Salen-like Chromium and Aluminum Complexes as Catalysts in the Copolymerization of Epoxides with Cyclic Anhydrides for the Synthesis of Polyesters
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of Complexes
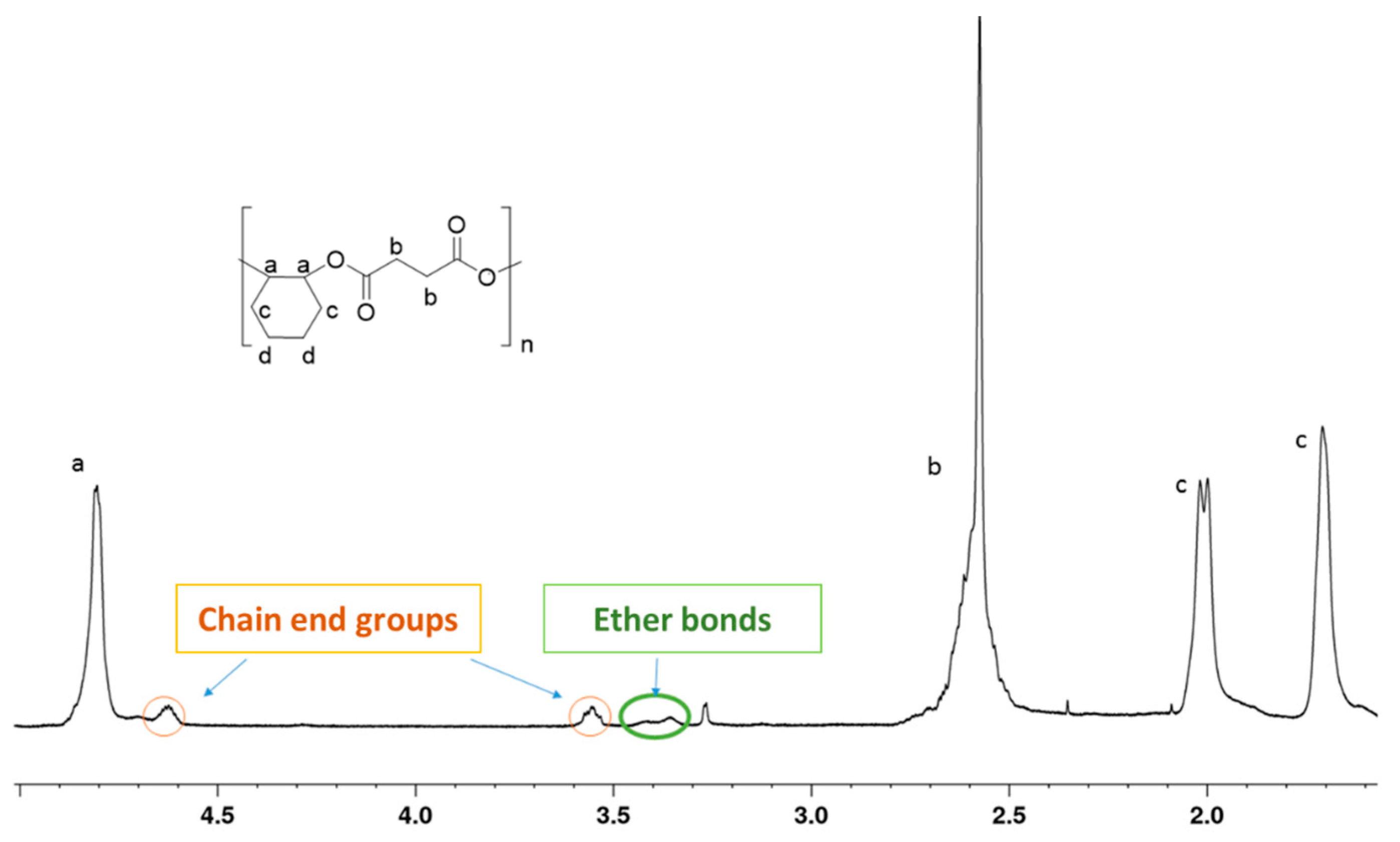
2.2. Polymerization of Epoxides and Anhydrides
| Entry | Cat | Ep. | Anhy. | T (°C) | Time (h) | Conv. [e] (%) | TOF (h−1) | Ester [e] (%) | Mn [f] (Kg/mol) | Đ [f] |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 [a] | Cr1 | CHO | SA | 110 | 2 | 95 | 119 | 88 | 1.3 | 1.31 |
| 2 [b] | Cr1 | CHO | SA | 110 | 2 | 28 | 35 | >99 | 1.1 | 1.42 |
| 3 [a] | Cr2 | CHO | SA | 110 | 2 | 98 | 122 | 88 | 1.5 | 1.32 |
| 4 [c] | Cr3 | CHO | SA | 130 | 2.5 | 100 | 100 | - | 1.9 | 1.25 |
| 5 [d] | Cr2 | CHO | SA | 110 | 1 | 93 | 232 | >99 | 2.3 | 1.18 |
| 6 [d] | Al2 | CHO | SA | 110 | 2 | 81 | 101 | >99 | 2.1 | 1.19 |
| 7 [d] | Cr2 | CHO | PA | 50 | 24 | 100 | 10 | >99 | 13.1 | 1.21 |
| 8 [d] | Al2 | CHO | PA | 50 | 24 | 78 | 8 | >99 | 9.9 | 1.11 |
| 9 [d] | Cr2 | PO | PA | 50 | 24 | 60 | 6 | >99 | 2.3 | 1.22 |
| 10 [d] | Al2 | PO | PA | 50 | 72 | 73 | 2 | >99 | 2.4 | 1.15 |
| 11 [d] | Cr2 | LO | PA | 100 | 17 | 91 | 13 | >99 | 3.6 | 1.21 |
| 12 [d] | Al2 | LO | PA | 100 | 17 | 81 | 12 | >99 | 3.0 | 1.10 |
2.3. Copolymerization of Maleic Anhydride and Propylene Oxide
3. Materials and Methods
3.1. Synthesis of Complex Cr2
3.2. Synthesis of Complex Al2
3.3. Representative Procedure for the Copolymerization of Epoxides with Anhydrides
3.4. Synthesis of Oly(propylene maleate)-block-polyglycolide
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albertsson, A.-C.; Varma, I.K. Recent Developments in Ring Opening Polymerization of Lactones for Biomedical Applications. Biomacromolecules 2003, 4, 1466–1486. [Google Scholar] [CrossRef] [PubMed]
- Pappalardo, D.; Mathisen, T.; Finne-Wistrand, A. Biocompatibility of Resorbable Polymers: A Historical Perspective and Framework for the Future. Biomacromolecules 2019, 20, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Albertsson, A.-C.; Hakkarainen, M. Designed to degrade. Science 2017, 358, 872–873. [Google Scholar] [CrossRef]
- Kirillova, A.; Yeazel, T.R.; Asheghali, D.; Petersen, S.R.; Dort, S.; Gall, K.; Becker, M.L. Fabrication of Biomedical Scaffolds Using Biodegradable Polymers. Chem. Rev. 2021, 121, 11238–11304. [Google Scholar] [CrossRef]
- Longo, J.M.; Sanford, M.J.; Coates, G.W. Ring-Opening Copolymerization of Epoxides and Cyclic Anhydrides with Discrete Metal Complexes: Structure–Property Relationships. Chem. Rev. 2016, 116, 15167–15197. [Google Scholar] [CrossRef]
- Paul, S.; Zhu, Y.; Romain, C.; Brooks, R.; Saini, P.K.; Williams, C.K. Ring-Opening Copolymerization (ROCOP): Synthesis and Properties of Polyesters and Polycarbonates. Chem. Commun. 2015, 51, 6459. [Google Scholar] [CrossRef]
- Strianese, M.; Pappalardo, D.; Mazzeo, M.; Lamberti, M.; Pellecchia, C. Salen-type aluminum and zinc complexes as two-faced Janus compounds: Contribution to molecular sensing and polymerization catalysis. Dalton Trans. 2020, 49, 16533–16550. [Google Scholar] [CrossRef]
- Stoesser, T.; Mulryan, D.; Williams, C.K. Switch Catalysis To Deliver Multi-Block Polyesters from Mixtures of Propene Oxide, Lactide, and Phthalic Anhydride. Angew. Chem. Int. Ed. 2018, 57, 16893–16897. [Google Scholar] [CrossRef] [PubMed]
- Romain, C.; Zhu, Y.; Dingwall, P.; Paul, S.; Rzepa, H.S.; Buchard, A.; Williams, C.K. Chemoselective Polymerizations from Mixtures of Epoxide, Lactone, Anhydride, and Carbon Dioxide. J. Am. Chem. Soc. 2016, 138, 4120–4131. [Google Scholar] [CrossRef]
- Guillaume, S.M.; Kirillov, E.; Sarazin, Y.; Carpentier, J.-F. Beyond Stereoselectivity, Switchable Catalysis: Some of the Last Frontier Challenges in Ring-Opening Polymerization of Cyclic Esters. Chem. Eur. J. 2015, 21, 7988–8003. [Google Scholar] [CrossRef]
- Isnard, F.; Lamberti, M.; Pellecchia, C.; Mazzeo, M. Ring-Opening Copolymerization of Epoxides with Cyclic Anhydrides Promoted by Bimetallic and Monometallic Phenoxy-Imine Aluminum complexes. ChemCatChem 2017, 9, 2972–2979. [Google Scholar] [CrossRef]
- Isnard, F.; Carratu, M.; Lamberti, M.; Venditto, V.; Mazzeo, M. Copolymerization of cyclic esters, epoxides and anhydrides: Evidence of the dual role of the monomers in the reaction mixture. Catal. Sci. Technol. 2018, 8, 5034–5043. [Google Scholar] [CrossRef]
- Santulli, F.; D’Auria, I.; Boggioni, L.; Losio, S.; Proverbio, M.; Costabile, C.; Mazzeo, M. Bimetallic Aluminum Complexes Bearing Binaphthyl-Based Iminophenolate Ligands as Catalysts for the Synthesis of Polyesters. Organometallics 2020, 39, 1213–1220. [Google Scholar] [CrossRef]
- Abel, B.A.; Lidston, C.A.L.; Coates, G.W. Mechanism-Inspired Design of Bifunctional Catalysts for the Alternating Ring-Opening Copolymerization of Epoxides and Cyclic Anhydrides. J. Am. Chem. Soc. 2019, 141, 12760. [Google Scholar] [CrossRef]
- Han, B.; Zhang, L.; Yang, M.; Liu, B.; Dong, X.; Theato, P. Highly Cis/Trans-Stereoselective (ONSO)CrCl-Catalyzed Ring-Opening Copolymerization of Norbornene Anhydrides and Epoxides. Macromolecules 2016, 49, 6232–6239. [Google Scholar] [CrossRef]
- Ryzhakov, D.; Printz, G.; Jacques, B.; Messaoudi, S.; Dumas, F.; Dagorne, S.; Le Bideau, F. Organo-catalyzed/initiated ring opening co-polymerization of cyclic anhydrides and epoxides: An emerging story. Polym. Chem. 2021, 12, 2932–2946. [Google Scholar] [CrossRef]
- He, G.-H.; Liu, Y.-L.; Liu, Y.; Lu, X.-B. Enantioselective Resolution Copolymerization of Racemic cis-Epoxides and Cyclic Anhydrides Mediated by Multichiral Bimetallic Chromium Complexes. Macromolecules 2022, 55, 3869–3876. [Google Scholar] [CrossRef]
- Li, J.; Ren, B.-H.; Chen, S.-Y.; He, G.-H.; Liu, Y.; Ren, W.-M.; Zhou, H.; Lu, X.-B. Development of Highly Enantioselective Catalysts for Asymmetric Copolymerization of meso-Epoxides and Cyclic Anhydrides: Subtle Modification Resulting in Superior Enantioselectivity. ACS Catal. 2019, 9, 1915–1922. [Google Scholar] [CrossRef]
- Cai, Z.; Wan, Y.; Becker, M.L.; Long, Y.-Z.; Dean, D. Poly(propylene fumarate)-based materials: Synthesis, functionalization, properties, device fabrication and biomedical applications. Biomaterials 2019, 208, 45–71. [Google Scholar] [CrossRef]
- Yaszemski, M.J.; Payne, R.G.; Hayes, W.C.; Langer, R.; Mikos, A.G. In vitro degradation of a poly(propylene fumarate)-based composite material. Biomaterials 1996, 17, 2127–2130. [Google Scholar] [CrossRef]
- Luo, Y.; Le Fer, G.; Dean, D.; Becker, M.L. 3D printing of poly(propylene fumarate) oligomers: Evaluation of resin viscosity, printing characteristics and mechanical properties. Biomacromolecules 2019, 20, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
- Yaszemski, M.J.; Payne, R.G.; Hayes, W.C.; Langer, R.; Mikos, A.G. Evolution of bone transplantation: Molecular, cellular and tissue strategies to engineer human bone. Biomaterials 1996, 17, 175–185. [Google Scholar] [CrossRef] [PubMed]
- DiCiccio, A.M.; Coates, G.W. Ring-Opening Copolymerization of Maleic Anhydride with Epoxides: A Chain-Growth Approach to Unsaturated Polyesters. J. Am. Chem. Soc. 2011, 133, 10724–10727. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.A.; Luong, D.; Kleinfehn, A.P.; Sallam, S.; Wesdemiotis, C.; Becker, M.L. Magnesium Catalyzed Polymerization of End Functionalized Poly(propylene maleate) and Poly(propylene fumarate) for 3D Printing of Bioactive Scaffolds. J. Am. Chem. Soc. 2018, 140, 277–284. [Google Scholar] [CrossRef]
- Petersen, S.R.; Wilson, J.A.; Becker, M.L. Versatile Ring-Opening Copolymerization and Postprinting Functionalization of Lactone and Poly(propylene fumarate) Block Copolymers: Resorbable Building Blocks for Additive Manufacturing. Macromolecules 2018, 51, 6202–6208. [Google Scholar] [CrossRef]
- Wannipurage, D.; D’Aniello, S.; Pappalardo, D.; Kulathungage, L.W.; Ward, C.L.; Anderson, D.P.; Groysman, S.; Mazzeo, M. Simple magnesium alkoxides: Synthesis, molecular structure, and catalytic behaviour in the ring-opening polymerization of lactide and macrolactones and for copolymerization of maleic anhydride and propylene oxide. Dalton Trans. 2023. [Google Scholar] [CrossRef]
- Van Zee, N.J.; Coates, G.W. Alternating Copolymerization of Propylene Oxide with Biorenewable Terpene-Based Cyclic Anhydrides: A Sustainable Route to Aliphatic Polyesters with High Glass Transition Temperatures. Angew. Chem. Int. Ed. 2015, 54, 2665–2668. [Google Scholar] [CrossRef]
- Bester, K.; Bukowska, A.; Myśliwiec, B.; Hus, K.; Tomczyk, D.; Urbaniak, P.; Bukowski, W. Alternating ring-opening copolymerization of phthalic anhydride with epoxides catalysed by salophen chromium(iii) complexes. An effect of substituents in salophen ligands. Polym. Chem. 2018, 9, 2147–2156. [Google Scholar] [CrossRef]
- Wang, L.Y.; Gu, G.G.; Yue, T.J.; Ren, M.W.; Lu, X.B. Semiaromatic Poly(thioesters) from the Copolymerization of Phthalic Thioanhydride and Epoxide: Synthesis, Structure, and Properties. Macromolecules 2019, 52, 2439. [Google Scholar] [CrossRef]
- Nejad, E.H.; van Melis, C.G.W.; Vermeer, T.J.; Koning, C.E.; Duchateau, R. Alternating Ring-Opening Polymerization of Cyclohexene Oxide and Anhydrides: Effect of Catalyst, Cocatalyst, and Anhydride Structure. Macromolecules 2012, 45, 1770–1776. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Poland, R.R.; Escobedo, C. Kinetic Studies of the Alternating Copolymerization of Cyclic Acid Anhydrides and Epoxides, and the Terpolymerization of Cyclic Acid Anhydrides, Epoxides, and CO2 Catalyzed by (salen)CrIIICl. Macromolecules 2012, 45, 2242–2248. [Google Scholar] [CrossRef]
- Liu, J.; Bao, Y.-Y.; Liu, Y.; Ren, W.-M.; Lu, X.-B. Binuclear chromium-salan complex catalyzed alternating copolymerization of epoxides and cyclic anhydrides. Polym. Chem. 2013, 4, 1439–1444. [Google Scholar] [CrossRef]
- Isnard, F.; Santulli, F.; Cozzolino, M.; Lamberti, M.; Pellecchia, C.; Mazzeo, M. Tetracoordinate aluminum complexes bearing phenoxy-based ligands as catalysts for epoxide/anhydride copolymerization: Some mechanistic insights. Catal. Sci. Technol. 2019, 9, 3090–3098. [Google Scholar] [CrossRef]
- D’Auria, I.; Santulli, F.; Ciccone, F.; Giannattasio, A.; Mazzeo, M.; Pappalardo, D. Synthesis of Semi-Aromatic Di-Block Polyesters by Terpolymerization of Macrolactones, Epoxides, and Anhydrides. ChemCatChem 2021, 13, 3303–3311. [Google Scholar] [CrossRef]
- D’Auria, I.; D’Aniello, S.; Viscusi, G.; Lamberti, E.; Gorrasi, G.; Mazzeo, M.; Pappalardo, D. One-Pot Terpolymerization of Macrolactones with Limonene Oxide and Phtalic Anhydride to Produce di-Block Semi-Aromatic Polyesters. Polymers 2022, 14, 4911. [Google Scholar] [CrossRef]
- Wannipurage, D.; Hollingsworth, T.S.; Santulli, F.; Cozzolino, M.; Lamberti, M.; Groysman, S.; Mazzeo, M. Synthesis of a mononuclear magnesium bis(alkoxide) complex and its reactivity in the ring-opening copolymerization of cyclic anhydrides with epoxides. Dalton Trans. 2020, 49, 2715–2723. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, I.; Santulli, F.; Lamberti, M.; Mazzeo, M. Chromium Complexes Supported by Salen-Type Ligands for the Synthesis of Polyesters, Polycarbonates, and Their Copolymers through Chemoselective Catalysis. Int. J. Mol. Sci. 2023, 24, 7642. [Google Scholar] [CrossRef]
- Li, B.; Wu, G.-P.; Ren, W.-M.; Wang, Y.-M.; Rao, D.-Y.; Lu, X.-B. Asymmetric, regio- and stereo-selective alternating copolymerization of CO2 and propylene oxide catalyzed by chiral chromium salan complexes. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 6102–6113. [Google Scholar] [CrossRef]
- Pilone, A.; Press, K.; Goldberg, I.; Kol, M.; Mazzeo, M.; Lamberti, M. Gradient Isotactic Multiblock Polylactides from Aluminum Complexes of Chiral Salalen Ligands. J. Am. Chem. Soc. 2014, 136, 2940–2943. [Google Scholar] [CrossRef]
- Winkler, M.; Romain, C.; Meier, M.A.R.; Williams, C.K. Renewable polycarbonates and polyesters from 1,4-cyclohexadiene. Green Chem. 2015, 17, 300–306. [Google Scholar] [CrossRef]
- Ciriminna, R.; Lomeli-Rodriguez, M.; Demma Carà, P.; Lopez-Sanchez, J.A.; Pagliaro, M. Limonene: A versatile chemical of the bioeconomy. Chem. Commun. 2014, 50, 15288–15296. [Google Scholar] [CrossRef] [PubMed]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Giarola, S.; Romain, C.; Williams, C.K.; Hallett, J.P.; Shah, N. Techno-economic assessment of the production of phthalic anhydride from corn stover. Chem. Eng. Res. Des. 2016, 107, 181–194. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Ren, W.-M.; Lu, X.-B. Asymmetric Alternating Copolymerization of Meso-epoxides and Cyclic Anhydrides: Efficient Access to Enantiopure Polyesters. J. Am. Chem. Soc. 2016, 138, 11493–11496. [Google Scholar] [CrossRef]
- Bernard, A.; Chatterjee, C.; Chisholm, M.H. The influence of the metal (Al, Cr and Co) and the substituents of the porphyrin in controlling the reactions involved in the copolymerization of propylene oxide and cyclic anhydrides by porphyrin metal(III) complexes. Polymer 2013, 54, 2639–2646. [Google Scholar] [CrossRef]
- Fuoco, T.; Meduri, A.; Lamberti, M.; Pellecchia, C.; Pappalardo, D. Copolymerization and terpolymerization of glycolide with lactones by dimethyl(salicylaldiminato)aluminum compounds. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Samantaray, P.K.; Little, A.; Haddleton, D.M.; McNally, T.; Tan, B.; Sun, Z.; Huang, W.; Ji, Y.; Wan, C. Poly(glycolic acid) (PGA): A versatile building block expanding high performance and sustainable bioplastic applications. Green Chem. 2020, 22, 4055–4081. [Google Scholar] [CrossRef]














| Entry | Cat | [PO] (eq) | [Cocat] (eq) | Solv | Time (h) | Conv [b] (%) | Ester [b] (%) | MnGPC [c] (KDa) | Ð [c] |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Cr2 | 200 | - | hexane | 17 | 18 | >99 | 2.3 | 1.2 |
| 2 | Cr2 | 200 | PPNCl | toluene | 24 | 18 | >99 | 1.3 | 1.5 |
| 3 | Cr4 | 200 | - | hexane | 24 | 13 | >99 | 1.9 | 1.2 |
| 4 | Cr4 | 200 | PPNCl | toluene | 24 | 8 | >99 | 1.2 | 1.1 |
| 5 | Cr2 | 1340 | PPNCl | - | 15 | 41 | >99 | 4.4 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santulli, F.; Grimaldi, I.; Pappalardo, D.; Lamberti, M.; Mazzeo, M. Salen-like Chromium and Aluminum Complexes as Catalysts in the Copolymerization of Epoxides with Cyclic Anhydrides for the Synthesis of Polyesters. Int. J. Mol. Sci. 2023, 24, 10052. https://doi.org/10.3390/ijms241210052
Santulli F, Grimaldi I, Pappalardo D, Lamberti M, Mazzeo M. Salen-like Chromium and Aluminum Complexes as Catalysts in the Copolymerization of Epoxides with Cyclic Anhydrides for the Synthesis of Polyesters. International Journal of Molecular Sciences. 2023; 24(12):10052. https://doi.org/10.3390/ijms241210052
Chicago/Turabian StyleSantulli, Federica, Ilaria Grimaldi, Daniela Pappalardo, Marina Lamberti, and Mina Mazzeo. 2023. "Salen-like Chromium and Aluminum Complexes as Catalysts in the Copolymerization of Epoxides with Cyclic Anhydrides for the Synthesis of Polyesters" International Journal of Molecular Sciences 24, no. 12: 10052. https://doi.org/10.3390/ijms241210052
APA StyleSantulli, F., Grimaldi, I., Pappalardo, D., Lamberti, M., & Mazzeo, M. (2023). Salen-like Chromium and Aluminum Complexes as Catalysts in the Copolymerization of Epoxides with Cyclic Anhydrides for the Synthesis of Polyesters. International Journal of Molecular Sciences, 24(12), 10052. https://doi.org/10.3390/ijms241210052







