Association of Vascular Endothelial Growth Factors (VEGFs) with Recurrent Miscarriage: A Systematic Review of the Literature
Abstract
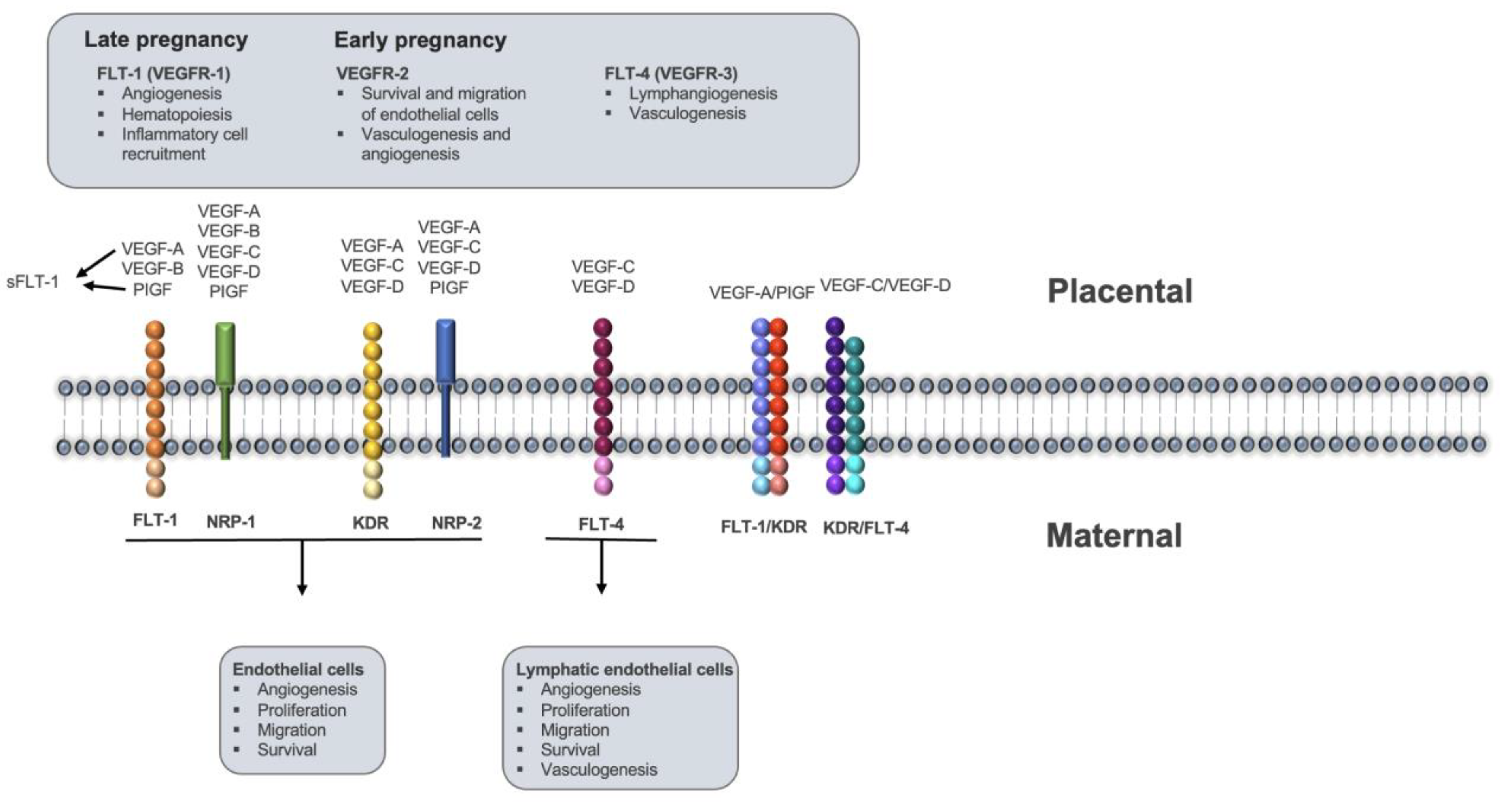
:1. Introduction
2. Methods and Research Design
2.1. Literature Search and Data Extraction
| Author, Year | VEGF Type/ Tissue Type | Methods | Statistics Analysis | Country/ Ethnicity | Patient Characteristic | Definition of Cases | Case (n) | Definition of Controls | Control (n) | Time of Menstrual Cycle/ Gestational Age and Confirmation Method |
|---|---|---|---|---|---|---|---|---|---|---|
| Almawi et al., 2013 [17] | Serum VEGF | ELISA | Unpaired student’s t-test for parametric data Pearson x2 or Fisher’s exact test was used to assess intergroup significance Mann–Whitney U-test for VEGF median comparisons | Bahrain/Middle Eastern |
Age (cases) mean of 31.6 + 5.4 year Age (control) mean of 31.6 + 4.9 year BMI (cases): 26.3 + 5.4 BMI (control): 25.2 + 4.3 |
Three or more consecutive spontaneous miscarriages of unknown etiology. Healthy women <40 years old at first. No significant medical history. Women were excluded if screening investigations revealed a possible contributing factor for their pregnancy losses. | 296 |
Multiparous non-pregnant women with at least two live births. No adverse pregnancy outcomes. No personal or family history of RM. | 305 | First 12 weeks of pregnancy |
| Amirchaghmaghi et al., 2014 [18] | Serum VEGF | RT-PCR ELISA | T-test or Mann–Whitney | Iran/Middle Eastern |
Age (cases) mean of 30.9 ± 1.24 Age (control) mean of 33.8 ± 1.10 BMI (cases): 25.52 ± 0.99 BMI (control): 26.1 ± 2.11 |
History of recurrent spontaneous miscarriage of unknown etiology. Women were excluded if screening investigations revealed a possible contributing factor for their pregnancy losses. | 10 |
Non-pregnant women with regular menstruation. At least one successful term pregnancy. | 6 | Weeks’ gestation (cases): Mean of 8.5 ± 1.014 |
| Atalay et al., 2016 [19] | Serum VEGF | ELISA | T-test and non- parametric Mann–Whitney U test were used in analysis between groups | Turkey /Middle Eastern | Age (cases): mean of 28.2 ± 5.2 Age (control) mean of 27.5 ± 4.4 BMI (cases): mean of 26.4 ± 5.1 BMI (control): mean of 25.1 ± 4.3 | Three or more consecutive spontaneous miscarriages. Excluded if late 1st trimester or 2nd trimester miscarriages. No significant medical history. Women were excluded if screening investigations revealed a possible contributing factor for their pregnancy losses. | 21 | Healthy ongoing pregnancies. Conceived spontaneously. | 24 | Between 5th and 10th week of pregnancy. Transvaginal obstetric ultrasonography for the evaluation of gestational age was conducted at the same day of sample collection. |
| Bagheri et al., 2013 [20] | Serum VEGF-A and VEGF-C | ELISA | Student’s t-test was used for parametric data; Pearson correlation coefficient for the relationship between quantitative variant | India/south Asian of Indian origin. | Age (cases): mean of 30.6667 Age (control/pregnant women): mean of 28. 6429 Age (control/healthy women): mean of 28.9571 BMI (cases): mean of 27.8207 BMI (control/pregnant women): mean of 24.1539 BMI (control/healthy non-pregnant women): mean of 24.9033 | Two or more spontaneous miscarriages. | 90 | Control group 1: Pregnant women, no history of RM, at least one child, women were excluded if screening investigations revealed a possible contributing factor for their pregnancy losses. Control group 2: Healthy non-pregnant women. | Control 1: 70 Control 2: 70 | RM: before 20 weeks of pregnancy |
| Banerjee et al., 2013 [13] | Endometrial biopsy for VEGF | ELISA IHC—Semi-quantitative scoring was performed independently by two observers to assess the staining intensity. A final IHC score was obtained by multiplying intensity score and extent of stained cells. | Unpaired Student’s t-test for parametric data | India/south Asian of Indian origin. | Age: cut-off of <35 years BMI: cut-off of ≤28 | Three or more consecutive spontaneous miscarriages of unknown etiology. No history of gynaecological disorders. Have not received any medication during the past three months. | 66 | Parity between 2 and 5 (2.5 ± 0.12). Normal regular menstrual cycles. No history of failed pregnancies or other significant clinical abnormalities. | 50 | First 12 weeks of pregnancy. Ultrasonography (USG) for serial folliculometry was performed at day 10 onwards in all cases to monitor follicular growth till ovulation occurred. Biopsies performed on day 18–22 of menstrual cycle. |
| Gupta et al., 2019 [21] | Serum VEGF | ELISA | Not defined | India/south Asian of Indian origin. | Age (cases): 28.9 Age (control): 28.2 BMI (cases): 25.2 BMI (control): 24.0 | Three or more consecutive spontaneous miscarriages. | 13 | At least one successful pregnancy. No personal history of miscarriage. | 30 | Not defined |
| He et al., 2016 [22] | Decidua and chorionic villi for VEGF | Western blot qRT-PCR IHC—Semi-quantitative scoring was performed independently by two observers to assess the staining intensity. A final IHC score was obtained by multiplying intensity of staining and percentage of positive staining. The results were generated by averaging the scores of the five views. | Student’s t-test for parametric data; Mann–Whitney test was applied for non-parametric data | China/Asian | Age (cases): Mean of 30.3 ± 4.0 Age (control): Mean of 28.7 ± 5.1 | Two or more spontaneous miscarriages. Diagnosis of unexplained recurrent miscarriage. | 28 | Normal early pregnancy with a surgical termination of pregnancy request for unwanted pregnancy. History of prior healthy live births. No abnormal pregnancy history including previous miscarriage, ectopic pregnancy, or still birth. | 28 | First 12 weeks of pregnancy. Gestational days (cases): Mean of 62.8 ± 8.3. Gestational days (control): Mean of 59.1 ± 7.6. Embryo death confirmed by ultrasound. |
| Lash et al., 2011 [23] | Endometrial biopsy for VEGF-A, VEGF-C, VEGF-D | qRT-PCR IHC—Semi-quantitative IHC score was obtained by multiplying intensity of staining percentage of cells and staining intensity. | ANOVA post-hoc Fischer unpaired Student’s t-test for parametric data | England/Multi-ethnic population | Age (cases): median of 38 (29–44) Age (control): median of 42 (28–51) | Three or more consecutive spontaneous miscarriages. Women were excluded if screening investigations revealed a possible contributing factor for their pregnancy losses. | 14 | No definition | 29 | LH + 7 (+2 days) |
| Pang et al., 2013 [24] | Chorionic villus tissues for VEGF Serum VEGF and VEGF-R1 (Flt) | ELISA IHC—Semi-quantitative IHC score was obtained by determining the percentage of positively stained cells (trophoblastic and interstitial cells) in all fields counted (10 fields for each specimen). | China/Asian | Age (cases): Mean of 33.2 ± 2.9 Age (control): Mean of 32.6 ± 2.1 BMI (cases): median of 20.3 BMI (control): median of 21.2 | Two or more spontaneous miscarriages. No history of adverse pregnancy outcomes, including preterm labour, had no current illnesses, did not use regular medication or smoke. Normal chromosome analyses of male and female partners. | 32 | Normal early pregnancy with a surgical termination of pregnancy request for unwanted pregnancy. No history of spontaneous abortion. | 50 | Between 6 and 12 weeks of pregnancy. Gestational weeks (cases): 10.9 ± 1.56. Gestational weeks (control): 10.1 ± 1.91. Embryonic death confirmed by ultrasound. | |
| Papamitsou et al., 2021 [25] | Decidua basalis, decidua parietalis and trophoblast for VEGF | IHC—Semi-quantitative IHC score was performed independently by two researchers and obtained by determining the intensity of staining evaluated as negative (–), weak (+), moderate (++), and strong (+++). | Mann–Whitney test. | Greek/European | Age (cases): range of 35–42 Age (control): range of 27–39 | Three or more spontaneous miscarriages. Women were excluded if screening investigations revealed a possible contributing factor for their pregnancy losses. | 20 | Normal early pregnancy with a surgical termination of pregnancy request for unwanted pregnancy. | 20 | First 12 weeks of pregnancy |
| Sadekova et al., 2015 [26] | Endometrial VEGF | IHC—Semi-quantitative IHC score was obtained by determining the intensity of staining evaluated on a 0–3 scale. | Mann–Whitney U test, chi square test | Russia/Asia | - | Two or more consecutive spontaneous miscarriages. | 24 | Fertile women. No history of concomitant gynaecological disease. At least one successful pregnancy. | 15 | First trimester of pregnancy. Ovulation confirmed by ultrasonography. Endometrial biopsies taken on day 8 after ovulation. |
| Scarpellini et al., 2019 [27] | Trophoblast Decidua VEGF and VEGFR-1 | IHC—Semi-quantitative IHC score was performed independently by two authors and was obtained by multiplying intensity of staining and percentage of cells stained for each intensity. | Mann–Whitney U test | Italy/European | Age: (cases): pregnancy started at 31.6 ± 2.3 Age: (control): pregnancy started at 30.8 ± 2.2 BMI (cases): 27.4 ± 1.9 BMI (control): 27.8 ± 1.8 | Women were excluded if screening investigations revealed a possible contributing factor for their pregnancy losses. Pregnancies not obtained by artificial reproductive technology. | 15 | Normal early pregnancy with a surgical termination of pregnancy request for unwanted pregnancy. No history of previous abortions. | 15 | Control: 9.4 ± 1.1 (7–10). Cases: 8.1 ± 1.2 (5–9). |
| Vuorela et al., 2000 [28] | Placental villi, decidua, endometrial glands, invading trophoblast for VEGF | IHC—Semi-quantitative IHC score was performed independently by two observers and was obtained by determining the staining intensity scored from negative (–) to faint (+), medium (++), and strong (+++) staining. | No statistical analysis | Finland/European | Age (cases/ missed abortion): 41.7 ± 1.3 Age (cases/blunted ovum): 29.7 ±1.7 Age (control): 28.6 ± 1.8 | Three or more consecutive spontaneous miscarriages. Women were excluded if screening investigations revealed a possible contributing factor for their pregnancy losses. | 18 | No definition. No miscarriage. | 12 | Cases/ missed abortion: 9.2 ± 0.3 (7–11) 8. Cases/blunted ovum: 8.3 ± 0.4 (7–10). Control: 9.4 ± 0.4 (7–11) |
| Author, Year | Aims | Summary of Results |
|---|---|---|
| Almawi et al., 2013 [17] |
|
|
| Atalay et al., 2016 [19] | Investigate whether maternal VEGF levels are associated with RM. |
|
| Amirchaghmaghi et al., 2014 [18] | Investigate the serum VEGF concentration in patients with a history of RM compared with normal fertile women. | Serum level of VEGF was higher in RM cases compared with the control group. |
| Bagheri et al., 2017 [20] | Investigate the relationships between serum level of VEGF-A and VEGF-C with clinical characteristic in women with RM and compare to pregnant and healthy women. |
|
| Banerjee et al., 2013 [13] | Identify the significant factor(s) responsible for vascular dysfunction in women with RM during window of implantation. |
|
| Gupta et al., 2019 [21] |
|
|
| He et al., 2016 [22] | Investigate whether the expressions of VEGF and Cx43 were altered in the first-trimester tissues (chorionic villi and decidua) collected from women with RM compared to those from healthy early pregnant women. | The immunoreactivity of VEGF in either chorionic villi or decidua was dramatically reduced in RM group compared to the control as revealed by immunostaining, Western blot, and VEGF mRNA. |
| Lash et al., 2011 [23] | Investigate the temporal and spatial expression of series of angiogenic growth factors (AGFs) and their receptors: vascular endothelial growth factor (VEGF)-A, VEGF-C, VEGF-D, VEGF-R1, VEGF-R2, VEGF-R3, platelet-derived growth factor (PDGF)-BB, PDGF-Ra, PDGF-Rb, transforming growth factor (TGF)-b1, TGF-bRI, TGF-bRII, angiopoietin (Ang)-1, Ang-2 and Tie-2, in the proliferative, early secretory and mid-late secretory phase endometrium from control women as well as in the mid-late secretory phase of women with a history of RM. Four cell types were investigated, namely, glandular epithelium, stromal cells, vascular smooth muscle cells (VSMCs), epithelial cells (ECs). |
|
| Pang et al., 2013 [24] | Investigate the levels of sFlt-1 and VEGF in serum and chorionic villus of RM patients compared to control. |
|
| Papamitsou et al., 2021 | Investigate the role of VEGF, BCL-2, and BCL-6 in RM. | Increased levels of VEGF, BCL-2, and BCL-6 in RM cases compared to control. |
| Sadekova et al., 2015 [26] |
|
|
| Scarpellini et al., 2019 [27] | Investigate the effects of G-CSF treatment on the maternal fetal interface using immunohistochemistry to assess the expression of G-CSF and its receptor, the VEGF and its receptor VEGFR-1, and Foxp3 in the trophoblast and decidua of first trimester miscarriages of RPL women treated with G-CSF that miscarried again despite the treatment, in no treated RPL and in normal first trimester pregnancies. |
|
| Vuorela etl al., 2000 [28] | Investigate whether the expression of VEGF, VEGFR-1, -2 or -3 or the Tie-1 or Tie- 2 receptors in the placenta or decidua are altered in RM compared to control. |
|
2.2. Inclusion Criteria
- Population
- Laboratory Methods
- (1)
- Enzyme-Linked Immunosorbent Assay (ELISA) to measure serum levels of the VEGFs.
- (2)
- Real-Time Quantitative Reverse Transcription PCR (qRT-PCR) to detect VEGF-gene expression.
- (3)
- Immunohistochemistry (IHC) of endometrial, decidua, and/or placental tissue to determine localization and distribution of VEGFs.
- (4)
- Western blot in conjunction with IHC and/or qRT-PCR, of endometrial, decidua, or fetoplacental tissue to determine localization, distribution, and levels of VEGFs.

- Outcome
2.3. Exclusion Criteria
3. Results and Discussion
3.1. Search Results
3.2. Terms and Definitions
- Recurrent Idiopathic Miscarriage
- Healthy Controls
- (1)
- Pregnant women with no current and/or previous pregnancy complications request a surgical termination of pregnancy for unwanted pregnancy.
- (2)
- Pregnant women with no personal and/or family history of miscarriage requesting a surgical termination of pregnancy for unwanted pregnancy.
- (3)
- Non-pregnant healthy multiparous women for endometrial sampling.
- Vascular Endothelial Growth Factors
3.3. VEGFs in Endometrial Tissue
3.4. VEGFs in the Maternal Decidua and Placental Tissues
3.5. VEGFs in Serum

3.6. Quality Assessment
3.7. Discussion
3.7.1. Clinical Variables
3.7.2. Sampling Variables
3.7.3. Analytical Variables
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ELISA | Enzyme-linked Immunosorbent Assay |
| FKBPL | FK506-binding protein like |
| HSP90 | Heat shock protein 90 |
| IHC | Immunohistochemistry |
| JBI | Joanna Briggs Institute |
| NRP-1 | Neuropilin-1 |
| PlGF | Placental Growth Factor |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-analysis |
| qRT-PCR | Real-Time Quantitative Reverse Transcription PCR |
| RM | Recurrent miscarriage |
| TGF-beta | Tumour Growth Factor Beta |
| VEGFs | Vascular Endothelial Growth Factors |
References
- The ESHRE Guideline Group on RPL; Bender Atik, R.; Christiansen, O.B.; Elson, J.; Kolte, A.M.; Lewis, S.; Middeldorp, S.; Nelen, W.; Peramo, B.; Quenby, S.; et al. ESHRE guideline: Recurrent pregnancy loss. Hum. Reprod. Open 2018, 2018, hoy004. [Google Scholar]
- Kaiser, J.; Branch, D.W. Recurrent Pregnancy Loss: Generally Accepted Causes and Their Management. Clin. Obs. Gynecol. 2016, 59, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Avagliano, L.; Garo, C.; Marconi, A.M. Placental amino acids transport in intrauterine growth restriction. J. Pregnancy 2012, 2012, 972562. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.J.; Bulmer, J.N.; Innes, B.A.; Broughton Pipkin, F. Possible roles for folic acid in the regulation of trophoblast invasion and placental development in normal early human pregnancy. Biol. Reprod. 2011, 84, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Gourvas, V.; Dalpa, E.; Konstantinidou, A.; Vrachnis, N.; Spandidos, D.A.; Sifakis, S. Angiogenic factors in placentas from pregnancies complicated by fetal growth restriction (review). Mol. Med. Rep. 2012, 6, 23–27. [Google Scholar]
- Connolly, D.T.; Olander, J.V.; Heuvelman, D.; Nelson, R.; Monsell, R.; Siegel, N.; Haymore, B.L.; Leimgruber, R.; Feder, J. Human vascular permeability factor. Isolation from U937 cells. J. Biol. Chem. 1989, 264, 20017–22002. [Google Scholar] [CrossRef]
- Ferrara, N.; Houck, K.; Jakeman, L.; Leung, D.W. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr. Rev. 1992, 13, 18–32. [Google Scholar] [CrossRef]
- Keck, P.J.; Hauser, S.D.; Krivi, G.; Sanzo, K.; Warren, T.; Feder, J.; Connolly, D.T. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science 1989, 246, 1309–1312. [Google Scholar] [CrossRef]
- Dvorak, H.F.; Brown, L.F.; Detmar, M.; Dvorak, A.M. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 1995, 146, 1029–1039. [Google Scholar]
- Grimwood, J.; Bicknell, R.; Rees, M.C. The isolation, characterization and culture of human decidual endothelium. Hum. Reprod. 1995, 10, 2142–2148. [Google Scholar] [CrossRef]
- Ng, E.H.; Chan, C.C.; Tang, O.S.; Yeung, W.S.; Ho, P.C. Comparison of endometrial and subendometrial blood flow measured by three-dimensional power Doppler ultrasound between stimulated and natural cycles in the same patients. Hum. Reprod. 2004, 19, 2385–2390. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Miyazawa, M.; Takanashi, Y.; Tanigawa, M.; Yasuda, K.; Onouchi, H.; Kawabe, N.; Mitsushita, J.; Hartman, P.S.; Ishii, N. Genetically induced oxidative stress in mice causes thrombocytosis, splenomegaly and placental angiodysplasia that leads to recurrent abortion. Redox Biol. 2014, 2, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Ghosh, S.; Dutta, M.; Subramani, E.; Khalpada, J.; RoyChoudhury, S.; Chakravarty, B.; Chaudhury, K. Identification of key contributory factors responsible for vascular dysfunction in idiopathic recurrent spontaneous miscarriage. PLoS ONE 2013, 8, e80940. [Google Scholar] [CrossRef] [PubMed]
- Yakkundi, A.; McCallum, L.; O’kane, A.; Dyer, H.; Worthington, J.; McKeen, H.D.; McClements, L.; Elliott, C.; McCarthy, H.; Hirst, D.G.; et al. The anti-migratory effects of FKBPL and its peptide derivative, AD-01: Regulation of CD44 and the cytoskeletal pathway. PLoS ONE 2013, 8, e55075. [Google Scholar] [CrossRef]
- The Jamovi Project (2020). Jamovi. (Version 2.3.21.0) [Computer Software]. Available online: https://www.jamovi.org (accessed on 2 May 2023).
- Review Manager (RevMan) [Computer Program]. Version 5.4. The Cochrane Collaboration: Sydney, Australia, 2020.
- Almawi, W.Y.; Saldanha, F.L.; Mahmood, N.A.; Al-Zaman, I.; Sater, M.S.; Mustafa, F.E. Relationship between VEGFA polymorphisms and serum VEGF protein levels and recurrent spontaneous miscarriage. Hum. Reprod. 2013, 28, 2628–2635. [Google Scholar] [CrossRef]
- Amirchaghmaghi, E.; Rezaei, A.; Moini, A.; Roghaei, M.A.; Hafezi, M.; Aflatoonian, R. Gene expression analysis of VEGF and its receptors and assessment of its serum level in unexplained recurrent spontaneous abortion. Cell J. 2015, 16, 538–545. [Google Scholar]
- Atalay, M.A.; Ugurlu, N.; Zulfikaroglu, E.; Danisman, N. Clinical significance of maternal serum vascular endothelial growth factor (VEGF) level in idiopathic recurrent pregnancy loss. Eur. Rev. Med. Pharm. Sci. 2016, 20, 2974–2982. [Google Scholar]
- Bagheri, A.; Kumar, P.; Kamath, A.; Rao, P. Association of angiogenic cytokines (VEGF-A and VEGF-C) and clinical characteristic in women with unexplained recurrent miscarriage. Bratisl. Med. J. 2017, 118, 258–264. [Google Scholar] [CrossRef]
- Gupta, P.; Deo, S.; Jaiswar, S.P.; Sankhwar, P.L. Case Control Study to Compare Serum Vascular Endothelial Growth Factor (VEGF) Level in Women with Recurrent Pregnancy Loss (RPL) Compared to Women with Term Pregnancy. J. Obstet. Gynaecol. India 2019, 69 (Suppl. 2), 95–102. [Google Scholar] [CrossRef]
- He, X.; Chen, Q. Reduced expressions of connexin 43 and VEGF in the first-trimester tissues from women with recurrent pregnancy loss. Reprod. Biol. Endocrinol. 2016, 14, 46. [Google Scholar] [CrossRef]
- Lash, G.E.; Innes, B.A.; Drury, J.A.; Robson, S.C.; Quenby, S.; Bulmer, J.N. Localization of angiogenic growth factors and their receptors in the human endometrium throughout the menstrual cycle and in recurrent miscarriage. Hum. Reprod. 2012, 27, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Wei, Z.; Li, O.; Huang, R.; Qin, J.; Chen, H.; Fan, X.; Chen, Z.-J. An increase in vascular endothelial growth factor (VEGF) and VEGF soluble receptor-1 (sFlt-1) are associated with early recurrent spontaneous abortion. PLoS ONE 2013, 8, e75759. [Google Scholar] [CrossRef] [PubMed]
- Papamitsou, K.; Fotiadou, S.; Papadopoulou, K.; Karachrysafi, S.; Papachristodoulou, A.; Kamperis; Tsaknakis, K.; Lialiaris, E.; Kavvadas, D.; Sioga, A. Evaluation of VEGF, BCL-2, BCL-6 by immunohistochemistry in the endometrial tissue of patients with recurrent pregnancy loss. Arch. Hell. Med. 2021, 38, 224–230. [Google Scholar]
- Sadekova, O.N.; Nikitina, L.A.; Rashidov, T.N.; Voloschuk, I.N.; Samokhodskaya, L.M.; Demidova, E.M.; Tkachuk, V.A. Luteal phase defect is associated with impaired VEGF mRNA expression in the secretory phase endometrium. Reprod. Biol. 2015, 15, 65–68. [Google Scholar] [CrossRef]
- Scarpellini, F.; Klinger, F.G.; Rossi, G.; Sbracia, M. Immunohistochemical Study on the Expression of G-CSF, G-CSFR, VEGF, VEGFR-1, Foxp3 in First Trimester Trophoblast of Recurrent Pregnancy Loss in Pregnancies Treated with G-CSF and Controls. Int. J. Mol. Sci. 2019, 21, 285. [Google Scholar] [CrossRef]
- Vuorela, P.; Carpen, O.; Tulppala, M.; Halmesmaki, E. VEGF, its receptors and the tie receptors in recurrent miscarriage. Mol. Hum. Reprod. 2000, 6, 276–282. [Google Scholar] [CrossRef]
- Tammela, T.; Enholm, B.; Alitalo, K.; Paavonen, K. The biology of vascular endothelial growth factors. Cardiovasc. Res. 2005, 65, 550–563. [Google Scholar] [CrossRef]
- Eller, A.G.; Branch, D.W.; Nelson, L.; Porter, T.F.; Silver, R.M. Vascular endothelial growth factor-A gene polymorphisms in women with recurrent pregnancy loss. J. Reprod. Immunol. 2011, 88, 48–52. [Google Scholar] [CrossRef]
- Reynolds, L.P.; Redmer, D.A. Angiogenesis in the placenta. Biol. Reprod. 2001, 64, 1033–1040. [Google Scholar] [CrossRef]
- Zygmunt, M.; Herr, F.; Münstedt, K.; Lang, U.; Liang, O.D. Angiogenesis and vasculogenesis in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 110, S8–S10. [Google Scholar] [CrossRef]
- Hoeben, A.; Landuyt, B.; Highley, M.S.; Wildiers, H.; Van Oosterom, A.T.; De Bruijn, E.A. Vascular endothelial growth factor and angiogenesis. Pharm. Rev. 2004, 56, 549–580. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Du, C.; Li, H.; Du, J.; Yan, X.; Peng, L.; Li, G.; Chen, Z.-J. Association of VEGF genetic polymorphisms with recurrent spontaneous abortion risk: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0123696. [Google Scholar] [CrossRef]
- Takahashi, H.; Ohkuchi, A.; Kuwata, T.; Usui, R.; Baba, Y.; Suzuki, H.; Kyi, T.T.C.; Matsubara, S.; Saito, S.; Takizawa, T. Endogenous and exogenous miR-520c-3p modulates CD44-mediated extravillous trophoblast invasion. Placenta 2017, 50, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Valentine, A.; O’Rourke, M.; Yakkundi, A.; Worthington, J.; Hookham, M.; Bicknell, R.; McCarthy, H.O.; McClelland, K.; McCallum, L.; Dyer, H.; et al. FKBPL and peptide derivatives: Novel biological agents that inhibit angiogenesis by a CD44-dependent mechanism. Clin. Cancer Res. 2011, 17, 1044–1056. [Google Scholar] [CrossRef] [PubMed]
- Duval, M.; Le Boeuf, F.; Huot, J.; Gratton, J.P. Src-mediated phosphorylation of Hsp90 in response to vascular endothelial growth factor (VEGF) is required for VEGF receptor-2 signaling to endothelial NO synthase. Mol. Biol. Cell 2007, 18, 4659–4668. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, S.; Song, T.; Yin, Y.; Tan, C. Placental Angiogenesis in Mammals: A Review of the Regulatory Effects of Signaling Pathways and Functional Nutrients. Adv. Nutr. 2021, 12, 2415–2434. [Google Scholar] [CrossRef]
- Seki, H. Balance of antiangiogenic and angiogenic factors in the context of the etiology of preeclampsia. Acta Obs. Gynecol. Scand. 2014, 93, 959–964. [Google Scholar] [CrossRef]

| Endometrial Vascular Endothelial Growth Factor | ||
|---|---|---|
| Clinical Variables for Cases and Controls | Sampling Variables | Analysis Variables |
| Age | Days following the last pregnancy | Correlation of tissue sample with ultrasonic or hysteroscopic features |
| BMI | Endometrial sampling techniques | Method of confirming menstrual phase |
| Ethnicity | Endometrial thickness | Criteria for endometrial histological dating |
| Parity and Gravity | Anatomical uterine location | Methodology of tissue fixation |
| Stage, day, and regularity of the menstrual cycle: menstrual phase, secretory phase, proliferative phase. | Histological uterine layer: uterine stratum compactum, stratum spongiosum and/or stratum basalis. | Methodology of endometrial analysis |
| Assessment of sperm DNA, meiotic alternations, and parameters | Size of tissue fragments | Isoform of VEGF sequenced |
| History of RM | Number of endometrial glands and presence of stroma | Analysis of immunostaining: utilisation of blinding, analysis of intensity of staining, analysis of the percentage of cells for each staining intensity |
| History of concomitant gynaecological diseases | Viability of the endometrial sampling | |
| Medical predispositions for RM * | ||
| Placental and Decidua Vascular Endothelial Growth Factor | ||
|---|---|---|
| Clinical Variables for Cases and Controls | Sampling Variables | Analysis Variables |
| Age | Type of tissue Placental villi Cytotrophoblasts Syncytiotrophoblast Stromal cells Vascular endothelium Decidua Stromal cells Vascular endothelium | Correlation of gestation age with ultrasonic or hysteroscopic features |
| BMI | Sampling techniques | Method of confirming termination of pregnancy |
| Ethnicity | Time of sampling following miscarriage/termination of pregnancy | Criteria of confirming gestation age |
| Parity and Gravity | Region of the sample analysed: central vs. peripheral | Methodology of tissue fixation |
| Gestation Age | Viability of the tissue sampled | Methodology of placental and decidua analysis |
| Assessment of sperm DNA, meiotic alternations, and parameters | Isoform of VEGF sequenced | |
| History of RM | Analysis of immunostaining: utilisation of blinding, analysis of intensity of staining, analysis of the percentage of cells for each staining intensity | |
| History of concomitant gynaecological diseases | ||
| Medical predispositions for RM * | ||
| Serum Vascular Endothelial Growth Factor | ||
|---|---|---|
| Clinical Variables for Cases and Controls | Sampling Variables | Analytical Variables |
| Age | Type of sample: serum vs. plasma | Correlation of gestation age with ultrasonic or hysteroscopic features |
| BMI | Time of sampling following miscarriage/termination of pregnancy | Method of confirming termination of pregnancy |
| Ethnicity | Viability of the tissue sampled | Criteria of confirming gestation age |
| Parity and Gravity | Methodology of analysis | |
| Gestation Age | VEGF isoforms | |
| Assessment of sperm DNA, meiotic alternations, and parameters | ||
| History of RM | ||
| History of concomitant gynaecological diseases | ||
| Medical predispositions for RM * | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Ghazaleh, N.; Brennecke, S.; Murthi, P.; Karanam, V. Association of Vascular Endothelial Growth Factors (VEGFs) with Recurrent Miscarriage: A Systematic Review of the Literature. Int. J. Mol. Sci. 2023, 24, 9449. https://doi.org/10.3390/ijms24119449
Abu-Ghazaleh N, Brennecke S, Murthi P, Karanam V. Association of Vascular Endothelial Growth Factors (VEGFs) with Recurrent Miscarriage: A Systematic Review of the Literature. International Journal of Molecular Sciences. 2023; 24(11):9449. https://doi.org/10.3390/ijms24119449
Chicago/Turabian StyleAbu-Ghazaleh, Nadine, Shaun Brennecke, Padma Murthi, and Vijaya Karanam. 2023. "Association of Vascular Endothelial Growth Factors (VEGFs) with Recurrent Miscarriage: A Systematic Review of the Literature" International Journal of Molecular Sciences 24, no. 11: 9449. https://doi.org/10.3390/ijms24119449
APA StyleAbu-Ghazaleh, N., Brennecke, S., Murthi, P., & Karanam, V. (2023). Association of Vascular Endothelial Growth Factors (VEGFs) with Recurrent Miscarriage: A Systematic Review of the Literature. International Journal of Molecular Sciences, 24(11), 9449. https://doi.org/10.3390/ijms24119449








