Comparative Analysis of a Human Neutralizing mAb Specific for SARS-CoV-2 Spike-RBD with Cilgavimab and Tixagevimab for the Efficacy on the Omicron Variant in Neutralizing and Detection Assays
Abstract
:1. Introduction
2. Results
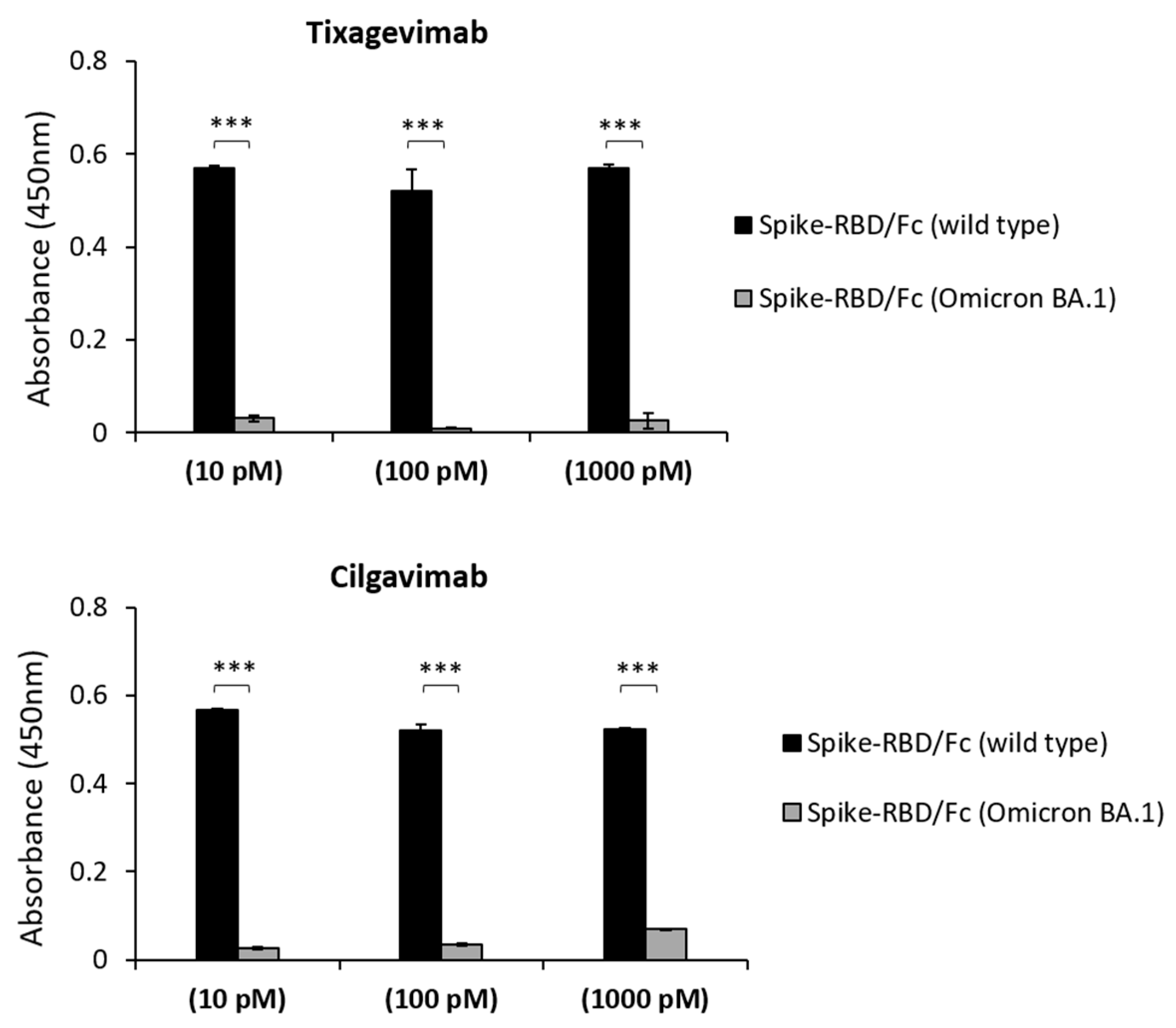
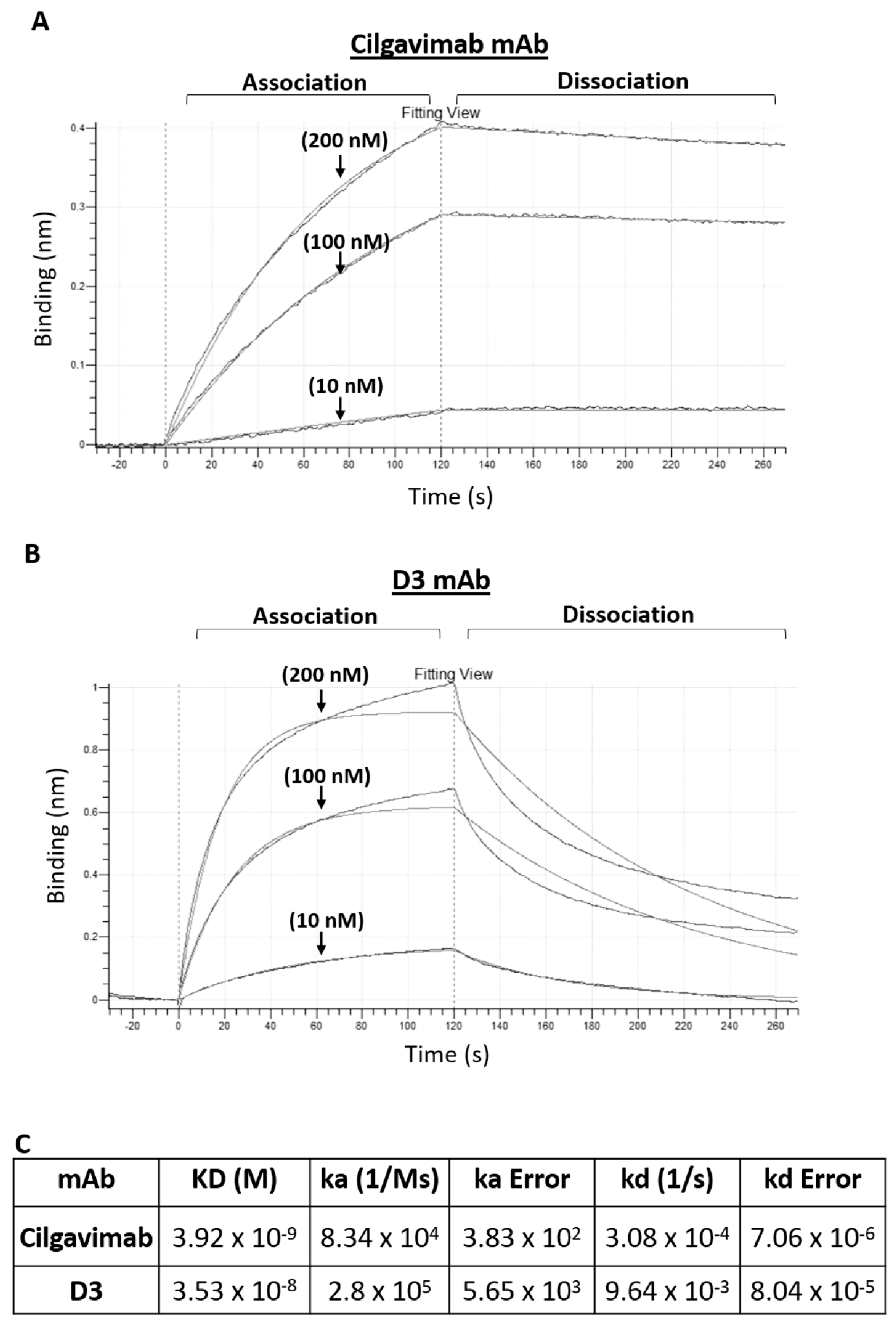
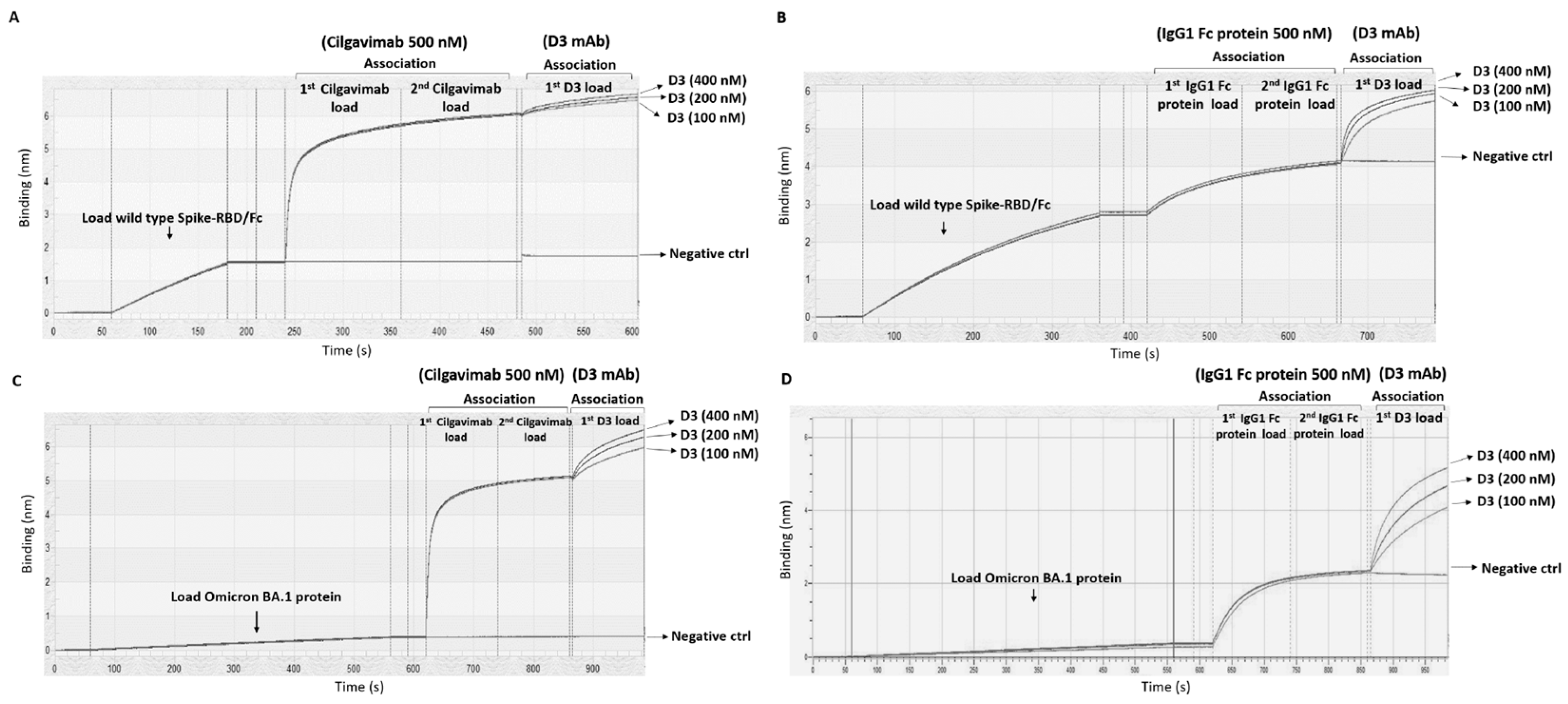
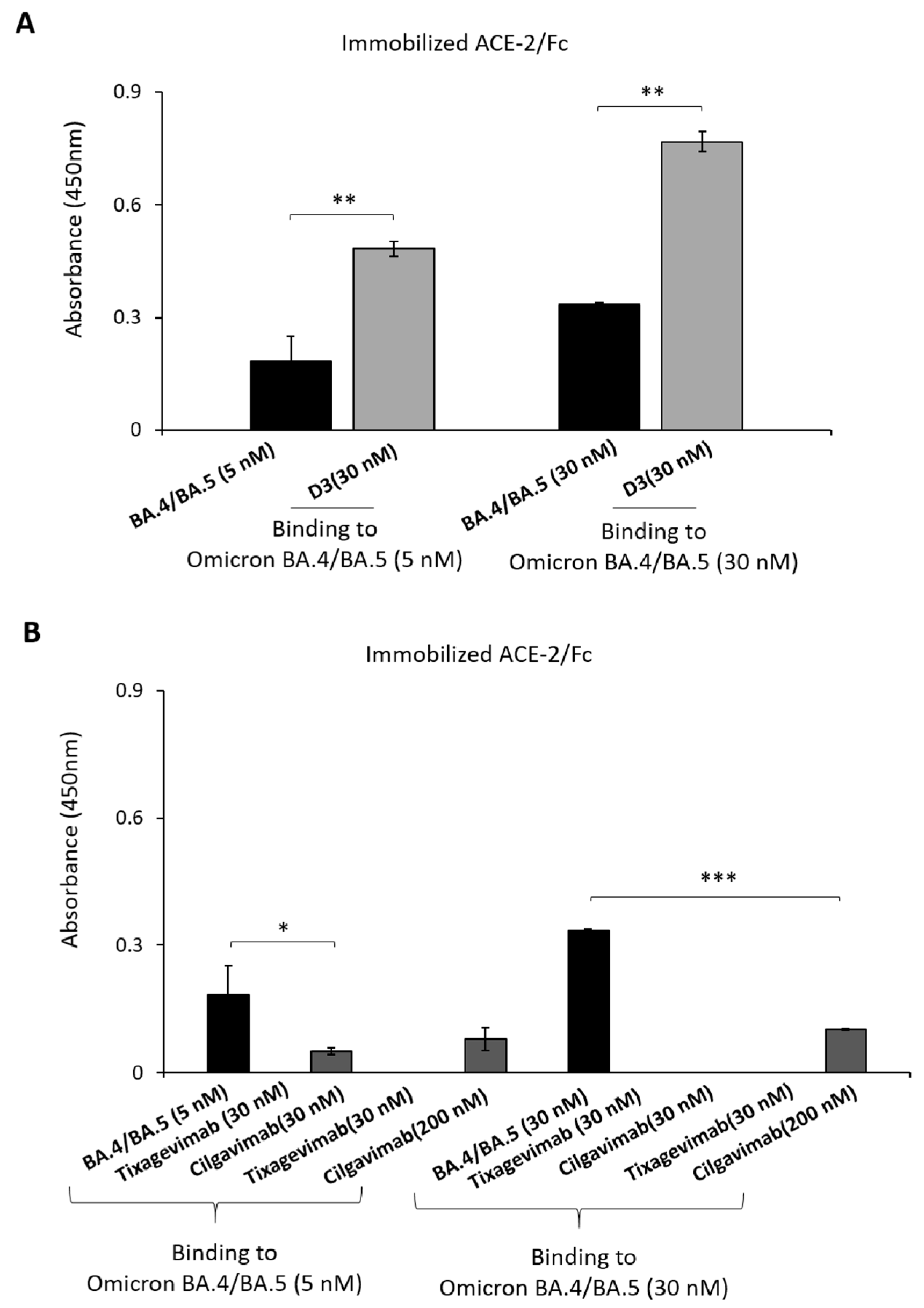
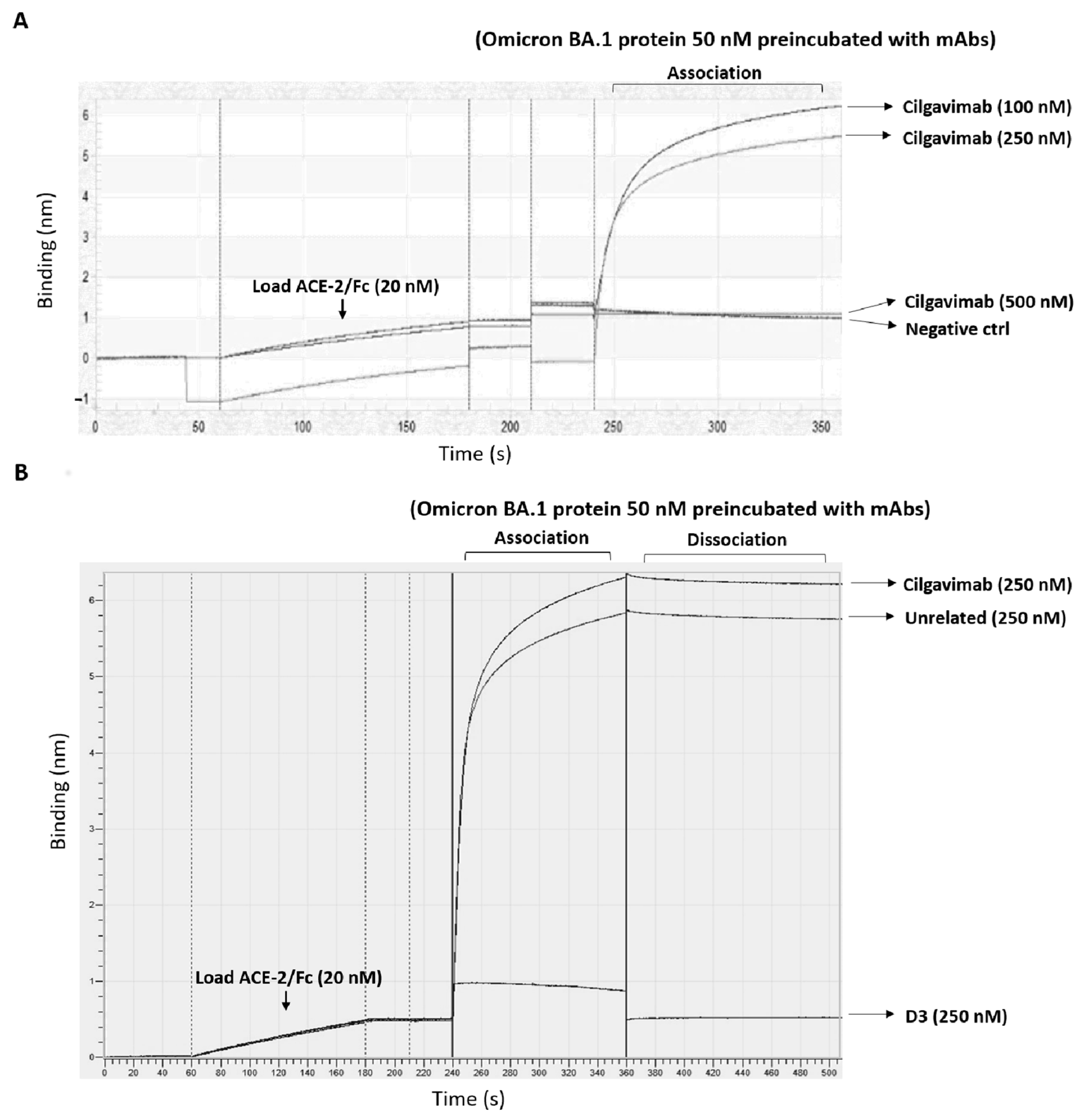
2.1. Binding to Spike-Omicron Variants of Novel D3 mAb Compared to Those of the Antibodies in Clinical Use for Prophylaxis and Therapy of COVID-19
2.2. Evaluation of the Anti-Spike mAbs for Diagnostic Applications
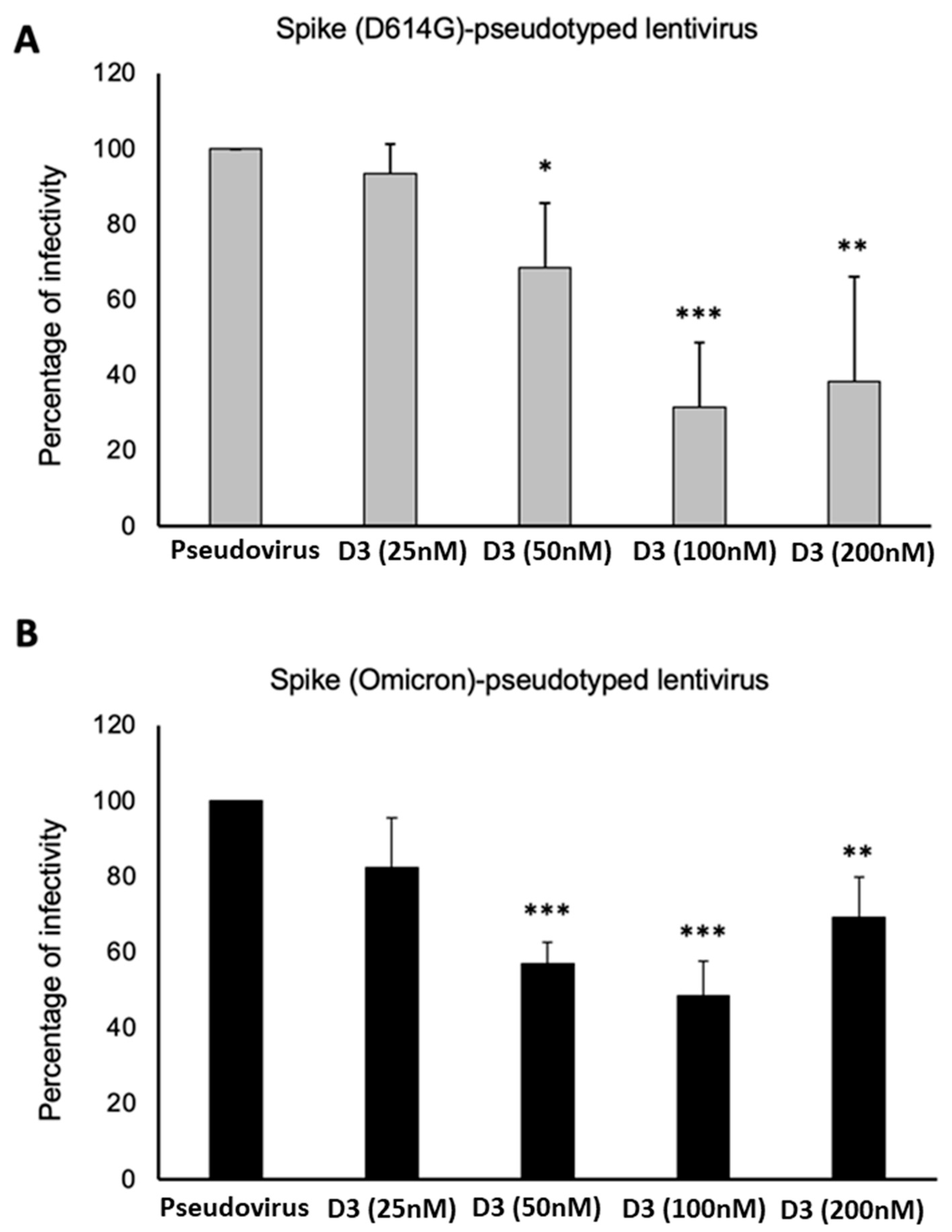
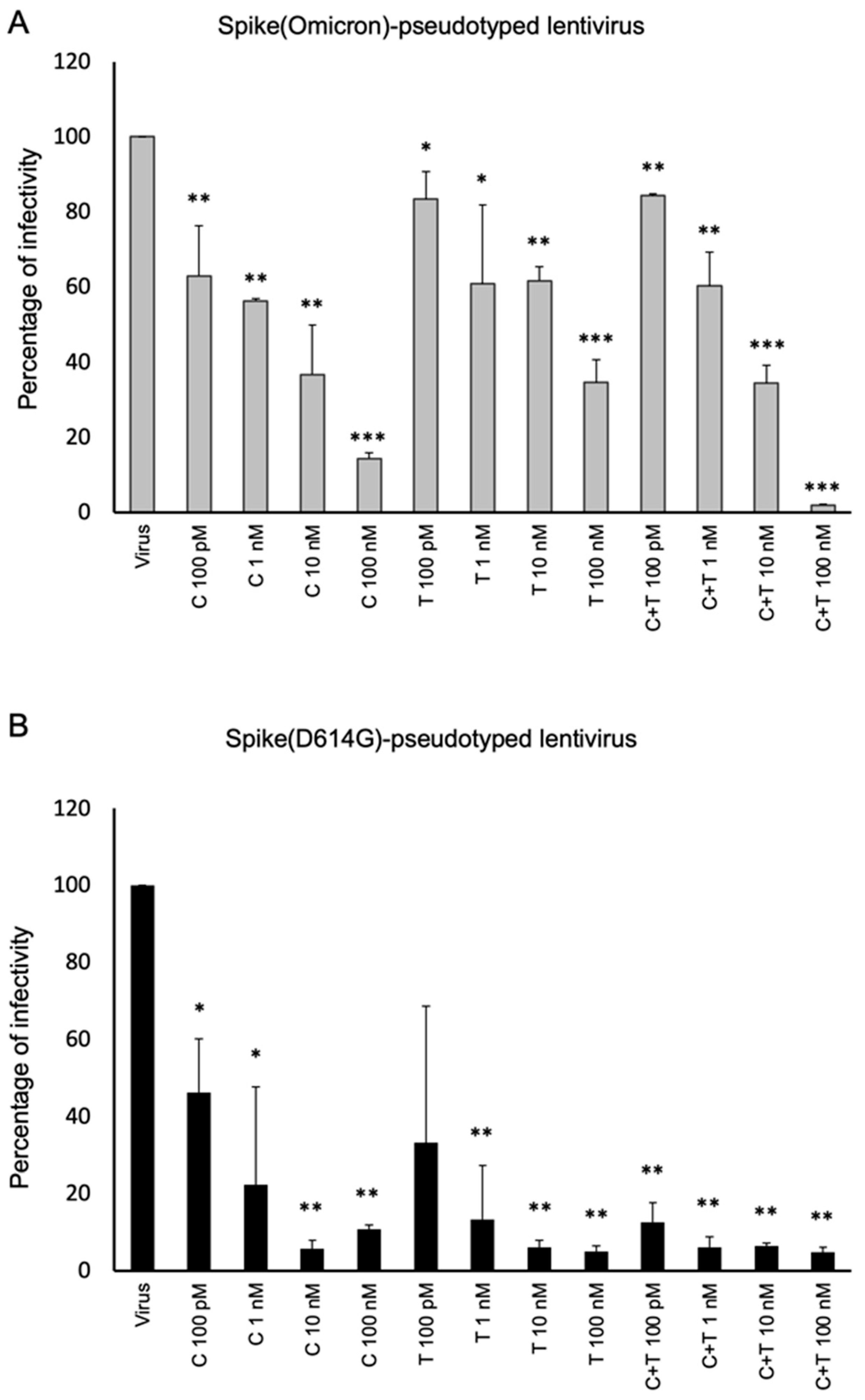
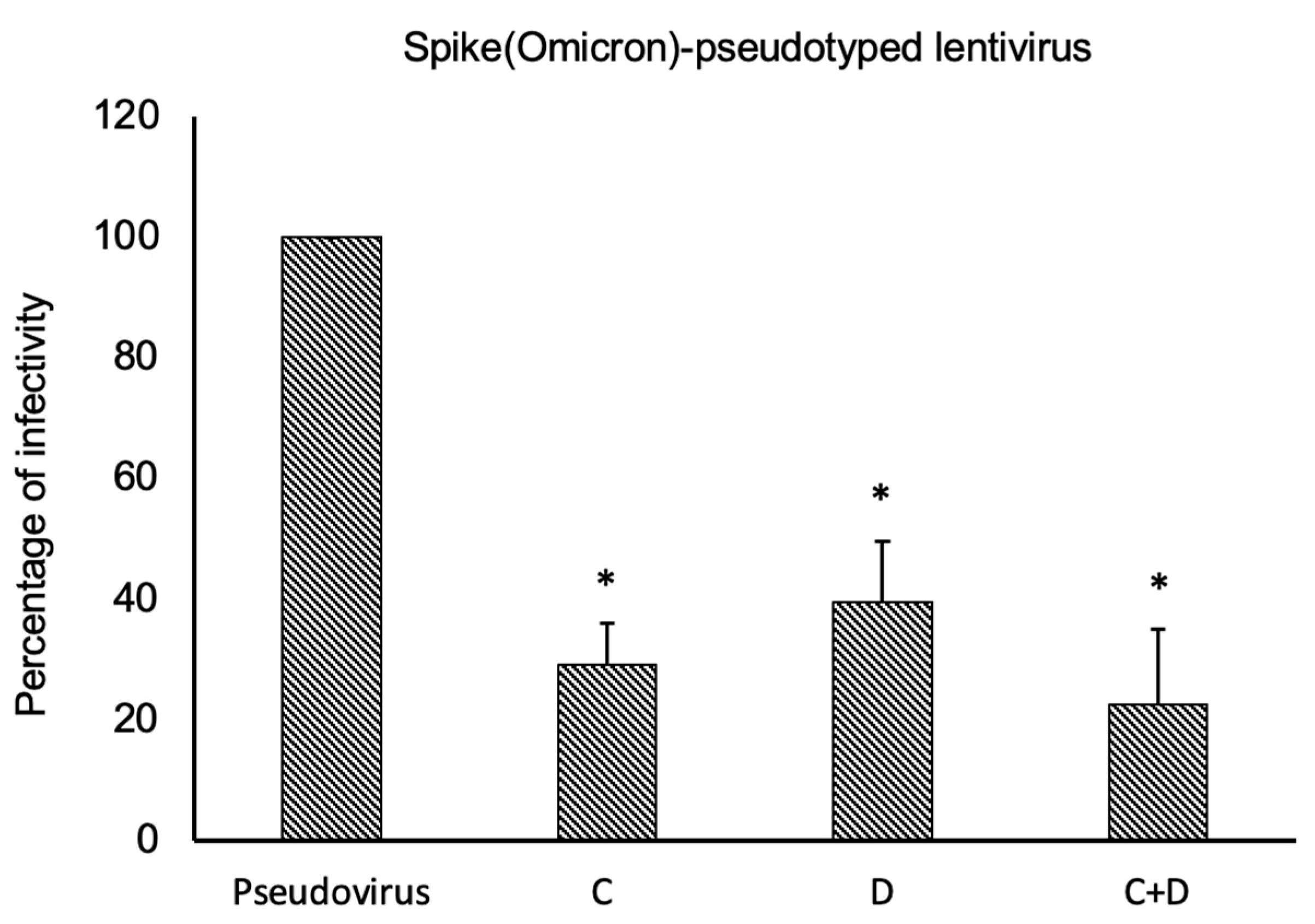
2.3. Neutralization Assays Using SARS-CoV-2 Spike-Pseudotyped Lentivirus
3. Discussion
4. Materials and Methods
4.1. Cell Line Cultures
4.2. Antibodies and Human Recombinant Proteins
4.3. Binding of mAbs to Spike-RBD from Wild-Type SARS-CoV-2 or Its Omicron Variants
4.4. Biolayer Interferometry (BLI) Analysis
4.5. Lentiviral Production and Cells Transduction
4.6. Quantization of Viral Particles by Droplet Digital PCR (ddPCR)
4.7. In Vitro Neutralization Assays of D614G/Omicron BA.1 SARS-CoV-2 Variant
4.8. Detection of Pseudoviral Particles Expressing Wild-Type Spike or Its Omicron Variant
4.9. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Wang, Q.; Ye, S.B.; Zhou, J.Y.; Lv, J.Z.; Hu, B.; Yuan, S.; Qiu, Y.; Ge, X.Y. Key mutations on spike protein altering ACE2 receptor utilization and potentially expanding host range of emerging SARS-CoV-2 variants. J. Med. Virol. 2023, 95, e28116. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, R.; Pan, Z.; Qian, C.; Yang, Y.; You, R.; Zhao, J.; Liu, P.; Gao, L.; Li, Z.; et al. Human monoclonal antibodies block the binding of SARS-CoV-2 spike protein to angiotensin converting enzyme 2 receptor. Cell. Mol. Immunol. 2020, 17, 647–649. [Google Scholar] [CrossRef]
- Kip, K.E.; McCreary, E.K.; Collins, K.; Minnier, T.E.; Snyder, G.M.; Garrard, W.; McKibben, J.C.; Yealy, D.M.; Seymour, C.W.; Huang, D.T.; et al. Evolving Real-World Effectiveness of Monoclonal Antibodies for Treatment of COVID-19: A Cohort Study. Ann. Intern. Med. 2023, 176, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.A.; Zhou, D.; Tan, T.K.; Chen, C.; Duyvesteyn, H.M.E.; Zhao, Y.; Ginn, H.M.; Qin, L.; Rijal, P.; Schimanski, L.; et al. Structures and therapeutic potential of anti-RBD human monoclonal antibodies against SARS-CoV-2. Theranostics 2022, 12, 1–17. [Google Scholar] [CrossRef]
- Regeneron Pharmaceuticals Inc. Fact Sheet for Health Care Providers: Emergency Use Authorization (EUA) of Casirivimab and Imdevimab. Available online: https://www.regeneron.com/sites/default/files/treatment-covid19-eua-fact-sheet-for-hcp.pdf (accessed on 20 March 2023).
- Regeneron’s Casirivimab and Imdevimab Antibody Cocktail for COVID-19 Is First Combination Therapy to Receive FDA Emergency Use Authorization. Available online: https://investor.regeneron.com/news-releases/news-release-details/regenerons-regen-cov2-first-antibody-cocktail-covid-19-receive/ (accessed on 20 March 2023).
- Dougan, M.; Nirula, A.; Azizad, M.; Mocherla, B.; Gottlieb, R.L.; Chen, P.; Hebert, C.; Perry, R.; Boscia, J.; Heller, B.; et al. Bamlanivimab plus Etesevimab in Mild or Moderate COVID-19. N. Engl. J. Med. 2021, 385, 1382–1392. [Google Scholar] [CrossRef]
- Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Monoclonal Antibody Bamlanivimab. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab (accessed on 22 March 2023).
- FDA Authorizes Bamlanivimab and Etesevimab Monoclonal Antibody Therapy for Post-Exposure Prophylaxis (Prevention) for COVID-19. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-authorizes-bamlanivimab-and-etesevimab-monoclonal-antibody-therapy-post-exposure-prophylaxis (accessed on 22 March 2023).
- Coronavirus (COVID-19) Update: FDA Authorizes Additional Monoclonal Antibody for Treatment of COVID-19. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-monoclonal-antibody-treatment-covid-19 (accessed on 22 March 2023).
- COVID-19: EMA Recommends Authorisation of Antibody Medicine Xevudy. Available online: https://www.ema.europa.eu/en/news/covid-19-ema-recommends-authorisation-antibody-medicine-xevudy (accessed on 27 March 2023).
- Evusheld Long-Acting Antibody Combination Approved in the EU for the Treatment of COVID-19. Available online: https://www.astrazeneca.com/media-centre/press-releases/2022/evusheld-approved-in-eu-for-covid-19-treatment.html (accessed on 27 March 2023).
- Zhou, Y.; Zhi, H.; Teng, Y. The outbreak of SARS-CoV-2 Omicron lineages, immune escape, and vaccine effectivity. J. Med. Virol. 2023, 95, e28138. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Iketani, S.; Li, Z.; Liu, L.; Guo, Y.; Huang, Y.; Bowen, A.D.; Liu, M.; Wang, M.; Yu, J.; et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell 2023, 186, 279–286. [Google Scholar] [CrossRef]
- Passariello, M.; Gentile, C.; Ferrucci, V.; Sasso, E.; Vetrei, C.; Fusco, G.; Viscardi, M.; Brandi, S.; Cerino, P.; Zambrano, N.; et al. Novel human neutralizing mAbs specific for Spike-RBD of SARS-CoV-2. Sci. Rep. 2021, 11, 11046. [Google Scholar] [CrossRef]
- Passariello, M.; Ferrucci, V.; Sasso, E.; Manna, L.; Rapuano Lembo, R.; Pascarella, S.; Fusco, G.; Zambrano, N.; Zollo, M.; De Lorenzo, C. A Novel Human Neutralizing mAb Recognizes Delta, Gamma and Omicron Variants of SARS-CoV-2 and Can Be Used in Combination with Sotrovimab. Int. J. Mol. Sci. 2022, 23, 5556. [Google Scholar] [CrossRef]
- Anti-SARS-CoV-2 Monoclonal Antibodies. Available online: https://www.idsociety.org/covid-19-real-time-learning-network/therapeutics-and-interventions/monoclonal-antibodies/ (accessed on 30 March 2023).
- Hwang, Y.C.; Lu, R.M.; Su, S.C.; Chiang, P.Y.; Ko, S.H.; Ke, F.Y.; Liang, K.H.; Hsieh, T.Y.; Wu, H.C. Monoclonal antibodies for COVID-19 therapy and SARS-CoV-2 detection. J. Biomed. Sci. 2022, 29, 1. [Google Scholar] [CrossRef]
- ACTIV-3–Therapeutics for Inpatients with COVID-19 (TICO) Study Group. Tixagevimab-cilgavimab for treatment of patients hospitalised with COVID-19: A randomised, double-blind, phase 3 trial. Lancet Respir. Med. 2022, 10, 972–984. [Google Scholar] [CrossRef]
- Focosi, D.; Casadevall, A. A Critical Analysis of the Use of Cilgavimab plus Tixagevimab Monoclonal Antibody Cocktail (Evusheld™) for COVID-19 Prophylaxis and Treatment. Viruses 2022, 14, 1999. [Google Scholar] [CrossRef] [PubMed]
- Boschi, C.; Colson, P.; Bancod, A.; Moal, V.; La Scola, B. Omicron Variant Escapes Therapeutic Monoclonal Antibodies (mAbs) Including Recently Released Evusheld®, Contrary to 8 Prior Main Variant of Concern (VOC). Clin. Infect. Dis. 2022, 75, e534–e535. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [Green Version]
- Karaba, A.H.; Kim, J.D.; Chiang, T.P.; Alejo, J.L.; Sitaras, I.; Abedon, A.T.; Eby, Y.; Johnston, T.S.; Li, M.; Aytenfisu, T.; et al. Neutralizing activity and 3-month durability of tixagevimab and cilgavimab prophylaxis against Omicron sublineages in transplant recipients. Am. J. Transplant. 2023, 23, 423–428. [Google Scholar] [CrossRef]
- Roe, T.L.; Brady, T.; Schuko, N.; Nguyen, A.; Beloor, J.; Guest, J.D.; Aksyuk, A.A.; Tuffy, K.M.; Zhang, T.; Streicher, K.; et al. Molecular Characterization of AZD7442 (Tixagevimab-Cilgavimab) Neutralization of SARS-CoV-2 Omicron Subvariants. Microbiol. Spectr. 2023, 11, e0033323. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, X.E. Construction and applications of SARS-CoV-2 pseudoviruses: A mini review. Int. J. Biol. Sci. 2021, 17, 1574–1580. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.; Oton, J.; Qu, K.; Cortese, M.; Zila, V.; McKeane, L.; Nakane, T.; Zivanov, J.; Neufeldt, C.J.; Cerikan, B.; et al. Structures and distributions of SARS-CoV-2 spike proteins on intact virions. Nature 2020, 588, 498–502. [Google Scholar] [CrossRef]
- Sasso, E.; D’Avino, C.; Passariello, M.; D’Alise, A.M.; Siciliano, D.; Esposito, M.L.; Froechlich, G.; Cortese, R.; Scarselli, E.; Zambrano, N.; et al. Massive parallel screening of phage libraries for the generation of repertoires of human immunomodulatory monoclonal antibodies. MAbs 2018, 10, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Passariello, M.; Yoshioka, A.; Takahashi, K.; Hashimoto, S.I.; Rapuano Lembo, R.; Manna, L.; Nakamura, K.; De Lorenzo, C. Novel Bi-Specific Immuno-Modulatory Tribodies Potentiate T Cell Activation and Increase Anti-Tumor Efficacy. Int. J. Mol. Sci. 2022, 23, 3466. [Google Scholar] [CrossRef] [PubMed]
- Passariello, M.; Vetrei, C.; Sasso, E.; Froechlich, G.; Gentile, C.; D’Alise, A.M.; Zambrano, N.; Scarselli, E.; Nicosia, A.; De Lorenzo, C. Isolation of Two Novel Human Anti-CTLA-4 mAbs with Intriguing Biological Properties on Tumor and NK Cells. Cancers 2020, 12, 2204. [Google Scholar] [CrossRef]
- Riccio, G.; Da Fonseca-Ricardo, A.R.; Passariello, M.; Cunnah, P.; Mertens, N.; De Lorenzo, C. Superior suppression of ErbB2-positive tumor cells by a novel human triparatopic tribody. J. Immunother. 2017, 40, 117–128. [Google Scholar] [CrossRef]
- Passariello, M.; D’Alise, A.M.; Esposito, A.; Vetrei, C.; Froechlich, G.; Scarselli, E.; Nicosia, A.; De Lorenzo, C. Novel Human Anti-PD-L1 mAbs Inhibit Immune-Independent Tumor Cell Growth and PD-L1 Associated Intracellular Signalling. Sci. Rep. 2019, 9, 13125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passariello, M.; Camorani, S.; Vetrei, C.; Cerchia, L.; De Lorenzo, C. Novel Human Bispecific Aptamer-Antibody Conjugates for Efficient Cancer Cell Killing. Cancers 2019, 11, 1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passariello, M.; Yoshioka, A.; Takahashi, K.; Hashimoto, S.I.; Inoue, T.; Nakamura, K.; De Lorenzo, C. Novel tri-specific tribodies induce strong T cell activation and anti-tumor effects in vitro and in vivo. J. Exp. Clin. Cancer Res. 2022, 41, 269. [Google Scholar] [CrossRef]
- Vetrei, C.; Passariello, M.; Froechlich, G.; Rapuano Lembo, R.; Zambrano, N.; De Lorenzo, C. Immunomodulatory mAbs as Tools to Investigate on Cis-Interaction of PD-1/PD-L1 on Tumor Cells and to Set Up Methods for Early Screening of Safe and Potent Combinatorial Treatments. Cancers 2021, 13, 2858. [Google Scholar] [CrossRef]


| Step | Temperature | Time | Ramp Rate | Cycles |
|---|---|---|---|---|
| Initial denaturation | 95 °C | 10 min | 2.5 °C/s | 1 |
| Denaturation | 95 °C | 30 s | 40 | |
| Annealing | 55 °C | 30 s | ||
| Extension | 72 °C | 15 s | ||
| Signal stabilization | 98 °C | 10′ | 1 old (optional) 12 °C | infinite |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passariello, M.; Esposito, S.; Manna, L.; Rapuano Lembo, R.; Zollo, I.; Sasso, E.; Amato, F.; De Lorenzo, C. Comparative Analysis of a Human Neutralizing mAb Specific for SARS-CoV-2 Spike-RBD with Cilgavimab and Tixagevimab for the Efficacy on the Omicron Variant in Neutralizing and Detection Assays. Int. J. Mol. Sci. 2023, 24, 10053. https://doi.org/10.3390/ijms241210053
Passariello M, Esposito S, Manna L, Rapuano Lembo R, Zollo I, Sasso E, Amato F, De Lorenzo C. Comparative Analysis of a Human Neutralizing mAb Specific for SARS-CoV-2 Spike-RBD with Cilgavimab and Tixagevimab for the Efficacy on the Omicron Variant in Neutralizing and Detection Assays. International Journal of Molecular Sciences. 2023; 24(12):10053. https://doi.org/10.3390/ijms241210053
Chicago/Turabian StylePassariello, Margherita, Speranza Esposito, Lorenzo Manna, Rosa Rapuano Lembo, Immacolata Zollo, Emanuele Sasso, Felice Amato, and Claudia De Lorenzo. 2023. "Comparative Analysis of a Human Neutralizing mAb Specific for SARS-CoV-2 Spike-RBD with Cilgavimab and Tixagevimab for the Efficacy on the Omicron Variant in Neutralizing and Detection Assays" International Journal of Molecular Sciences 24, no. 12: 10053. https://doi.org/10.3390/ijms241210053






