Symbiotic Bacteria Modulate Lymantria dispar Immunity by Altering Community Proportions after Infection with LdMNPV
Abstract
:1. Introduction
2. Results
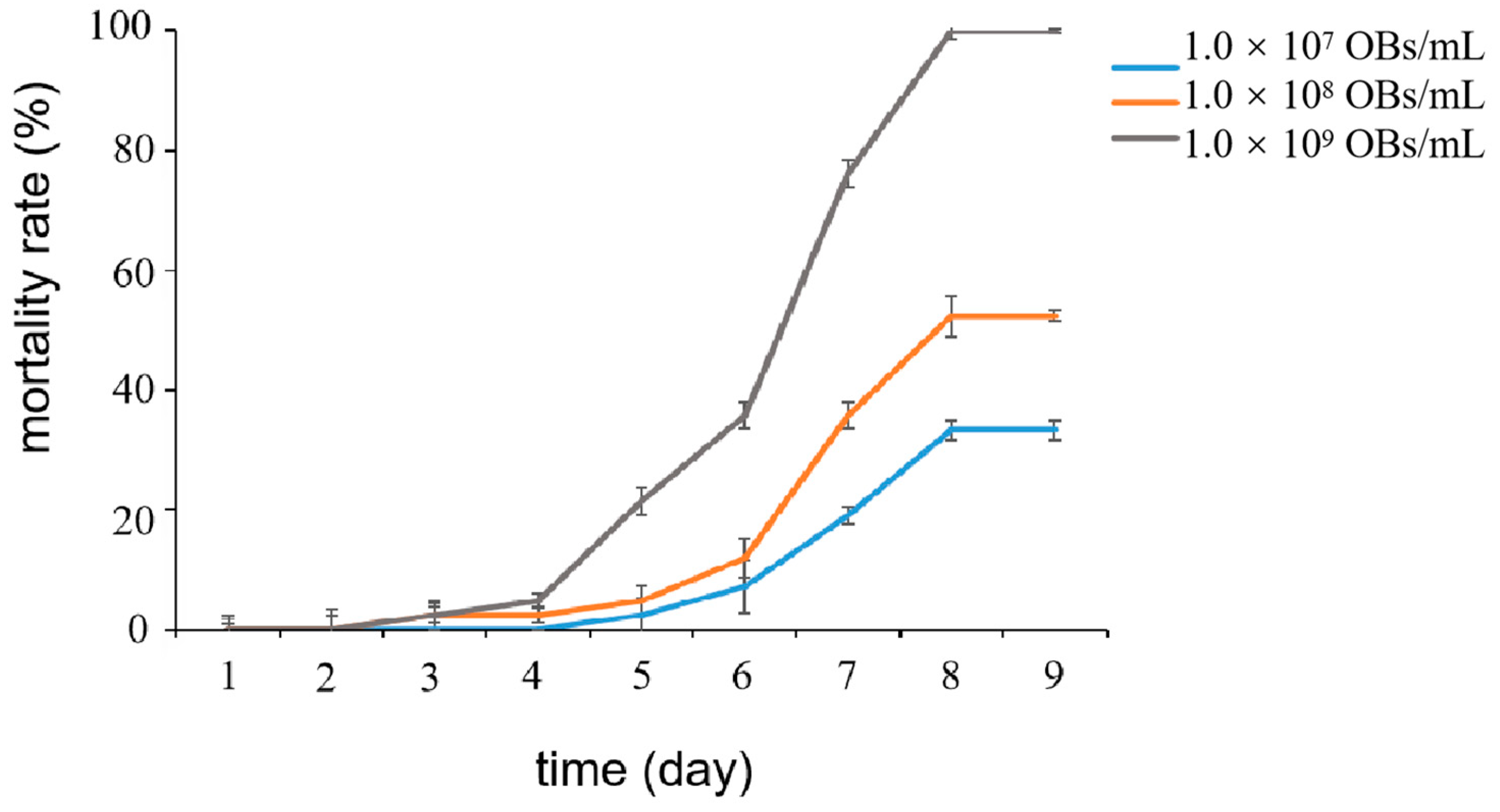
2.1. Effect of LdMNPV Solution with Different Concentrations on Mortality
2.2. Sequencing Information
2.3. Composition and Structure of Microbial Community of Symbiotic Bacteria
2.4. Host Immunity-Related Genes Differentially Expressed following LdMNPV Infection
3. Discussion
4. Materials and Methods
4.1. Insect and Virus
4.2. Bioassays
4.3. Sample Preparation
4.4. DNA Extraction, PCR Amplification and Miseq Sequencing
4.5. 16S rRNA Gene Sequence Analysis
4.6. Quantitative Real-Time RT-PCR Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slack, J.; Arif, B.M. The Baculoviruses Occlusion-Derived Virus: Virion Structure and Function. Adv. Virus Res. 2006, 69, 99–165. [Google Scholar]
- Lacey, L.; Grzywacz, D.; Shapiro-Ilan, D.; Frutos, R.; Brownbridge, M.; Goettel, M. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X. History and Current Status of Development and Use of Viral Insecticides in China. Viruses 2015, 7, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, B.; Hoyos-Carvajal, L.; Paluszek, M.; Skrzecz, W.; de Souza, M.L. Baculoviruses-re-emerging biopesticides. Biotechnol. Adv. 2006, 24, 143–160. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.R.; McClintock, J.T. Baculoviridae. Nuclear Polyhedrosis Viruses. Part 1. In Nuclear Polyhedrosis Viruses of Insects; CRC Press: Boca Raton, FL, USA, 1991; pp. 87–204. [Google Scholar]
- Terra, W.R.; Ferreira, C. Insect digestive enzymes: Properties, compartmentalization and function. Comp. Biochem. Physiol. Part B Comp. Biochem. 1994, 109, 1–62. [Google Scholar] [CrossRef]
- Cory, J.S.; Myers, J.H. The ecology and evolution of insect baculoviruses. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 239–272. [Google Scholar] [CrossRef] [Green Version]
- Engelhard, E.K.; Kam-Morgan, L.N.; Washburn, J.O.; Volkman, L.E. The insect tracheal system: A conduit for the systemic spread of Autographa californica M nuclear polyhedrosis virus. Proc. Natl. Acad. Sci. USA 1994, 91, 3224–3227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkman, L.E.; Goldsmith, P.A. Mechanism of neutralization of budded Autographa californica nuclear polyhedrosis virus by a monoclonal antibody: Inhibition of entry by adsorptive endocytosis. Virology 1985, 143, 185–195. [Google Scholar] [CrossRef]
- Trudeau, D.; Washburn, J.O.; Volkman, L.E. Central role of hemocytes in Autographa californica M nucleopolyhedrovirus pathogenesis in Heliothis virescens and Helicoverpa zea. J. Virol. 2001, 75, 996–1003. [Google Scholar] [CrossRef] [Green Version]
- Goulson, D. Wipfelkrankheit: Modification of host behaviour during baculoviral infection. Oecologia 1997, 109, 219–228. [Google Scholar] [CrossRef]
- Smirnoff, W.A. Observations on the effect of virus infection on insect behavior. J. Invertebr. Pathol. 1965, 7, 387–388. [Google Scholar] [CrossRef]
- Xu, J.P.; Chen, K.P.; Yao, Q.; Liu, M.H.; Gao, G.T.; Zhao, Y. Identification and characterization of an NPV infection-related gene Bmsop2 in Bombyx mori L. J. Appl. Entomol. 2005, 129, 425–431. [Google Scholar] [CrossRef]
- Li, P.; Jiang, X.F.; Guo, W.B.; Yan, J.; Zhou, K.Y. Expression patterns of two heat-shock cognate 70 genes during immune responses and larval development of the Chinese mitten crab Eriocheir sinensis. Genet. Mol. Res. 2016, 15, 15036319. [Google Scholar] [CrossRef]
- Cai, K.; Chen, K.; Liu, X.; Yao, Q.; Li, J. Differential expression of haemolymph proteome of resistant strain and susceptible strain for BmNPV in Bombyx mori L. Chin. J. Biotechnol. 2008, 24, 285–290. [Google Scholar]
- Tsakas, S.; Marmaras, V. Insect immunity and its signaling: An overview. Invertebr. Surviv. J. 2010, 7, 228–238. [Google Scholar]
- Nappi, A.J.; Kohler, L.; Mastore, M. Signaling pathways implicated in the cellular innate immune responses of Drosophila. Invertebr. Surviv. J. 2004, 1, 5–33. [Google Scholar]
- Mavrouli, M.; Tsakas, S.; Theodorou, G.; Lampropoulou, M.; Marmaras, V. MAP kinases mediate phagocytosis and melanization via prophenoloxidase activation in medfly hemocytes. Biochim. Biophys. Acta 2005, 1744, 145–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, O.; Theopold, U.; Strand, M. Innate immunity and its evasion and suppression by hymenopteran endoparasitoids. BioEssays 2001, 23, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Strand, M.R.; Pech, L.L. Immunological Basis for Compatibility in Parasitoid-Host Relationships. Hubei Plant Prot. 1997, 40, 31–56. [Google Scholar] [CrossRef] [PubMed]
- Christensen, B.; Li, J.; Chen, C.; Nappi, A. Melanization immune responses in mosquito vectors. Trends Parasitol. 2005, 21, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, B.; Hoffmann, J. The Host Defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Zhang, B.; Zhang, H.; Xu, A.; Qian, H. Integration of Transcriptomic and Proteomic Analyses Reveals New Insights into the Regulation of Immune Pathways in Midgut of Samia ricini upon SariNPV Infection. Insects 2022, 13, 294. [Google Scholar] [CrossRef] [PubMed]
- Katsuma, S.; Mita, K.; Shimada, T. ERK and JNK-dependent signaling pathways contribute to Bombyx mori nucleopolyhedrovirus infection. J. Virol. 2007, 81, 13700–13709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Liu, J.; Lu, Y.; Gong, Y.; Zhu, M.; Chen, F.; Liang, Z.; Zhu, L.; Kuang, S.; Hu, X. Immune signaling pathways activated in response to different pathogenic micro-organisms in Bombyx mori. Mol. Immunol. 2015, 65, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Schnettler, E.; Tykalová, H.; Watson, M.; Sharma, M.; Sterken, M.G.; Obbard, D.J.; Lewis, S.H.; McFarlane, M.; Bell-Sakyi, L.; Barry, G. Induction and suppression of tick cell antiviral RNAi responses by tick-borne flaviviruses. Nucleic Acids Res. 2014, 42, 9436–9446. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Vargas, I.; Scott, J.C.; Poole-Smith, B.K.; Franz, A.W.E.; Barbosa-Solomieu, V.; Wilusz, J.; Olson, K.E.; Blair, C.D. Dengue Virus Type 2 Infections of Aedes aegypti Are Modulated by the Mosquito’s RNA Interference Pathway. PLoS Pathog. 2009, 5, e1000299. [Google Scholar] [CrossRef] [Green Version]
- Jayachandran, B.; Hussain, M.; Asgari, S. RNA Interference as a Cellular Defense Mechanism against the DNA Virus Baculovirus. J. Virol. 2012, 86, 13729–13734. [Google Scholar] [CrossRef] [Green Version]
- Carissimo, G.; Pondeville, E.; McFarlane, M.; Dietrich, I.; Mitri, C.; Bischoff, E.; Antoniewski, C.; Bourgouin, C.; Failloux, A.B.; Kohl, A. Antiviral immunity of Anopheles gambiae is highly compartmentalized, with distinct roles for RNA interference and gut microbiota. Proc. Natl. Acad. Sci. USA 2015, 112, E176–E185. [Google Scholar] [CrossRef] [Green Version]
- Galiana-Arnoux, D.; Dostert, C.; Schneemann, A.; Hoffmann, J.A.; Imler, J.L. Essential function in vivo for Dicer-2 in host defense against RNA viruses in Drosophila. Nat. Immunol. 2006, 7, 590–597. [Google Scholar] [CrossRef]
- Russell, T.A.; Ayaz, A.; Davidson, A.D.; Fernandez-Sesma, A.; Maringer, K. Imd pathway-specific immune assays reveal NF-κB stimulation by viral RNA PAMPs in Aedes aegypti Aag2 cells. PLoS Negl. Trop. Dis. 2021, 15, e0008524. [Google Scholar] [CrossRef]
- Azzami, K.; Ritter, W.; Tautz, J.; Beier, H. Infection of honey bees with acute bee paralysis virus does not trigger humoral or cellular immune responses. Arch. Virol. 2012, 157, 689–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, H.; Tago, K.; Hayatsu, M.; Kikuchi, Y. Detoxifying symbiosis: Microbe-mediated detoxification of phytotoxins and pesticides ininsects. Nat. Prod. Rep. 2018, 35, 434–454. [Google Scholar] [CrossRef] [PubMed]
- Malke, H. Paul Buchner, Endosymbiosis of Animals with Plant Microorganisms. J. Basic Microbiol. 2010, 7, 168. [Google Scholar]
- Zchorifein, E.; Bourtzis, K. Manipulative Tenants: Bacteria Associated with Arthropods; CRC Press: Boca Raton, FL, USA, 2012; pp. 45–73. [Google Scholar]
- Sudakaran, S.; Kost, C.; Kaltenpoth, M. Symbiont Acquisition and Replacement as a Source of Ecological Innovation. Trends Microbiol. 2017, 25, 375–390. [Google Scholar] [CrossRef]
- Baumann, P. Biology of bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu. Rev. Microbiol. 2005, 59, 155–189. [Google Scholar] [CrossRef]
- Cheng, Q.; Aksoy, S. Tissue tropism, transmission and expression of foreign genes in vivo in midgut symbionts of tsetse flies. Insect Mol. Biol. 2010, 8, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Engl, T.; Kaltenpoth, M. Influence of microbial symbionts on insect pheromones. Nat. Prod. Rep. 2018, 35, 386–397. [Google Scholar] [CrossRef]
- Goodacre, S.L.; Martin, O.Y. Modification of Insect and Arachnid Behaviours by Vertically Transmitted Endosymbionts: Infections as Drivers of Behavioural Change and Evolutionary Novelty. Insects 2012, 3, 246–261. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Wang, Z.; Chen, M.; Qu, Y.; Li, J.; Zhou, A.; Xie, S.; Zeng, F.; Zou, J. Microbiota comparison of Pacific white shrimp intestine and sediment at freshwater and marine cultured environment. Sci. Total Environ. 2018, 657, 1194–1204. [Google Scholar] [CrossRef]
- Zeng, J.Y.; Vuong, T.M.D.; Shi, J.H.; Shi, Z.B.; Guo, J.X.; Zhang, G.C.; Bi, B. Avermectin stress varied structure and function of gut microbial community in Lymantria dispar asiatica (Lepidoptera: Lymantriidae) larvae. Pestic. Biochem. Physiol. 2020, 164, 196–202. [Google Scholar] [CrossRef]
- Mohr, K.I.; Tebbe, C.C. Diversity and phylotype consistency of bacteria in the guts of three bee species (Apoidea) at an oilseed rape field. Environ. Microbiol. 2006, 8, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Li, X.; Tang, L.; Yao, H.; Wang, H. 16S rRNA gene sequencing reveals the relationship between gut microbiota and ovarian development in the swimming crab Portunus trituberculatus. Chemosphere 2020, 254, 126891. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.K.; Moran, N.A. The impact of microbial symbionts on host plant utilization by herbivorous insects. Mol. Ecol. 2014, 23, 1473–1496. [Google Scholar] [CrossRef] [PubMed]
- Pontin, J. The Leafcutter Ants: Civilization by Instinct. Zool. J. Linn. Soc. 2011, 163, 317. [Google Scholar] [CrossRef] [Green Version]
- Aylward, F.O.; Burnum, K.E.; Scott, J.J.; Suen, G.; Tringe, S.G.; Adams, S.M.; Barry, K.W.; Nicora, C.D.; Piehowski, P.D.; Purvine, S.O. Metagenomic and metaproteomic insights into bacterial communities in leaf-cutter ant fungus gardens. ISME J. 2012, 6, 1688. [Google Scholar] [CrossRef] [Green Version]
- Pinto-Tomas, A.A.; Anderson, M.A.; Suen, G.; Stevenson, D.M.; Chu, F.; Cleland, W.W.; Weimer, P.J.; Currie, C.R. Symbiotic Nitrogen Fixation in the Fungus Gardens of Leaf-Cutter Ants. Science 2009, 326, 1120–1123. [Google Scholar] [CrossRef]
- Dillon, R. Chemical Barriers to Gut Infection in the Desert Locust: In Vivo Production of Antimicrobial Phenols Associated with the Bacterium Pantoea agglomerans. J. Invertebr. Pathol. 1995, 66, 72–75. [Google Scholar] [CrossRef]
- Florez, L.; Scherlach, K.; Gaube, P.; Ross, C.; Sitte, E.; Hermes, C.; Rodrigues, A.; Hertweck, C.; Kaltenpoth, M. Antibiotic-producing symbionts dynamically transition between plant pathogenicity and insect-defensive mutualism. Nat. Commun. 2017, 8, 15172. [Google Scholar] [CrossRef]
- Mattoso, T.C.; Moreira, D.D.O.; Samuels, R.I. Symbiotic bacteria on the cuticle of the leaf-cutting ant Acromyrmex subterraneus subterraneus protect workers from attack by entomopathogenic fungi. Biol. Lett. 2012, 8, 461–464. [Google Scholar] [CrossRef] [Green Version]
- Scarborough, C.; Ferrari, J.; Godfray, C. Aphid Protected from Pathogen by Endosymbiont. Science 2005, 310, 1781. [Google Scholar] [CrossRef]
- Wang, S.; Dos-santos, A.; Huang, W.; Liu, K.; Oshaghi, M.; Wei, G.; Agre, P.; Jacobs-Lorena, M. Driving mosquito refractoriness to Plasmodium falciparum with engineered symbiotic bacteria. Science 2017, 357, 1399–1402. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; Wang, L.; Vega-Rodriguez, J.; Wang, G.; Wang, S. A Gut Symbiotic Bacterium Serratia marcescens Renders Mosquito Resistance to Plasmodium Infection Through Activation of Mosquito Immune Responses. Front. Microbiol. 2019, 10, 1580. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.K.; Lee, B.L. Insect Symbiosis and Immunity: The Bean Bug–Burkholderia Interaction as a Case Study. Adv. Insect Physiol. 2017, 52, 179–197. [Google Scholar]
- López-Madrigal, S.; Maire, J.; Balmand, S.; Zaidman-Rémy, A.; Heddi, A. Effects of symbiotic status on cellular immunity dynamics in Sitophilus oryzae. Dev. Comp. Immunol. 2017, 77, 259–269. [Google Scholar] [CrossRef]
- Hernández-Martínez, P.; Naseri, B.; Navarro-Cerrillo, G.; Escriche, B.; Ferré, J.; Herrero, S. Increase in midgut microbiota load induces an apparent immune priming and increases tolerance to Bacillus thuringiensis. Environ. Microbiol. 2010, 12, 2730–2737. [Google Scholar] [CrossRef] [PubMed]
- Benyacoub, J.; Bosco, N.; Blanchard, C.; Demont Chuat, A.; Philippe, D.; Castiel-Higounenc, I.; Guéniche, A. Immune modulation property of Lactobacillus paracasei NCC2461 (ST11) strain and impact on skin defences. Benef. Microbes 2014, 5, 129–136. [Google Scholar] [CrossRef]
- Futo, M.; Sell, M.; Kutzer, M.; Kurtz, J. Specificity of oral immune priming in the red flour beetle Tribolium castaneum. Biol. Lett. 2017, 13, 20170632. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, J.; Brayner, F.; Alves, L.; Dixit, R.; Barillas-Mury, C. Hemocyte Differentiation Mediates Innate Immune Memory in Anopheles gambiae Mosquitoes. Science 2010, 329, 1353–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, J.; Vavre, F. Bacterial symbionts in insects or the story of communities affecting communities. Biol. Sci. 2011, 366, 1389–1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douglas, A.E. Nutritional Interactions in Insect-Microbial Symbioses: Aphids and Their Symbiotic Bacteria Buchnera. Annu. Rev. Entomol. 1998, 43, 17–37. [Google Scholar] [CrossRef] [Green Version]
- Eichler, S.; Schaub, G. Development of Symbionts in Triatomine Bugs and the Effects of Infections with Trypanosomatids. Exp. Parasitol. 2002, 100, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Hongoh, Y.; Sharma, V.; Prakash, T.; Noda, S.; Taylor, T.; Kudo, T.; Sakaki, Y.; Toyoda, A.; Hattori, M.; Ohkuma, M. Complete genome of the uncultured Termite Group I bacteria in a single host protist cell. Proc. Natl. Acad. Sci. USA 2008, 105, 5555–5560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaio, A.; Gusmao, D.; Santos, A.; Berbert-Molina, M.; Pimenta, P.; Lemos, F. Contribution of midgut bacteria to blood digestion and egg production in Aedes aegypti (Diptera: Culicidae) (L.). Parasites Vectors 2011, 4, 105. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Manfredini, F.; Dimopoulos, G.; Schneider, D.S. Implication of the Mosquito Midgut Microbiota in the Defense against Malaria Parasites. PLoS Pathog. 2009, 5, e1000423. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.D.; Guo, H.F. Importance of endosymbionts Wolbachia and Rickettsia in insect resistance development—ScienceDirect. Curr. Opin. Insect Sci. 2019, 33, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Pietri, J.E.; Liang, D. The Links Between Insect Symbionts and Insecticide Resistance: Causal Relationships and Physiological Tradeoffs. Ann. Entomol. Soc. Am. 2018, 3, 92–97. [Google Scholar] [CrossRef]
- Wei, G.; Lai, Y.; Wang, G.; Chen, H.; Li, F.; Wang, S. Insect pathogenic fungus interacts with the gut microbiota to accelerate mosquito mortality. Proc. Natl. Acad. Sci. USA 2017, 114, 5994–5999. [Google Scholar] [CrossRef] [Green Version]
- Blum, J.E.; Fischer, C.N.; Miles, J.; Handelsman, J. Frequent Replenishment Sustains the Beneficial Microbiome of Drosophila melanogaster. mBio 2013, 4, e00860. [Google Scholar] [CrossRef] [Green Version]
- Glittenberg, M.T.; Kounatidis, I.; Christensen, D.; Kostov, M.; Ligoxygakis, P. Pathogen and host factors are needed to provoke a systemic host response to gastrointestinal infection of Drosophila larvae by Candida albicans. Dis. Model. Mech. 2011, 4, 515. [Google Scholar] [CrossRef] [Green Version]
- Cavalier-Smith, T. Symbiosis as a source of evolutionary innovation: Speciation and morphogenesis. Trends Ecol. Evol. 1992, 7, 422–423. [Google Scholar] [CrossRef]
- Feldhaar, H. Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecol. Entomol. 2011, 36, 533–543. [Google Scholar] [CrossRef]
- Janson, E.M.; Stireman, J.O.; Abbot, S.P.; Singer, M.S.; Abbot, P. Phytophagous insect-microbe mutualisms and adaptive evolutionary diversification. Evolution 2010, 62, 997–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schluter, D. Ecological Character Displacement in Adaptive Radiation. Am. Nat. 2000, 156, S4–S16. [Google Scholar] [CrossRef]
- Takiya, D.M.; Tran, P.L.; Dietrich, C.H.; Moran, N.A. Co-cladogenesis spanning three phyla: Leafhoppers (Insecta: Hemiptera: Cicadellidae) and their dual bacterial symbionts. Mol. Ecol. 2010, 15, 4175–4191. [Google Scholar] [CrossRef]
- Ramya, S.L.; Venkatesan, T.; Murthy, K.S.; Jalali, S.K.; Verghese, A. Detection of carboxylesterase and esterase activity in culturable gut bacterial flora isolated from diamondback moth, Plutella xylostella (Linnaeus), from India and its possible role in indoxacarb degradation. Braz. J. Microbiol. 2016, 47, 327–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakubowska, A.K.; Vogel, H.; Herrero, S. Increase in gut microbiota after immune suppression in baculovirus-infected larvae. PLoS Pathog 2013, 9, e1003379. [Google Scholar] [CrossRef]
- Zeng, J.Y.; Guo, J.; Shi, J.; Shi, Z.; Zhang, G.; Zhang, J. Stress response of Lymantria dispar asiatica (Lepidoptera: Erebidae) larvae and its gut microbiota to manganese ion. J. For. Res. 2020, 32, 8. [Google Scholar] [CrossRef]
- Kumar, D.; Sun, Z.; Xue, R.; Cao, G.; Hu, X.; Cheng, G. Bombyx mori bidensovirus infection alters the intestinal microflora of fifth instar silkworm (Bombyx mori) larvae. J. Invertebr. Pathol. 2019, 163, 48–63. [Google Scholar] [CrossRef]
- Yuan, C.; Xing, L.; Wang, M.; Hu, Z.; Zou, Z. Microbiota modulates gut immunity and promotes baculovirus infection in Helicoverpa armigera. Insect Sci. 2021, 28, 1766–1779. [Google Scholar] [CrossRef]
- Zeng, J.Y.; Wu, D.D.; Shi, Z.B.; Yang, J.; Zhang, G.C.; Zhang, J. Influence of dietary aconitine and nicotine on the gut microbiota of two lepidopteran herbivores. Arch. Insect Biochem. Physiol. 2020, 104, e21676. [Google Scholar] [CrossRef]
- Cherry, S.; Perrimon, N. Entry is a rate-limiting step for viral infection in a Drosophila melanogaster model of pathogenesis. Nat. Immunol. 2004, 5, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, A.; Bhatnagar, N.B.; Bhatnagar, R. Bacterial insecticidal toxins. Crit. Rev. Microbiol. 2004, 30, 33–54. [Google Scholar] [CrossRef] [PubMed]
- Itoh, J.; Omoto, S.; Shomura, T.; Nishizawa, N.; Miyado, S.; Yuda, Y.; Shibata, U.; Inouye, S. Amicoumacin-A, a new antibiotic with strong antiinflammatory and antiulcer activity. J. Antibiot. 1981, 34, 611–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, J.; Shomura, T.; Omoto, S.; Miyado, S.; Yuda, Y.; Shibata, U.; Inouye, S. Isolation, Physicochemical Properties and Biological Activities of Amicoumacins Produced by Bacillus pumilus. Agric. Biol. Chem. 1982, 46, 1255–1259. [Google Scholar] [CrossRef]
- Pinchuk, I.V.; Bressollier, P.; Verneuil, B.; Fenet, B.; Urdaci, M.C. In vitro anti-Helicobacter pylori activity of the probiotic strain Bacillus subtilis 3 is due to secretion of antibiotics. Antimicrob. Agents Chemother. 2001, 45, 3156–3161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paniagua, V.L.R.; Enric, F.; Martin, K.; Monika, H.; Fatouros, N.E. Bacterial Symbionts in Lepidoptera: Their Diversity, Transmission, and Impact on the Host. Front. Microbiol. 2018, 9, 556. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Feng, H.; He, J.; Liang, X.; Zhang, N.; Shao, Y.; Zhang, F.; Lu, X. The gut commensal bacterium Enterococcus faecalis LX10 contributes to defending against Nosema bombycis infection in Bombyx mori. Pest Manag. Sci. 2022, 78, 6. [Google Scholar] [CrossRef]
- Broderick, N.A.; Raffa, K.F.; Goodman, R.M.; Handelsman, J. Census of the Bacterial Community of the Gypsy Moth Larval Midgut by Using Culturing and Culture-Independent Methods. Appl. Environ. Microbiol. 2004, 70, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Chart, H. Vibrio, mobiluncus, gardnerella and spirillum. In Medical Microbiology; Churchill Livingstone: London, UK, 2012; pp. 314–323. [Google Scholar]
- Kemp, C.; Imler, J.L. Antiviral immunity in drosophila. Curr. Opin. Immunol. 2009, 21, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Sabin, L.R.; Hanna, S.L.; Cherry, S. Innate antiviral immunity in Drosophila. Curr. Opin. Immunol. 2010, 22, 4–9. [Google Scholar] [CrossRef] [Green Version]
- Luplertlop, N.; Surasombatpattana, P.; Patramool, S.; Dumas, E.; Wasinpiyamongkol, L.; Sauné, L.; Hamel, R.; Bernard, E.; Denis, S.; Thomas, F. Induction of a Peptide with Activity against a Broad Spectrum of Pathogens in the Aedes aegypti Salivary Gland, following Infection with Dengue Virus. PLoS Pathog. 2011, 7, e1001252. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Liu, Y.; Zhang, X.; Wang, J.; Li, Z.; Pang, X.; Wang, P.; Cheng, G. Complement-Related Proteins Control the Flavivirus Infection of Aedes aegypti by Inducing Antimicrobial Peptides. PLoS Pathog. 2014, 10, e1004027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avadhanula, V.; Weasner, B.P.; Hardy, G.G.; Kumar, J.P.; Hardy, R.W.; Ding, S.W. A Novel System for the Launch of Alphavirus RNA Synthesis Reveals a Role for the Imd Pathway in Arthropod Antiviral Response. PLoS Pathog. 2009, 5, e1000582. [Google Scholar] [CrossRef] [Green Version]
- Zaghloul, H.; Hice, R.; Bideshi, D.K.; Arensburger, P.; Federici, B.A. Mitochondrial and Innate Immunity Transcriptomes from Spodoptera frugiperda Larvae Infected with the Spodoptera frugiperda Ascovirus. J. Virol. 2020, 94, e01985-19. [Google Scholar] [CrossRef]
- Bischoff, V.; Vignal, C.; Duvic, B.; Boneca, I.G.; Hoffmann, J.A.; Royet, J. Downregulation of the Drosophila Immune Response by Peptidoglycan-Recognition Proteins SC1 and SC2. PLoS Pathog. 2006, 2, e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haine, E.R.; Moret, Y.; Siva-Jothy, M.T.; Rolff, J. Antimicrobial Defense and Persistent Infection in Insects. Science 2008, 322, 1257–1259. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, L.; Yu, X.; Rensing, C.; Wang, D. The PI3K/AKT Pathway and PTEN Gene are Involved in “Tree-Top Disease” of Lymantria dispar. Genes 2022, 13, 247. [Google Scholar] [CrossRef]
- Zheng, J.; Xiao, X.; Zhang, Q.; Mao, L.; Yu, M.; Xu, J. The Placental Microbiome Varies in Association with Low Birth Weight in Full-Term Neonates. Nutrients 2015, 7, 6924–6937. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.; Bokulich, N.; Abnet, C.; Al-Ghalith, G.; Alexander, H.; Alm, E.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 1. [Google Scholar] [CrossRef]
- Lyu, F.; Han, F.; Ge, C.; Mao, W.; Chen, L.; Hu, H.; Chen, G.; Lang, Q.; Fang, C. OmicStudio: A composable bioinformatics cloud platform with real-time feedback that can generate high-quality graphs for publication. iMeta 2023, 2, e85. [Google Scholar] [CrossRef]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. Jvenn: An interactive Venn diagram viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schefe, J.H.; Lehmann, K.E.; Buschmann, I.R.; Unger, T.; Funke-Kaiser, H. Quantitative real-time RT-PCR data analysis: Current concepts and the novel “gene expression’s CT difference” formula. J. Mol. Med. 2006, 84, 901–910. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, P.; Rensing, C.; Wang, D. Symbiotic Bacteria Modulate Lymantria dispar Immunity by Altering Community Proportions after Infection with LdMNPV. Int. J. Mol. Sci. 2023, 24, 9694. https://doi.org/10.3390/ijms24119694
Zhao P, Rensing C, Wang D. Symbiotic Bacteria Modulate Lymantria dispar Immunity by Altering Community Proportions after Infection with LdMNPV. International Journal of Molecular Sciences. 2023; 24(11):9694. https://doi.org/10.3390/ijms24119694
Chicago/Turabian StyleZhao, Peixu, Christopher Rensing, and Dun Wang. 2023. "Symbiotic Bacteria Modulate Lymantria dispar Immunity by Altering Community Proportions after Infection with LdMNPV" International Journal of Molecular Sciences 24, no. 11: 9694. https://doi.org/10.3390/ijms24119694






