Chromogranin A: An Endocrine Factor of Pregnancy
Abstract
1. Introduction
2. Hormonal Balance of Pregnancy
| Hormone Name | Mainsource in Pregnancy | Role in Pregnancy |
|---|---|---|
| Progesterone | Corpus luteum Placenta |
|
| Estrogens | Corpus luteum Placenta |
|
| Human placental lactogen (hPL) | Placenta (syncytiotrophoblast) |
|
| Human chorionic gonadotropin (hCG) | Placenta (trophoblast) |
|
| Relaxin | Corpus luteum |
|
| Placental growth hormone (HPGH) | Placenta (syncytiotrophoblast) |
|
| Leptin | Placenta (syncytiotrophoblast) |
|
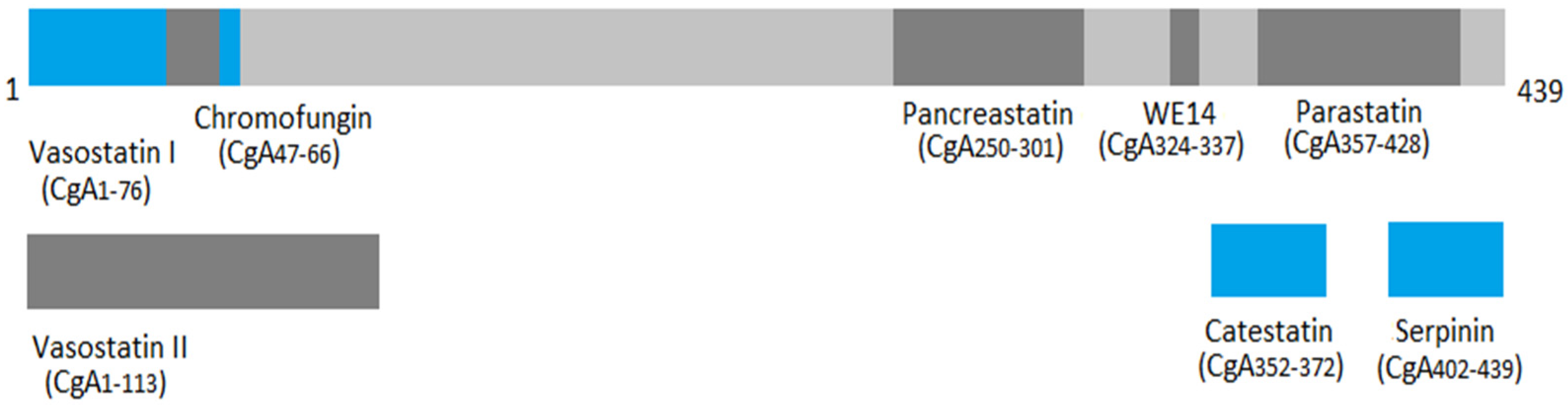
3. Chromogranin A Family and Structure
| CgA-Derived Peptide | Position | Biological Function(s) |
|---|---|---|
| Chromostatin | CgA1-20 | |
| Vasostatin I and II (VS-I and VS-II) | VS-I: CgA176 VS-II: CgA1-113 |
|
| Chromofungin (CHR) | CgA47-66 |
|
| Chromacin | CgA173-194 |
|
| Pancreastatin (PST) | CgA250-301 |
|
| Catestatin (CST) | CgA352-372 |
|
| WE14 | CgA324-337 |
|
| GE25 | CgA347-365 | - |
| Parastatin | CgA357-428 |
|
| Serpinin | CgA402-439 |
|
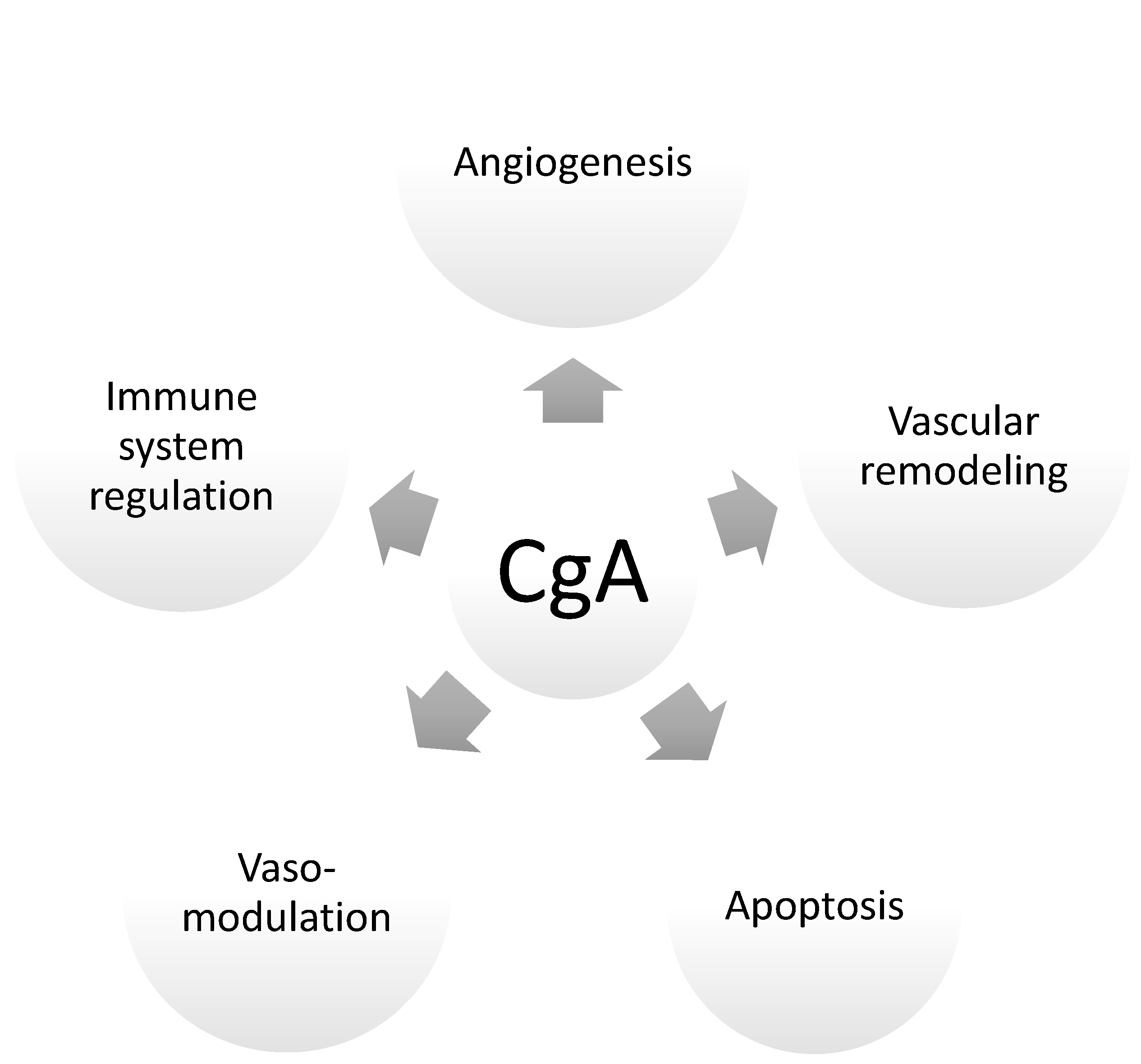
4. Crucial Pregnancy Adaptations: Chromogranin A and CgA-Derived Peptides as Endocrine Factors of Pregnancy
4.1. Cardiovascular Adaptations in Pregnancy
4.2. Renal Adaptations, Blood Pressure, and Vasomodulatory Effects during Preganancy
4.3. Maternal Immune Tolerance Adaptations
4.4. Apoptosis: Trophoblast Differentiation and Vascular Remodeling
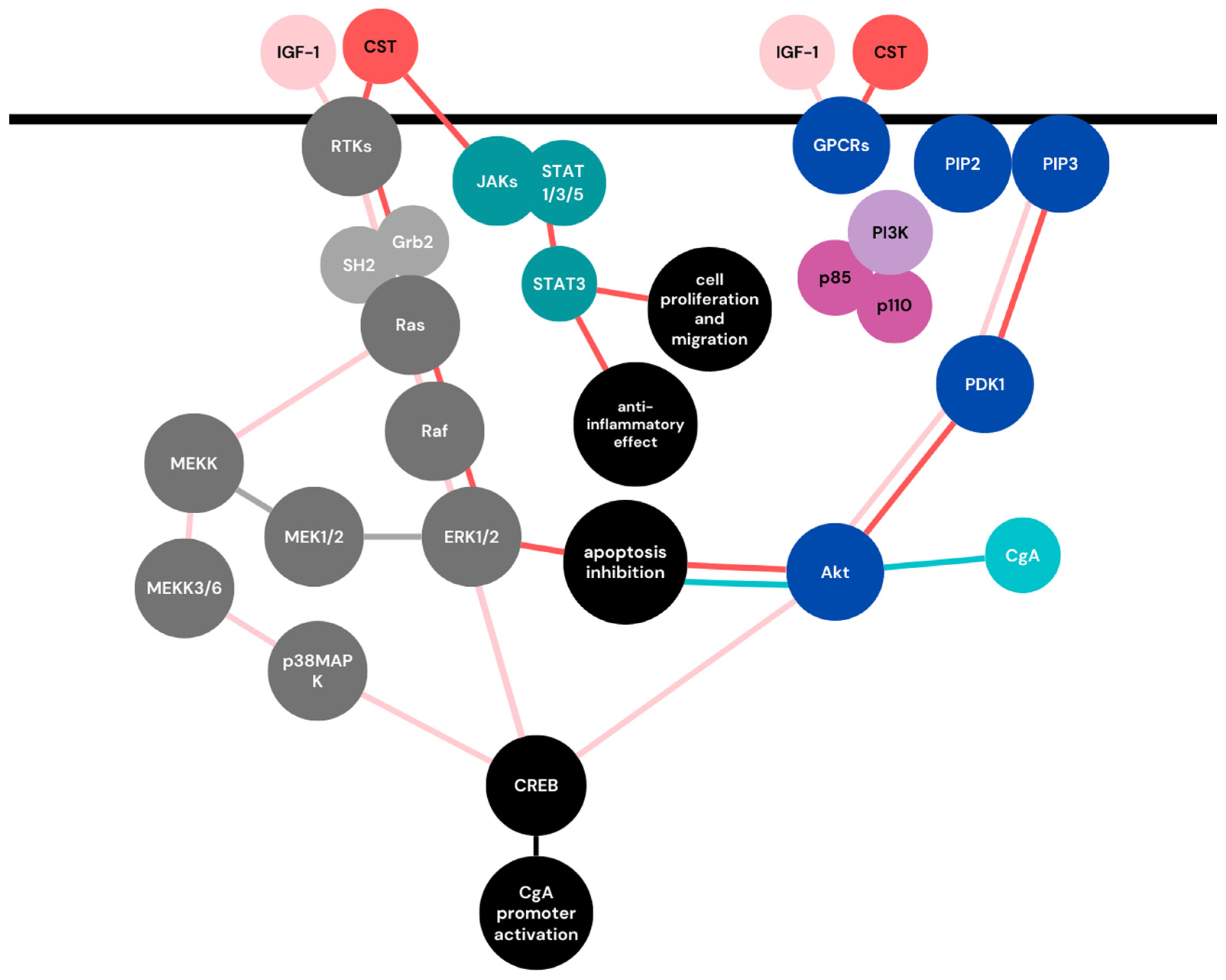
5. Chromogranin A as a Player in Cell Signaling Pathways of Pregnancy
5.1. Mitogen-Activated Protein Kinases (MAPKs) Signaling Pathway
5.2. Phosphoinositide 3-Kinase (PI3K)/AKT Signaling Pathway
5.3. JAK-STAT Signaling Pathway
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, P.; Magon, N. Hormones in pregnancy. Niger. Med. J. 2012, 53, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Florio, P.; Mezzesimi, A.; Turchetti, V.; Severi, F.M.; Ticconi, C.; Forconi, S.; Petraglia, F. High Levels of Human Chromogranin A in Umbilical Cord Plasma and Amniotic Fluid at Parturition. J. Soc. Gynecol. Investig. 2002, 9, 32–36. [Google Scholar] [CrossRef]
- Syversen, U.; Opsjøn, S.L.; Stridsberg, M.; Sandvik, A.K.; Dimaline, R.; Tingulstad, S.; Arntzen, K.J.; Brenna, E.; Waldum, H.L. Chromogranin A and pancreastatin-like immunoreactivity in normal pregnancies. J. Clin. Endocrinol. Metab. 1996, 81, 4470–4475. [Google Scholar] [CrossRef] [PubMed]
- Jiaur, G.; Gayen, R.; Zhang, K.; Ramachandrarao, S.P.; Mahata, M.; Chen, Y.; Kim, H.-S.; Naviaux, R.K.; Sharma, K.; Mahata, S.K.; et al. Role of Reactive Oxygen Species in Hyper-Adrenergic Hypertension: Biochemical, Physiological, and Pharmacological Evidence from Targeted Ablation of the Chromogranin a (Chga) gene. Circ. Cardiovasc. Genet. 2010, 3, 414–425. [Google Scholar] [CrossRef]
- Gong, J.; Lee, J.; Akio, H.; Schlegel, P.N.; Shen, R. Attenuation of Apoptosis by Chromogranin A-Induced Akt and Survivin Pathways in Prostate Cancer Cells. Endocrinology 2007, 148, 4489–4499. [Google Scholar] [CrossRef][Green Version]
- Muntjewerff, E.M.; Dunkel, G.; Nicolasen, M.J.T.; Mahata, S.K.; Van Den Bogaart, G. Catestatin as a target for treatment of inflammatory diseases. Front. Immunol. 2018, 9, 2199. [Google Scholar] [CrossRef]
- Bílek, R.; Vlček, P.; Šafařík, L.; Michalský, D.; Novák, K.; Dušková, J.; Václavíková, E.; Widimský, J.; Zelinka, T. Chromogranin a in the laboratory diagnosis of pheochromocytoma and paraganglioma. Cancers 2019, 11, 586. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Matute Teresa, F.; Mercader-Cidoncha, E.; Mitjavila-Casanovas, M.; Robledo, M.; Tena, I.; Alvarez-Escola, C.; Arístegui, M.; Bella-Cueto, M.R.; Ferrer-Albiach, C.; et al. Multidisciplinary practice guidelines for the diagnosis, genetic counseling and treatment of pheochromocytomas and paragangliomas. Clin. Transl. Oncol. 2021, 23, 1995–2019. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Redman, C.W.; Roberts, J.M.; Moffett, A. Pre-eclampsia: Pathophysiology and clinical implications. BMJ 2019, 366, l2381. [Google Scholar] [CrossRef]
- Costa, M.A. The endocrine function of human placenta: An overview. Reprod. Biomed. Online 2016, 32, 14–43. [Google Scholar] [CrossRef]
- Miyaura, H.; Iwata, M. Direct and indirect inhibition of Th1 development by progesterone and glucocorticoids. J. Immunol. 2002, 168, 1087–1094. [Google Scholar] [CrossRef]
- Halasz, M.; Szekeres-Bartho, J. The role of progesterone in implantation and trophoblast invasion. J. Reprod. Immunol. 2013, 97, 43–50. [Google Scholar] [CrossRef]
- Fluhr, H.; Bischof-Islami, D.; Krenzer, S.; Licht, P.; Bischof, P.; Zygmunt, M. Human chorionic gonadotropin stimulates matrix metalloproteinases-2 and -9 in cytotrophoblastic cells and decreases tissue inhibitor of metalloproteinases-1, -2, and -3 in decidualized endometrial stromal cells. Fertil. Steril. 2008, 90, 1390–1395. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, L.T.; Weiss, G. Relaxin in human pregnancy. Ann. N. Y. Acad. Sci. 2009, 1160, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Bartolomucci, A.; Possenti, R.; Mahata, S.K.; Fischer-Colbrie, R.; Loh, Y.P.; Salton, S.R.J. The extended granin family: Structure, function, and biomedical implications. Endocr. Rev. 2011, 32, 755–797. [Google Scholar] [CrossRef]
- Ottesen, A.H.; Carlson, C.R.; Louch, W.E.; Dahl, M.B.; Sandbu, R.A.; Johansen, R.F.; Jarstadmarken, H.; Bjørås, M.; Høiseth, A.D.; Brynildsen, J.; et al. Glycosylated Chromogranin A in Heart Failure: Implications for Processing and Cardiomyocyte Calcium Homeostasis. Circ. Heart Fail. 2017, 10, e003675. [Google Scholar] [CrossRef] [PubMed]
- Gut, P.; Czarnywojtek, A.; Fischbach, J.; Bączyk, M.; Ziemnicka, K.; Wrotkowska, E.; Gryczyńska, M.; Ruchała, M. Chromogranin A—Unspecific neuroendocrine marker. Clinical utility and potential diagnostic pitfalls. Arch. Med. Sci. 2016, 12, 1–9. [Google Scholar] [CrossRef]
- D’amico, M.A.; Ghinassi, B.; Izzicupo, P.; Manzoli, L.; Di Baldassarre, A. Biological function and clinical relevance of chromogranin A and derived peptides. Endocr. Connect. 2014, 3, R45–R54. [Google Scholar] [CrossRef] [PubMed]
- Eskeland, N.L.; Zhou, A.; Dinh, T.Q.; Wu, H.; Parmer, R.J.; Mains, R.E.; O’Connor, D.T. Chromogranin A processing and secretion: Specific role of endogenous and exogenous prohormone convertases in the regulated secretory pathway. J. Clin. Investig. 1996, 98, 148–156. [Google Scholar] [CrossRef][Green Version]
- Jiang, Q.; Taupenot, L.; Mahata, S.K.; Mahata, M.; O’Connor, D.T.; Miles, L.A.; Parmer, R.J. Proteolytic cleavage of chromogranin A (CgA) by plasmin: Selective liberation of a specific bioactive CgA fragment that regulates catecholamine release. J. Biol. Chem. 2001, 276, 25022–25029. [Google Scholar] [CrossRef] [PubMed]
- Taupenot, L.; Harper, K.L. The Chromogranin–Secretogranin Family. New Engl. J. Med. 2013, 348, 1134–1149. [Google Scholar] [CrossRef]
- Koshimizu, H.; Cawley, N.X.; Kim, T.; Yergey, A.L.; Loh, Y.P. Serpinin: A Novel Chromogranin A-Derived, Secreted Peptide Up-Regulates Protease Nexin-1 Expression and Granule Biogenesis in Endocrine Cells. Mol. Endocrinol. 2011, 25, 732–744. [Google Scholar] [CrossRef]
- Niezgoda, M.; Kasacka, I. Chromogranin A: Its known and possible roles in obstetrics and gynecology. J. Mother Child 2018, 22, 297–300. [Google Scholar]
- Galindo, E.; Rill, A.; Bader, M.F.; Aunis, D. Chromostatin, a 20-amino acid peptide derived from chromogranin A, inhibits chromaffin cell secretion. Proc. Natl. Acad. Sci. USA 1991, 88, 1426–1430. [Google Scholar] [CrossRef]
- Helle, K.B. The chromogranin A-derived peptides vasostatin-I and catestatin as regulatory peptides for cardiovascular functions. Cardiovasc. Res. 2010, 85, 9–16. [Google Scholar] [CrossRef]
- Belloni, D.; Scabini, S.; Foglieni, C.; Veschini, L.; Giazzon, A.; Colombo, B.; Fulgenzi, A.; Helle, K.B.; Ferrero, M.E.; Corti, A.; et al. The vasostatin-I fragment of chromogranin A inhibits VEGF-induced endothelial cell proliferation and migration. FASEB J. 2007, 21, 3052–3062. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, K.; Eissa, N.; Tshikudi, D.; Bernstein, C.N.; Ghia, J.E. Impact of intrarectal chromofungin treatment on dendritic cells-related markers in different immune compartments in colonic inflammatory conditions. World J. Gastroenterol. 2021, 27, 8138. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T. The emerging roles of chromogranins and derived polypeptides in atherosclerosis, diabetes, and coronary heart disease. Int. J. Mol. Sci. 2021, 22, 6118. [Google Scholar] [CrossRef] [PubMed]
- Mahata, S.K.; Mahata, M.; Fung, M.M.; O’Connor, D.T. Catestatin: A multifunctional peptide from chromogranin A. Regul. Pept. 2010, 162, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Curry, W.J.; Barkatullah, S.C.; Johansson, A.N.; Quinn, J.G.; Norlen, P.; Connolly, C.K.; McCollum, A.P.; McVicar, C.M. WE-14, a chromogranin a-derived neuropeptide. Ann. N. Y. Acad. Sci. 2002, 971, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Koshimizu, H.; Cawley, N.X.; Yergy, A.L.; Loh, Y.P.; Loh, Y.P. Role of pGlu-Serpinin, a Novel Chromogranin A-Derived Peptide in Inhibition of Cell Death HHS Public Access. J. Mol. Neurosci. 2011, 45, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Soma-Pillay, P.; Nelson-Piercy, C.; Tolppanen, H.; Mebazaa, A. Physiological changes in pregnancy. Cardiovasc. J. Afr. 2016, 27, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Sanghavi, M.; Rutherford, J.D. Cardiovascular Management in Pregnancy. Circulation 2014, 130, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Tan, H.; Zhou, S.; Smith, G.N.; Walker, M.C.; Wen, S.W. Trajectory of blood pressure change during pregnancy and the role of pre-gravid blood pressure: A functional data analysis approach. Sci. Rep. 2017, 7, 1–6. [Google Scholar] [CrossRef]
- St, G.; Whitley, J.; Cartwright, J.E. Trophoblast-mediated spiral artery remodelling: A role for apoptosis. J. Anat. 2009, 215, 21–26. [Google Scholar] [CrossRef]
- Sato, Y.; Fujiwara, H.; Konishi, I. Mechanism of maternal vascular remodeling during human pregnancy; Mechanism of maternal vascular remodeling during human pregnancy. Reprod. Med. Biol. 2011, 11, 27–36. [Google Scholar] [CrossRef]
- Smith, S.D.; Dunk, C.E.; Aplin, J.D.; Harris, L.K.; Jones, R.L. Evidence for Immune Cell Involvement in Decidual Spiral Arteriole Remodeling in Early Human Pregnancy. Am. J. Pathol. 2009, 174, 1959–1971. [Google Scholar] [CrossRef]
- Kim, M.; Ju Park, H.; Won Seol, J.; Yeob Jang, J.; Cho, Y.-S.; Rae Kim, K.; Choi, Y.; Lydon, J.P.; DeMayo, F.J.; Shibuya, M.; et al. VEGF-A regulated by progesterone governs uterine angiogenesis and vascular remodelling during pregnancy. EMBO Mol. Med. 2013, 5, 1415–1430. [Google Scholar] [CrossRef]
- Veschini, L.; Crippa, L.; Dondossola, E.; Doglioni, C.; Corti, A.; Ferrero, E. The vasostatin-1 fragment of chromogranin A preserves a quiescent phenotype in hypoxia-driven endothelial cells and regulates tumor neovascularization. FASEB J. 2011, 25, 3906–3914. [Google Scholar] [CrossRef]
- Theurl, M.; Schgoer, W.; Albrecht, K.; Jeschke, J.; Egger, M.; Beer, A.G.E.; Vasiljevic, D.; Rong, S.; Wolf, A.M.; Bahlmann, F.H.; et al. The neuropeptide catestatin acts as a novel angiogenic cytokine via a basic fibroblast growth factor-dependent mechanism. Circ. Res. 2010, 107, 1326–1335. [Google Scholar] [CrossRef]
- Xu, W.; Yu, H.; Li, W.; Gao, W.; Guo, L.; Wang, G. Plasma Catestatin: A Useful Biomarker for Coronary Collateral Development with Chronic Myocardial Ischemia. PLoS ONE 2016, 11, e0149062. [Google Scholar] [CrossRef][Green Version]
- Özalp, M.; Yaman, H.; Demir, Ö.; Garip, S.A.; Aran, T.; Osmanağaoğlu, M.A. The role of maternal serum catestatin in the evaluation of preeclampsia and fetal cardiac functions. Turk. J. Obstet. Gynecol. 2021, 18, 272. [Google Scholar] [CrossRef]
- Hussein, W.; Lafayette, R.A. Renal function in normal and disordered pregnancy. Curr. Opin. Nephrol. Hypertens. 2014, 23, 46–53. [Google Scholar] [CrossRef]
- Chen, Y.; Mahata, M.; Rao, F.; Khandrika, S.; Courel, M.; Fung, M.M.; Zhang, K.; Stridsberg, M.; Ziegler, M.G.; Hamilton, B.A.; et al. Chromogranin A Regulates Renal Function by Triggering Weibel-Palade Body Exocytosis. J. Am. Soc. Nephrol. 2009, 20, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Fischer-Colbrie, R.; Schmid, K.W.; Mahata, S.K.; Mahata, M.; Laslop, A.; Bauer, J.W. Sex-Related Differences in Chromogranin A, Chromogranin B and Secretogranin II Gene Expression in Rat Pituitary. J. Neuroendocrinol. 1992, 4, 125–130. [Google Scholar] [CrossRef]
- Maul, H.; Longo, M.; Saade, G.; Garfield, R. Nitric Oxide and its Role During Pregnancy: From Ovulation to Delivery. Curr. Pharm. Des. 2003, 9, 359–380. [Google Scholar] [CrossRef] [PubMed]
- Bassino, E.; Gallo, M.P.; Levi, R. Endothelium Dependent Cardiovascular Effects of the Chromogranin A-Derived Peptides Vasostatin-1 and Catestatin Endothelium Dependent Cardiovascular Effects of the Chromogranin A-Derived. Curr. Med. Chem. 2012, 19, 4059–4067. [Google Scholar] [CrossRef]
- Pia Gallo, M.; Levi, R.; Ramella, R.; Brero, A.; Boero, O.; Tota, B.; Alloatti, G. Endothelium-derived nitric oxide mediates the antiadrenergic effect of human vasostatin-1 in rat ventricular myocardium. Am. J. Physiol.-Heart Circ. Physiol. 2007, 292, 2906–2912. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.A.; Shahid, I.Z.; Fong, A.Y.; Hammond, A.M.; Pilowsky, P.M. Vasostatin I (CgA17–76) vasoconstricts rat splanchnic vascular bed but does not affect central cardiovascular function. Auton. Neurosci. 2012, 166, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Fung, M.M.; Salem, R.M.; Mehtani, P.; Thomas, B.; Lu, C.F.; Perez, B.; Rao, F.; Stridsberg, M.; Ziegler, M.G.; Mahata, S.K.; et al. Direct Vasoactive Effects of the Chromogranin A (CHGA) Peptide Catestatin in Humans In Vivo. Clin. Exp. Hypertens. 2010, 32, 278–287. [Google Scholar] [CrossRef]
- Bralewska, M.; Biesiada, L.; Grzesiak, M.; Rybak-Krzyszkowska, M.; Huras, H.; Gach, A.; Pietrucha, T.; Sakowicz, A. Chromogranin A demonstrates higher expression in preeclamptic placentas than in normal pregnancy. BMC Pregnancy Childbirth 2021, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tüten, N.; Güralp, O.; Gök, K.; Hamzaoglu, K.; Oner, Y.O.; Makul, M.; Bulut, H.; Irmak, K.; Tüten, A.; Malik, E. Serum catestatin level is increased in women with preeclampsia. J. Obstet. Gynaecol. 2022, 42, 55–60. [Google Scholar] [CrossRef]
- Morelli, S.; Mandal, M.; Goldsmith, L.T.; Kashani, B.N.; Ponzio, N.M. The maternal immune system during pregnancy and its influence on fetal development. Res. Rep. Biol. 2015, 6, 171–189. [Google Scholar] [CrossRef]
- Fong, C.H.Y.; Bebien, M.; Didierlaurent, A.; Nebauer, R.; Hussell, T.; Broide, D.; Karin, M.; Lawrence, T. An antiinflammatory role for IKKβ through the inhibition of “classical” macrophage activation. J. Exp. Med. 2008, 205, 1269–1276. [Google Scholar] [CrossRef]
- Walter, U.; Santamaria, P. CD8+ T cells in autoimmunity. Curr. Opin. Immunol. 2005, 17, 624–631. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, L.; Li, Y.; Zhang, J.; Guo, B.; Meng, G.; Chen, X.; Zheng, Q.; Zhang, L.; Zhang, M.; et al. Identification of autoreactive CD8+ T cell responses targeting chromogranin A in humanized NOD mice and type 1 diabetes patients. Clin. Immunol. 2015, 159, 63–71. [Google Scholar] [CrossRef]
- Yockey, L.J.; Iwasaki, A. Interferons and Proinflammatory Cytokines in Pregnancy and Fetal Development. Immunity 2018, 49, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, W.; Liu, C. Increased expression of IFN-γ in preeclampsia impairs human trophoblast invasion via a SOCS1/JAK/STAT1 feedback loop. Exp. Ther. Med. 2021, 21, 112. [Google Scholar] [CrossRef]
- El Shahaway, A.A.; Elhady, R.R.A.; Abdelrhman, A.A.; Yahia, S. Role of maternal serum interleukin 17 in preeclampsia: Diagnosis and prognosis. J. Inflamm. Res. 2019, 12, 175. [Google Scholar] [CrossRef]
- Shao, L.; Jacobs, A.R.; Johnson, V.V.; Mayer, L. Activation of CD8+ Regulatory T Cells by Human Placental Trophoblasts. J. Immunol. 2005, 174, 7539–7547. [Google Scholar] [CrossRef] [PubMed]
- Margni, A.; Tometten, M.; Klapp, B.F.; Blois, S.M.; Arck, P.C.; Joachim, R.; Kandil, J. Altering the Th1/Th2 Cytokine Profile Substitution with Dydrogesterone in Mice by Pregnancy Protective Effect of Progesterone Cells Abolishes the + Depletion of CD8. J. Immunol. Ref. 2018, 172, 5893–5899. [Google Scholar] [CrossRef]
- Goand, U.K.; Verma, S.; Patel, I.; Tasneem, S.; Garg, R.; Gayen, J.R. Immuno-metabolic effect of pancreastatin inhibitor PSTi8 in diet induced obese mice: In vitro and in vivo findings. Life Sci. 2023, 316, 121415. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Xu, X.H.; Jin, L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front. Immunol. 2019, 10, 792. [Google Scholar] [CrossRef] [PubMed]
- Mahata, S.K.; Corti, A. Chromogranin a and its fragments in cardiovascular, immunometabolic, and cancer regulation. In Annals of the New York Academy of Sciences; Blackwell Publishing Inc.: Malden, MA, USA, 2019; Volume 1455, pp. 34–58. [Google Scholar]
- Sakowicz, A.; Bralewska, M.; Pietrucha, T.; Habrowska-Górczyńska, D.E.; Piastowska-Ciesielska, A.W.; Gach, A.; Rybak-Krzyszkowska, M.; Witas, P.J.; Huras, H.; Grzesiak, M.; et al. Canonical, non-canonical and atypical pathways of nuclear factor кB activation in preeclampsia. Int. J. Mol. Sci. 2020, 21, 5574. [Google Scholar] [CrossRef]
- Straszewski-Chavez, S.L.; Abrahams, V.M.; Mor, G. The Role of Apoptosis in the Regulation of Trophoblast Survival and Differentiation during Pregnancy. Endocr. Rev. 2005, 26, 877–897. [Google Scholar] [CrossRef]
- Yu, D.; Hsieh, D.; Chang, S. Modulation of Prostate Carcinoma Cell Growth and Apoptosis By Chromogranin A. J. Urol. 2003, 170, 2031–2035. [Google Scholar] [CrossRef] [PubMed]
- Hooper, C.; Pocock, J.M. Chromogranin A activates diverse pathways mediating inducible nitric oxide expression and apoptosis in primary microglia. Neurosci. Lett. 2007, 413, 227–232. [Google Scholar] [CrossRef]
- Kingham, P.J.; Pocock, J.M. Microglial Apoptosis Induced by Chromogranin A Is Mediated by Mitochondrial Depolarisation and the Permeability Transition but Not by Cytochrome c Release. J. Neurochem. 2000, 74, 1452–1462. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Malhotra, S.S.; Malik, A.; Verma, S.; Chaudhary, P. Cell Signaling Pathways Involved During Invasion and Syncytialization of Trophoblast Cells. Am. J. Reprod. Immunol. 2016, 75, 361–371. [Google Scholar] [CrossRef]
- Chung, E.; Yeung, F.; Leinwand, L.A. Akt and MAPK signaling mediate pregnancy-induced cardiac adaptation. J. Appl. Physiol. 2012, 112, 1564. [Google Scholar] [CrossRef]
- Zhang, J.; Mei, F.; Zhao, L.; Zuo, T.; Hong, Y.; Li, M.; Yu, J.; Wang, W. Inhibition of the p38 MAPK pathway attenuates renal injury in pregnant rats with acute necrotizing pancreatitis. Immunol. Res. 2021, 69, 295–306. [Google Scholar] [CrossRef]
- Menon, R.; Papaconstantinou, J. p38 Mitogen activated protein kinase (MAPK): A new therapeutic target for reducing the risk of adverse pregnancy outcomes. Expert Opin. Ther. Targets 2016, 20, 1397–1412. [Google Scholar] [CrossRef]
- Quan, D.; Li, L.; Zuo, M. Efficacy of Low Molecular Heparin on Preeclampsia by Inhibiting Apoptosis of Trophoblasts via the p38MAPK Signaling Pathway. Comput. Math. Methods Med. 2021, 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pugazhenthi, S.; Boras, T.; Kay Meintzer, M.; Heidenreich, K.A.; Reusch, J.E.-B. Insulin-like Growth Factor I-mediated Activation of the Transcription Factor cAMP Response Element-binding Protein in PC12 Cells Involvement of P38 Mitogen-Activated Protein Kinase-Mediated Pathway. J. Biol. Chem. 1999, 274, 2829–2837. [Google Scholar] [CrossRef] [PubMed]
- Briest, F.; Grabowski, P. PI3K-AKT-mTOR-Signaling and beyond: The Complex Network in Gastroenteropancreatic Neuroendocrine Neoplasms. Theranostics 2014, 4, 336. [Google Scholar] [CrossRef]
- Münzberg, C.; Höhn, K.; Krndija, D.; Maaß, U.; Bartsch, D.K.; Slater, E.P.; Oswald, F.; Walther, P.; Seufferlein, T.; von Wichert, G. IGF-1 drives chromogranin A secretion via activation of Arf1 in human neuroendocrine tumour cells. J. Cell. Mol. Med. 2015, 19, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Armistead, B.; Kadam, L.; Drewlo, S.; Kohan-Ghadr, H.R. The Role of NFκB in Healthy and Preeclamptic Placenta: Trophoblasts in the Spotlight. Int. J. Mol. Sci. 2020, 21, 1775. [Google Scholar] [CrossRef]
- Fabi, F.; Grenier, K.; Parent, S.; Adam, P.; Tardif, L.; Leblanc, V.; Asselin, E. Regulation of the PI3K/Akt pathway during decidualization of endometrial stromal cells. PLoS ONE 2017, 12, e0177387. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Yu, Q. The PI3K/Akt signaling pathway exerts effects on the implantation of mouse embryos by regulating the expression of RhoA. Int. J. Mol. Med. 2014, 33, 1089–1096. [Google Scholar] [CrossRef]
- Liao, F.; Zheng, Y.; Cai, J.; Fan, J.; Wang, J.; Yang, J.; Cui, Q.; Xu, G.; Tang, C.; Geng, B. Catestatin attenuates endoplasmic reticulum induced cell apoptosis by activation type 2 muscarinic acetylcholine receptor in cardiac ischemia/reperfusion. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef]
- Pitt, S.C.; Chen, H.; Kunnimalaiyaan, M. Inhibition of Phosphatidylinositol 3-Kinase/Akt Signaling Suppresses Tumor Cell Proliferation and Neuroendocrine Marker Expression in GI Carcinoid Tumors. Ann. Surg. Oncol. 2009, 16, 2936. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sharp, A.N.; Heazell, A.E.P.; Crocker, I.P.; Mor, G. Placental Apoptosis in Health and Disease. Am. J. Reprod. Immunol. 2010, 64, 159. [Google Scholar] [CrossRef] [PubMed]
- Rabbi, M.F.; Eissa, N.; Munyaka, P.M.; Kermarrec, L.; Elgazzar, O.; Khafipour, E.; Bernstein, C.N.; Ghia, J.E. Reactivation of intestinal inflammation is suppressed by catestatin in a murine model of colitis via M1 macrophages and not the gut microbiota. Front. Immunol. 2017, 8, 985. [Google Scholar] [CrossRef] [PubMed]
- Lokeswara, A.W.; Hiksas, R.; Irwinda, R.; Wibowo, N. Preeclampsia: From Cellular Wellness to Inappropriate Cell Death, and the Roles of Nutrition. Front. Cell Dev. Biol. 2021, 9, 3031. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bralewska, M.; Pietrucha, T.; Sakowicz, A. Chromogranin A: An Endocrine Factor of Pregnancy. Int. J. Mol. Sci. 2023, 24, 4986. https://doi.org/10.3390/ijms24054986
Bralewska M, Pietrucha T, Sakowicz A. Chromogranin A: An Endocrine Factor of Pregnancy. International Journal of Molecular Sciences. 2023; 24(5):4986. https://doi.org/10.3390/ijms24054986
Chicago/Turabian StyleBralewska, Michalina, Tadeusz Pietrucha, and Agata Sakowicz. 2023. "Chromogranin A: An Endocrine Factor of Pregnancy" International Journal of Molecular Sciences 24, no. 5: 4986. https://doi.org/10.3390/ijms24054986
APA StyleBralewska, M., Pietrucha, T., & Sakowicz, A. (2023). Chromogranin A: An Endocrine Factor of Pregnancy. International Journal of Molecular Sciences, 24(5), 4986. https://doi.org/10.3390/ijms24054986






