The Utility of Peptide Ligand-Functionalized Liposomes for Subcutaneous Drug Delivery for Arthritis Therapy
Abstract
1. Introduction
2. Results and Discussion
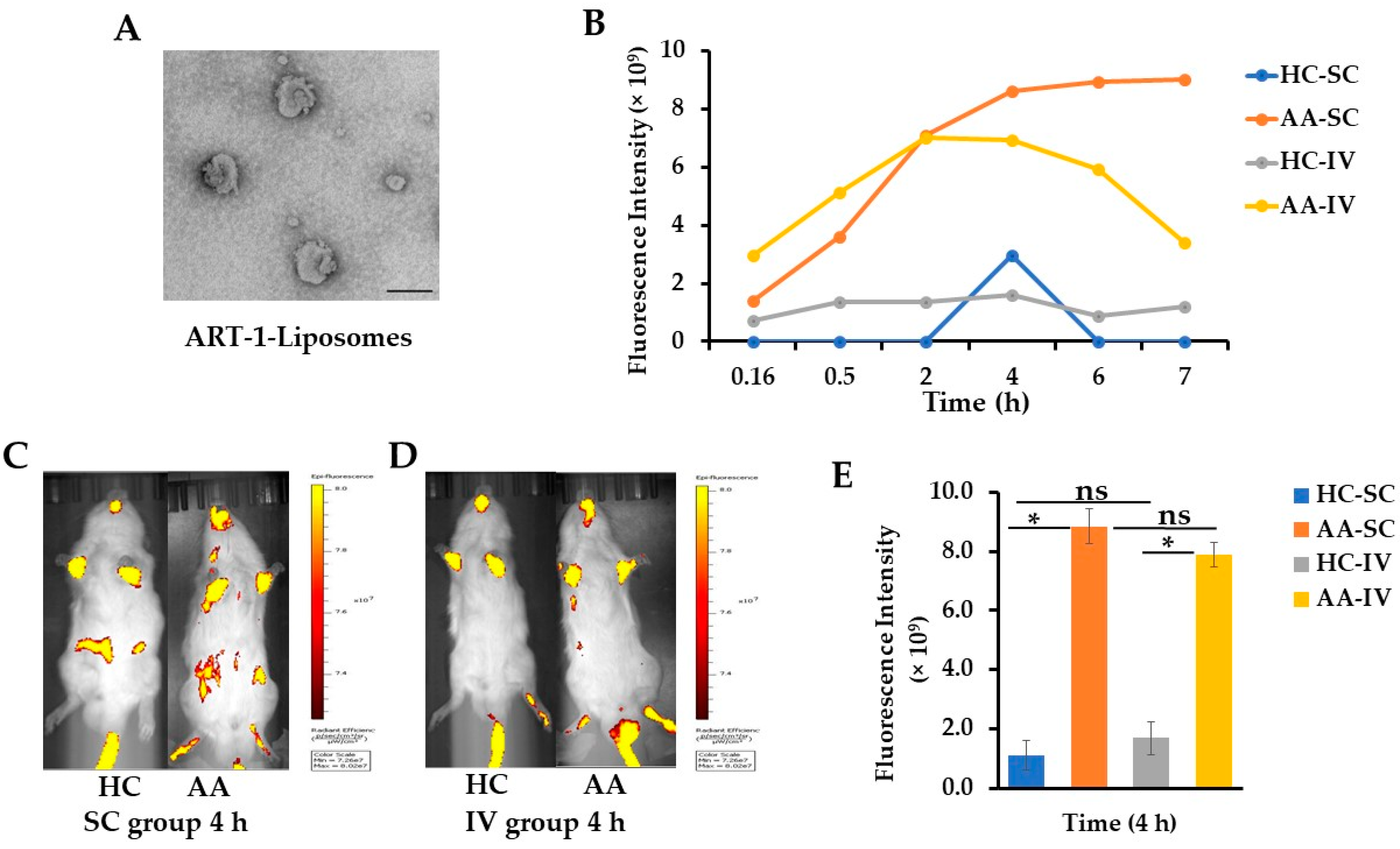
2.1. Characterization of ART-1-Functionalized Liposomes Entrapping Cyanine 7 (Cy7) or Dexamethasone (Dex)
2.2. Live Imaging of Arthritic and Control Rats at Different Time Points after Injection of Cy7-Liposomes SC or IV
2.3. Ex Vivo Imaging of Various Internal Organs and Hind Paws Harvested at 4 h Post-Liposome Injection of AA and Control Rats
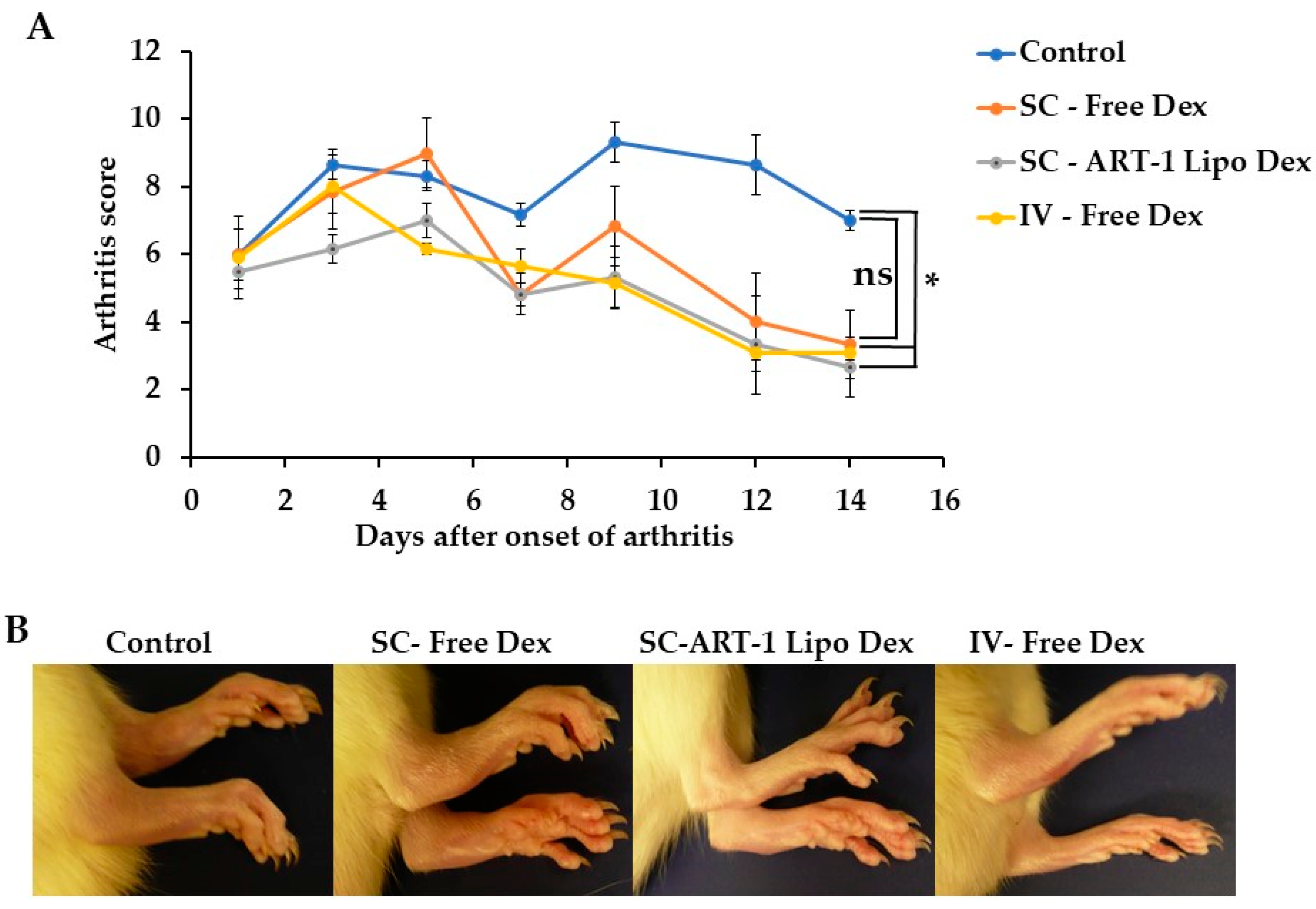
2.4. The Use of SC-Administered Dex-Entrapping ART-1-Functionalized Liposomes for Arthritis Therapy in Rats
3. Materials and Methods
3.1. Peptide-Functionalized Liposomes for Live Imaging and Arthritis Therapy
3.2. Animal Model of Arthritis
3.3. Live Imaging of Rats and Ex Vivo Imaging of Harvested Organs and Hind Paws
3.4. Treatment of Arthritic Rats Using Liposomal/Free Dex
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heath, J.R. Nanotechnologies for biomedical science and translational medicine. Proc. Natl. Acad. Sci. USA 2015, 112, 14436–14443. [Google Scholar] [CrossRef] [PubMed]
- Contera, S.; Bernardino de la Serna, J.; Tetley, T.D. Biotechnology, nanotechnology and medicine. Emerg. Top. Life Sci. 2020, 4, 551–554. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, T.; Todeschini, A.R.; Santos-Oliveira, R.; de Souza de Bustamante Monteiro, M.S.; de Souza, V.T.; Ricci-Júnior, E. Trends in Nanomedicines for Cancer Treatment. Curr. Pharm. Des. 2020, 26, 3579–3600. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef]
- Devalapally, H.; Chakilam, A.; Amiji, M.M. Role of nanotechnology in pharmaceutical product development. J. Pharm. Sci. 2007, 96, 2547–2565. [Google Scholar] [CrossRef]
- Gokce, E.H.; Ozyazici, M.; Souto, E.B. Nanoparticulate strategies for effective delivery of poorly soluble therapeutics. Ther. Deliv. 2010, 1, 149–167. [Google Scholar] [CrossRef]
- Onoue, S.; Yamada, S.; Chan, H.K. Nanodrugs: Pharmacokinetics and safety. Int. J. Nanomed. 2014, 9, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Piazzini, V.; Risaliti, L.; Vanti, G.; Casamonti, M.; Wang, M.; Bergonzi, M.C. Nanocarriers: A Successful Tool to Increase Solubility, Stability and Optimise Bioefficacy of Natural Constituents. Curr. Med. Chem. 2019, 26, 4631–4656. [Google Scholar] [CrossRef]
- Abd Ellah, N.H.; Abouelmagd, S.A. Surface functionalization of polymeric nanoparticles for tumor drug delivery: Approaches and challenges. Expert Opin. Drug Deliv. 2017, 14, 201–214. [Google Scholar] [CrossRef]
- Berillo, D.; Yeskendir, A.; Zharkinbekov, Z. Peptide-Based Drug Delivery Systems. Medicina 2021, 57, 1209. [Google Scholar] [CrossRef]
- Säälik, P.; Lingasamy, P.; Toome, K.; Mastandrea, I.; Rousso-Noori, L.; Tobi, A.; Simón-Gracia, L.; Hunt, H.; Paiste, P.; Kotamraju, V.R.; et al. Peptide-guided nanoparticles for glioblastoma targeting. J. Control Release 2019, 308, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Deane, K.D. Rheumatoid Arthritis Pathogenesis, Prediction, and Prevention: An Emerging Paradigm Shift. Nat. Immunol. 2021, 73, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Kwon, E.J. Rheumatoid Arthritis: Pathogenic Roles of Diverse Immune Cells. Int. J. Mol. Sci. 2022, 23, 905. [Google Scholar] [CrossRef] [PubMed]
- Cush, J.J. Rheumatoid Arthritis: Early Diagnosis and Treatment. Rheum. Dis. Clin. N. Am. 2022, 48, 537–547. [Google Scholar] [CrossRef]
- Radu, A.F.; Bungau, S.G. Management of Rheumatoid Arthritis: An Overview. Cells 2021, 10, 2857. [Google Scholar] [CrossRef]
- Lee, L.; Buckley, C.; Blades, M.C.; Panayi, G.; George, A.J.; Pitzalis, C. Identification of synovium-specific homing peptides by in vivo phage display selection. Arthritis Rheum. 2002, 46, 2109–2120. [Google Scholar] [CrossRef]
- Paulos, C.M.; Turk, M.J.; Breur, G.J.; Low, P.S. Folate receptor-mediated targeting of therapeutic and imaging agents to activated macrophages in rheumatoid arthritis. Adv. Drug Deliv. Rev. 2004, 56, 1205–1217. [Google Scholar] [CrossRef]
- Vanniasinghe, A.S.; Manolios, N.; Schibeci, S.; Lakhiani, C.; Kamali-Sarvestani, E.; Sharma, R.; Kumar, V.; Moghaddam, M.; Ali, M.; Bender, V. Targeting fibroblast-like synovial cells at sites of inflammation with peptide targeted liposomes results in inhibition of experimental arthritis. Clin. Immunol. 2014, 151, 43–54. [Google Scholar] [CrossRef]
- Yang, Y.H.; Rajaiah, R.; Ruoslahti, E.; Moudgil, K.D. Peptides targeting inflamed synovial vasculature attenuate autoimmune arthritis. Proc. Natl. Acad. Sci. USA 2011, 108, 12857–12862. [Google Scholar] [CrossRef]
- Meka, R.R.; Venkatesha, S.H.; Moudgil, K.D. Peptide-directed liposomal delivery improves the therapeutic index of an immunomodulatory cytokine in controlling autoimmune arthritis. J. Control Release 2018, 286, 279–288. [Google Scholar] [CrossRef]
- Bianchi, G.; Caporali, R.; Todoerti, M.; Mattana, P. Methotrexate and Rheumatoid Arthritis: Current Evidence Regarding Subcutaneous Versus Oral Routes of Administration. Adv. Ther. 2016, 33, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Bittner, B.; Richter, W.; Schmidt, J. Subcutaneous Administration of Biotherapeutics: An Overview of Current Challenges and Opportunities. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2018, 32, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhuang, S.; Qi, X.R. Comparative study of the in vitro and in vivo characteristics of cationic and neutral liposomes. Int. J. Nanomed. 2011, 6, 3087–3098. [Google Scholar] [CrossRef]
- Zou, P.; Xu, S.; Povoski, S.P.; Wang, A.; Johnson, M.A.; Martin, E.W., Jr.; Subramaniam, V.; Xu, R.; Sun, D. Near-infrared fluorescence labeled anti-TAG-72 monoclonal antibodies for tumor imaging in colorectal cancer xenograft mice. Mol. Pharm. 2009, 6, 428–440. [Google Scholar] [CrossRef]
- Moudgil, K.D.; Chang, T.T.; Eradat, H.; Chen, A.M.; Gupta, R.S.; Brahn, E.; Sercarz, E.E. Diversification of T cell responses to carboxy-terminal determinants within the 65-kD heat-shock protein is involved in regulation of autoimmune arthritis. J. Exp. Med. 1997, 185, 1307–1316. [Google Scholar] [CrossRef]

| Liposome Preparation | Size (nm) (Mean ± SEM) | PDI (Mean ± SEM) | ZP (mV) (Mean ± SEM) | |
|---|---|---|---|---|
| 1 | Liposome-control | 113.5 ± 3.03 | 0.2 ± 0.01 | −3.4 ± 2.42 |
| 2 | Liposome-Dex | 98.9 ± 1.08 | 0.3 ± 0.26 | −6.0 ± 2.34 |
| 3 | ART-1-Liposome-control | 123.7 ± 3.88 | 0.2 ± 0.01 | 22.6 ± 1.33 * |
| 4 | ART-1-Liposome-Cy7 | 147.7 ± 2.44 | 0.3 ± 0.02 | 19.3 ± 0.46 * |
| 5 | ART-1-Liposome-Dex | 160.2 ± 4.46 | 0.3 ± 0.02 | 26.9 ± 1.85 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nanjaiah, H.; Moudgil, K.D. The Utility of Peptide Ligand-Functionalized Liposomes for Subcutaneous Drug Delivery for Arthritis Therapy. Int. J. Mol. Sci. 2023, 24, 6883. https://doi.org/10.3390/ijms24086883
Nanjaiah H, Moudgil KD. The Utility of Peptide Ligand-Functionalized Liposomes for Subcutaneous Drug Delivery for Arthritis Therapy. International Journal of Molecular Sciences. 2023; 24(8):6883. https://doi.org/10.3390/ijms24086883
Chicago/Turabian StyleNanjaiah, Hemalatha, and Kamal D. Moudgil. 2023. "The Utility of Peptide Ligand-Functionalized Liposomes for Subcutaneous Drug Delivery for Arthritis Therapy" International Journal of Molecular Sciences 24, no. 8: 6883. https://doi.org/10.3390/ijms24086883
APA StyleNanjaiah, H., & Moudgil, K. D. (2023). The Utility of Peptide Ligand-Functionalized Liposomes for Subcutaneous Drug Delivery for Arthritis Therapy. International Journal of Molecular Sciences, 24(8), 6883. https://doi.org/10.3390/ijms24086883






