MoCbp7, a Novel Calcineurin B Subunit-Binding Protein, Is Involved in the Calcium Signaling Pathway and Regulates Fungal Development, Virulence, and ER Homeostasis in Magnaporthe oryzae
Abstract
1. Introduction
2. Results
2.1. Identification of MoCbp7
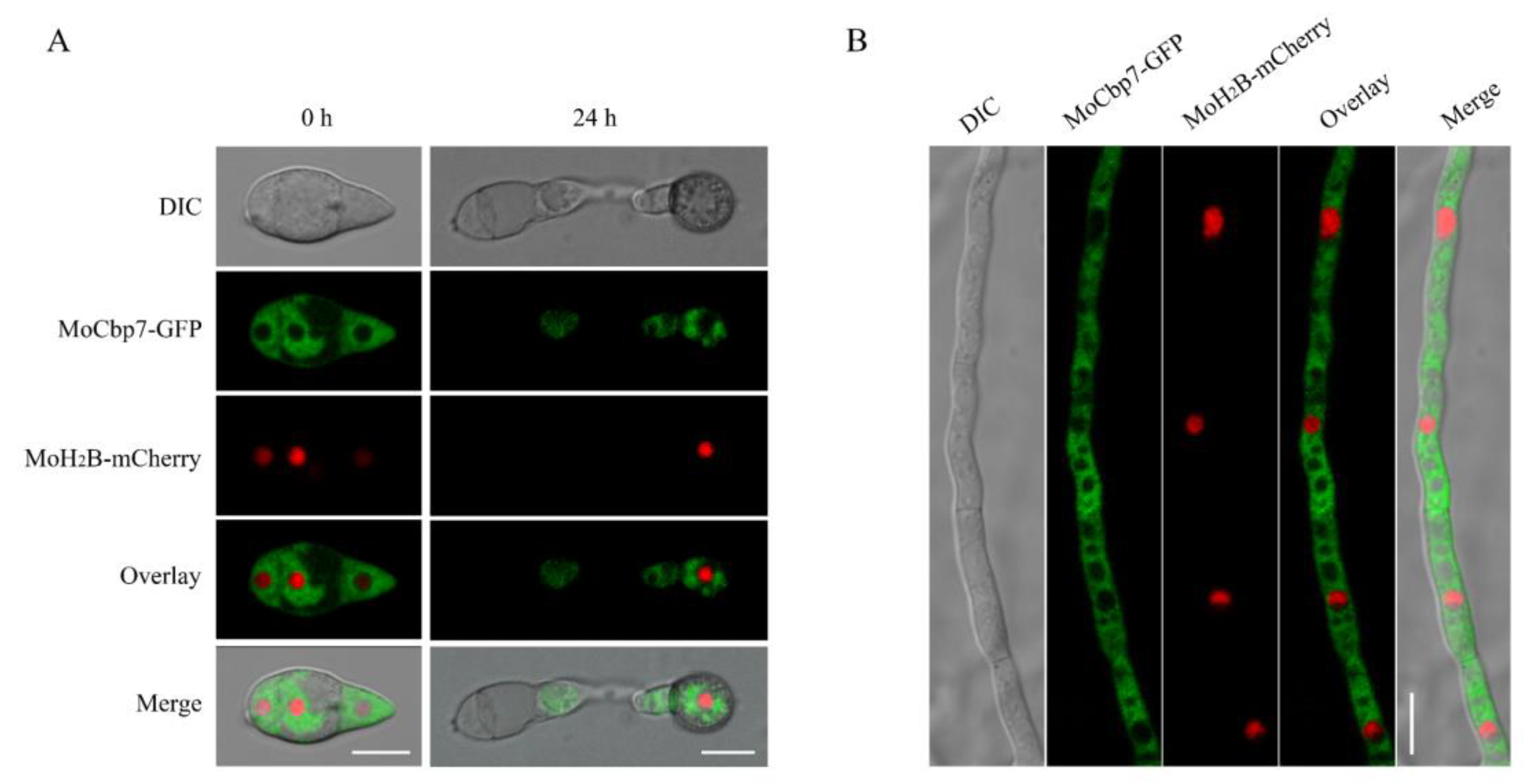
2.2. MoCbp7 Is Localized in the Cytoplasm of M. oryzae
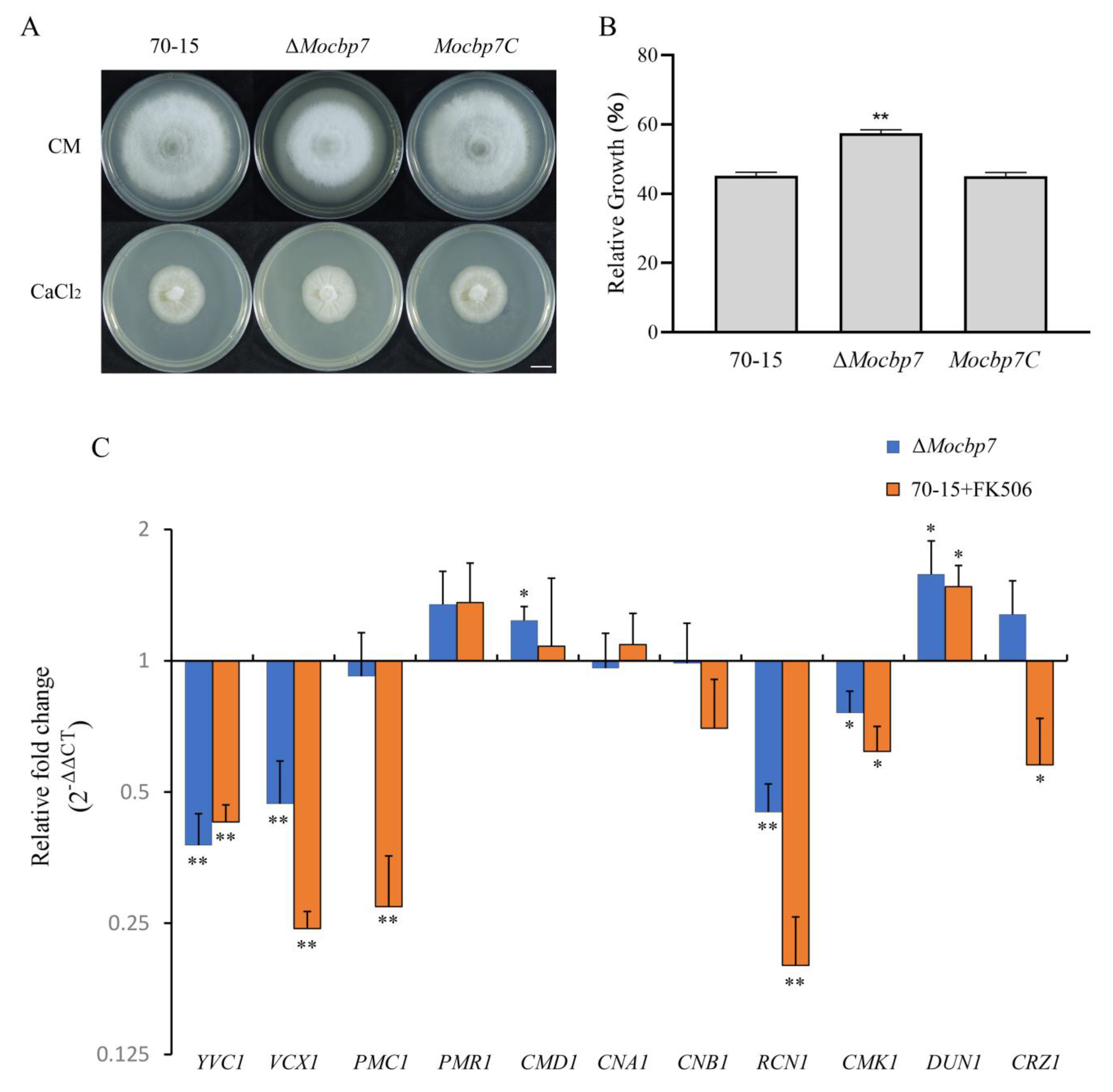
2.3. MoCbp7 Is Involved in the Calcium Signaling Pathway
2.4. MoCbp7 Is Essential for Asexual Growth, Conidiation, and Appressorium Formation
2.5. MoCbp7 Is Required for Virulence
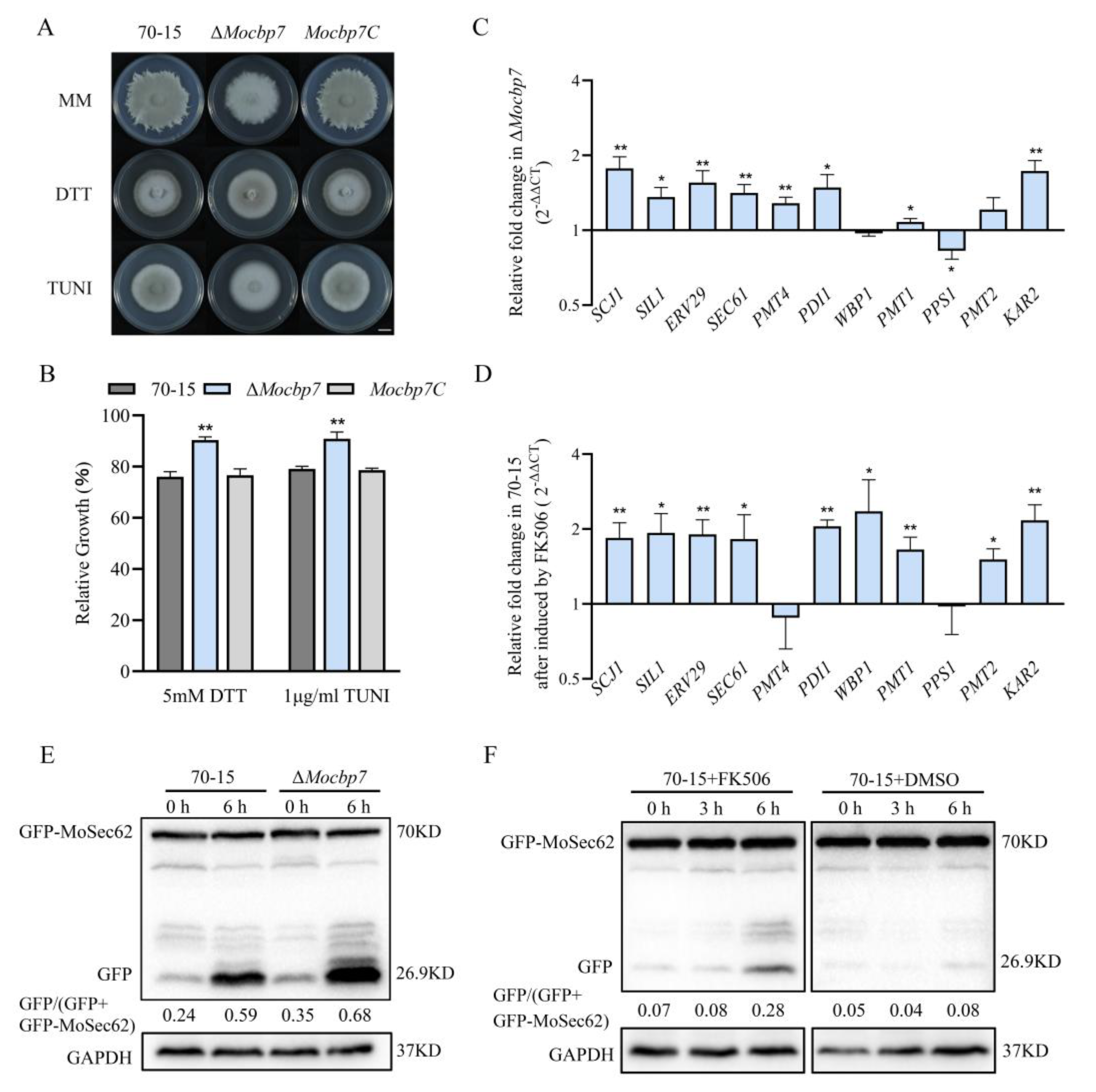
2.6. MoCbp7 Cooperates with Calcineurin to Regulate ER Homeostasis in M. oryzae
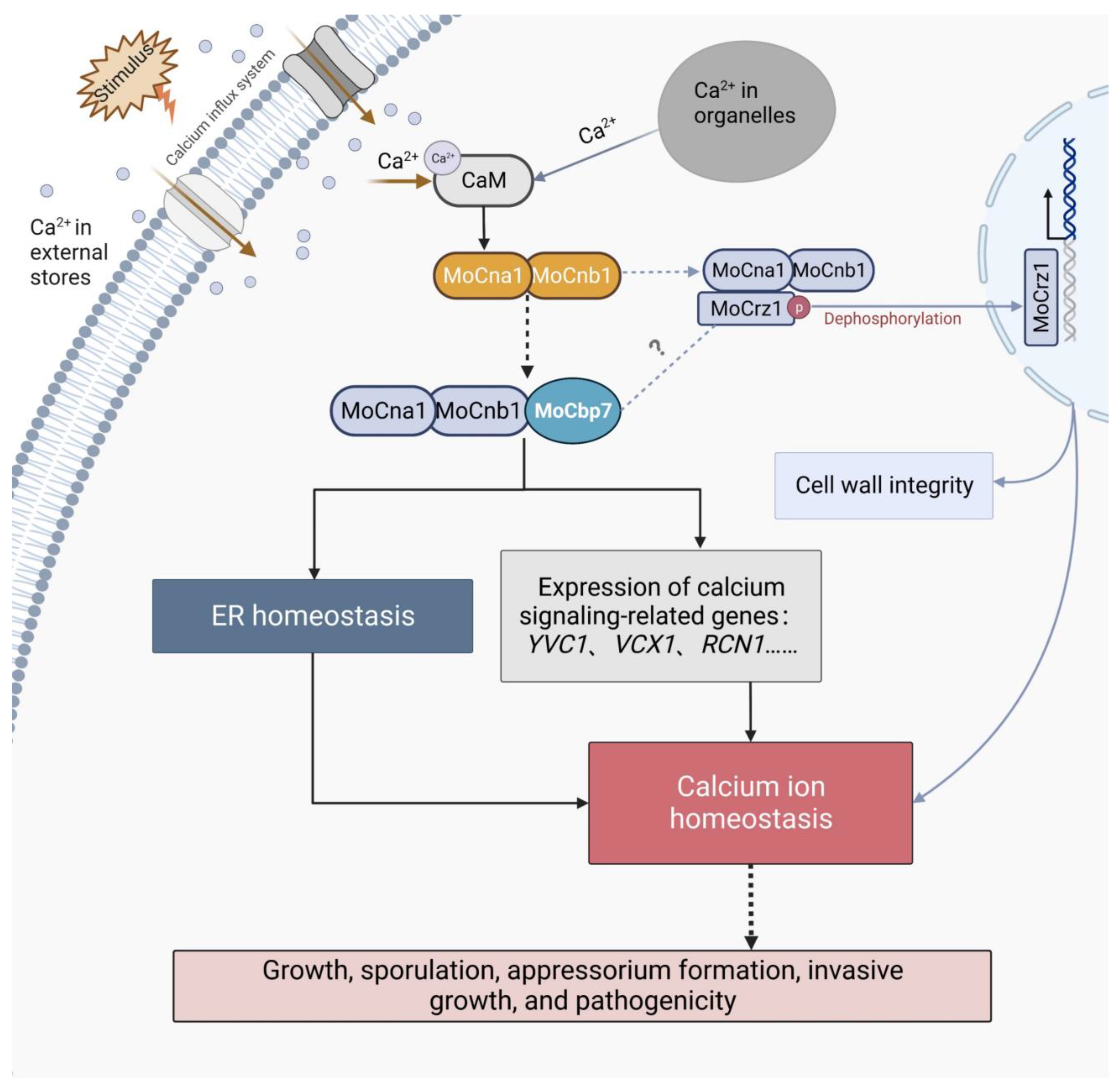
3. Discussion
4. Materials and Methods
4.1. Fungal Strains and Culture Conditions
4.2. Generation and Complementation of the Null Mutant
4.3. Quantitative Real-Time PCR Analysis
4.4. Protein Extraction and Western Blot Analysis
4.5. Yeast Two-Hybrid Assays
4.6. Pull-Down Assays
4.7. Fluorescence Observation
4.8. Phenotype Assays
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef]
- Carbó, N.; Tarkowski, N.; Ipiña, E.P.; Dawson, S.P.; Aguilar, P.S. Sexual pheromone modulates the frequency of cytosolic Ca2+ bursts in Saccharomyces cerevisiae. Mol. Biol. Cell 2017, 28, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hou, Y.; Liu, W.; Lu, C.; Wang, W.; Sun, S. Components of the calcium-calcineurin signaling pathway in fungal cells and their potential as antifungal targets. Eukaryot. Cell 2015, 14, 324–334. [Google Scholar] [CrossRef]
- Zelter, A.; Bencina, M.; Bowman, B.J.; Yarden, O.; Read, N.D. A comparative genomic analysis of the calcium signaling machinery in Neurospora crassa, Magnaporthe grisea, and Saccharomyces cerevisiae. Fungal Genet. Biol. 2004, 41, 827–841. [Google Scholar] [CrossRef]
- Lange, M.; Peiter, E. Calcium transport proteins in fungi: The phylogenetic diversity of their relevance for growth, virulence, and stress resistance. Front. Microbiol. 2020, 10, 3100. [Google Scholar] [CrossRef] [PubMed]
- Klee, C.; Crouch, T.; Krinks, M. Calcineurin: A calcium-and calmodulin-binding protein of the nervous system. Proc. Natl. Acad. Sci. USA 1979, 76, 6270–6273. [Google Scholar] [CrossRef] [PubMed]
- Rusnak, F.; Mertz, P. Calcineurin: Form and function. Physiol. Rev. 2000, 80, 1483–1521. [Google Scholar] [CrossRef]
- Thewes, S. Calcineurin-Crz1 signaling in lower eukaryotes. Eukaryot. Cell 2014, 13, 694–705. [Google Scholar] [CrossRef]
- Choi, J.; Kim, Y.; Kim, S.; Park, J.; Lee, Y.-H. MoCRZ1, a gene encoding a calcineurin-responsive transcription factor, regulates fungal growth and pathogenicity of Magnaporthe oryzae. Fungal Genet. Biol. 2009, 46, 243–254. [Google Scholar] [CrossRef]
- Fox, D.S.; Cox, G.M.; Heitman, J. Phospholipid-binding protein Cts1 controls septation and functions coordinately with calcineurin in Cryptococcus neoformans. Eukaryot. Cell 2003, 2, 1025–1035. [Google Scholar] [CrossRef]
- Liu, C.; Liu, T.; Lv, Z.; Qin, M.; Qu, Z.; Zhang, Z.; Li, F.; Chen, D.; Zhang, X.; Chen, X.-L. A Calcineurin Regulator MoRCN1 Is Important for Asexual Development, Stress Response, and Plant Infection of Magnaporthe oryzae. Front. Plant Sci. 2022, 13, 925645. [Google Scholar] [CrossRef] [PubMed]
- Carreras-Sureda, A.; Pihán, P.; Hetz, C. Calcium signaling at the endoplasmic reticulum: Fine-tuning stress responses. Cell Calcium 2018, 70, 24–31. [Google Scholar] [CrossRef]
- Strayle, J.; Pozzan, T.; Rudolph, H.K. Steady-state free Ca2+ in the yeast endoplasmic reticulum reaches only 10 μM and is mainly controlled by the secretory pathway pump Pmr1. EMBO J. 1999, 18, 4733–4743. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, M.; Nastase, K.K.; Cunningham, K.W. Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J. 2002, 21, 2343–2353. [Google Scholar] [CrossRef]
- Qi, Z.; Chen, L. Endoplasmic reticulum stress and autophagy. In Autophagy: Biology and Diseases: Basic Science; Springer: Singapore, 2019; pp. 167–177. [Google Scholar]
- Bernales, S.; Schuck, S.; Walter, P. ER-phagy: Selective autophagy of the endoplasmic reticulum. Autophagy 2007, 3, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.-P.; Vu, K.; Bautos, J.; Gelli, A. Cch1 restores intracellular Ca2+ in fungal cells during endoplasmic reticulum stress. J. Biol. Chem. 2010, 285, 10951–10958. [Google Scholar] [CrossRef]
- Dudgeon, D.D.; Zhang, N.; Ositelu, O.O.; Kim, H.; Cunningham, K.W. Nonapoptotic death of Saccharomyces cerevisiae cells that is stimulated by Hsp90 and inhibited by calcineurin and Cmk2 in response to endoplasmic reticulum stresses. Eukaryot. Cell 2008, 7, 2037–2051. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, H.; Chen, Q.; Chen, Y.; Wang, S.; Lu, L.; Zhang, S. The lectin chaperone calnexin is involved in the endoplasmic reticulum stress response by regulating Ca2+ homeostasis in Aspergillus nidulans. Appl. Environ. Microbiol. 2017, 83, e00673-17. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, J.; Zhu, X.; Dong, B.; Liu, X.; Lu, J.; Lin, F. The P5-type ATPase Spf1 is required for development and virulence of the rice blast fungus Pyricularia oryzae. Curr. Genet. 2020, 66, 385–395. [Google Scholar] [CrossRef]
- Talbot, N.J. On the trail of a cereal killer: Exploring the biology of Magnaporthe grisea. Annu. Rev. Microbiol. 2003, 57, 177–202. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.A.; Talbot, N.J. Under pressure: Investigating the biology of plant infection by Magnaporthe oryzae. Nat. Rev. Microbiol. 2009, 7, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Talbot, N.J. Appressoria. Curr. Biol. 2019, 29, R144–R146. [Google Scholar] [CrossRef] [PubMed]
- Balhadère, P.V.; Foster, A.J.; Talbot, N.J. Identification of pathogenicity mutants of the rice blast fungus Magnaporthe grisea by insertional mutagenesis. Mol. Plant-Microbe Interact. 1999, 12, 129–142. [Google Scholar] [CrossRef]
- Talbot, N.J. Having a blast: Exploring the pathogenicity of Magnaporthe grisea. Trends Microbiol. 1995, 3, 9–16. [Google Scholar] [CrossRef]
- Liu, S.; Dean, R.A. G protein α subunit genes control growth, development, and pathogenicity of Magnaporthe grisea. Mol. Plant-Microbe Interact. 1997, 10, 1075–1086. [Google Scholar] [CrossRef]
- Adachi, K.; Hamer, J.E. Divergent cAMP signaling pathways regulate growth and pathogenesis in the rice blast fungus Magnaporthe grisea. Plant Cell 1998, 10, 1361–1373. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, X.; Li, G.; Li, L.; Kong, L.; Wang, C.; Zhang, H.; Xu, J.-R. Multiple plant surface signals are sensed by different mechanisms in the rice blast fungus for appressorium formation. PLoS Pathog. 2011, 7, e1001261. [Google Scholar] [CrossRef]
- Osés-Ruiz, M.; Cruz-Mireles, N.; Martin-Urdiroz, M.; Soanes, D.M.; Eseola, A.B.; Tang, B.; Derbyshire, P.; Nielsen, M.; Cheema, J.; Were, V. Appressorium-mediated plant infection by Magnaporthe oryzae is regulated by a Pmk1-dependent hierarchical transcriptional network. Nat. Microbiol. 2021, 6, 1383–1397. [Google Scholar] [CrossRef]
- Kershaw, M.J.; Talbot, N.J. Genome-wide functional analysis reveals that infection-associated fungal autophagy is necessary for rice blast disease. Proc. Natl. Acad. Sci. USA 2009, 106, 15967–15972. [Google Scholar] [CrossRef] [PubMed]
- Mehrabi, R.; Ding, S.; Xu, J.-R. MADS-box transcription factor Mig1 is required for infectious growth in Magnaporthe grisea. Eukaryot. Cell 2008, 7, 791–799. [Google Scholar] [CrossRef]
- Qi, Z.; Wang, Q.; Dou, X.; Wang, W.; Zhao, Q.; Lv, R.; Zhang, H.; Zheng, X.; Wang, P.; Zhang, Z. MoSwi6, an APSES family transcription factor, interacts with MoMps1 and is required for hyphal and conidial morphogenesis, appressorial function and pathogenicity of Magnaporthe oryzae. Mol. Plant Pathol. 2012, 13, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-M.; Li, L.; Cai, Y.-Y.; Wu, X.-Y.; Shi, H.-B.; Liang, S.; Qu, Y.-M.; Naqvi, N.I.; Del Poeta, M.; Dong, B. A VASt-domain protein regulates autophagy, membrane tension, and sterol homeostasis in rice blast fungus. Autophagy 2021, 17, 2939–2961. [Google Scholar] [CrossRef]
- Marroquin-Guzman, M.; Sun, G.; Wilson, R.A. Glucose-ABL1-TOR signaling modulates cell cycle tuning to control terminal appressorial cell differentiation. PLoS Genet. 2017, 13, e1006557. [Google Scholar] [CrossRef]
- Nguyen, Q.B.; Kadotani, N.; Kasahara, S.; Tosa, Y.; Mayama, S.; Nakayashiki, H. Systematic functional analysis of calcium-signalling proteins in the genome of the rice-blast fungus, Magnaporthe oryzae, using a high-throughput RNA-silencing system. Mol. Microbiol. 2008, 68, 1348–1365. [Google Scholar] [CrossRef]
- Xiong, Q.; Li, W.; Li, P.; Yang, M.; Wu, C.; Eichinger, L. The role of ATG16 in autophagy and the ubiquitin proteasome system. Cells 2018, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, F.; Hallin, U.; Uchino, H. Calcineurin as a multifunctional regulator. J. Biochem. 2002, 131, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kudla, J.; Becker, D.; Grill, E.; Hedrich, R.; Hippler, M.; Kummer, U.; Parniske, M.; Romeis, T.; Schumacher, K. Advances and current challenges in calcium signaling. New Phytol. 2018, 218, 414–431. [Google Scholar] [CrossRef]
- Tang, W.; Ru, Y.; Hong, L.; Zhu, Q.; Zuo, R.; Guo, X.; Wang, J.; Zhang, H.; Zheng, X.; Wang, P. System-wide characterization of bZIP transcription factor proteins involved in infection-related morphogenesis of Magnaporthe oryzae. Environ. Microbiol. 2015, 17, 1377–1396. [Google Scholar] [CrossRef]
- Urra, H.; Dufey, E.; Lisbona, F.; Rojas-Rivera, D.; Hetz, C. When ER stress reaches a dead end. Biochim. Et Biophys. Acta (BBA) Mol. Cell Res. 2013, 1833, 3507–3517. [Google Scholar] [CrossRef]
- Tisi, R.; Rigamonti, M.; Groppi, S.; Belotti, F. Calcium homeostasis and signaling in fungi and their relevance for pathogenicity of yeasts and filamentous fungi. AIMS Mol. Sci. 2016, 3, 505–549. [Google Scholar] [CrossRef]
- Prokisch, H.; Yarden, O.; Dieminger, M.; Tropschug, M.; Barthelmess, I. Impairment of calcineurin function in Neurospora crassa reveals its essential role in hyphal growth, morphology and maintenance of the apical Ca2+ gradient. Mol. Gen. Genet. MGG 1997, 256, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Viaud, M.C.; Balhadère, P.V.; Talbot, N.J. A Magnaporthe grisea cyclophilin acts as a virulence determinant during plant infection. Plant Cell 2002, 14, 917–930. [Google Scholar] [CrossRef]
- Miyakawa, T.; Mizunuma, M. Physiological roles of calcineurin in Saccharomyces cerevisiae with special emphasis on its roles in G2/M cell-cycle regulation. Biosci. Biotechnol. Biochem. 2007, 71, 633–645. [Google Scholar] [CrossRef]
- Koike, A.; Kato, T.; Sugiura, R.; Ma, Y.; Tabata, Y.; Ohmoto, K.; Sio, S.O.; Kuno, T. Genetic screening for regulators of Prz1, a transcriptional factor acting downstream of calcineurin in fission yeast. J. Biol. Chem. 2012, 287, 19294–19303. [Google Scholar] [CrossRef]
- Kim, S.; Hu, J.; Oh, Y.; Park, J.; Choi, J.; Lee, Y.-H.; Dean, R.A.; Mitchell, T.K. Combining ChIP-chip and expression profiling to model the MoCRZ1 mediated circuit for Ca2+/calcineurin signaling in the rice blast fungus. PLoS Pathog. 2010, 6, e1000909. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, M.; Cunningham, K.W. Mitogen-activated protein kinase stimulation of Ca2+ signaling is required for survival of endoplasmic reticulum stress in yeast. Mol. Biol. Cell 2003, 14, 4296–4305. [Google Scholar] [CrossRef]
- Kim, H.; Kim, A.; Cunningham, K.W. Vacuolar H+-ATPase (V-ATPase) promotes vacuolar membrane permeabilization and nonapoptotic death in stressed yeast. J. Biol. Chem. 2012, 287, 19029–19039. [Google Scholar] [CrossRef]
- Tang, W.; Jiang, H.; Aron, O.; Wang, M.; Wang, X.; Chen, J.; Lin, B.; Chen, X.; Zheng, Q.; Gao, X. Endoplasmic reticulum-associated degradation mediated by MoHrd1 and MoDer1 is pivotal for appressorium development and pathogenicity of Magnaporthe oryzae. Environ. Microbiol. 2020, 22, 4953–4973. [Google Scholar] [CrossRef]
- Wei, Y.-Y.; Liang, S.; Zhang, Y.-R.; Lu, J.-P.; Lin, F.-C.; Liu, X.-H. MoSec61β, the beta subunit of Sec61, is involved in fungal development and pathogenicity, plant immunity, and ER-phagy in Magnaporthe oryzae. Virulence 2020, 11, 1685–1700. [Google Scholar] [CrossRef]
- Giraldo, M.C.; Dagdas, Y.F.; Gupta, Y.K.; Mentlak, T.A.; Yi, M.; Martinez-Rocha, A.L.; Saitoh, H.; Terauchi, R.; Talbot, N.J.; Valent, B. Two distinct secretion systems facilitate tissue invasion by the rice blast fungus Magnaporthe oryzae. Nat. Commun. 2013, 4, 1996. [Google Scholar] [CrossRef] [PubMed]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef]
- Knupp, J.; Arvan, P.; Chang, A. Increased mitochondrial respiration promotes survival from endoplasmic reticulum stress. Cell Death Differ. 2019, 26, 487–501. [Google Scholar] [CrossRef]
- Ryder, L.S.; Talbot, N.J. The Rice Blast Fungus Magnaporthe oryzae Uses a Turgor-Dependent, Septin-Mediated Mechanism to Invade Rice. In Plant Relationships: Fungal-Plant Interactions; Springer: Cham, Switzerland, 2022; pp. 307–327. [Google Scholar]
- Son, K.; Hussain, A.; Sehmi, R.; Janssen, L. The Cycling of Intracellular Calcium Released in Response to Fluid Shear Stress Is Critical for Migration-Associated Actin Reorganization in Eosinophils. Cells 2021, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Kyozuka, K.; Chun, J.T.; Puppo, A.; Gragnaniello, G.; Garante, E.; Santella, L. Actin cytoskeleton modulates calcium signaling during maturation of starfish oocytes. Dev. Biol. 2008, 320, 426–435. [Google Scholar] [CrossRef]
- Brand, A.; Gow, N.A. Mechanisms of hypha orientation of fungi. Curr. Opin. Microbiol. 2009, 12, 350–357. [Google Scholar] [CrossRef]
- Takeshita, N. Control of actin and calcium for chitin synthase delivery to the hyphal tip of aspergillus. In The Fungal Cell Wall: An Armour and a Weapon for Human Fungal Pathogens; Springer: Cham, Switzerland, 2020; pp. 113–129. [Google Scholar]
- Rho, H.S.; Jeon, J.; Lee, Y.H. Phospholipase C-mediated calcium signalling is required for fungal development and pathogenicity in Magnaporthe oryzae. Mol. Plant Pathol. 2009, 10, 337–346. [Google Scholar] [CrossRef]
- Talbot, N.J.; Ebbole, D.J.; Hamer, J.E. Identification and characterization of MPG1, a gene involved in pathogenicity from the rice blast fungus Magnaporthe grisea. Plant Cell 1993, 5, 1575–1590. [Google Scholar]
- Lu, J.; Cao, H.; Zhang, L.; Huang, P.; Lin, F. Systematic analysis of Zn2Cys6 transcription factors required for development and pathogenicity by high-throughput gene knockout in the rice blast fungus. PLoS Pathog. 2014, 10, e1004432. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, H.; Zhu, S.; Wang, J.; Liu, X.; Lin, F.; Lu, J. The methylcitrate cycle is required for development and virulence in the rice blast fungus Pyricularia oryzae. Mol. Plant-Microbe Interact. 2019, 32, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, Z.; Huang, P.; Wang, Q.; Li, Y.; Liu, X.-H.; Lin, F.-C.; Lu, J. The Plant Homeodomain Protein Clp1 Regulates Fungal Development, Virulence, and Autophagy Homeostasis in Magnaporthe oryzae. Microbiol. Spectr. 2022, 10, e01021–e01022. [Google Scholar] [CrossRef] [PubMed]
- Molinari, C.; Talbot, N.J. A Basic Guide to the Growth and Manipulation of the Blast Fungus, Magnaporthe oryzae. Curr. Protoc. 2022, 2, e523. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.-H.; Shen, Z.-F.; Wang, J.-Y.; Cai, Y.-Y.; Li, L.; Liao, J.; Lu, J.-P.; Zhu, X.-M.; Lin, F.-C.; Liu, X.-H. MoCbp7, a Novel Calcineurin B Subunit-Binding Protein, Is Involved in the Calcium Signaling Pathway and Regulates Fungal Development, Virulence, and ER Homeostasis in Magnaporthe oryzae. Int. J. Mol. Sci. 2023, 24, 9297. https://doi.org/10.3390/ijms24119297
Wang Z-H, Shen Z-F, Wang J-Y, Cai Y-Y, Li L, Liao J, Lu J-P, Zhu X-M, Lin F-C, Liu X-H. MoCbp7, a Novel Calcineurin B Subunit-Binding Protein, Is Involved in the Calcium Signaling Pathway and Regulates Fungal Development, Virulence, and ER Homeostasis in Magnaporthe oryzae. International Journal of Molecular Sciences. 2023; 24(11):9297. https://doi.org/10.3390/ijms24119297
Chicago/Turabian StyleWang, Zi-He, Zi-Fang Shen, Jing-Yi Wang, Ying-Ying Cai, Lin Li, Jian Liao, Jian-Ping Lu, Xue-Ming Zhu, Fu-Cheng Lin, and Xiao-Hong Liu. 2023. "MoCbp7, a Novel Calcineurin B Subunit-Binding Protein, Is Involved in the Calcium Signaling Pathway and Regulates Fungal Development, Virulence, and ER Homeostasis in Magnaporthe oryzae" International Journal of Molecular Sciences 24, no. 11: 9297. https://doi.org/10.3390/ijms24119297
APA StyleWang, Z.-H., Shen, Z.-F., Wang, J.-Y., Cai, Y.-Y., Li, L., Liao, J., Lu, J.-P., Zhu, X.-M., Lin, F.-C., & Liu, X.-H. (2023). MoCbp7, a Novel Calcineurin B Subunit-Binding Protein, Is Involved in the Calcium Signaling Pathway and Regulates Fungal Development, Virulence, and ER Homeostasis in Magnaporthe oryzae. International Journal of Molecular Sciences, 24(11), 9297. https://doi.org/10.3390/ijms24119297






