Human GBP1 Is Involved in the Repair of Damaged Phagosomes/Endolysosomes
Abstract
:1. Introduction
2. Results
2.1. Human GBP1/2 Are Recruited to Intracellular Mycobacterium tuberculosis and Listeria monocytogenes
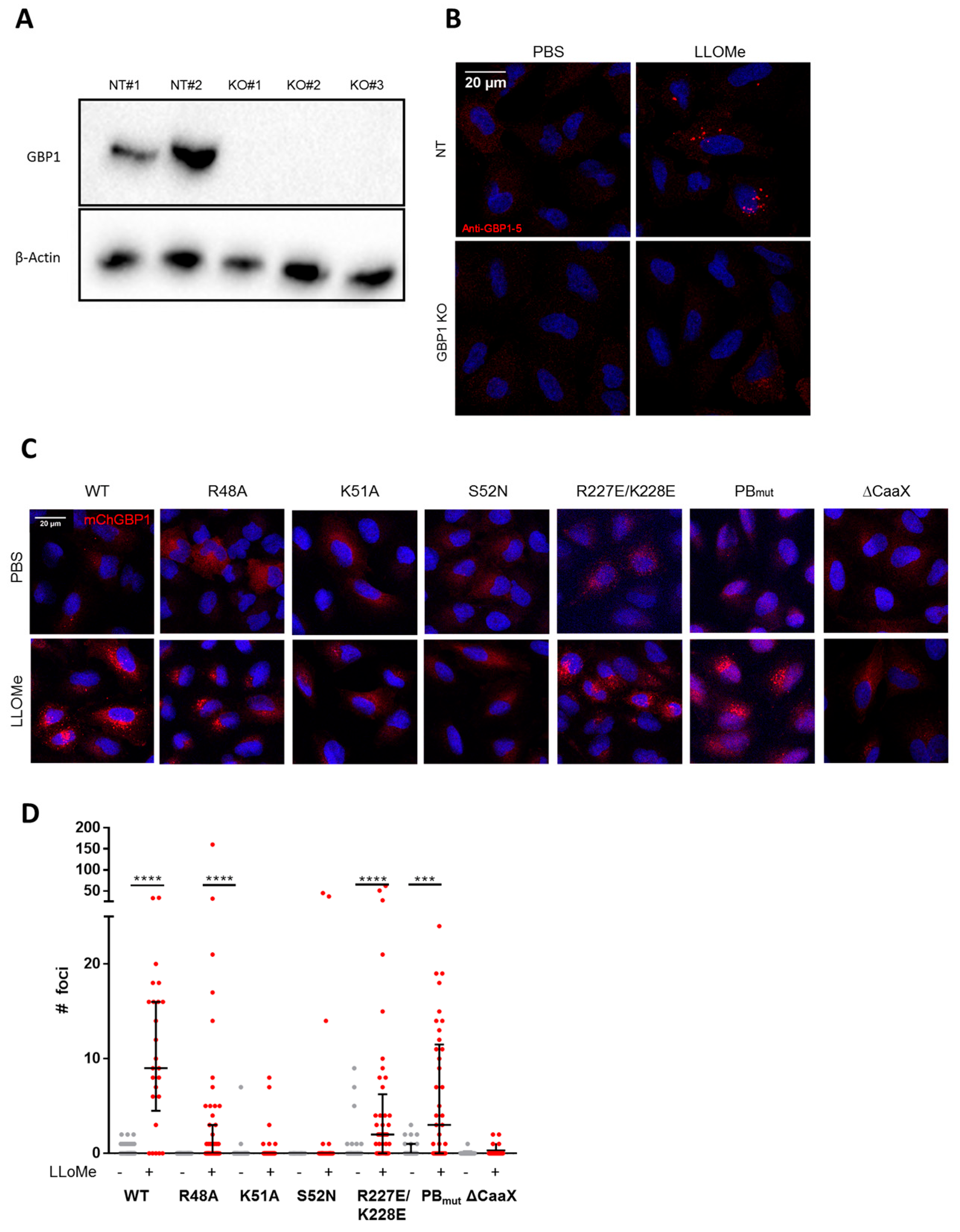
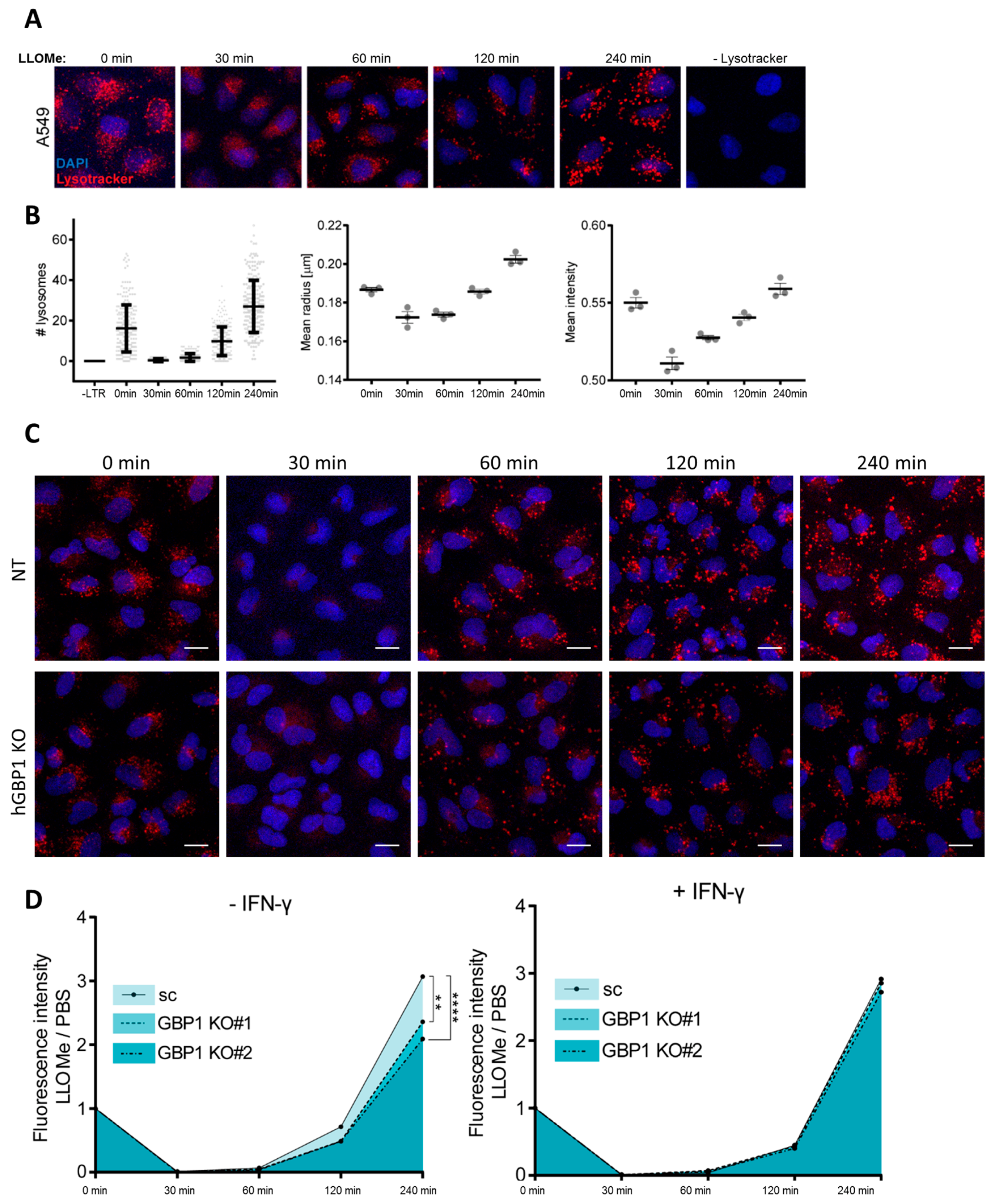
2.2. hGBP1 Forms Puncta Structures upon Endolysosomal Damage
2.3. GTP-Binding and Isoprenylation of hGBP1 Are Critical for Its Puncta Formation

2.4. hGBP1 Mediates Recovery of Endolysosomal Integrity
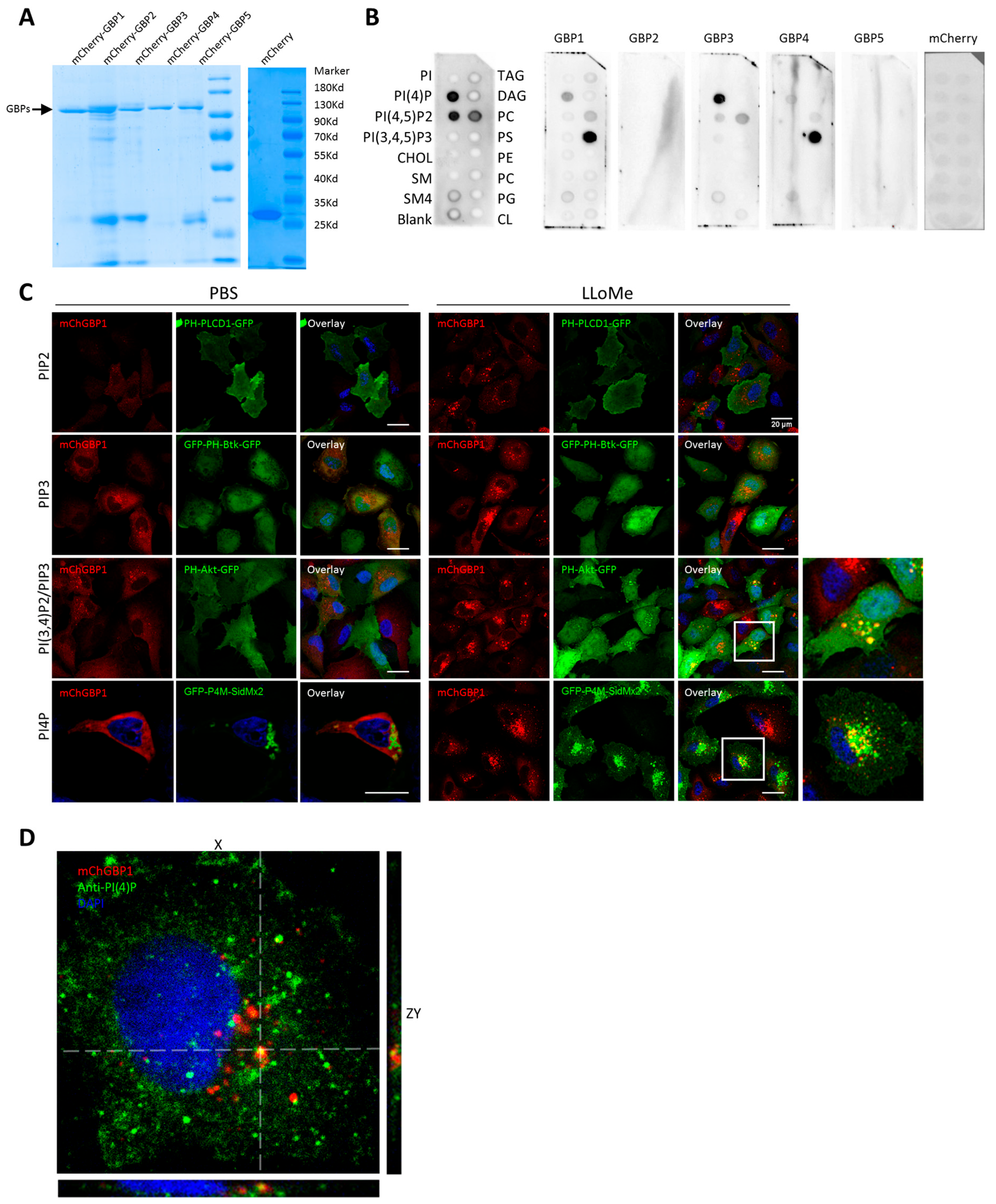
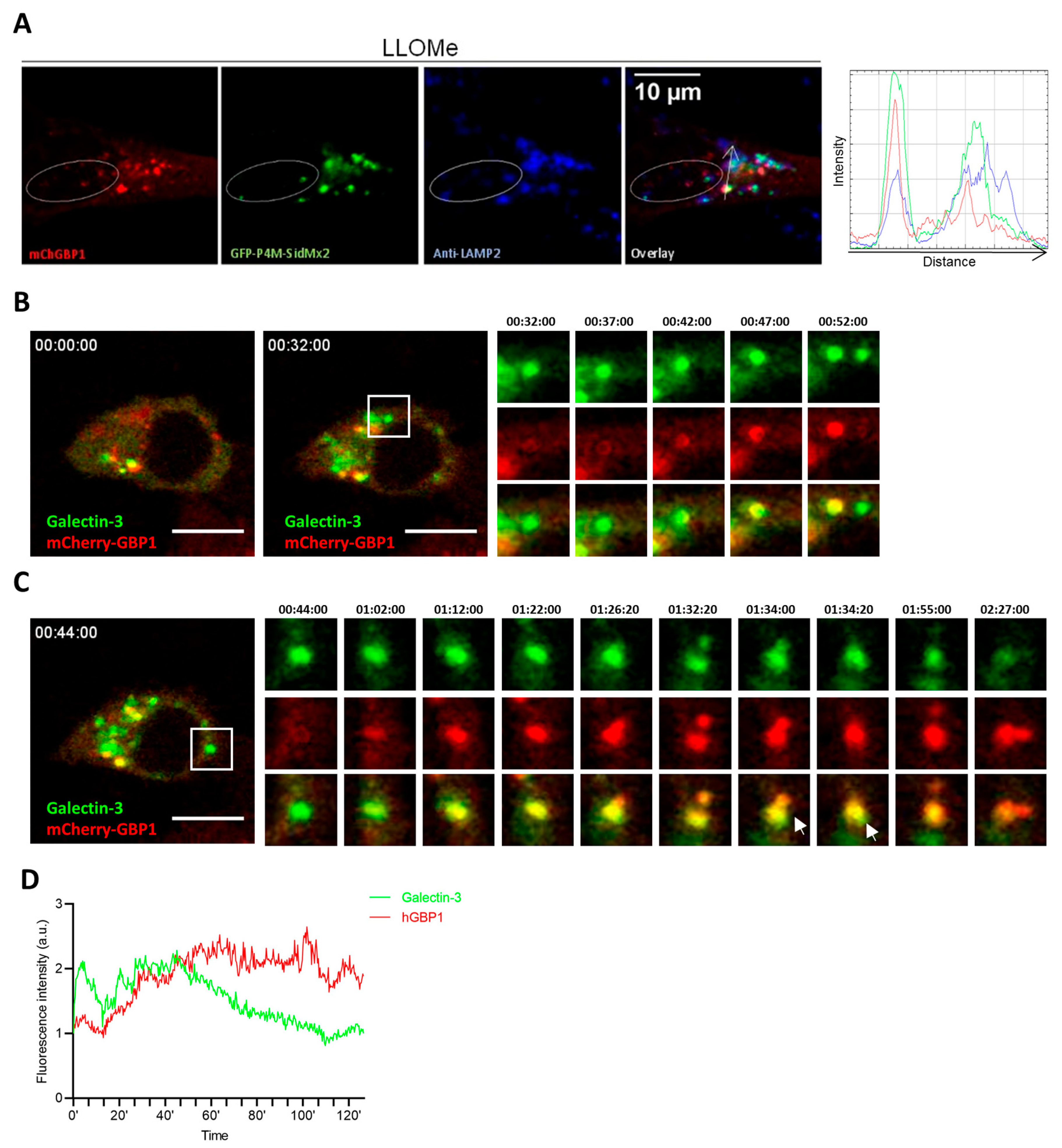
2.5. hGBP1 Is Targeted to Damaged Endolysosomes Likely via Binding to the Membrane Lipids, PI4P and PI(3,4)P2
2.6. hGBP1 Contributes to the Repair of Damaged Endolysosomes
2.7. hGBP1 Does Not Affect Intracellular Survival of Mtb and Lm
3. Discussion
4. Material and Methods
4.1. Cell and Bacteria Culture
4.2. Plasmids, Antibodies, and Reagents
4.3. mCherry-hGBPs Lentiviral Vector Cloning
4.4. hGBP1 Mutagenesis
4.5. Lentivirus Production
4.6. hGBP1 KO Using CRISPR/Cas9 Technology
4.7. Western Blot
4.8. mCherry-hGBPs Protein Purification
4.9. In Vitro Lipid-Binding Assay of hGBPs
4.10. LysoTracker Red Assay
4.11. Confocal Microscopy, Automatic Image Acquisition, and Image Analysis
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, Y.S.; Colonno, R.J.; Yin, F.H. Interferon induction of fibroblast proteins with guanylate binding activity. J. Biol. Chem. 1983, 258, 7746–7750. [Google Scholar]
- Tretina, K.; Park, E.S.; Maminska, A.; MacMicking, J.D. Interferon-induced guanylate-binding proteins: Guardians of host defense in health and disease. J. Exp. Med. 2019, 216, 482–500. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.H.; Chee, J.D.; Bradfield, C.J.; Park, E.S.; Kumar, P.; MacMicking, J.D. Interferon-induced guanylate-binding proteins in inflammasome activation and host defense. Nat. Immunol. 2016, 17, 481–489. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Zhang, J.; Sun, Y.; Wang, H.; Wang, Y. The evolutionarily dynamic IFN-inducible GTPase proteins play conserved immune functions in vertebrates and cephalochordates. Mol. Biol. Evol. 2009, 26, 1619–1630. [Google Scholar] [CrossRef] [Green Version]
- Lubeseder-Martellato, C.; Guenzi, E.; Jorg, A.; Topolt, K.; Naschberger, E.; Kremmer, E.; Zietz, C.; Tschachler, E.; Hutzler, P.; Schwemmle, M.; et al. Guanylate-binding protein-1 expression is selectively induced by inflammatory cytokines and is an activation marker of endothelial cells during inflammatory diseases. Am. J. Pathol. 2002, 161, 1749–1759. [Google Scholar] [CrossRef] [Green Version]
- Naschberger, E.; Werner, T.; Vicente, A.B.; Guenzi, E.; Topolt, K.; Leubert, R.; Lubeseder-Martellato, C.; Nelson, P.J.; Sturzl, M. Nuclear factor-kappaB motif and interferon-alpha-stimulated response element co-operate in the activation of guanylate-binding protein-1 expression by inflammatory cytokines in endothelial cells. Biochem. J. 2004, 379, 409–420. [Google Scholar] [CrossRef]
- Prakash, B.; Praefcke, G.J.; Renault, L.; Wittinghofer, A.; Herrmann, C. Structure of human guanylate-binding protein 1 representing a unique class of GTP-binding proteins. Nature 2000, 403, 567–571. [Google Scholar] [CrossRef]
- Shydlovskyi, S.; Zienert, A.Y.; Ince, S.; Dovengerds, C.; Hohendahl, A.; Dargazanli, J.M.; Blum, A.; Gunther, S.D.; Kladt, N.; Sturzl, M.; et al. Nucleotide-dependent farnesyl switch orchestrates polymerization and membrane binding of human guanylate-binding protein 1. Proc. Natl. Acad. Sci. USA 2017, 114, E5559–E5568. [Google Scholar] [CrossRef] [Green Version]
- Sistemich, L.; Kutsch, M.; Hamisch, B.; Zhang, P.; Shydlovskyi, S.; Britzen-Laurent, N.; Sturzl, M.; Huber, K.; Herrmann, C. The Molecular Mechanism of Polymer Formation of Farnesylated Human Guanylate-binding Protein 1. J. Mol. Biol. 2020, 432, 2164–2185. [Google Scholar] [CrossRef]
- Modiano, N.; Lu, Y.E.; Cresswell, P. Golgi targeting of human guanylate-binding protein-1 requires nucleotide binding, isoprenylation, and an IFN-gamma-inducible cofactor. Proc. Natl. Acad. Sci. USA 2005, 102, 8680–8685. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.H.; Shenoy, A.R.; Kumar, P.; Das, R.; Tiwari, S.; MacMicking, J.D. A family of IFN-gamma-inducible 65-kD GTPases protects against bacterial infection. Science 2011, 332, 717–721. [Google Scholar] [CrossRef]
- Meunier, E.; Dick, M.S.; Dreier, R.F.; Schurmann, N.; Kenzelmann Broz, D.; Warming, S.; Roose-Girma, M.; Bumann, D.; Kayagaki, N.; Takeda, K.; et al. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature 2014, 509, 366–370. [Google Scholar] [CrossRef]
- Meunier, E.; Wallet, P.; Dreier, R.F.; Costanzo, S.; Anton, L.; Ruhl, S.; Dussurgey, S.; Dick, M.S.; Kistner, A.; Rigard, M.; et al. Guanylate-binding proteins promote activation of the AIM2 inflammasome during infection with Francisella novicida. Nat. Immunol. 2015, 16, 476–484. [Google Scholar] [CrossRef] [Green Version]
- Piro, A.S.; Hernandez, D.; Luoma, S.; Feeley, E.M.; Finethy, R.; Yirga, A.; Frickel, E.M.; Lesser, C.F.; Coers, J. Detection of Cytosolic Shigella flexneri via a C-Terminal Triple-Arginine Motif of GBP1 Inhibits Actin-Based Motility. MBio 2017, 8, e01979-17. [Google Scholar] [CrossRef] [Green Version]
- Place, D.E.; Briard, B.; Samir, P.; Karki, R.; Bhattacharya, A.; Guy, C.S.; Peters, J.L.; Frase, S.; Vogel, P.; Neale, G.; et al. Interferon inducible GBPs restrict Burkholderia thailandensis motility induced cell-cell fusion. PLoS Pathog. 2020, 16, e1008364. [Google Scholar] [CrossRef] [Green Version]
- Wandel, M.P.; Pathe, C.; Werner, E.I.; Ellison, C.J.; Boyle, K.B.; von der Malsburg, A.; Rohde, J.; Randow, F. GBPs Inhibit Motility of Shigella flexneri but Are Targeted for Degradation by the Bacterial Ubiquitin Ligase IpaH9.8. Cell Host Microbe 2017, 22, 507–518.e5. [Google Scholar] [CrossRef] [Green Version]
- Feeley, E.M.; Pilla-Moffett, D.M.; Zwack, E.E.; Piro, A.S.; Finethy, R.; Kolb, J.P.; Martinez, J.; Brodsky, I.E.; Coers, J. Galectin-3 directs antimicrobial guanylate binding proteins to vacuoles furnished with bacterial secretion systems. Proc. Natl. Acad. Sci. USA 2017, 114, E1698–E1706. [Google Scholar] [CrossRef] [Green Version]
- Costa Franco, M.M.; Marim, F.; Guimaraes, E.S.; Assis, N.R.G.; Cerqueira, D.M.; Alves-Silva, J.; Harms, J.; Splitter, G.; Smith, J.; Kanneganti, T.D.; et al. Brucella abortus Triggers a cGAS-Independent STING Pathway To Induce Host Protection That Involves Guanylate-Binding Proteins and Inflammasome Activation. J. Immunol. 2018, 200, 607–622. [Google Scholar] [CrossRef] [Green Version]
- Degrandi, D.; Konermann, C.; Beuter-Gunia, C.; Kresse, A.; Wurthner, J.; Kurig, S.; Beer, S.; Pfeffer, K. Extensive characterization of IFN-induced GTPases mGBP1 to mGBP10 involved in host defense. J. Immunol. 2007, 179, 7729–7740. [Google Scholar] [CrossRef] [Green Version]
- Sohrabi, Y.; Volkova, V.; Kobets, T.; Havelkova, H.; Krayem, I.; Slapnickova, M.; Demant, P.; Lipoldova, M. Genetic Regulation of Guanylate-Binding Proteins 2b and 5 during Leishmaniasis in Mice. Front. Immunol. 2018, 9, 130. [Google Scholar] [CrossRef] [Green Version]
- Selleck, E.M.; Fentress, S.J.; Beatty, W.L.; Degrandi, D.; Pfeffer, K.; Virgin, H.W.T.; Macmicking, J.D.; Sibley, L.D. Guanylate-binding protein 1 (Gbp1) contributes to cell-autonomous immunity against Toxoplasma gondii. PLoS Pathog. 2013, 9, e1003320. [Google Scholar] [CrossRef]
- Kravets, E.; Degrandi, D.; Ma, Q.; Peulen, T.O.; Klumpers, V.; Felekyan, S.; Kuhnemuth, R.; Weidtkamp-Peters, S.; Seidel, C.A.; Pfeffer, K. Guanylate binding proteins directly attack Toxoplasma gondii via supramolecular complexes. Elife 2016, 5, e11479. [Google Scholar] [CrossRef]
- Shenoy, A.R.; Wellington, D.A.; Kumar, P.; Kassa, H.; Booth, C.J.; Cresswell, P.; MacMicking, J.D. GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science 2012, 336, 481–485. [Google Scholar] [CrossRef]
- Pilla, D.M.; Hagar, J.A.; Haldar, A.K.; Mason, A.K.; Degrandi, D.; Pfeffer, K.; Ernst, R.K.; Yamamoto, M.; Miao, E.A.; Coers, J. Guanylate binding proteins promote caspase-11-dependent pyroptosis in response to cytoplasmic LPS. Proc. Natl. Acad. Sci. USA 2014, 111, 6046–6051. [Google Scholar] [CrossRef] [Green Version]
- Man, S.M.; Karki, R.; Malireddi, R.K.; Neale, G.; Vogel, P.; Yamamoto, M.; Lamkanfi, M.; Kanneganti, T.D. The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat. Immunol. 2015, 16, 467–475. [Google Scholar] [CrossRef] [Green Version]
- Fisch, D.; Bando, H.; Clough, B.; Hornung, V.; Yamamoto, M.; Shenoy, A.R.; Frickel, E.M. Human GBP1 is a microbe-specific gatekeeper of macrophage apoptosis and pyroptosis. EMBO J. 2019, 38, e100926. [Google Scholar] [CrossRef]
- Kutsch, M.; Sistemich, L.; Lesser, C.F.; Goldberg, M.B.; Herrmann, C.; Coers, J. Direct binding of polymeric GBP1 to LPS disrupts bacterial cell envelope functions. EMBO J. 2020, 39, e104926. [Google Scholar] [CrossRef]
- Berry, M.P.; Graham, C.M.; McNab, F.W.; Xu, Z.; Bloch, S.A.; Oni, T.; Wilkinson, K.A.; Banchereau, R.; Skinner, J.; Wilkinson, R.J.; et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 2010, 466, 973–977. [Google Scholar] [CrossRef] [Green Version]
- Bloom, C.I.; Graham, C.M.; Berry, M.P.; Rozakeas, F.; Redford, P.S.; Wang, Y.; Xu, Z.; Wilkinson, K.A.; Wilkinson, R.J.; Kendrick, Y.; et al. Transcriptional blood signatures distinguish pulmonary tuberculosis, pulmonary sarcoidosis, pneumonias and lung cancers. PLoS ONE 2013, 8, e70630. [Google Scholar] [CrossRef] [Green Version]
- Maertzdorf, J.; McEwen, G.; Weiner, J., III; Tian, S.; Lader, E.; Schriek, U.; Mayanja-Kizza, H.; Ota, M.; Kenneth, J.; Kaufmann, S.H. Concise gene signature for point-of-care classification of tuberculosis. EMBO Mol. Med. 2016, 8, 86–95. [Google Scholar] [CrossRef]
- Maertzdorf, J.; Repsilber, D.; Parida, S.K.; Stanley, K.; Roberts, T.; Black, G.; Walzl, G.; Kaufmann, S.H. Human gene expression profiles of susceptibility and resistance in tuberculosis. Genes Immun. 2011, 12, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Verhagen, L.M.; Zomer, A.; Maes, M.; Villalba, J.A.; Del Nogal, B.; Eleveld, M.; van Hijum, S.A.; de Waard, J.H.; Hermans, P.W. A predictive signature gene set for discriminating active from latent tuberculosis in Warao Amerindian children. BMC Genom. 2013, 14, 74. [Google Scholar] [CrossRef] [Green Version]
- Houben, D.; Demangel, C.; van Ingen, J.; Perez, J.; Baldeon, L.; Abdallah, A.M.; Caleechurn, L.; Bottai, D.; van Zon, M.; de Punder, K.; et al. ESX-1-mediated translocation to the cytosol controls virulence of mycobacteria. Cell Microbiol. 2012, 14, 1287–1298. [Google Scholar] [CrossRef]
- Van der Wel, N.; Hava, D.; Houben, D.; Fluitsma, D.; van Zon, M.; Pierson, J.; Brenner, M.; Peters, P.J.M. Tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell 2007, 129, 1287–1298. [Google Scholar] [CrossRef] [Green Version]
- Portnoy, D.A.; Auerbuch, V.; Glomski, I.J. The cell biology of Listeria monocytogenes infection: The intersection of bacterial pathogenesis and cell-mediated immunity. J. Cell Biol. 2002, 158, 409–414. [Google Scholar] [CrossRef] [Green Version]
- Meyer-Morse, N.; Robbins, J.R.; Rae, C.S.; Mochegova, S.N.; Swanson, M.S.; Zhao, Z.; Virgin, H.W.; Portnoy, D. Listeriolysin O is necessary and sufficient to induce autophagy during Listeria monocytogenes infection. PLoS ONE 2010, 5, e8610. [Google Scholar] [CrossRef]
- Thiele, D.L.; Lipsky, P.E. Mechanism of L-leucyl-L-leucine methyl ester-mediated killing of cytotoxic lymphocytes: Dependence on a lysosomal thiol protease, dipeptidyl peptidase I, that is enriched in these cells. Proc. Natl. Acad. Sci. USA 1990, 87, 83–87. [Google Scholar] [CrossRef] [Green Version]
- Praefcke, G.J.; Kloep, S.; Benscheid, U.; Lilie, H.; Prakash, B.; Herrmann, C. Identification of residues in the human guanylate-binding protein 1 critical for nucleotide binding and cooperative GTP hydrolysis. J. Mol. Biol. 2004, 344, 257–269. [Google Scholar] [CrossRef]
- Britzen-Laurent, N.; Bauer, M.; Berton, V.; Fischer, N.; Syguda, A.; Reipschlager, S.; Naschberger, E.; Herrmann, C.; Sturzl, M. Intracellular trafficking of guanylate-binding proteins is regulated by heterodimerization in a hierarchical manner. PLoS ONE 2010, 5, e14246. [Google Scholar] [CrossRef] [Green Version]
- Skowyra, M.L.; Schlesinger, P.H.; Naismith, T.V.; Hanson, P.I. Triggered recruitment of ESCRT machinery promotes endolysosomal repair. Science 2018, 360, eaar5078. [Google Scholar] [CrossRef] [Green Version]
- Radulovic, M.; Schink, K.O.; Wenzel, E.M.; Nahse, V.; Bongiovanni, A.; Lafont, F.; Stenmark, H. ESCRT-mediated lysosome repair precedes lysophagy and promotes cell survival. EMBO J. 2018, 37, e99753. [Google Scholar] [CrossRef]
- Repnik, U.; Cesen, M.H.; Turk, B. The Use of Lysosomotropic Dyes to Exclude Lysosomal Membrane Permeabilization. Cold Spring Harb Protoc. 2016, 2016, pdb-rot087106. [Google Scholar] [CrossRef] [Green Version]
- Jia, J.Y.; Abudu, Y.P.; Claude-Taupin, A.; Gu, Y.X.; Kumar, S.; Choi, S.W.; Peters, R.; Mudd, M.H.; Allers, L.; Salemi, M.; et al. Galectins Control mTOR in Response to Endomembrane Damage. Mol. Cell 2018, 70, 120–135.e8. [Google Scholar] [CrossRef] [Green Version]
- Jia, J.Y.; Claude-Taupin, A.; Gu, Y.X.; Choi, S.W.; Peters, R.; Bissa, B.; Mudd, M.H.; Allers, L.; Pallikkuth, S.; Lidke, K.A.; et al. Galectin-3 Coordinates a Cellular System for Lysosomal Repair and Removal. Dev. Cell 2020, 52, 69–87. [Google Scholar] [CrossRef]
- Repnik, U.; Borg Distefano, M.; Speth, M.T.; Ng, M.Y.W.; Progida, C.; Hoflack, B.; Gruenberg, J.; Griffiths, G. L-leucyl-L-leucine methyl ester does not release cysteine cathepsins to the cytosol but inactivates them in transiently permeabilized lysosomes. J. Cell Sci. 2017, 130, 3124–3140. [Google Scholar] [CrossRef] [Green Version]
- Morlot, S.; Roux, A. Mechanics of dynamin-mediated membrane fission. Annu. Rev. Biophys. 2013, 42, 629–649. [Google Scholar] [CrossRef] [Green Version]
- Hammond, G.R.; Machner, M.P.; Balla, T. A novel probe for phosphatidylinositol 4-phosphate reveals multiple pools beyond the Golgi. J. Cell Biol. 2014, 205, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Varnai, P.; Balla, T. Visualization of phosphoinositides that bind pleckstrin homology domains: Calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J. Cell Biol. 1998, 143, 501–510. [Google Scholar] [CrossRef]
- Clayton, E.L.; Minogue, S.; Waugh, M.G. Mammalian phosphatidylinositol 4-kinases as modulators of membrane trafficking and lipid signaling networks. Prog. Lipid Res. 2013, 52, 294–304. [Google Scholar] [CrossRef] [Green Version]
- Czech, M.P. PIP2 and PIP3: Complex roles at the cell surface. Cell 2000, 100, 603–606. [Google Scholar] [CrossRef] [Green Version]
- Di Paolo, G.; De Camilli, P. Phosphoinositides in cell regulation and membrane dynamics. Nature 2006, 443, 651–657. [Google Scholar] [CrossRef]
- Schink, K.O.; Tan, K.W.; Stenmark, H. Phosphoinositides in Control of Membrane Dynamics. Annu. Rev. Cell Dev. Biol. 2016, 32, 143–171. [Google Scholar] [CrossRef]
- Gozzelino, L.; De Santis, M.C.; Gulluni, F.; Hirsch, E.; Martini, M. PI(3,4)P2 Signaling in Cancer and Metabolism. Front. Oncol. 2020, 10, 360. [Google Scholar] [CrossRef] [Green Version]
- Sridhar, S.; Patel, B.; Aphkhazava, D.; Macian, F.; Santambrogio, L.; Shields, D.; Cuervo, A.M. The lipid kinase PI4KIIIbeta preserves lysosomal identity. EMBO J. 2013, 32, 324–339. [Google Scholar] [CrossRef]
- Jovic, M.; Kean, M.J.; Szentpetery, Z.; Polevoy, G.; Gingras, A.C.; Brill, J.A.; Balla, T. Two phosphatidylinositol 4-kinases control lysosomal delivery of the Gaucher disease enzyme, beta-glucocerebrosidase. Mol. Biol. Cell 2012, 23, 1533–1545. [Google Scholar] [CrossRef]
- Paz, I.; Sachse, M.; Dupont, N.; Mounier, J.; Cederfur, C.; Enninga, J.; Leffler, H.; Poirier, F.; Prevost, M.C.; Lafont, F.; et al. Galectin-3, a marker for vacuole lysis by invasive pathogens. Cell Microbiol. 2010, 12, 530–544. [Google Scholar] [CrossRef]
- Thurston, T.L.; Wandel, M.P.; von Muhlinen, N.; Foeglein, A.; Randow, F. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 2012, 482, 414–418. [Google Scholar] [CrossRef] [Green Version]
- Maejima, I.; Takahashi, A.; Omori, H.; Kimura, T.; Takabatake, Y.; Saitoh, T.; Yamamoto, A.; Hamasaki, M.; Noda, T.; Isaka, Y.; et al. Autophagy sequesters damaged lysosomes to control lysosomal biogenesis and kidney injury. EMBO J. 2013, 32, 2336–2347. [Google Scholar] [CrossRef] [Green Version]
- Haldar, A.K.; Saka, H.A.; Piro, A.S.; Dunn, J.D.; Henry, S.C.; Taylor, G.A.; Frickel, E.M.; Valdivia, R.H.; Coers, J. IRG and GBP host resistance factors target aberrant, “non-self” vacuoles characterized by the missing of “self” IRGM proteins. PLoS Pathog. 2013, 9, e1003414. [Google Scholar] [CrossRef] [Green Version]
- Haldar, A.K.; Foltz, C.; Finethy, R.; Piro, A.S.; Feeley, E.M.; Pilla-Moffett, D.M.; Komatsu, M.; Frickel, E.M.; Coers, J. Ubiquitin systems mark pathogen-containing vacuoles as targets for host defense by guanylate binding proteins. Proc. Natl. Acad. Sci. USA 2015, 112, E5628–E5637. [Google Scholar] [CrossRef] [Green Version]
- Fisch, D.; Clough, B.; Domart, M.C.; Encheva, V.; Bando, H.; Snijders, A.P.; Collinson, L.M.; Yamamoto, M.; Shenoy, A.R.; Frickel, E.M. Human GBP1 Differentially Targets Salmonella and Toxoplasma to License Recognition of Microbial Ligands and Caspase-Mediated Death. Cell Rep. 2020, 32, 108008. [Google Scholar] [CrossRef]
- Yamamoto, M.; Okuyama, M.; Ma, J.S.; Kimura, T.; Kamiyama, N.; Saiga, H.; Ohshima, J.; Sasai, M.; Kayama, H.; Okamoto, T.; et al. A cluster of interferon-gamma-inducible p65 GTPases plays a critical role in host defense against Toxoplasma gondii. Immunity 2012, 37, 302–313. [Google Scholar] [CrossRef] [Green Version]
- Degrandi, D.; Kravets, E.; Konermann, C.; Beuter-Gunia, C.; Klumpers, V.; Lahme, S.; Rasch, E.; Mausberg, A.K.; Beer-Hammer, S.; Pfeffer, K. Murine guanylate binding protein 2 (mGBP2) controls Toxoplasma gondii replication. Proc. Natl. Acad. Sci. USA 2013, 110, 294–299. [Google Scholar] [CrossRef] [Green Version]
- Wandel, M.P.; Kim, B.H.; Park, E.S.; Boyle, K.B.; Nayak, K.; Lagrange, B.; Herod, A.; Henry, T.; Zilbauer, M.; Rohde, J.; et al. Guanylate-binding proteins convert cytosolic bacteria into caspase-4 signaling platforms. Nat. Immunol. 2020, 21, 880–891. [Google Scholar] [CrossRef]
- Kohler, K.M.; Kutsch, M.; Piro, A.S.; Wallace, G.D.; Coers, J.; Barber, M.F. A Rapidly Evolving Polybasic Motif Modulates Bacterial Detection by Guanylate Binding Proteins. MBio 2020, 11, e00340-20. [Google Scholar] [CrossRef]
- Lopez-Jimenez, A.T.; Cardenal-Munoz, E.; Leuba, F.; Gerstenmaier, L.; Barisch, C.; Hagedorn, M.; King, J.S.; Soldati, T. The ESCRT and autophagy machineries cooperate to repair ESX-1-dependent damage at the Mycobacterium-containing vacuole but have opposite impact on containing the infection. PLoS Pathog. 2018, 14, e1007501. [Google Scholar] [CrossRef] [Green Version]
- Mittal, E.; Skowyra, M.L.; Uwase, G.; Tinaztepe, E.; Mehra, A.; Koster, S.; Hanson, P.I.; Philips, J.A. Mycobacterium tuberculosis Type VII Secretion System Effectors Differentially Impact the ESCRT Endomembrane Damage Response. MBio 2018, 9, e01765-18. [Google Scholar] [CrossRef] [Green Version]
- Herbst, S.; Campbell, P.; Harvey, J.; Bernard, E.M.; Papayannopoulos, V.; Wood, N.W.; Morris, H.R.; Gutierrez, M.G. LRRK2 activation controls the repair of damaged endomembranes in macrophages. EMBO J. 2020, 39, e104494. [Google Scholar] [CrossRef]
- Chauhan, S.; Kumar, S.; Jain, A.; Ponpuak, M.; Mudd, M.H.; Kimura, T.; Choi, S.W.; Peters, R.; Mandell, M.; Bruun, J.A.; et al. TRIMs and Galectins Globally Cooperate and TRIM16 and Galectin-3 Co-direct Autophagy in Endomembrane Damage Homeostasis. Dev. Cell 2016, 39, 13–27. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, Y.; Yasuda, S.; Fujita, T.; Hamasaki, M.; Murakami, A.; Kawawaki, J.; Iwai, K.; Saeki, Y.; Yoshimori, T.; Matsuda, N.; et al. Ubiquitination of exposed glycoproteins by SCF(FBXO27) directs damaged lysosomes for autophagy. Proc. Natl. Acad. Sci. USA 2017, 114, 8574–8579. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, G.; Chen, C.; Portnoy, D.A. Strategies Used by Bacteria to Grow in Macrophages. Microbiol. Spectr. 2016, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Olive, A.J.; Smith, C.M.; Baer, C.E.; Coers, J.; Sassetti, C.M. Mycobacterium tuberculosis Evasion of Guanylate Binding Protein-Mediated Host Defense in Mice Requires the ESX1 Secretion System. Int. J. Mol. Sci. 2023, 24, 2861. [Google Scholar] [CrossRef]
- Pei, G.; Buijze, H.; Liu, H.; Moura-Alves, P.; Goosmann, C.; Brinkmann, V.; Kawabe, H.; Dorhoi, A.; Kaufmann, S.H.E. The E3 ubiquitin ligase NEDD4 enhances killing of membrane-perturbing intracellular bacteria by promoting autophagy. Autophagy 2017, 13, 2041–2055. [Google Scholar] [CrossRef]

| hGBP1 | Fw: AGTAGGATCCGCATCAGAGATCCACATGACAG |
| Rv: TACTGCGGCCGCTTATTAGCTTATGGTACATGCCTTTCG | |
| hGBP2 | Fw: AGTAGGATCCGCTCCAGAGATCAACTTGCCG |
| Rv: TACTGCGGCCGCTTATTAGAGTATGTTACATATTGGCTC | |
| hGBP3 | Fw: AGTAGGATCCGCTCCAGAGATCCACATGACAG |
| Rv: TACTGCGGCCGCTTATTAGATCTTTAGCTTATGCGAC | |
| hGBP4 | Fw: AGTAGGATCCGGTGAGAGAACTCTTCACGCTG |
| Rv: TACTGCGGCCGCTTATTAAATACGTGAGCCAAGATATTTTG | |
| hGBP5 | Fw: AGTAGGATCCGCTTTAGAGATCCACGCTTTAG |
| Rv: TACTGCGGCCGCTTATTAGAGTAAAACACATGGATCATC |
| hGBP1 R48A | Fw: GGGCCTCTACgcCACAGGCAAATC |
| Rv: ACAATTGCCACCACCACC | |
| hGBP1 K51A | Fw: CCGCACAGGCgcATCCTACCTG |
| Rv: TAGAGGCCCACAATTGCC | |
| hGBP1 S52N | Fw: CACAGGCAAAaaCTACCTGATGAACAAG |
| Rv: CGGTAGAGGCCCACAATT | |
| hGBP1 R227E/K228E | Fw: gcagcagcgGCATGTACCATAAGCTAATAAG |
| Rv: tgccgctgcCGTCTGGAGATCCTGTATC | |
| hGBP1 ΔCaaX | Fw: TAATAAGCGGCCGCACTC |
| Rv: TGCCTTTCGTCGTCTCATTTTC | |
| hGBP1 PBmut | Fw: ACTCTGTATCgaggaaTTCTTCCCAAAGAAAAAATG |
| Rv: CTGGGCAGGTTAAAAGTTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buijze, H.; Brinkmann, V.; Hurwitz, R.; Dorhoi, A.; Kaufmann, S.H.E.; Pei, G. Human GBP1 Is Involved in the Repair of Damaged Phagosomes/Endolysosomes. Int. J. Mol. Sci. 2023, 24, 9701. https://doi.org/10.3390/ijms24119701
Buijze H, Brinkmann V, Hurwitz R, Dorhoi A, Kaufmann SHE, Pei G. Human GBP1 Is Involved in the Repair of Damaged Phagosomes/Endolysosomes. International Journal of Molecular Sciences. 2023; 24(11):9701. https://doi.org/10.3390/ijms24119701
Chicago/Turabian StyleBuijze, Hellen, Volker Brinkmann, Robert Hurwitz, Anca Dorhoi, Stefan H. E. Kaufmann, and Gang Pei. 2023. "Human GBP1 Is Involved in the Repair of Damaged Phagosomes/Endolysosomes" International Journal of Molecular Sciences 24, no. 11: 9701. https://doi.org/10.3390/ijms24119701
APA StyleBuijze, H., Brinkmann, V., Hurwitz, R., Dorhoi, A., Kaufmann, S. H. E., & Pei, G. (2023). Human GBP1 Is Involved in the Repair of Damaged Phagosomes/Endolysosomes. International Journal of Molecular Sciences, 24(11), 9701. https://doi.org/10.3390/ijms24119701






