After Ischemic Stroke, Minocycline Promotes a Protective Response in Neurons via the RNA-Binding Protein HuR, with a Positive Impact on Motor Performance
Abstract
:1. Introduction
2. Results
2.1. Characterization of the Peri-Infarct Area following Ischemic Stroke
2.2. Effect of Minocycline on Inflammation in the Peri-Infarct Area following Ischemic Stroke
2.3. Effect of Minocycline on the Level of Motor Performance in Rats after FCI
3. Discussion
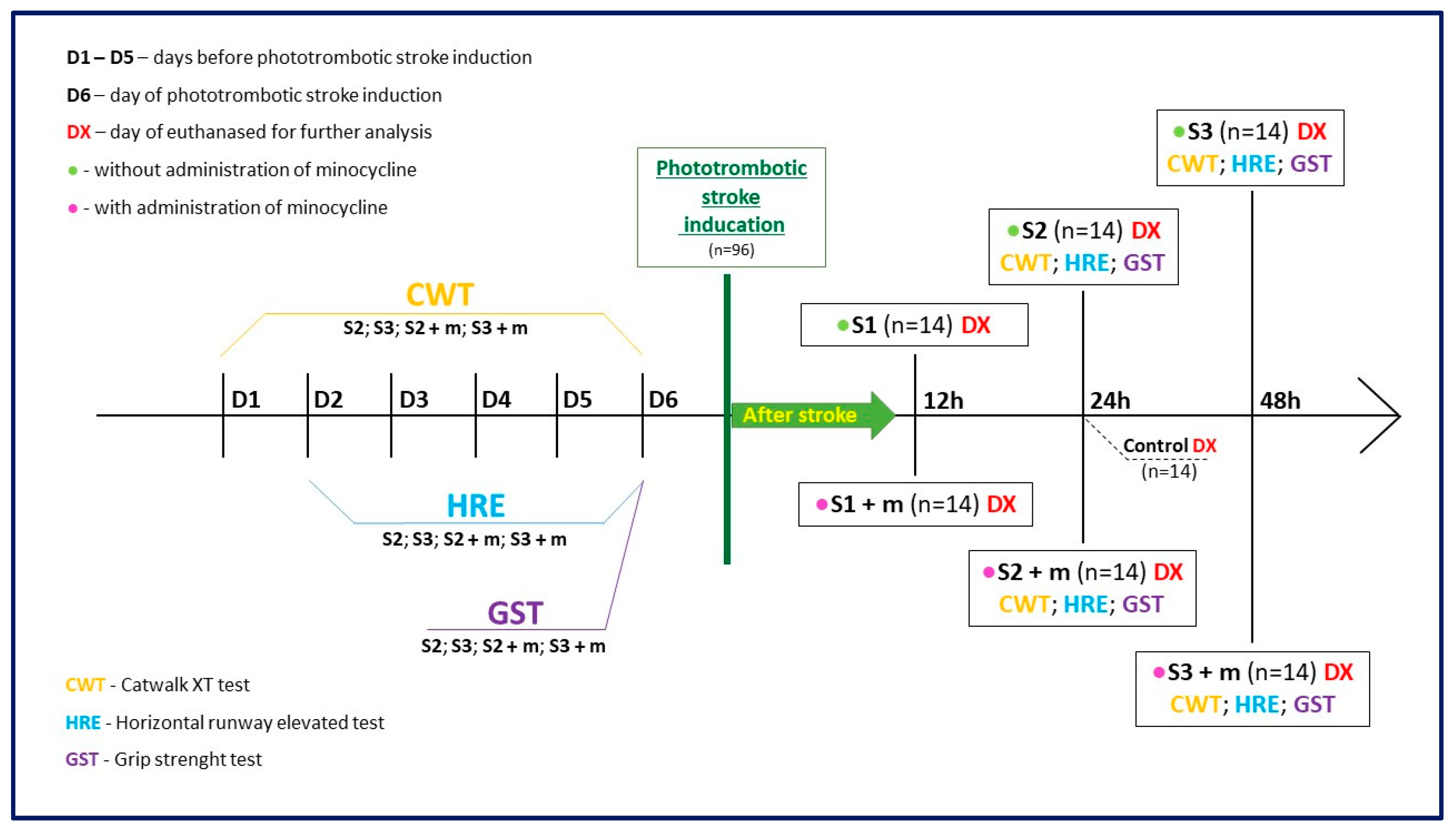
4. Materials and Methods
4.1. Animals
4.2. Animal Model
4.3. Treatment with Minocycline
4.4. Horizontal Runway Elevated Test
4.5. Grip Strength Test
4.6. CatWalk™ XT
4.7. Histological Analysis
4.7.1. Nissl Staining
4.7.2. Immunohistochemistry
4.8. Western Blotting
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BR | the Bengal Rose |
| CWT | CatWalk™ XT |
| D1–D4 | days of training |
| D6 | days of photothrombotic ischemic stroke induction |
| DX | day of euthanasia |
| FCI | focal cerebral ischemia |
| GST | forelimb Grip Strength test |
| HRE | horizontal runway elevated test |
| HSP70 | 70 kilodalton heat shock proteins |
| HuR | human antigen R protein |
| IQR | the quartile range |
| IL-6 | interleukin 6 |
| LE | Long-Evans rats |
| MCAO | Middle Cerebral Artery Occlusion |
| PBS | phosphate-buffered saline |
| TNFα | tumor necrosis factor alpha |
| RBP | RNA-binding proteins |
| RH | right hind paw |
| ROI | region of interesting |
| RF | right front paw |
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report from the American Heart Association. Circulation 2022, 145, e153–e639, Erratum in Circulation 2022, 146, e141. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Lu, X.; Pan, Q.; Wang, B.; Pong U, K.; Yang, Y.; Wang, H.; Lin, S.; Feng, L.; Wang, Y.; et al. Cranial Bone Transport Promotes Angiogenesis, Neurogenesis, and Modulates Meningeal Lymphatic Function in Middle Cerebral Artery Occlusion Rats. Stroke 2022, 53, 1373–1385. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, Regional, and National Burden of Stroke and Its Risk Factors, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Donkor, E.S. Stroke in the 21st Century: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res. Treat. 2018, 2018, 3238165. [Google Scholar]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics—2018 Update: A Report from the American Heart Association. Circulation 2018, 137, E67–E492. [Google Scholar] [CrossRef]
- Song, J.; Zhao, Y.; Yu, C.; Xu, J. DUSP14 Rescues Cerebral Ischemia/Reperfusion (IR) Injury by Reducing Inflammation and Apoptosis via the Activation of Nrf-2. Biochem. Biophys. Res. Commun. 2019, 509, 713–721. [Google Scholar] [CrossRef]
- McMeekin, P.; White, P.; James, M.A.; Price, C.I.; Flynn, D.; Ford, G.A. Estimating the Number of UK Stroke Patients Eligible for Endovascular Thrombectomy. Eur. Stroke J. 2017, 2, 319–326. [Google Scholar] [CrossRef]
- Kim, J.S. TPA Helpers in the Treatment of Acute Ischemic Stroke: Are They Ready for Clinical Use? J. Stroke 2019, 21, 160–174. [Google Scholar] [CrossRef]
- Sun, M.S.; Jin, H.; Sun, X.; Huang, S.; Zhang, F.L.; Guo, Z.N.; Yang, Y. Free Radical Damage in Ischemia-Reperfusion Injury: An Obstacle in Acute Ischemic Stroke after Revascularization Therapy. Oxid. Med. Cell. Longev. 2018, 2018, 3804979. [Google Scholar] [CrossRef]
- Dirnagl, U.; Iadecola, C.; Moskowitz, M.A. Pathobiology of Ischaemic Stroke: An Integrated View. Trends Neurosci. 1999, 22, 391–397. [Google Scholar] [CrossRef]
- Fluri, F.; Schuhmann, M.K.; Kleinschnitz, C. Animal Models of Ischemic Stroke and Their Application in Clinical Research. Drug Des. Dev. Ther. 2015, 9, 3445–3454. [Google Scholar] [CrossRef]
- Kurniawan, N.A.; Grimbergen, J.; Koopman, J.; Koenderink, G.H. Factor XIII Stiffens Fibrin Clots by Causing Fiber Compaction. J. Thromb. Haemost. 2014, 12, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Fagan, S.C.; Cronic, L.E.; Hess, D.C. Minocycline Development for Acute Ischemic Stroke. Transl. Stroke Res. 2011, 2, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Yew, W.P.; Djukic, N.D.; Jayaseelan, J.S.P.; Walker, F.R.; Roos, K.A.A.; Chataway, T.K.; Muyderman, H.; Sims, N.R. Early Treatment with Minocycline Following Stroke in Rats Improves Functional Recovery and Differentially Modifies Responses of Peri-Infarct Microglia and Astrocytes. J. Neuroinflamm. 2019, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Hoppen, M.; Strecker, J.K.; Diederich, K.; Schäbitz, W.R.; Schilling, M.; Minnerup, J. Photochemically Induced Ischemic Stroke in Rats. Exp. Transl. Stroke Med. 2012, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Ostrova, I.V.; Kalabushev, S.N.; Ryzhkov, I.A.; Tsokolaeva, Z.I. A Novel Thromboplastin-Based Rat Model of Ischemic Stroke. Brain Sci. 2021, 11, 1475. [Google Scholar] [CrossRef]
- Pawletko, K.; Jędrzejowska-Szypułka, H.; Bogus, K.; Pascale, A.; Fahmideh, F.; Marchesi, N.; Grajoszek, A.; Olakowska, E.; Barski, J.J. A Novel Improved Thromboembolism-Based Rat Stroke Model That Meets the Latest Standards in Preclinical Studies. Brain Sci. 2022, 12, 1671. [Google Scholar] [CrossRef]
- Benowitz, L.I.; Carmichael, S.T. Promoting Axonal Rewiring to Improve Outcome after Stroke. Neurobiol. Dis. 2010, 37, 259–266. [Google Scholar] [CrossRef]
- Uzdensky, A.B. Photothrombotic Stroke as a Model of Ischemic Stroke. Transl. Stroke Res. 2018, 9, 437–451. [Google Scholar] [CrossRef]
- Lasek-Bal, A.; Jedrzejowska-Szypulka, H.; Student, S.; Warsz-Wianecka, A.; Zareba, K.; Puz, P.; Bal, W.; Pawletko, K.; Lewin-Kowalik, J. The Importance of Selected Markers of Inflammation and Blood-Brain Barrier Damage for Short-Term Ischemic Stroke Prognosis. J. Physiol. Pharmacol. 2019, 70, 209–217. [Google Scholar] [CrossRef]
- Chen, Y.; Won, S.J.; Xu, Y.; Swanson, R.A. Targeting Microglial Activation in Stroke Therapy: Pharmacological Tools and Gender Effects. Curr. Med. Chem. 2014, 21, 2146–2155. [Google Scholar] [CrossRef]
- Yang, C.; Hawkins, K.E.; Doré, S.; Candelario-Jalil, X.E. Neuroinflammatory Mechanisms of Blood-Brain Barrier Damage in Ischemic Stroke. Am. J. Physiol. Cell Physiol. 2019, 316, 135–153. [Google Scholar] [CrossRef]
- Ramiro, L.; Simats, A.; García-Berrocoso, T.; Montaner, J. Inflammatory Molecules Might Become Both Biomarkers and Therapeutic Targets for Stroke Management. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418789340. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, N.; Patial, S. Tumor Necrosis Factor-α Signaling in Macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sato, K.; Hayashi, T.; Omori, N.; Nagano, I.; Kato, S.; Horiuchi, S.; Abe, K. Extension of Ischemic Therapeutic Time Window by a Free Radical Scavenger, Edaravone, Reperfused with TPA in Rat Brain. Neurol. Res. 2004, 26, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, C.; Brihmat, N.; Castel-Lacanal, E.; Le Friec, A.; Barbieux-Guillot, M.; Raposo, N.; Pariente, J.; Viguier, A.; Simonetta-Moreau, M.; Albucher, J.F.; et al. Post-Stroke Remodeling Processes in Animal Models and Humans. J. Cereb. Blood Flow Metab. 2020, 40, 3–22. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Wang, Y.; Yang, G.Y. The Biphasic Function of Microglia in Ischemic Stroke. Prog. Neurobiol. 2017, 157, 247–272. [Google Scholar] [CrossRef]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and Foe for Ischemic Stroke. J. Neuroinflamm. 2019, 16, 142. [Google Scholar] [CrossRef]
- Alano, C.C.; Kauppinen, T.M.; Valls, A.V.; Swanson, R.A. Minocycline Inhibits Poly(ADP-Ribose) Polymerase-1 at Nanomolar Concentrations. Proc. Natl. Acad. Sci. USA 2006, 103, 9685–9690. [Google Scholar] [CrossRef]
- Abbaszadeh, A.; Darabi, S.; Hasanvand, A.; Amini-Khoei, H.; Abbasnezhad, A.; Choghakhori, R.; Aaliehpour, A. Minocycline through Attenuation of Oxidative Stress and Inflammatory Response Reduces the Neuropathic Pain in a Rat Model of Chronic Constriction Injury. Iran. J. Basic Med. Sci. 2018, 21, 138–144. [Google Scholar] [CrossRef]
- Soliman, S.; Ishrat, T.; Fouda, A.Y.; Patel, A.; Pillai, B.; Fagan, S.C. Sequential Therapy with Minocycline and Candesartan Improves Long-Term Recovery After Experimental Stroke. Transl. Stroke Res. 2015, 6, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Faheem, H.; Mansour, A.; Elkordy, A.; Rashad, S.; Shebl, M.; Madi, M.; Elwy, S.; Niizuma, K.; Tominaga, T. Neuroprotective Effects of Minocycline and Progesterone on White Matter Injury after Focal Cerebral Ischemia. J. Clin. Neurosci. 2019, 64, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Liang, J.; Wang, J.; Kolattukudy, P.E. MCP-Induced Protein 1 Mediates the Minocycline-Induced Neuroprotection against Cerebral Ischemia/Reperfusion Injury in Vitro and in Vivo. J. Neuroinflamm. 2015, 12, 39. [Google Scholar] [CrossRef]
- Yrjänheikki, J.; Tikka, T.; Keinänen, R.; Goldsteins, G.; Chan, P.H.; Koistinaho, J. A Tetracycline Derivative, Minocycline, Reduces Inflammation and Protects against Focal Cerebral Ischemia with a Wide Therapeutic Window. Proc. Natl. Acad. Sci. USA 1999, 96, 13496–13500. [Google Scholar] [CrossRef] [PubMed]
- Marques, B.L.; Carvalho, G.A.; Freitas, E.M.M.; Chiareli, R.A.; Barbosa, T.G.; Di Araújo, A.G.P.; Nogueira, Y.L.; Ribeiro, R.I.; Parreira, R.C.; Vieira, M.S.; et al. The Role of Neurogenesis in Neurorepair after Ischemic Stroke. Semin. Cell Dev. Biol. 2019, 95, 98–110. [Google Scholar] [CrossRef]
- Stone, M.J.; Hayward, J.A.; Huang, C.; Huma, Z.E.; Sanchez, J. Mechanisms of Regulation of the Chemokine-Receptor Network. Int. J. Mol. Sci. 2017, 18, 342. [Google Scholar] [CrossRef]
- Kondo, M.; Okazaki, H.; Nakayama, K.; Hohjoh, H.; Nakagawa, K.; Segi-Nishida, E.; Hasegawa, H. Characterization of Astrocytes in the Minocycline-Administered Mouse Photothrombotic Ischemic Stroke Model. Neurochem. Res. 2022, 47, 2839–2855. [Google Scholar] [CrossRef]
- Offner, H.; Subramanian, S.; Parker, S.M.; Afentoulis, M.E.; Vandenbark, A.A.; Hurn, P.D. Experimental Stroke Induces Massive, Rapid Activation of the Peripheral Immune System. J. Cereb. Blood Flow Metab. 2006, 26, 654–665. [Google Scholar] [CrossRef]
- Murakami, Y.; Saito, K.; Hara, A.; Zhu, Y.; Sudo, K.; Niwa, M.; Fujii, H.; Wada, H.; Ishiguro, H.; Mori, H.; et al. Increases in Tumor Necrosis Factor-α Following Transient Global Cerebral Ischemia Do Not Contribute to Neuron Death in Mouse Hippocampus. J. Neurochem. 2005, 93, 1616–1622. [Google Scholar] [CrossRef]
- Van Molle, W.; Wielockx, B.; Mahieu, T.; Takada, M.; Taniguchi, T.; Sekikawa, K.; Libert, C. HSP70 Protects against TNF-Induced Lethal Inflammatory Shock. Immunity 2002, 16, 685–695. [Google Scholar] [CrossRef]
- Zheng, Z.; Yenari, M.A. Post-Ischemic Inflammation: Molecular Mechanisms and Therapeutic Implications. Neurol. Res. 2004, 26, 884–892. [Google Scholar] [CrossRef]
- Abdelmohsen, K.; Kuwano, Y.; Kim, H.H.; Gorospe, M. Posttranscriptional Gene Regulation by RNA-Binding Proteins during Oxidative Stress: Implications for Cellular Senescence. Biol. Chem. 2008, 389, 243–255. [Google Scholar] [CrossRef]
- Kim, J.Y.; Han, Y.; Lee, J.E.; Yenari, M.A. The 70-KDa Heat Shock Protein (Hsp70) as a Therapeutic Target for Stroke. Expert Opin. Ther. Targets 2018, 22, 191–199. [Google Scholar] [CrossRef]
- Giffard, R.G.; Yenari, M.A. Many Mechanisms for Hsp70 Protection from Cerebral Ischemia. J. Neurosurg. Anesthesiol. 2003, 16, 53–61. [Google Scholar] [CrossRef]
- Pascale, A.; Govoni, S. The Complex World of Post-Transcriptional Mechanisms: Is Their Deregulation a Common Link for Diseases? Focus on ELAV-like RNA-Binding Proteins. Cell. Mol. Life Sci. 2012, 69, 501–517. [Google Scholar] [CrossRef]
- Yi, J.; Chang, N.; Liu, X.; Guo, G.; Xue, L.; Tong, T.; Gorospe, M.; Wang, W. Reduced Nuclear Export of HuR MRNA by HuR Is Linked to the Loss of HuR in Replicative Senescence. Nucleic Acids Res. 2009, 38, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Doller, A.; Akool, E.-S.; Huwiler, A.; Müller, R.; Radeke, H.H.; Pfeilschifter, J.; Eberhardt, W. Posttranslational Modification of the AU-Rich Element Binding Protein HuR by Protein Kinase Cδ Elicits Angiotensin II-Induced Stabilization and Nuclear Export of Cyclooxygenase 2 MRNA. Mol. Cell. Biol. 2008, 28, 2608–2625. [Google Scholar] [CrossRef] [PubMed]
- Gallouzi, I.-E.; Brennan, C.M.; Stenberg, M.G.; Swanson, M.S.; Eversole, A.; Maizels, N.; Steitz, J.A. HuR Binding to Cytoplasmic MRNA Is Perturbed by Heat Shock. Proc. Natl. Acad. Sci. USA 2000, 97, 3073–3078. [Google Scholar] [CrossRef]
- Gallouzi, I.-E.; Brennan, C.M.; Steitz, J.A. Protein Ligands Mediate the CRM1-Dependent Export of HuR in Response to Heat Shock. RNA 2001, 7, 1348–1361. [Google Scholar] [CrossRef] [PubMed]
- Amadio, M.; Scapagnini, G.; Laforenza, U.; Intrieri, M.; Romeo, L.; Govoni, S.; Pascale, A. Post-Transcriptional Regulation of HSP70 Expression Following Oxidative Stress in SH-SY5Y Cells: The Potential Involvement of the RNA-Binding Protein HuR. Curr. Pharm. Des. 2008, 14, 2651–2658. [Google Scholar] [CrossRef] [PubMed]
- Jamison, J.T.; Kayali, F.; Rudolph, J.; Marshall, M.; Kimball, S.R.; Degracia, D.J. Persistent Redistribution of Poly-Adenylated MRNAs Correlates with Translation Arrest and Cell Death Following Global Brain Ischemia and Reperfusion. Neuroscience 2008, 154, 504–520. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.G.; Collinge, M.; Ramgolam, V.; Ayalon, O.; Fan, X.C.; Pardi, R.; Bender, J.R. LFA-1-Dependent HuR Nuclear Export and Cytokine MRNA Stabilization in T Cell Activation. J. Immunol. 2006, 176, 2105–2113. [Google Scholar] [CrossRef]
- Sung, S.C.; Kim, K.; Lee, K.A.; Choi, K.H.; Kim, S.M.; Son, Y.H.; Moon, Y.S.; Eo, S.K.; Rhim, B.Y. 7-Ketocholesterol Upregulates Interleukin-6 via Mechanisms That Are Distinct from Those of Tumor Necrosis Factor-α, in Vascular Smooth Muscle Cells. J. Vasc. Res. 2008, 46, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Grammatikakis, I.; Abdelmohsen, K.; Gorospe, M. Posttranslational Control of HuR Function. Wiley Interdiscip. Rev. RNA 2017, 8, e1372. [Google Scholar] [CrossRef]
- Srikantan, S.; Gorospe, M. HuR Function in Disease. Front. Biosci. Landmark Ed. 2012, 17, 189. [Google Scholar] [CrossRef] [PubMed]
- Schmider, E.; Ziegler, M.; Danay, E.; Beyer, L.; Bühner, M. Is It Really Robust? Reinvestigating the Robustness of ANOVA against Violations of the Normal Distribution Assumption. Methodology 2010, 6, 147–151. [Google Scholar] [CrossRef]
- Blanca, M.J.; Alarcón, R.; Arnau, J.; Bono, R.; Bendayan, R. Datos No Normales: ¿es El ANOVA Una Opción Válida? Psicothema 2017, 29, 552–557. [Google Scholar] [CrossRef]
- Liu, N.W.; Ke, C.C.; Zhao, Y.; Chen, Y.A.; Chan, K.C.; Tan, D.T.W.; Lee, J.S.; Chen, Y.Y.; Hsu, T.W.; Hsieh, Y.J.; et al. Evolutional Characterization of Photochemically Induced Stroke in Rats: A Multimodality Imaging and Molecular Biological Study. Transl. Stroke Res. 2017, 8, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhou, M.; Li, Y.; Li, Y.; Hua, Y.; Fan, Y. Minocycline Promotes Functional Recovery in Ischemic Stroke by Modulating Microglia Polarization through STAT1/STAT6 Pathways. Biochem. Pharmacol. 2021, 186, 114464. [Google Scholar] [CrossRef]
- Tanaka, M.; Ishihara, Y.; Mizuno, S.; Ishida, A.; Vogel, C.F.; Tsuji, M.; Yamazaki, T.; Itoh, K. Progression of Vasogenic Edema Induced by Activated Microglia under Permanent Middle Cerebral Artery Occlusion. Biochem. Biophys. Res. Commun. 2018, 496, 582–587. [Google Scholar] [CrossRef]
- Lu, Y.; Xiao, G.; Luo, W. Minocycline Suppresses NLRP3 Inflammasome Activation in Experimental Ischemic Stroke. Neuroimmunomodulation 2017, 23, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Park, S.I.; Park, S.K.; Jang, K.S.; Han, Y.M.; Kim, C.H.; Oh, S.J. Preischemic Neuroprotective Effect of Minocycline and Sodium Ozagrel on Transient Cerebral Ischemic Rat Model. Brain Res. 2015, 1599, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Lakhan, S.E.; Kirchgessner, A.; Hofer, M. Inflammatory Mechanisms in Ischemic Stroke: Therapeutic Approaches. J. Transl. Med. 2009, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Dugue, R.; Nath, M.; Dugue, A.; Barone, F.C. Roles of Pro- and Anti-Inflammatory Cytokines in Traumatic Brain Injury and Acute Ischemic Stroke. In Mechanisms of Neuroinflammation; IntechOpen: London, UK, 2017. [Google Scholar]
- Maida, C.D.; Norrito, R.L.; Daidone, M.; Tuttolomondo, A.; Pinto, A. Neuroinflammatory Mechanisms in Ischemic Stroke: Focus on Cardioembolic Stroke, Background, and Therapeutic Approaches. Int. J. Mol. Sci. 2020, 21, 6454. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Yang, G.; Li, G. Inflammatory Mechanisms in Ischemic Stroke: Role of Inflammatory Cells. J. Leukoc. Biol. 2010, 87, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Ceulemans, A.G.; Zgavc, T.; Kooijman, R.; Hachimi-Idrissi, S.; Sarre, S.; Michotte, Y. The Dual Role of the Neuroinflammatory Response after Ischemic Stroke: Modulatory Effects of Hypothermia. J. Neuroinflamm. 2010, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Tan, G.; Zhang, S.; Zhu, H.; Liu, F.; Huang, C.; Zhang, F.; Wang, Z. Minocycline Reduces Reactive Gliosis in the Rat Model of Hydrocephalus. BMC Neurosci. 2012, 13, 148. [Google Scholar] [CrossRef]
- Emerich, D.F.; Dean, R.L.; Bartus, R.T. The Role of Leukocytes Following Cerebral Ischemia: Pathogenic Variable or Bystander Reaction to Emerging Infarct? Exp. Neurol. 2002, 173, 168–181. [Google Scholar] [CrossRef]
- Camargos, Q.M.; Silva, B.C.; Silva, D.G.; de Brito Toscano, E.C.; da Silva Oliveira, B.; Bellozi, P.M.Q.; de Oliveira Jardim, B.L.; Vieira, É.L.M.; de Oliveira, A.C.P.; Sousa, L.P.; et al. Minocycline Treatment Prevents Depression and Anxiety-like Behaviors and Promotes Neuroprotection after Experimental Ischemic Stroke. Brain Res. Bull. 2020, 155, 1–10. [Google Scholar] [CrossRef]
- Naderi, Y.; Sabetkasaei, M.; Parvardeh, S.; Zanjani, T.M. Neuroprotective Effects of Pretreatment with Minocycline on Memory Impairment Following Cerebral Ischemia in Rats. Behav. Pharmacol. 2017, 28, 214–222. [Google Scholar] [CrossRef]
- Pfau, M.L.; Russo, S.J. Neuroinflammation Regulates Cognitive Impairment in Socially Defeated Mice. Trends Neurosci. 2016, 39, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yi, Q.; Liu, G.; Shen, X.; Xuan, L.; Tian, Y. Cerebral White Matter Injury and Damage to Myelin Sheath Following Whole-Brain Ischemia. Brain Res. 2013, 1495, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Clark, R.K.; McDonnell, P.C.; Young, P.R.; White, R.F.; Barone, F.C.; Feuerstein, G.Z. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke 1994, 25, 1481–1488. [Google Scholar] [CrossRef]
- Botchkina, G.I.; Meistrell, M.E.; Botchkina, I.L.; Tracey, K.J. Expression of TNF and TNF Receptors (P55 and P75) in the Rat Brain after Focal Cerebral Ischemia. Mol. Med. 1997, 3, 765–781. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Zhao, H.; Cao, X.; Hao, J.; Zhang, H.; Liu, Y.; Zhu, M.; Fan, L.; Weng, L.; Qian, L.; et al. Double-Negative T Cells Remarkably Promote Neuroinflammation after Ischemic Stroke. Proc. Natl. Acad. Sci. USA 2019, 116, 5558–5563. [Google Scholar] [CrossRef] [PubMed]
- Sotgiu, S.; Zanda, B.; Marchetti, B.; Fois, M.L.; Arru, G.; Pes, G.M.; Salaris, F.S.; Arru, A.; Pirisi, A.; Rosati, G. Inflammatory Biomarkers in Blood of Patients with Acute Brain Ischemia. Eur. J. Neurol. 2006, 13, 505–513. [Google Scholar] [CrossRef]
- Li, J.; Hao, M.; Liu, M.; Wang, J.; Gao, D.; Han, S.; Yu, D. Transcutaneous Electrical Acupoint Stimulation Pretreatment Alleviates Cerebral Ischemia–Reperfusion Injury in Rats by Modulating Microglia Polarization and Neuroinflammation Through Nrf2/HO-1 Signaling Pathway. Neurochem. Res. 2023, 48, 862–873. [Google Scholar] [CrossRef]
- Yang, Y.; Salayandia, V.M.; Thompson, J.F.; Yang, L.Y.; Estrada, E.Y.; Yang, Y. Attenuation of Acute Stroke Injury in Rat Brain by Minocycline Promotes Blood-Brain Barrier Remodeling and Alternative Microglia/Macrophage Activation during Recovery. J. Neuroinflamm. 2015, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Biagioni, F.; Mastroiacovo, F.; Lenzi, P.; Puglisi-Allegra, S.; Busceti, C.L.; Ryskalin, L.; Ferese, R.; Bucci, D.; Frati, A.; Nicoletti, F.; et al. The Autophagy-Related Organelle Autophagoproteasome Is Suppressed within Ischemic Penumbra. Int. J. Mol. Sci. 2021, 22, 10364. [Google Scholar] [CrossRef]
- Liu, F.; Lu, Z.; Li, Z.; Wang, S.; Zhuang, L.; Hong, M.; Huang, K. Electroacupuncture Improves Cerebral Ischemic Injury by Enhancing the Epo-Jak2-Stat5 Pathway in Rats. Neuropsychiatr. Dis. Treat. 2021, 17, 2489–2498. [Google Scholar] [CrossRef]
- Kim, H.J.; Wei, Y.; Wojtkiewicz, G.R.; Lee, J.Y.; Moskowitz, M.A.; Chen, J.W. Reducing Myeloperoxidase Activity Decreases Inflammation and Increases Cellular Protection in Ischemic Stroke. J. Cereb. Blood Flow Metab. 2019, 39, 1864–1877. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Yang, W.; Ren, Z.; Zhang, H.; Shi, D.; Li, Y.; Yu, Z.; Guo, Q.; Yang, G.; Gu, Y.; et al. Neuroprotective and Angiogenesis Effects of Levetiracetam Following Ischemic Stroke in Rats. Front. Pharmacol. 2021, 12, 638209. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Pu, J.; Wang, Z.; Wang, R.; Guo, Z.; Liu, C.; Sun, J.; Gao, L.; Zhou, R. Effects of Minocycline on the Expression of NGF and HSP70 and Its Neuroprotection Role Following Intracerebral Hemorrhage in Rats. J. Biomed. Res. 2011, 25, 292–298. [Google Scholar]
- Shi, W.; Wang, Z.; Pu, J.; Wang, R.; Guo, Z.; Liu, C.; Sun, J.; Gao, L.; Zhou, R. Changes of Blood-Brain Barrier Permeability Following Intracerebral Hemorrhage and the Therapeutic Effect of Minocycline in Rats. In Early Brain Injury or Cerebral Vasospasm; Acta Neurochirurgica, Supplementum; Springer: Wien, Austria, 2011; Volume 110, pp. 61–67. [Google Scholar]
- Bollaerts, I.; Van Houcke, J.; Andries, L.; De Groef, L.; Moons, L. Neuroinflammation as Fuel for Axonal Regeneration in the Injured Vertebrate Central Nervous System. Mediat. Inflamm. 2017, 2017, 9478542. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Badoni, H.; Abu-Izneid, T.; Olatunde, A.; Rahman, M.M.; Painuli, S.; Semwal, P.; Wilairatana, P.; Mubarak, M.S. Neuroinflammatory Markers: Key Indicators in the Pathology of Neurodegenerative Diseases. Molecules 2022, 27, 3194. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Selvakumar, G.P.; Zaheer, S.; Ahmed, M.E.; Raikwar, S.P.; Zahoor, H.; Saeed, D.; Natteru, P.A.; Iyer, S.; et al. Brain and Peripheral Atypical Inflammatory Mediators Potentiate Neuroinflammation and Neurodegeneration. Front. Cell. Neurosci. 2017, 11, 216. [Google Scholar] [CrossRef]
- Lucas, S.M.; Rothwell, N.J.; Gibson, R.M. The Role of Inflammation in CNS Injury and Disease. Br. J. Pharmacol. 2006, 147, S232–S240. [Google Scholar] [CrossRef]
- Li, S.; Hua, X.; Zheng, M.; Wu, J.; Ma, Z.; Xing, X.; Ma, J.; Zhang, J.; Shan, C.; Xu, J. PLXNA2 Knockdown Promotes M2 Microglia Polarization through MTOR/STAT3 Signaling to Improve Functional Recovery in Rats after Cerebral Ischemia/Reperfusion Injury. Exp. Neurol. 2021, 346, 113854. [Google Scholar] [CrossRef]
- Jickling, G.C.; Sharp, F.R. Improving the Translation of Animal Ischemic Stroke Studies to Humans. Metab. Brain Dis. 2015, 30, 461–467. [Google Scholar] [CrossRef]
- Narayan, S.K.; Cherian, S.G.; Phaniti, P.B.; Chidambaram, S.B.; Vasanthi, A.H.R.; Arumugam, M. Preclinical Animal Studies in Ischemic Stroke: Challenges and Some Solutions. Anim. Model. Exp. Med. 2021, 4, 104–115. [Google Scholar] [CrossRef]
- Boboc, I.K.S.; Rotaru-Zavaleanu, A.D.; Calina, D.; Albu, C.V.; Catalin, B.; Turcu-Stiolica, A. A Preclinical Systematic Review and Meta-Analysis of Behavior Testing in Mice Models of Ischemic Stroke. Life 2023, 13, 567. [Google Scholar] [CrossRef]
- Watson, B.D.; Dietrich, W.D.; Busto, R.; Wachtel, M.S.; Ginsberg, M.D. Induction of Reproducible Brain Infarction by Photochemically Initiated Thrombosis. Ann. Neurol. 1985, 17, 497–504. [Google Scholar] [CrossRef]
- Hoda, N.; Fagan, S.C.; Khan, M.B.; Vaibhav, K.; Chaudhary, A.; Wang, P.; Dhandapani, K.M.; Waller, J.L.; Hess, D.C. A 2 × 2 Factorial Design for the Combination Therapy of Minocycline and Remote Ischemic Perconditioning: Efficacy in a Preclinical Trial in Murine Thromboembolic Stroke Model. Exp. Transl. Stroke Med. 2014, 6, 10. [Google Scholar] [CrossRef]
- Metz, G.A.; Whishaw, I.Q. The Ladder Rung Walking Task: A Scoring System and Its Practical Application. J. Vis. Exp. 2009, 28, e1204. [Google Scholar] [CrossRef]
- Metz, G.A.; Whishaw, I.Q. Cortical and Subcortical Lesions Impair Skilled Walking in the Ladder Rung Walking Test: A New Task to Evaluate Fore- and Hindlimb Stepping, Placing, and Co-Ordination. J. Neurosci. Methods 2002, 115, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Alaverdashvili, M.; Moon, S.K.; Beckman, C.D.; Virag, A.; Whishaw, I.Q. Acute but Not Chronic Differences in Skilled Reaching for Food Following Motor Cortex Devascularization vs. Photothrombotic Stroke in the Rat. Neuroscience 2008, 157, 297–308. [Google Scholar] [CrossRef]
- Qian, H.Z.; Zhang, H.; Yin, L.L.; Zhang, J.J. Postischemic Housing Environment on Cerebral Metabolism and Neuron Apoptosis after Focal Cerebral Ischemia in Rats. Curr. Med. Sci. 2018, 38, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Ishrat, T.; Pillai, B.; Soliman, S.; Fouda, A.Y.; Kozak, A.; Johnson, M.H.; Ergul, A.; Fagan, S.C. Low-Dose Candesartan Enhances Molecular Mediators of Neuroplasticity and Subsequent Functional Recovery After Ischemic Stroke in Rats. Mol. Neurobiol. 2015, 51, 1542–1553. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, P.; Ali, M.; Tabassum, H.; Parvez, S. Post-Ischemic Administration of Dopamine D2 Receptor Agonist Reduces Cell Death by Activating Mitochondrial Pathway Following Ischemic Stroke. Life Sci. 2020, 261, 118349. [Google Scholar] [CrossRef]
- Li, C.; Sun, R.; Chen, J.; Hong, J.; Sun, J.; Zeng, Y.; Zhang, X.; Dou, Z.; Wen, H. Different Training Patterns at Recovery Stage Improve Cognitive Function in Ischemic Stroke Rats through Regulation of the Axonal Growth Inhibitor Pathway. Behav. Brain Res. 2022, 421, 113730. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, C.; Yang, X.; Liu, Y.; Yang, H.; Yuan, L.; Liu, Y.; Sun, S.; Yang, J. Pseudoginsenoside-F11 Protects against Transient Cerebral Ischemia Injury in Rats Involving Repressing Calcium Overload. Neuroscience 2019, 411, 86–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bontempi, B.; Hong, S.M.; Mehta, K.; Weinstein, P.R.; Abrams, G.M.; Liu, J. A Comprehensive Analysis of Gait Impairment after Experimental Stroke and the Therapeutic Effect of Environmental Enrichment in Rats. J. Cereb. Blood Flow Metab. 2008, 28, 1936–1950. [Google Scholar] [CrossRef] [PubMed]
- Encarnacion, A.; Horie, N.; Keren-Gill, H.; Bliss, T.M.; Steinberg, G.K.; Shamloo, M. Long-Term Behavioral Assessment of Function in an Experimental Model for Ischemic Stroke. J. Neurosci. Methods 2011, 196, 247–257. [Google Scholar] [CrossRef]
- Fan, Q.Y.; Liu, J.J.; Zhang, G.L.; Wu, H.Q.; Zhang, R.; Zhan, S.Q.; Liu, N. Inhibition of SNK-SPAR Signaling Pathway Promotes the Restoration of Motor Function in a Rat Model of Ischemic Stroke. J. Cell. Biochem. 2018, 119, 1093–1110. [Google Scholar] [CrossRef]
- Wu, J.; Lin, B.; Liu, W.; Huang, J.; Shang, G.; Lin, Y.; Wang, L.; Chen, L.; Tao, J. Roles of Electro-Acupuncture in Glucose Metabolism as Assessed by 18F-FDG/PET Imaging and AMPKα Phosphorylation in Rats with Ischemic Stroke. Int. J. Mol. Med. 2017, 40, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Orgah, J.O.; Ren, J.; Liu, X.; Orgah, E.A.; Gao, X.M.; Zhu, Y. Danhong Injection Facilitates Recovery of Post-Stroke Motion Deficit via Parkin-Enhanced Mitochondrial Function. Restor. Neurol. Neurosci. 2019, 37, 375–395. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lei, J.F.; Ouyang, J.Y.; Li, M.Z.; Zhan, Y.; Feng, X.F.; Lu, Y.; Li, M.C.; Wang, L.; Zou, H.Y.; et al. Effect of Neurorepair for Motor Functional Recovery Enhanced by Total Saponins from Trillium Tschonoskii Maxim. Treatment in a Rat Model of Focal Ischemia. Front. Pharmacol. 2021, 12, 763181. [Google Scholar] [CrossRef]
- de Prisco, N.; Chemiakine, A.; Lee, W.; Botta, S.; Gennarino, V.A. Protocol to Assess the Effect of Disease-Driving Variants on Mouse Brain Morphology and Primary Hippocampal Neurons. STAR Protoc. 2022, 3, 101244. [Google Scholar] [CrossRef]
- Schroeder, A.B.; Dobson, E.T.A.; Rueden, C.T.; Tomancak, P.; Jug, F.; Eliceiri, K.W. The ImageJ Ecosystem: Open-Source Software for Image Visualization, Processing, and Analysis. Protein Sci. 2021, 30, 234–249. [Google Scholar] [CrossRef]







| Antibody | Abbreviation | Type | Company | Catalog Number | Dilution | Order |
|---|---|---|---|---|---|---|
| HuR/ELAV1 | HuR | mouse monoclonal | Santa Cruz Biotechnology | sc-5261 | for IHCP we used 1:1000 | primary antibodies |
| Neuronal Marker | NeuN | rabbit monoclonal | Abcam | ab177487 | for IHCP we used 1:1000 | primary antibodies |
| Anti-TNFα | TNFα | mouse monoclonal | Santa Cruz Biotechnology | sc-52746 | for IHCP we used 1:1000 | primary antibodies |
| Anti-Hsp70 | HSP70 | mouse monoclonal | Abcam | ab2787S | for IHCP we used 1:500 | primary antibodies |
| HuR/ELAV1 | HuR | mouse monoclonal | Santa Cruz Biotechnology | sc-5261 | for WB we used 1:1000 | primary antibodies |
| Anti-TNFα | TNFα | rabbit monoclonal | Abcam | ab205587 | for WB we uded 1:500 | primary antibodies |
| HSC70/HSP70 | HSP70 | mouse monoclonal | Santa Cruz Biotechnology | sc-24 | for WB we uded 1:500 | primary antibodies |
| Anti-α-Tubulin | α-Tubulin | mouse monoclonal | Sigma-Aldrich | T0198 | for WB we udes 1:1000 | primary antibodies |
| Goat anti-mouse Alexa Fluor® 488 | green Alexa | goat anti-mouse IgG | Abcam | ab150113 | for IHCP we used 1:500 | secondary antibodies |
| Goat anti-rabbit Alexa Fluor® 594 | red Alexa | goat anti-rabbit IgG | Abcam | ab150080 | for IHCP we used 1:500 | secondary antibodies |
| Goat anti-mouse IgG-HRP | anti mouse | goat anti-mouse IgG | Sigma-Aldrich | A4416 | for WB we used 1:3000 | secondary antibodies |
| Goat anti-rabbit IgG-HRP | anti rabbit | goat anti-rabbit IgG | Merck Millipore | AP156P | for WB we used 1:3000 | secondary antibodies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawletko, K.; Jędrzejowska-Szypułka, H.; Bogus, K.; Pascale, A.; Fahmideh, F.; Marchesi, N.; Grajoszek, A.; Gendosz de Carrillo, D.; Barski, J.J. After Ischemic Stroke, Minocycline Promotes a Protective Response in Neurons via the RNA-Binding Protein HuR, with a Positive Impact on Motor Performance. Int. J. Mol. Sci. 2023, 24, 9446. https://doi.org/10.3390/ijms24119446
Pawletko K, Jędrzejowska-Szypułka H, Bogus K, Pascale A, Fahmideh F, Marchesi N, Grajoszek A, Gendosz de Carrillo D, Barski JJ. After Ischemic Stroke, Minocycline Promotes a Protective Response in Neurons via the RNA-Binding Protein HuR, with a Positive Impact on Motor Performance. International Journal of Molecular Sciences. 2023; 24(11):9446. https://doi.org/10.3390/ijms24119446
Chicago/Turabian StylePawletko, Katarzyna, Halina Jędrzejowska-Szypułka, Katarzyna Bogus, Alessia Pascale, Foroogh Fahmideh, Nicoletta Marchesi, Aniela Grajoszek, Daria Gendosz de Carrillo, and Jarosław Jerzy Barski. 2023. "After Ischemic Stroke, Minocycline Promotes a Protective Response in Neurons via the RNA-Binding Protein HuR, with a Positive Impact on Motor Performance" International Journal of Molecular Sciences 24, no. 11: 9446. https://doi.org/10.3390/ijms24119446
APA StylePawletko, K., Jędrzejowska-Szypułka, H., Bogus, K., Pascale, A., Fahmideh, F., Marchesi, N., Grajoszek, A., Gendosz de Carrillo, D., & Barski, J. J. (2023). After Ischemic Stroke, Minocycline Promotes a Protective Response in Neurons via the RNA-Binding Protein HuR, with a Positive Impact on Motor Performance. International Journal of Molecular Sciences, 24(11), 9446. https://doi.org/10.3390/ijms24119446







