Abstract
Lytic polysaccharide monooxygenases (LPMOs) can oxidatively break the glycosidic bonds of crystalline cellulose, providing more actionable sites for cellulase to facilitate the conversion of cellulose to cello-oligosaccharides, cellobiose and glucose. In this work, a bioinformatics analysis of BaLPMO10 revealed that it is a hydrophobic, stable and secreted protein. By optimizing the fermentation conditions, the highest protein secretion level was found at a IPTG concentration of 0.5 mM and 20 h of fermentation at 37 °C, with a yield of 20 mg/L and purity > 95%. The effect of metal ions on the enzyme activity of BaLPMO10 was measured, and it was found that 10 mM Ca2+ and Na+ increased the enzyme activity by 47.8% and 98.0%, respectively. However, DTT, EDTA and five organic reagents inhibited the enzyme activity of BaLPMO10. Finally, BaLPMO10 was applied in biomass conversion. The degradation of corn stover pretreated with different steam explosions was performed. BaLPMO10 and cellulase had the best synergistic degradation effect on corn stover pretreated at 200 °C for 12 min, improving reducing sugars by 9.2% compared to cellulase alone. BaLPMO10 was found to be the most efficient for ethylenediamine-pretreated Caragana korshinskii by degrading three different biomasses, increasing the content of reducing sugars by 40.5% compared to cellulase alone following co-degradation with cellulase for 48 h. The results of scanning electron microscopy revealed that BaLPMO10 disrupted the structure of Caragana korshinskii, making its surface coarse and poriferous, which increased the accessibility of other enzymes and thus promoted the process of conversion. These findings provide guidance for improving the efficiency of enzymatic digestion of lignocellulosic biomass.
1. Introduction
Cello-oligosaccharide, cellobiose and glucose are the main degradation products of cellulose. Cello-oligosaccharide is a functional oligosaccharide that can strengthen human immunity to prevent the occurrence of diseases [1,2], and can also change the bacterial composition of animal intestines to promote intestinal development [3]. Because of its low sweetness properties, cellobiose is often used as a food additive in the beverage industry [4,5]. It is a sweetener very friendly to people who suffer from diabetes. In addition, glucose, the smallest unit of cellulose, can alleviate the energy shortage by converting it into bioethanol through fermentation [6].
Among the methods for the preparation of cello-oligosaccharide, cellobiose and glucose, enzymatic techniques are widely used because of their low cost, mild conditions and environmental friendliness. The cellulose structure is complex and mostly crystalline, while the current cellulases have low catalytic efficiency for crystalline cellulose, which limits the conversion efficiency of cellulose [7,8]. Recently, a class of copper ion-dependent lytic polysaccharide monooxygenases (LPMOs) was identified which can disrupt crystalline polysaccharides and improve substrate accessibility [9,10]. In the presence of an electron donor, the divalent copper ion in the active center of the LPMO receives an electron, thus becoming a monovalent copper ion, which in turn breaks the glycosidic bond of the polysaccharide [11,12]. Therefore, the synergistic effect of LPMO and cellulase can significantly improve the conversion efficiency of cellulose [10,13,14,15].
Based on amino acid sequences and functional similarities, LPMOs are currently classified into eight auxiliary activities (AA) families, including AA9–AA11 [16,17] and AA13-AA17 [18,19,20,21,22]. Among them, AA9 and AA10 are the two earliest discovered and most studied families [17,23,24]. In our previous study, a new AA10 protein (BaLPMO10) was obtained by screening and characterization, and showed catalytic activity for both cellulose and chitin, which is important for the conversion of biomass [25]. BaLPMO10 was expressed extracellularly in Escherichia coli (E. coli) using signal peptides, but in low yields [25]. The fermentation process is critical to the level of protein secretion, so optimizing the fermentation conditions can improve the level of protein secretion.
In order to further investigate the biochemical properties of BaLPMO10, in this study, we analyzed its bioinformatics and then improved its production by optimizing the fermentation conditions, including isopropyl β-D-1-thiogalactopyranoside (IPTG) concentration, fermentation temperature and time. In addition, the effects of metal ions, reducing agent DTT, chelating agent EDTA and organic reagents on the enzymatic activity and kinetic parameters of BaLPMO10 were determined. Finally, the degradation ability of BaLPMO10 for pretreated corn stover, rice straw, Caragana korshinskii and pulp was analyzed, and the surface structure of the biomass was observed in order to determine the mode of action of BaLPMO10.
2. Results and Discussion
2.1. Bioinformatics Analysis of BaLPMO10
To explore the bioinformatics of BaLPMO10, its physicochemical properties were analyzed using the ProtParam website (https://web.expasy.org/protparam/) (accessed on 10 March 2021) [26]. The results indicated that it was composed of 206 amino acids, with a molecular formula of C1017H1534N272O301S2 and a theoretical molecular weight of 22451.13 Da. The isoelectric point was determined to be 8.62, with 20 positively charged amino acid residues (R + K) and 18 negatively charged amino acid residues (D + E). BaLPMO10 had a total average hydrophilicity of −0.388 (<0), indicating that it was a hydrophilic protein. The instability index was calculated to be 30.72 (<40), suggesting that BaLPMO10 was a stable protein. Additionally, the TMHMM Server 2.0 website (http://www.cbs.dtu.dk/services/TMHMM-2.0/) (accessed on 10 March 2021) [27] was used to predict whether BaLPMO10 contained any transmembrane regions, and the results shown in Figure S1 indicate that BaLPMO10 did not have any transmembrane domains. To predict whether BaLPMO10 contained a signal peptide, its amino acid sequence was analyzed using the SignalP 5.0 Server (http://www.cbs.dtu.dk/services/SignalP-5.0/) (accessed on 10 March 2021) [28]. The results, as shown in Figure S2, revealed that BaLPMO10 contained a 27-amino-acid signal peptide (MKGLVKAAVLTVTLGIGGAFYSSDASA), indicating that it was a secretory protein.
2.2. Optimization of BaLPMO10 Fermentation Conditions
In the previous study, the gene of BaLPMO10 was successfully constructed into the vector pET-22b and achieved secretory expression using its native signal peptide [25]. In order to improve the secretion level of BaLPMO10 and reduce the cost of industrial downstream processing, its fermentation conditions were optimized.
2.2.1. Optimization of IPTG Induction Concentration
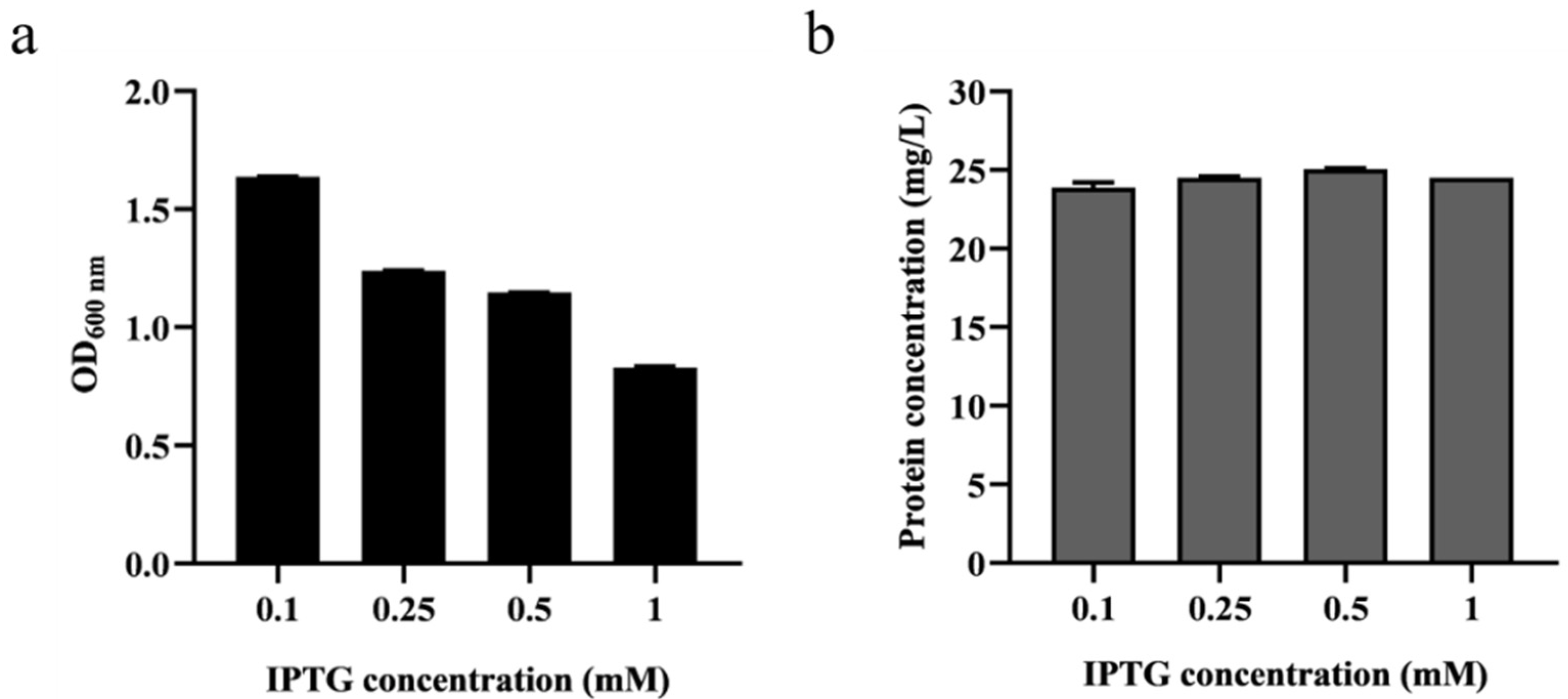
Due to the fact that pET-22b operates through an inducible T7 promoter, the concentration of the IPTG inducer plays a critical role in the expression of the target protein. Therefore, to obtain the appropriate IPTG concentration, it was added to final concentrations of 0.1, 0.25, 0.5 and 1 mM for induction during the fermentation process of BaLPMO10. Following fermentation, we measured the cell density at OD600 (Figure 1a) and collected the purified protein from the supernatant to determine its concentration (Figure 1b). Figure 1a demonstrates that, as the IPTG concentration increased, the cell concentration at OD600 decreased, suggesting that IPTG had a toxic effect that inhibited bacterial growth [29]. Figure 1b reveals that the secretion level of the BaLPMO10 was highest when the IPTG induction concentration was 0.5 mM, indicating that this was the optimal concentration for IPTG induction. However, the concentration of BaLPMO10 was reduced in the system with the addition of 1 mM IPTG. Higher concentrations of IPTG may adversely affect bacterial growth and thus interfere with the expression and secretion levels of the target protein. Similar results were found in previous studies [30], suggesting that an IPTG concentration of 0.5 mM may be the most appropriate when using E. coli to express exogenous proteins.
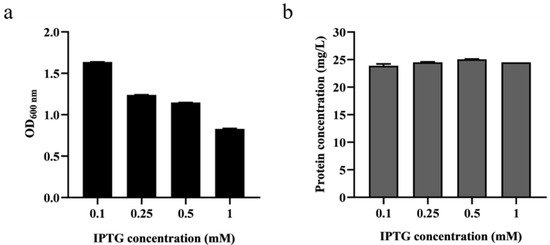
Figure 1.
Effect of various IPTG concentrations on cell growth and BaLPMO10 expression. (a) Cell concentration; (b) Production of BaLPMO10.
2.2.2. Optimization of Fermentation Temperature and Time
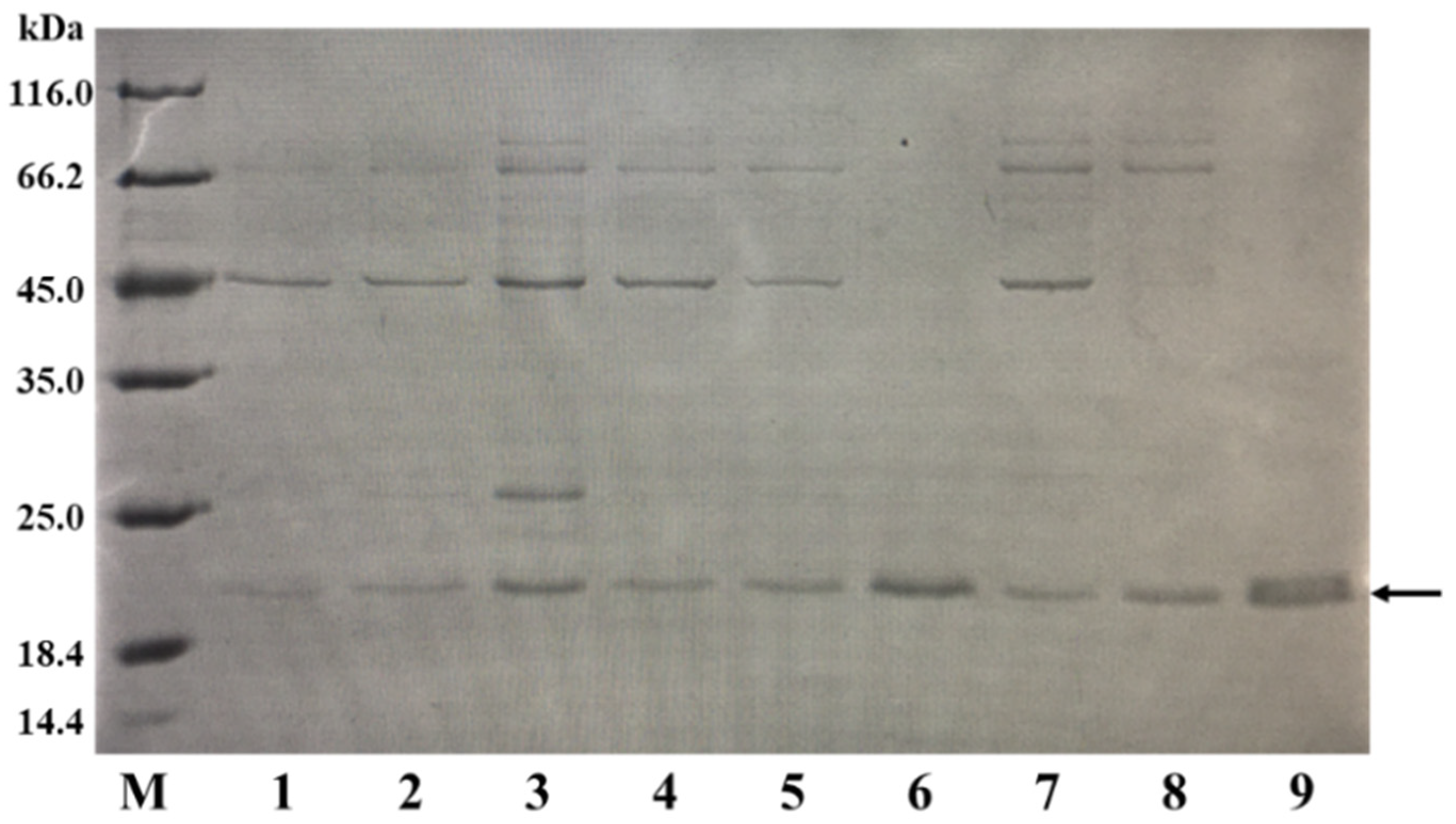
When expressing different exogenous proteins in a host, the optimal induction temperature and time can vary. To determine the ideal temperature and time for the expression of BaLPMO10, we investigated the effects of various fermentation temperatures and times on the secretory expression of BaLPMO10. After the addition of an IPTG inducer to the fermentation medium, the fermentation was carried out at 16 °C, 30 °C and 37 °C for 4 h, 8 h and 20 h, respectively. BaLPMO10 in the supernatant was purified at the end of fermentation, and the protein purity was analyzed by SDS-PAGE under different fermentation conditions. The results are shown in Figure 2. The results demonstrated that the highest expression level of BaLPMO10, with the least amount of impurities, was obtained after 20 h of fermentation at 37 °C (lane 9). The protein yield from purifying 1 L of fermentation broth was 20 mg. These findings highlight the critical role of IPTG concentration, fermentation temperature and time in regulating the expression of target proteins, and underscore the importance of careful optimization for maximizing protein expression.
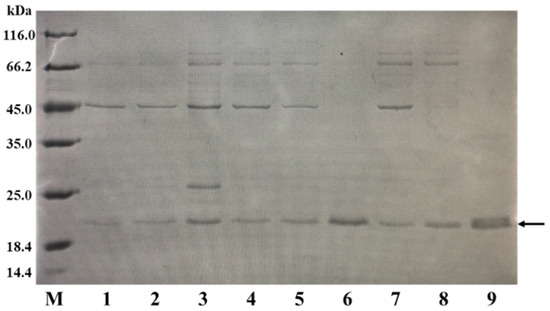
Figure 2.
Comparison of the BaLPMO10 production at various temperatures and times. The band indicated by the arrow is BaLPMO10. 1: 16 °C, 4 h; 2: 16 °C, 8 h; 3: 16 °C, 20 h; 4: 30 °C, 4 h; 5: 30 °C, 8 h; 6: 30 °C, 20 h; 7: 37 °C, 4 h; 8: 37 °C, 8 h; 9: 37 °C, 20 h.
2.3. Enzymatic Properties of BaLPMO10
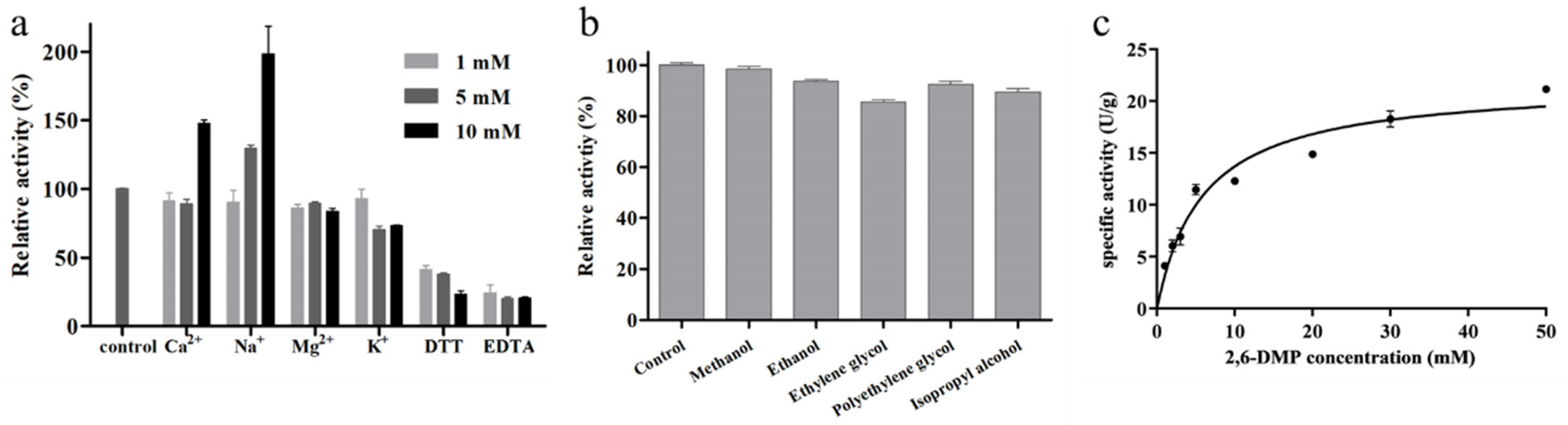
In previous studies, we investigated the effects of temperature and pH on the activity of BaLPMO10 and found that its optimal temperature was 70 °C, its optimal pH was 6.0 and its maximum specific activity was 91.4 U/g [25]. As BaLPMO10 was a metalloenzyme, the effects of different metal ions (K+, Na+, Ca2+, Ba2+, Mg2+, Cu2+, Mn2+, Co2+ and Fe3+), DTT and EDTA on its activity were examined. However, when Ba2+, Cu2+, Mn2+, Co2+ and Fe3+ were added, the reaction mixture became turbid, so these five metal ions were not studied further. The effects of the other four metal ions on BaLPMO10 activity are shown in Figure 3a. High concentrations of Ca2+ promoted enzyme activity; 10 mM Ca2+ increased BaLPMO10 activity by 47.8%, and 5 mM and 10 mM Na+ increased BaLPMO10 activity by 29.5% and 98.0%, respectively. However, different concentrations of Mg2+ and K+ inhibited enzyme activity. By comparison with other LPMOs, it was found that different metal ions showed inconsistent results regarding their enzyme activity. For example, different concentrations of Mg2+ were able to increase the enzyme activity of MtC1LPMO; 10 mM of Mg2+ increased the enzyme activity of MtC1LPMO by 37.6% [31]. In addition, the reducing agent DTT and chelating agent EDTA both inhibited enzyme activity in a concentration-dependent manner (Figure 3a). DTT and EDTA at 10 mM inhibited the enzyme activity of BaLPMO10 by 76.9% and 79.6%, respectively. Both of these are commonly used protease inhibitors. EDTA is supposed to chelate the copper ions in the active center of BaLPMO10, resulting in a more pronounced decrease in enzyme activity.
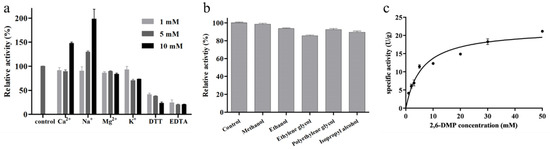
Figure 3.
Enzymatic properties of BaLPMO10. (a) Effect of metal ions, DTT and EDTA on the activity of BaLPMO10. (b) Effect of organic reagents on the activity of BaLPMO10. (c) Kinetic plot of BaLPMO10.
The impacts of five organic reagents, namely, methanol, ethanol, ethylene glycol, polyethylene glycol and isopropyl alcohol, on the enzyme activity of BaLPMO10 were analyzed, and the results are presented in Figure 3b. It can be seen that all five organic reagents reduced the enzyme activity to different degrees. Among them, ethylene glycol exhibited the greatest impact on enzyme activity, with a 14.5% decrease in BaLPMO10 activity. Methanol had the lowest impact on BaLPMO10 activity, with only a 1.7% decrease in activity. Ethanol, polyethylene glycol and isopropyl alcohol showed intermediate impacts on BaLPMO10 activity, with the activity decreasing by 6.4%, 7.6% and 10.6%, respectively.
To clarify the enzyme kinetics of BaLPMO10, its enzymatic activity was determined using different concentrations of 2,6-DMP as a substrate. The results of the kinetic curves are shown in Figure 3c. Based on the kinetic curves, the kinetic parameters of BaLPMO10 were calculated, and the results are shown in Table 1. The maximum velocity (Vmax), the substrate affinity (Km) and the catalytic efficiency (kcat/Km) of BaLPMO10 were 21.78 ± 2.23 U/g, 5.89 ± 1.95 mmol/L and 18.48 s−1 mM−1, respectively. The Km of BaLPMO10 was lower than that of NcLPMO9C (245 ± 74 mmol/L) [32], indicating a higher affinity between BaLPMO10 and the substrate.

Table 1.
Kinetic parameters of BaLPMO10.
2.4. Synergistic Degradation of Steam-Exploded Corn Stover by BaLPMO10 and Cellulase
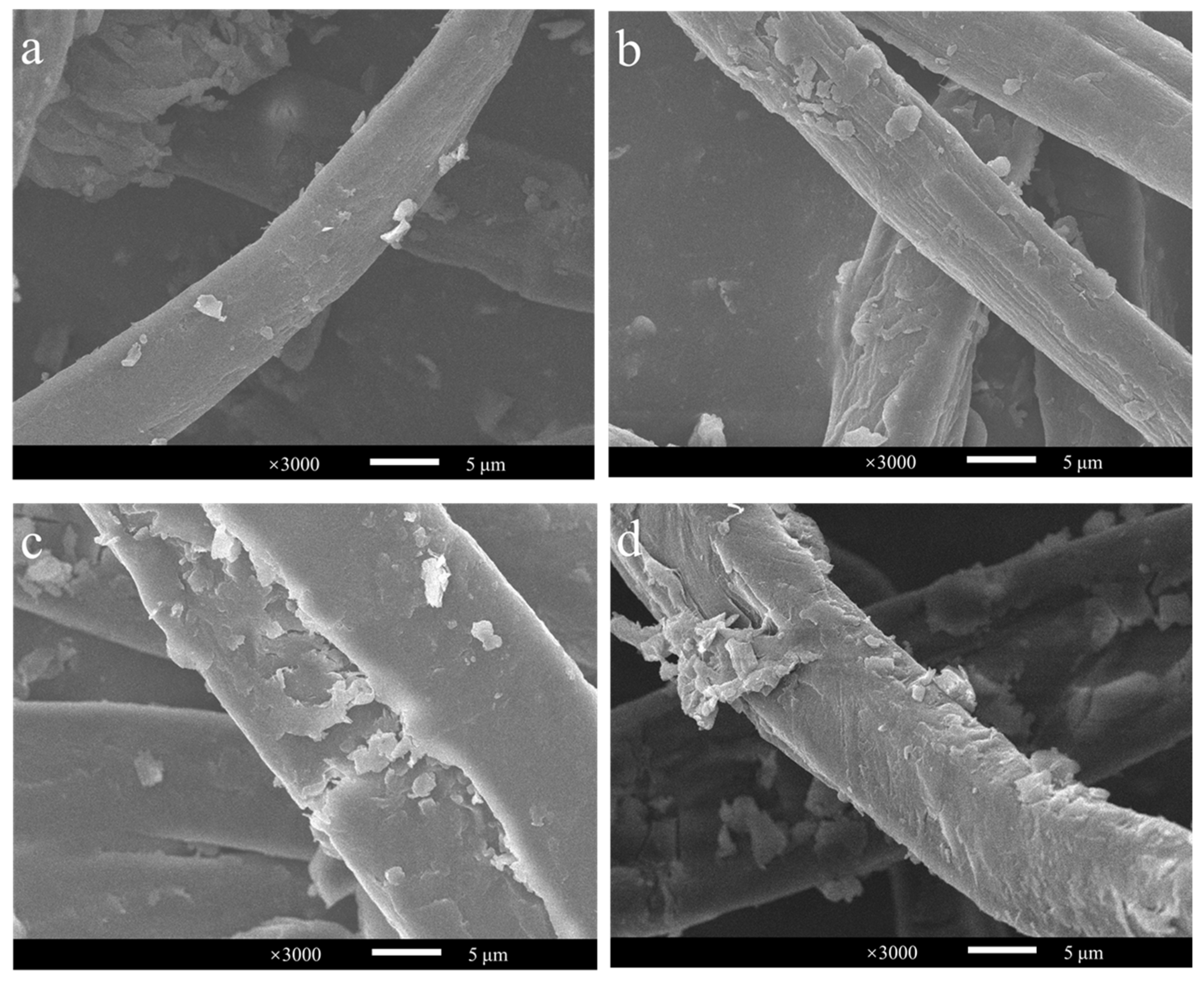
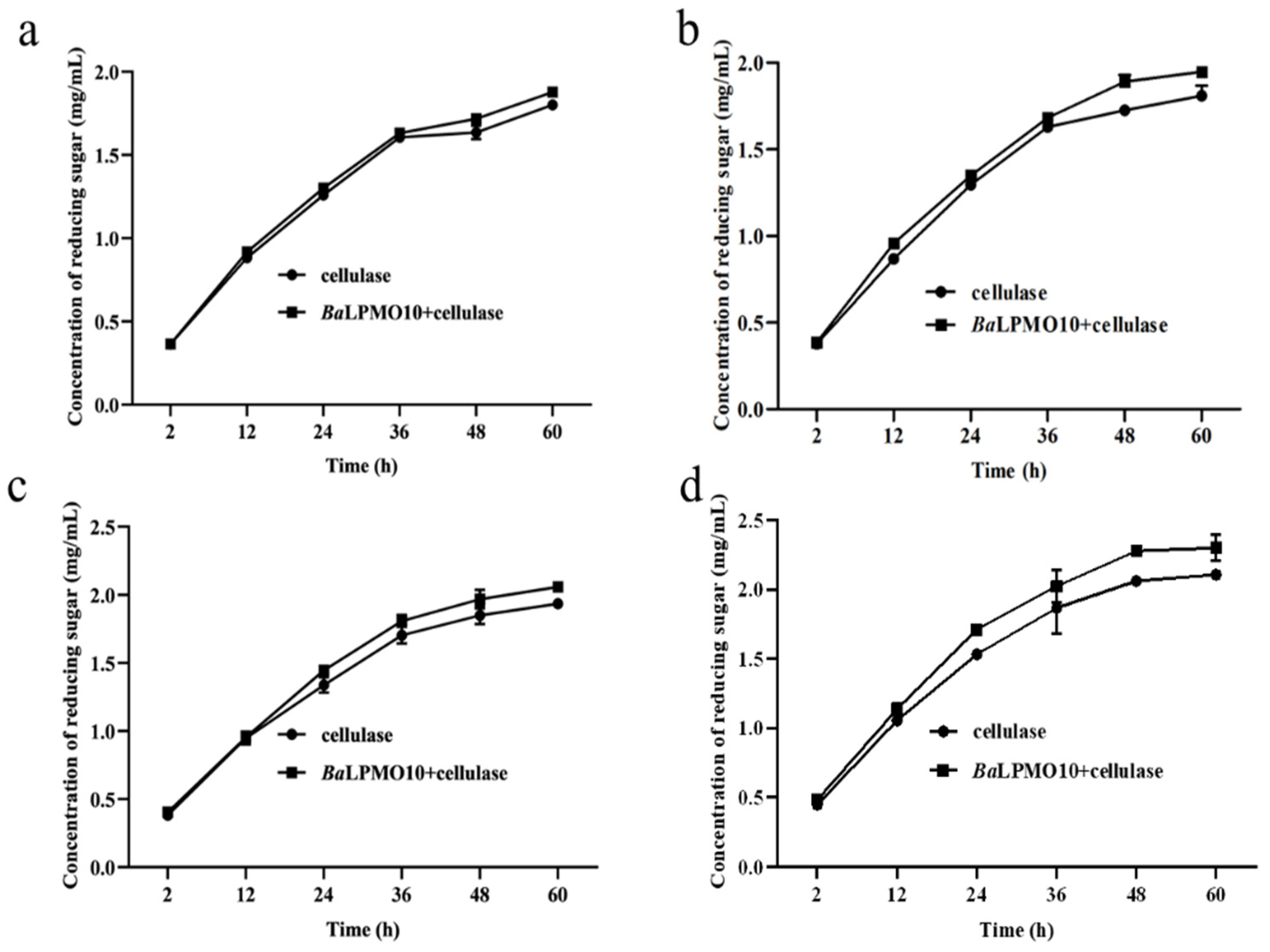
Approximately hundreds of millions of tons of corn stover are wasted each year in China, and it has a very high potential for conversion into cello-oligosaccharides, cellobiose and glucose [33,34]. Its structure is complex, and pretreatment would greatly improve its bioavailability. Compared with acid and alkali pretreatment, steam explosion can reduce the use of organic chemicals, making it a green pretreatment method [35,36]. Therefore, corn stover with steam explosions at 200 °C for 3, 6, 9, and 12 min was chosen as the substrate with which to investigate the conversion efficiency of BaLPMO10 on corn stover. The morphology of the cellulose in the four pretreated corn stover samples was observed using scanning electron microscopy (SEM) (Figure 4), and obvious differences in cellulose morphology were observed. The surface morphology of the cellulose in the sample pretreated for 3 min showed the smallest changes (Figure 4a), indicating the lowest degree of disruption. The sample which showed the most severe destruction was that pretreated for 12 min (Figure 4d), which was able to increase the accessibility of the enzyme. As the pretreatment time increased, the cellulose surface clearly continued to rupture. This is because the longer pretreatment time allowed more hot steam to penetrate into the internal voids of the fibers, enhancing the effect of the steam explosion pretreatment and further decomposing the fiber structure [35,37,38].
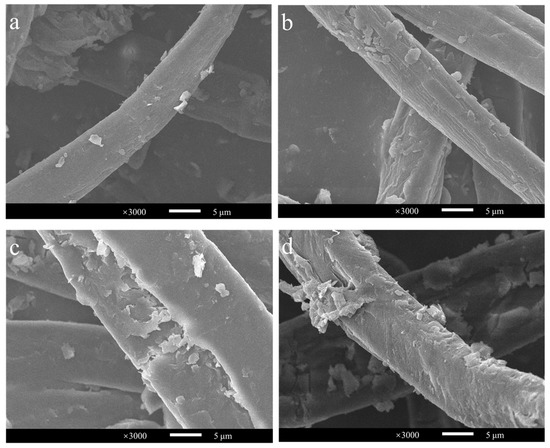
Figure 4.
Observation of the morphology of steam-exploded corn stover. (a) 200 °C, 3 min; (b) 200 °C, 6 min; (c) 200 °C, 9 min; (d) 200 °C, 12 min.
In order to select the optimal pretreatment condition for BaLPMO10, the synergistic degradation of corn stover with different pretreatments by BaLPMO10 and cellulase was investigated, and the results are shown in Figure 5. It was found that the synergistic actions of BaLPMO10 and cellulase on corn stover under different pretreatment conditions were different. As the pretreatment time increased, the reducing sugar content produced by enzymatic digestion increased. For the corn stover pretreated for 12 min, the highest reducing sugar concentration was produced after 60 h of degradation by either cellulase alone or the two enzymes working together, with concentrations of 2.1 mg/mL and 2.3 mg/mL, respectively (Figure 5d). However, the degree of reducing sugars enhanced by the synergistic action of the two enzymes did not increase along with the substrate pretreatment time. The reducing sugar content of corn stover pretreated for 3, 6, 9 and 12 min was increased by 4.2% (Figure 5a), 7.6% (Figure 5b), 6.3% (Figure 5c) and 9.2% (Figure 5d), respectively, after 60 h of synergistic degradation by the two enzymes rather than cellulase alone. The corn stover pretreated for 3 min released the lowest amount of reducing sugar (Figure 5a), possibly due to the low degree of structural damage caused by the short pretreatment time (Figure 4a), which made it difficult for the enzyme to contact the substrate. Surprisingly, the reducing sugar content raised by BaLPMO10 in synergy with the cellulase degradation of corn stover pretreated for 6 min (7.6%) was higher than that with the 9 min treatment (6.3%), probably due to the incomplete pretreatment resulting in a high lignin content. Lignin can provide electrons to LPMO, thereby increasing its catalytic efficiency [13,15,39,40]. The reducing sugars released from the corn stover with pretreatment for12 min were the highest, which is likely due to the more thorough destruction of the lignocellulosic structure and the higher exposure of cellulose caused by the longer pretreatment time, which made it easier for the enzyme to contact the cellulose. However, the longer the pretreatment time, the higher the energy consumption. Because the pretreatment temperature was 200 °C, both equipment and material were required to be heated from room temperature to 200 °C until the end of the pretreatment, and the steam consumption was very high. Therefore, a comprehensive consideration of economic costs is also a future consideration.
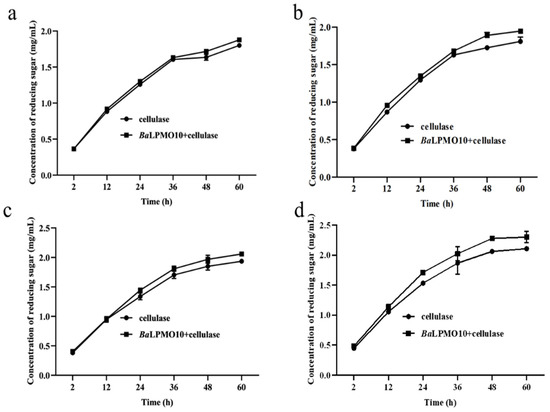
Figure 5.
Synergism activity of BaLPMO10 and cellulase on corn stover with different pretreatments. (a) 200 °C, 3 min; (b) 200 °C, 6 min; (c) 200 °C, 9 min; (d) 200 °C, 12 min.
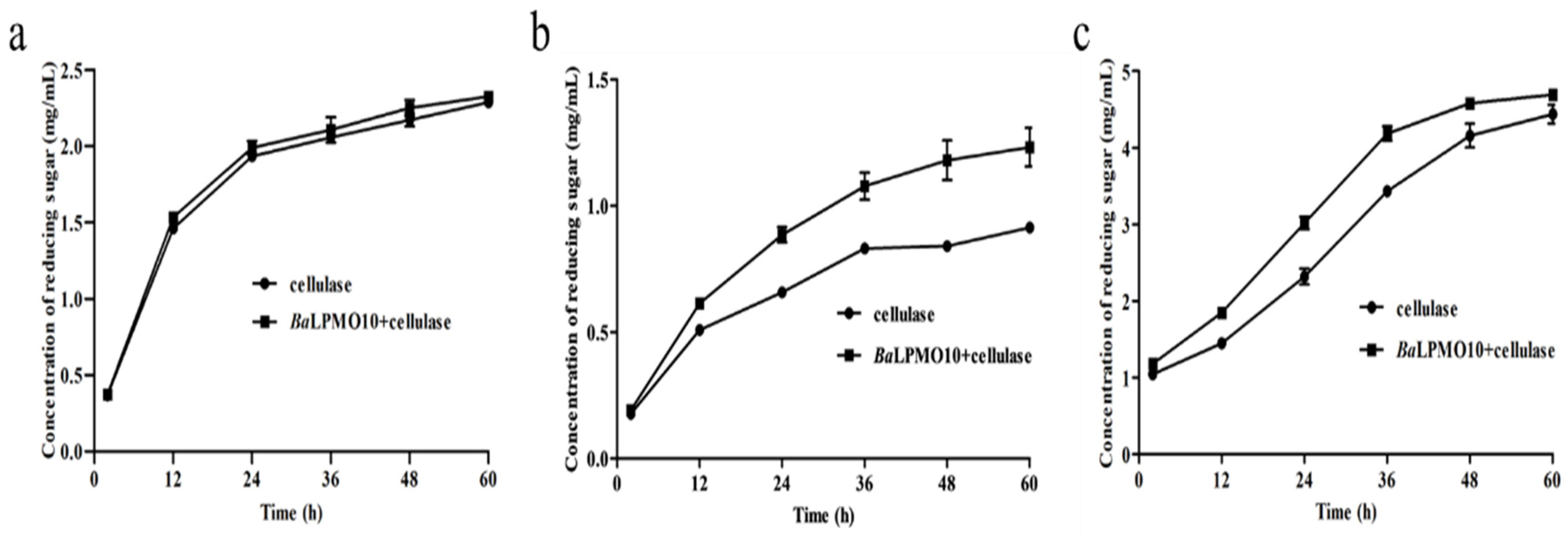
2.5. Synergistic Degradation of Pretreated Rice Straw, Caragana Korshinskii and Pulp by BaLPMO10 and Cellulase
Due to the complex composition of lignocellulosic substrates, different substrates were often chosen for the study of the activity of LPMO. To investigate the catalytic ability of BaLPMO10 on various forms of plant biomass, experiments were conducted using rice straw treated with ethylenediamine, Caragana korshinskii treated with ethylenediamine and pulp treated with NaOH as substrates, which have frequently been used in previous studies [37,41,42,43]. The reducing sugar yield was increased after the synergistic degradation of all three substrates by BaLPMO10 and cellulase compared to cellulase alone (Figure 6). Specifically, compared to the degradation of cellulose alone, the co-degradation of BaLPMO10 and cellulase significantly increased the production of reducing sugars in Caragana korshinskii and pulp. However, the synergistic effect on rice straw was not significant, with only a 1.7–5.0% increase in reducing sugar production during the 2–60 h of synergistic degradation (Figure 6a). During the degradation of Caragana korshinskii, the production of reducing sugars gradually increased with the prolongation of the reaction time. Compared to cellulase alone, the efficiency of the synergistic degradation of the two enzymes reached a maximum at 48 h, with a 40.5% increase in reducing sugar production (Figure 6b). At 60 h, the efficiency decreased to 34.7%, possibly due to the gradual decrease in enzyme activity over time (Figure 6b). When degrading pulp, the best synergistic degradation effect was observed at 24 h, with a 30.1% increase in reducing sugar production compared to cellulase alone (Figure 6c). However, the synergistic degradation only increased the reducing sugar production by 5.7% at 60 h, with a concentration of 4.7 mg/mL (Figure 6c). At this point, the reducing sugar yield had already approached the initial substrate concentration of 5.0 mg/mL, indicating that most of the cellulose had been converted to reducing sugars. In summary, it could be seen that BaLPMO10 demonstrated significant differences in conversion efficiency for these three biomasses, with the highest catalytic efficiency observed for Caragana korshinskii.
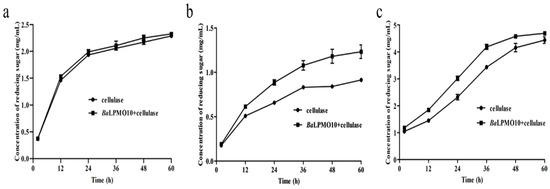
Figure 6.
Cellulolytic activity of BaLPMO10 on biomass. (a) Ethylenediamine-pretreated rice straw; (b) ethylenediamine-pretreated Caragana korshinskii; (c) NaOH-pretreated pulp.
It can be seen that the degradation ability of BaLPMO10 was different for various biomasses. This was similar to other LPMOs. For example, ViLPMO10B synergized with cellulase on corn stalk, sugarcane bagasse and rice straw to increase the yields of reducing sugars by 17%, 16% and 22%, respectively, compared to cellulase alone [42]. Both MtC1LPMO and its mutant R17L degraded rice straw more efficiently than corn straw and Caragana korshinskii [41]. The addition of PMO_07920 from Aspergillus terreus increased the conversion efficiency of oxalic acid-treated bagasse and rice straw by 4.68% and 17.10%, respectively [44]. LPMO9 from Thermoascus aurantiacus increased the conversion of organosolv-pretreated and steam-pretreated corn stover by 25% and 14%, respectively [15]. Additionally, the degradation capacity of BaLPMO10 for biomass was higher than that of some LPMOs. For example, the synergistic action of LPMO with cellulase increased the conversion of the corn stover by 3–10% compared to cellulase alone [45]. Additionally, LPMO derived from Chaetomium globosum improved the conversion efficiency of filter paper by 5% [43].
Although commercial cellulase Celluclast 1.5 L has been widely used in the literature for the synergistic conversion of biomass with LPMO [10,13,37,43], the efficiency of the final reducing sugars produced in this study was still not high enough. As observed in previous studies [46], this may be due to the fact that Celluclast 1.5 L was not the most efficient enzyme for converting biomass. Therefore, it is also very important to optimize the enzyme preparation in future research. Moreover, the current reaction system produced low levels of reducing sugars, and unsuitable substrate concentrations may have also been a contributing factor. Currently, there is a trend towards hydrolysis at high concentrations of substrates [47]. Therefore, the conversion of suitable concentrations of biomass substrates into fermentable sugars using optimized enzyme preparations is well worthy of in-depth study.
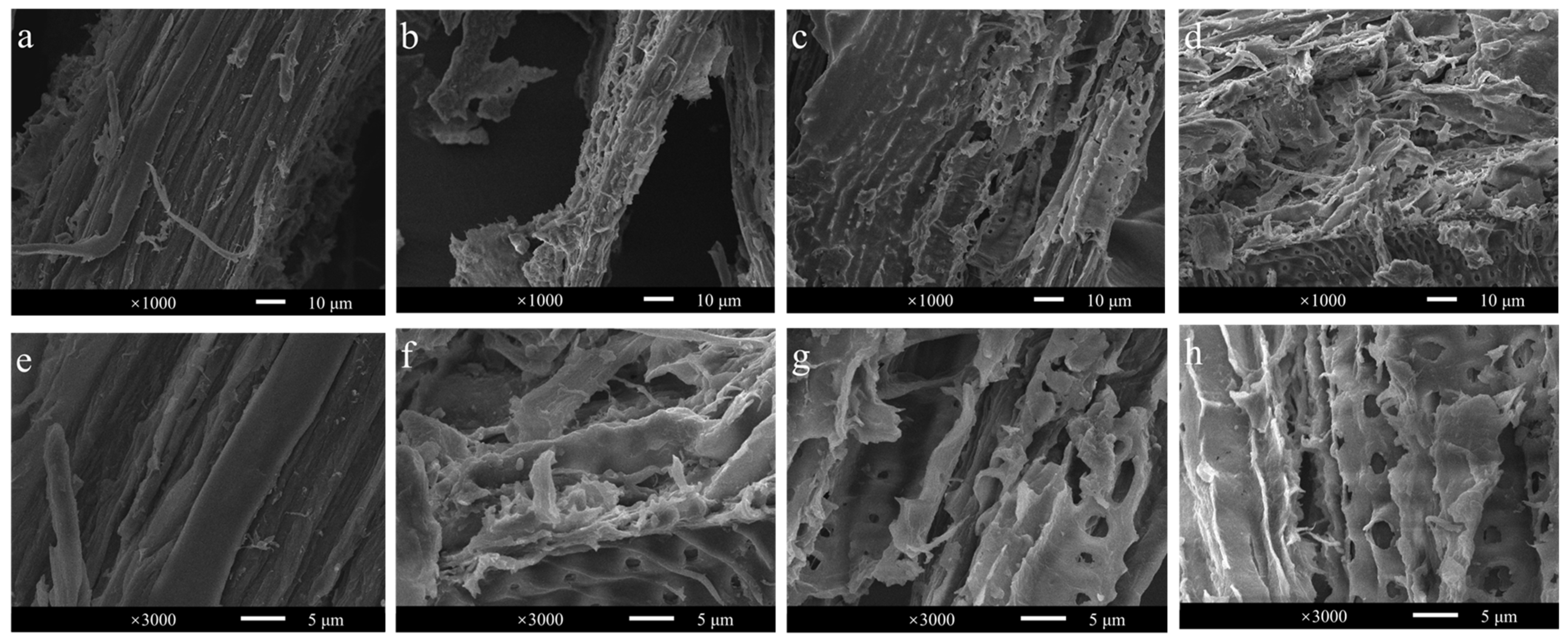
Since BaLPMO10 had the highest degradation efficiency on Caragana korshinskii, to further analyze the structural changes of Caragana korshinskii during enzymatic digestion, the surface morphology of the substrate in four different reaction systems (without enzyme, BaLPMO10 alone, cellulase alone, BaLPMO10 and cellulase) was observed using SEM (Figure 7). The surface morphology of Caragana korshinskii at 1000× magnification is shown in Figure 7a–d. It can be seen that the surface of the unenzymatically degraded Caragana korshinskii was relatively flat (Figure 7a). After degradation of BaLPMO10 (Figure 7b), the structure showed obvious porosity and fragmentation, indicating that BaLPMO10 is able to disrupt the structure of Caragana korshinskii. After cellulase hydrolysis alone (Figure 7c), the surface of the Caragana korshinskii showed porosity, indicating that cellulase degraded it as well. The fracture and fragmentation of the structure of Caragana korshinskii, degraded synergistically by BaLPMO10 and cellulase, were more pronounced (Figure 7d), indicating that the pretreatment of BaLPMO10 increased the actionable zone of cellulase, thus making the structure of cellulose more susceptible to disruption.
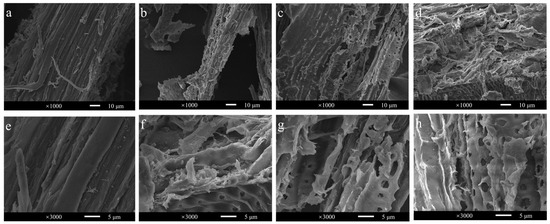
Figure 7.
Observation of the morphology of ethylenediamine-pretreated Caragana korshinskii after enzymolysis. (a,e) Without enzyme; (b,f) with BaLPMO10; (c,g) with cellulase; (d,h) both with BaLPMO10 and cellulase. Figures (a–d) and (e–h) show the surface morphology of Caragana korshinskii at 1000× and 3000× magnification, respectively.
To observe the surface morphology of Caragana korshinskii under different enzymatic conditions more clearly, observations were made at 3000× magnification, as shown in Figure 7e–h. The surface of untreated Caragana korshinskii was relatively smooth (Figure 7e), while the surface after BaLPMO10 degradation was rough and porous (Figure 7f). In addition, the surface of the substrate was broken for both cellulase alone (Figure 7g) and BaLPMO10 and cellulase synergistically (Figure 7h), but the substrate degraded by the two enzymes synergistically was more damaged, with a higher number of pores and larger pore sizes on the surface (Figure 7h). Many studies have reported that LPMOs can break the glycosidic bonds of polysaccharides [9,48], suggesting that the pretreatment with BaLPMO10 broke the glycosidic bonds of cellulose and provided more action sites for other cellulase enzymes, promoting the efficient conversion of cellulose. Similar phenomena were also described in other studies. For example, microscopic observations revealed that GcLPMO9s disrupted birch fibers [49]. The results of atomic force microscopy demonstrated the degradation of cellulose nanofibers into shorter and thinner insoluble fragments by LPMO [50,51]. SEM results showed that LPMO-R17L disrupted the structure of corn stover, making it broken and porous [37].
3. Materials and Methods
3.1. Optimization of IPTG Induction Concentration
The expression host used in this paper was the previously constructed E. coli BL21 (DE3) with the recombinant plasmid [25]. A single colony was selected and incubated in 5 mL of lysogeny broth (LB) containing 100 mg/L ampicillin at 37 °C and 220 rpm for 12 h to obtain the seed medium. Subsequently, 1 mL of the seed culture was transferred into 50 mL LB medium containing 100 mg/L ampicillin for fermentation. When the OD600 of the culture reached 0.6, IPTG was added at final concentrations of 0.1, 0.25, 0.5 and 1 mM, respectively. In addition, a final concentration of 1 mM CuSO4 was added to each medium, and the cultures were fermented at 16 °C for 20 h. After fermentation, the culture was centrifuged at 8000 rpm and 4 °C for 10 min to obtain the supernatant, which was then purified using Ni+-NTA affinity chromatography (Qiagen, Hilden, Germany) to obtain the target protein as previously described [25]. Protein concentrations were measured with a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) at 280 nm.
3.2. Optimization of Fermentation Temperature and Time
First, 1 mL of the seed culture from Section 2.1 was inoculated in 50 mL of LB medium containing 100 mg/L ampicillin. When the OD600 of the medium reached 0.6, IPTG and CuSO4 were added for final concentrations of 0.5 mM and 1 mM, respectively. The medium was then fermented at 16 °C, 30 °C and 37 °C for 4 h, 8 h and 20 h, respectively. After fermentation, the supernatant was separated from the medium by centrifugation (8000 rpm, 4 °C, 10 min) and purified using Ni+-NTA affinity chromatography (Qiagen, Hilden, Germany) to obtain the target protein, as previously described [25]. The protein purity was analyzed by SDS-PAGE.
3.3. SDS-PAGE Analysis
A 20 μL sample was mixed with 5 μL loading buffer, boiled for 10 min and centrifuged at 5000 rpm for 2 min. Then, 10–15 μL of the supernatant was loaded onto SDS-PAGE gels (5% for stacking gels and 12% for separating gels), and gel electrophoresis was performed under constant pressure. After electrophoresis, the gels were placed in Coomassie Brilliant Blue, microwaved for 30 s to fix the color and stained with slight shaking at room temperature for 1 h. After removal of the Coomassie Brilliant Blue, water was added to cover the stained gels and heated for about 10 min for decolorization until the protein bands were clear.
3.4. Enzyme Activity Assay
The enzymatic activity of BaLPMO10 was determined using 2,6-dimethoxyphenol (2,6-DMP) as substrate and H2O2 as co-substrate [32]. The 250 μL reaction system included 195 μL of 50 mM pH 6.0 sodium phosphate buffer, 25 μL of 10 mM 2,6-DMP solution, 5 μL of 50 mM H2O2 stock solution and 25 μL of purified BaLPMO10 at a concentration of 0.5 mg/mL. The enzyme activity was calculated by measuring the absorbance value after 5 min of reaction at 469 nm using multimode reader. One unit of enzyme activity is defined as the amount of enzyme required to generate 1 µmol of oxidation product (ε469 = 53,200 M−1 cm−1) per minute under reaction conditions.
3.5. Effect of Metal Ions, DTT, EDTA and Organic Reagents on the Enzyme Activity of BaLPMO10
Metal ions (K+, Na+, Ca2+, Ba2+, Mg2+, Cu2+, Mn2+, Co2+ and Fe3+), reducing agent DTT and chelating agent EDTA were added to the reaction system at final concentrations of 1 mM, 5 mM and 10 mM, respectively, and the enzyme activity of BaLPMO10 was determined at the optimum temperature and pH. Methanol, ethanol, ethylene glycol, polyethylene glycol and isopropyl alcohol were added to the reaction system at 10%, and the residual activity of BaLPMO10 was determined under optimum reaction conditions. The enzyme activity of BaLPMO10 without the above added substances was taken as 100%. All experiments were repeated three times.
3.6. Determination of Enzymatic Kinetics of BaLPMO10
The Km and Vmax values of BaLPMO10 were determined through reactions with different concentrations of 2,6-DMP (1–50 mM) at 45 °C for 5 min in 50 mM sodium phosphate buffer (pH 6.0). All reactions were performed in triplicate. The curves and kinetic parameters were analyzed using GraphPad Prism software.
3.7. Enzymatic Degradation of Different Biomasses by BaLPMO10
To evaluate the degradation activity of BaLPMO10 on different pretreated biomasses, two groups of experiments were conducted. One group utilized corn stover pretreated by steam explosion at 200 °C for 3, 6, 9 and 12 min, while the other group used three types of biomasses: rice stover pretreated with ethylenediamine, Caragana korshinskii pretreated with ethylenediamine and bleached softwood kraft pulp pretreated with dilute NaOH. The reaction system consisted of 1 mL of 50 mM pH 6.0 sodium phosphate buffer, including 5 mg substrate, 20 μL 1 mg/mL BaLPMO10 and 10 μL 100 mM ascorbic acid. A reaction system without LPMO was used as a blank control. The system was reacted for 24 h at 30 °C and 900 rpm on a shaker, followed by the addition of 1 μL of commercial cellulase Celluclast 1.5 L (Novozymes, Bagsvaerd, Denmark), and the reaction was continued for 2–60 h. The reaction was terminated by boiling for 10 min. The supernatant was obtained by centrifugation at 12,000 rpm for 10 min, and the concentration of reducing sugar was measured using dinitrosalicylic acid (DNS) reagent [52]. A 100 μL sample of the supernatant was added to a 1.5-mL Ep tube and mixed with 300 μL of DNS. The mixture was reacted accurately in boiling water for 5 min, and then the reaction was terminated in an ice bath for 2 min. After centrifugation at 5000 rpm for 2 min, 200 μL was taken into a 96-well plate, and the absorbance value at OD540 nm was measured using a multimode reader to calculate the reducing sugar content.
3.8. Surface Morphology Analysis
The surface morphology of the biomass was examined using a scanning electron microscopy (SEM). The samples to be observed were freeze-dried and fixed on a sample stage using conductive adhesive. To remove the surface charge, a layer of gold particles was sprayed onto the sample surface, and then the surface morphology was observed with a SU1510 scanning electron microscope under an accelerating voltage of 5 kV.
4. Conclusions
In this study, the bioinformatics of BaLPMO10 were analyzed, and it was found to be a secreted protein. Its highest secretion level was induced by 0.5 mM IPTG and fermented at 37 °C for 20 h, and 20 mg of protein with >95% purity was obtained by purifying 1 L of supernatant. It was found that 10 mM of Ca2+ and Na+ increased the enzyme activity of BaLPMO10 by 47.8% and 98.0%, respectively. However, DTT, EDTA and five organic reagents inhibited the enzyme activity of BaLPMO10. BaLPMO10 had the highest synergistic degradation efficiency with cellulase on steam-exploded corn stover at 200 °C for 12 min, increasing the reducing sugars by 9.2% compared to cellulase alone. The degradation of three different biomasses revealed that BaLPMO10 had the highest activity against ethylenediamine-pretreated Caragana korshinskii, and the reducing sugar content increased by 40.5% after 48 h of degradation by BaLPMO10 in synergy with cellulase compared to cellulase alone. The observation of the surface morphology revealed that BaLPMO10 was able to disrupt the cellulose structure and increase the accessibility of cellulase, thus facilitating the conversion process. Considering the importance of LPMO in the conversion of lignocellulose, this study provides theoretical support for achieving efficient conversion of plant biomass.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24119710/s1.
Author Contributions
Conceptualization, methodology: X.G., F.L. (Fuping Lu), F.L. (Fufeng Liu) and B.W.; validation, formal analysis, investigation, resources: X.G. and Y.A.; data curation, visualization: X.G. and Y.A.; writing—original draft: X.G.; writing—review and editing: F.L. (Fufeng Liu) and X.G.; funding acquisition: F.L. (Fufeng Liu), X.G. and B.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Key Research and Development Program of China (2021YFC2102700); the Shandong Province Natural Science Foundation (ZR2022QC199); the National Natural Science Foundation of China (21868011); the Project Program of Key Laboratory of Industrial Fermentation Microbiology, Ministry of Education; Tianjin Key Laboratory of Industrial Microbiology, China (2021KF001); and the financial support from the talent youth program of Shandong University of Science and Technology.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data analyzed in this study are included in the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Song, J.; Jiao, L.F.; Xiao, K.; Luan, Z.S.; Hu, C.H.; Shi, B.; Zhan, X.A. Cello-oligosaccharide ameliorates heat stress-induced impairment of intestinal microflora, morphology and barrier integrity in broilers. Anim. Feed. Sci. Technol. 2013, 185, 175–181. [Google Scholar] [CrossRef]
- Li, J.; Chen, Q. Recent advances in research on the role of bifidobacteria in regulating and improving gut-associated diseases. Food Sci. 2011, 23, 333–339. [Google Scholar]
- Jiao, L.F.; Song, Z.H.; Ke, Y.L.; Xiao, K.; Hu, C.H.; Shi, B. Cello-oligosaccharide influences intestinal microflora, mucosal architecture and nutrient transport in weaned pigs. Anim. Feed. Sci. Technol. 2014, 195, 85–91. [Google Scholar] [CrossRef]
- Nakamura, S.; Oku, T.; Ichinose, M. Bioavailability of cellobiose by tolerance test and breath hydrogen excretion in humans. Nutrition 2004, 20, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Kulka, H.; Ungureanu, I.M. Sweetness Enhancement. WO/2017/068034, 27 April 2017. [Google Scholar]
- Fang, Z.; Liu, K.; Chen, F. Research development of bioethanol preparation from lignocellulosic biomass. Mod. Chem. Ind. 2013, 33, 452–461. [Google Scholar] [CrossRef]
- Payne, C.M.; Knott, B.C.; Mayes, H.B.; Hansson, H.; Himmel, M.E.; Sandgren, M.; Stahlberg, J.; Beckham, G.T. Fungal cellulases. Chem. Rev. 2015, 115, 1308–1448. [Google Scholar] [CrossRef]
- Beguin, P.; Aubert, J.P. The biological degradation of cellulose. FEMS Microbiol. Rev. 1994, 13, 25–58. [Google Scholar] [CrossRef]
- Vaaje-Kolstad, G.; Westereng, B.; Horn, S.J.; Liu, Z.; Zhai, H.; Sorlie, M.; Eijsink, V.G. An oxidative enzyme boosting the enzymatic conversion of recalcitrant polysaccharides. Science 2010, 330, 219–222. [Google Scholar] [CrossRef]
- Guo, X.; An, Y.; Liu, F.; Lu, F.; Wang, B. Lytic polysaccharide monooxygenase—A new driving force for lignocellulosic biomass degradation. Bioresour. Technol. 2022, 362, 127803. [Google Scholar] [CrossRef]
- Meier, K.K.; Jones, S.M.; Kaper, T.; Hansson, H.; Koetsier, M.J.; Karkehabadi, S.; Solomon, E.I.; Sandgren, M.; Kelemen, B. Oxygen activation by Cu LPMOs in recalcitrant carbohydrate polysaccharide conversion to monomer sugars. Chem. Rev. 2018, 118, 2593–2635. [Google Scholar] [CrossRef]
- Quinlan, R.J.; Sweeney, M.D.; Lo Leggio, L.; Otten, H.; Poulsen, J.C.; Johansen, K.S.; Krogh, K.B.; Jorgensen, C.I.; Tovborg, M.; Anthonsen, A.; et al. Insights into the oxidative degradation of cellulose by a copper metalloenzyme that exploits biomass components. Proc. Natl. Acad. Sci. USA 2011, 108, 15079–15084. [Google Scholar] [CrossRef]
- Dimarogona, M.; Topakas, E.; Olsson, L.; Christakopoulos, P. Lignin boosts the cellulase performance of a GH-61 enzyme from Sporotrichum thermophile. Bioresour. Technol. 2012, 110, 480–487. [Google Scholar] [CrossRef]
- Chylenski, P.; Bissaro, B.; Sorlie, M.; Rohr, A.K.; Varnai, A.; Horn, S.J.; Eijsink, V.G.H. Lytic polysaccharide monooxygenases in enzymatic processing of lignocellulosic biomass. ACS Catal. 2019, 9, 4970–4991. [Google Scholar] [CrossRef]
- Hu, J.; Arantes, V.; Pribowo, A.; Gourlay, K.; Saddler, J.N. Substrate factors that influence the synergistic interaction of AA9 and cellulases during the enzymatic hydrolysis of biomass. Energy Environ. Sci. 2014, 7, 2308–2315. [Google Scholar] [CrossRef]
- Hemsworth, G.R.; Henrissat, B.; Davies, G.J.; Walton, P.H. Discovery and characterization of a new family of lytic polysaccharide monooxygenases. Nat. Chem. Biol. 2014, 10, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.J.; Vaaje-Kolstad, G.; Westereng, B.; Eijsink, V.G. Novel enzymes for the degradation of cellulose. Biotechnol. Biofuels 2012, 5, 45. [Google Scholar] [CrossRef]
- Lo Leggio, L.; Simmons, T.J.; Poulsen, J.C.; Frandsen, K.E.; Hemsworth, G.R.; Stringer, M.A.; von Freiesleben, P.; Tovborg, M.; Johansen, K.S.; De Maria, L.; et al. Structure and boosting activity of a starch-degrading lytic polysaccharide monooxygenase. Nat. Commun. 2015, 6, 5961. [Google Scholar] [CrossRef]
- Voshol, G.P.; Vijgenboom, E.; Punt, P.J. The discovery of novel LPMO families with a new Hidden Markov model. BMC Res. Notes 2017, 10, 105. [Google Scholar] [CrossRef]
- Sabbadin, F.; Hemsworth, G.R.; Ciano, L.; Henrissat, B.; Dupree, P.; Tryfona, T.; Marques, R.D.S.; Sweeney, S.T.; Besser, K.; Elias, L.; et al. An ancient family of lytic polysaccharide monooxygenases with roles in arthropod development and biomass digestion. Nat. Commun. 2018, 9, 756. [Google Scholar] [CrossRef]
- Filiatrault-Chastel, C.; Navarro, D.; Haon, M.; Grisel, S.; Herpoel-Gimbert, I.; Chevret, D.; Fanuel, M.; Henrissat, B.; Heiss-Blanquet, S.; Margeot, A.; et al. AA16, a new lytic polysaccharide monooxygenase family identified in fungal secretomes. Biotechnol. Biofuels 2019, 12, 55. [Google Scholar] [CrossRef]
- Sabbadin, F.; Urresti, S.; Henrissat, B.; Avrova, A.O.; Welsh, L.R.J.; Lindley, P.J.; Csukai, M.; Squires, J.N.; Walton, P.H.; Davies, G.J.; et al. Secreted pectin monooxygenases drive plant infection by pathogenic oomycetes. Science 2021, 373, 774–779. [Google Scholar] [CrossRef]
- Monclaro, A.V.; Filho, E.X.F. Fungal lytic polysaccharide monooxygenases from family AA9: Recent developments and application in lignocelullose breakdown. Int. J. Biol. Macromol. 2017, 102, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Hemsworth, G.R.; Johnston, E.M.; Davies, G.J.; Walton, P.H. Lytic polysaccharide monooxygenases in biomass conversion. Trends Biotechnol. 2015, 33, 747–761. [Google Scholar] [CrossRef]
- Guo, X.; An, Y.; Jiang, L.; Zhang, J.; Lu, F.; Liu, F. The discovery and enzymatic characterization of a novel AA10 LPMO from Bacillus amyloliquefaciens with dual substrate specificity. Int. J. Biol. Macromol. 2022, 203, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar] [CrossRef]
- Viklund, H.; Elofsson, A. Best alpha-helical transmembrane protein topology predictions are achieved using hidden Markov models and evolutionary information. Protein Sci. 2004, 13, 1908–1917. [Google Scholar] [CrossRef]
- Armenteros, J.J.A.; Tsirigos, K.D.; Sonderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Dvorak, P.; Chrast, L.; Nikel, P.I.; Fedr, R.; Soucek, K.; Sedlackova, M.; Chaloupkova, R.; de Lorenzo, V.; Prokop, Z.; Damborsky, J. Exacerbation of substrate toxicity by IPTG in Escherichia coli BL21(DE3) carrying a synthetic metabolic pathway. Microb. Cell Factories 2015, 14, 201. [Google Scholar] [CrossRef]
- Guo, X.; An, Y.; Chai, C.; Lu, F.; Liu, F. Fermentation condition optimization of recombinant lytic polysaccharide monooxygenase extracellularly expressed in Escherichia coli. Food Ferment. Ind. 2020, 46, 31–37. [Google Scholar]
- Guo, X.; Sang, J.; Chai, C.; An, Y.; Wei, Z.; Zhang, H.; Ma, L.; Dai, Y.; Lu, F.; Liu, F. A lytic polysaccharide monooxygenase from Myceliophthora thermophila C1 and its characterization in cleavage of glycosidic chain of cellulose. Biochem. Eng. J. 2020, 165, 107712. [Google Scholar] [CrossRef]
- Breslmayr, E.; Hanzek, M.; Hanrahan, A.; Leitner, C.; Kittl, R.; Santek, B.; Oostenbrink, C.; Ludwig, R. A fast and sensitive activity assay for lytic polysaccharide monooxygenase. Biotechnol. Biofuels 2018, 11, 79–91. [Google Scholar] [CrossRef]
- Ou, L.W.; Brown, T.R.; Thilakaratne, R.; Hu, G.P.; Brown, R.C. Techno-economic analysis of co-located corn grain and corn stover ethanol plants. Biofuels Bioprod. Biorefin. 2014, 8, 412–422. [Google Scholar] [CrossRef]
- Zhong, C.; Cao, Y.X.; Li, B.Z.; Yuan, Y.J. Biofuels in China: Past, present and future. Biofuels Bioprod. Biorefin. 2010, 4, 326–342. [Google Scholar] [CrossRef]
- Chen, H.Z.; Liu, Z.H. Steam explosion and its combinatorial pretreatment refining technology of plant biomass to bio-based products. Biotechnol. J. 2015, 10, 866–885. [Google Scholar] [CrossRef] [PubMed]
- Avellar, B.K.; Glasser, W.G. Steam-assisted biomass fractionation. I. Process considerations and economic evaluation. Biomass Bioenergy 1998, 14, 205–218. [Google Scholar] [CrossRef]
- Guo, X.; An, Y.; Lu, F.; Liu, F. Optimization of synergistic degradation of steam exploded corn straw by lytic polysaccharide monooxygenase R17L and cellulase. Ind. Crops Prod. 2022, 182, 114924. [Google Scholar] [CrossRef]
- Dechao, N.; Zhuo, Z.; Chen, Z.; Yanling, L. Research progress on steam explosion technology in feed utilization of corn straw. China Anim. Husb. Vet. Med. 2021, 48, 4488–4496. [Google Scholar]
- Rodríguez-Zúñiga, U.F.; Cannella, D.; Giordano, R.d.C.; Giordano, R.d.L.C.; Jørgensen, H.; Felby, C. Lignocellulose pretreatment technologies affect the level of enzymatic cellulose oxidation by LPMO. Green Chem. 2015, 17, 2896–2903. [Google Scholar] [CrossRef]
- Westereng, B.; Cannella, D.; Wittrup Agger, J.; Jorgensen, H.; Larsen Andersen, M.; Eijsink, V.G.; Felby, C. Enzymatic cellulose oxidation is linked to lignin by long-range electron transfer. Sci. Rep. 2015, 5, 18561. [Google Scholar] [CrossRef]
- Guo, X.; An, Y.; Chai, C.; Sang, J.; Jiang, L.; Lu, F.; Dai, Y.; Liu, F. Construction of the R17L mutant of MtC1LPMO for improved lignocellulosic biomass conversion by rational point mutation and investigation of the mechanism by molecular dynamics simulations. Bioresour. Technol. 2020, 317, 124024. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xu, Z.; He, J.; Li, Y.; Pan, C.; Wang, C.; Deng, M.R.; Zhu, H. A myxobacterial LPMO10 has oxidizing cellulose activity for promoting biomass enzymatic saccharification of agricultural crop straws. Bioresour. Technol. 2020, 318, 124217. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.J.; Nam, K.H.; Yun, E.J.; Kim, S.; Youn, H.J.; Lee, H.J.; Choi, I.G.; Kim, K.H. Optimization of synergism of a recombinant auxiliary activity 9 from Chaetomium globosum with cellulase in cellulose hydrolysis. Appl. Microbiol. Biotechnol. 2015, 99, 8537–8547. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, D.; Kaur, B.; Kaur Brar, K.; Chadha, B.S. An innovative approach of priming lignocellulosics with lytic polysaccharide mono-oxygenases prior to saccharification with glycosyl hydrolases can economize second generation ethanol process. Bioresour. Technol. 2020, 308, 123257. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chandra, R.; Arantes, V.; Gourlay, K.; Susan van Dyk, J.; Saddler, J.N. The addition of accessory enzymes enhances the hydrolytic performance of cellulase enzymes at high solid loadings. Bioresour. Technol. 2015, 186, 149–153. [Google Scholar] [CrossRef]
- Aksenov, A.S.; Tyshkunova, I.V.; Poshina, D.N.; Guryanova, A.A.; Chukhchin, D.G.; Sinelnikov, I.G.; Terentyev, K.Y.; Skorik, Y.A.; Novozhilov, E.V.; Synitsyn, A.P. Biocatalysis of industrial kraft pulps: Similarities and differences between hardwood and softwood pulps in hydrolysis by enzyme complex of Penicillium verruculosum. Catalysts 2020, 10, 536. [Google Scholar] [CrossRef]
- Sun, C.; Meng, X.; Sun, F.; Zhang, J.; Tu, M.; Chang, J.S.; Reungsang, A.; Xia, A.; Ragauskas, A.J. Advances and perspectives on mass transfer and enzymatic hydrolysis in the enzyme-mediated lignocellulosic biorefinery: A review. Biotechnol. Adv. 2023, 62, 108059. [Google Scholar] [CrossRef]
- Forsberg, Z.; Mackenzie, A.K.; Sorlie, M.; Rohr, A.K.; Helland, R.; Arvai, A.S.; Vaaje-Kolstad, G.; Eijsink, V.G. Structural and functional characterization of a conserved pair of bacterial cellulose-oxidizing lytic polysaccharide monooxygenases. Proc. Natl. Acad. Sci. USA 2014, 111, 8446–8551. [Google Scholar] [CrossRef]
- Ladeveze, S.; Haon, M.; Villares, A.; Cathala, B.; Grisel, S.; Herpoel-Gimbert, I.; Henrissat, B.; Berrin, J.G. The yeast Geotrichum candidum encodes functional lytic polysaccharide monooxygenases. Biotechnol. Biofuels 2017, 10, 215. [Google Scholar] [CrossRef]
- Eibinger, M.; Ganner, T.; Bubner, P.; Rosker, S.; Kracher, D.; Haltrich, D.; Ludwig, R.; Plank, H.; Nidetzky, B. Cellulose surface degradation by a lytic polysaccharide monooxygenase and its effect on cellulase hydrolytic efficiency. J. Biol. Chem. 2014, 289, 35929–35938. [Google Scholar] [CrossRef]
- Eibinger, M.; Sattelkow, J.; Ganner, T.; Plank, H.; Nidetzky, B. Single-molecule study of oxidative enzymatic deconstruction of cellulose. Nat. Commun. 2017, 8, 894. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).