Genomic Assessment of the Contribution of the Wolbachia Endosymbiont of Eurosta solidaginis to Gall Induction
Abstract
1. Introduction
2. Results
2.1. Parsing wEsol and E. solidaginis Contigs
2.2. Features of the wEsol Genome
2.3. Horizontal Gene Transfer of wEsol DNA into Eurosta Contigs
2.4. Phylogenetic Placement of wEsol and Strain Origin
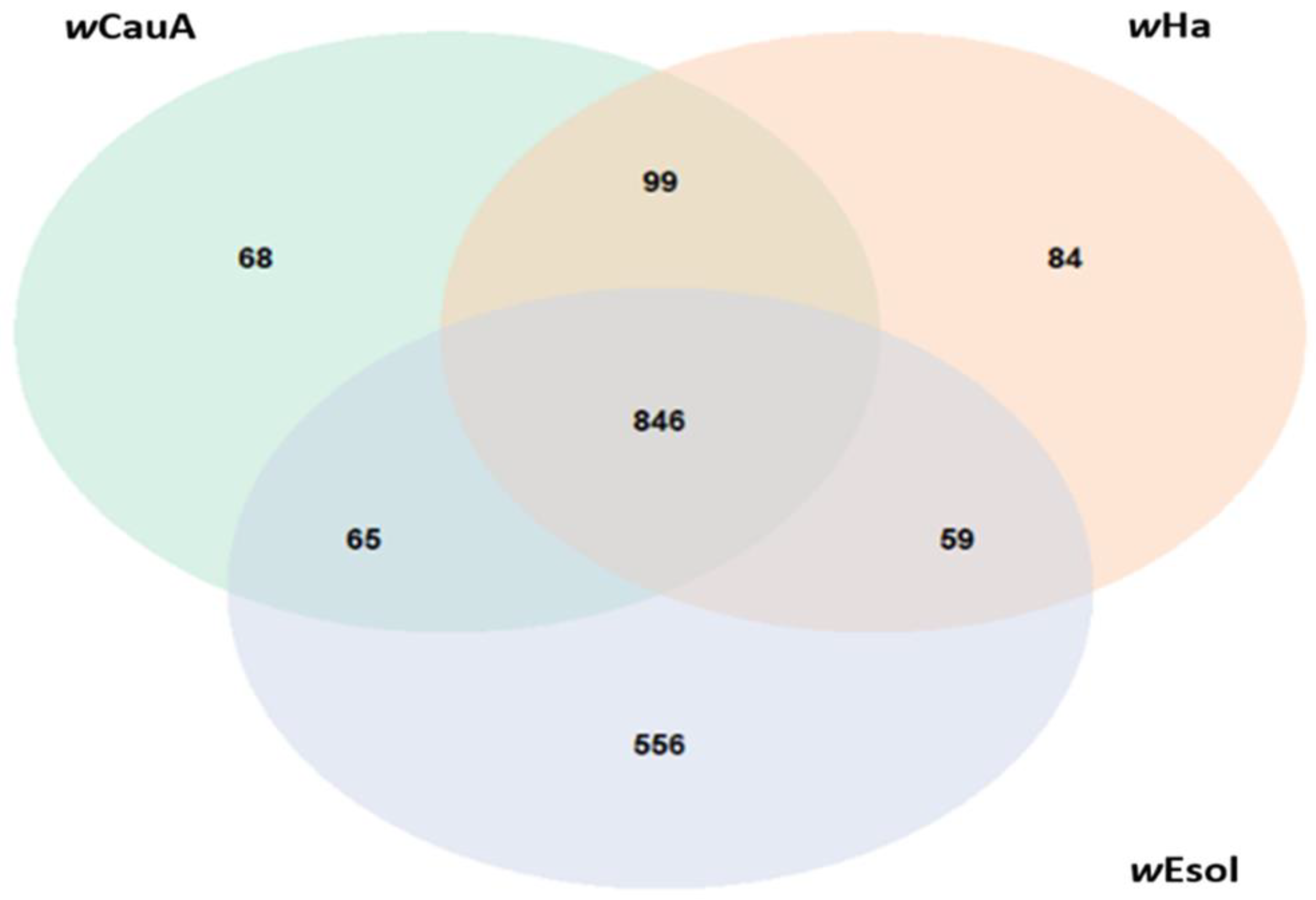
2.5. Comparative Genomics
2.6. Potential Contribution of wEsol to Gall Induction by E. solidaginis
2.6.1. Phytohormone Biosynthesis Proteins
2.6.2. Secreted Effector Proteins
3. Discussion
3.1. General Features of the wEsol Genome
3.2. Does wEsol Contribute to Gall Induction by Eurosta solidaginis?
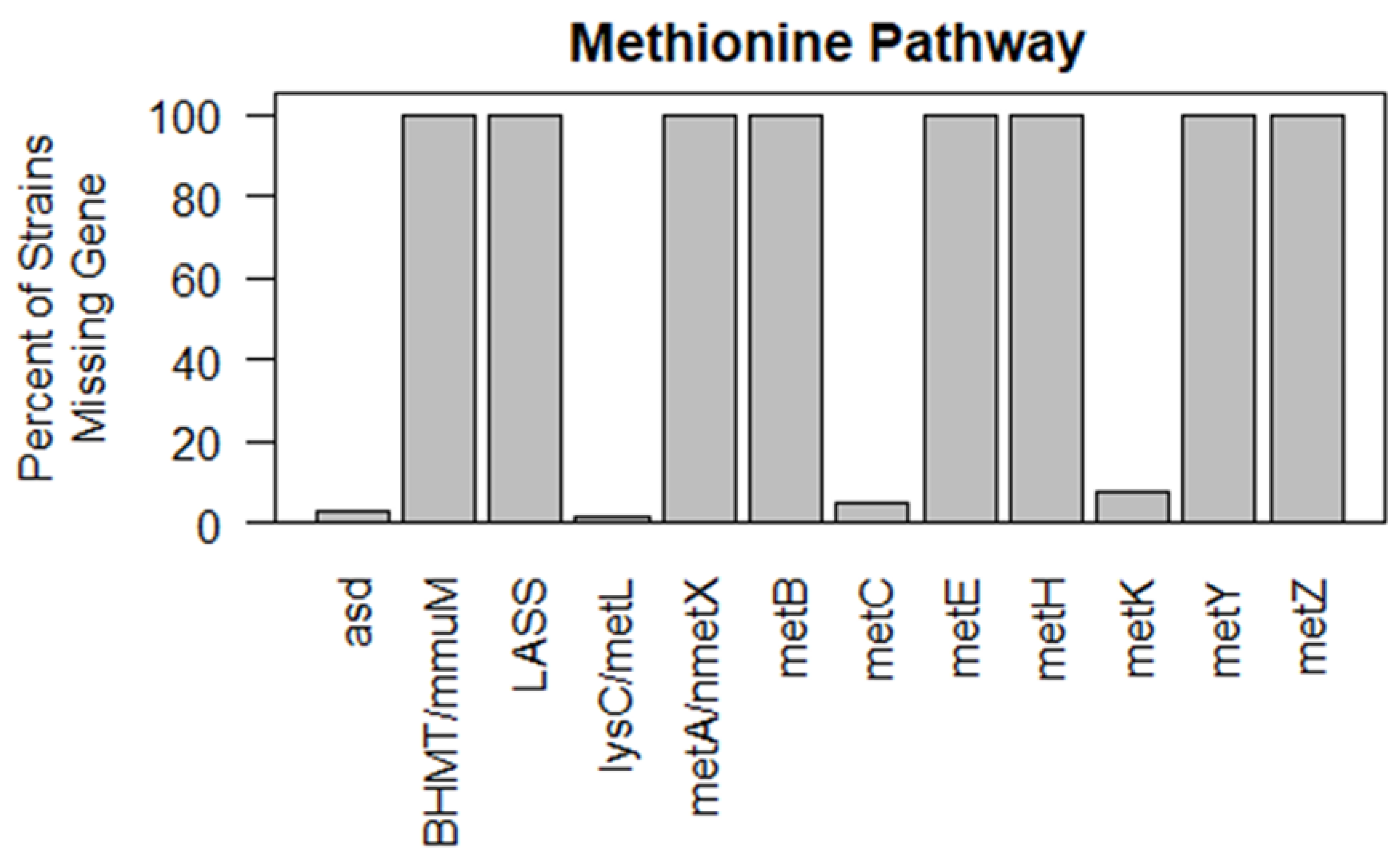
3.2.1. wEsol and Phytohormone Synthesis
3.2.2. Bacterial Effectors and Gall Induction
4. Materials and Methods
4.1. Genome Assembly
4.2. Identification of wEsol Contigs and Putative Instances of HGT
4.3. Genome Annotation
4.4. Coverage Patterns
4.5. Phylogenetic Placement of wEsol and Host Relationships
4.6. Genome Comparisons
4.7. Potential Contribution of wEsol to Gall Induction by E. solidaginis
4.7.1. Phytohormone Biosynthesis Proteins
4.7.2. Effector Proteins
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foster, J.; Ganatra, M.; Kamal, I.; Ware, J.; Makarova, K.; Ivanova, N.; Bhattacharyya, A.; Kapatral, V.; Kumar, S.; Posfai, J.; et al. The Wolbachia genome of Brugia malayi: Endosymbiont evolution within a human pathogenic nematode. PLoS Biol. 2005, 3, e121. [Google Scholar] [CrossRef] [PubMed]
- Moran, N. Symbiosis as an adaptive process and source of phenotypic complexity. Proc. Natl. Acad. Sci. USA 2007, 104, 8627–8633. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, A.C.; Cesar, C.S.; Martins, M.; Cogni, R. The antiviral effects of the symbiont bacteria Wolbachia in insects. Front. Immunol. 2021, 11, 626329. [Google Scholar] [CrossRef] [PubMed]
- Yen, J.H.; Barr, A.R. New hypothesis of the cause of cytoplasmic incompatibility in Culex pipiens L. Nature 1971, 232, 657–658. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H. Biology of Wolbachia. Annu. Rev. Entomol. 1997, 42, 587–609. [Google Scholar] [CrossRef]
- Stouthamer, R.; Breeuwer, J.A.; Hurst, G.D. Wolbachia pipientis: Microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 1999, 53, 71–102. [Google Scholar] [CrossRef]
- Hussain, M.; Frentiu, F.D.; Moreira, L.A.; O’Neill, S.L.; Asgari, S. Wolbachia uses host microRNAs to manipulate host gene expression and facilitate colonization of the dengue vector Aedes aegypti. Proc. Natl. Acad. Sci. USA 2011, 108, 9250–9255. [Google Scholar] [CrossRef]
- Goodacre, S.L.; Martin, O.Y. Modification of insect and arachnid behaviours by vertically transmitted endosymbionts: Infections as drivers of behavioral change and evolutionary novelty. Insects 2012, 3, 246–261. [Google Scholar] [CrossRef]
- Kozek, W.; Rao, R.U. The discovery of Wolbachia in arthropods and nematodes: A historical perspective. In Wolbachia: Issues in Infective Diseases; Hoerauf, A., Rao, R.U., Eds.; Karger: Basel, Switzerland, 2007; Volume 5, pp. 1–14. [Google Scholar] [CrossRef]
- Werren, J.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef]
- Zug, R.; Hammerstein, P. Still a host of hosts for Wolbachia: Analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE 2012, 7, e38544. [Google Scholar] [CrossRef]
- Weinert, L.A.; Araujo-Jnr, E.V.; Ahmed, M.Z.; Welch, J.J. The incidence of bacterial endosymbionts in terrestrial arthropods. Proc. R. Soc. B Biol. Sci. 2015, 282, 20150249. [Google Scholar] [CrossRef]
- Mani, M.S. Ecology of Plant Galls; Springer: Dordrecht, The Netherlands, 1964. [Google Scholar] [CrossRef]
- MacDonald, E.M.S.; Powell, G.K.; Regier, D.A.; Glass, N.L.; Roberto, F.; Kosuge, T.; Morris, R.O. Secretion of zeatin, ribosylzeatin, and ribosyl-1”-methylzeatin by Pseudomonas savastanoi. Plant Physiol. 1986, 82, 742–747. [Google Scholar] [CrossRef]
- Zhu, J.; Oger, P.M.; Schrammeijer, B.; Hooykaas, P.J.J.; Farrand, S.K.; Winans, S.C. The bases of crown gall tumorigenesis. J. Bacteriol. 2000, 182, 3885–3895. [Google Scholar] [CrossRef]
- Stone, G.; Schönrogge, K. The adaptive significance of insect gall morphology. Trends Ecol. Evol. 2003, 18, 512–522. [Google Scholar] [CrossRef]
- De Lillo, E.; Montfreda, R. ‘Salivary secretions’ of eriophyoids (Acari: Eriophyoidea): First results of an experimental model. Exp. Appl. Acarol. 2004, 34, 291–306. [Google Scholar] [CrossRef]
- Reineke, G.; Heinze, B.; Schiraski, J.; Buettner, H.; Kahmann, R.; Basse, C.W. Indole-3-acetic acid (IAA) biosynthesis in the smut fungus Ustilago maydis and its relevance for increased IAA levels in infected tissue and host tumor formation. Mol. Plant Pathol. 2008, 9, 339–355. [Google Scholar] [CrossRef]
- Ludwig-Müller, J.; Prinsen, E.; Rolfe, S.A.; Scholes, J.D. Metabolism and plant hormone action during clubroot disease. J. Plant Growth Regul. 2009, 28, 229–244. [Google Scholar] [CrossRef]
- Bruce, S.A.; Saville, B.J.; Emery, R.J.N. Ustilago maydis produces cytokinins and abscisic acid for potential regulation of tumor formation in maize. J. Plant Growth Regul. 2011, 30, 51–63. [Google Scholar] [CrossRef]
- Dodueva, I.E.; Lebedeva, M.A.; Kuznetsova, K.A.; Gancheva, M.S.; Paponova, S.S.; Lutova, L.L. Plant tumors: A hundred years of study. Planta 2020, 251, 82. [Google Scholar] [CrossRef]
- Hammer, T.J.; De Clerck-Floate, R.; Tooker, J.F.; Price, P.W.; Miller, D.G., III; Connor, E.F. Are bacterial symbionts associated with gall induction in insects? Arthropod-Plant Interact. 2021, 15, 1–12. [Google Scholar] [CrossRef]
- Giron, D.; Frago, E.; Glevarec, G.; Pieterse, C.M.J.; Dicke, M. Cytokinins as key regulators in plant–microbe–insect interactions: Connecting plant growth and defence. Funct. Ecol. 2013, 27, 599–609. [Google Scholar] [CrossRef]
- Bartlett, L.; Connor, E.F. Exogenous phytohormones and the induction of plant galls by insects. Arthropod-Plant Interact. 2014, 8, 339–348. [Google Scholar] [CrossRef]
- Best, V.M.; Vasanthakumar, A.; McManus, P.S. Anatomy of cranberry stem gall and localization of bacteria in galls. Phytopathology 2004, 94, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Contreras, M.; Vlisidou, I. The diversity of insect-bacteria interactions and its applications for disease control. Biotechnol. Genet. Eng. Rev. 2008, 25, 203–244. [Google Scholar] [CrossRef]
- Zeidan, M.; Crosnek, H. Acquisition and transmission of Agrobacterium by the whitefly Bemisia tabaci. Mol. Plant-Microbe Interact. 1994, 7, 792–798. [Google Scholar] [CrossRef]
- Wells, M.L.; Gitaitis, R.D.; Sanders, F.H. Association of tobacco thrips, Frankliniella fusca (Thysanoptera: Thripidae) with two species of bacteria of the genus Pantoea. Ann. Entomol. Soc. Am. 2002, 95, 719–723. [Google Scholar] [CrossRef]
- Dillon, R.; Charnley, K. Mutualism between the desert locust Schistocerca gregaria and its gut microbiota. Res. Microbiol. 2002, 153, 503–509. [Google Scholar] [CrossRef]
- Giron, D.; Kaiser, W.; Imbault, N.; Casas, J. Cytokinin-mediated leaf manipulation by a leafminer caterpillar. Biol. Lett. 2007, 3, 340–343. [Google Scholar] [CrossRef]
- Kaiser, W.; Huguet, E.; Casas, J.; Commin, C.; Giron, D. Plant green-island phenotype induced by leaf-miners is mediated by bacterial symbionts. Proc. R. Soc. B Biol. Sci. 2010, 277, 2311–2319. [Google Scholar] [CrossRef]
- Body, M.; Kaiser, W.; Dubreuil, G.; Casas, J.; Giron, D. Leaf-miners co-opt microorganisms to enhance their nutritional environment. J. Chem. Ecol. 2013, 39, 969–977. [Google Scholar] [CrossRef]
- Zhang, H.; Guiguet, A.; Dubreil, G.; Kisiala, A.; Andreas, P.; Emery, R.J.N.; Body, M.; Giron, D. Dynamics and origin of cytokinins involved in plant manipulation by a leaf-mining insect. Insect Sci. 2017, 24, 1065–1078. [Google Scholar] [CrossRef]
- Lichter, A.; Barash, I.; Valinsky, L.; Manulis, S. The genes involved in cytokinin biosynthesis in Erwinia herbicola pv. gypsophilae: Characterization and role in gall formation. J. Bacteriol. 1995, 177, 4457–4465. [Google Scholar] [CrossRef]
- Jameson, P. Cytokinins and auxins in plant pathogen interactions—An overview. Plant Growth Regul. 2000, 32, 369–380. [Google Scholar] [CrossRef]
- Barash, I.; Manulis-Sasson, S. Virulence mechanisms and host-specificity of gall forming Pantoea agglomerans. Trends Microbiol. 2007, 15, 538–545. [Google Scholar] [CrossRef]
- Barash, I.; Manulis-Sasson, S. Recent evolution of bacterial pathogens: The gall-forming Pantoea agglomerans case. Annu. Rev. Phytopath. 2009, 47, 133–152. [Google Scholar] [CrossRef]
- Joshi, M.V.; Loria, R. Streptomyces turgidiscabies possesses a functional cytokinin biosynthetic pathway and produces leafy galls. Mol. Plant-Microbe Interact. 2007, 20, 751–758. [Google Scholar] [CrossRef]
- Jameson, P.E.; Dhandapani, P.; Song, J.; Zatloukal, M.; Strnad, M.; Remus-Emsermann, M.N.P.; Schlechter, R.O.; Novák, O. The cytokinin complex associated with Rhodococcus fascians: Which compounds are critical for virulence? Front. Plant Sci. 2019, 10, 674. [Google Scholar] [CrossRef]
- Mapes, C.C.; Davies, P.J. Indole-3-acetic acid and ball gall development on Solidago altissima. New Phytol. 2001, 151, 195–202. [Google Scholar] [CrossRef]
- Mapes, C.C.; Davies, P.J. Cytokinins in the ball gall of Solidago altissima and the gall forming larvae of Eurosta solidaginis. New Phytol. 2001, 151, 203–212. [Google Scholar] [CrossRef]
- Dorchin, N.; Hoffman, J.; Stirk, W.; Novák, O.; Strnad, M.; van Staden, J. Sexually dimorphic gall structures correspond to differential phytohormone contents in male and female wasps. Physiol. Entomol. 2009, 34, 359–369. [Google Scholar] [CrossRef]
- Straka, J.; Hayward, A.; Emery, R. Gall-inducing Pachypsylla celtidis (Psyllidae) infiltrate hackberry trees with high concentrations of phytohormones. J. Plant Interact. 2010, 5, 197–203. [Google Scholar] [CrossRef]
- Tooker, J.F.; De Moraes, C.M. Feeding by a gall-inducing caterpillar species alters levels of indole-3-acetic acid and abscisic acid in Solidago altissima (Asteraceae) stems. Arthropod-Plant Interact. 2011, 5, 115–124. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Tanaka, H.; Hasegawa, M.; Tokuda, M.; Asami, T.; Suzuki, Y. Phytohormones and willow gall induction by a gall-inducing sawfly. New Phytol. 2012, 196, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Okada, K.; Asami, T.; Suzuki, Y. Phytohormones in Japanese mugwort gall induction by a gall-inducing gall midge. Biosci. Biotechnol. Biochem. 2013, 9, 1942–1948. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Yokokaura, J.; Ito, T.; Arai, R.; Yokoyama, C.; Toshima, H.; Nagat, S.; Asami, T.; Suzuki, Y. Biosynthetic pathway of the phytohormone auxin in insects and screening of its inhibitors. Insect Biochem. Mol. Biol. 2014, 53, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Takei, M.; Yoshida, S.; Kawai, T.; Hasegawa, M.; Suzuki, Y. Adaptive significance of gall formation for a gall-inducing aphids on Japanese elm trees. J. Insect Physiol. 2015, 72, 43–51. [Google Scholar] [CrossRef]
- Kai, S.; Kumashiro, S.; Adachi, S.; Suzuki, Y.; Shiomi, Y.; Matsunaga, K.; Gyoutoku, N.; Asami, T.; Tokuda, M. Life history of Stenopsylla nigricornis (Hemiptera: Psylloidea: Triozidae) and phytohormones involved in gall induction. Arthropod-Plant Interact. 2017, 11, 99–108. [Google Scholar] [CrossRef]
- Andreas, P.; Kisiala, A.; Emery, R.J.N.; De Clerck-Floate, R.; Tooker, J.F.; Price, P.W.; Miller, D.G., III; Chen, M.S.; Connor, E.F. Cytokinins are abundant and widespread among insect species. Plants 2020, 9, 208. [Google Scholar] [CrossRef]
- Jia, M.; Li, Q.; Hua, J.; Liu, J.; Zhou, W.; Qu, B.; Luo, S. Phytohormones regulate both “fish scale” galls and cones on Picea koraiensis. Front. Plant Sci. 2020, 11, 580155. [Google Scholar] [CrossRef]
- Tokuda, M.; Suzuki, Y.; Fujita, S.; Matsuda, H.; Adachi-Fukunaga, S.; Elsayed, A.K. Terrestrial arthropods broadly possess endogenous phytohormones auxin and cytokinins. Sci. Rep. 2022, 12, 4750. [Google Scholar] [CrossRef]
- Wang, W.; Guo, W.; Tang, J.; Li, X. Phytohormones in galls on eucalypt trees and in the gall-forming wasp Leptocybe invasa (Hymenoptera: Eulophidae). Agric. For. Entomol. 2022, 24, 609–617. [Google Scholar] [CrossRef]
- Siddique, S.; Radakovic, Z.S.; De La Torre, C.M.; Chronis, D.; Novák, O.; Ramireddy, E.; Holbein, J.; Matera, C.; Hütten, M.; Gutbrod, P.; et al. A parasitic nematode releases cytokinin that controls cell division and orchestrates feeding site formation in host plants. Proc. Natl. Acad. Sci. USA 2015, 112, 12669–12674. [Google Scholar] [CrossRef] [PubMed]
- Chanclud, E.; Kisiala, A.; Emery, R.J.N.; Chalvon, V.; Ducasse, A.; Romiti-Michel, C.; Gravot, A.; Kroj, T.; Morel, J. Cytokinin production by the rice blast fungus is a pivotal requirement for full virulence. PLoS Pathog. 2016, 12, e1005457. [Google Scholar] [CrossRef]
- Wu, J.; Baldwin, I.T. New insights into plant responses to the attack from insect herbivores. Annu. Rev. Genet. 2010, 44, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Navarro Escalante, L.; Chen, H.; Benatti, T.R.; Qu, J.; Chellapilla, S.; Waterhouse, R.M.; Wheeler, D.; Andersson, M.N.; Bao, R.; et al. A massive expansion of effector genes underlies gall-formation in the wheat pest Mayetiola destructor. Curr. Biol. 2015, 25, 613–620. [Google Scholar] [CrossRef]
- Cambier, S.; Ginis, O.; Moreau, S.J.M.; Gayral, P.; Hearn, J.; Stone, G.N.; Giron, D.; Huguet, E.; Drezen, J.M. Gall wasp transcriptomes unravel potential effectors involved in molecular dialogues with oak and rose. Front. Physiol. 2019, 10, 926. [Google Scholar] [CrossRef]
- Korgaonkar, A.; Han, C.; Lemire, A.L.; Siwanowicz, I.; Bennouna, D.; Kopec, R.E.; Andolfatto, P.; Shigenobu, S.; Stern, D.L. A novel family of secreted insect proteins linked to plant gall development. Curr. Biol. 2021, 31, 1836–1849. [Google Scholar] [CrossRef]
- Doehlemann, G.; Wahl, R.; Horst, R.J.; Voll, L.M.; Usadel, B.; Poree, F.; Stitt, M.; Pons-Kühnemann, J.; Sonnewald, U.; Kahmann, R.; et al. Reprogramming a maize plant: Transcriptional and metabolic changes induced by the fungal biotroph Ustilago maydis. Plant J. 2008, 56, 181–195. [Google Scholar] [CrossRef]
- Redkar, A.; Hoser, R.; Schilling, L.; Zechmann, B.; Krzymowska, M.; Walbot, V.; Doehlemann, G.A. secreted effector protein of Ustilago maydis guides maize leaf cells to form tumors. Plant Cell 2015, 27, 1332–1351. [Google Scholar] [CrossRef]
- Jimenez, N.E.; Gerdtzen, Z.P.; Olivera-Nappa, A.; Salgado, J.C.; Conca, C. A systems biology approach for studying Wolbachia metabolism reveals points of interaction with its host in the context of arboviral infection. PLoS Negl. Trop. Dis. 2019, 13, e0007678. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef]
- Pascar, J.; Chandler, C.H. A bioinformatics approach to identifying Wolbachia infections in arthropods. PeerJ 2018, 6, e5486. [Google Scholar] [CrossRef]
- Michonneau, F.; Brown, J.W.; Winter, D.J. rotl: An R package to interact with the Open Tree of Life data. Methods Ecol. Evol. 2016, 7, 1476–1481. [Google Scholar] [CrossRef]
- Mooi, N.; Roy, S.; Connor, E.F. A bioinformatic examination of cytokinin biosynthesis in insects. Arthropod-Plant Interact. 2022; in prep. [Google Scholar]
- Xie, G.; Keyhani, N.O.; Bonner, C.A.; Jensen, R.A. Ancient origin of the tryptophan operon and the dynamics of evolutionary change. Microbiol. Mol. Biol. Rev. 2003, 67, 303–342. [Google Scholar] [CrossRef]
- Yu, N.Y.; Wagner, J.R.; Laird, M.R.; Melli, G.; Rey, S.; Lo, R.; Dao, P.; Sahinalp, S.C.; Ester, M.; Foster, L.J.; et al. PSORTb 3.0: Improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 2010, 26, 1608–1615. [Google Scholar] [CrossRef]
- Sun, L.V.; Foster, J.M.; Tzertzinis, G.; Ono, M.; Bandi, C.; Slatko, B.E.; O’Neill, S.L. Determination of Wolbachia genome size by pulsed-field gel electrophoresis. J. Bacteriol. 2001, 183, 2219–2225. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Genome Table. Available online: https://www.ncbi.nlm.nih.gov/genome/browse/#!/prokaryotes/Wolbachia (accessed on 10 May 2020).
- Wu, M.; Sun, L.V.; Vamathevan, J.; Riegler, M.; Deboy, R.; Brownlie, J.C.; McGraw, E.A.; Martin, W.; Esser, C.; Ahmadinejad, N.; et al. Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: A streamlined genome overrun by mobile genetic elements. PLoS Biol. 2004, 2, E69. [Google Scholar] [CrossRef]
- Scholz, M.; Albanese, D.; Tuohy, K.; Donati, C.; Segata, N.; Rota-Stabelli, O. Large scale genome reconstruction illuminate Wolbachia evolution. Nat. Commun. 2020, 11, 5235. [Google Scholar] [CrossRef]
- Haine, E.R.; Cook, J.M. Convergent incidences of Wolbachia infection in fig wasp communities from two continents. Proc. R. Soc. B Biol. Sci. 2005, 272, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-H.; Zhu, D.-H.; Liu, Z.; Zhao, L.; Su, C.-Y. High levels of multiple infections, recombination and horizontal transmission of Wolbachia in the Andricus mukaigawae (Hymenoptera; Cynipidae) Communities. PLoS ONE 2013, 8, e78970. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.Z.; Breinholt, J.W.; Kawahara, A.Y. Evidence for common horizontal transmission of Wolbachia among butterflies and moths. BMC Evol. Biol. 2016, 16, 118. [Google Scholar] [CrossRef] [PubMed]
- Tolley, S.J.A.; Nonacs, P.; Sapountzis, P. Wolbachia horizontal transmission events in ants: What do we know and what can we learn? Front. Microbiol. 2019, 10, 296. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Nikoh, N.; Ijichi, N.; Shimada, M.; Fukatsu, T. Genome fragment of Wolbachia endosymbiont transferred to X chromosome of host insect. Proc. Natl. Acad. Sci. USA 2002, 99, 14280–14285. [Google Scholar] [CrossRef]
- Nikoh, N.; Tanaka, K.; Shibata, F.; Kondo, N.; Hizume, M.; Shimada, M.; Fukatsu, T. Wolbachia genome integrated in an insect chromosome: Evolution and fate of laterally transferred endosymbiont genes. Genome Res. 2008, 8, 272–280. [Google Scholar] [CrossRef]
- Klasson, L.; Kambris, Z.; Cook, P.E.; Walker, T.; Sinkins, S.P. Horizontal gene transfer between Wolbachia and the mosquito Aedes aegypti. BMC Genomics 2009, 10, 33. [Google Scholar] [CrossRef]
- Brelsfoard, C.; Tsiamis, G.; Falchetto, M.; Gomulski, L.M.; Telleria, E.; Alam, U.; Doudoumis, V.; Scolari, F.; Benoit, J.B.; Swain, M.; et al. Presence of extensive Wolbachia symbiont insertions discovered in the genome of its host Glossina morsitans morsitans. PLoS. Negl. Trop. Dis. 2014, 8, e2728. [Google Scholar] [CrossRef]
- Hotopp, J.C.D.; Clark, M.E.; Oliveira, D.C.S.G.; Foster, J.M.; Fischer, P.; Muñoz Torres, M.C.; Giebel, J.D.; Kumar, N.; Ishmael, N.; Wang, S.; et al. Widespread lateral gene transfer from intracellular bacteria to multicellular eukaryotes. Science 2007, 317, 1753–1756. [Google Scholar] [CrossRef]
- Hotopp, J.C.D.; Slatko, B.E.; Foster, J.M. Targeted enrichment and sequencing of recent endosymbiont-host lateral gene transfers. Sci. Rep. 2017, 7, 857. [Google Scholar] [CrossRef]
- Bordenstein, S.R.; Bordenstein, S.R. Eukaryotic association module in phage WO genomes from Wolbachia. Nat. Commun. 2016, 7, 13155. [Google Scholar] [CrossRef]
- Ponce, G.E.; Fuse, M.; Chan, A.; Connor, E.F. The localization of phytohormones within the gall-inducing insect Eurosta solidaginis (Diptera: Tephritidae). Arthropod-Plant Interact. 2021, 15, 375–385. [Google Scholar] [CrossRef]
- Ferla, M.P.; Patrick, W.M. Bacterial methionine synthesis. Microbiology 2014, 160, 1571–1584. [Google Scholar] [CrossRef]
- Driscoll, T.P.; Verhoeve, V.I.; Guillotte, M.L.; Lehman, S.S.; Rennoll, S.A.; Beier-Sexton, M.; Rahman, M.S.; Azad, M.F.; Gillespie, J.J. Wholly Rickettsia! Reconstructed metabolic profile of the quintessential bacterial parasite of eukaryotic cells. mBio 2017, 8, e00859-17. [Google Scholar] [CrossRef]
- Ahyong, V.; Berdan, C.A.; Burke, T.P.; Nomura, D.K.; Welch, M.D. A metabolic dependency for host isoprenoids in the obligate intracellular pathogen Rickettsia parkeri underlies a sensitivity to the statin class of host-targeted therapeutics. mSphere 2019, 4, e00536-19. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, B.; Yan, H.; Wen, F.; Qin, D.; Jander, G.; Xia, Q.; Wang, G. Systemic disruption of the homeostasis of transfer RNA isopentenyltransferase causes growth and development abnormalities in Bombyx mori. Insect Mol. Biol. 2019, 28, 380–391. [Google Scholar] [CrossRef]
- Bellés, X.; Martín, D.; Piulachs, M.-D. The mevalonate pathway and the synthesis of juvenile hormone in insects. Annu. Rev. Entomol. 2005, 50, 181–199. [Google Scholar] [CrossRef]
- Hayashi, Y.; Kashio, S.; Murotomi, K.; Hino, S.; Kang, W.; Miyado, K.; Nakao, M.; Miura, M.; Kobayashi, S.; Namihira, M. Biosynthesis of S-adenosyl-methionine enhances aging-related defects in Drosophila oogenesis. Sci. Rep. 2022, 12, 5593. [Google Scholar] [CrossRef]
- Schweizer, U.; Bohleber, S.; Fradejas-Villar, N. The modified base isopentenyladenosine and its derivatives in tRNA. RNA Biol. 2017, 14, 1197–1208. [Google Scholar] [CrossRef]
- Reiter, V.; Matschal, D.M.S.; Wagner, M.; Globisch, D.; Kneuttinger, A.C.; Muller, M.; Carell, T. The CDK5 repressor CDK5RAP1 is a methylthiotransferase acting on nuclear and mitochondrial RNA. Nucleic Acids Res. 2012, 40, 6235–6240. [Google Scholar] [CrossRef]
- Gibb, M.; Kisiala, A.B.; Morrison, E.N.; Emery, R.J.N. The origins and roles of methylthiolated cytokinins: Evidence from among life kingdoms. Front. Cell Dev. Biol. 2020, 8, 605672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Jin, T.; Sahi, S.K.; Xu, J.; Shi, Q.; Liu, H.; Wang, Y. The distribution of tryptophan-dependent indole-3-acetic acid synthesis pathways in bacteria unraveled by large-scale genomic analysis. Molecules 2019, 24, 1411. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, C.; Takei, M.; Kozuma, Y.; Nagata, S.; Suzuki, Y. Novel tryptophan metabolic pathways in auxin biosynthesis in silkworm. J. Insect Physiol. 2017, 101, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Takei, M.; Kogure, S.; Yokoyama, C.; Kouzuma, Y.; Suzuki, Y. Identification of an aldehyde oxidase involved in indole-3-acetic acid synthesis in Bombyx mori silk gland. Biosci. Biotech. Biochem. 2019, 83, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Miyata, U.; Arakawa, K.; Takei, M.; Asami, T.; Asanbou, K.; Oshima, H.; Suzuki, Y. Identification of an aromatic aldehyde synthase involved in indole-3-acetic acid biosynthesis in the galling sawfly (Pontania sp.) and screening of an inhibitor. Insect Biochem. Mol. Biol. 2021, 137, 103639. [Google Scholar] [CrossRef] [PubMed]
- Mayoral, J.G.; Hussain, M.; Joubert, D.A.; Iturbe-Ormaetxeb, I.; O’Neill, S.L.; Asgari, S. Wolbachia small noncoding RNAs and their role in cross-kingdom communications. Proc. Natl. Acad. Sci. USA 2014, 111, 18721–18726. [Google Scholar] [CrossRef] [PubMed]
- Jernigan, K.K.; Bordenstein, S.R. Ankyrin domains across the tree of life. PeerJ 2014, 2, e264. [Google Scholar] [CrossRef]
- Rice, D.W.; Sheehan, K.B.; Newton, I.L.G. Large-scale identification of Wolbachia pipientis effectors. Genome Biol. Evol. 2017, 9, 1925–1937. [Google Scholar] [CrossRef]
- Mateos, M.; Martinez Montoya, H.; Lanzavecchia, S.B.; Conte, C.; Guillén, K.; Morán-Aceves, B.M.; Toledo, J.; Liedo, P.; Asimakis, E.D.; Doudoumis, V.; et al. Wolbachia pipientis associated with Tephritid fruit fly pests: From basic research to applications. Front. Microbiol. 2020, 11, 1080. [Google Scholar] [CrossRef]
- Bing, X.L.; Xia, C.B.; Ye, Q.T.; Gong, X.; Cui, J.R.; Peng, C.W.; Hong, X.Y. Wolbachia manipulates reproduction of spider mites by influencing herbivore salivary proteins. Pest Manag. Sci. 2023, 79, 315–323. [Google Scholar] [CrossRef]
- Zhao, D.X.; Zhang, X.F.; Chen, D.S.; Zhang, Y.K.; Hong, X.Y. Wolbachia-host interactions: Host mating patterns affect Wolbachia density dynamics. PLoS ONE 2013, 8, e66373. [Google Scholar] [CrossRef]
- Hearn, J.; Blaxter, M.; Schönrogge, K.; Nieves-Aldrey, J.L.; Pujade-Villar, J.; Huguet, E.; Drezen, J.M.; Shorthouse, J.D.; Stone, G.N. Genomic dissection of an extended phenotype: Oak galling by a cynipid gall wasp. PLoS Genet. 2019, 15, e1008398. [Google Scholar] [CrossRef]
- Hou, H.Q.; Zhao, G.Z.; Su, C.Y.; Zhu, D.H. Wolbachia prevalence patterns: Horizontal transmission, recombination, and multiple infections in chestnut gall wasp-parasitoid communities. Entomol. Exp. Appl. 2020, 168, 752–765. [Google Scholar] [CrossRef]
- Klimov, P.B.; Chetverikov, P.E.; Dodueva, I.E.; Vishnyakov, A.E.; Bolton, S.J.; Poponova, S.S.; Lutova, L.A.; Tolstikov, A.V. Symbiotic bacteria of the gall-inducing mite Fragariocoptes setiger (Eriophyoidea) and phylogenomic resolution of the eriophyoid position among Acari. Sci. Rep. 2022, 12, 3811. [Google Scholar] [CrossRef]
- Kolmogorov, M.; Yuan, J.; Lin, Y.; Pevzner, P. Assembly of long error-prone reads using repeat graphs. Nat. Biotechnol 2019, 37, 540–546. [Google Scholar] [CrossRef]
- Laczny, C.C.; Kiefer, C.; Galata, V.; Fehlmann, T.; Backes, C.; Keller, A. BusyBee WeB: Metagenomic data analysis by bootstrapped supervised binning and annotation. Nucleic Acids Res. 2017, 3, W171–W179. [Google Scholar] [CrossRef]
- Sharp, P.M.; Li, W.H. The codon adaptation index—A measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 1987, 15, 1281–1295. [Google Scholar] [CrossRef]
- Hyatt, D.; Chen, G.L.; LoCascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef]
- Lowe, T.M.; Eddy, S.R. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997, 25, 955–964. [Google Scholar] [CrossRef]
- Nawrocki, E.P. Structural RNA Homology Search and Alignment Using Covariance Models. Ph.D. Dissertation, Washington University, St. Louis, MO, USA, 2009. [Google Scholar]
- Huang, Y.; Gilna, P.; Li, W. Identification of ribosomal RNA genes in metagenomic fragments. Bioinformatics 2009, 25, 1338–1340. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using Diamond. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Tatusov, R.L.; Natale, D.A.; Garkavtsev, I.V.; Tatusova, T.A.; Shankavaram, U.T.; Rao, B.S.; Kiryutin, B.; Galperin, M.Y.; Fedorova, N.D.; Koonin, E.V. The COG database: New developments in phylogenetic classification of proteins from complete genomes. Nucleic Acids Res. 2001, 29, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Haft, D.H.; Loftus, B.J.; Richardson, D.L.; Yang, F.; Eisen, J.A.; Paulsen, I.T.; White, O. TIGRFAMs: A protein family resource for the functional identification of proteins. Nucleic Acids Res. 2001, 29, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Eddy, S.R. Accelerated profile HMM searches. PLoS Comput. Biol. 2011, 7, e1002195. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Li, H.; Durban, R. Fast and accurate short read alignment with Burrows-Wheeler algorithm. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar] [CrossRef]
- Faddeeva-Vakhrusheva, A.; Kraaijeveld, K.; Derks, M.F.L.; Anvar, S.Y.; Agamennone, V.; Suring, W.; Kampfraath, A.A.; Ellers, J.; Ngoc, G.L.; van Gestel, C.A.M.; et al. Coping with living in the soil: The genome of the parthenogenetic springtail Folsomia candida. BMC Genom. 2017, 18, 493. [Google Scholar] [CrossRef]
- Parks, D.H.; Rinke, C.; Chuvochina, M.; Chaumeil, P.; Woodcroft, B.J.; Evans, P.N.; Waite, D.W.; Hugenholtz, P.; Tyson, G.W. Recovery of nearly 8,000 metagenome-assembled genomes substantially expands the tree of life. Nat. Microbiol. 2017, 2, 1533–1542. [Google Scholar] [CrossRef]
- Parks, D.H.; Chuvochina, M.; Waite, D.W.; Rinke, C.; Skarshewski, A.; Chaumeil, P.; Hugenholtz, P. A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat. Biotechnol. 2018, 36, 996–1004. [Google Scholar] [CrossRef]
- Parks, D.H.; Chuvochina, M.; Waite, D.W.; Rinke, C.; Mussig, A.J.; Hugenholtz, P. A complete domain-to-species taxonomy for Bacteria and Archaea. Nat. Biotechnol. 2020, 38, 1079–1086. [Google Scholar] [CrossRef]
- Chaumeil, P.A.; Mussig, A.J.; Hugenholtz, P.; Parks, D.H. GTDB-Tk: A toolkit to classify genomes with the Genome Taxonomy Database. Bioinformatics 2019, 36, 1925–1927. [Google Scholar] [CrossRef]
- Minh, B.Q.; Nguyen, M.A.T.; von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 30 January 2023).
- McTavish, E.J.; Hinchliff, C.E.; Allman, J.F.; Brown, J.W.; Cranston, K.A.; Holder, M.T.; Rees, J.A.; Smith, S.A. Phylesystem: A git-based data store for community curated phylogenetic estimates. Bioinformatics 2015, 31, 2794–2800. [Google Scholar] [CrossRef]
- Rees, J.; Cranston, K. Automated assembly of a reference taxonomy for phylogenetic data synthesis. Biodivers. Data J. 2017, 5, e12581. [Google Scholar] [CrossRef]
- Redelings, B.D.; Holder, M.T. A supertree pipeline for summarizing phylogenetic and taxonomic information for millions of species. PeerJ 2017, 5, e3058. [Google Scholar] [CrossRef]
- Revell, L.J. phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 2012, 3, 217–223. [Google Scholar] [CrossRef]
- Li, L.; Stoeckert, C.J., Jr.; Roos, D.S. OrthoMCL: Identification of ortholog groups for eukaryotic genomes. Genome Res. 2003, 13, 2178–2189. [Google Scholar] [CrossRef]
- Contreras-Moreira, B.; Vinuesa, P. Get_homologues, a versatile software packages for scalable and robust microbial pangenome analysis. Appl. Environ. Microbiol. 2013, 79, 7696–7701. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Petty, N.K.; Beatson, S.A. Easyfig: A genome comparison visualizer. Bioinformatics 2011, 27, 1009–1010. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019, 28, 1947–1951. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Ishiguro-Watanabe, M.; Tanabe, M. KEGG: Integrating viruses and cellular organisms. Nucleic Acids Res. 2021, 49, D545–D551. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, H. Cytokinins: Activity, biosynthesis, and translocation. Annu. Rev. Plant Biol. 2006, 57, 431–449. [Google Scholar] [CrossRef]
- Galuszka, P.; Spíchal, L.; Kopečný, D.; Tarkowski, P.; Frébortová, J.; Šebela, M.; Frébort, I. Metabolism of plant hormones cytokinins and their functions signaling, cell differentiation and plant development. St. Nat. Prod. Chem. 2008, 34, 203–263. [Google Scholar] [CrossRef]
- Frébort, I.; Kowalska, M.; Hluska, T.; Frébortová, J.; Galuszka, T. Evolution of cytokinin biosynthesis and degradation. J. Exp. Bot. 2011, 62, 2431–2452. [Google Scholar] [CrossRef]
- Morrison, E.N.; Knowles, S.; Thorn, R.G.; Saville, B.J.; Emery, R.J.N. Detection of phytohormones in temperate forest fungi predicts consistent abscisic acid production and a common pathway for cytokinin biosynthesis. Mycologia 2015, 107, 245–257. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Nguyen, T.Q.; Kisiala, A.B.; Emery, R.J.N. Beyond transport: Cytokinin ribosides are translocated and active in regulating the development and environmental responses of plants. Planta 2021, 254, 45. [Google Scholar] [CrossRef]
- Mok, D.W.S.; Mok, M.C. Cytokinin metabolism and action. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 89–118. [Google Scholar] [CrossRef]
- Frébortová, J.; Frébort, I. Biochemical and structural aspects of cytokinin biosynthesis and degradation in bacteria. Microorganisms 2021, 9, 1314. [Google Scholar] [CrossRef]
- Bartel, B. Auxin biosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 51–66. [Google Scholar] [CrossRef]
- Woodward, A.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef]
- Wang, B.; Chu, J.; Tianying, Y.; Xu, Q.; Sun, X.; Yuan, J.; Xiong, G.; Wang, G.; Wang, Y.; Jiayang, L. Tryptophan-independent auxin biosynthesis contributes to early embryogenesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2015, 122, 4821–4826. [Google Scholar] [CrossRef]
- Nonhebel, H.M. Tryptophan-independent indole-3-acetic acid synthesis: Critical evaluation of the evidence. Plant Physiol. 2015, 169, 1001–1005. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Xiong, Y.; Wang, H.; Deng, Z.; Song, J. T4SEfinder: A bioinformatics tool for genome-scale prediction of bacterial type IV secreted effectors using pre-trained protein language model. Brief. Bioinform. 2022, 23, bbab420. [Google Scholar] [CrossRef]




| Genome size | 1,664,961 bp |
| Predicted CDSs | 1878 |
| Average gene length | 723 bp |
| Percent coding | 81.5 |
| Assigned function | 1406 |
| Multispecies hypothetical | 184 |
| Unknown function | 472 |
| Hypothetical | 245 |
| Transfer RNAs | 35 |
| Ribosomal RNAs | 1 each of 5S, 16S, and 23S |
| Prophage | 7 |
| GC content | 35.3% |
| Ankyrin-repeat-domain-containing proteins | 63 |
| Proteins encoded by mobile genetic elements (transposase, recombinase, integrase) | 307 |
| Species | Strain | Super Group | GenBank Accession |
|---|---|---|---|
| Pseudomyrmex sp. PSW-54 PC | - | A | SRR1742977 |
| Acromyrmex echinatior PC | - | A | ERR034186, ERR034187 |
| Formica exsecta | wFex | A | GCA_003704235.1 |
| Cardiocondyla obscurior | wCobs | A | GCA_902713635.1 |
| Nomada panzeri | wNpa | A | GCA_001675775.1 |
| Nomada ferruginata | wNfe | A | GCA_001675785.1 |
| Pediaspis aceris 1 PC | - | A | ERR1355090 |
| Biorhiza pallida 1 PC | - | A | ERR233308 |
| Nasonia vitripennis | wVitA | A | GCA_001983615.1 |
| Mucidifurax uniraptor | wUni | A | GCA_000174095.1 |
| Diachasma alloeum PC | - | A | SRR2042503, SRR2046752 |
| Drosophila ananassae | wRi | A | GCA_002907405.1 |
| Drosophila triauraria 1 PC | - | A | DRR061000 |
| Drosophila simulans 1 PC | - | A | ERR1597896 |
| Drosophila simulans | wHa | A | GCA_000376605.1 |
| Drosophila simulans | wAu | A | GCA_018690095.1 |
| Eurosta solidaginis | wEsol | A | GCA_029238795.1 |
| Drosophila melanogaster | wMel | A | GCA_000008025.1 |
| Drosophila yakuba 1 PC | - | A | SRR2318687 |
| Drosophila santomea | wSan | A | GCA_005862095.1 |
| Drosophila recens | wRec | A | GCA_000742435.1 |
| Rhagoletis zephyria PC | - | A | SRR3670117, SRR3670118, SRR3670120 |
| Rhagoletis pomonella PC | - | A | SRR3900841, SRR3901027 |
| Drosophila incompta | wInc cu | A | GCA_001758565.1 |
| Drosophila sturtevanti | wStv | A | GCA_014107475.1 |
| Dactylopius coccus | wDacA | A | GCA_001648025.1 |
| Megacopta cribraria PC | - | A | SRR1145746 |
| Diabrotica virgifera virgifera 1 PC | - | A | SRR1106544, SRR1106897, SRR1106898 |
| Carposina sasakii | wCauA | A | GCA_006542295.1 |
| Nasonia vitripennis | wVitB | B | GCA_000204545.1 |
| Isocolus centaureae 1 PC | - | B | ERR1359249 |
| Diplolepis spinosa 1 PC | - | B | ERR1359308 |
| Leptopilina clavipes | wLcla | B | GCA_006334525.1 |
| Trichogramma pretiosum | wTpre | B | GCA_001439985.1 |
| Gerris buenoi 1 PC | - | B | SRR1197265 |
| Mycopsylla fici 1 PC | - | B | SRR2954433 |
| Nilaparvata lugens | wLug | B | GCA_007115045.1 |
| Homalodisca vitripennis 1 PC | - | B | SRR941995, SRR941996, SRR9419967 |
| Laodelphax striatellus | wStri | B | GCA_001637495.1 |
| Diaphorina citri 1 PC | - | B | SRR183690, SRR189238 |
| Maconellicoccus hirsutus PC | - | B | ERR1189167 |
| Bemesia tabaci China 1 | - | B | GCA_003999585.1 |
| Dactylopius coccus | wDacB | B | GCA_001648015.1 |
| Ecitophya simulans PC | - | B | SRR4301374 |
| Diploeciton nevermanni PC | - | B | SRR4342174 |
| Callosobruchus chinensis PC | - | B | SRR949786, SRR952345 |
| Bembidion lapponicum PC | - | B | SRR2939026 |
| Operothoptera brumata | wBa | B | GCA_001266585.1 |
| Plutella australiana | wAus | B | GCA_002318985.1 |
| Delias oraia PC | - | B | SRR4341246 |
| Drosophila simulans | wNo | B | GCA_000376585.1 |
| Aedes albopictus | wAlbB | B | GCA_000242415.3 |
| Culex quinquefasciatus | wPipB | B | GCA_000156735.1 |
| Culex pipiens molestus | wPip Mol | B | GCA_000208785.1 |
| Anopheles gambiae PC | - | B | ERR1554834, ERR1554870, ERR1554906 |
| Onchocerca volvulus | wOv | C | GCA_000338375.1 |
| Onchocerca ochingi | wOo | C | GCA_000306885.1 |
| Cimex lectularius | wCle | F | GCA_000829315.1 |
| Brugia malayi | wBm | D | GCA_000008385.1 |
| Wuchereria bancrofti | wWb | D | GCA_002204235.2 |
| Folsomia candida Berlin | - | E | GCA_001931755.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiutek, N.; Couger, M.B.; Pirro, S.; Roy, S.W.; de la Torre, J.R.; Connor, E.F. Genomic Assessment of the Contribution of the Wolbachia Endosymbiont of Eurosta solidaginis to Gall Induction. Int. J. Mol. Sci. 2023, 24, 9613. https://doi.org/10.3390/ijms24119613
Fiutek N, Couger MB, Pirro S, Roy SW, de la Torre JR, Connor EF. Genomic Assessment of the Contribution of the Wolbachia Endosymbiont of Eurosta solidaginis to Gall Induction. International Journal of Molecular Sciences. 2023; 24(11):9613. https://doi.org/10.3390/ijms24119613
Chicago/Turabian StyleFiutek, Natalie, Matthew B. Couger, Stacy Pirro, Scott W. Roy, José R. de la Torre, and Edward F. Connor. 2023. "Genomic Assessment of the Contribution of the Wolbachia Endosymbiont of Eurosta solidaginis to Gall Induction" International Journal of Molecular Sciences 24, no. 11: 9613. https://doi.org/10.3390/ijms24119613
APA StyleFiutek, N., Couger, M. B., Pirro, S., Roy, S. W., de la Torre, J. R., & Connor, E. F. (2023). Genomic Assessment of the Contribution of the Wolbachia Endosymbiont of Eurosta solidaginis to Gall Induction. International Journal of Molecular Sciences, 24(11), 9613. https://doi.org/10.3390/ijms24119613







