Functionalization of and through Melanin: Strategies and Bio-Applications
Abstract
:1. Introduction
1.1. Melanin and Melanin-like Materials
1.1.1. Natural Melanin
1.1.2. Synthetic Melanin-like Materials
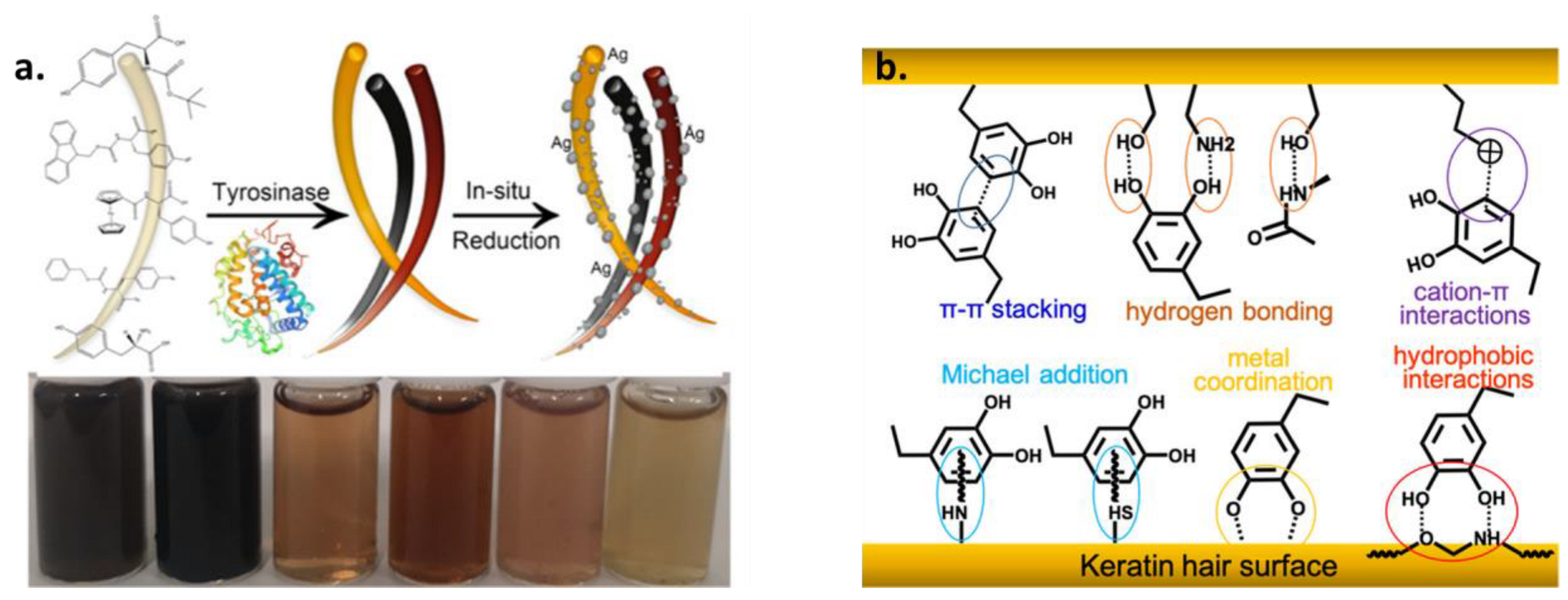
1.2. Melanin Surface Chemistry Governs the Functionalization Capability
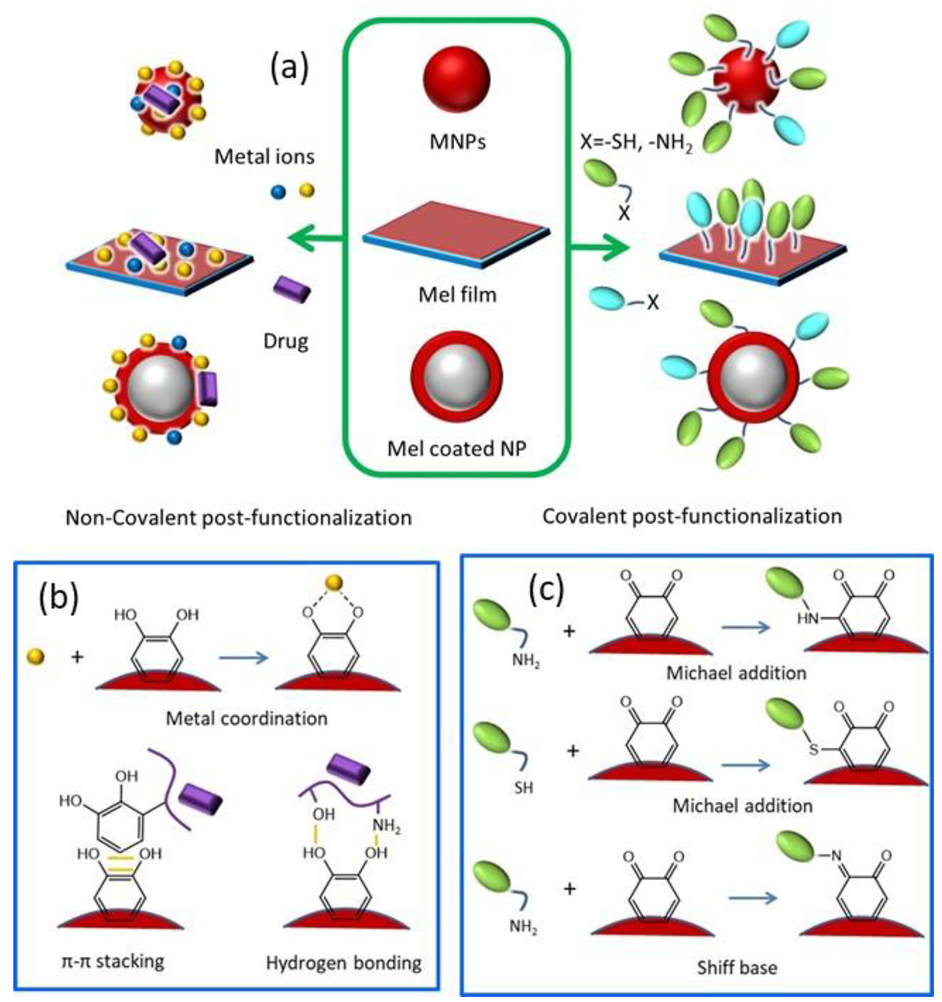
1.3. Pre- and Post-Functionalization of Melanin
2. Functionalization
2.1. Post-Functionalization of Melanin NPs and MNPs
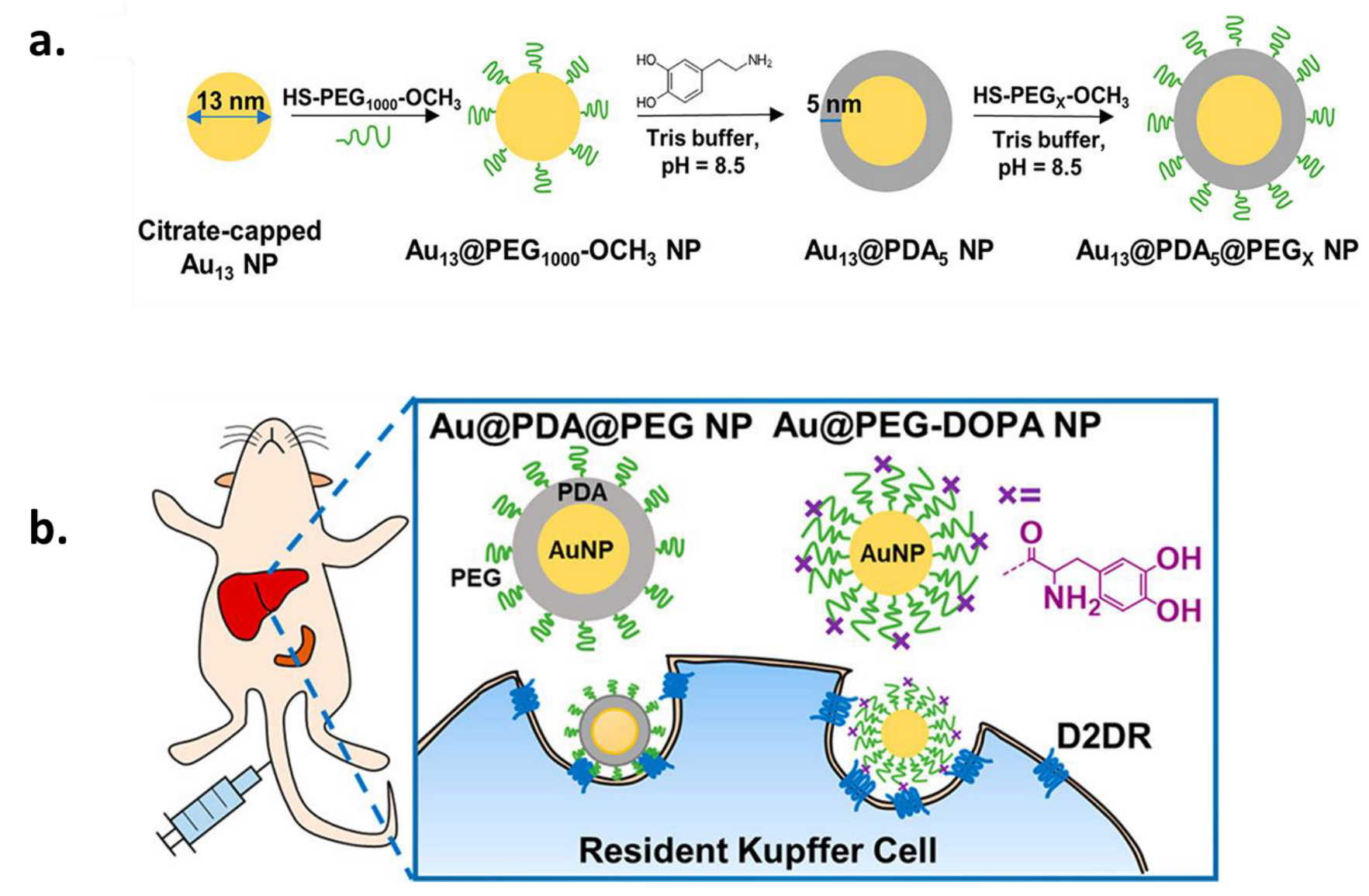
2.1.1. PEGylation for Increased Circulation Time and Improved Target Specificity
2.1.2. Metal-Functionalization
2.1.3. Gene Functionalization
2.1.4. Peptide-Grafting
2.2. Pre-Functionalization of Synthetic MNPs with Advanced Surface Properties: Co-Polymers
2.3. Functionalization through PDA: PDA as a Functional Coating of NPs and Surfaces
3. Perspectives
3.1. Purification and Characterization of the Produced Materials
3.2. Quantification and Localization of the Functional Unit
3.3. Dispersity of the Composition and Properties
3.4. Comparison of the Performances
3.5. Effect of the Functionalization Strategy
3.6. Effect of the Functionalization Order
3.7. Cross-Talk between the Functions
3.8. Possible Use of Different Melanin-like Materials
3.9. Cost Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fytianos, G.; Rahdar, A.; Kyzas, G.Z. Nanomaterials in Cosmetics: Recent Updates. Nanomaterials 2020, 10, 979. [Google Scholar] [CrossRef]
- Paramasivam, G.; Palem, V.V.; Sundaram, T.; Sundaram, V.; Kishore, S.C.; Bellucci, S. Nanomaterials: Synthesis and Applications in Theranostics. Nanomaterials 2021, 11, 3228. [Google Scholar] [CrossRef]
- Huang, R.; Shen, Y.W.; Guan, Y.Y.; Jiang, Y.X.; Wu, Y.; Rahman, K.; Zhang, L.J.; Liu, H.J.; Luan, X. Mesoporous silica nanoparticles: Facile surface functionalization and versatile biomedical applications in oncology. Acta Biomater. 2020, 116, 1–15. [Google Scholar] [CrossRef]
- Cao, K.; Cai, J.M.; Shan, B.; Chen, R. Surface functionalization on nanoparticles via atomic layer deposition. Sci. Bull. 2020, 65, 678–688. [Google Scholar] [CrossRef] [Green Version]
- Bahrami, A.; Delshadi, R.; Jafari, S.M. Active delivery of antimicrobial nanoparticles into microbial cells through surface functionalization strategies. Trends Food Sci. Technol. 2020, 99, 217–228. [Google Scholar] [CrossRef]
- Jiang, S.; Win, K.Y.; Liu, S.; Teng, C.P.; Zheng, Y.; Han, M.-Y. Surface-functionalized nanoparticles for biosensing and imaging-guided therapeutics. Nanoscale 2013, 5, 3127–3148. [Google Scholar] [CrossRef]
- Wang, C.; Fan, W.; Zhang, Z.; Wen, Y.; Xiong, L.; Chen, X. Advanced Nanotechnology Leading the Way to Multimodal Imaging-Guided Precision Surgical Therapy. Adv. Mater. 2019, 31, 1904329. [Google Scholar] [CrossRef]
- Escudé Martinez de Castilla, P.; Tong, L.; Huang, C.; Sofias, A.M.; Pastorin, G.; Chen, X.; Storm, G.; Schiffelers, R.M.; Wang, J.-W. Extracellular vesicles as a drug delivery system: A systematic review of preclinical studies. Adv. Drug Deliv. Rev. 2021, 175, 113801. [Google Scholar] [CrossRef]
- Rimpela, A.K.; Reinisalo, M.; Hellinen, L.; Grazhdankin, E.; Kidron, H.; Urtti, A.; del Amo, E.M. Implications of melanin binding in ocular drug delivery. Adv. Drug Deliv. Rev. 2018, 126, 23–43. [Google Scholar] [CrossRef]
- Liu, R.; Meng, X.; Mo, C.; Wei, X.; Ma, A. Melanin of fungi: From classification to application. World J. Microbiol. Biotechnol. 2022, 38, 228. [Google Scholar] [CrossRef]
- Kim, M.A.; Yoon, S.D.; Kim, E.M.; Jeong, H.J.; Lee, C.M. Natural melanin-loaded nanovesicles for near-infrared mediated tumor ablation by photothermal conversion. Nanotechnology 2018, 29, 415101. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, Y.; Liu, Y.; Pan, J.; Wang, J.; Man, F.; Zhang, W.; Liu, G. Melanin-Like Nanomaterials for Advanced Biomedical Applications: A Versatile Platform with Extraordinary Promise. Adv. Sci. 2020, 7, 1903129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavridi-Printezi, A.; Menichetti, A.; Guernelli, M.; Montalti, M. The Photophysics and Photochemistry of Melanin- Like Nanomaterials Depend on Morphology and Structure. Chem. A Eur. J. 2021, 27, 16309–16319. [Google Scholar] [CrossRef] [PubMed]
- Ghattavi, K.; Homaei, A.; Kamrani, E.; Kim, S.K. Melanin pigment derived from marine organisms and its industrial applications. Dye. Pigment. 2022, 201, 110214. [Google Scholar] [CrossRef]
- Tran-Ly, A.N.; Reyes, C.; Schwarze, F.; Ribera, J. Microbial production of melanin and its various applications. World J. Microbiol. Biotechnol. 2020, 36, 170. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. New insight into melanin for food packaging and biotechnology applications. Crit. Rev. Food Sci. Nutr. 2022, 62, 4629–4655. [Google Scholar] [CrossRef]
- Dube, T.; Kumar, N.; Bishnoi, M.; Panda, J.J. Dual Blood-Brain Barrier-Glioma Targeting Peptide-Poly (levodopamine) Hybrid Nanoplatforms as Potential Near Infrared Phototheranostic Agents in Glioblastoma. Bioconjugate Chem. 2021, 32, 2014–2031. [Google Scholar] [CrossRef]
- Yang, L.; Guo, X.; Jin, Z.; Guo, W.; Duan, G.; Liu, X.; Li, Y. Emergence of melanin-inspired supercapacitors. Nano Today 2021, 37, 101075. [Google Scholar] [CrossRef]
- Marcovici, I.; Coricovac, D.; Pinzaru, I.; Macasoi, I.G.; Popescu, R.; Chioibas, R.; Zupko, I.; Dehelean, C.A. Melanin and Melanin-Functionalized Nanoparticles as Promising Tools in Cancer Research—A Review. Cancers 2022, 14, 1838. [Google Scholar]
- Cheng, W.; Zeng, X.; Chen, H.; Li, Z.; Zeng, W.; Mei, L.; Zhao, Y. Versatile Polydopamine Platforms: Synthesis and Promising Applications for Surface Modification and Advanced Nanomedicine. ACS Nano 2019, 13, 8537–8565. [Google Scholar] [CrossRef]
- Yang, S.T.; Liu, Y.; Wang, Y.W.; Cao, A.N. Biosafety and Bioapplication of Nanomaterials by Designing ProteinNanoparticle Interactions. Small 2013, 9, 1635–1653. [Google Scholar] [CrossRef]
- Gao, M.; Fan, F.; Li, D.D.; Yu, Y.; Mao, K.R.; Sun, T.M.; Qian, H.S.; Tao, W.; Yang, X.Z. Tumor acidity-activatable TAT targeted nanomedicine for enlarged fluorescence/magnetic resonance imaging-guided photodynamic therapy. Biomaterials 2017, 133, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.Y.; Zhou, F.J.; Sheng, S.H.; Wei, Y.; Chen, X.; Su, J.C. Intra-articular nanodrug delivery strategies for treating osteoarthritis. Drug Discov. Today 2023, 28, 103482. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Cai, H.F.; Cui, Y.B.; Liu, W.G.; Xiao, C.S. Nanomedicine-Based Therapeutics to Combat Acute Lung Injury. Int. J. Nanomed. 2021, 16, 2247–2269. [Google Scholar] [CrossRef]
- Wu, W.C.; Pu, Y.Y.; Shi, J.L. Nanomedicine-enabled chemotherapy-based synergetic cancer treatments. J. Nanobiotechnol. 2022, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.T.; Zhang, Q.; Li, J.C.; Peng, S.J.; Wang, X.L.; Cai, R. Near-infrared photoactivated nanomedicines for photothermal synergistic cancer therapy. Nano Today 2021, 37, 101073. [Google Scholar] [CrossRef]
- Yang, H.Y.; Liu, R.F.; Xu, Y.X.; Qian, L.X.; Dai, Z.F. Photosensitizer Nanoparticles Boost Photodynamic Therapy for Pancreatic Cancer Treatment. Nano Micro Lett. 2021, 13, 35. [Google Scholar] [CrossRef]
- Yang, N.; Cao, C.Y.; Li, H.; Hong, Y.; Cai, Y.; Song, X.J.; Wang, W.N.; Mou, X.Z.; Dong, X.C. Polymer-Based Therapeutic Nanoagents for Photothermal-Enhanced Combination Cancer Therapy. Small Struct. 2021, 2, 2100110. [Google Scholar] [CrossRef]
- Gobin, A.M.; Lee, M.H.; Halas, N.J.; James, W.D.; Drezek, R.A.; West, J.L. Near-Infrared Resonant Nanoshells for Combined Optical Imaging and Photothermal Cancer Therapy. Nano Lett. 2007, 7, 1929–1934. [Google Scholar] [CrossRef]
- Czarnecka-Czapczynska, M.; Aebisher, D.; Oles, P.; Sosna, B.; Krupka-Olek, M.; Dynarowicz, K.; Latos, W.; Cieslar, G.; Kawczyk-Krupka, A. The role of photodynamic therapy in breast cancer-A review of in vitro research. Biomed. Pharmacother. 2021, 144, 112342. [Google Scholar] [CrossRef]
- Dennahy, I.S.; Han, Z.; MacCuaig, W.M.; Chalfant, H.M.; Condacse, A.; Hagood, J.M.; Claros-Sorto, J.C.; Razaq, W.; Holter-Chakrabarty, J.; Squires, R.; et al. Nanotheranostics for Image-Guided Cancer Treatment. Pharmaceutics 2022, 14, 917. [Google Scholar] [CrossRef]
- Shubayev, V.I.; Pisanic, T.R.; Jin, S.H. Magnetic nanoparticles for theragnostics. Adv. Drug Deliv. Rev. 2009, 61, 467–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, H.; Wu, F. Image Guided Biodistribution and Pharmacokinetic Studies of Theranostics. Theranostics 2012, 2, 1040–1053. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.Y.; Chen, Y. Computational Biomaterials: Computational Simulations for Biomedicine. Adv. Mater. 2023, 35, 2204798. [Google Scholar] [CrossRef] [PubMed]
- Zor, F.; Selek, F.N.; Orlando, G.; Williams, D.F. Biocompatibility in regenerative nanomedicine. Nanomedicine 2019, 14, 2763–2775. [Google Scholar] [CrossRef]
- Jin, A.; Wang, Y.; Lin, K.; Jiang, L. Nanoparticles modified by polydopamine: Working as “drug” carriers. Bioact. Mater. 2020, 5, 522–541. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.Y.; Cheng, H.; Wang, Z.; Miao, Z.Y.; Zhang, F.; Chen, J.; Wang, G.; Tao, L.; Zhou, J.P.; Zhang, H.Q.; et al. “Carrier-drug” layer-by-layer hybrid assembly of biocompatible polydopamine nanoparticles to amplify photo-chemotherapy. Nanoscale 2022, 14, 13740–13754. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Chu, J.C.H.; Chan, C.K.W.; Choi, C.H.J.; Ng, D.K.P. Reactive oxygen species-responsive polydopamine nanoparticles for targeted and synergistic chemo and photodynamic anticancer therapy. Nanoscale 2021, 13, 15899–15915. [Google Scholar] [CrossRef]
- Hashemi-Moghaddam, H.; Kazemi-Bagsangani, S.; Jamili, M.; Zavareh, S. Evaluation of magnetic nanoparticles coated by 5-fluorouracil imprinted polymer for controlled drug delivery in mouse breast cancer model. Int. J. Pharm. 2016, 497, 228–238. [Google Scholar] [CrossRef]
- Cao, W.; Zhou, X.; McCallum, N.C.; Hu, Z.; Ni, Q.Z.; Kapoor, U.; Heil, C.M.; Cay, K.S.; Zand, T.; Mantanona, A.J.; et al. Unraveling the Structure and Function of Melanin through Synthesis. J. Am. Chem. Soc. 2021, 143, 2622–2637. [Google Scholar] [CrossRef]
- D’Ischia, M.; Napolitano, A.; Pezzella, A.; Meredith, P.; Buehler, M. Melanin Biopolymers: Tailoring Chemical Complexity for Materials Design. Angew. Chem. Int. Ed. 2020, 59, 11196–11205. [Google Scholar] [CrossRef] [PubMed]
- Mavridi-Printezi, A.; Guernelli, M.; Menichetti, A.; Montalti, M. Bio-Applications of Multifunctional Melanin Nanoparticles: From Nanomedicine to Nanocosmetics. Nanomaterials 2020, 10, 2276. [Google Scholar] [CrossRef] [PubMed]
- D’Ischia, M.; Wakamatsu, K.; Napolitano, A.; Briganti, S.; Garcia-Borron, J.C.; Kovacs, D.; Meredith, P.; Pezzella, A.; Picardo, M.; Sarna, T.; et al. Melanins and melanogenesis: Methods, standards, protocols. Pigment Cell Melanoma Res. 2013, 26, 616–633. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.A.N.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Zhang, Q.L.; Zheng, D.W.; Zhang, C.; Zhang, Y.; Ye, J.J.; Cheng, H.; Zhang, X.Z. Enzyme Mimicking Based on the Natural Melanin Particles from Human Hair. Iscience 2020, 23, 100778. [Google Scholar] [CrossRef] [Green Version]
- Dossou, S.S.K.; Luo, Z.S.; Wang, Z.J.; Zhou, W.Y.; Zhou, R.; Zhang, Y.X.; Li, D.H.; Liu, A.L.; Dossa, K.; You, J.; et al. The Dark Pigment in the Sesame (Sesamum indicum L.) Seed Coat: Isolation, Characterization, and Its Potential Precursors. Front. Nutr. 2022, 28, 858673. [Google Scholar] [CrossRef]
- Eom, T.; Woo, K.; Shim, B.S. Melanin: A Naturally Existing Multifunctional Material. Appl. Chem. Eng. 2016, 27, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Zadlo, A.; Szewczyk, G.; Sarna, M.; Camenisch, T.G.; Sidabras, J.W.; Ito, S.; Wakamatsu, K.; Sagan, F.; Mitoraj, M.; Sarna, T. Photobleaching of pheomelanin increases its phototoxic potential: Physicochemical studies of synthetic pheomelanin subjected to aerobic photolysis. Pigment Cell Melanoma Res. 2019, 32, 359–372. [Google Scholar] [CrossRef]
- Deng, R.-H.; Zou, M.-Z.; Zheng, D.; Peng, S.-Y.; Liu, W.; Bai, X.-F.; Chen, H.-S.; Sun, Y.; Zhou, P.-H.; Zhang, X.-Z. Nanoparticles from Cuttlefish Ink Inhibit Tumor Growth by Synergizing Immunotherapy and Photothermal Therapy. ACS Nano 2019, 13, 8618–8629. [Google Scholar] [CrossRef]
- El-Naggar, N.E.; Saber, W.I.A. Natural Melanin: Current Trends, and Future Approaches, with Especial Reference to Microbial Source. Polymers 2022, 14, 1339. [Google Scholar] [CrossRef]
- Wu, J.X.; Zheng, Y.J.; Jiang, S.B.; Qu, Y.C.; Wei, T.; Zhan, W.J.; Wang, L.; Yu, Q.; Chen, H. Two-in-One Platform for High-Efficiency Intracellular Delivery and Cell Harvest: When a Photothermal Agent Meets a Thermoresponsive Polymer. ACS Appl. Mater. Interfaces 2019, 11, 12357–12366. [Google Scholar] [CrossRef] [PubMed]
- Ju, K.-Y.; Fischer, M.C.; Warren, W.S. Understanding the Role of Aggregation in the Broad Absorption Bands of Eumelanin. ACS Nano 2018, 12, 12050–12061. [Google Scholar] [CrossRef]
- Liu, Y.N.; Yu, B.R.; Dai, X.G.; Zhao, N.N.; Xu, F.J. Biomineralized calcium carbonate nanohybrids for mild photothermal heating-enhanced gene therapy. Biomaterials 2021, 274, 120885. [Google Scholar] [CrossRef]
- Xie, B.B.; Zhao, H.C.; Shui, M.J.; Ding, Y.F.; Sun, C.; Wang, Z.Y.; Gao, C.; Chen, G.S.; Wang, R.B. Spermine-Responsive Intracellular Self-Aggregation of Gold Nanocages for Enhanced Chemotherapy and Photothermal Therapy of Breast Cancer. Small 2022, 18, 2201971. [Google Scholar] [CrossRef]
- Liebscher, J. Chemistry of Polydopamine–Scope, Variation, and Limitation. Eur. J. Org. Chem. 2019, 2019, 4976–4994. [Google Scholar] [CrossRef]
- Almeida, L.C.; Correia, R.D.; Marta, A.; Squillaci, G.; Morana, A.; La Cara, F.; Correia, J.P.; Viana, A.S. Electrosynthesis of polydopamine films-tailored matrices for laccase-based biosensors. Appl. Surf. Sci. 2019, 480, 979–989. [Google Scholar] [CrossRef]
- Kohl, F.R.; Grieco, C.; Kohler, B. Ultrafast spectral hole burning reveals the distinct chromophores in eumelanin and their common photoresponse. Chem. Sci. 2020, 11, 1248–1259. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Liu, J.; Wang, Y.; Qi, W.; Su, R.; He, Z. Colorful Pigments for Hair Dyeing Based on Enzymatic Oxidation of Tyrosine Derivatives. ACS Appl. Mater. Interfaces 2021, 13, 34851–34864. [Google Scholar] [CrossRef]
- He, S.; Fang, J.; Zhong, C.X.; Ren, F.Z.; Wang, M. Controlled pVEGF delivery via a gene-activated matrix comprised of a peptide-modified non-viral vector and a nanofibrous scaffold for skin wound healing. Acta Biomater. 2022, 140, 149–162. [Google Scholar] [CrossRef]
- Bernsmann, F.; Ball, V.; Addiego, F.; Ponche, A.; Michel, M.; Gracio, J.J.d.A.; Toniazzo, V.; Ruch, D. Dopamine−Melanin Film Deposition Depends on the Used Oxidant and Buffer Solution. Langmuir 2011, 27, 2819–2825. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, Z.; Gao, D.; Luo, G.; Ma, T.; Wang, Y.; Lu, L.; Gao, X. Stepwise Coordination-Driven Metal-Phenolic Nanoparticle as a Neuroprotection Enhancer for Alzheimer’s Disease Therapy. ACS Appl. Mater. Interfaces 2023, 14, 524–540. [Google Scholar] [CrossRef]
- Ouyang, Z.X.; Tan, T.T.; Liu, C.F.; Duan, J.; Wang, W.C.; Guo, X.N.; Zhang, Q.; Li, Z.H.; Huang, Q.L.; Dou, P.C.; et al. Targeted delivery of hesperetin to cartilage attenuates osteoarthritis by bimodal imaging with Gd-2(CO3)(3)@PDA nanoparticles via TLR-2/NF-kappa B/Akt signaling. Biomaterials 2019, 205, 50–63. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Y.; Hu, Z.; Yue, X.; Proetto, M.T.; Jones, Y.; Gianneschi, N.C. Mimicking Melanosomes: Polydopamine Nanoparticles as Artificial Microparasols. ACS Cent. Sci. 2017, 3, 564–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, C.-C.; Ding, S.-J. The pH-controlled nanoparticles size of polydopamine for anti-cancer drug delivery. J. Mater. Sci. Mater. Med. 2013, 24, 2381–2390. [Google Scholar] [CrossRef] [PubMed]
- Nieto, C.; Vega, M.A.; Marcelo, G.; del Valle, E.M.M. Polydopamine nanoparticles kill cancer cells. RSC Adv. 2018, 8, 36201–36208. [Google Scholar] [CrossRef] [Green Version]
- Supanakorn, G.; Thiramanas, R.; Mahatnirunkul, T.; Wongngam, Y.; Polpanich, D. Polydopamine-Based Nanoparticles for Safe Sunscreen Protection Factor Products with Enhanced Performance. ACS Appl. Nano Mater. 2022, 5, 9084–9095. [Google Scholar] [CrossRef]
- Ju, K.-Y.; Lee, Y.; Lee, S.; Park, S.B.; Lee, J.-K. Bioinspired Polymerization of Dopamine to Generate Melanin-Like Nanoparticles Having an Excellent Free-Radical-Scavenging Property. Biomacromolecules 2011, 12, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Ge, Y.; Ye, Z.; Pan, X.; Yan, Y.; Mao, Z.; Ye, J. Anti-oxidative and mucin-compensating dual-functional nano eye drops for synergistic treatment of dry eye disease. Appl. Mater. Today 2022, 27, 101411. [Google Scholar] [CrossRef]
- Shi, H.; Jin, L.; Li, J.; Liang, K.; Li, X.; Ye, Z.; Zhu, X.; Oliveira, J.M.; Reis, R.L.; Mao, Z.; et al. Mesoporous polydopamine nanoparticles for sustained release of rapamycin and reactive oxygen species scavenging to synergistically accelerate neurogenesis after spinal cord injury. J. Mater. Chem. B 2022, 10, 6351–6359. [Google Scholar] [CrossRef]
- Wang, Y.; Shang, B.; Liu, M.; Shi, F.; Peng, B.; Deng, Z. Hollow polydopamine colloidal composite particles: Structure tuning, functionalization and applications. J. Colloid Interface Sci. 2018, 513, 43–52. [Google Scholar] [CrossRef]
- Zhou, X.; McCallum, N.C.; Hu, Z.; Cao, W.; Gnanasekaran, K.; Feng, Y.; Stoddart, J.F.; Wang, Z.; Gianneschi, N.C. Artificial Allomelanin Nanoparticles. ACS Nano 2019, 13, 10980–10990. [Google Scholar] [CrossRef]
- Zhou, X.; Gong, X.; Cao, W.; Forman, C.J.; Oktawiec, J.; D’Alba, L.; Sun, H.; Thompson, M.P.; Hu, Z.; Kapoor, U.; et al. Anisotropic Synthetic Allomelanin Materials via Solid-State Polymerization of Self-Assembled 1,8-Dihydroxynaphthalene Dimers. Angew. Chem. Int. Ed. 2021, 60, 17464–17471. [Google Scholar] [CrossRef]
- McCallum, N.C.; Son, F.A.; Clemons, T.D.; Weigand, S.J.; Gnanasekaran, K.; Battistella, C.; Barnes, B.E.; Abeyratne-Perera, H.; Siwicka, Z.E.; Forman, C.J.; et al. Allomelanin: A Biopolymer of Intrinsic Microporosity. J. Am. Chem. Soc. 2021, 143, 4005–4016. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Sci. N.Y. 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.D.; Hu, R.; Song, Y.C.; Gao, F.; Peng, W.X.; Zhang, Y.; Xie, Z.H.; Kang, J.; Zheng, Z.; Cao, Y.; et al. Hydrophilic modified polydopamine tailored heterogeneous polyamide in thin-film nanocomposite membranes for enhanced separation performance and anti-fouling properties. J. Membr. Sci. 2023, 666, 121124. [Google Scholar] [CrossRef]
- Xiao, M.; Shawkey, M.D.; Dhinojwala, A. Bioinspired Melanin-Based Optically Active Materials. Adv. Opt. Mater. 2020, 8, 2000932. [Google Scholar] [CrossRef]
- Svoboda, J.; Král, M.; Dendisová, M.; Matějka, P.; Pop-Georgievski, O. Unraveling the influence of substrate on the growth rate, morphology and covalent structure of surface adherent polydopamine films. Colloids Surf. B Biointerfaces 2021, 205, 111897. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Chen, J.; Lu, J.X.; Xi, J.Q.; Xu, Z.L.; Fan, L.; Dai, H.; Gao, L.Z. Metal ions/nucleotide coordinated nanoparticles comprehensively suppress tumor by synergizing ferroptosis with energy metabolism interference. J. Nanobiotechnol. 2022, 20, 199. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhou, J.J.; Chen, X.H.; Chen, Y.H.; Hou, S.; Qian, H.H.; Zhang, L.F.; Tang, G.P.; Chen, Z.; Ping, Y.; et al. Mesoporous polydopamine with built-in plasmonic core: Traceable and NIR triggered delivery of functional proteins. Biomaterials 2020, 238, 119847. [Google Scholar] [CrossRef]
- Gao, Y.F.; Cheng, Y.X.; Chen, J.P.; Lin, D.M.; Liu, C.; Zhang, L.K.; Yin, L.; Yang, R.C.; Guan, Y.Q. NIR-Assisted MgO-Based Polydopamine Nanoparticles for Targeted Treatment of Parkinson’s Disease through the Blood-Brain Barrier. Adv. Healthc. Mater. 2022, 11, 2201655. [Google Scholar] [CrossRef] [PubMed]
- Mancebo-Aracil, J.; Casagualda, C.; Moreno-Villaécija, M.Á.; Nador, F.; García-Pardo, J.; Franconetti-García, A.; Busqué, F.; Alibés, R.; Esplandiu, M.J.; Ruiz-Molina, D.; et al. Bioinspired Functional Catechol Derivatives through Simple Thiol Conjugate Addition. Chem. A Eur. J. 2019, 25, 12367–12379. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.A.; Park, E.; Lee, H. Polydopamine and Its Derivative Surface Chemistry in Material Science: A Focused Review for Studies at KAIST. Adv. Mater. 2020, 32, 1907505. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Xia, Z.; Deng, N.; Chen, L.; Zhang, H.; Lu, Y.; Liu, Y.; Gao, H. Eumelanin-inspired nanomaterials in electrochemical energy storage devices: A review. Chem. Eng. J. 2023, 452, 138607. [Google Scholar] [CrossRef]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine Surface Chemistry: A Decade of Discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, C.; Vitiello, G.; Adinolfi, B.; Silvestri, B.; Armanetti, P.; Manini, P.; Pezzella, A.; d’Ischia, M.; Luciani, G.; Menichetti, L. Melanin and Melanin-Like Hybrid Materials in Regenerative Medicine. Nanomaterials 2020, 10, 1518. [Google Scholar] [CrossRef]
- Wang, L.C.; Wang, Z.Q.; Pan, Y.Y.; Chen, S.S.; Fan, X.; Li, X.L.; Chen, G.Q.; Ma, Y.H.; Cai, Y.J.; Zhang, J.X.; et al. Polycatechol-Derived Mesoporous Polydopamine Nanoparticles for Combined ROS Scavenging and Gene Interference Therapy in Inflammatory Bowel Disease. ACS Appl. Mater. Interfaces 2022, 14, 19975–19987. [Google Scholar] [CrossRef]
- Davidsen, M.B.; Teixeira, J.F.L.; Dehli, J.; Karlsson, C.; Kraft, D.; Souza, P.P.C.; Foss, M. Post-treatments of polydopamine coatings influence cellular response. Colloids Surf. B Biointerfaces 2021, 207, 111972. [Google Scholar] [CrossRef]
- Tian, L.; Li, X.; Ji, H.; Yu, Q.; Yang, M.; Guo, L.; Huang, L.; Gao, W. Melanin-like nanoparticles: Advances in surface modification and tumour photothermal therapy. J. Nanobiotechnol. 2022, 20, 485. [Google Scholar] [CrossRef]
- Niezni, D.; Harris, Y.; Sason, H.; Avrashami, M.; Shamay, Y. Polydopamine Copolymers for Stable Drug Nanoprecipitation. Int. J. Mol. Sci. 2022, 23, 12420. [Google Scholar] [CrossRef]
- Xu, Y.N.; Fourniols, T.; Labrak, Y.; Preat, V.; Beloqui, A.; des Rieux, A. Surface Modification of Lipid-Based Nanoparticles. ACS Nano 2022, 16, 7168–7196. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, W.; Ji, H.; Tian, L.; Yang, M.; Wang, J.; Li, X.; Huang, L.; Guo, L.; Gao, W. Surface Membrane Coating as a Versatile Platform for Modifying Antitumor Nanoparticles. ACS Mater. Lett. 2022, 4, 2019–2050. [Google Scholar] [CrossRef]
- Qi, C.; Fu, L.H.; Xu, H.; Wang, T.F.; Lin, J.; Huang, P. Melanin/polydopamine-based nanomaterials for biomedical applications. Sci. China Chem. 2019, 62, 162–188. [Google Scholar] [CrossRef]
- Poinard, B.; Kamaluddin, S.; Tan, A.Q.Q.; Neoh, K.G.; Kah, J.C.Y. Polydopamine Coating Enhances Mucopenetration and Cell Uptake of Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 4777–4789. [Google Scholar] [CrossRef]
- Chen, A.Q.; Sun, J.H.; Liu, S.J.; Li, L.P.; Peng, X.Y.; Ma, L.X.; Zhang, R.P. The effect of metal ions on endogenous melanin nanoparticles used as magnetic resonance imaging contrast agents. Biomater. Sci. 2020, 8, 379–390. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Zheng, Y.; Zhang, H.; Sun, J.H.; Tan, C.P.; He, L.; Zhang, W.; Ji, L.N.; Mao, Z.W. Delivery of Phosphorescent Anticancer Iridium(III) Complexes by Polydopamine Nanoparticles for Targeted Combined Photothermal-Chemotherapy and Thermal/Photoacoustic/Lifetime Imaging. Adv. Sci. 2018, 5, 1800581. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Sun, J.; Li, L.; Peng, X.; Zhang, R.; Wang, B. Melanin-manganese nanoparticles with ultrahigh efficient clearance in vivo for tumor-targeting T1 magnetic resonance imaging contrast agent. Biomater. Sci. 2018, 6, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Zmerli, I.; Michel, J.P.; Makky, A. Bioinspired polydopamine nanoparticles: Synthesis, nanomechanical properties, and efficient PEGylation strategy. J. Mater. Chem. B 2020, 8, 4489–4504. [Google Scholar] [CrossRef]
- Luong, D.; Kesharwani, P.; Deshmukh, R.; Amin, M.; Gupta, U.; Greish, K.; Iyer, A.K. PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 2016, 43, 14–29. [Google Scholar] [CrossRef]
- Grigoletto, A.; Tedeschini, T.; Canato, E.; Pasut, G. The evolution of polymer conjugation and drug targeting for the delivery of proteins and bioactive molecules. WIREs Nanomed. Nanobiotechnol. 2021, 13, e1689. [Google Scholar] [CrossRef]
- Mishra, P.; Nayak, B.; Dey, R.K. PEGylation in anti-cancer therapy: An overview. Asian J. Pharm. Sci. 2016, 11, 337–348. [Google Scholar] [CrossRef] [Green Version]
- Ding, F.; Gao, X.H.; Huang, X.G.; Ge, H.; Xie, M.; Qian, J.W.; Song, J.; Li, Y.H.; Zhu, X.Y.; Zhang, C. Polydopamine-coated nucleic acid nanogel for siRNA-mediated low-temperature photothermal therapy. Biomaterials 2020, 245, 119976. [Google Scholar] [CrossRef]
- Poinard, B.; Lam, S.A.E.; Neoh, K.G.; Kah, J.C.Y. Mucopenetration and biocompatibility of polydopamine surfaces for delivery in an Ex Vivo porcine bladder. J. Control. Release 2019, 300, 161–173. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, Y.; Guo, R.; Yao, X.; Sung, S.; Pang, Z.; Yang, W. Erythrocyte-cancer hybrid membrane-camouflaged melanin nanoparticles for enhancing photothermal therapy efficacy in tumors. Biomaterials 2019, 192, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yue, Y.; Pan, D.; Yang, R.; Xu, Y.; Wang, L.; Yan, J.; Li, X.; Yang, M. Pharmacokinetics study of Zr-89-labeled melanin nanoparticle in iron-overload mice. Nucl. Med. Biol. 2016, 43, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Veronese, F.M.; Pasut, G. PEGylation, successful approach to drug delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Han, W.Y.; Han, X.; Liu, Z.Q.; Zhang, S.T.; Li, Y.; Lu, J.Y.; Chen, J.; Ou, L.L.; Fu, G.Q. Facile modification of protein-imprinted polydopamine coatings over nanoparticles with enhanced binding selectivity. Chem. Eng. J. 2020, 385, 123463. [Google Scholar] [CrossRef]
- Li, M.; Liang, Y.P.; Liang, Y.Q.; Pan, G.Y.; Guo, B.L. Injectable stretchable self-healing dual dynamic network hydrogel as adhesive anti-oxidant wound dressing for photothermal clearance of bacteria and promoting wound healing of MRSA infected motion wounds. Chem. Eng. J. 2022, 427, 132039. [Google Scholar] [CrossRef]
- Sunoqrot, S.; Mahmoud, N.N.; Ibrahim, L.H.; Al-Dabash, S.; Raschke, H.; Hergenroder, R. Tuning the Surface Chemistry of Melanin-Mimetic Polydopamine Nanoparticles Drastically Enhances Their Accumulation into Excised Human Skin. ACS Biomater. Sci. Eng. 2020, 6, 4424–4432. [Google Scholar] [CrossRef]
- Xu, N.; Hu, A.; Pu, X.M.; Wang, J.; Liao, X.M.; Huang, Z.B.; Yin, G.F. Cu-Chelated polydopamine nanoparticles as a photothermal medium and “immunogenic cell death” inducer for combined tumor therapy. J. Mater. Chem. B 2022, 10, 3104–3118. [Google Scholar] [CrossRef]
- Ho, L.W.C.; Liu, Y.; Han, R.F.; Bai, Q.Q.; Choi, C.H.J. Nano-Cell Interactions of Non-Cationic Bionanomaterials. Acc. Chem. Res. 2019, 52, 1519–1530. [Google Scholar] [CrossRef]
- Xiao, S.Y.; Tang, Y.F.; Lin, Y.M.; Lv, Z.; Chen, L. In vivo nano contrast-enhanced photoacoustic imaging for dynamically lightening the molecular changes of rheumatoid arthritis. Mater. Des. 2021, 207, 109862. [Google Scholar] [CrossRef]
- Zhou, Z.J.; Yan, Y.; Wang, L.; Zhang, Q.; Cheng, Y.Y. Melanin-like nanoparticles decorated with an autophagy-inducing peptide for efficient targeted photothermal therapy. Biomaterials 2019, 203, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Duan, Y.; Duan, Y.W. Application of polydopamine in tumor targeted drug delivery system and its drug release behavior. J. Control. Release 2018, 290, 56–74. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Y.; Zhu, P.; Xu, Z.H.; Chen, J. Dual-Responsive Dual-Drug-Loaded Bioinspired Polydopamine Nanospheres as an Efficient Therapeutic Nanoplatform against Drug-Resistant Cancer Cells. ACS Appl. Bio Mater. 2020, 3, 5730–5740. [Google Scholar] [CrossRef]
- Zmerli, I.; Ibrahim, N.; Cressey, P.; Denis, S.; Makky, A. Design and Synthesis of New PEGylated Polydopamine-Based Nanoconstructs Bearing ROS-Responsive Linkers and a Photosensitizer for Bimodal Photothermal and Photodynamic Therapies against Cancer. Mol. Pharm. 2021, 18, 3623–3637. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Zhang, J.; Chen, F.; Liu, J.; Cai, K. Mesoporous polydopamine nanoparticles with co-delivery function for overcoming multidrug resistance via synergistic chemo-photothermal therapy. Nanoscale 2017, 9, 8781–8790. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.P.; Gan, Y.; Zhu, P.D.; Li, S.S.; Lin, C.; Yu, S.L.; Zhao, S.; Shi, J.H.; Li, R.M.; Yuan, J.F. Hollow mesoporous polydopamine nanospheres: Synthesis, biocompatibility and drug delivery. Nanotechnology 2021, 32, 285602. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Liu, Y.; Zhou, J.E.; Lin, L.Z.; Jia, C.; Wang, J.; Yu, L.; Wang, Y.T.; Yan, Z.Q. Controllable hydrogen release for gas-assisted chemotherapy and ultrasonic imaging of drug-resistant tumors. Chem. Eng. J. 2021, 421, 129917. [Google Scholar] [CrossRef]
- Zhou, X.; Su, S.; Vanthournout, B.; Hu, Z.; Son, F.A.; Zhang, K.; Siwicka, Z.E.; Gong, X.; Paul, N.; Gnanasekaran, K.; et al. Hydrophobic Melanin via Post-Synthetic Modification for Controlled Self-Assembly. ACS Nano 2022, 16, 19087–19095. [Google Scholar] [CrossRef]
- Kapoor, U.; Jayaraman, A. Self-Assembly of Allomelanin Dimers and the Impact of Poly(ethylene glycol) on the Assembly: A Molecular Dynamics Simulation Study. J. Phys. Chem. B 2020, 124, 2702–2714. [Google Scholar] [CrossRef]
- Mo, X.H.; Xiang, H.J.; Wei, L.; Xia, L.H.; Chen, X.Y.; Chen, Y.; Zhang, B. Nature-inspired allomelanin nanomedicine alleviates cardiac ischemia/reperfusion injury via scavenging free radicals and ameliorating myocardial microenvironment. Nano Today 2022, 46, 101589. [Google Scholar] [CrossRef]
- Yu, H.L.; Yan, J.Q.; Li, Z.P.; Song, T.T.; Ning, F.; Tan, J.S.; Sun, Y. Enhanced photothermal-ferroptosis effects based on RBCm-coated PDA nanoparticles for effective cancer therapy. J. Mater. Chem. B 2023, 11, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zou, Y.; Li, Y.; Cheng, Y. Metal-Containing Polydopamine Nanomaterials: Catalysis, Energy, and Theranostics. Small 2020, 16, 1907042. [Google Scholar] [CrossRef]
- Zhan, Q.; Shi, X.; Zhou, J.; Zhou, L.; Wei, S. Drug-Controlled Release Based on Complementary Base Pairing Rules for Photodynamic–Photothermal Synergistic Tumor Treatment. Small 2019, 15, 1803926. [Google Scholar] [CrossRef]
- Lemaster, J.E.; Wang, Z.; Hariri, A.; Chen, F.; Hu, Z.Y.; Huang, Y.R.; Barback, C.V.; Cochran, R.; Gianneschi, N.C.; Jokerst, J.V. Gadolinium Doping Enhances the Photoacoustic Signal of Synthetic Melanin Nanoparticles: A Dual Modality Contrast Agent for Stem Cell Imaging. Chem. Mater. 2019, 31, 251–259. [Google Scholar] [CrossRef]
- Sun, T.; Jiang, D.; Rosenkrans, Z.T.; Ehlerding, E.B.; Ni, D.; Qi, C.; Kutyreff, C.J.; Barnhart, T.E.; Engle, J.W.; Huang, P.; et al. A Melanin-Based Natural Antioxidant Defense Nanosystem for Theranostic Application in Acute Kidney Injury. Adv. Funct. Mater. 2019, 29, 1904833. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xu, W.; Li, L.; Fan, B.; Peng, X.; Qu, B.; Wang, L.; Li, T.; Li, S.; Zhang, R. Ultrasmall endogenous biopolymer nanoparticles for magnetic resonance/photoacoustic dual-modal imaging-guided photothermal therapy. Nanoscale 2018, 10, 10584–10595. [Google Scholar] [CrossRef]
- Liu, H.; Chu, C.; Liu, Y.; Pang, X.; Wu, Y.; Zhou, Z.; Zhang, P.; Zhang, W.; Liu, G.; Chen, X. Novel Intrapolymerization Doped Manganese-Eumelanin Coordination Nanocomposites with Ultrahigh Relaxivity and Their Application in Tumor Theranostics. Adv. Sci. 2018, 5, 1800032. [Google Scholar] [CrossRef]
- Zou, Y.; Wu, T.; Li, N.; Guo, X.T.; Li, Y.W. Photothermal-enhanced synthetic melanin inks for near-infrared imaging. Polymer 2020, 186, 122042. [Google Scholar] [CrossRef]
- Xu, N.; Hu, A.; Pu, X.M.; Li, J.F.; Wang, X.M.; Wang, J.; Huang, Z.B.; Liao, X.M.; Yin, G.F. Fe(III)-Chelated Polydopamine Nanoparticles for Synergistic Tumor Therapies of Enhanced Photothermal Ablation and Antitumor Immune Activation. ACS Appl. Mater. Interfaces 2022, 14, 15894–15910. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Q.; He, X.; Chen, H.; Zou, Y.; Li, Y.; Lin, K.; Cai, X.; Xiao, J.; Zhang, Q.; et al. Multifunctional melanin-like nanoparticles for bone-targeted chemo-photothermal therapy of malignant bone tumors and osteolysis. Biomaterials 2018, 183, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, H.; Liu, Y.; Chu, C.; Yang, Y.; Zeng, Y.; Zhang, W.; Liu, G. Eumelanin–Fe3O4 hybrid nanoparticles for enhanced MR/PA imaging-assisted local photothermolysis. Biomater. Sci. 2018, 6, 586–595. [Google Scholar] [CrossRef]
- Liang, Y.; Li, R.; Sun, H.; Dan, J.; Su, Z.; Kang, Y.; Zhang, Q.; Shi, S.; Wang, J.; Zhang, W. Functionalized natural melanin nanoparticle mimics natural peroxidase for total antioxidant capacity determination. Sens. Actuators B Chem. 2022, 359, 131541. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, N.; Yu, W.; Xu, H.; Li, X.; Li, M.; Peng, C.; Wang, Q.; Zhu, M.; Chen, Z. In situ growth of Au nanoparticles on natural melanin as biocompatible and multifunctional nanoagent for efficient tumor theranostics. J. Mater. Chem. B 2019, 7, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Zandieh, M.; Liu, J.W. Metal-Doped Polydopamine Nanoparticles for Highly Robust and Efficient DNA Adsorption and Sensing. Langmuir 2021, 37, 8953–8960. [Google Scholar] [CrossRef]
- Cheng, W.Q.; Lin, Z.; Zhao, L.A.; Fan, N.K.; Bai, H.J.; Cheng, W.; Zhao, M.; Ding, S.J. CeO2/MXene heterojunction-based ultrasensitive electrochemiluminescence biosensing for BCR-ABL fusion gene detection combined with dual-toehold strand displacement reaction for signal amplification. Biosens. Bioelectron. 2022, 210, 114287. [Google Scholar] [CrossRef]
- Battistella, C.; McCallum, N.C.; Gnanasekaran, K.; Zhou, X.; Caponetti, V.; Montalti, M.; Gianneschi, N.C. Mimicking Natural Human Hair Pigmentation with Synthetic Melanin. ACS Cent. Sci. 2020, 6, 1179–1188. [Google Scholar] [CrossRef]
- Im, K.M.; Kim, T.-W.; Jeon, J.-R. Metal-Chelation-Assisted Deposition of Polydopamine on Human Hair: A Ready-to-Use Eumelanin-Based Hair Dyeing Methodology. ACS Biomater. Sci. Eng. 2017, 3, 628–636. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.F.; Wang, X.Y.; Gao, J.B.; Xia, F. Rapid preparation of polydopamine coating as a multifunctional hair dye. RSC Adv. 2019, 9, 20492–20496. [Google Scholar] [CrossRef] [Green Version]
- Alvi, S.B.; Sridharan, D.; Sanghvi, S.K.; Mergaye, M.; Ahmed, U.; Mikula, S.K.; Singh, H.; Khan, M. Modulation of Mitochondrial Bioenergetics by Polydopamine Nanoparticles in Human iPSC-Derived Cardiomyocytes. ACS Appl. Mater. Interfaces 2022, 14, 53451–53461. [Google Scholar] [CrossRef]
- Mao, W.; Hu, C.; Zheng, H.; Xie, J.; Shi, X.; Du, Y.; Wang, F. A Functionalized Polydopamine Theranostic Nanoprobe for Efficient Imaging of miRNA-21 and In Vivo Synergetic Cancer Therapy. Mol. Ther. Nucleic Acids 2020, 22, 27–37. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Yang, G.Z.; Zhu, D.Z.; Kong, H.; Corrales-Urena, Y.R.; Ciacchi, L.C.; Wei, G. Biomolecule-mimetic nanomaterials for photothermal and photodynamic therapy of cancers: Bridging nanobiotechnology and biomedicine. J. Nanobiotechnol. 2022, 20, 483. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Fan, B.; Gao, W.; Li, L.; Li, T.; Sun, J.; Peng, X.; Li, X.; Wang, Z.; Wang, B.; et al. Enhanced endosomal escape by photothermal activation for improved small interfering RNA delivery and antitumor effect. Int. J. Nanomed. 2018, 13, 4333–4344. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.Y.; Tang, Z.; Zhang, Y.; Wang, Q.X.; Liang, Z.G.; Zeng, X.W. Mussel-Inspired Polydopamine: The Bridge for Targeting Drug Delivery System and Synergistic Cancer Treatment. Macromol. Biosci. 2020, 20, 2000222. [Google Scholar] [CrossRef]
- Feng, J.; Yu, W.Q.; Xu, Z.; Hu, J.L.; Liu, J.; Wang, F.A. Multifunctional siRNA-Laden Hybrid Nanoplatform for Noninvasive PA/IR Dual-Modal Imaging-Guided Enhanced Photogenetherapy. ACS Appl. Mater. Interfaces 2020, 12, 22613–22623. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Zhang, M.; Wei, A.; Yin, F.; Wang, Y.; Hu, K.; Jiang, J. Doxorubicin and PD-L1 siRNA co-delivery with stem cell membrane-coated polydopamine nanoparticles for the targeted chemoimmunotherapy of PCa bone metastases. Nanoscale 2021, 13, 8998–9008. [Google Scholar] [CrossRef]
- Fan, B.; Yang, X.; Li, X.; Lv, S.; Zhang, H.; Sun, J.; Li, L.; Wang, L.; Qu, B.; Peng, X.; et al. Photoacoustic-imaging-guided therapy of functionalized melanin nanoparticles: Combination of photothermal ablation and gene therapy against laryngeal squamous cell carcinoma. Nanoscale 2019, 11, 6285–6296. [Google Scholar] [CrossRef]
- Yang, W.; Yan, J.Q.; Zhuang, P.Z.; Ding, T.; Chen, Y.; Zhang, Y.; Zhang, H.B.; Cui, W.G. Progress of delivery methods for CRISPR-Cas9. Expert Opin. Drug Deliv. 2022, 19, 913–926. [Google Scholar] [CrossRef]
- Ma, K.; Li, W.Z.; Zhu, G.; Sun, S.B.; Chi, H.; Yin, Y.L.; Diao, H.; Xing, X.J.; Guo, Z.M.; Wang, L.; et al. Functionalized PDA/DEX-PEI@HA nanoparticles combined with sleeping-beauty transposons for multistage targeted delivery of CRISPR/Cas9 gene. Biomed. Pharmacother. 2021, 142, 112061. [Google Scholar] [CrossRef]
- Wang, J.; Chen, P.; Dong, Y.; Xie, H.; Wang, Y.; Soto, F.; Ma, P.; Feng, X.; Du, W.; Liu, B.-F. Designer exosomes enabling tumor targeted efficient chemo/gene/photothermal therapy. Biomaterials 2021, 276, 121056. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, W.; Li, R.-Q.; Qiu, W.-X.; Zhuang, Z.-N.; Cheng, S.-X.; Zhang, X.-Z. Peptide-Based Multifunctional Nanomaterials for Tumor Imaging and Therapy. Adv. Funct. Mater. 2018, 28, 1804492. [Google Scholar] [CrossRef]
- Xue, S.; Zhou, X.J.; Sang, W.L.; Wang, C.; Lu, H.M.; Xu, Y.M.; Zhong, Y.M.; Zhu, L.B.; He, C.L.; Ma, J.Z. Cartilage-targeting peptide-modified dual-drug delivery nanoplatform with NIR laser response for osteoarthritis therapy. Bioact. Mater. 2021, 6, 2372–2389. [Google Scholar] [CrossRef] [PubMed]
- Cuzzubbo, S.; Roch, B.; Darrasse-Jeze, G.; Hosten, B.; Leclercq, M.; Vignal, N.; Banissi, C.; Tartour, E.; Carpentier, A.F. Synthetic Melanin Acts as Efficient Peptide Carrier in Cancer Vaccine Strategy. Int. J. Mol. Sci. 2022, 23, 14975. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Wang, X.L.; Zhou, J.F.; Shi, S.B.; Shen, T.F.; Chen, L.L.; Zhang, M.; Liao, C.S.; Wang, C. Development of PDA Nanoparticles for H9N2 Avian Influenza BPP-V/BP-IV Epitope Peptide Vaccines: Immunogenicity and Delivery Efficiency Improvement. Front. Immunol. 2021, 12, 693972. [Google Scholar] [CrossRef]
- Bao, B.Q.; Tong, L.; Xu, Y.; Zhang, J.J.; Zhai, X.; Su, P.; Weng, L.X.; Wang, L.H. Mussel-inspired functionalization of semiconducting polymer nanoparticles for amplified photoacoustic imaging and photothermal therapy. Nanoscale 2019, 11, 14727–14733. [Google Scholar] [CrossRef]
- Yuan, Z.; Tao, B.L.; He, Y.; Mu, C.Y.; Liu, G.H.; Zhang, J.X.; Liao, Q.; Liu, P.; Cai, K.Y. Remote eradication of biofilm on titanium implant via near-infrared light triggered photothermal/photodynamic therapy strategy. Biomaterials 2019, 223, 119479. [Google Scholar] [CrossRef]
- He, T.; He, J.; Younis, M.R.; Blum, N.T.; Lei, S.; Zhang, Y.L.; Huang, P.; Lin, J. Dual-Stimuli-Responsive Nanotheranostics for Dual-Targeting Photothermal-Enhanced Chemotherapy of Tumor. ACS Appl. Mater. Interfaces 2021, 13, 22204–22212. [Google Scholar] [CrossRef]
- Liu, X.; Wang, F.; Liu, L.; Li, T.T.; Zhong, X.Y.; Lin, H.S.; Zhang, Y.; Xue, W. Functionalized polydopamine nanospheres as in situ spray for photothermal image-guided tumor precise surgical resection. Biosens. Bioelectron. 2023, 222, 114995. [Google Scholar] [CrossRef]
- Jing, L.; Qu, H.; Wu, D.; Zhu, C.; Yang, Y.; Jin, X.; Zheng, J.; Shi, X.; Yan, X.; Wang, Y. Platelet-camouflaged nanococktail: Simultaneous inhibition of drug-resistant tumor growth and metastasis via a cancer cells and tumor vasculature dual-targeting strategy. Theranostics 2018, 8, 2683–2695. [Google Scholar] [CrossRef]
- Chen, R.; Zhu, C.Q.; Xu, L.; Gu, Y.; Ren, S.J.; Bai, H.; Zhou, Q.; Liu, X.; Lu, S.F.; Bi, X.L.; et al. An injectable peptide hydrogel with excellent self-healing ability to continuously release salvianolic acid B for myocardial infarction. Biomaterials 2021, 274, 120855. [Google Scholar] [CrossRef]
- Andoy, N.M.O.; Jeon, K.; Kreis, C.T.; Sullan, R.M.A. Multifunctional and Stimuli-Responsive Polydopamine Nanoparticle-Based Platform for Targeted Antimicrobial Applications. Adv. Funct. Mater. 2020, 30, 2004503. [Google Scholar] [CrossRef]
- Fan, W.J.; Han, H.J.; Lu, Z.Y.; Huang, Y.; Zhang, Y.; Chen, Y.Y.; Zhang, X.B.; Ji, J.; Yao, K. epsilon-poly-L-lysine-modified polydopamine nanoparticles for targeted photothermal therapy of drug-resistant bacterial keratitis. Bioeng. Transl. Med. 2023, 8, e10380. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.J.; Tan, H.Q.; Liu, H.; Jin, D.W.; Yin, M.; Lin, H.D.; Qu, X.; Liu, C.S. A reduced polydopamine nanoparticle-coupled sprayable PEG hydrogel adhesive with anti-infection activity for rapid wound sealing. Biomater. Sci. 2020, 8, 6946–6956. [Google Scholar] [CrossRef]
- Sun, H.L.; Yang, Y.; Wu, Y.T.; Fu, Z.; Zhang, Y.; Liu, Y.X.; Nie, J.X.; Wang, Y.L.; Wang, H.C.; Mai, B.J.; et al. Zinc alginate hydrogels with embedded RL-QN15 peptide-loaded hollow polydopamine nanoparticles for diabetic wound healing therapy. Mater. Des. 2022, 222, 111085. [Google Scholar] [CrossRef]
- Qin, P.; Meng, Y.; Yang, Y.; Gou, X.Y.; Liu, N.X.; Yin, S.G.; Hu, Y.; Sun, H.L.; Fu, Z.; Wang, Y.L.; et al. Mesoporous polydopamine nanoparticles carrying peptide RL-QN15 show potential for skin wound therapy. J. Nanobiotechnol. 2021, 19, 309. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.L.; Wang, Y.; He, T.T.; He, D.W.; Hu, Y.; Fu, Z.; Wang, Y.L.; Sun, D.D.; Wang, J.S.; Liu, Y.X.; et al. Hollow polydopamine nanoparticles loading with peptide RL-QN15: A new pro-regenerative therapeutic agent for skin wounds. J. Nanobiotechnol. 2021, 19, 304. [Google Scholar] [CrossRef]
- Su, H.; Zhao, F. Recent Advances in Intrinsically Fluorescent Polydopamine Materials. Appl. Sci. 2022, 12, 4560. [Google Scholar] [CrossRef]
- Golabchi, A.; Wu, B.; Cao, B.; Bettinger, C.J.; Cui, X.T. Zwitterionic polymer/polydopamine coating reduce acute inflammatory tissue responses to neural implants. Biomaterials 2019, 225, 119519. [Google Scholar] [CrossRef]
- Addisu, K.D.; Hailemeskel, B.Z.; Mekuria, S.L.; Andrgie, A.T.; Lin, Y.-C.; Tsai, H.-C. Bioinspired, Manganese-Chelated Alginate–Polydopamine Nanomaterials for Efficient in Vivo T1-Weighted Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2018, 10, 5147–5160. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, S.; Chen, X.; Liu, X.; Wang, Z.; Li, Y. Recent developments in polydopamine fluorescent nanomaterials. Mater. Horiz. 2020, 7, 746–761. [Google Scholar] [CrossRef]
- Liu, M.; Ji, J.; Zhang, X.; Zhang, X.; Yang, B.; Deng, F.; Li, Z.; Wang, K.; Yang, Y.; Wei, Y. Self-polymerization of dopamine and polyethyleneimine: Novel fluorescent organic nanoprobes for biological imaging applications. J. Mater. Chem. B 2015, 3, 3476–3482. [Google Scholar] [CrossRef]
- Zhao, C.; Zuo, F.; Liao, Z.; Qin, Z.; Du, S.; Zhao, Z. Mussel-inspired one-pot synthesis of a fluorescent and water-soluble polydopamine-polyethyleneimine copolymer. Macromol. Rapid Commun. 2015, 36, 909–915. [Google Scholar] [CrossRef]
- Zhong, Z.; Jia, L. Room temperature preparation of water-soluble polydopamine-polyethyleneimine copolymer dots for selective detection of copper ions. Talanta 2019, 197, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.M.; Du, B.; Liu, X.F.; Mei, W.J.; Wang, H.Q.; Zheng, Y.; Wang, Q.; Yang, X.H.; Wang, K.M. Copolymer Polydopamine Nanoparticles as Multifunctional Nanoinhibitors for Modulating beta-Amyloid Aggregation. ACS Appl. Nano Mater. 2022, 5, 16912–16922. [Google Scholar] [CrossRef]
- Sharma, M.; Tiwari, V.; Shukla, S.; Panda, J.J. Fluorescent Dopamine-Tryptophan Nanocomposites as Dual-Imaging and Antiaggregation Agents: New Generation of Amyloid Theranostics with Trimeric Effects. ACS Appl. Mater. Interfaces 2020, 12, 44180–44194. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Jia, F.; Zhang, P.; Sun, X.H.; Qiao, Y.S.; Chen, X.L.; Wang, Y.X.; Chen, J.Y.; Lei, Y. A miRNA stabilizing polydopamine nano-platform for intraocular delivery of miR-21-5p in glaucoma therapy. J. Mater. Chem. B 2021, 9, 3335–3345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xu, Q.; Du, J.; Wang, Y. Polydopamine-based nanoparticles with excellent biocompatibility for photothermally enhanced gene delivery. RSC Adv. 2018, 8, 34596–34602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Li, X.; Xu, Q.; Wang, Y.; Ji, J. Polydopamine nanoparticles with different sizes for NIR-promoted gene delivery and synergistic photothermal therapy. Colloids Surf. B Biointerfaces 2021, 208, 112125. [Google Scholar] [CrossRef]
- Morgan, W.F.; Sowa, M.B. Non-targeted effects induced by ionizing radiation: Mechanisms and potential impact on radiation induced health effects. Cancer Lett. 2015, 356, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Cao, W.; McCallum, N.C.; Ni, Q.Z.; Li, W.; Boyce, H.; Mao, H.; Zhou, X.; Sun, H.; Thompson, M.P.; Battistella, C.; et al. Selenomelanin: An Abiotic Selenium Analogue of Pheomelanin. J. Am. Chem. Soc. 2020, 142, 12802–12810. [Google Scholar] [CrossRef]
- Schweitzer, A.D.; Howell, R.C.; Jiang, Z.; Bryan, R.A.; Gerfen, G.; Chen, C.C.; Mah, D.; Cahill, S.; Casadevall, A.; Dadachova, E. Physico-chemical evaluation of rationally designed melanins as novel nature-inspired radioprotectors. PLoS ONE 2009, 4, e7229. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Mantanona, A.J.; Mao, H.; McCallum, N.C.; Jiao, Y.; Battistella, C.; Caponetti, V.; Zang, N.; Thompson, M.P.; Montalti, M.; et al. Radical-Enriched Artificial Melanin. Chem. Mater. 2020, 32, 5759–5767. [Google Scholar] [CrossRef]
- Mollica, F.; Lucernati, R.; Amorati, R. Expanding the spectrum of polydopamine antioxidant activity by nitroxide conjugation. J. Mater. Chem. B 2021, 9, 9980–9988. [Google Scholar] [CrossRef]
- Moosaipour, M.; Pakinia, S.; Izadi, Z.; Khalilzadeh, B.; Jaymand, M.; Samadian, H. Nanofibrous electroconductive nerve guide conduits based on polyaniline-co-polydopamine random copolymer for peripheral nerve regeneration. J. Appl. Polym. Sci. 2022, 139, e52365. [Google Scholar] [CrossRef]
- Massoumi, B.; Abbasian, M.; Jahanban-Esfahlan, R.; Mohammad-Rezaei, R.; Khalilzadeh, B.; Samadian, H.; Rezaei, A.; Derakhshankhah, H.; Jaymand, M. A novel bio-inspired conductive, biocompatible, and adhesive terpolymer based on polyaniline, polydopamine, and polylactide as scaffolding biomaterial for tissue engineering application. Int. J. Biol. Macromol. 2020, 147, 1174–1184. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Y.; Li, Z.; Chen, J.X.; Su, L.C.; Wang, J.Q.; Chen, D.S.; Ye, J.M.; Liao, N.S.; Yang, H.H.; Song, J.B.; et al. Site-Specific Biomimicry of Antioxidative Melanin Formation and Its Application for Acute Liver Injury Therapy and Imaging. Adv. Mater. 2021, 33, 2102391. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.L.; Choi, C.K.K.; Choi, C.H.J.; Fong, W.P.; Ng, D.K.P. Glutathione-degradable polydopamine nanoparticles as a versatile platform for fabrication of advanced photosensitisers for anticancer therapy. Biomater. Sci. 2021, 10, 189–201. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Saeb, M.R.; Thomas, S. Functionalized theranostic nanocarriers with bio-inspired polydopamine for tumor imaging and chemo-photothermal therapy. J. Control. Release 2019, 309, 203–219. [Google Scholar] [CrossRef]
- Liu, P.; Peng, Y.; Zhou, Y.B.; Shi, X.Y.; Li, Q.N.; Ding, J.S.; Gao, Y.; Zhou, W.H. Rapamycin as a “One-Stone-Three-Birds” Agent for Cooperatively Enhanced Phototherapies Against Metastatic Breast Cancer. ACS Appl. Mater. Interfaces 2021, 13, 25674–25684. [Google Scholar] [CrossRef]
- Liu, Y.; Choi, C.K.K.; Hong, H.; Xiao, Y.; Kwok, M.L.; Liu, H.; Tian, X.Y.; Choi, C.H.J. Dopamine Receptor-Mediated Binding and Cellular Uptake of Polydopamine-Coated Nanoparticles. ACS Nano 2021, 15, 13871–13890. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhang, Y.; Zhao, Y.W.; Cao, Y.; Zhang, Y.J.; Chong, Y.; Pei, R.J. Dual-Stimuli-Responsive Multifunctional Gd2Hf2O7 Nanoparticles for MRI-Guided Combined Chemo-/Photothermal-/Radiotherapy of Resistant Tumors. ACS Appl. Mater. Interfaces 2020, 12, 35928–35939. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Zhang, L.; Huang, C.L.; Guo, Q.; Zuo, Y.Y.; Wang, N.N.; Jin, X.; Zhang, L.H.; Zhu, D.W. Gas-generating mesoporous silica nanoparticles with rapid localized drug release for enhanced chemophotothermal tumor therapy. Biomater. Sci. 2020, 8, 6754–6763. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.C.; Frade, T.; Correia, R.D.; Niu, Y.; Jin, G.; Correia, J.P.; Viana, A.S. Electrosynthesis of polydopamine-ethanolamine films for the development of immunosensing interfaces. Sci. Rep. 2021, 11, 2237. [Google Scholar] [CrossRef] [PubMed]
- Sadrearhami, Z.; Shafiee, F.N.; Ho, K.K.K.; Kumar, N.; Krasowska, M.; Blencowe, A.; Wong, E.H.H.; Boyer, C. Antibiofilm Nitric Oxide-Releasing Polydopamine Coatings. ACS Appl. Mater. Interfaces 2019, 11, 7320–7329. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.Z.; Xia, Y.J.; Wang, L.; Nan, X.R.; Hou, J.X.; Guo, Y.Q.; Meng, K.J.; Lian, J.; Zhang, Y.F.; Wu, F.; et al. Polydopamine-assisted immobilization of silk fibroin and its derived peptide on chemically oxidized titanium to enhance biological activity in vitro. Int. J. Biol. Macromol. 2021, 185, 1022–1035. [Google Scholar] [CrossRef]
- Pralea, I.E.; Moldovan, R.C.; Petrache, A.M.; Ilieș, M.; Hegheș, S.C.; Ielciu, I.; Nicoară, R.; Moldovan, M.; Ene, M.; Radu, M.; et al. From Extraction to Advanced Analytical Methods: The Challenges of Melanin Analysis. Int. J. Mol. Sci. 2019, 20, 3943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsouko, E.; Tolia, E.; Sarris, D. Microbial Melanin: Renewable Feedstock and Emerging Applications in Food-Related Systems. Sustainability 2023, 15, 7516. [Google Scholar] [CrossRef]







| Post-Functionality | References | |
|---|---|---|
| PEG | mPEG | [120,121] |
| PEG-NH2 | [102,114,118] | |
| PEG-SH | [108,110,112,126,155] | |
| NH2-PEG-NH2 | [94,96,108,111] | |
| HS-PEG-COOH | [97,108,115] | |
| PEG-Chol | [108] | |
| FA-PEG-NH2 | [109] | |
| Metal | Cd3+ | [94,124,125,129,135] |
| Mn2+ | [94,96,126,127,128] | |
| Mn3+ | [129] | |
| Fe3+ | [94,129,133,134,135,138] | |
| Fe2+ | [138] | |
| Cu2+ | [109,129,138,139] | |
| Zn2+ | [129,135] | |
| Ga3+ | [129] | |
| Ca2+ | [135] | |
| Ni2+ | [135] | |
| AgNPs | [58] | |
| Pt4+ | [136] | |
| Gene | siRNA | [86,110,143,145,146] |
| CRISPR/Cas9 | [149] | |
| miRNA | [150] | |
| Peptide | RGD | [37,111,112,156,157,158,159] |
| beclin 1 | [112] | |
| ε-poly-L-lysine | [143,162] | |
| RL-QN15 | [164,165,166] | |
| Pre-Functionalization | References | |
| Precursor | Functionality | |
| DA * | PEI | [171,172,173,174,176] |
| Tryptophan | [175] | |
| 4-amino-TEMPO | [181,183] | |
| Arginine | [75] | |
| PANI | [184,185] | |
| L-DOPA | Selenocysteine | [180] |
| PEG-NH2 | [186] | |
| pOVA30 **,gp100 ***, Acetyl-R VIYRYYGL | [153] | |
| DA and 1,1,2-Trimethyl-3-(4 sulfobutyl) benz[e]indolium | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavridi-Printezi, A.; Menichetti, A.; Mordini, D.; Montalti, M. Functionalization of and through Melanin: Strategies and Bio-Applications. Int. J. Mol. Sci. 2023, 24, 9689. https://doi.org/10.3390/ijms24119689
Mavridi-Printezi A, Menichetti A, Mordini D, Montalti M. Functionalization of and through Melanin: Strategies and Bio-Applications. International Journal of Molecular Sciences. 2023; 24(11):9689. https://doi.org/10.3390/ijms24119689
Chicago/Turabian StyleMavridi-Printezi, Alexandra, Arianna Menichetti, Dario Mordini, and Marco Montalti. 2023. "Functionalization of and through Melanin: Strategies and Bio-Applications" International Journal of Molecular Sciences 24, no. 11: 9689. https://doi.org/10.3390/ijms24119689





