total Magnetization (μB) | Magnetization on AB Molecule (μB) | Total Charge on AB Molecule (e) | Adsorption Mode |
|---|---|---|---|---|---|
| MoS2-Cus-H2 | 9.35/0.45 | 0.67 | 0.00 (0.00) | 0.02 | Cu-H2 |
| MoS2-Cus-O2 | 24.88/1.07 | 0.95 | 0.34 (0.34) | −0.50 | Cu-O2 |
| MoS2-Cus-N2 | 15.21/0.66 | 0.46 | 0.00 (0.02) | −0.09 | Cu-N-N |
| MoS2-Cus-CO | 28.54/1.24 | 0.43 | 0.01 (0.02) | −0.10 | Cu-C-O |
| MoS2-Cus-NO | 33.18/1.44 | 2.07 | 0.47 (0.70) | −0.28 | Cu-N-O |
| MoS2-Ags-H2 | 6.12/0.27 | 0.89 | 0.00 (0.00) | 0.05 | Ag-H2 |
| MoS2-Ags-O2 | 13.16/0.57 | 0.94 | 0.62 (0.64) | −0.42 | Ag-O2 |
| MoS2-Ags-N2 | 7.67/0.33 | 0.82 | 0.00 (0.01) | −0.02 | Ag-N-N |
| MoS2-Ags-CO | 17.85/0.77 | 0.69 | 0.01 (0.02) | −0.01 | Ag-C-O |
| MoS2-Ags-NO | 7.95/0.35 | 1.75 | 0.35 (0.88) | −0.19 | Ag-O-N |
| MoS2-Aus-H2 | 5.20/0.23 | 0.57 | 0.00 (0.00) | 0.06 | Au-H2 |
| MoS2-Aus-O2 | 11.74/0.51 | 0.86 | 0.42 (0.61) | −0.40 | Au-O-O |
| MoS2-Aus-N2 | 6.69/0.29 | 0.26 | 0.00 (0.01) | −0.02 | Au-N-N |
| MoS2-Aus-CO | 2.95/0.13 | 0.66 | 0.00 (0.00) | 0.00 | Au-O-C |
| MoS2-Aus-NO | 2.48/0.11 | 1.53 | 0.44 (0.58) | −0.25 | Au-N-O |
| System | Eads (kcal·mol−1)/eV | Total Magnetization (μB) | Magnetization on AB Molecule (μB) | Total Charge on AB Molecule (e) | Adsorption Mode |
|---|---|---|---|---|---|
| MoS2-Cu2S2-H2 | 9.09/0.395 | 0.03 | 0.00 (0.00) | 0.03 | Cu-H2 |
| MoS2-Cu2S2-O2 | 38.04/1.65 | 0.26 | 0.21 (0.21) | −0.85 | Cu-O-O-Cu |
| MoS2-Cu2S2-N2 | 15.24/0.66 | 0.04 | 0.00 (0.01) | −0.12 | Cu-N-N |
| MoS2-Cu2S2-CO | 30.21/1.31 | 0.03 | 0.00 (0.00) | −0.12 | Cu-C-O |
| MoS2-Cu2S2-NO | 38.89/1.69 | 0.94 | 0.45 (0.45) | −0.51 | Cu-N-O |
| MoS2-Ag2S2-H2 | 5.306/0.23 | 0.02 | 0.00 (0.00) | 0.04 | Ag-H2 |
| MoS2-Ag2S2-O2 | 22.41/0.97 | 0.94 | 0.48 (0.48) | −0.60 | Ag-O-O-Ag |
| MoS2-Ag2S2-N2 | 7.35/0.32 | 0.02 | 0.00 (0.00) | −0.02 | Ag-N-N |
| MoS2-Ag2S2-CO | 18.55/0.80 | 0.02 | 0.00 (0.00) | −0.03 | Ag-C-O |
| MoS2-Ag2S2-NO | 21.41/0.93 | 1.15 | 0.41 (0.66) | −0.25 | Ag-N-O |
| MoS2-Au2S2-H2 | 5.102/0.22 | 0.03 | 0.01 (0.01) | 0.06 | Au-H2 |
| MoS2-Au2S2-O2 | 22.078/0.96 | 0.28 | 0.25 (0.25) | −0.71 | Au-O-O-Au |
| MoS2-Au2S2-N2 | 5.903/0.26 | 0.04 | 0.00 (0.00) | −0.02 | Au-N-N |
| MoS2-Au2S2-CO | 24.82/1.08 | 0.03 | 0.00 (0.00) | −0.05 | Au-C-O |
| MoS2-Au2S2-NO | 25.65/1.11 | 0.87 | 0.33 (0.36) | −0.37 | Au-N-O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez-Rodriguez, J.; Castro, M.; Nieto-Jalil, J.M.; Medina, D.I.; Montes de Oca, S.; García-González, J.A.; Rangel-Cortes, E.; Miralrio, A. Substitutional Coinage Metals as Promising Defects for Adsorption and Detection of Gases on MoS2 Monolayers: A Computational Approach. Int. J. Mol. Sci. 2023, 24, 10284. https://doi.org/10.3390/ijms241210284
Gutierrez-Rodriguez J, Castro M, Nieto-Jalil JM, Medina DI, Montes de Oca S, García-González JA, Rangel-Cortes E, Miralrio A. Substitutional Coinage Metals as Promising Defects for Adsorption and Detection of Gases on MoS2 Monolayers: A Computational Approach. International Journal of Molecular Sciences. 2023; 24(12):10284. https://doi.org/10.3390/ijms241210284
Chicago/Turabian StyleGutierrez-Rodriguez, Josue, Miguel Castro, Jose Manuel Nieto-Jalil, Dora Iliana Medina, Saul Montes de Oca, José Andrés García-González, Eduardo Rangel-Cortes, and Alan Miralrio. 2023. "Substitutional Coinage Metals as Promising Defects for Adsorption and Detection of Gases on MoS2 Monolayers: A Computational Approach" International Journal of Molecular Sciences 24, no. 12: 10284. https://doi.org/10.3390/ijms241210284






