DNA Aptamer Beacon Probe (ABP) for Monitoring of Adenosine Triphosphate Level in SW480 Cancer Cells Treated with Glycolysis Inhibitor 2-Deoxyglucose
Abstract
:1. Introduction
2. Results and Discussion
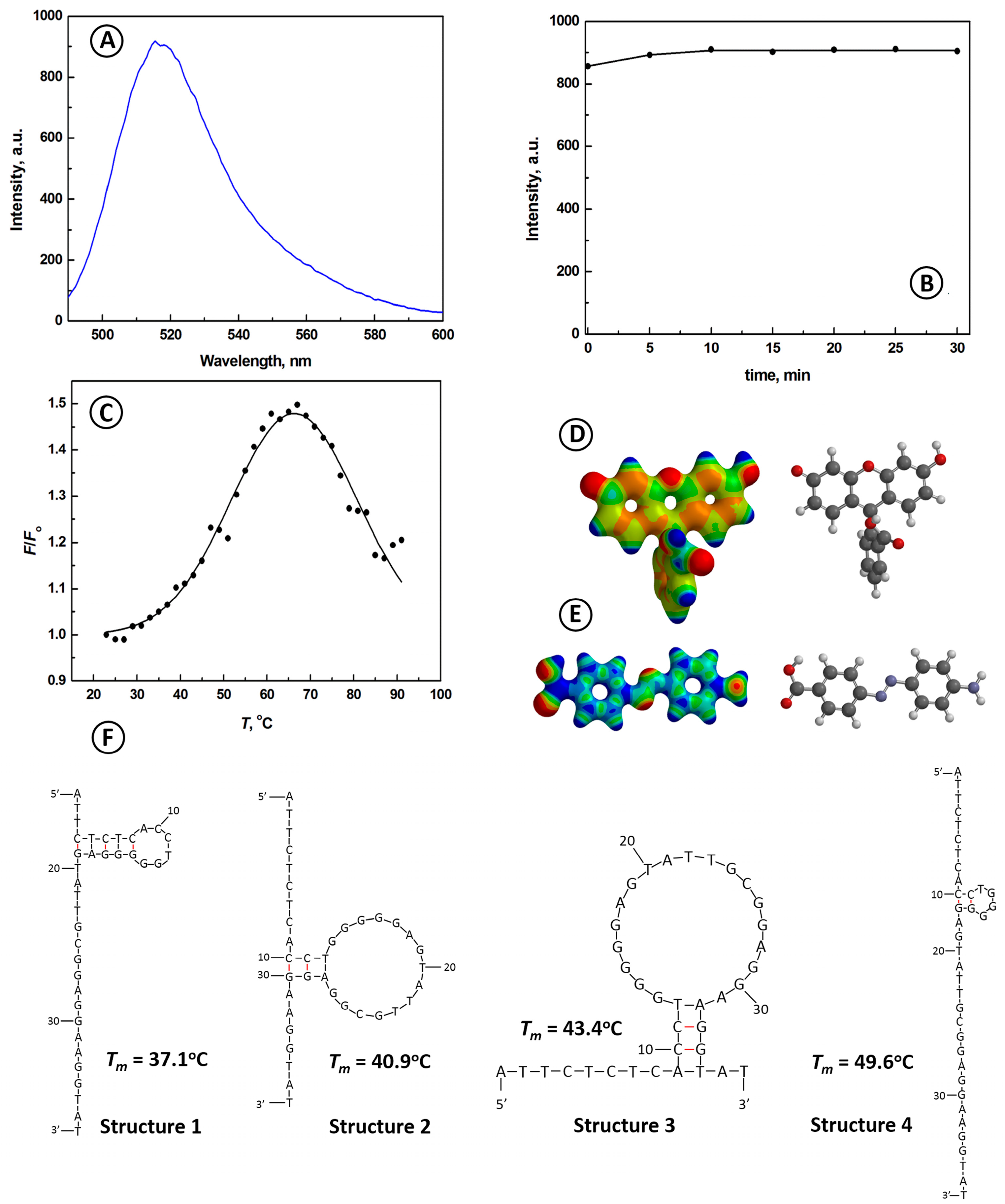
2.1. Characterization of the ATP Aptamer Beacon Probe
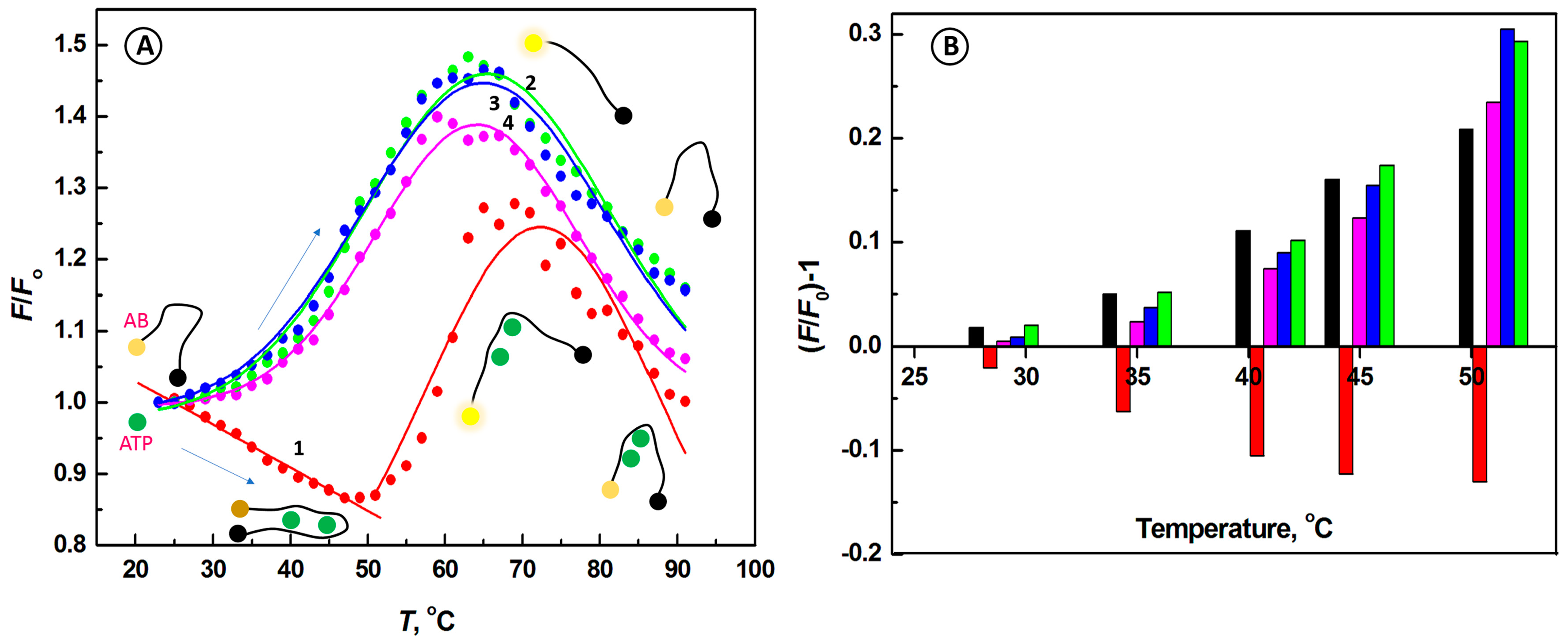
2.2. Temperature Dependence of the Interactions of Aptamer Beacon Probe with ATP and Other Nucleotides
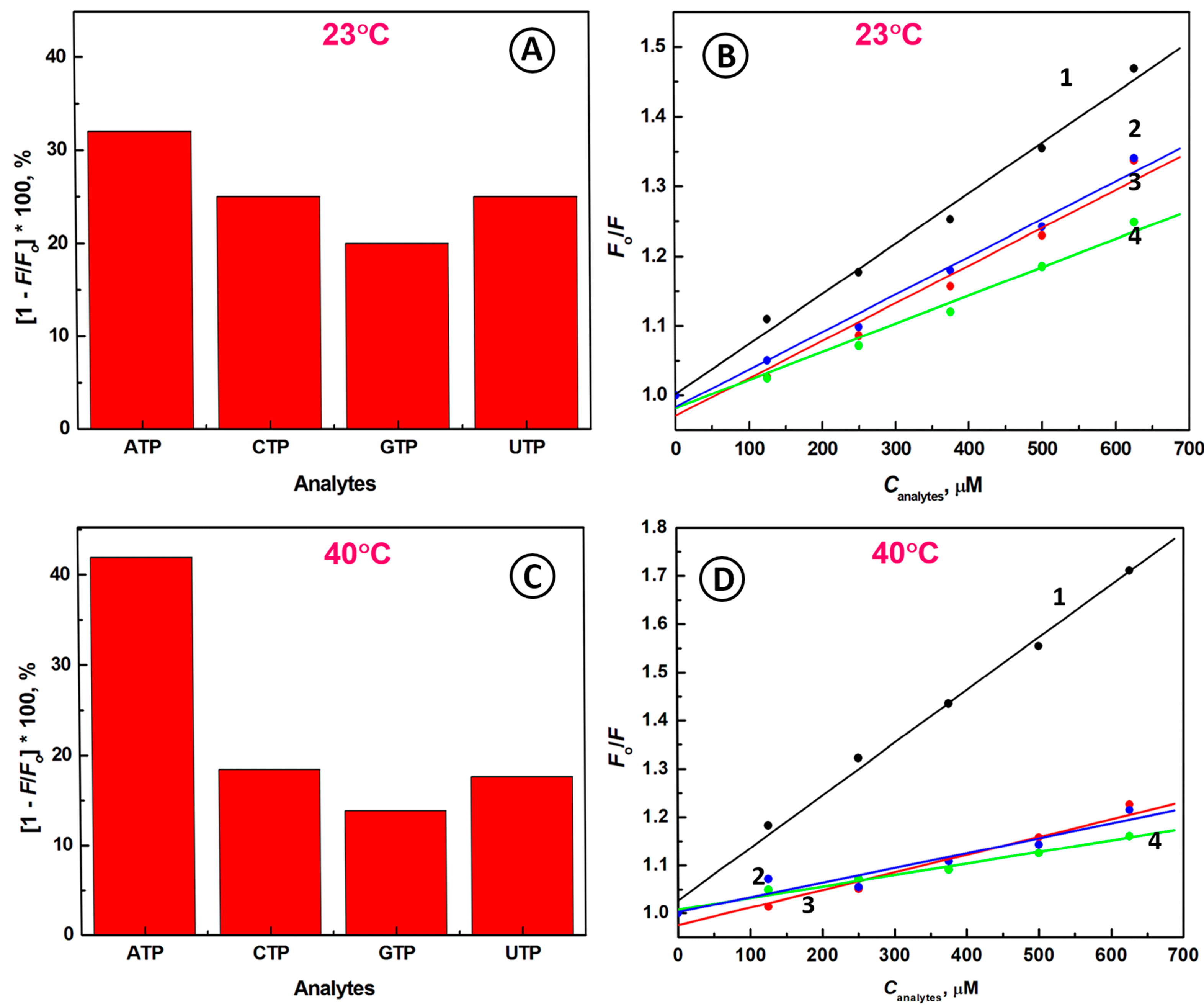
2.3. Selectivity of the Biosensing System
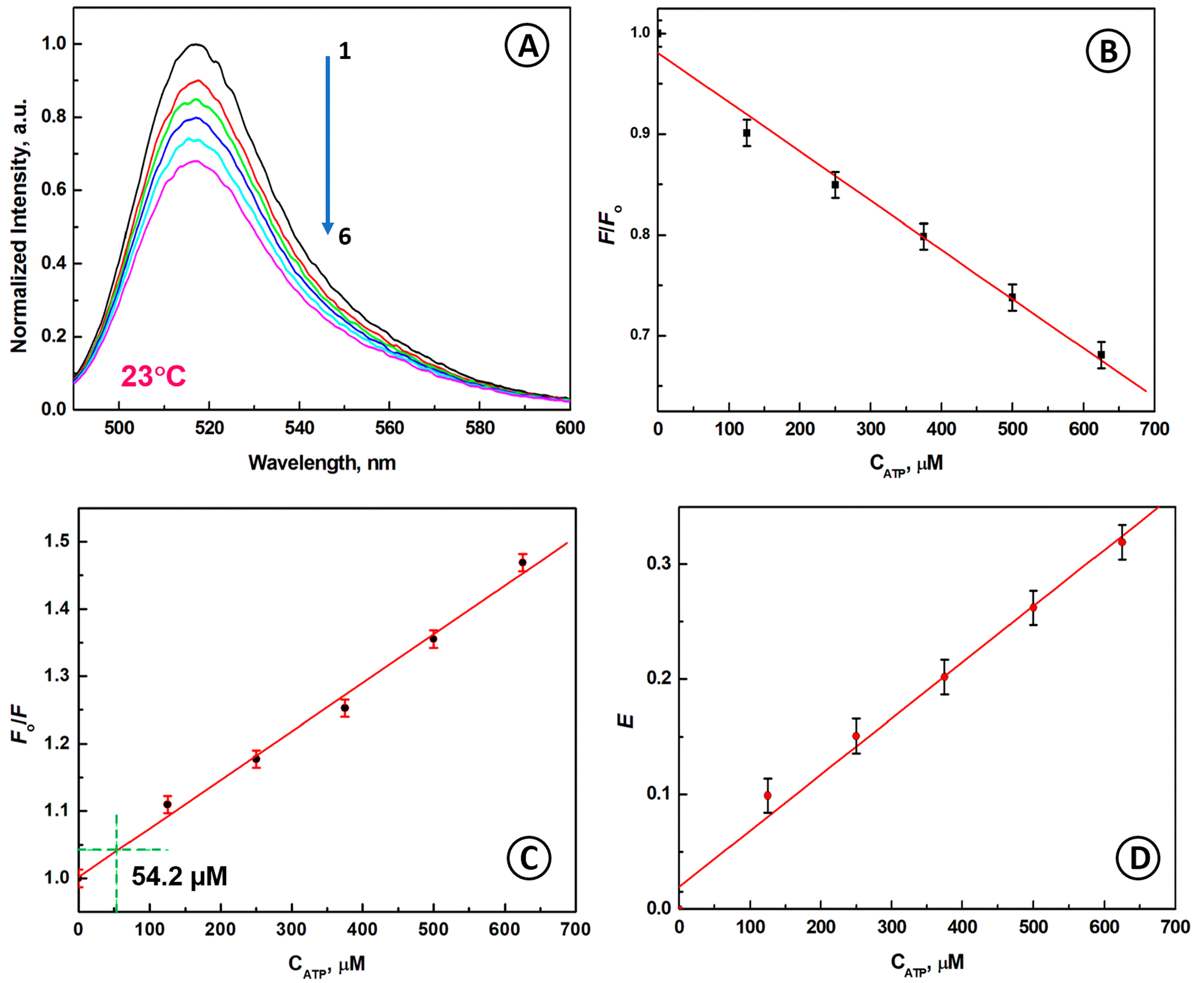
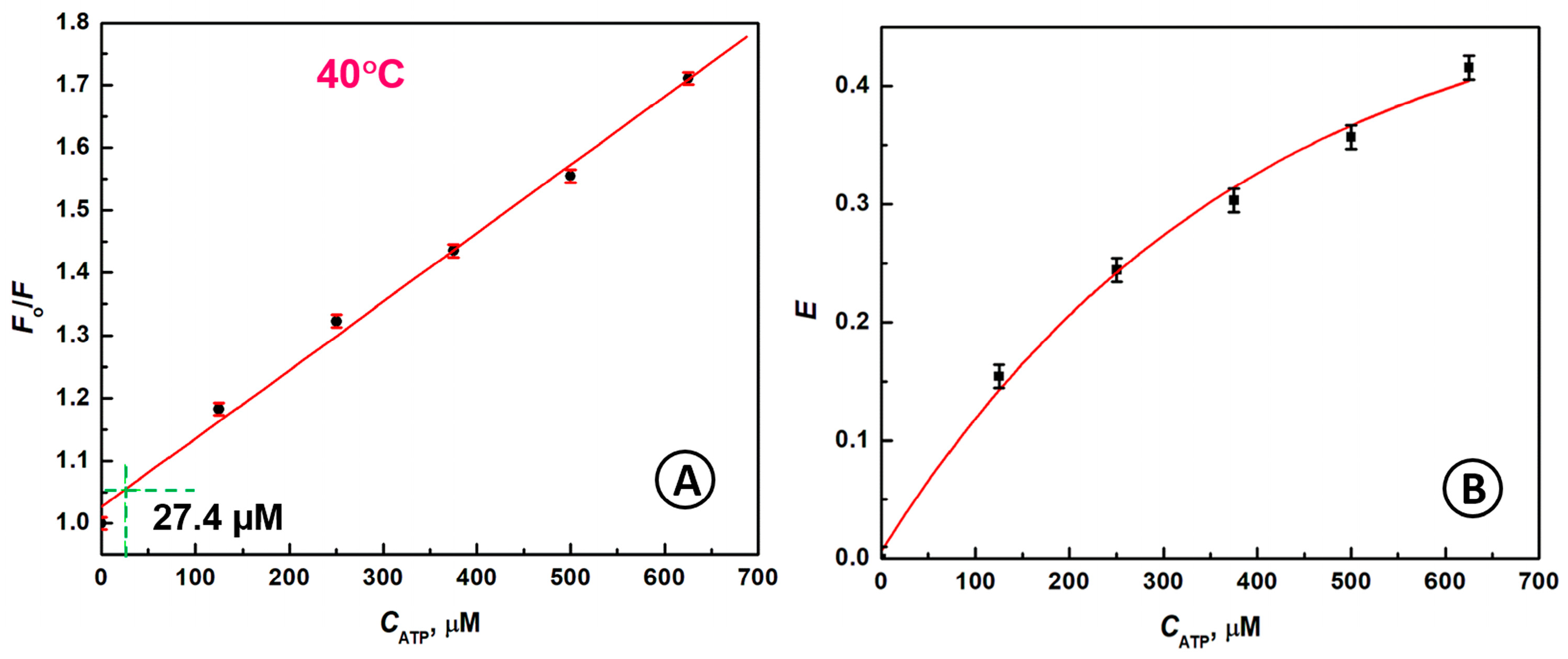
2.4. Detection of ATP Molecules
2.5. Determination of ATP in Cell Lysate Matrix Using Standard Addition Procedure
3. Materials and Methods
3.1. Chemicals
3.2. Instrumentation
3.3. Cell Culture
3.4. Cell Lysate
3.5. Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef]
- Stobiecka, M.; Ratajczak, K.; Jakiela, S. Toward early cancer detection: Focus on biosensing systems and biosensors for an anti-apoptotic protein survivin and survivin mRNA. Biosens. Bioelectron. 2019, 137, 58–71. [Google Scholar] [CrossRef]
- Ratajczak, K.; Krazinski, B.E.; Kowalczyk, A.E.; Dworakowska, B.; Jakiela, S.; Stobiecka, M. Hairpin–Hairpin Molecular Beacon Interactions for Detection of Survivin mRNA in Malignant SW480 Cells. ACS Appl. Mater. Interfaces 2018, 10, 17028–17039. [Google Scholar] [CrossRef]
- Ratajczak, K.; Krazinski, B.E.; Kowalczyk, A.E.; Dworakowska, B.; Jakiela, S.; Stobiecka, M. Optical Biosensing System for the Detection of Survivin mRNA in Colorectal Cancer Cells Using a Graphene Oxide Carrier-Bound Oligonucleotide Molecular Beacon. Nanomaterials 2018, 8, 510. [Google Scholar] [CrossRef]
- Stobiecka, M.; Dworakowska, B.; Jakiela, S.; Lukasiak, A.; Chalupa, A.; Zembrzycki, K. Sensing of Survivin mRNA in Malignant Astrocytes Using Graphene Oxide Nanocarrier-Supported Oligonucleotide Molecular Beacons. Sens. Actuat. B 2016, 235, 136–145. [Google Scholar] [CrossRef]
- Stobiecka, M.; Chalupa, A.; Dworakowska, B. Piezometric biosensors for anti-apoptotic protein survivin based on buried positive-potential barrier and immobilized monoclonal antibodies. Biosens. Bioelectron. 2016, 84, 37–43. [Google Scholar] [CrossRef]
- Ratajczak, K.; Stobiecka, M. Ternary Interactions and Energy Transfer Between Fluorescein Isothiocyanate, Adenosine Triphosphate, and Graphene Oxide Nanocarriers. J. Phys. Chem. B 2017, 121, 6822–6830. [Google Scholar] [CrossRef]
- Coppi, E.; Pugliese, A.M.; Urbani, S.; Melani, A.; Cerbai, E.; Mazzanti, B.; Bosi, A.; Saccardi, R.; Pedata, F. ATP Modulates Cell Proliferation and Elicits Two Different Electrophysiological Responses in Human Mesenchymal Stem Cells. Stem Cells 2007, 25, 1840–1849. [Google Scholar] [CrossRef]
- Rajendran, M.; Dane, E.; Conley, J.; Tantama, M. Imaging Adenosine Triphosphate (ATP). Biol. Bull. 2016, 231, 73–84. [Google Scholar] [CrossRef]
- Rigoulet, M.; Bouchez, C.L.; Paumard, P.; Ransac, S.; Cuvellier, S.; Duvezin-Caubet, S.; Mazat, J.P.; Devin, A. Cell energy metabolism: An update. Biochim. Biophys. Acta (BBA) Bioenerg. 2020, 1861, 148276. [Google Scholar] [CrossRef]
- Martinello, T.; Baldoin, M.C.; Morbiato, L.; Paganin, M.; Tarricone, E.; Schiavo, G.; Bianchini, E.; Sandonà, D.; Betto, R. Extracellular ATP signaling during differentiation of C2C12 skeletal muscle cells: Role in proliferation. Mol. Cell. Biochem. 2011, 351, 183–196. [Google Scholar] [CrossRef]
- Salvestrini, V.; Orecchioni, S.; Talarico, G.; Reggiani, F.; Mazzetti, C.; Bertolini, F.; Orioli, E.; Adinolfi, E.; Di Virgilio, F.; Pezzi, A.; et al. Extracellular ATP induces apoptosis through P2X7R activation in acute myeloid leukemia cells but not in normal hematopoietic stem cells. Oncotarget 2017, 8, 5895–5908. [Google Scholar] [CrossRef]
- Fiorillo, M.; Ózsvári, B.; Sotgia, F.; Lisanti, M.P. High ATP Production Fuels Cancer Drug Resistance and Metastasis: Implications for Mitochondrial ATP Depletion Therapy. Front. Oncol. 2021, 11, 3875. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Sarti, A.C.; Falzoni, S.; De Marchi, E.; Adinolfi, E. Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat. Rev. Cancer 2018, 18, 601–618. [Google Scholar] [CrossRef]
- Vultaggio-Poma, V.; Sarti, A.C.; Di Virgilio, F. Extracellular ATP: A Feasible Target for Cancer Therapy. Cells 2020, 9, 2496. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Adinolfi, E. Extracellular purines, purinergic receptors and tumor growth. Oncogene 2017, 36, 293–303. [Google Scholar] [CrossRef]
- Yang, H.; Geng, Y.-H.; Wang, P.; Zhou, Y.-T.; Yang, H.; Huo, Y.-F.; Zhang, H.-Q.; Li, Y.; He, H.-Y.; Tian, X.-X.; et al. Extracellular ATP promotes breast cancer invasion and epithelial-mesenchymal transition via hypoxia-inducible factor 2α signaling. Cancer Sci. 2019, 110, 2456–2470. [Google Scholar] [CrossRef]
- Yang, H.; Geng, Y.-H.; Wang, P.; Zhang, H.-Q.; Fang, W.-G.; Tian, X.-X. Extracellular ATP promotes breast cancer chemoresistance via HIF-1α signaling. Cell Death Dis. 2022, 13, 199. [Google Scholar] [CrossRef]
- Fiorillo, M.; Scatena, C.; Naccarato, A.G.; Sotgia, F.; Lisanti, M.P. Bedaquiline, an FDA-approved drug, inhibits mitochondrial ATP production and metastasis in vivo, by targeting the gamma subunit (ATP5F1C) of the ATP synthase. Cell Death Differ. 2021, 28, 2797–2817. [Google Scholar] [CrossRef]
- Zhou, Y.; Tozzi, F.; Chen, J.; Fan, F.; Xia, L.; Wang, J.; Gao, G.; Zhang, A.; Xia, X.; Brasher, H.; et al. Intracellular ATP levels are a pivotal determinant of chemoresistance in colon cancer cells. Cancer Res. 2012, 72, 304–314. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Pelicano, H.; Martin, D.; Xu, R.-H.; Huang, P. Glycolysis inhibition for anticancer treatment. Oncogene 2006, 25, 4633–4646. [Google Scholar] [CrossRef]
- Zhao, Y.; Butler, E.; Tan, M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 2013, 4, e532. [Google Scholar] [CrossRef]
- Martin, D.S.; Bertino, J.R.; Koutcher, J.A. ATP Depletion + Pyrimidine Depletion Can Markedly Enhance Cancer Therapy: Fresh Insight for a New Approach1. Cancer Res. 2000, 60, 6776–6783. [Google Scholar]
- Martin, D.S.; Stolfi, R.L.; Colofiore, J.R.; Nord, L.D.; Sternberg, S. Biochemical Modulation of Tumor Cell Energy in Vivo: II. A Lower Dose of Adriamycin is Required and a Greater Antitumor Activity is Induced when Cellular Energy is Depressed. Cancer Investig. 1994, 12, 296–307. [Google Scholar] [CrossRef]
- Stolfi, R.L.; Colofiore, J.R.; Nord, L.D.; Martin, D.S. Enhanced antitumor activity of an adriamycin + 5-fluorouracil combination when preceded by biochemical modulation. Anti-Cancer Drugs 1996, 7, 100–104. [Google Scholar] [CrossRef]
- Stolfi, R.L.; Colofiore, J.R.; Nord, L.D.; Koutcher, J.A.; Martin, D.S. Biochemical Modulation of Tumor Cell Energy: Regression of Advanced Spontaneous Murine Breast Tumors with a 5-Fluorouracil-containing Drug Combination1. Cancer Res. 1992, 52, 4074–4081. [Google Scholar]
- Wang, H.; Li, Y.; Zhang, M.; Wu, D.; Shen, Y.; Tang, G.; Ping, Y. Redox-Activatable ATP-Depleting Micelles with Dual Modulation Characteristics for Multidrug-Resistant Cancer Therapy. Adv. Healthc. Mater. 2017, 6, 1601293. [Google Scholar] [CrossRef]
- Song, X.-R.; Li, S.-H.; Guo, H.; You, W.; Tu, D.; Li, J.; Lu, C.-H.; Yang, H.-H.; Chen, X. Enhancing Antitumor Efficacy by Simultaneous ATP-Responsive Chemodrug Release and Cancer Cell Sensitization Based on a Smart Nanoagent. Adv. Sci. 2018, 5, 1801201. [Google Scholar] [CrossRef]
- Kaushik, N.; Lee, S.J.; Choi, T.G.; Baik, K.Y.; Uhm, H.S.; Kim, C.H.; Kaushik, N.K.; Choi, E.H. Non-thermal plasma with 2-deoxy-D-glucose synergistically induces cell death by targeting glycolysis in blood cancer cells. Sci. Rep. 2015, 5, 8726. [Google Scholar] [CrossRef]
- Chow, L.W.C.; Cheng, K.-S.; Leong, F.; Cheung, C.-W.; Shiao, L.-R.; Leung, Y.-M.; Wong, K.-L. Enhancing tetrandrine cytotoxicity in human lung carcinoma A549 cells by suppressing mitochondrial ATP production. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 427–436. [Google Scholar] [CrossRef]
- Maximchik, P.; Abdrakhmanov, A.; Inozemtseva, E.; Tyurin-Kuzmin, P.A.; Zhivotovsky, B.; Gogvadze, V. 2-Deoxy-D-glucose has distinct and cell line-specific effects on the survival of different cancer cells upon antitumor drug treatment. FEBS J. 2018, 285, 4590–4601. [Google Scholar] [CrossRef]
- Ben Sahra, I.; Laurent, K.; Giuliano, S.; Larbret, F.; Ponzio, G.; Gounon, P.; Le Marchand-Brustel, Y.; Giorgetti-Peraldi, S.; Cormont, M.; Bertolotto, C.; et al. Targeting cancer cell metabolism: The combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res. 2010, 70, 2465–2475. [Google Scholar] [CrossRef]
- Javaherian, S.; Musheev, M.U.; Kanoatov, M.; Berezovski, M.V.; Krylov, S.N. Selection of aptamers for a protein target in cell lysate and their application to protein purification. Nucleic Acids Res. 2009, 37, e62. [Google Scholar] [CrossRef]
- Aljohani, M.M.; Cialla-May, D.; Popp, J.; Chinnappan, R.; Al-Kattan, K.; Zourob, M. Aptamers: Potential Diagnostic and Therapeutic Agents for Blood Diseases. Molecules 2022, 27, 383. [Google Scholar] [CrossRef]
- Grabowska, I.; Sharma, N.; Vasilescu, A.; Iancu, M.; Badea, G.; Boukherroub, R.; Ogale, S.; Szunerits, S. Electrochemical Aptamer-Based Biosensors for the Detection of Cardiac Biomarkers. ACS Omega 2018, 3, 12010–12018. [Google Scholar] [CrossRef]
- Huizenga, D.E.; Szostak, J.W. A DNA aptamer that binds adenosine and ATP. Biochemistry 1995, 34, 656–665. [Google Scholar] [CrossRef]
- Zhang, Z.; Oni, O.; Liu, J. New insights into a classic aptamer: Binding sites, cooperativity and more sensitive adenosine detection. Nucleic Acids Res. 2017, 45, 7593–7601. [Google Scholar] [CrossRef]
- Biniuri, Y.; Luo, G.-F.; Fadeev, M.; Wulf, V.; Willner, I. Redox-Switchable Binding Properties of the ATP–Aptamer. J. Am. Chem. Soc. 2019, 141, 15567–15576. [Google Scholar] [CrossRef]
- Ng, S.; Lim, H.S.; Ma, Q.; Gao, Z. Optical Aptasensors for Adenosine Triphosphate. Theranostics 2016, 6, 1683–1702. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, X.; Mozneb, M.; Nagahara, L.; Hu, T.Y.; Li, C.-Z. Lighting up ATP in cells and tissues using a simple aptamer-based fluorescent probe. Microchim. Acta 2021, 188, 352. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, J.; Ju, H. Target-driven DNA association to initiate cyclic assembly of hairpins for biosensing and logic gate operation. Chem. Sci. 2015, 6, 4318–4323. [Google Scholar] [CrossRef]
- Zhou, C.; Yu, Z.; Yu, W.; Liu, H.; Zhang, H.; Guo, C. Split aptamer-based detection of adenosine triphosphate using surface enhanced Raman spectroscopy and two kinds of gold nanoparticles. Microchim. Acta 2019, 186, 251. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, C.; Wang, C.; Wang, P.; Chang, X.; Han, L.; Zhang, Y. Multiple Biomarker Simultaneous Detection in Serum via a Nanomaterial-Functionalized Biosensor for Ovarian Tumor/Cancer Diagnosis. Micromachines 2022, 13, 2046. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, J.; Liu, J.; Sun, S.; Xiong, Y.; Ma, Y.; Yan, S.; Yang, Y.; Yin, H.; Cai, X. Label-free microfluidic paper-based electrochemical aptasensor for ultrasensitive and simultaneous multiplexed detection of cancer biomarkers. Biosens. Bioelectron. 2019, 136, 84–90. [Google Scholar] [CrossRef]
- Kuntamung, K.; Jakmunee, J.; Ounnunkad, K. A label-free multiplex electrochemical biosensor for the detection of three breast cancer biomarker proteins employing dye/metal ion-loaded and antibody-conjugated polyethyleneimine-gold nanoparticles. J. Mater. Chem. B 2021, 9, 6576–6585. [Google Scholar] [CrossRef]
- Ratajczak, K.; Lukasiak, A.; Grel, H.; Dworakowska, B.; Jakiela, S.; Stobiecka, M. Monitoring of dynamic ATP level changes by oligomycin-modulated ATP synthase inhibition in SW480 cancer cells using fluorescent “On-Off” switching DNA aptamer. Anal. Bioanal. Chem. 2019, 411, 6899–6911. [Google Scholar] [CrossRef]
- Markham, N.R.; Zuker, M. DINAMelt Web Server for Nucleic Acid Melting Prediction. Nucleic Acids Res. 2005, 33, W577–W581. [Google Scholar] [CrossRef]
- Markham, N.; Zuker, M. UNAFold: Software for Nucleic Acid Folding and Hybridization. In Bioinformatics: Structure, Function and Applications; Keith, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2008; Volume 453, pp. 3–31. [Google Scholar]
- Tippana, R.; Chen, M.C.; Demeshkina, N.A.; Ferré-D’Amaré, A.R.; Myong, S. RNA G-quadruplex is resolved by repetitive and ATP-dependent mechanism of DHX36. Nat. Commun. 2019, 10, 1855. [Google Scholar] [CrossRef]
- Stobiecka, M.; Chalupa, A. DNA Strand Replacement Mechanism in Molecular Beacons Encoded for Detection of Cancer Biomarkers. J. Phys. Chem. B 2016, 120, 4782–4790. [Google Scholar] [CrossRef]
- Slavkovic, S.; Zhu, Y.; Churcher, Z.R.; Shoara, A.A.; Johnson, A.E.; Johnson, P.E. Thermodynamic analysis of cooperative ligand binding by the ATP-binding DNA aptamer indicates a population-shift binding mechanism. Sci. Rep. 2020, 10, 18944. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Liu, B. Fluorescent detection of ATP based on signaling DNA aptamer attached silica nanoparticles. Nanotechnology 2008, 19, 415605. [Google Scholar] [CrossRef]
- Wang, D.; Geng, F.; Wang, Y.; Ma, Y.; Li, G.; Qu, P.; Shao, C.; Xu, M. Design of a Fluorescence Turn-on and Label-free Aptasensor Using the Intrinsic Quenching Power of G-Quadruplex to AMT. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2020, 36, 965–970. [Google Scholar] [CrossRef]
- Bing, T.; Mei, H.; Zhang, N.; Qi, C.; Liu, X.; Shangguan, D. Exact tailoring of an ATP controlled streptavidin binding aptamer. RSC Adv. 2014, 4, 15111–15114. [Google Scholar] [CrossRef]
- Park, Y.; Nim-anussornkul, D.; Vilaivan, T.; Morii, T.; Kim, B.H. Facile conversion of ATP-binding RNA aptamer to quencher-free molecular aptamer beacon. Bioorganic Med. Chem. Lett. 2018, 28, 77–80. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, S.; Liu, B.; Wu, J.; Zhou, Y.; He, L.; Ding, J.; Liu, J. Intracellular Detection of ATP Using an Aptamer Beacon Covalently Linked to Graphene Oxide Resisting Nonspecific Probe Displacement. Anal. Chem. 2014, 86, 12229–12235. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, J.H. The combination of tephrosin with 2-deoxy-D-glucose enhances the cytotoxicity via accelerating ATP depletion and blunting autophagy in human cancer cells. Cancer Biol. Ther. 2011, 12, 989–996. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, Y.; Zhang, Y.; Fu, B.; Wu, X.; Li, Q.; Cai, G.; Chen, X.; Bai, X.-Y. Low-dose 2-deoxyglucose and metformin synergically inhibit proliferation of human polycystic kidney cells by modulating glucose metabolism. Cell Death Discov. 2019, 5, 76. [Google Scholar] [CrossRef]
- Dunn, J.; Grider, M.H. Physiology, Adenosine Triphosphate. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]


| No. | Fluorescence Aptaprobe | Cprobe, µM | Buffer Solution | ATP Detection (LOD), [µM] | References |
|---|---|---|---|---|---|
| 1. | Cy5-TGGAAGGAGGCG TTA TGA GGG GGT CCA | 3.3 | 300 mM NaCl, 20 mM Tris-HCl, 0.1% Tween 20, pH = 8.3 | 34 | [55] |
| 2. | DNA1-GGGTTGGG/iSp9/ACCTGGGGGAGTA | 12 | Tris-HCl, pH 9.0 | 28 | [56] |
| DNA2-CCCTGCGGAGGAAGGT/iSp9/GGGTAGGG | 4 | ||||
| 3. | TCTCTCACCTGGGGGAGTATTGCGGAGGAAGGT-FAM | 0.0667 | 20 mM Tris-HCl, 100 mM NaCl, 5 mM MgCl2, pH 7.4 | 17 | [49] |
| 4. | FAM-ATTCTCTCACCTGGGGGAGTATTGCGGAGGAAGGTAT-Dabcyl | 0.133 | 20 mM Tris-HCl buffer, 100 mM NaCl 5 mM MgCl2, pH 7.4 | 27 | This work |
| 5. | ATACCTGGGGAGTATAGCTGTGTGACGCAACACTAGCGGAGGAAGGTAT | 0.5 | 25 mM Tris-HCl, 120 mM NaCl, 5 mM KCl, 3 mM MgCl2, 0.02% Tween20, pH 7.4 | 40 | [57] |
| 6. | GUCGCACGUAGUGG-PyUGUGUGUGUGGUGCGAC | 1.5 | 10 mM Tris-HCl buffer, pH 7.2, 100 mM NaCl, 10 mM MgCl2 | 90 | [58] |
| 7. | GUCGCACGPyUAGUGG-UGUGUGUGUGGUGCGAC | 1.5 | 10 mM Tris-HCl buffer, pH 7.2, 100 mM NaCl, 10 mM MgCl2 | 820 | [58] |
| 8. | FAM-ACCTGGGGGAGTATTGCGGAGGAAGGT T10 | 0.5 | 15 mM Tris buffer, pH 8.5 | 28 | [59] |
| 9. | FAM-ACC TGG GGG AGT ATT GCG GAG GAA GGT-NH2 | 20 | 15 mM Tris buffer, pH 8.5 | 19 | [59] |
| Samples | Added, µM | RSD (%) |
|---|---|---|
| lysate from untreated SW480 cancer cells | 125 | 5.87 |
| 250 | 9.01 | |
| 375 | 9.88 | |
| lysate from SW480 cancer cells treated with 2-DG | 125 | 4.17 |
| 250 | 3.86 | |
| 375 | 4.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratajczak, K.; Stobiecka, M. DNA Aptamer Beacon Probe (ABP) for Monitoring of Adenosine Triphosphate Level in SW480 Cancer Cells Treated with Glycolysis Inhibitor 2-Deoxyglucose. Int. J. Mol. Sci. 2023, 24, 9295. https://doi.org/10.3390/ijms24119295
Ratajczak K, Stobiecka M. DNA Aptamer Beacon Probe (ABP) for Monitoring of Adenosine Triphosphate Level in SW480 Cancer Cells Treated with Glycolysis Inhibitor 2-Deoxyglucose. International Journal of Molecular Sciences. 2023; 24(11):9295. https://doi.org/10.3390/ijms24119295
Chicago/Turabian StyleRatajczak, Katarzyna, and Magdalena Stobiecka. 2023. "DNA Aptamer Beacon Probe (ABP) for Monitoring of Adenosine Triphosphate Level in SW480 Cancer Cells Treated with Glycolysis Inhibitor 2-Deoxyglucose" International Journal of Molecular Sciences 24, no. 11: 9295. https://doi.org/10.3390/ijms24119295
APA StyleRatajczak, K., & Stobiecka, M. (2023). DNA Aptamer Beacon Probe (ABP) for Monitoring of Adenosine Triphosphate Level in SW480 Cancer Cells Treated with Glycolysis Inhibitor 2-Deoxyglucose. International Journal of Molecular Sciences, 24(11), 9295. https://doi.org/10.3390/ijms24119295






