Heat-Priming during Somatic Embryogenesis Increased Resilience to Drought Stress in the Generated Maritime Pine (Pinus pinaster) Plants
Abstract
1. Introduction
2. Results
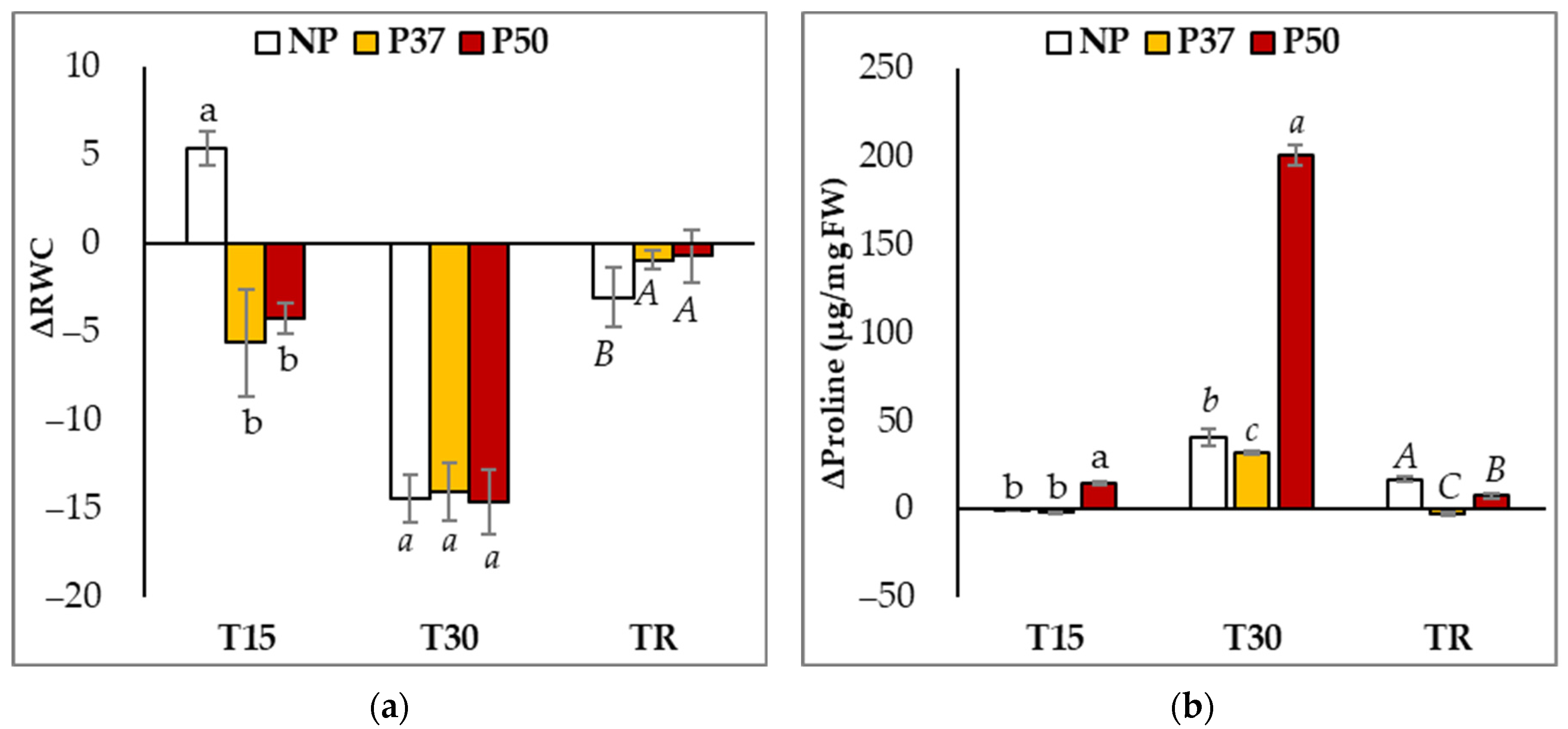
2.1. Osmotic Adjustment in Maritime Pine Plants under Drought Stress
2.2. Photosynthesis Parameters during Hydric Stress
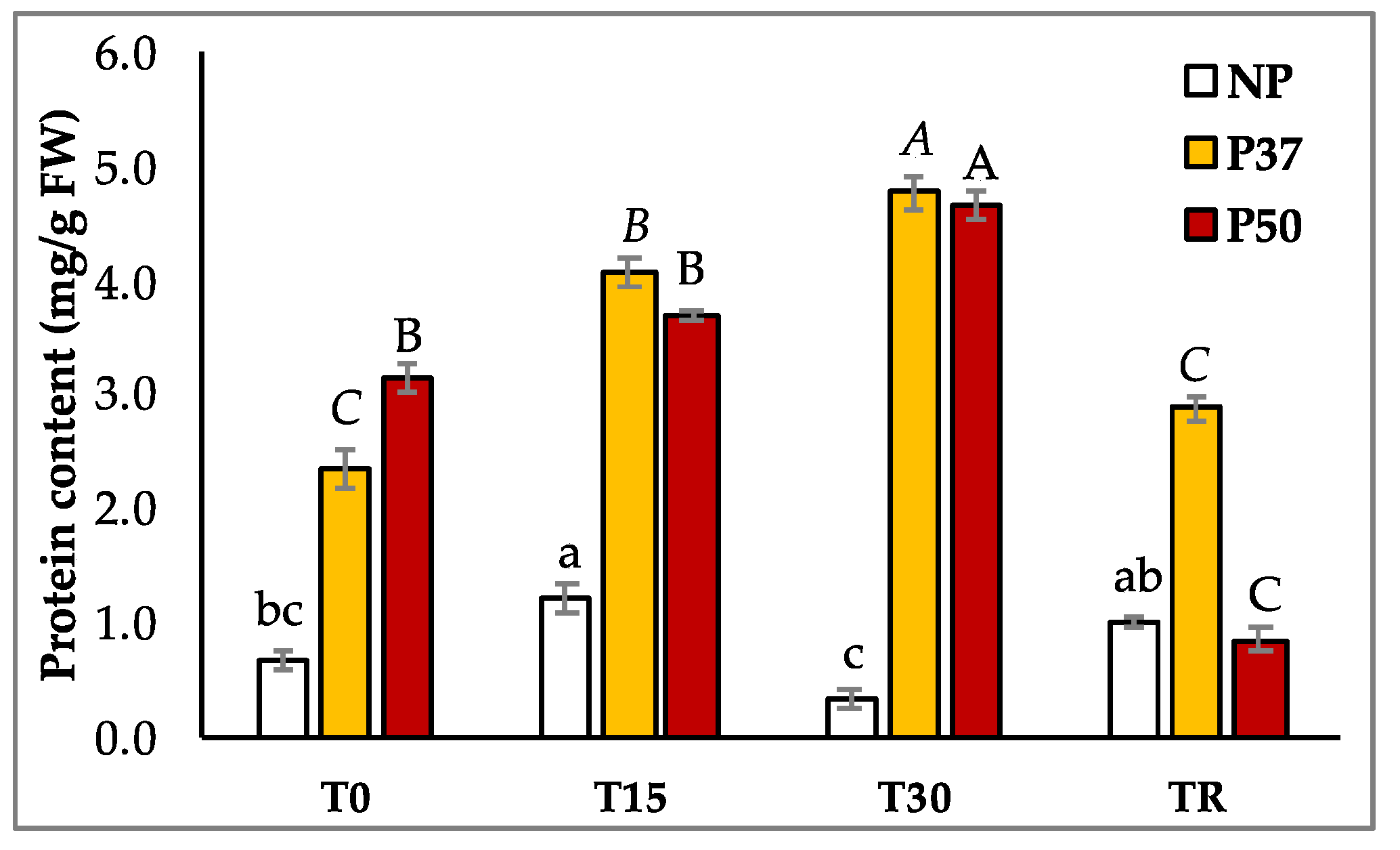
2.3. Carbohydrates and Protein Contents of Maritime Pine Plants
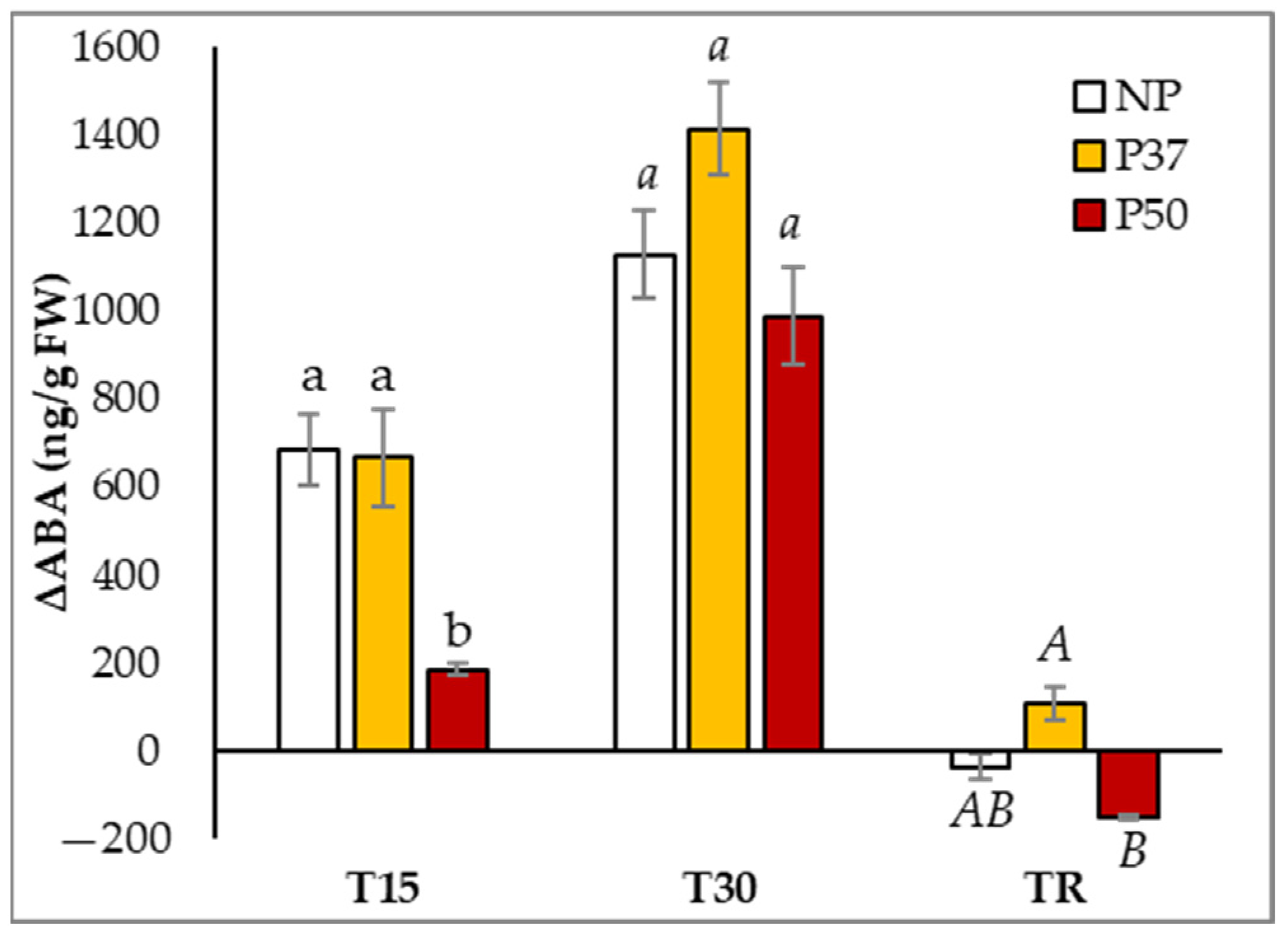
2.4. ABA Content in Maritime Pine Needles
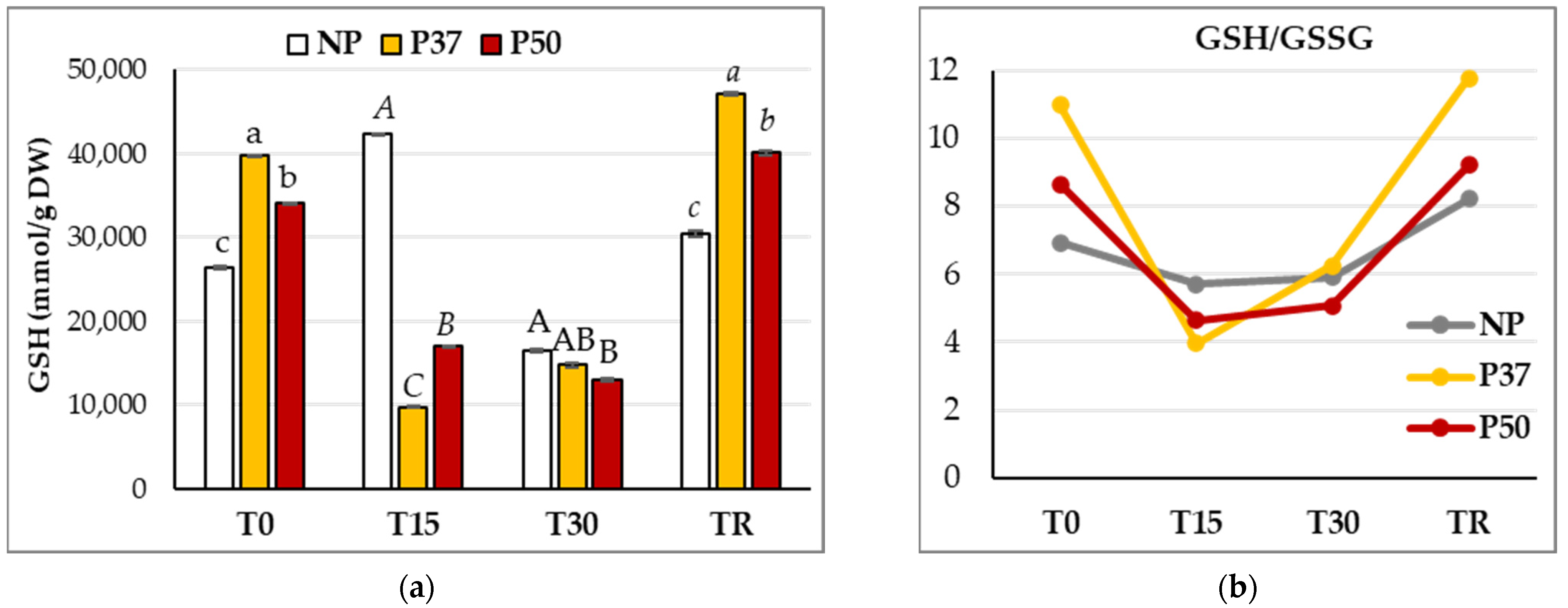
2.5. Glutathione Metabolism in Maritime Pine Needles
2.6. Gene Expression Analyses
3. Discussion
4. Materials and Methods
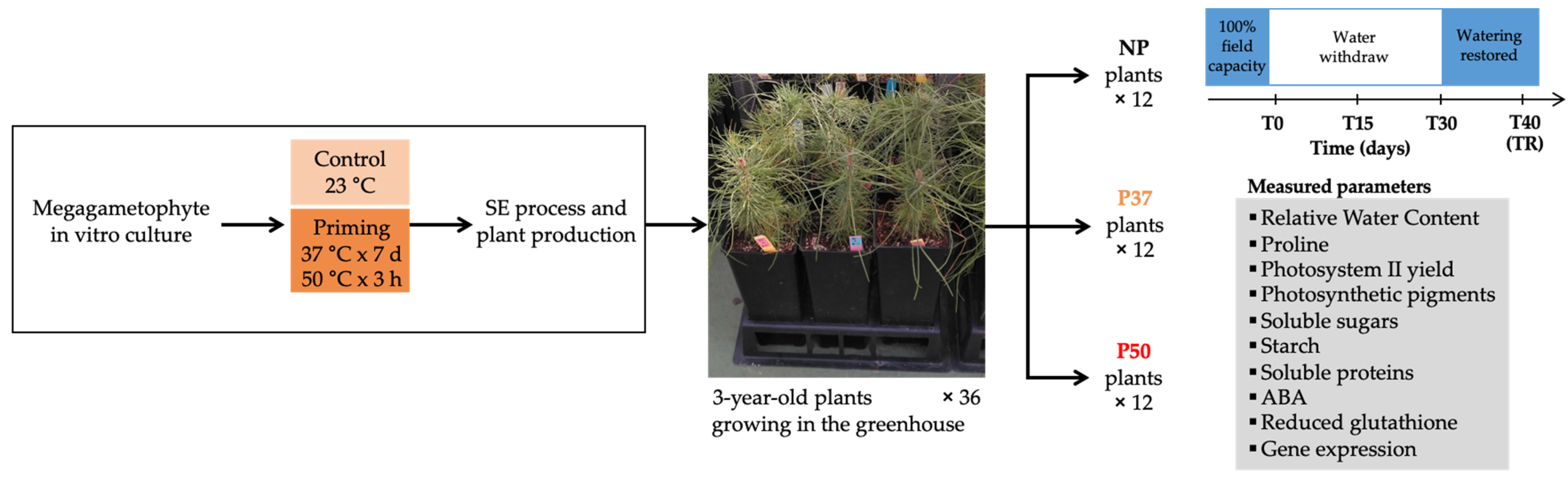
4.1. Plant Material and Drought Experiment Performed in Greenhouse
4.2. Physiological Characterization of 3-Year-Old Maritime Plants Subjected to Drought Stress
4.3. Gene Expression Analyses
4.4. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gustafson, E.J.; Sturtevant, B.R. Modeling forest mortality caused by drought stress: Implications for climate change. Ecosystems 2013, 16, 60–74. [Google Scholar] [CrossRef]
- Férriz, M.; Martin-Benito, D.; Cañellas, I.; Gea-izquierdo, G. Sensitivity to water stress drives differential decline and mortality dynamics of three co-occurring conifers with different drought tolerance. For. Ecol. Manag. 2021, 486, 118964. [Google Scholar] [CrossRef]
- Giorgi, F.; Lionello, P. Climate change projections for the Mediterranean region. Glob. Planet. Change 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Wang, W.; Singh, V.P.; Ren, L. A global perspective on the probability of propagation of drought: From meteorological to soil moisture. J. Hydrol. 2021, 603, 126907. [Google Scholar] [CrossRef]
- Ray, D.; Berlin, M.; Alia, R.; Sanchez, L.; Hynynen, J.; González-Martinez, S.; Bastien, C. Transformative changes in tree breeding for resilient forest restoration. Front. For. Glob. Change 2022, 5, 1005761. [Google Scholar] [CrossRef]
- Moran, E.; Lauder, J.; Musser, C.; Stathos, A.; Shu, M. The genetics of drought tolerance in conifers. New Phytol. 2017, 216, 1034–1048. [Google Scholar] [CrossRef]
- Brodribb, T.J.; McAdam, S.A.; Jordan, G.J.; Martins, S.C. Conifer species adapt to low-rainfall climates by following one of two divergent pathways. Proc. Natl. Acad. Sci. USA 2014, 111, 14489–14493. [Google Scholar] [CrossRef] [PubMed]
- Polle, A.; Chen, S.L.; Eckert, C.; Harfouche, A. Engineering drought resistance in forest trees. Front. Plant Sci. 2019, 9, 1875. [Google Scholar] [CrossRef]
- Liu, X.; Quan, W.; Bartels, D. Stress memory responses and seed priming correlate with drought tolerance in plants: An overview. Planta 2022, 255, 45. [Google Scholar] [CrossRef]
- Hossain, M.A.; Li, Z.G.; Hoque, T.S.; Burrit, D.J.; Fujita, M.; Munné-Bosch, S. Heat or cold priming-induced cross-tolerance to abiotic stresses in plants: Key regulators and possible mechanisms. Protoplasma 2018, 255, 399–412. [Google Scholar] [CrossRef]
- García-Campa, L.; Guerrero, S.; Lamelas, L.; Meijón, M.; Hasbúnc, R.; Cañal, M.J.; Valledor, L. Chloroplast proteomics reveals transgenerational cross-stress priming in Pinus radiata. Environ. Exp. Bot. 2022, 202, 105009. [Google Scholar] [CrossRef]
- Amaral, J.; Ribeyre, Z.; Vigneaud, J.; Sow, M.D.; Fichot, R.; Messier, C.; Pinto, G.; Nolet, P.; Maury, S. Advances and promises of epigenetics for forest trees. Forests 2020, 11, 976. [Google Scholar] [CrossRef]
- Menéndez-Gutiérrez, M.; Alonso, M.; Toval, G.; Díaz, R. Variation in pinewood nematode susceptibility among Pinus pinaster Ait. provenances from the Iberian Peninsula and France. Ann. For. Sci. 2017, 74, 76. [Google Scholar] [CrossRef]
- Zas, R.; Sampedro, L.; Solla, A.; Vivas, M.; Lombardero, M.J.; Alía, R.; Rozas, V. Dendroecology in common gardens: Population differentiation and plasticity in resistance, recovery and resilience to extreme drought events in Pinus pinaster. Agric. For. Meteorol. 2020, 291, 108060. [Google Scholar] [CrossRef]
- Hurel, A.; de Miguel, M.; Dutech, C.; Desprez-Loustau, M.L.; Plomion, C.; Rodríguez-Quilón, I.; Cyrille, A.; Guzman, T.; Alía, R.; González-Martínez, S.C.; et al. Genetic basis of growth, spring phenology, and susceptibility to biotic stressors in maritime pine. Evol. Appl. 2021, 14, 2750–2772. [Google Scholar] [CrossRef] [PubMed]
- Corcuera, L.; Gil-Pelegrín, E.; Notivol, E. Differences in hydraulic architecture between mesic and xeric Pinus pinaster populations at the seedling stage. Tree Physiol. 2012, 32, 1442–1457. [Google Scholar] [CrossRef]
- Correia, I.; Almeida, M.H.; Aguiar, A.; Alía, R.; Soares David, T.; Santos Pereira, J. Variations in growth, survival and carbon isotope composition (d13C) among Pinus pinaster populations of different geographic origins. Tree Physiol. 2008, 28, 1545–1552. [Google Scholar] [CrossRef]
- Perdiguero, P.; Barbero, M.; Cervera, M.; Collada, C.; Soto, Á. Molecular response to water stress in two contrasting Mediterranean pines (Pinus pinaster and Pinus pinea). Plant Physiol. Biochem. 2013, 67, 199–208. [Google Scholar] [CrossRef]
- Cañas, R.A.; Feito, I.; Fuente-Maqueda, J.F.; Ávila, C.; Majada, J.; Cánovas, F.M. Transcriptome-wide analysis supports environmental adaptations of two Pinus pinaster populations from contrasting habitats. BMC Genom. 2015, 16, 909. [Google Scholar] [CrossRef]
- de María, N.; Guevara, M.A.; Perdiguero, P.; Vélez, M.D.; Cabezas, J.A.; López-Hinojosa, M.; Li, Z.; Díaz, L.M.; Pizarro, A.; Mancha, J.A.; et al. Molecular study of drought response in the Mediterranean conifer Pinus pinaster Ait.: Differential transcriptomic profiling reveals constitutive water deficit-independent drought tolerance mechanisms. Ecol. Evol. 2020, 10, 9788–9807. [Google Scholar] [CrossRef]
- López-Hidalgo, C.; Lamelas, L.; Cañal, M.J.; Valledor, L.; Meijón, M. Untargeted metabolomics revealed essential biochemical rearrangements towards combined heat and drought stress acclimatization in Pinus pinaster. Environ. Exp. Bot. 2023, 208, 105261. [Google Scholar] [CrossRef]
- Valeriano, C.; Gazol, A.; Colangelo, M.; Camarero, J.J. Drought drives growth and mortality rates in three pine species under Mediterranean conditions. Forests 2021, 12, 1700. [Google Scholar] [CrossRef]
- Valeriano, C.; Gazol, A.; Colangelo, M.; González de Andrés, E.; Camarero, J.J. Modeling climate impacts on tree growth to assess tree vulnerability to drought during forest dieback. Front. Plant Sci. 2021, 12, 672855. [Google Scholar] [CrossRef] [PubMed]
- de Miguel, M.; Rodríguez-Quilón, I.; Heuertz, M.; Hurel, A.; Grivet, D.; Jaramillo-Correa, J.P.; Vendramin, G.G.; Plomion, C.; Majada, J.; Alía, R.; et al. Polygenic adaptation and negative selection across traits, years and environments in a long-lived plant species (Pinus pinaster Ait.; Pinaceae). Mol. Ecol. 2022, 31, 2089–2105. [Google Scholar] [CrossRef]
- Yakovlev, I.A.; Carneros, E.; Lee, Y.; Olsen, J.E.; Fossdal, C.G. Transcriptional profiling of epigenetic regulators in somatic embryos during temperature induced formation of an epigenetic memory in Norway spruce. Planta 2016, 243, 1237–1249. [Google Scholar] [CrossRef]
- Pereira, C.; Castander-Olarieta, A.; Sales, E.; Montalbán, I.A.; Canhoto, J.; Moncaleán, P. Heat stress in Pinus halepensis somatic embryogenesis induction: Effect in DNA methylation and differential expression of stress-related genes. Plants 2021, 10, 2333. [Google Scholar] [CrossRef]
- Castander-Olarieta, A.; Pereira, C.; Sales, E.; Meijón, M.; Arrillaga, I.; Cañal, M.J.; Goicoa, T.; Ugarte, M.D.; Moncaleán, P.; Montalbán, I.A. Induction of radiata pine somatic embryogenesis at high temperatures provokes a long-term decrease in DNA methylation/hydroxymethylation and differential expression of stress-related genes. Plants 2020, 9, 1762. [Google Scholar] [CrossRef]
- Castander-Olarieta, A.; Montalbán, I.A.; De Medeiros Oliveira, E.; Dell’Aversana, E.; D’Amelia, L.; Carillo, P.; Steiner, N.; Fraga, H.P.D.F.; Guerra, M.P.; Goicoa, T.; et al. Effect of thermal stress on tissue ultrastructure and metabolite profiles during initiation of radiata pine somatic embryogenesis. Front. Plant Sci. 2019, 9, 2004. [Google Scholar] [CrossRef]
- Castander-Olarieta, A.; Moncaleán, P.; Pereira, C.; Pencík, A.; Petrík, I.; Pavlovic, I.; Novák, O.; Strnad, M.; Goicoa, T.; Ugarte, M.D.; et al. Cytokinins are involved in drought tolerance of Pinus radiata plants originating from embryonal masses induced at high temperatures. Tree Physiol. 2021, 41, 912–926. [Google Scholar] [CrossRef]
- Pérez-Oliver, M.A.; Haro, J.G.; Pavlović, I.; Novák, O.; Segura, J.; Sales, E.; Arrillaga, I. Priming maritime pine megagametophytes during somatic embryogenesis improved plant adaptation to heat stress. Plants 2021, 10, 446. [Google Scholar] [CrossRef]
- Marques Do Nascimento, A.M.; Montalbán, I.A.; Llamazares De Miguel, D.; Goicoa, T.; Ugarte, M.D.; Moncaleán, P. High temperature and water deficit cause epigenetic changes in somatic plants of Pinus radiata D. Don. Plant Cell Tiss. Org. Cult. 2022, 151, 107–121. [Google Scholar] [CrossRef]
- Arrillaga, I.; Morcillo, M.; Zanón, I.; Lario, F.; Segura, J.; Sales, E. New approaches to optimize somatic embryogenesis in maritime pine. Front. Plant Sci. 2019, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Sales, E.; Cañizares, E.; Pereira, C.; Pérez-Oliver, M.A.; Nebauer, S.G.; Pavlović, I.; Novák, O.; Segura, J.; Arrillaga, I. Changing temperature conditions during somatic embryo maturation result in Pinus pinaster plants with altered response to heat stress. Int. J. Mol. Sci. 2022, 23, 1318. [Google Scholar] [CrossRef] [PubMed]
- Gessler, A.; Bottero, A.; Marshall, J.D.; Arend, M. The way back: Recovery of trees from drought and its implication for acclimation. New Phytol. 2020, 228, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- DeSoto, L.; Cailleret, M.; Sterck, F.; Jansen, S.; Kramer, K.; Robert, E.M.R.; Aakala, T.; Amoroso, M.M.; Bigler, C.; Camarero, J.J.; et al. Low growth resilience to drought is related to future mortality risk in trees. Nat. Commun. 2020, 11, 545. [Google Scholar] [CrossRef]
- Baldi, P.; La Porta, N. Toward the genetic improvement of drought tolerance in conifers: An integrated approach. Forests 2022, 13, 2016. [Google Scholar] [CrossRef]
- Shao, C.; Duan, H.; Ding, G.; Luo, X.; Fu, Y.; Lou, Q. Physiological and biochemical dynamics of Pinus massoniana Lamb. seedlings under extreme drought stress and during recovery. Forests 2022, 13, 65. [Google Scholar] [CrossRef]
- Xiong, S.; Wang, Y.; Chen, Y.; Gao, M.; Zhao, Y.; Wu, L. Effects of drought stress and rehydration on physiological and biochemical properties of four oak species in China. Plants 2022, 11, 679. [Google Scholar] [CrossRef]
- Thalmann, M.; Santelia, D. Starch as a determinant of plant fitness under abiotic stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef]
- Correia, B.; Hancock, R.D.; Amaral, J.; Gomez-Cadenas, A.; Valledor, L.; Pinto, G. Combined drought and heat activates protective responses in Eucalyptus globulus that are not activated when subjected to drought or heat stress alone. Front. Plant Sci. 2018, 9, 819. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Rivero, R.M.; Martínez, V.; Gómez-Cadenas, A.; Arbona, V. Tolerance of citrus plants to the combination of high temperatures and drought is associated to the increase in transpiration modulated by a reduction in abscisic acid levels. BMC Plant Biol. 2016, 16, 105. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, Q.P.; Li, D.X.; Yang, X.H.; Li, D.Q. Multifunctional roles of plant dehydrins in response to environmental stresses. Front Plant Sci. 2017, 8, 1018. [Google Scholar] [CrossRef] [PubMed]
- Priya, M.; Dhanker, O.P.; Siddique, K.H.M.; HanumanthaRao, B.; Nair, R.M.; Pandey, S.; Sadhana, S.; Varshney, R.K.; Prasad, P.V.; Nayyar, H. Drought and heat stress-related proteins: An update about their functional relevance in imparting stress tolerance in agricultural crops. Theor. Appl. Genet. 2019, 132, 1607–1638. [Google Scholar] [CrossRef] [PubMed]
- Riyazuddin, R.; Nisha, N.; Singh, K.; Verma, R.; Gupta, R. Involvement of dehydrin proteins in mitigating the negative effects of drought stress in plants. Plant Cell Rep. 2022, 41, 519–533. [Google Scholar] [CrossRef]
- Baum, S.; Reimer-Michalski, E.-M.; Bolger, A.; Andrea, J.; Mantai, A.J.; Benes, V.; Usadel, B.; Conratha, U. Isolation of Open Chromatin Identifies Regulators of Systemic Acquired Resistance. Plant Physiol. 2019, 181, 817–833. [Google Scholar] [CrossRef] [PubMed]
- Phukan, U.J.; Jeena, G.S.; Shukla, R.K. WRKY Transcription factors: Molecular regulation and stress responses in plants. Front. Plant Sci. 2016, 7, 760. [Google Scholar] [CrossRef]
- Matus, J.T.; Aquea, F.; Espinoza, C.; Vega, A.; Cavallini, E.; Santo, S.D.; Cañón, P.; Rodríguez-Hoces de la Guardia, A.; Serrano, J.; Tornielli, G.B.; et al. Inspection of the grapevine BURP superfamily highlights an expansion of RD22 genes with distinctive expression features in berry development and ABA-mediated stress responses. PLoS ONE 2014, 9, e110372. [Google Scholar] [CrossRef]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. The plant hormone abscisic acid mediates the drought-induced expression but not the seed-specific expression of RD22, a gene responsive to dehydration stress in Arabidopsis thaliana. Mol. Gen. Genet. 1993, 238, 17–25. [Google Scholar] [CrossRef]
- Pulido, P.; Leister, D. Novel DNAJ-related proteins in Arabidopsis thaliana. New Phytol. 2018, 217, 480–490. [Google Scholar] [CrossRef]
- Dong, A.; Zenda, T.; Liu, X.; Wang, Y.; Li, J.; Yang, Y.; Liu, S.; Duan, H. Transcriptome analysis of drought tolerance and utility assessment of DnaJ gene expression as a potential index for drought resistance evaluation in maize. Euphytica 2022, 218, 136. [Google Scholar] [CrossRef]
- Alonso, R.; Elvira, S.; Castillo, F.J.; Gimeno, B.S. Interactive effects of ozone and drought stress on pigments and activities of antioxidative enzymes in Pinus halepensis. Plant Cell Environ. 2001, 24, 905–916. [Google Scholar] [CrossRef]
- Chun, H.J.; Lim, L.H.; Cheong, M.S.; Baek, D.; Park, M.S.; Cho, H.M.; Lee, S.H.; Jin, B.J.; No, D.H.; Cha, Y.J.; et al. Arabidopsis CCoAOMT1 plays a role in drought stress response via ROS- and ABA-dependent manners. Plants 2021, 10, 831. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Trivedi, P.K. Glutathione S-Transferases: Role in combating abiotic stresses including arsenic detoxification in plants. Front. Plant Sci. 2018, 9, 751. [Google Scholar] [CrossRef] [PubMed]
- Hernández Estévez, I.; Rodríguez Hernández, M. Plant Glutathione S-transferases: An overview. Plant Gene 2020, 23, 100233. [Google Scholar] [CrossRef]
- Husaini, A.M. High-value pleiotropic genes for developing multiple stress-tolerant biofortified crops for 21st-century challenges. Heredity 2021, 128, 460–472. [Google Scholar] [CrossRef]
- Liu, W.; Huang, L.; Liang, X.; Liu, L.; Sun, C.; Lin, X. Heat shock induces cross adaptation to aluminum stress through enhancing ascorbate-glutathione cycle in wheat seedlings. Chemosphere 2021, 278, 130397. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef]
- Szalai, G.; Kellős, T.; Galiba, G.; Kocsy, G. Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. J. Plant Growth Regul. 2009, 28, 66–80. [Google Scholar] [CrossRef]
- Escandón, M.; Cañal, M.; Pascual, J.; Pinto, G.; Correia, B.; Amaral, J.; Meijón, M. Integrated physiological and hormonal profile of heat-induced thermotolerance in Pinus radiata. Tree Physiol. 2016, 36, 63–77. [Google Scholar] [CrossRef]
- Bates, L.; Waldren, R.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–208. [Google Scholar] [CrossRef]
- Nebauer, S.; Renau-Morata, B.; Guardiola, J.; Molina, R. Photosynthesis down-regulation precedes carbohydrate accumulation under sink limitation in Citrus. Tree Physiol. 2011, 31, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1997, 148, 350–382. [Google Scholar] [CrossRef]
- Rodríguez, O.; Vilasó, J.E.; Aguilera, I.; Pérez, R.M.; Ábalos, A. Validación por verificación del método colorimétrico de la antrona para la cuantificación de ramnolípidos. Rev. Cub. Quím. 2013, 25, 287–294. [Google Scholar]
- Šimura, J.; Antoniadi, I.; Široká, J.; Tarkowská, D.; Strnad, M.; Ljung, K.; Novák, O. Plant hormonomics: Multiple phytohormone profiling by targeted metabolomics. Plant Physiol. 2018, 177, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Téllez, S.; Anoman, A.D.; Alcántara-Enguídanos, A.; Garza-Aguirre, R.A.; Alseekh, S.; Ros, R. PGDH family genes differentially affect Arabidopsis tolerance to salt stress. Plant Sci. 2020, 290, 110284. [Google Scholar] [CrossRef] [PubMed]
- Canales, J.; Ávila, C.; Cantón, F.R.; Pacheco-Villalobos, D.; Díaz-Moreno, S.; Ariza, D.; Molina-Rueda, J.J.; Navarro-Cerrillo, R.M.; Gonzalo Claros, M.; Cánovas, F.M. Gene expression profiling in the stem of young maritime pine trees: Detection of ammonium stress-responsive genes in the apex. Trees 2012, 26, 609–619. [Google Scholar] [CrossRef]
- Velasco-Conde, T.; Yakovlev, I.; Majada, J.P.; Aranda, I.; Johnsen, Ø. Dehydrins in maritime pine (Pinus pinaster) clones and their expression related to drought stress response. Tree Genet. Genomes 2012, 8, 957–973. [Google Scholar] [CrossRef]
- Vega-Bartol, J.J.; Santos, R.R.; Simões, M.; Miguel, C.M. Normalizing gene expression by quantitative PCR during somatic embryogenesis in two representative conifer species: Pinus pinaster and Picea abies. Plant Cell Rep. 2013, 32, 715–729. [Google Scholar] [CrossRef]



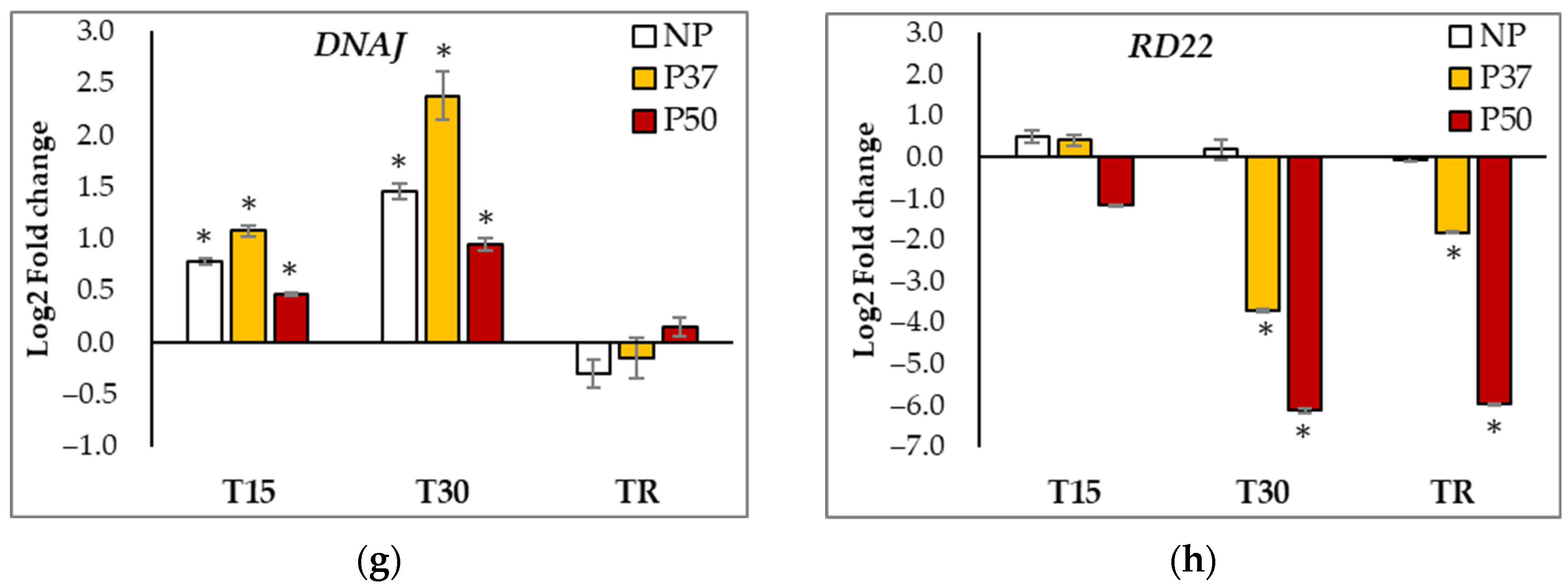
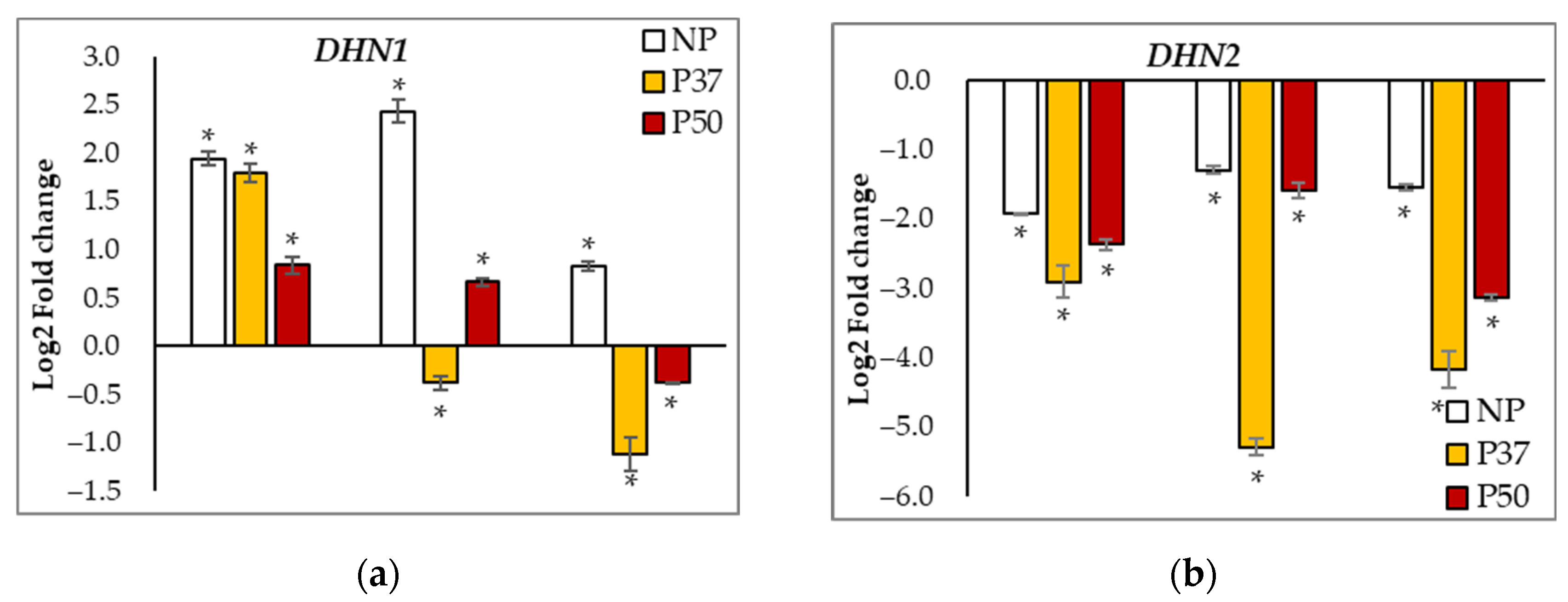
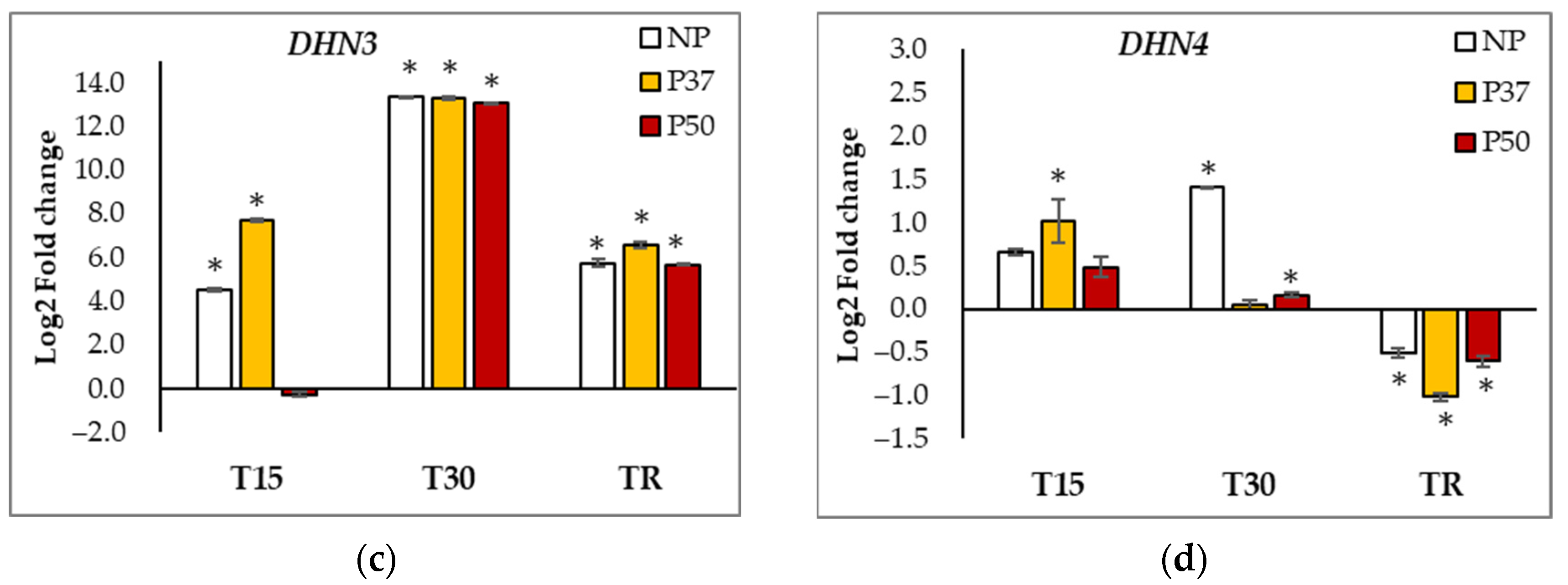
| APX | SOD | CCOMT | HSP70 | GST | WRKY | DNAJ | RD22 | DHN1 | DHN2 | DHN3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SOD | −0.377 * | ||||||||||
| CCOMT | 0.127 | −0.105 | |||||||||
| HSP70 | −0.290 | 0.445 ** | −0.317 | ||||||||
| GST | −0.541 ** | 0.321 | −0.288 | 0.498 ** | |||||||
| WRKY | 0.250 | −0.050 | 0.665 ** | −0.237 | −0.407 * | ||||||
| DNAJ | 0.031 | 0.022 | 0.526 ** | −0.202 | −0.396 * | 0.896 ** | |||||
| RD22 | 0.387 * | 0.387 * | −0.076 | −0.037 | −0.137 | 0.007 | −0.036 | ||||
| DHN1 | −0.305 | 0.277 | 0.697 ** | 0.244 | 0.283 | 0.551 ** | 0.467 ** | −0.135 | |||
| DHN2 | −0.019 | −0.069 | 0.328 | −0.480 ** | −0.026 | −0.120 | −0.184 | 0.138 | 0.120 | ||
| DHN3 | 0.274 | −0.119 | 0.238 | 0.138 | −0.502 ** | 0.658 ** | 0.603 ** | −0.190 | 0.201 | −0.540 ** | |
| DHN4 | −0.042 | 0.310 | 0.675 ** | −0.090 | −0.095 | 0.675 ** | 0.661 ** | 0.289 | 0.743 ** | 0.297 | 0.130 |
| Gene | Gene Annotation | Reference |
|---|---|---|
| APX | Ascorbate Peroxidase | [30] |
| CCOMT | Caffeoyl-CoA O-Methyltransferase | |
| HSP70 | Heat Shock Protein 70 (HSP70) | |
| SOD | Cu-Zn-superoxide dismutase precursor | |
| WRKY | Transcription factor WRKY11 | |
| DNAJ | Chaperone protein dnaj chloroplastic-like | [20] |
| GST | Glutathione S-transferase | |
| RD22 | Dehydration-responsive protein RD22 | |
| DHN1 | Ppindhn1 | [67] |
| DHN2 | Ppindhn2 | |
| DHN3 | Ppindhn3 | |
| DHN4 | Ppindhn4 | |
| HIS3 | Histone 3 (HIS3) | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Oliver, M.A.; González-Mas, M.d.C.; Renau-Morata, B.; Arrillaga, I.; Sales, E. Heat-Priming during Somatic Embryogenesis Increased Resilience to Drought Stress in the Generated Maritime Pine (Pinus pinaster) Plants. Int. J. Mol. Sci. 2023, 24, 9299. https://doi.org/10.3390/ijms24119299
Pérez-Oliver MA, González-Mas MdC, Renau-Morata B, Arrillaga I, Sales E. Heat-Priming during Somatic Embryogenesis Increased Resilience to Drought Stress in the Generated Maritime Pine (Pinus pinaster) Plants. International Journal of Molecular Sciences. 2023; 24(11):9299. https://doi.org/10.3390/ijms24119299
Chicago/Turabian StylePérez-Oliver, María Amparo, María del Carmen González-Mas, Begoña Renau-Morata, Isabel Arrillaga, and Ester Sales. 2023. "Heat-Priming during Somatic Embryogenesis Increased Resilience to Drought Stress in the Generated Maritime Pine (Pinus pinaster) Plants" International Journal of Molecular Sciences 24, no. 11: 9299. https://doi.org/10.3390/ijms24119299
APA StylePérez-Oliver, M. A., González-Mas, M. d. C., Renau-Morata, B., Arrillaga, I., & Sales, E. (2023). Heat-Priming during Somatic Embryogenesis Increased Resilience to Drought Stress in the Generated Maritime Pine (Pinus pinaster) Plants. International Journal of Molecular Sciences, 24(11), 9299. https://doi.org/10.3390/ijms24119299






