The Therapeutic Potential of Multipotent Mesenchymal Stromal Cell—Derived Extracellular Vesicles in Endometrial Regeneration
Abstract
1. Introduction
2. Endometrial Regeneration
3. Pathology of the Endometrium Associated with Its Impaired Regeneration
3.1. “Thin” Endometrium
3.2. Asherman’s Syndrome
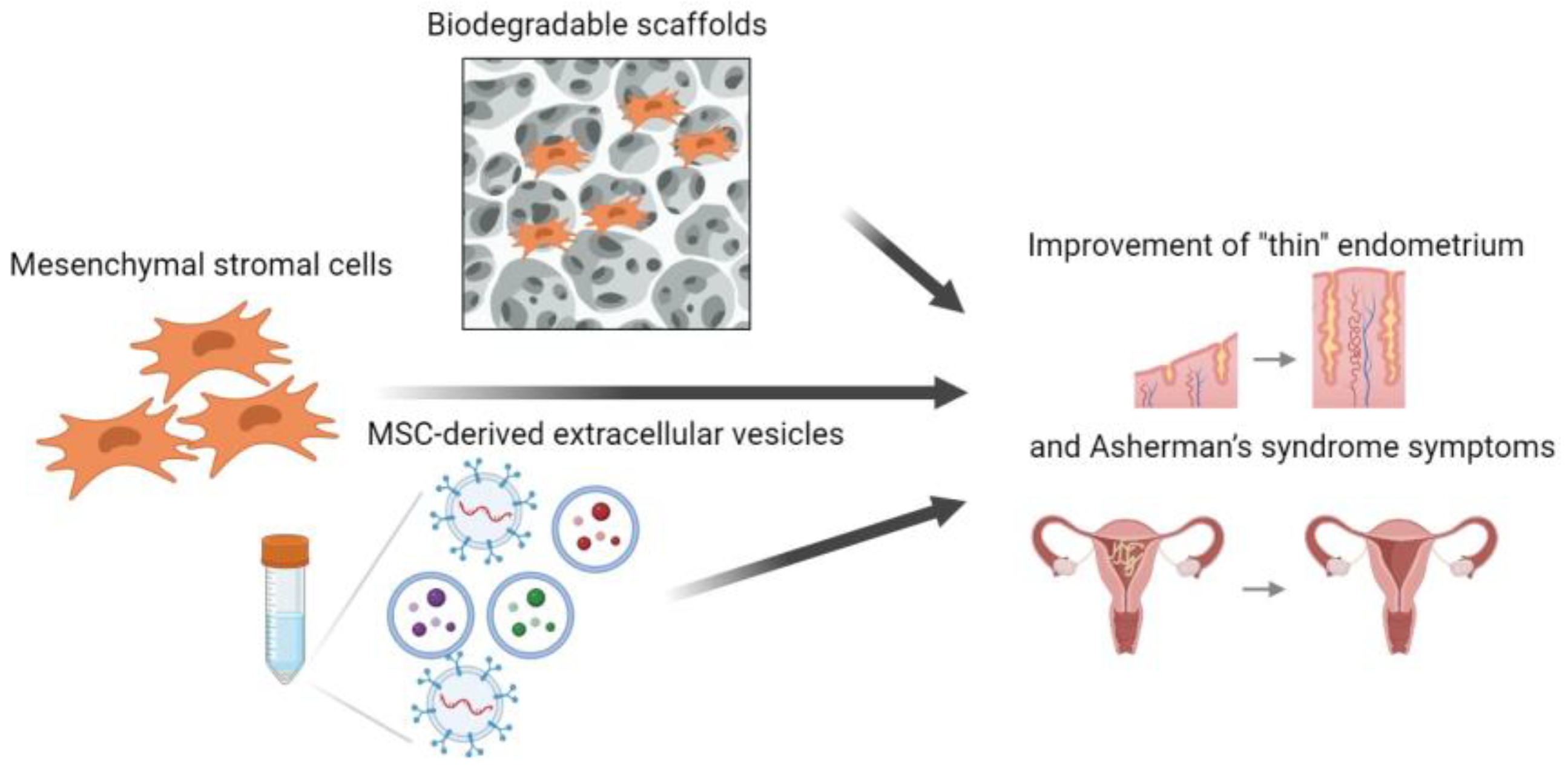
4. Effect of Multipotent Mesenchymal Stromal Cells on Endometrial Regeneration
5. Effect of MMSC-Derived Extracellular Vesicles on Endometrial Regeneration
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAGL | American Association of Gynecologic Laparoscopists |
| ART | assisted reproductive technique |
| AS | Asherman’s syndrome |
| AXIN2 | axis inhibition protein 2 |
| BMDSC | bone marrow-derived stem cell |
| EGF | epithelial growth factor |
| ERK | extracellular-signal-regulated kinase |
| ERα | estrogen receptor α |
| ET | endometrial thickness |
| EV | extracellular vesicle |
| FGF2 | fibroblast growth factor 2 |
| GRO | growth-regulating oncogene |
| IL | interleukin |
| MARK | mitogen-activated protein kinase |
| MET | mesenchymalmal-epithelial transition |
| MMP | matrix metalloproteinase |
| MMSC | multipotent mesenchymal stromal cell |
| NF-κB | nuclear factor-κB |
| NLM | National Library of Medicine |
| PR | progesterone receptor |
| ROS | reactive oxygen species |
| SHG | sonohysterography |
| Snai 1,2,3 | Snail family transcriptional repressor 1,2,3 |
| SSEA-1 | stage-specific embryonic anti-gene-1 |
| TAZ | transcriptional coactivator with PDZ-binding motif |
| TGF-1 | transforming growth factor 1 |
| TGFα | transforming growth factor α |
| TIE-2 | angiopoietin receptor |
| TIMP-1/2 | tissue matrix metalloproteinase inhibitor |
| VEGFB | vascular endothelial growth factor |
| WT1 | Wilms’ tumour 1 |
| YAP | Yes-associated protein |
| NIH | National Institutes of Health |
References
- Eremichev, R.; Kulebyakina, M.; Alexandrushkina, N.; Nimiritsky, P.; Basalova, N.; Grigorieva, O.; Egiazaryan, M.; Dyikanov, D.; Tkachuk, V.; Makarevich, P. Scar-Free Healing of Endometrium: Tissue-Specific Program of Stromal Cells and Its Induction by Soluble Factors Produced after Damage. Front. Cell Dev. Biol. 2021, 9, 616893. [Google Scholar] [CrossRef]
- Schenker, J.G.; Margalioth, E.J. Intrauterine Adhesions: An Updated Appraisal. Fertil. Steril. 1982, 37, 593–610. [Google Scholar] [CrossRef] [PubMed]
- Hooker, A.B.; Lemmers, M.; Thurkow, A.L.; Heymans, M.W.; Opmeer, B.C.; Brölmann, H.A.M.; Mol, B.W.; Huirne, J.A.F. Systematic Review and Meta-Analysis of Intrauterine Adhesions after Miscarriage: Prevalence, Risk Factors and Long-Term Reproductive Outcome. Hum. Reprod. Update 2014, 20, 262–278. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Parolini, O.; Deng, L. The Potential Role of Microvesicles in Mesenchymal Stem Cell-Based Therapy. Stem Cells Dev. 2013, 22, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Guo, E.J.; Chung, J.P.W.; Poon, L.C.Y.; Li, T.C. Reproductive Outcomes after Surgical Treatment of Asherman Syndrome: A Systematic Review. Best. Pract. Res. Clin. Obs. Gynaecol. 2019, 59, 98–114. [Google Scholar] [CrossRef]
- Alawadhi, F.; Du, H.; Cakmak, H.; Taylor, H.S. Bone Marrow-Derived Stem Cell (BMDSC) Transplantation Improves Fertility in a Murine Model of Asherman’s Syndrome. PLoS ONE 2014, 9, e96662. [Google Scholar] [CrossRef]
- Gan, L.; Duan, H.; Xu, Q.; Tang, Y.-Q.; Li, J.-J.; Sun, F.-Q.; Wang, S. Human Amniotic Mesenchymal Stromal Cell Transplantation Improves Endometrial Regeneration in Rodent Models of Intrauterine Adhesions. Cytotherapy 2017, 19, 603–616. [Google Scholar] [CrossRef]
- Nagori, C.B.; Panchal, S.Y.; Patel, H. Endometrial Regeneration Using Autologous Adult Stem Cells Followed by Conception by in Vitro Fertilization in a Patient of Severe Asherman’s Syndrome. J. Hum. Reprod. Sci. 2011, 4, 43–48. [Google Scholar] [CrossRef]
- Tang, Y.-Q.; Gan, L.; Xu, Q.; Wang, S.; Li, J.-J.; Duan, H. Effects of Human Umbilical Cord Mesenchymal Stem Cells on Intrauterine Adhesions in a Rat Model. Int. J. Clin. Exp. Pathol. 2016, 9, 12119–12129. [Google Scholar]
- Jing, Z.; Qiong, Z.; Yonggang, W.; Yanping, L. Rat Bone Marrow Mesenchymal Stem Cells Improve Regeneration of Thin Endometrium in Rat. Fertil. Steril. 2014, 101, 587–594. [Google Scholar] [CrossRef]
- Gowen, A.; Shahjin, F.; Chand, S.; Odegaard, K.E.; Yelamanchili, S.V. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Challenges in Clinical Applications. Front. Cell Dev. Biol. 2020, 8, 149. [Google Scholar] [CrossRef] [PubMed]
- Machtinger, R.; Baccarelli, A.A.; Wu, H. Extracellular Vesicles and Female Reproduction. J. Assist. Reprod. Genet. 2021, 38, 549–557. [Google Scholar] [CrossRef]
- Baraggino, E.; Dalla Pria, S.; Cuberli, C.; Bortolotti, S. Scanning Electron Microscopy of the Human Normal Endometrium. Clin. Exp. Obs. Gynecol. 1980, 7, 66–70. [Google Scholar]
- Evans-Hoeker, E.A.; Young, S.L. Endometrial Receptivity and Intrauterine Adhesive Disease. Semin. Reprod. Med. 2014, 32, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Garry, R.; Hart, R.; Karthigasu, K.A.; Burke, C. Structural Changes in Endometrial Basal Glands during Menstruation. BJOG Int. J. Obstet. Gynaecol. 2010, 117, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Spencer, T.E.; Hayashi, K.; Hu, J.; Carpenter, K.D. Comparative Developmental Biology of the Mammalian Uterus. Curr. Top. Dev. Biol. 2005, 68, 85–122. [Google Scholar] [CrossRef]
- Prianishnikov, V.A. On the Concept of Stem Cell and a Model of Functional-Morphological Structure of the Endometrium. Contraception 1978, 18, 213–223. [Google Scholar] [CrossRef]
- Valentijn, A.J.; Palial, K.; Al-Lamee, H.; Tempest, N.; Drury, J.; Von Zglinicki, T.; Saretzki, G.; Murray, P.; Gargett, C.E.; Hapangama, D.K. SSEA-1 Isolates Human Endometrial Basal Glandular Epithelial Cells: Phenotypic and Functional Characterization and Implications in the Pathogenesis of Endometriosis. Hum. Reprod. 2013, 28, 2695–2708. [Google Scholar] [CrossRef]
- Tempest, N.; Baker, A.M.; Wright, N.A.; Hapangama, D.K. Does Human Endometrial LGR5 Gene Expression Suggest the Existence of Another Hormonally Regulated Epithelial Stem Cell Niche? Hum. Reprod. 2018, 33, 1052–1062. [Google Scholar] [CrossRef]
- Lv, Q.; Wang, L.; Luo, X.; Chen, X. Adult Stem Cells in Endometrial Regeneration: Molecular Insights and Clinical Applications. Mol. Reprod. Dev. 2021, 88, 379–394. [Google Scholar] [CrossRef]
- Evans, J.; Salamonsen, L.A.; Winship, A.; Menkhorst, E.; Nie, G.; Gargett, C.E.; Dimitriadis, E. Fertile Ground: Human Endometrial Programming and Lessons in Health and Disease. Nat. Rev. Endocrinol. 2016, 12, 654–667. [Google Scholar] [CrossRef]
- Garry, R.; Hart, R.; Karthigasu, K.A.; Burke, C. A Re-Appraisal of the Morphological Changes within the Endometrium during Menstruation: A Hysteroscopic, Histological and Scanning Electron Microscopic Study. Hum. Reprod. 2009, 24, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Goncalves, R.; Mosser, D.M. The Isolation and Characterization of Murine Macrophages. Curr. Protoc. Immunol. 2008, 83, 14.1.1–14.1.14. [Google Scholar] [CrossRef]
- Santamaria, X.; Mas, A.; Cervelló, I.; Taylor, H.; Simon, C. Uterine Stem Cells: From Basic Research to Advanced Cell Therapies. Hum. Reprod. Update 2018, 24, 673–693. [Google Scholar] [CrossRef] [PubMed]
- Strakova, Z.; Kruss, S.; Morris, K.; Reed, J. Members of the Hippo Pathway Are Regulated in the Uterus During the Menstrual Cycle. Biol. Reprod. 2010, 83, 363. [Google Scholar] [CrossRef]
- Waghmare, I.; Page-McCaw, A. Wnt Signaling in Stem Cell Maintenance and Differentiation in the Drosophila Germarium. Genes 2018, 9, 127. [Google Scholar] [CrossRef]
- Nguyen, H.P.T.; Sprung, C.N.; Gargett, C.E. Differential Expression of Wnt Signaling Molecules between Pre- and Postmenopausal Endometrial Epithelial Cells Suggests a Population of Putative Epithelial Stem/Progenitor Cells Reside in the Basalis Layer. Endocrinology 2012, 153, 2870–2883. [Google Scholar] [CrossRef]
- Chan, R.W.S.; Gargett, C.E. Identification of Label-Retaining Cells in Mouse Endometrium. Stem Cells 2006, 24, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Lee, Y.H.; Lucas, E.S.; Chan, Y.-W.; Durairaj, R.P.; Takeda, S.; Moore, J.D.; Tan, B.K.; Quenby, S.; Chan, J.K.Y.; et al. Decidualization Induces a Secretome Switch in Perivascular Niche Cells of the Human Endometrium. Endocrinology 2014, 155, 4542–4553. [Google Scholar] [CrossRef]
- Spitzer, T.L.B.; Rojas, A.; Zelenko, Z.; Aghajanova, L.; Erikson, D.W.; Barragan, F.; Meyer, M.; Tamaresis, J.S.; Hamilton, A.E.; Irwin, J.C.; et al. Perivascular Human Endometrial Mesenchymal Stem Cells Express Pathways Relevant to Self-Renewal, Lineage Specification, and Functional Phenotype. Biol. Reprod. 2012, 86, 58. [Google Scholar] [CrossRef]
- Masuda, H.; Anwar, S.S.; Bühring, H.-J.; Rao, J.R.; Gargett, C.E. A Novel Marker of Human Endometrial Mesenchymal Stem-like Cells. Cell Transpl. 2012, 21, 2201–2214. [Google Scholar] [CrossRef] [PubMed]
- Gargett, C.E.; Schwab, K.E.; Deane, J.A. Endometrial Stem/Progenitor Cells: The First 10 Years. Hum. Reprod. Update 2016, 22, 137–163. [Google Scholar] [CrossRef]
- Tsuji, S.; Yoshimoto, M.; Takahashi, K.; Noda, Y.; Nakahata, T.; Heike, T. Side Population Cells Contribute to the Genesis of Human Endometrium. Fertil. Steril. 2008, 90, 1528–1537. [Google Scholar] [CrossRef]
- Cervelló, I.; Mas, A.; Gil-Sanchis, C.; Peris, L.; Faus, A.; Saunders, P.T.K.; Critchley, H.O.D.; Simón, C. Reconstruction of Endometrium from Human Endometrial Side Population Cell Lines. PLoS ONE 2011, 6, e21221. [Google Scholar] [CrossRef]
- Miyazaki, K.; Maruyama, T.; Masuda, H.; Yamasaki, A.; Uchida, S.; Oda, H.; Uchida, H.; Yoshimura, Y. Stem Cell-like Differentiation Potentials of Endometrial Side Population Cells as Revealed by a Newly Developed in Vivo Endometrial Stem Cell Assay. PLoS ONE 2012, 7, e50749. [Google Scholar] [CrossRef]
- Bratincsák, A.; Brownstein, M.J.; Cassiani-Ingoni, R.; Pastorino, S.; Szalayova, I.; Tóth, Z.E.; Key, S.; Németh, K.; Pickel, J.; Mezey, E. CD45-Positive Blood Cells Give Rise to Uterine Epithelial Cells in Mice. Stem Cells 2007, 25, 2820–2826. [Google Scholar] [CrossRef]
- Taylor, H.S. Endometrial Cells Derived from Donor Stem Cells in Bone Marrow Transplant Recipients. JAMA 2004, 292, 81–85. [Google Scholar] [CrossRef]
- Ong, Y.R.; Cousins, F.L.; Yang, X.; Mushafi, A.A.A.A.; Breault, D.T.; Gargett, C.E.; Deane, J.A. Bone Marrow Stem Cells Do Not Contribute to Endometrial Cell Lineages in Chimeric Mouse Models. Stem Cells 2018, 36, 91–102. [Google Scholar] [CrossRef]
- Ibrahim, M.I.; Raafat, T.A.; Ellaithy, M.I.; Aly, R.T. Risk of Postpartum Uterine Synechiae Following Uterine Compression Suturing during Postpartum Haemorrhage. Aust. N. Z. J. Obs. Gynaecol. 2013, 53, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Mara, M.; Fucikova, Z.; Kuzel, D.; Maskova, J.; Dundr, P.; Zizka, Z. Hysteroscopy after Uterine Fibroid Embolization in Women of Fertile Age. J. Obs. Gynaecol. Res. 2007, 33, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wong, Y.-M.; Cheong, Y.; Xia, E.; Li, T.-C. Asherman Syndrome--One Century Later. Fertil. Steril. 2008, 89, 759–779. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Akyaw, A.; Krishnamoorthy, K.; Goldsmith, L.T.; Morelli, S.S. The Role of Mesenchymal-Epithelial Transition in Endometrial Function. Hum. Reprod. Update 2019, 25, 114–133. [Google Scholar] [CrossRef] [PubMed]
- Ayyaz, A.; Attisano, L.; Wrana, J.L. Recent Advances in Understanding Contextual TGFβ Signaling. F1000Results 2017, 6, 749. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.E.; Hartman, M.; Hartman, A.; Luo, Z.-C.; Mahutte, N. The Impact of a Thin Endometrial Lining on Fresh and Frozen-Thaw IVF Outcomes: An Analysis of over 40 000 Embryo Transfers. Hum. Reprod. 2018, 33, 1883–1888. [Google Scholar] [CrossRef] [PubMed]
- Kasius, A.; Smit, J.G.; Torrance, H.L.; Eijkemans, M.J.C.; Mol, B.W.; Opmeer, B.C.; Broekmans, F.J.M. Endometrial Thickness and Pregnancy Rates after IVF: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2014, 20, 530–541. [Google Scholar] [CrossRef]
- Mouhayar, Y.; Franasiak, J.M.; Sharara, F.I. Obstetrical Complications of Thin Endometrium in Assisted Reproductive Technologies: A Systematic Review. J. Assist. Reprod. Genet. 2019, 36, 607–611. [Google Scholar] [CrossRef]
- Chen, M.; He, Y.; Zhang, P.; Geng, Q.; Liu, Q.; Kong, L.; Chen, Y.; Wei, Q.; Liu, J.; Guo, S.; et al. Comparison of Uterine Receptivity between Fertile and Unexplained Infertile Women by Assessment of Endometrial and Subendometrial Perfusion Using Contrast-Enhanced Ultrasound: Which Index Is Better—Peak Intensity or Area under the Curve? Ultrasound Med. Biol. 2016, 42, 654–663. [Google Scholar] [CrossRef]
- Alfer, J.; Happel, L.; Dittrich, R.; Beckmann, M.W.; Hartmann, A.; Gaumann, A.; Buck, V.U.; Classen-Linke, I. Insufficient Angiogenesis: Cause of Abnormally Thin Endometrium in Subfertile Patients? Geburtshilfe Frauenheilkd. 2017, 77, 756–764. [Google Scholar] [CrossRef]
- Maekawa, R.; Taketani, T.; Mihara, Y.; Sato, S.; Okada, M.; Tamura, I.; Jozaki, K.; Kajimura, T.; Asada, H.; Tamura, H.; et al. Thin Endometrium Transcriptome Analysis Reveals a Potential Mechanism of Implantation Failure. Reprod. Med. Biol. 2017, 16, 206–227. [Google Scholar] [CrossRef]
- Sundström, P. Establishment of a Successful Pregnancy Following In-Vitro Fertilization with an Endometrial Thickness of No More than 4 Mm. Hum. Reprod. 1998, 13, 1550–1552. [Google Scholar] [CrossRef]
- Check, J.H.; Dietterich, C.; Check, M.L.; Katz, Y. Successful Delivery despite Conception with a Maximal Endometrial Thickness of 4 Mm. Clin. Exp. Obs. Gynecol. 2003, 30, 93–94. [Google Scholar]
- Jacobs, E.A.; Van Voorhis, B.; Kawwass, J.F.; Kondapalli, L.A.; Liu, K.; Dokras, A. Endometrial Thickness: How Thin Is Too Thin? Fertil. Steril. 2022, 118, 249–259. [Google Scholar] [CrossRef]
- Gonen, Y.; Casper, R.F. Sonographic Determination of a Possible Adverse Effect of Clomiphene Citrate on Endometrial Growth. Hum. Reprod. 1990, 5, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Casper, R.F. It’s Time to Pay Attention to the Endometrium. Fertil. Steril. 2011, 96, 519–521. [Google Scholar] [CrossRef]
- Talukdar, N.; Bentov, Y.; Chang, P.T.; Esfandiari, N.; Nazemian, Z.; Casper, R.F. Effect of Long-Term Combined Oral Contraceptive Pill Use on Endometrial Thickness. Obs. Gynecol. 2012, 120, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Le, A.W.; Wang, Z.H.; Yuan, R.; Shan, L.L.; Xiao, T.H.; Zhuo, R.; Shen, Y. Association of the Estrogen Receptor-β Gene RsaI and AluI Polymorphisms with Human Idiopathic Thin Endometrium. Genet. Mol. Res. 2013, 12, 5978–5985. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Le, A.-W. A Study on the Estrogen Receptor α Gene Polymorphism and Its Expression in Thin Endometrium of Unknown Etiology. Gynecol. Obs. Invest. 2012, 74, 13–20. [Google Scholar] [CrossRef]
- Shaffer, W. Role of Uterine Adhesions in the Cause of Multiple Pregnancy Losses. Clin. Obs. Gynecol. 1986, 29, 912–924. [Google Scholar] [CrossRef]
- Conforti, A.; Alviggi, C.; Mollo, A.; De Placido, G.; Magos, A. The Management of Asherman Syndrome: A Review of Literature. Reprod. Biol. Endocrinol. 2013, 11, 118. [Google Scholar] [CrossRef]
- Deans, R.; Abbott, J. Review of Intrauterine Adhesions. J. Minim. Invasive Gynecol. 2010, 17, 555–569. [Google Scholar] [CrossRef]
- Toaff, R.; Ballas, S. Traumatic Hypomenorrhea-Amenorrhea (Asherman’s Syndrome). Fertil. Steril. 1978, 30, 379–387. [Google Scholar] [CrossRef] [PubMed]
- March, C.M.; Israel, R.; March, A.D. Hysteroscopic Management of Intrauterine Adhesions. Am. J. Obs. Gynecol. 1978, 130, 653–657. [Google Scholar] [CrossRef] [PubMed]
- The American Fertility Society Classifications of Adnexal Adhesions, Distal Tubal Occlusion, Tubal Occlusion Secondary to Tubal Ligation, Tubal Pregnancies, Müllerian Anomalies and Intrauterine Adhesions. Fertil. Steril. 1988, 49, 944–955. [CrossRef] [PubMed]
- Sutton, C.; Sutton, C.J.G.; Diamond, M.P. Endoscopic Surgery for Gynecologists, 2nd ed.; W. B. Saunders: London, UK, 1998; ISBN 978-0-7020-2250-0. [Google Scholar]
- Nasr, A.L.; Al-Inany, H.G.; Thabet, S.M.; Aboulghar, M. A Clinicohysteroscopic Scoring System of Intrauterine Adhesions. Gynecol. Obs. Invest. 2000, 50, 178–181. [Google Scholar] [CrossRef] [PubMed]
- AAGL Worldwild. AAGL Advancing Minimally Invasive Gynecology Worldwide AAGL Practice Report: Practice Guidelines for Management of Intrauterine Synechiae. J. Minim. Invasive Gynecol. 2010, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Xu, D. The Effect of Adjuvant Treatment to Prevent and Treat Intrauterine Adhesions: A Network Meta-Analysis of Randomized Controlled Trials. J. Minim. Invasive Gynecol. 2018, 25, 589–599. [Google Scholar] [CrossRef]
- Ranisavljevic, N.; Raad, J.; Anahory, T.; Grynberg, M.; Sonigo, C. Embryo Transfer Strategy and Therapeutic Options in Infertile Patients with Thin Endometrium: A Systematic Review. J. Assist. Reprod. Genet. 2019, 36, 2217–2231. [Google Scholar] [CrossRef]
- Sukhikh, G.T.; Chernukha, G.E.; Tabeeva, G.I.; Goryunov, G.V.; Sylachev, D.N. Current possibilities of cell therapy for Asherman’s syndrome. Akusherstvo Ginekol. Obstet. Gynecol. 2018, 5, 20–29. [Google Scholar] [CrossRef]
- Sukhikh, G.T.; Silachyov, D.N.; Pevzner, I.B.; Zorova, L.D.; Babenko, V.A.; Popkov, V.A.; Yankauskas, S.S.; Zubkov, V.V.; Zorov, D.B.; Plotnikov, E.Y. Prospects for using stem and progenitor cells in the therapy of consequences of neonatal hypoxic-ischemic encephalopathy. Akusherstvo Ginekol. Obstet. Gynecol. 2016, 5, 55–66. [Google Scholar] [CrossRef]
- Mahla, R.S. Stem Cells Applications in Regenerative Medicine and Disease Therapeutics. Int. J. Cell Biol. 2016, 2016, 6940283. [Google Scholar] [CrossRef]
- Narayanan, R.; Huang, C.-C.; Ravindran, S. Hijacking the Cellular Mail: Exosome Mediated Differentiation of Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 3808674. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Kilic, S.; Yuksel, B.; Pinarli, F.; Albayrak, A.; Boztok, B.; Delibasi, T. Effect of Stem Cell Application on Asherman Syndrome, an Experimental Rat Model. J. Assist. Reprod. Genet. 2014, 31, 975–982. [Google Scholar] [CrossRef]
- Pekarev, O.G.; Maiborodin, I.V.; Pozdnyakov, I.M.; Onoprienko, N.V.; Pekareva, E.O.; Anikeev, A.A. Experimental rationale for the use of cell technologies for the correction of myometrial scar. Akusherstvo Ginekol. Obstet. Gynecol. 2016, 8, 79–85. [Google Scholar] [CrossRef]
- Transplantation of Human Umbilical Cord Mesenchymal Stem Cells Induces Angiogenesis and Promotes Repair of Uterine Scars in Rats. Available online: https://www.researchsquare.com (accessed on 14 March 2023).
- Zhu, H.; Pan, Y.; Jiang, Y.; Li, J.; Zhang, Y.; Zhang, S. Activation of the Hippo/TAZ Pathway Is Required for Menstrual Stem Cells to Suppress Myofibroblast and Inhibit Transforming Growth Factor β Signaling in Human Endometrial Stromal Cells. Hum. Reprod. 2019, 34, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Q.; Wang, Y.; Li, Y. Uterine Infusion with Bone Marrow Mesenchymal Stem Cells Improves Endometrium Thickness in a Rat Model of Thin Endometrium. Reprod. Sci. 2015, 22, 181–188. [Google Scholar] [CrossRef]
- Search of: Mesenchymal Stem Cells—List Results—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/results?cond=mesenchymal+stem+cells&term=&cntry=&state=&city=&dist= (accessed on 20 March 2023).
- Zhao, Y.; Wang, A.; Tang, X.; Li, M.; Yan, L.; Shang, W.; Gao, M. Intrauterine Transplantation of Autologous Bone Marrow Derived Mesenchymal Stem Cells Followed by Conception in a Patient of Severe Intrauterine Adhesions. OJOG Open J. Obstet. Gynecol. 2013, 03, 377–380. [Google Scholar] [CrossRef]
- Tan, J.; Li, P.; Wang, Q.; Li, Y.; Li, X.; Zhao, D.; Xu, X.; Kong, L. Autologous Menstrual Blood-Derived Stromal Cells Transplantation for Severe Asherman’s Syndrome. Hum. Reprod. 2016, 31, 2723–2729. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.; Jung, Y.; Kim, S.H. Insight on Stem Cell Preconditioning and Instructive Biomaterials to Enhance Cell Adhesion, Retention, and Engraftment for Tissue Repair. Biomaterials 2016, 90, 85–115. [Google Scholar] [CrossRef] [PubMed]
- Roche, E.T.; Hastings, C.L.; Lewin, S.A.; Shvartsman, D.; Brudno, Y.; Vasilyev, N.V.; O’Brien, F.J.; Walsh, C.J.; Duffy, G.P.; Mooney, D.J. Comparison of Biomaterial Delivery Vehicles for Improving Acute Retention of Stem Cells in the Infarcted Heart. Biomaterials 2014, 35, 6850–6858. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Sun, H.; Zhu, H.; Zhu, X.; Tang, X.; Yan, G.; Wang, J.; Bai, D.; Wang, J.; Wang, L.; et al. Allogeneic Cell Therapy Using Umbilical Cord MSCs on Collagen Scaffolds for Patients with Recurrent Uterine Adhesion: A Phase I Clinical Trial. Stem Cell Res. 2018, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, L.; Lin, X.; Zhou, F.; Xin, L.; Xu, W.; Yu, H.; Li, J.; Pan, M.; Pan, Y.; et al. Unresponsive Thin Endometrium Caused by Asherman Syndrome Treated with Umbilical Cord Mesenchymal Stem Cells on Collagen Scaffolds: A Pilot Study. Stem Cell Res. 2021, 12, 420. [Google Scholar] [CrossRef]
- Hocking, A.M. The Role of Chemokines in Mesenchymal Stem Cell Homing to Wounds. Adv. Wound Care New Rochelle 2015, 4, 623–630. [Google Scholar] [CrossRef]
- Gargett, C.E.; Ye, L. Endometrial Reconstruction from Stem Cells. Fertil. Steril. 2012, 98, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Toward Cell-Free Therapeutic Applications. Mol. Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhu, X.-Y.; Lerman, A.; Lerman, L.O. Extracellular Vesicles as Theranostic Tools in Kidney Disease. Clin. J. Am. Soc. Nephrol. 2022, 17, 1418–1429. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bayado, N.; He, D.; Li, J.; Chen, H.; Li, L.; Li, J.; Long, X.; Du, T.; Tang, J.; et al. Therapeutic Applications of Extracellular Vesicles for Myocardial Repair. Front. Cardiovasc. Med. 2021, 8, 758050. [Google Scholar] [CrossRef]
- Turovsky, E.A.; Golovicheva, V.V.; Varlamova, E.G.; Danilina, T.I.; Goryunov, K.V.; Shevtsova, Y.A.; Pevzner, I.B.; Zorova, L.D.; Babenko, V.A.; Evtushenko, E.A.; et al. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Afford Neuroprotection by Modulating PI3K/AKT Pathway and Calcium Oscillations. Int. J. Biol. Sci. 2022, 18, 5345–5368. [Google Scholar] [CrossRef]
- Gong, Q.; Zeng, Z.; Jiang, T.; Bai, X.; Pu, C.; Hao, Y.; Guo, Y. Anti-Fibrotic Effect of Extracellular Vesicles Derived from Tea Leaves in Hepatic Stellate Cells and Liver Fibrosis Mice. Front. Nutr. 2022, 9, 1009139. [Google Scholar] [CrossRef]
- Pekarev, O.G.; Pekareva, E.O.; Mayborodin, I.V.; Silachev, D.N.; Baranov, I.I.; Pozdnyakov, I.M.; Bushueva, N.S.; Novikov, A.M.; Sukhikh, G.T. The Potential of Extracellular Microvesicles of Mesenchymal Stromal Cells in Obstetrics. J. Matern. Fetal. Neonatal. Med. 2022, 35, 7523–7525. [Google Scholar] [CrossRef]
- Avalos, P.N.; Forsthoefel, D.J. An Emerging Frontier in Intercellular Communication: Extracellular Vesicles in Regeneration. Front. Cell Dev. Biol. 2022, 10, 849905. [Google Scholar] [CrossRef]
- Shao, C.; Yang, F.; Miao, S.; Liu, W.; Wang, C.; Shu, Y.; Shen, H. Role of Hypoxia-Induced Exosomes in Tumor Biology. Mol. Cancer 2018, 17, 120. [Google Scholar] [CrossRef]
- Blonda, M.; Amoruso, A.; Martino, T.; Avolio, C. New Insights Into Immune Cell-Derived Extracellular Vesicles in Multiple Sclerosis. Front. Neurol. 2018, 9, 604. [Google Scholar] [CrossRef]
- Vilette, D.; Courte, J.; Peyrin, J.M.; Coudert, L.; Schaeffer, L.; Andréoletti, O.; Leblanc, P. Cellular Mechanisms Responsible for Cell-to-Cell Spreading of Prions. Cell Mol. Life Sci. 2018, 75, 2557–2574. [Google Scholar] [CrossRef]
- Salomon, C.; Torres, M.J.; Kobayashi, M.; Scholz-Romero, K.; Sobrevia, L.; Dobierzewska, A.; Illanes, S.E.; Mitchell, M.D.; Rice, G.E. A Gestational Profile of Placental Exosomes in Maternal Plasma and Their Effects on Endothelial Cell Migration. PLoS ONE 2014, 9, e98667. [Google Scholar] [CrossRef]
- Khodzhaeva, Z.S.; Abramova, M.E.; Muminova, K.T.; Gorina, K.A.; Frolova, E.R.; Goryunov, K.V.; Silachev, D.N.; Shevtsova, Y.A. The role of plasma extracellular vesicles as predictors of gestational diabetes mellitus in the first trimester of pregnancy. Akusherstvo Ginekol. Obstet. Gynecol. 2022, 4, 76–83. [Google Scholar] [CrossRef]
- Xu, R.; Greening, D.W.; Zhu, H.-J.; Takahashi, N.; Simpson, R.J. Extracellular Vesicle Isolation and Characterization: Toward Clinical Application. J. Clin. Investig. 2016, 126, 1152–1162. [Google Scholar] [CrossRef]
- Bebelman, M.P.; Bun, P.; Huveneers, S.; van Niel, G.; Pegtel, D.M.; Verweij, F.J. Real-Time Imaging of Multivesicular Body–Plasma Membrane Fusion to Quantify Exosome Release from Single Cells. Nat. Protoc. 2020, 15, 102–121. [Google Scholar] [CrossRef]
- Battistelli, M.; Falcieri, E. Apoptotic Bodies: Particular Extracellular Vesicles Involved in Intercellular Communication. Biology 2020, 9, 21. [Google Scholar] [CrossRef]
- Anand, S.; Samuel, M.; Mathivanan, S. Exomeres: A New Member of Extracellular Vesicles Family. Subcell. Biochem. 2021, 97, 89–97. [Google Scholar] [CrossRef]
- Huang, C.; Quinn, D.; Sadovsky, Y.; Suresh, S.; Hsia, K.J. Formation and Size Distribution of Self-Assembled Vesicles. Proc. Natl. Acad. Sci. USA 2017, 114, 2910–2915. [Google Scholar] [CrossRef]
- Kang, K.; Ma, R.; Cai, W.; Huang, W.; Paul, C.; Liang, J.; Wang, Y.; Zhao, T.; Kim, H.W.; Xu, M.; et al. Exosomes Secreted from CXCR4 Overexpressing Mesenchymal Stem Cells Promote Cardioprotection via Akt Signaling Pathway Following Myocardial Infarction. Stem Cells Int. 2015, 2015, 659890. [Google Scholar] [CrossRef]
- Salamonsen, L.A.; Hutchison, J.C.; Gargett, C.E. Cyclical Endometrial Repair and Regeneration. Development 2021, 148, dev199577. [Google Scholar] [CrossRef]
- Fonseka, P.; Marzan, A.L.; Mathivanan, S. Introduction to the Community of Extracellular Vesicles. Subcell. Biochem. 2021, 97, 3–18. [Google Scholar] [CrossRef]
- Kraevaya, E.E.; Makarova, N.O.; Sysoeva, A.P.; Kalinina, E.A.; Silachev, D.N. Therapeutic opportunities of extraceccular vesicles in reproductive medicine. Akusherstvo Ginekol. Obstet. Gynecol. 2021, 7, 5–9. [Google Scholar]
- Hart, A.R.; Khan, N.L.A.; Godakumara, K.; Dissanayake, K.; Piibor, J.; Muhandiram, S.; Eapen, S.; Heath, P.R.; Fazeli, A. The Role of Extracellular Vesicles in Endometrial Receptivity and Their Potential in Reproductive Therapeutics and Diagnosis. Reprod. Biol. 2022, 22, 100645. [Google Scholar] [CrossRef]
- Kasvandik, S.; Saarma, M.; Kaart, T.; Rooda, I.; Velthut-Meikas, A.; Ehrenberg, A.; Gemzell, K.; Lalitkumar, P.G.; Salumets, A.; Peters, M. Uterine Fluid Proteins for Minimally Invasive Assessment of Endometrial Receptivity. J. Clin. Endocrinol. Metab. 2020, 105, dgz019. [Google Scholar] [CrossRef]
- Uyar, Y.; Özgül, M.; Gökap, S.; Ok, G.; Tan, A.; Vatansever, H.S. The Correlation between Unexplained Infertility and Exosomes. Ginekol. Pol. 2020, 91, 240–246. [Google Scholar] [CrossRef]
- Governini, L.; Luongo, F.P.; Haxhiu, A.; Piomboni, P.; Luddi, A. Main Actors behind the Endometrial Receptivity and Successful Implantation. Tissue Cell 2021, 73, 101656. [Google Scholar] [CrossRef]
- Chen, K.; Liang, J.; Qin, T.; Zhang, Y.; Chen, X.; Wang, Z. The Role of Extracellular Vesicles in Embryo Implantation. Front. Endocrinol. 2022, 13, 809596. [Google Scholar] [CrossRef]
- Ranjbaran, A.; Latifi, Z.; Nejabati, H.R.; Abroon, S.; Mihanfar, A.; Sadigh, A.R.; Fattahi, A.; Nouri, M.; Raffel, N. Exosome-Based Intercellular Communication in Female Reproductive Microenvironments. J. Cell Physiol. 2019, 234, 19212–19222. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Shi, S.; Liang, J.; Zhang, X.; Cao, D.; Wang, Z. MicroRNAs in Small Extracellular Vesicles Indicate Successful Embryo Implantation during Early Pregnancy. Cells 2020, 9, 645. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Czerniawska-Piątkowska, E.; Wrzecińska, M. The Importance of Interferon-Tau in the Diagnosis of Pregnancy. BioMed Res. Int. 2021, 2021, 9915814. [Google Scholar] [CrossRef]
- Hajipour, H.; Farzadi, L.; Roshangar, L.; Latifi, Z.; Kahroba, H.; Shahnazi, V.; Hamdi, K.; Ghasemzadeh, A.; Fattahi, A.; Nouri, M. A Human Chorionic Gonadotropin (HCG) Delivery Platform Using Engineered Uterine Exosomes to Improve Endometrial Receptivity. Life Sci. 2021, 275, 119351. [Google Scholar] [CrossRef]
- Yuana, Y.; Sturk, A.; Nieuwland, R. Extracellular Vesicles in Physiological and Pathological Conditions. Blood Rev. 2013, 27, 31–39. [Google Scholar] [CrossRef]
- Kletukhina, S.; Neustroeva, O.; James, V.; Rizvanov, A.; Gomzikova, M. Role of Mesenchymal Stem Cell-Derived Extracellular Vesicles in Epithelial-Mesenchymal Transition. Int. J. Mol. Sci. 2019, 20, 4813. [Google Scholar] [CrossRef]
- Chen, D.; Qiao, H.; Wang, Y.; Zhou, L.; Yin, N.; Fang, L.; Wang, Z. Adenomyosis-Derived Extracellular Vesicles Endow Endometrial Epithelial Cells with an Invasive Phenotype through Epithelial-Mesenchymal Transition. Genes Dis. 2020, 7, 636–648. [Google Scholar] [CrossRef]
- Xin, L.; Lin, X.; Zhou, F.; Li, C.; Wang, X.; Yu, H.; Pan, Y.; Fei, H.; Ma, L.; Zhang, S. A Scaffold Laden with Mesenchymal Stem Cell-Derived Exosomes for Promoting Endometrium Regeneration and Fertility Restoration through Macrophage Immunomodulation. Acta Biomater. 2020, 113, 252–266. [Google Scholar] [CrossRef]
- Zhao, S.; Qi, W.; Zheng, J.; Tian, Y.; Qi, X.; Kong, D.; Zhang, J.; Huang, X. Exosomes Derived from Adipose Mesenchymal Stem Cells Restore Functional Endometrium in a Rat Model of Intrauterine Adhesions. Reprod. Sci. 2020, 27, 1266–1275. [Google Scholar] [CrossRef]
- Xiao, B.; Zhu, Y.; Huang, J.; Wang, T.; Wang, F.; Sun, S. Exosomal Transfer of Bone Marrow Mesenchymal Stem Cell-Derived MiR-340 Attenuates Endometrial Fibrosis. Biol. Open 2019, 8, bio039958. [Google Scholar] [CrossRef]
- Tan, Q.; Xia, D.; Ying, X. MiR-29a in Exosomes from Bone Marrow Mesenchymal Stem Cells Inhibit Fibrosis during Endometrial Repair of Intrauterine Adhesion. Int. J. Stem Cells 2020, 13, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Saribas, G.S.; Ozogul, C.; Tiryaki, M.; Alpaslan Pinarli, F.; Hamdemir Kilic, S. Effects of Uterus Derived Mesenchymal Stem Cells and Their Exosomes on Asherman’s Syndrome. Acta Histochem. 2020, 122, 151465. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, R.; Wang, G.; Zhang, Y.; Liu, F. Exosomes Derived from Mesenchymal Stem Cells Reverse EMT via TGF-Β1/Smad Pathway and Promote Repair of Damaged Endometrium. Stem Cell Res. 2019, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Tang, S.; Zhu, Y.; Chen, D.; Huang, J.; Lin, J. Exosomes Derived from CTF1-Modified Bone Marrow Stem Cells Promote Endometrial Regeneration and Restore Fertility. Front. Bioeng. Biotechnol. 2022, 10, 868734. [Google Scholar] [CrossRef]
- Xin, L.; Wei, C.; Tong, X.; Dai, Y.; Huang, D.; Chen, J.; Ma, L.; Zhang, S. In Situ Delivery of Apoptotic Bodies Derived from Mesenchymal Stem Cells via a Hyaluronic Acid Hydrogel: A Therapy for Intrauterine Adhesions. Bioact. Mater. 2022, 12, 107–119. [Google Scholar] [CrossRef]

| Authors | Type of MMSC | Model | Results | References |
|---|---|---|---|---|
| Kilic, S. et al. | MMSCs from the bone marrow and umbilical cord | Rat model of AS | Increased endometrial vascularization and decreased fibrosis | [74] |
| Pekarev, O.G. et al. | Human umbilical cord MSC | Rat model of a uterine scar | Stimulating effect on vascular remodeling and the formation of de novo formed vessels in the uterine scar | [75] |
| Zhu, H. et al. | Menstrual stem cells | In vitro | Increased proliferation of endometrial stromal cells, suppression of myofibroblast differentiation | [77] |
| Zhao, J. et al. | Autologous bone marrow derived MSC | Rat model of “thin” endometrium | Increased the ET, activated the expression of markers of regeneration and receptivity, anti-inflammatory effects | [78] |
| Tan, J. et al. | Autologous menstrual blood-derived stromal cells | Patient’s with severe AS | Increased the ET (71%), pregnancy (71%), live birth (29%) | [81] |
| Zhao, Y. et al. | Autologous bone marrow derived MSC | Patient’s with intrauterine adhesions | Restoration of the endometrium in a woman with refractory AS | [80] |
| Cao, Y. et al. | Umbilical cord MSCs on collagen scaffolds | Patients with Recurrent Uterine Adhesions | Increased in the ET (100%), pregnancy (38%) | [84] |
| Zhang, Y. et al. | Umbilical cord MSC on collagen scaffolds | Patients with AS | Increase in ET (100%), pregnancy (31%), live birth (12%) | [85] |
| Authors | Type of Extracellular Vesicles | Model | Results | References |
|---|---|---|---|---|
| Xin, L. et al. | Umbilical cord MMSCs-derived exosomes on a collagen scaffold | Injured rat uterus In vitro | Induction of endometrial regeneration, collagen remodeling, increased ER α/RP expression and restoration of fertility. | [121] |
| Zhao, S. et al. | Exosomes Derived from Adipose MSCs | Rat Model of Intrauterine Adhesions | Maintenance of normal uterine structure, activation of endometrial regeneration and collagen remodeling, increased expression of integrin-β3, LIF and VEGF, increased endometrial receptivity and fertility | [122] |
| Xiao, B. et al. | Bone marrow MMSCs-derived exosomes | Injured rat uterus In vitro | Antifibrotic effect (improved functional recovery and suppression of collagen 1α1, α-SMA and TGF-β1,suppression of increased expression of fibrotic genes induced by TGF-β1) | [123] |
| Tan, Q. et al. | Bone marrow MSC-derived exosomes | Mouse model of intrauterine adhesions In vitro | Activation of cell proliferation and cell migration in vitro, repair of damaged endometrium in a mouse model | [124] |
| Saribas, G.S. et al. | Exosomes from uterus derived MSC | Rat model of AS | Increase in proliferation and vascularization, decrease in fibrosis in the uterus | [125] |
| Yao, Y. et al. | MMSC-derived exosomes | Rabbit model of intrauterine adhesions | Repair of damaged endometrium by reversing EMT via the TGF-β1/Smad signaling pathway | [126] |
| Zhu, Q. et al. | Exosomes derived from CTF1-modified bone marrow stem cells | Injured rat uterus | Activation of tissue regeneration of the endometrium and myometrium, improvement of endometrial receptivity, stimulating neovascularization | [127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabeeva, G.; Silachev, D.; Vishnyakova, P.; Asaturova, A.; Fatkhudinov, T.; Smetnik, A.; Dumanovskaya, M. The Therapeutic Potential of Multipotent Mesenchymal Stromal Cell—Derived Extracellular Vesicles in Endometrial Regeneration. Int. J. Mol. Sci. 2023, 24, 9431. https://doi.org/10.3390/ijms24119431
Tabeeva G, Silachev D, Vishnyakova P, Asaturova A, Fatkhudinov T, Smetnik A, Dumanovskaya M. The Therapeutic Potential of Multipotent Mesenchymal Stromal Cell—Derived Extracellular Vesicles in Endometrial Regeneration. International Journal of Molecular Sciences. 2023; 24(11):9431. https://doi.org/10.3390/ijms24119431
Chicago/Turabian StyleTabeeva, Gyuzyal, Denis Silachev, Polina Vishnyakova, Alexandra Asaturova, Timur Fatkhudinov, Antonina Smetnik, and Madina Dumanovskaya. 2023. "The Therapeutic Potential of Multipotent Mesenchymal Stromal Cell—Derived Extracellular Vesicles in Endometrial Regeneration" International Journal of Molecular Sciences 24, no. 11: 9431. https://doi.org/10.3390/ijms24119431
APA StyleTabeeva, G., Silachev, D., Vishnyakova, P., Asaturova, A., Fatkhudinov, T., Smetnik, A., & Dumanovskaya, M. (2023). The Therapeutic Potential of Multipotent Mesenchymal Stromal Cell—Derived Extracellular Vesicles in Endometrial Regeneration. International Journal of Molecular Sciences, 24(11), 9431. https://doi.org/10.3390/ijms24119431









