Screening of Mono-, Di- and Trivalent Cationic Dopants for the Enhancement of Thermal Behavior, Kinetics, Structural, Morphological, Surface and Magnetic Properties of CoFe2O4-SiO2 Nanocomposites
Abstract
1. Introduction
- (i)
- A study of the influence of the SiO2 matrix and dopant ion on forming metallic succinates and their decomposition into ferrites embedded in the SiO2 matrix.
- (ii)
- A comparative study of the impact of monovalent, divalent, and trivalent ion doping of CoFe2O4 embedded in the SiO2 matrix on the morphological, magnetic and structural properties in order to find new strategies to increase their potential for existing and new possible applications.
- (iii)
- Filling the gap in the existing literature on the effect of Na+, Ag+, Cd2+, La3+, and Ca2+ ion doping on the physicochemical properties of CoFe2O4 embedded in the SiO2 matrix; the embedding of CoFe2O4 nanoparticles in the non-toxic, inert SiO2 matrix via the sol-gel method allows the control of the particle growth, reduces the nanoparticle agglomeration and enhances the chemical stability and magnetic guidance [11].
- (iv)
- The elucidation of the thermal behavior under isothermal and non-isothermal conditions and the formation kinetics of undoped and doped CoFe2O4.
- (v)
- Obtaining single crystalline ferrites at low calcination temperatures (400 and 700 °C) by doping CoFe2O4 with mono-, di- and trivalent ions.
2. Results and Discussion
2.1. Thermal Stability of CoFe2O4 and Role of Doping Metals
2.2. Kinetics of Doped and Undoped CoFe2O4 Formation
2.3. FT-IR Spectroscopy
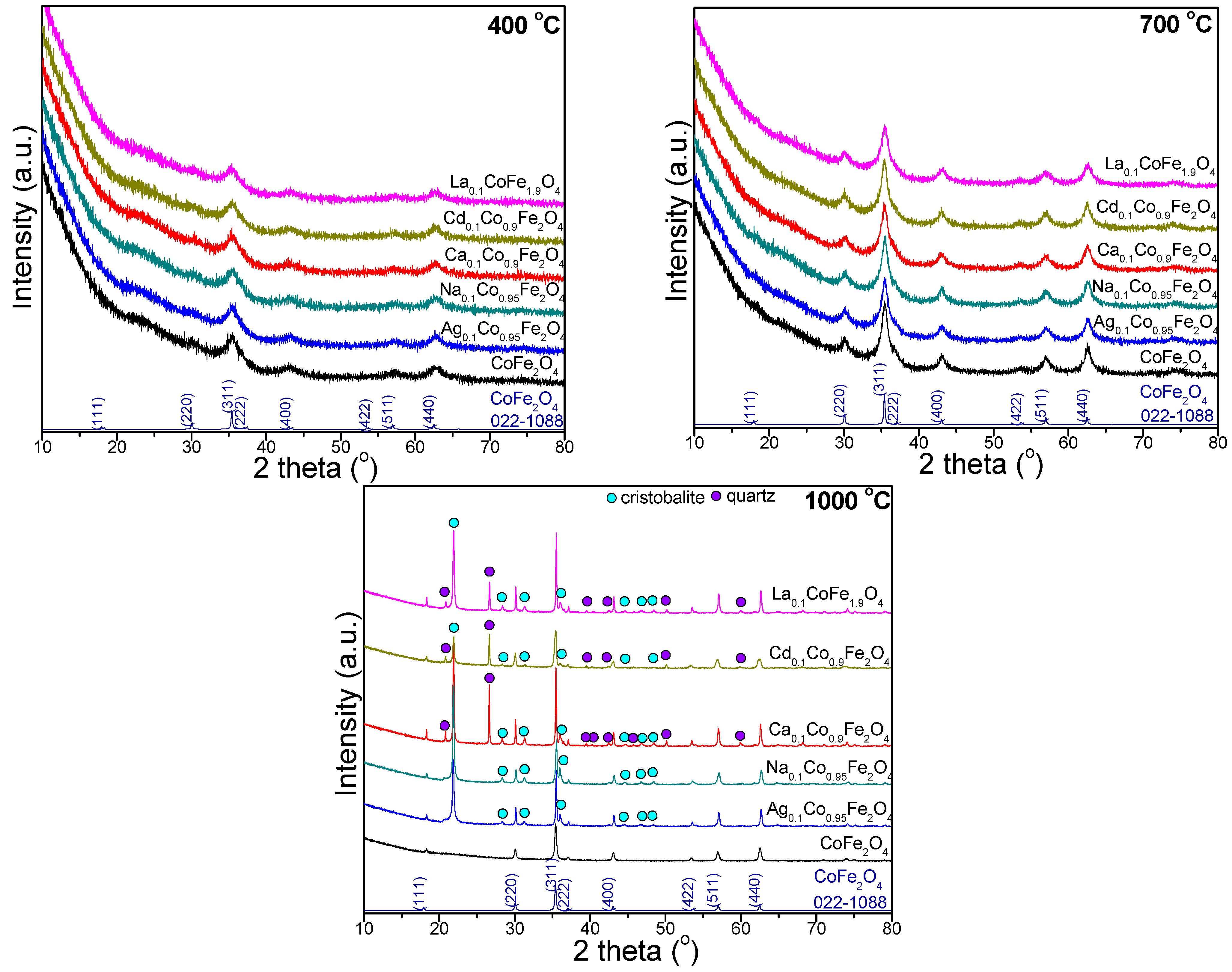
2.4. X-ray Diffraction of Undoped and Doped CoFe2O4
2.5. Elemental Composition of Undoped and Doped CoFe2O4
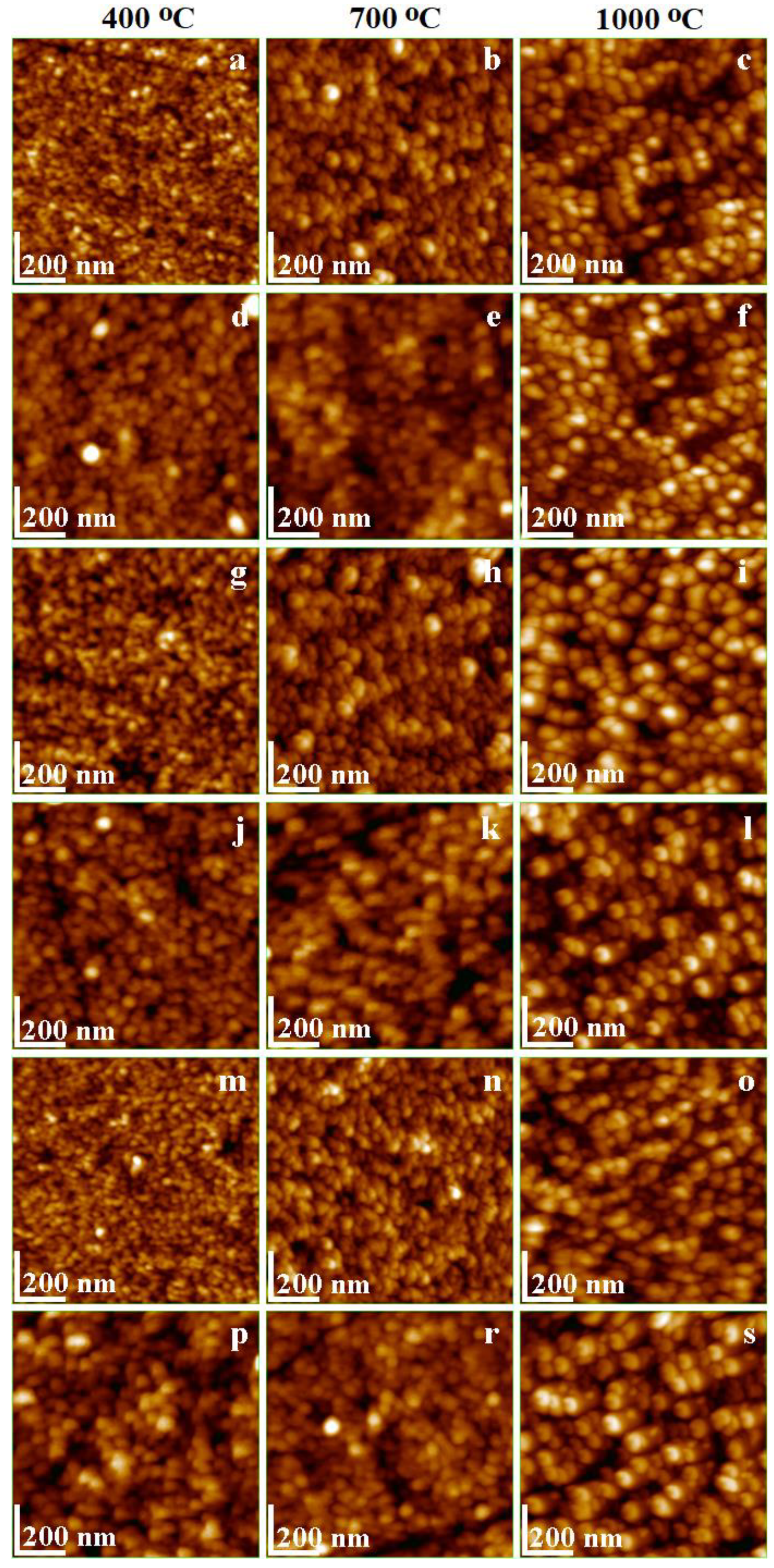
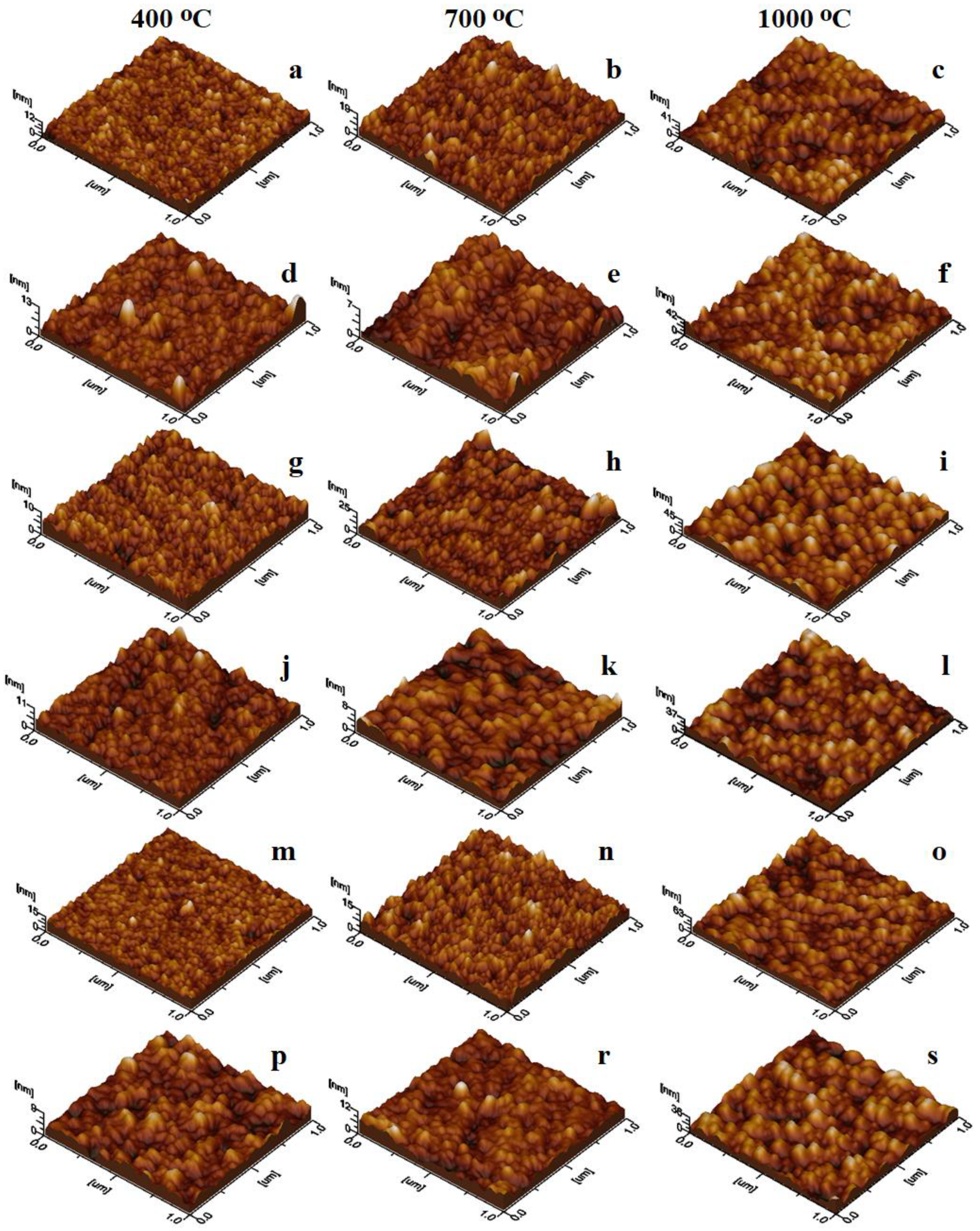
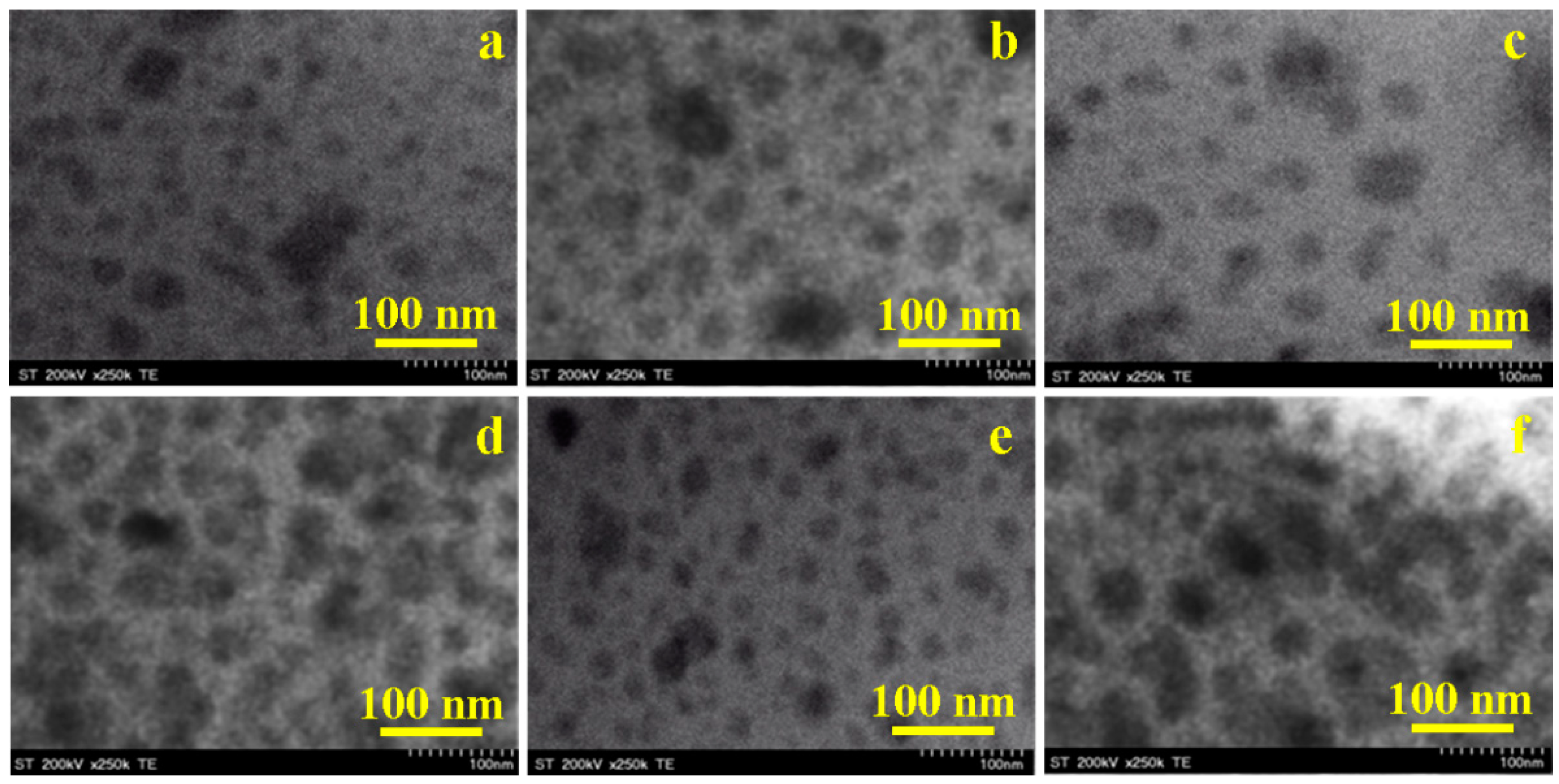
2.6. Morphology and Surface Parameters of Undoped and Doped CoFe2O4
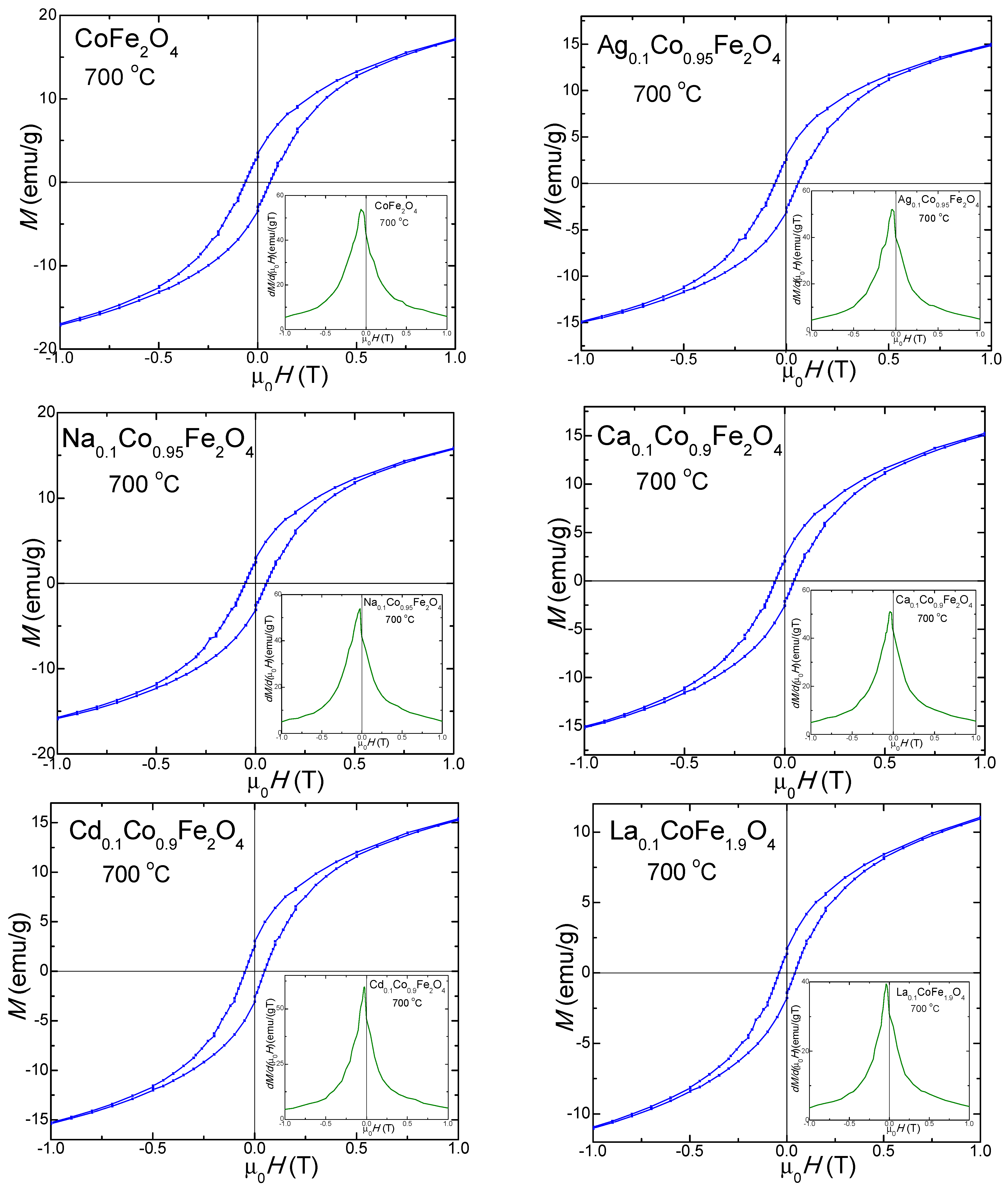
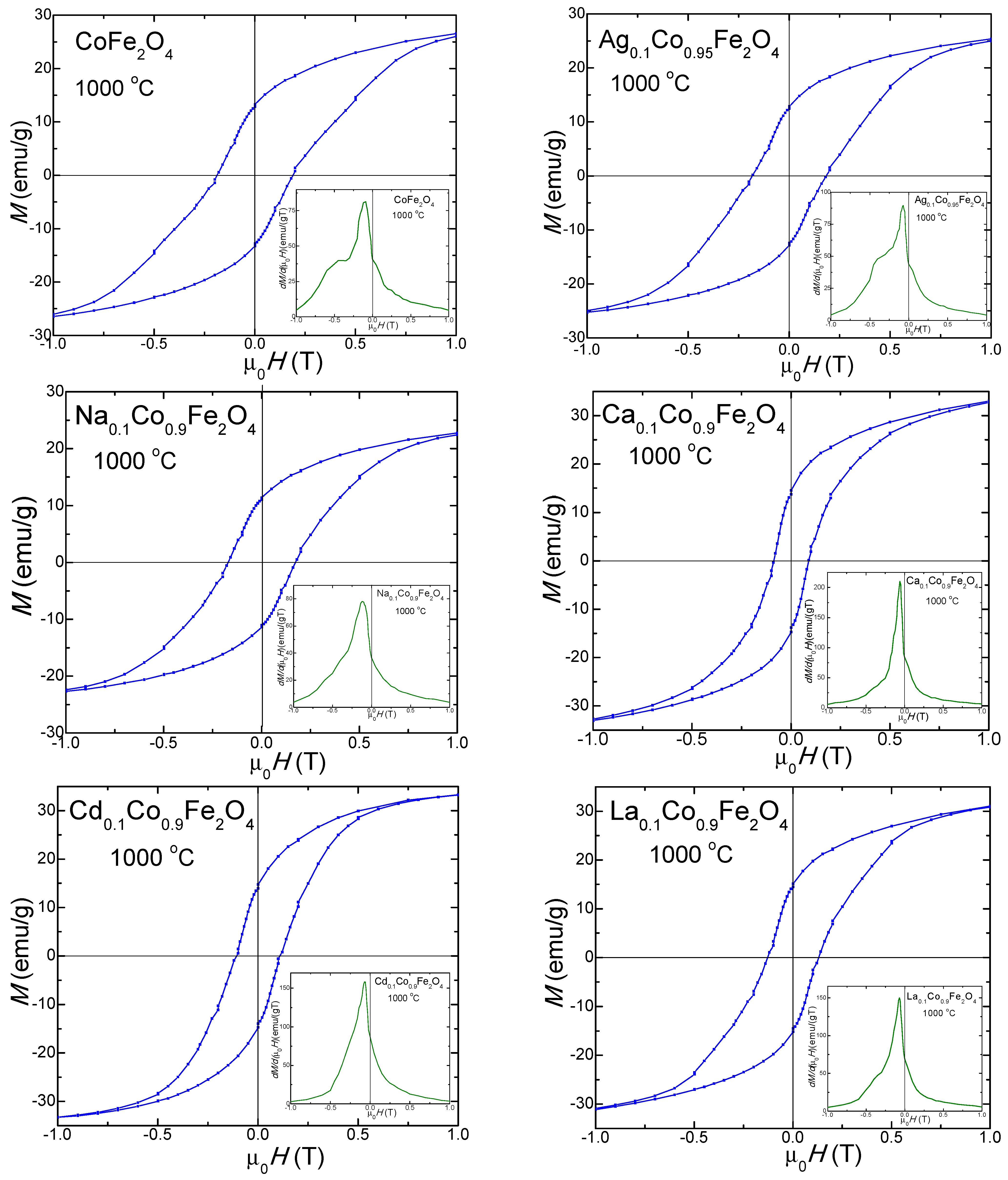
2.7. Magnetic Properties of Undoped and Doped CoFe2O4
3. Materials and Methods
3.1. Reagents
3.2. Synthesis
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, H.; Singh, A.; Kumar, V.; Ahlawat, D.S. Structural, thermal and magnetic investigations of cobalt ferrite doped with Zn2+ and Cd2+ synthesized by auto combustion method. J. Magn. Magn. Mater. 2019, 474, 505–511. [Google Scholar] [CrossRef]
- Ahmad, R.; Gul, I.H.; Zarrar, M.; Anwar, H.; Niazi, M.B.K.; Khan, A. Improved electrical properties of cadmium substituted cobalt ferrites nano-particles for microwave application. J. Magn. Magn. Mater. 2016, 405, 28–35. [Google Scholar] [CrossRef]
- Ghorbani, H.; Eshraghi, M.; Dodaran, A.A.S. Structural and magnetic properties of cobalt ferrite nanoparticles doped with cadmium. Phys. B Condens. Matter. 2022, 634, 413816. [Google Scholar] [CrossRef]
- Sharifianjazia, F.; Moradi, M.; Parvin, N.; Nemati, A.; Rad, A.J.; Sheysi, N.; Abouchenari, A.; Mohammadi, A.; Karbasi, S.; Ahmadi, Z.; et al. Magnetic CoFe2O4 nanoparticles doped with metal ions: A review. Ceram. Int. 2020, 46, 18391–18412. [Google Scholar] [CrossRef]
- Pinheiro, A.V.B.; da Silva, R.B.; Morales, M.A.; Silva Filho, E.D.; Soares, J.M. Exchange bias and superspin glass behavior in nanostructured CoFe2O4−Ag composites. J. Magn. Magn. Mater. 2020, 497, 165940. [Google Scholar] [CrossRef]
- Mariosi, F.R.; Venturini, J.; da Cas Viegas, A.; Bergmann, C.P. Lanthanum-doped spinel cobalt ferrite (CoFe2O4) nanoparticles for environmental applications. Ceram. Int. 2020, 462, 772–2779. [Google Scholar] [CrossRef]
- Waki, T.; Takao, K.; Tabata, Y.; Nakamura, H. Single crystal synthesis and magnetic properties of Co2+-substituted and non-substituted magnetoplumbite-type Na–La ferrite. J. Solid State Chem. 2020, 282, 121071. [Google Scholar] [CrossRef]
- Mansour, S.F.; Hemeda, O.M.; El-Dek, S.I.; Salem, B.I. Influence of La doping and synthesis method on the properties of CoFe2O4 nanocrystals. J. Magn. Magn. Mater. 2016, 420, 7–18. [Google Scholar] [CrossRef]
- Shang, T.; Lu, Q.; Chao, L.; Qin, Y.; Yun, Y.; Yun, G. Effects of ordered mesoporous structure and La-doping on the microwave absorbing properties of CoFe2O4. Appl. Surf. Sci. 2018, 434, 232–242. [Google Scholar] [CrossRef]
- Thakur, P.; Gahlawat, N.; Punia, P.; Kharbanda, S.; Ravelo, B.; Thakur, A. Cobalt nanoferrites: A review on synthesis, characterization, and applications. J. Supercond. Nov. Magn. 2022, 35, 2639–2669. [Google Scholar] [CrossRef]
- Dippong, T.; Levei, E.A.; Cadar, O. Recent advances in synthesis and applications of MFe2O4 (M = Co, Cu, Mn, Ni, Zn) Nanoparticles. Nanomaterials 2021, 11, 1560. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.I. Nano cobalt ferrites: Doping, Structural, Low-temperature, and room temperature magnetic and dielectric properties–A comprehensive review. J. Magn. Magn. Mater. 2022, 562, 169840. [Google Scholar] [CrossRef]
- Hua, J.; Cheng, Z.; Chen, Z.; Dong, H.; Li, P.; Wang, J. Tuning the microstructural and magnetic properties of CoFe2O4/SiO2 nanocomposites by Cu2+ doping. RSC Adv. 2021, 11, 26336–26343. [Google Scholar] [CrossRef]
- Gupta, M.; Das, A.; Das, D.; Mohapatra, S.; Datta, A. Chemical synthesis of rare earth (La, Gd) doped cobalt ferrite and a comparative analysis of their magnetic properties. J. Nanosci. Nanotechnol. 2020, 20, 5239–5245. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singhal, S. Transition metal doped cobalt ferrite nanoparticles: Efficient photocatalyst for photodegradation of textile dye. Mater. Today Proc. 2019, 14, 453–460. [Google Scholar] [CrossRef]
- Abbas, N.; Rubab, N.; Sadiq, N.; Manzoor, S.; Khan, M.I.; Fernandez Garcia, J.; Barbosa Aragao, I.; Tariq, M.; Akhtar, Z.; Yasmin, G. Aluminum-doped cobalt ferrite as an efficient photocatalyst for the abatement of methylene blue. Water 2020, 12, 2285. [Google Scholar] [CrossRef]
- Aslam, A.; Razzaq, A.; Naz, S.; Amin, N.; Arshad, M.I.; Nabi, M.A.U.; Nawaz, A.; Mahmood, K.; Bibi, A.; Iqbal, F.; et al. Impact of lanthanum-doping on the physical and electrical properties of cobalt ferrites. J. Supercond. Nov. Magn. 2021, 34, 1855–1864. [Google Scholar] [CrossRef]
- Kashid, P.; Suresh, H.K.; Mathad, S.N.; Shedam, R.; Shedam, M. A review on synthesis, properties and applications on cobalt ferrite. Int. J. Adv. Sci. Eng. 2022, 9, 2567–2583. [Google Scholar] [CrossRef]
- Sakhawat, K.; Ullah, F.; Ahmed, I.; Iqbal, T.; Maqbool, F.; Khan, M.; Tabassum, H.; Abrar, M. Simple synthesis of Ag-doped ferrites nanoparticles for its application as bactericidal activity. Appl. Nanosci. 2021, 11, 2801–2809. [Google Scholar] [CrossRef]
- El Nahrawy, A.A.; Soliman, A.A.; Sakr, E.M.M.; El Attar, H.A. Sodium–cobalt ferrite nanostructure study: Sol–gel synthesis, characterization, and magnetic properties. J. Ovonic Res. 2018, 14, 193–200. [Google Scholar]
- Dippong, T.; Deac, I.G.; Cadar, O.; Levei, E.A. Effect of silica embedding on the structure, morphology and magnetic behavior of (Zn0.6Mn0.4Fe2O4)δ/(SiO2)(100−δ) Nanoparticles. Nanomaterials 2021, 11, 2232. [Google Scholar] [CrossRef] [PubMed]
- Dippong, T.; Levei, E.A.; Deac, I.G.; Petean, I.; Cadar, O. Dependence of structural, morphological and magnetic properties of manganese ferrite on Ni-Mn substitution. Int. J. Mol. Sci. 2022, 23, 3097. [Google Scholar] [CrossRef]
- Dippong, T.; Levei, E.A.; Cadar, O.; Deac, I.G.; Lazar, M.; Borodi, G.; Petean, I. Effect of amorphous SiO2 matrix on structural and magnetic properties of Cu0.6Co0.4Fe2O4/SiO2 nanocomposites. J. Alloys Compd. 2020, 849, 156695. [Google Scholar] [CrossRef]
- Ajeesha, T.L.; Manikandan, A.; Anantharaman, A.; Jansi, S.; Durka, M.; Almessiere, M.A.; Slimani, Y.; Baykal, A.; Asiri, A.M.; Kasmery, H.A.; et al. Structural investigation of Cu doped calcium ferrite (Ca1-xCuxFe2O4; x = 0, 0.2, 0.4, 0.6, 0.8, 1) nanomaterials prepared by co-precipitation method. J. Mater. Res. Technol. 2022, 18, 705–719. [Google Scholar] [CrossRef]
- Sadegh, F.; Tavakol, H. Synthesis of Ag/CoFe2O4 magnetic aerogel for catalytic reduction of nitroaromatics. Res. Chem. 2022, 4, 100592. [Google Scholar] [CrossRef]
- He, S.; Yang, C.; Niu, M.; Wei, D.; Chu, S.; Zhong, M.; Wang, J.; Su, X.; Wang, L. Coordination adsorption of Ag(I) on cobalt-ferrous oxalates and their derived Ag/CoFe2O4 for catalytic hydrogenation reactions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 124007. [Google Scholar] [CrossRef]
- Satheeshkumar, M.K.; Kumar, E.R.; Srinivas, C.; Suriyanarayanan, N.; Deepty, M.; Prajapat, C.L.; Rao, T.V.C.; Sastry, D.L. Study of structural, morphological and magnetic properties of Ag substituted cobalt ferrite nanoparticles prepared by honey assisted combustion method and evaluation of their antibacterial activity. J. Magn. Magn. Mater. 2019, 469, 691–697. [Google Scholar] [CrossRef]
- Guo, L.; Okinaka, N.; Zhang, L.; Watanabe, S. Molten salt-assisted shape modification of CaFe2O4 nanorods for highly efficient photocatalytic degradation of methylene blue. Opt. Mater. 2021, 119, 111295. [Google Scholar] [CrossRef]
- Dippong, T.; Levei, E.A.; Cadar, O. Investigation of structural, morphological and magnetic properties of MFe2O4 (M = Co, Ni, Zn, Cu, Mn) obtained by thermal decomposition. Int. J. Mol. Sci. 2022, 23, 8483. [Google Scholar] [CrossRef] [PubMed]
- Dippong, T.; Cadar, O.; Levei, E.A.; Deac, I.G.; Goga, F.; Borodi, G.; Barbu Tudoran, L. Influence of polyol structure and molecular weight on the shape and properties of Ni0.5Co0.5Fe2O4 nanoparticles obtained by sol-gel synthesis. Ceram. Int. 2019, 45, 7458–7467. [Google Scholar] [CrossRef]
- Malyshev, A.V.; Vlasov, V.A.; Nikolaev, E.V.; Surzhikov, A.P.; Lysenko, E.N. Microstructure and thermal analysis of lithium ferrite pre-milled in a high-energy ball mill. J. Therm. Anal. Calorim. 2018, 134, 127–133. [Google Scholar]
- Becherescu, D.; Cristea, V.; Marx, F.; Menessy, I.; Winter, F. Metode Fizice in Chimia Silicatilor; Editura Stiintifica si Enciclopedica: Bucuresti, Romania, 1977. [Google Scholar]
- Kumar, H.; Singh, J.P.; Srivastava, R.C.; Negi, P.; Agrawal, H.M.; Asokan, K. FTIR and electrical study of dysprosium doped cobalt ferrite nanoparticles. J. Nanosci. 2014, 2014, 862415. [Google Scholar] [CrossRef]
- Xu, Z.; Tang, Z.; Chen, F.; Bo, X.; Wu, H.; Li, Z.; Jiang, S. Study of lateral assembly of magnetic particles in magnetorheological fluids under magnetic fields. J. Magn. Magn. Mater. 2023, 556, 170293. [Google Scholar] [CrossRef]
- Singh, R.; Sharma, V. Investigations on sintering mechanism of nano tungsten carbide powder based on molecular dynamics simulation and experimental validation. Adv. Powder Technol. 2022, 33, 103724. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, W.Y.; Zhao, X.J.; Xiao, L.R.; Cai, Z.Y.; Pu, R.; Xu, X.Q. Enhancing dispersion of ultra-fine WC powders in aqueous media. Colloids Surf. A Physicochem. Eng. Asp. 2019, 567, 63–68. [Google Scholar] [CrossRef]
- Damm, C.; Peukert, W. Chapter 3-Particle dispersions in liquid media. In Handbooks in Separation Science; Contado, C., Ed.; Particle Separation Techniques; Elsevier: Amsterdam, The Netherlands, 2022; pp. 27–62. [Google Scholar]
- Shao, Y.; Tian, M.; Zhen, Z.; Cui, J.; Xiao, M.; Qi, B.; Wang, T.; Hou, X. Adsorption of Ag/Au nanoparticles by ordered macro-microporous ZIF-67, and their synergistic catalysis application. J. Clean. Prod. 2022, 346, 131032. [Google Scholar] [CrossRef]
- Zhao, C.; Fu, H.; Yang, X.; Xiong, S.; Han, D.; An, X. Adsorption and photocatalytic performance of Au nanoparticles decorated porous Cu2O nanospheres under simulated solar light irradiation. Appl. Surf. Sci. 2021, 545, 149014. [Google Scholar] [CrossRef]
- Johan, A.; Setiabudidaya, D.; Arsyad, F.S.; Ramlan; Adi, W.A. Strong and weak ferromagnetic of cobalt ferrite: Structural, magnetic properties and reflection loss characteristic. Mater. Chem. Phys. 2023, 295, 127086. [Google Scholar] [CrossRef]
- Lakshmi, K.; Bharadwaj, S.; Chanda, S.; Reddy, C.V.K.; Pola, S.; Kumar, K.V.S. Iron ion non-stoichiometry and its effect on structural, magnetic and dielectric properties of cobalt ferrites prepared using oxalate precursor method. Mater. Chem. Phys. 2023, 295, 127172. [Google Scholar] [CrossRef]
- Prabagar, C.J.; Anand, S.; Janifer, M.A.; Pauline, S.; Agastian, P.; Theoder, S. Effect of metal substitution (Zn, Cu and Ag) in cobalt ferrite nanocrystallites for antibacterial activities. Mater. Today Proc. 2021, 16, 1999–2006. [Google Scholar] [CrossRef]
- Mahajan, P.; Sharma, A.; Kaur, B.; Goyal, N.; Gautam, S. Green synthesized (Ocimum sanctum and Allium sativum) Ag-doped cobalt ferrite nanoparticles for antibacterial application. Vacuum 2019, 16, 1389–1397. [Google Scholar] [CrossRef]
- Soltani, N.; Salavati, H.; Moghadasi, A. The role of Na-montmorillonite/cobalt ferrite nanoparticles in the corrosion of epoxy coated AA 3105 aluminum alloy. Surf. Interfaces 2019, 15, 89–99. [Google Scholar] [CrossRef]
- Kumar, R.; Kar, M. Correlation between lattice strain and magnetic behavior in non-magnetic Ca substituted nano-crystalline cobalt ferrite. Ceram. Int. 2016, 42, 6640–6647. [Google Scholar] [CrossRef]
- Jauhar, S.; Kaur, J.; Goyala, A.; Singhal, S. Tuning the properties of cobalt ferrite: A road towards diverse applications. RSC Adv. 2016, 6, 97694–97719. [Google Scholar] [CrossRef]
- Shakil, M.; Inayat, U.; Arshad, M.I.; Nabi, G.; Khalid, N.R.; Tariq, N.H.; Shah, A.; Iqbal, M.Z. Influence of zinc and cadmium co-doping on optical and magnetic properties of cobalt ferrites. Ceram. Int. 2020, 46, 7767–7773. [Google Scholar] [CrossRef]
- Kamran, M.; Anisur-Rehman, M. Influence of La3+ substitutions on structural, dielectric and electrical properties of spinel cobalt ferrite. Ceram. Int. 2023, 49, 7017–7029. [Google Scholar] [CrossRef]
- Kumar, L.; Kumar, P.; Kar, M. Non-linear behavior of coercivity to the maximum applied magnetic field in La substituted nanocrystalline cobalt ferrite. Phys. B Condens. Matter 2014, 448, 38–42. [Google Scholar] [CrossRef]
- Channagoudra, G.; Nunez, J.P.J.; Hadimani, R.L.; Dayal, V. Study of cation distribution in La3+ and Eu3+ substituted cobalt ferrite and its effect on magnetic properties. J. Magn. Magn. Mater. 2022, 559, 169550. [Google Scholar] [CrossRef]
- Jiang, C.; Leung, C.W.; Pong, P.W.T. Self-assembled thin films of Fe3O4-Ag composite nanoparticles for spintronic applications. Appl. Surf. Sci. 2017, 419, 692–696. [Google Scholar] [CrossRef]
- Zang, D.; Huo, Z.; Yang, S.; Li, Q.; Dai, G.; Zeng, M.; Ruhlmann, L.; Wei, Y. Layer by layer self-assembled hybrid thin films of Porphyrin/Polyoxometalates@Pt nanoparticles for photo & electrochemical application. Mater. Today Commun. 2022, 31, 103811. [Google Scholar]
- Li, X.; Sun, Y.; Zong, Y.; Wei, Y.; Liu, X.; Li, X.; Peng, Y.; Zheng, X. Size-effect induced cation redistribution on the magnetic properties of well-dispersed CoFe2O4 nanocrystals. J. Alloys Compd. 2020, 841, 155710. [Google Scholar] [CrossRef]
- Nadeem, K.; Traussnig, T.; Letofsky-Papst, I.; Krenn, H.; Brossmann, U. Sol-gel synthesis and characterization of single-phase Ni ferrite nanoparticles dispersed in SiO2 matrix. J. Alloys Compd. 2010, 493, 385–390. [Google Scholar] [CrossRef]
- Haque, S.U.; Saikia, K.K.; Murugesan, G.; Kalainathan, S. A study on dielectric and magnetic properties of lanthanum substituted cobalt ferrite. J. Alloys Compd. 2017, 701, 612–618. [Google Scholar] [CrossRef]
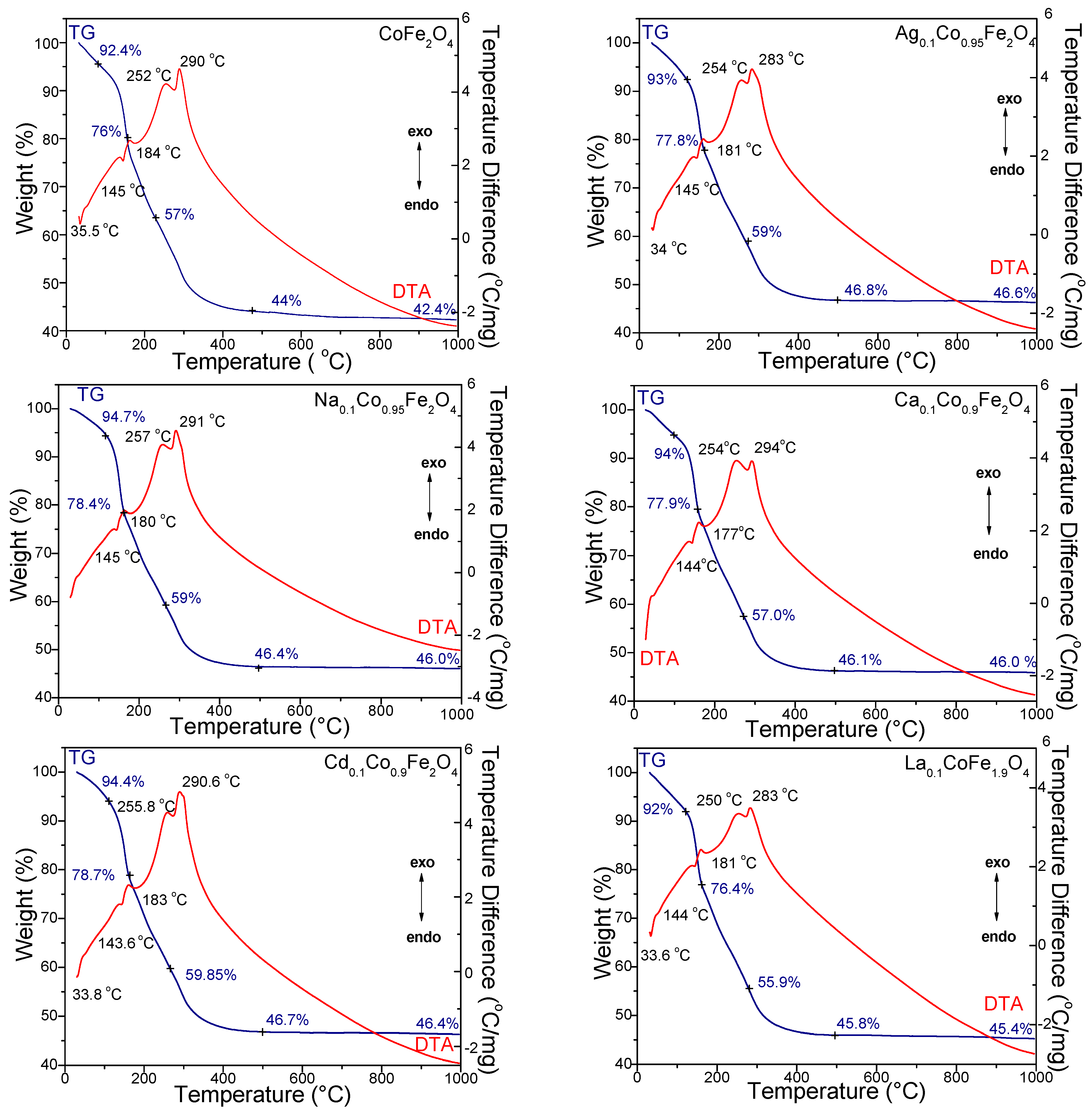
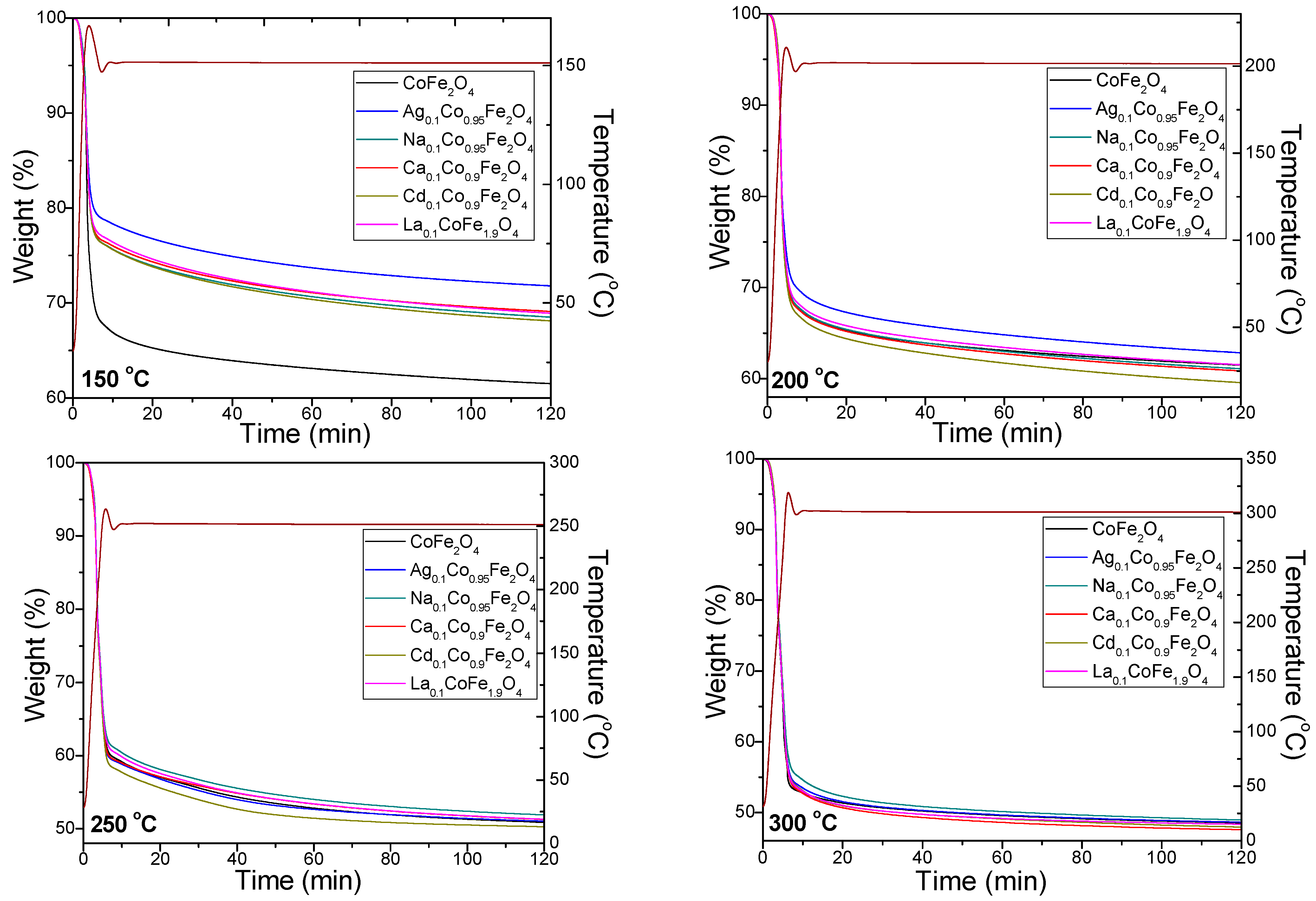
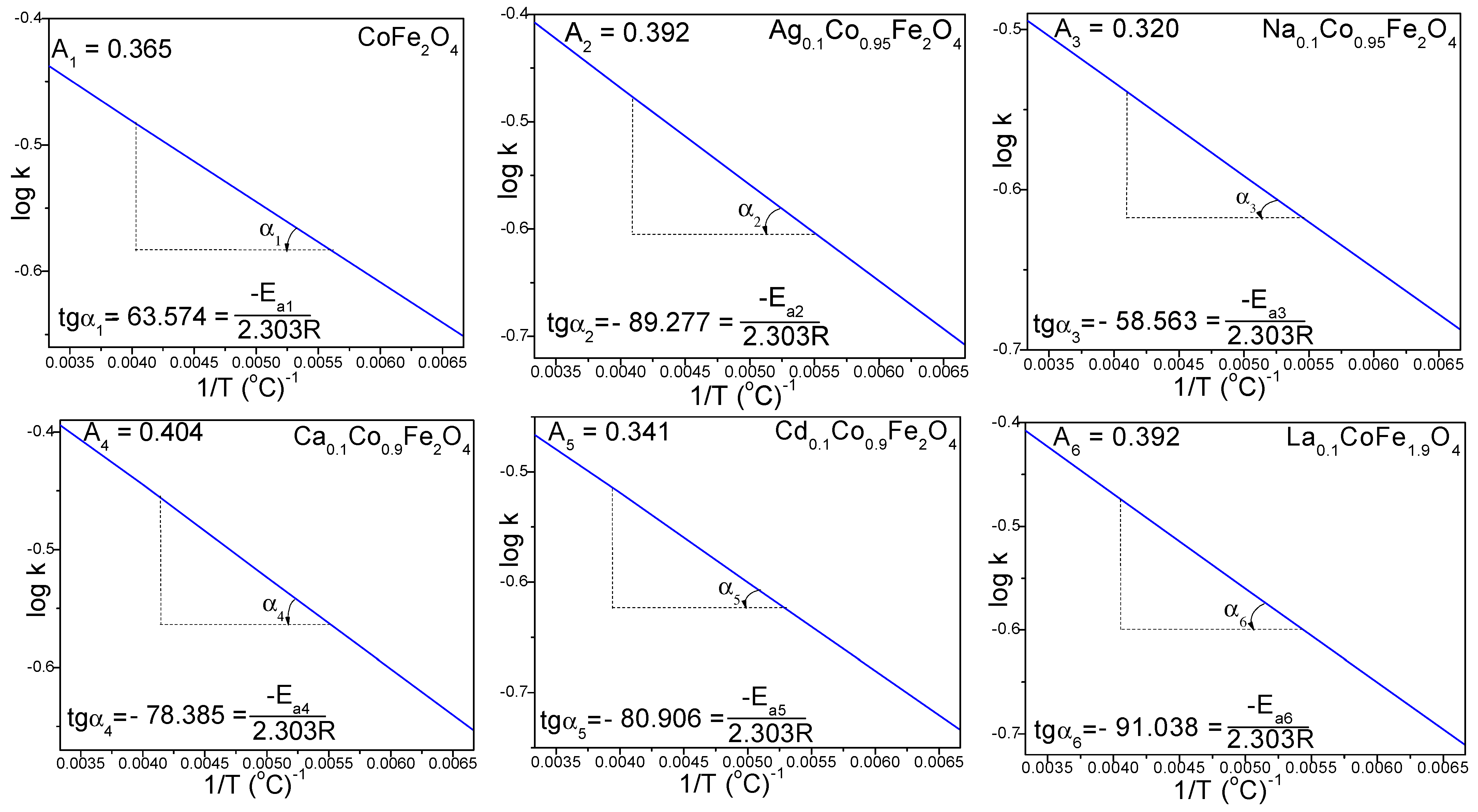
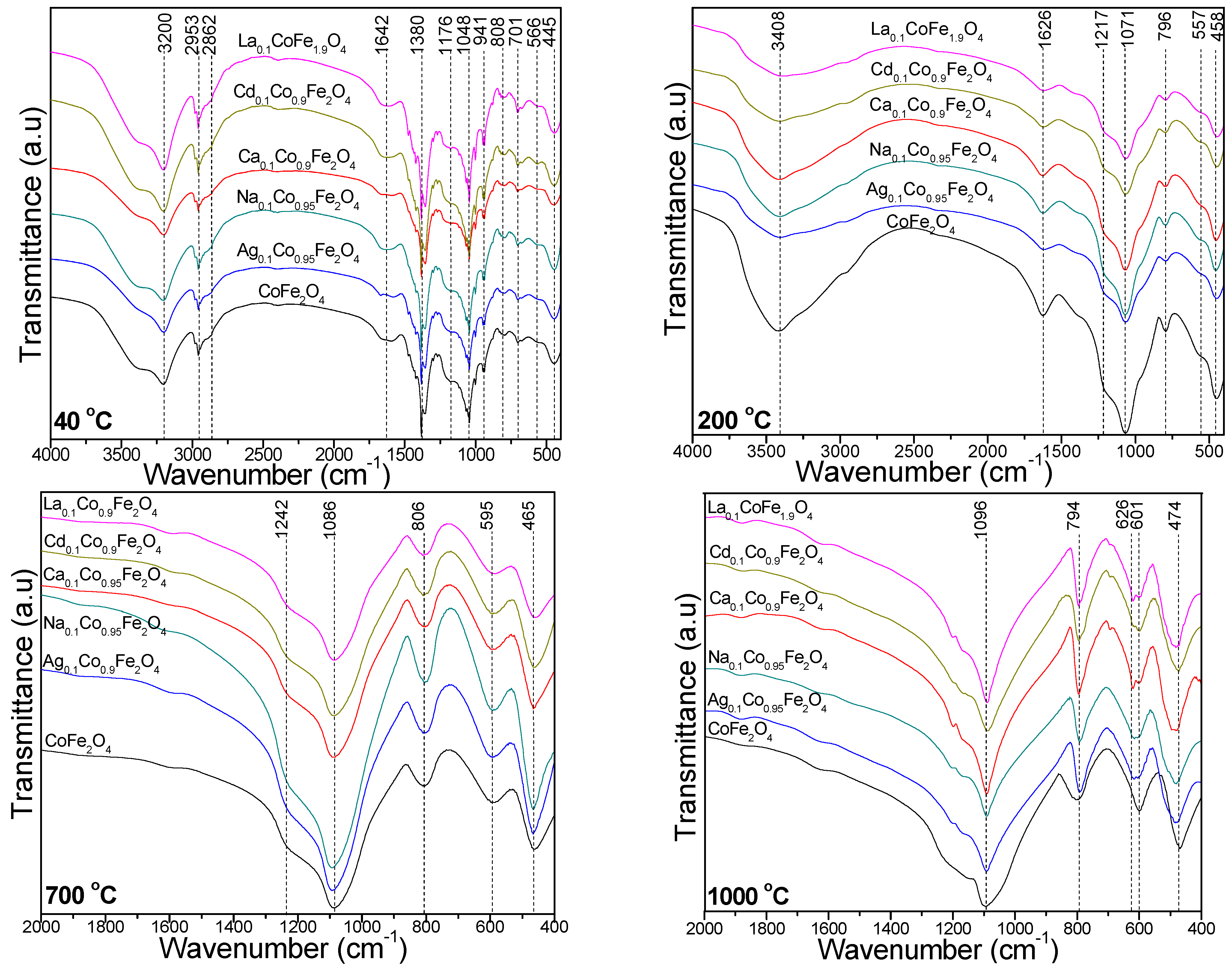
| Gels | k (s−1) | Ea (kJ/mol) | |||
|---|---|---|---|---|---|
| 150 °C | 200 °C | 250 °C | 300 °C | ||
| CoFe2O4 | 0.223 | 0.285 | 0.330 | 0.365 | 1.217 |
| Ag0.1Co0.95Fe2O4 | 0.196 | 0.271 | 0.336 | 0.391 | 1.709 |
| Na0.1Co0.95Fe2O4 | 0.205 | 0.256 | 0.293 | 0.320 | 1.121 |
| Ca0.1Co0.9Fe2O4 | 0.222 | 0.300 | 0.359 | 0.404 | 1.501 |
| Cd0.1Co0.9Fe2O4 | 0.184 | 0.251 | 0.303 | 0.341 | 1.549 |
| La0.1CoFe1.9O4 | 0.194 | 0.275 | 0.340 | 0.391 | 1.743 |
| Parameter | Temp (°C) | CoFe2O4 | Ag0.1Co0.95Fe2O4 | Na0.1Co0.95Fe2O4 | Ca0.1Co0.9Fe2O4 | Cd0.1Co0.9Fe2O4 | La0.1CoFe1.9O4 |
|---|---|---|---|---|---|---|---|
| DXRD (nm) | 400 | 13.2 | 14.0 | 13.6 | 14.6 | 12.3 | 15.5 |
| 700 | 23.4 | 24.7 | 23.7 | 25.2 | 22.5 | 26.1 | |
| 1000 | 37.2 | 65.1 | 47.6 | 73.7 | 35.0 | 81.8 | |
| DC (%) | 400 | 58.8 | 62.7 | 64.2 | 63.7 | 64.8 | 60.2 |
| 700 | 71.7 | 65.2 | 69.6 | 67.8 | 72.9 | 75.4 | |
| 1000 | 85.7 | 86.9 | 87.8 | 92.5 | 88.9 | 90.6 | |
| a (Å) | 400 | 8.391 | 8.380 | 8.377 | 8.374 | 8.400 | 8.408 |
| 700 | 8.395 | 8.400 | 8.390 | 8.388 | 8.412 | 8.411 | |
| 1000 | 8.403 | 8.410 | 8.400 | 8.397 | 8.422 | 8.423 | |
| V (Å3) | 400 | 590.8 | 588.5 | 587.8 | 587.2 | 592.7 | 594.4 |
| 700 | 591.6 | 592.7 | 590.6 | 590.2 | 595.2 | 595.0 | |
| 1000 | 593.3 | 594.8 | 592.7 | 592.1 | 597.4 | 597.6 | |
| dA (Å) | 400 | 3.633 | 3.629 | 3.627 | 3.626 | 3.637 | 3.641 |
| 700 | 3.635 | 3.637 | 3.633 | 3.632 | 3.643 | 3.642 | |
| 1000 | 3.639 | 3.642 | 3.637 | 3.636 | 3.647 | 3.647 | |
| dB (Å) | 400 | 2.967 | 2.963 | 2.962 | 2.961 | 2.970 | 2.973 |
| 700 | 2.968 | 2.970 | 2.966 | 2.966 | 2.974 | 2.974 | |
| 1000 | 2.971 | 2.973 | 2.970 | 2.969 | 2.978 | 2.978 | |
| dp (g/cm3) | 400 | 4.433 | 4.805 | 4.472 | 4.445 | 4.755 | 4.685 |
| 700 | 4.453 | 4.833 | 4.494 | 4.462 | 4.771 | 4.706 | |
| 1000 | 4.471 | 4.861 | 4.517 | 4.491 | 4.794 | 4.721 | |
| dXRD (g/cm3) | 400 | 5.276 | 5.473 | 5.288 | 5.265 | 5.445 | 5.429 |
| 700 | 5.269 | 5.435 | 5.263 | 5.239 | 5.422 | 5.424 | |
| 1000 | 5.253 | 5.415 | 5.244 | 5.222 | 5.402 | 5.400 | |
| P (%) | 400 | 16.0 | 12.2 | 15.4 | 15.6 | 12.7 | 13.7 |
| 700 | 15.5 | 11.1 | 14.6 | 14.8 | 12.0 | 13.2 | |
| 1000 | 14.9 | 10.2 | 13.9 | 14.0 | 11.3 | 12.6 | |
| M/Co/Fe molar ratio | 400 | 0/0.95/1.97 | 0.08/0.91/1.95 | 0.08/0.91/1.98 | 0.08/0.86/1.96 | 0.08/0.84/1.96 | 0.07/0.94/1.85 |
| 700 | 0/0.96/1.97 | 0.08/0.92/1.97 | 0.08/0.92/1.96 | 0.08/0.86/1.97 | 0.08/0.86/1.96 | 0.07/0.97/1.86 | |
| 1000 | 0/0.99/2.01 | 0.09/0.94/1.98 | 0.11/0.96/1.98 | 0.11/0.89/2.01 | 0.09/0.89/2.02 | 0.09/0.98/1.89 |
| Parameter | Temp. (°C) | CoFe2O4 | Ag0.1Co0.95Fe2O4 | Na0.1Co0.95Fe2O4 | Ca0.1Co0.9Fe2O4 | Cd0.1Co0.9Fe2O4 | La0.1CoFe1.9O4 |
|---|---|---|---|---|---|---|---|
| DAFM (nm) | 400 | 25 | 27 | 24 | 28 | 20 | 30 |
| 700 | 30 | 31 | 35 | 33 | 28 | 38 | |
| 1000 | 40 | 70 | 52 | 75 | 39 | 90 | |
| DTEM (nm) | 1000 | 38 | 67 | 50 | 76 | 37 | 84 |
| Height (nm) | 400 | 12 | 13 | 10 | 11 | 15 | 9 |
| 700 | 19 | 7 | 25 | 8 | 15 | 12 | |
| 1000 | 41 | 42 | 45 | 37 | 63 | 36 | |
| Rg (nm) | 400 | 0.81 | 0.95 | 0.97 | 0.77 | 1.10 | 1.04 |
| 700 | 1.95 | 0.83 | 2.42 | 0.75 | 1.50 | 1.10 | |
| 1000 | 5.80 | 6.16 | 6.21 | 5.35 | 7.82 | 5.58 | |
| Powder surface area (nm2) | 400 | 1017 | 1013 | 1016 | 1015 | 1018 | 1012 |
| 700 | 1028 | 1021 | 1032 | 1023 | 1024 | 1026 | |
| 1000 | 1083 | 1095 | 1094 | 1062 | 1160 | 1069 |
| Parameter | Temp (°C) | CoFe2O4 | Ag0.1Co0.95Fe2O4 | Na0.1Co0.95Fe2O4 | Ca0.1Co0.9Fe2O4 | Cd0.1Co0.9Fe2O4 | La0.1CoFe1.9O4 |
|---|---|---|---|---|---|---|---|
| MS (emu/g) | 700 | 30.0 | 25.4 | 27.7 | 26.7 | 26.2 | 26.5 |
| 1000 | 31.5 | 29.0 | 31.2 | 39.4 | 36.3 | 36.6 | |
| MR (emu/g) | 700 | 3.5 | 7.7 | 7.9 | 2.7 | 3.0 | 1.8 |
| 1000 | 13.4 | 13.8 | 11.3 | 14.5 | 17.0 | 15.1 | |
| Hc (Oe) | 700 | 600 | 530 | 360 | 410 | 440 | 385 |
| 1000 | 1750 | 1850 | 1760 | 840 | 1070 | 1300 | |
| nB | 700 | 0.935 | 0.815 | 0.857 | 0.821 | 0.831 | 0.821 |
| 1000 | 0.977 | 0.814 | 0.965 | 0.917 | 1.151 | 1.175 | |
| K·103 (erg/cm3) | 700 | 1.13 | 0.84 | 0.63 | 0.68 | 0.72 | 0.64 |
| 1000 | 3.46 | 2.90 | 3.45 | 2.08 | 2.44 | 2.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dippong, T.; Levei, E.A.; Petean, I.; Deac, I.G.; Mereu, R.A.; Cadar, O. Screening of Mono-, Di- and Trivalent Cationic Dopants for the Enhancement of Thermal Behavior, Kinetics, Structural, Morphological, Surface and Magnetic Properties of CoFe2O4-SiO2 Nanocomposites. Int. J. Mol. Sci. 2023, 24, 9703. https://doi.org/10.3390/ijms24119703
Dippong T, Levei EA, Petean I, Deac IG, Mereu RA, Cadar O. Screening of Mono-, Di- and Trivalent Cationic Dopants for the Enhancement of Thermal Behavior, Kinetics, Structural, Morphological, Surface and Magnetic Properties of CoFe2O4-SiO2 Nanocomposites. International Journal of Molecular Sciences. 2023; 24(11):9703. https://doi.org/10.3390/ijms24119703
Chicago/Turabian StyleDippong, Thomas, Erika Andrea Levei, Ioan Petean, Iosif Grigore Deac, Raluca Anca Mereu, and Oana Cadar. 2023. "Screening of Mono-, Di- and Trivalent Cationic Dopants for the Enhancement of Thermal Behavior, Kinetics, Structural, Morphological, Surface and Magnetic Properties of CoFe2O4-SiO2 Nanocomposites" International Journal of Molecular Sciences 24, no. 11: 9703. https://doi.org/10.3390/ijms24119703
APA StyleDippong, T., Levei, E. A., Petean, I., Deac, I. G., Mereu, R. A., & Cadar, O. (2023). Screening of Mono-, Di- and Trivalent Cationic Dopants for the Enhancement of Thermal Behavior, Kinetics, Structural, Morphological, Surface and Magnetic Properties of CoFe2O4-SiO2 Nanocomposites. International Journal of Molecular Sciences, 24(11), 9703. https://doi.org/10.3390/ijms24119703











