Polyaromatic Hydrocarbon (PAH)-Based Aza-POPOPs: Synthesis, Photophysical Studies, and Nitroanalyte Sensing Abilities
Abstract
:1. Introduction
2. Results
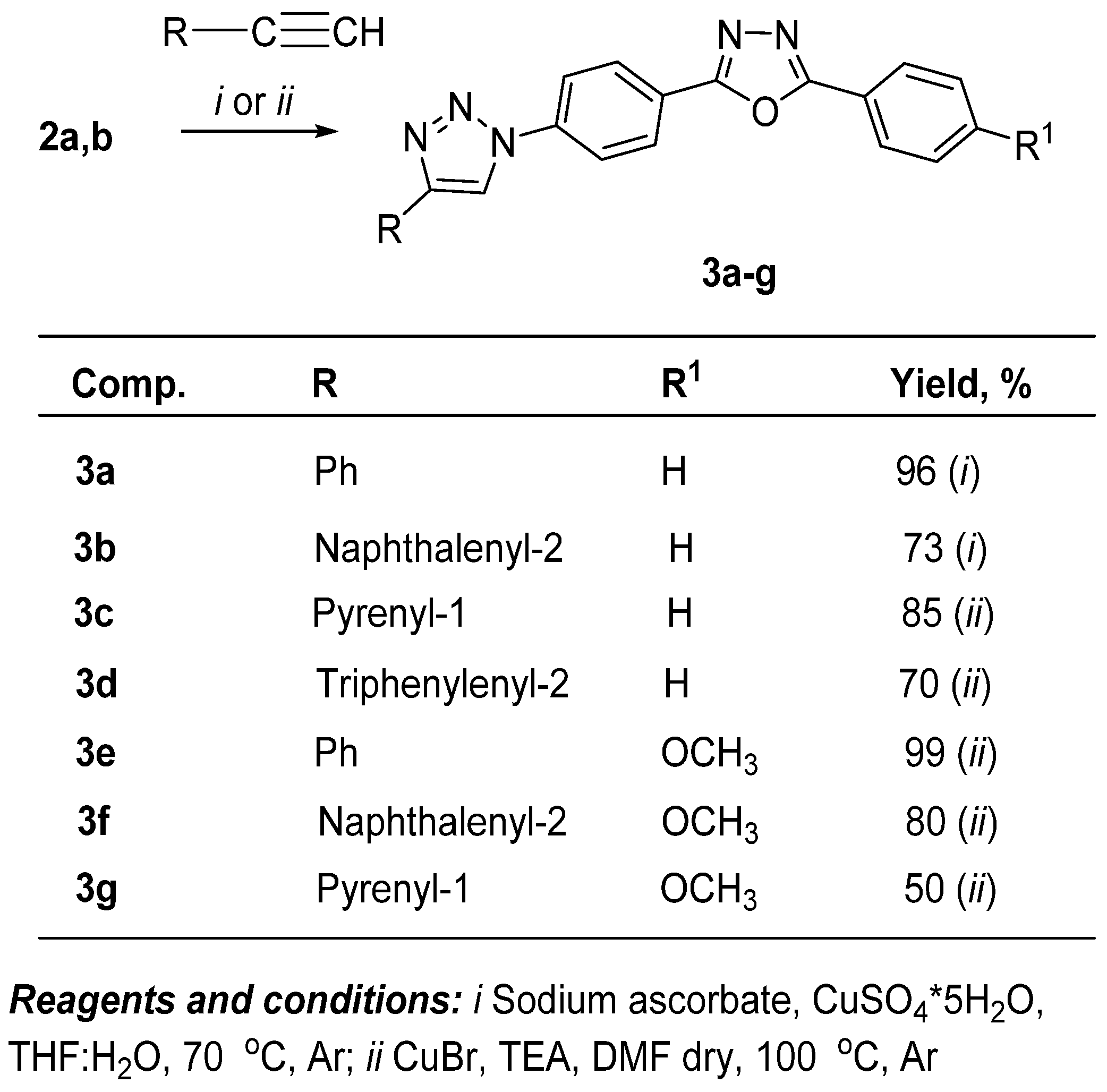
2.1. Synthesis of Target Fluorophores
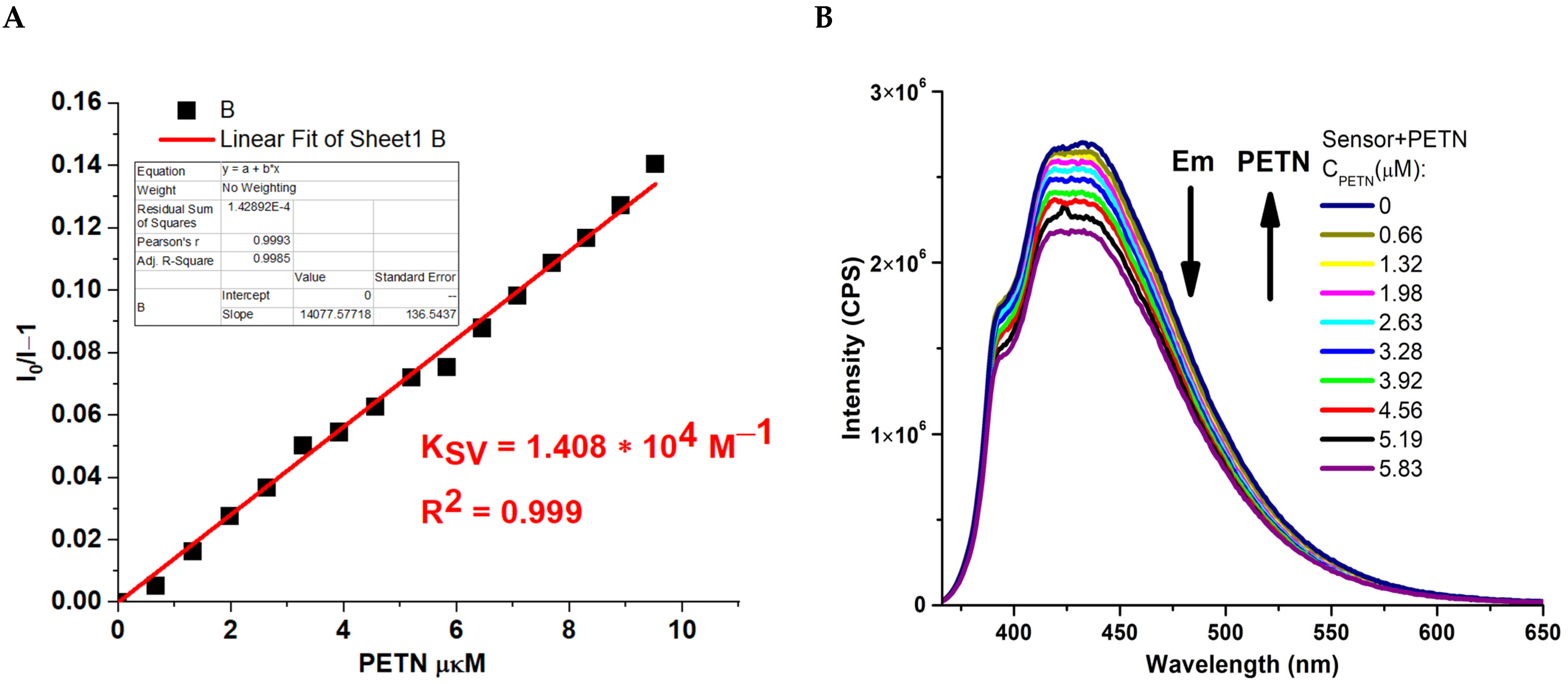
2.2. Photophysical and Sensing Properties of the Obtained Compounds
3. Materials and Methods
3.1. Synthesis
3.1.1. 2-(4-Azidophenyl)-5-phenyl-1,3,4-oxadiazoles 2a–b
3.1.2. 2-Phenyl-5-(4-(4-arenyl-1H-1,2,3-triazol-1-yl)phenyl)-1,3,4-oxadiazoles 3a–g
3.2. Photophysical Investigations
Materials and Equipment
3.3. Experimental Methods
3.3.1. Fluorometric Titration
3.3.2. Limit of Detection Calculation
3.3.3. Time-Resolved Fluorescence Measurement
3.3.4. Excitation Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adadurov, A.F.; Zhmurin, P.N.; Lebedev, V.N.; Titskaya, V.D. Optimizing concentration of shifter additive for plastic scintillators of different size. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2009, 599, 167–170. [Google Scholar] [CrossRef]
- Patterson, M.S.; Greene, R.C. Measurement of Low Energy Beta-Emitters in Aqueous Solution by Liquid Scintillation Counting of Emulsions. Anal. Chem. 1965, 37, 854–857. [Google Scholar] [CrossRef]
- Mardelli, M.; Olmsted, J. Calorimetric determination of the 9,10-diphenyl-anthracene fluorescence quantum yield. J. Photochem. 1977, 7, 277–285. [Google Scholar] [CrossRef]
- Shank, C.V. Physics of dye lasers. Rev. Mod. Phys. 1975, 47, 649–657. [Google Scholar] [CrossRef]
- Basov, N.G.; Logunov, O.A.; Startsev, A.V.; Stoilov, Y.Y.; Zuev, V.S. Vapour phase dye lasers of the visible range. J. Mol. Struct. 1982, 79, 119–123. [Google Scholar] [CrossRef]
- Desai, N.; Monapara, J.; Jethawa, A.; Khedkar, V.; Shingate, B. Oxadiazole: A highly versatile scaffold in drug discovery. Arch. Pharm. 2022, 355, 2200123. [Google Scholar] [CrossRef]
- Luczynski, M.; Kudelko, A. Synthesis and Biological Activity of 1,3,4-Oxadiazoles Used in Medicine and Agriculture. Appl. Sci. 2022, 12, 3756. [Google Scholar] [CrossRef]
- Siwach, A.; Verma, P.K. Therapeutic potential of oxadiazole or furadiazole containing compounds. BMC Chem. 2020, 14, 70. [Google Scholar] [CrossRef]
- Li, Z.; Li, W.; Keum, C.; Archer, E.; Zhao, B.; Slawin, A.M.Z.; Huang, W.; Gather, M.C.; Samuel, I.D.W.; Zysman-Colman, E. 1,3,4-Oxadiazole-based Deep Blue Thermally Activated Delayed Fluorescence Emitters for Organic Light Emitting Diodes. J. Phys. Chem. C 2019, 123, 24772–24785. [Google Scholar] [CrossRef]
- Mayder, D.M.; Tonge, C.M.; Hudson, Z.M. Thermally activated delayed fluorescence in 1,3,4-oxadiazoles with π-extended donors. J. Org. Chem. 2020, 85, 11094–11103. [Google Scholar] [CrossRef]
- Zhou, J.-A.; Tang, X.-L.; Cheng, J.; Ju, J.-H.; Yang, L.-Z.; Liu, W.-S.; Chen, C.-Y.; Bai, D.-C. An 1,3,4-oxadiazole-based OFF-ON fluorescent chemosensor for Zn2+ in aqueous solution and imaging application in living cells. Dalt. Trans. 2012, 41, 10626–10632. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Gryczynski, I.; Malak, H.; Gryczynski, Z. Fluorescence spectral properties of 2,5-diphenyl-1,3,4-oxadiazole with two-color two-photon excitation. J. Phys. Chem. 1996, 100, 19406–19411. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chemie Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Sudeep, P.; Vagish, C.B.; Kumar, A.D.; Kumar, K.A. 1,2,3-Triazoles: A Review on Current Trends in Synthetic and Biological Applications. J. Appl. Chem. 2020, 13, 22–40. [Google Scholar] [CrossRef]
- Hein, C.D.; Liu, X.M.; Wang, D. Click chemistry, a powerful tool for pharmaceutical sciences. Pharm. Res. 2008, 25, 2216–2230. [Google Scholar] [CrossRef] [Green Version]
- Neeraja, P.; Srinivas, S.; Mukkanti, K.; Dubey, P.K.; Pal, S. 1H-1,2,3-Triazolyl-substituted 1,3,4-oxadiazole derivatives containing structural features of ibuprofen/naproxen: Their synthesis and antibacterial evaluation. Bioorg. Med. Chem. Lett. 2016, 26, 5212–5217. [Google Scholar] [CrossRef]
- Shang, J.Q.; Fu, H.; Li, Y.; Yang, T.; Gao, C.; Li, Y.M. Copper-catalyzed decarboxylation/cycloaddition cascade of alkynyl carboxylic acids with azide. Tetrahedron 2019, 75, 253–259. [Google Scholar] [CrossRef]
- Lauria, A.; Delisi, R.; Mingoia, F.; Terenzi, A.; Martorana, A.; Barone, G.; Almerico, A.M. 1,2,3-Triazole in Heterocyclic Compounds, Endowed With Biological Activity, Through 1,3-Dipolar Cycloadditions. Eur. J. Org. Chem. 2014, 2014, 3289–3306. [Google Scholar] [CrossRef]
- Farooq, T.; Haug, B.E.; Sydnes, L.K.; Törnroos, K.W. 1,3-Dipolar cycloaddition of benzyl azide to two highly functionalized alkynes. Mon. Fur Chemie. 2012, 143, 505–512. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-F.; Wang, C.-J.; Feng, Q.-Z.; Zhai, J.-J.; Qi, S.-S.; Zhong, A.-G.; Chu, M.M.; Xu, D.-Q. Copper-catalyzed asymmetric 1,6-conjugate addition of in situ generated para-quinone methides with β-ketoesters. Chem. Commun. 2022, 58, 6653–6656. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, J.J.; Li, Y.; Yuan, X.; Xiong, T.; Zhang, Q. Copper-Catalyzed Asymmetric Conjugate Addition of Alkene-Derived Nucleophiles to Alkenyl-Substituted Heteroarenes. ACS Catal. 2022, 12, 9611–9620. [Google Scholar] [CrossRef]
- Pan, Z.-Z.; Pan, D.; Li, J.-H.; Xue, X.-S.; Yin, L. Copper(I)-Catalyzed Asymmetric Conjugate Addition of 1,4-Dienes to β-Substituted Alkenyl Azaarenes. J. Am. Chem. Soc. 2023, 145, 1749–1758. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Liu, J.-H.; Zhang, Y.-P.; Zhao, J.-Q.; You, Y.; Zhou, M.-Q.; Han, W.-Y.; Yuan, W.-C. Cu-Catalyzed Asymmetric 1,3-Dipolar Cycloaddition of N-2,2,2-Trifluoroethylisatin Ketimines Enables the Desymmetrization of N-Arylmaleimides: Access to Enantioenriched F3C-Containing Octahydropyrrolo[3,4-c]pyrrole. Org. Lett. 2022, 24, 4052–4057. [Google Scholar] [CrossRef]
- Zhang, D.-Y.; Shao, L.; Xu, J.; Hu, X.-P. Copper-Catalyzed Asymmetric Formal [3 + 2] Cycloaddition of Propargylic Acetates with Hydrazines: Enantioselective Synthesis of Optically Active 2-Pyrazolines. ACS Catal. 2015, 5, 5026–5030. [Google Scholar] [CrossRef]
- Zeng, L.; Li, J.; Cui, S. Rhodium-Catalyzed Atroposelective Click Cycloaddition of Azides and Alkynes. Angew. Chem. Int. Ed. 2022, 134, e202205037. [Google Scholar] [CrossRef]
- Luvino, D.; Amalric, C.; Smietana, M.; Vasseur, J.J. Sequential Seyferth-Gilbert/CuAAC reactions: Application to the one-pot synthesis of triazoles from aldehydes. Synlett 2007, 2007, 3037–3041. [Google Scholar] [CrossRef]
- Lauko, J.; Kouwer, P.H.J.; Rowan, A.E. 1H-1,2,3-Triazole: From Structure to Function and Catalysis. J. Heterocycl. Chem. 2017, 54, 1677–1699. [Google Scholar] [CrossRef]
- Thomas, J.; John, J.; Parekh, N.; Dehaen, W. A Metal-Free Three-Component Reaction for the Regioselective Synthesis of 1,4,5-Trisubstituted 1,2,3-Triazoles. Angew. Chem. 2014, 126, 10319–10323. [Google Scholar] [CrossRef]
- Totobenazara, J.; Burke, A.J. New click-chemistry methods for 1,2,3-triazoles synthesis: Recent advances and applications. Tetrahedron Lett. 2015, 56, 2853–2859. [Google Scholar] [CrossRef]
- Dubey, N.; Sharma, P.; Kumar, A. Clay-Supported Cu(II) Catalyst: An Efficient, Heterogeneous, and Recyclable Catalyst for Synthesis of 1,4-Disubstituted 1,2,3-Triazoles from Alloxan-Derived Terminal Alkyne and Substituted Azides Using Click Chemistry. Synth. Commun. 2015, 45, 2608–2626. [Google Scholar] [CrossRef]
- Kushwaha, D.; Dwivedi, P.; Kuanar, S.K.; Tiwari, V.K. Click Reaction in Carbohydrate Chemistry: Recent Developments and Future Perspective. Curr. Org. Synth. 2013, 10, 90–135. [Google Scholar] [CrossRef]
- Kang, M.; Ying, Q. Synthesis and Fluorescence Properties of Bisbranched 1,3,4-Oxadiazole Derivatives. Chin. J. Org. Chem. 2009, 29, 71–77. Available online: http://sioc-journal.cn/Jwk_yjhx/EN/abstract/article_326517.shtml (accessed on 15 March 2023).
- Li, A.F.; Ruan, Y.B.; Jiang, Q.Q.; He, W.B.; Jiang, Y.B. Molecular logic gates and switches based on 1,3,4-oxadiazoles triggered by metal ions. Chem. Eur. J. 2010, 16, 5794–5802. [Google Scholar] [CrossRef] [Green Version]
- Kutonova, K.V.; Trusova, M.E.; Postnikov, P.; Filimonov, V.D.; Parello, J. A simple and effective synthesis of aryl azides via arenediazonium tosylates. Synthesis 2013, 45, 2706–2710. [Google Scholar] [CrossRef] [Green Version]
- Huisgen, R. 1.3-Dipolare Cycloadditionen Ruckschau und Ausblick. Angew. Chem. 1963, 75, 604–637. [Google Scholar] [CrossRef]
- Liang, L.; Astruc, D. The copper(I)-catalysed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coord. Chem. Rev. 2011, 255, 2933–2945. [Google Scholar] [CrossRef]
- De Silva, T.P.D.; Youm, S.G.; Fronczek, F.R.; Sahasrabudhe, G.; Nesterov, E.E.; Warner, I.M. Pyrene-benzimidazole derivatives as novel blue emitters for OLEDs. Molecules 2021, 26, 6523. [Google Scholar] [CrossRef] [PubMed]
- Türel, T.; Mahadevan, G.; Valiyaveettil, S. Modular Synthesis and Structure–Property Correlation of Pyrene—Rylene Dyes for Cellular Imaging. Eur. J. Org. Chem. 2020, 2020, 3303–3311. [Google Scholar] [CrossRef]
- Sadieva, L.K.; Kovalev, I.S.; Taniya, O.S.; Platonov, V.A.; Novikov, A.S.; Berseneva, V.S.; Santra, S.; Zyryanov, G.V.; Ranu, B.C.; Charushin, V.N. Bola-type PEG-linked polyaromatic hydrocarbon-based chemosensors for the ‘turn-off’ excimer fluorescence detection of nitro-analytes/explosives in aqueous solutions. Dyes Pig. 2023, 210, 111014. [Google Scholar] [CrossRef]
- Thomas III, S.W.; Joly, G.D.; Swager, T.M. Chemical Sensors Based on Amplifying Fluorescent Conjugated Polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef]
- Shaw, P.E.; Burn, P.L. Real-time fluorescence quenching-based detection of nitro-containing explosive vapours: What are the key processes? Phys. Chem. Chem. Phys. 2017, 19, 29714–29730. [Google Scholar] [CrossRef] [PubMed]
- Curnrning, C.; Fisher, M.; Sikes, J. Amplifying fluorescent polymer arrays for chemical detection of explosives. In Electronic Noses & Sensors for the Detection of Explosives. NATO Science Series II: Mathematics, Physics and Chemistry; Gardner, J.W., Yinon, J., Eds.; Springer: Dordrecht, The Netherlands, 2004; Volume 159. [Google Scholar] [CrossRef]
- Salinas, Y.; Agostini, A.; Pérez-Esteve, É.; Martínez-Máñez, R.; Sancenón, F.; Marcos, M.D.; Soto, J.; Costero, A.M.; Gil, S.; Parra, M.; et al. Fluorogenic detection of Tetryl and TNT explosives using nanoscopic-capped mesoporous hybrid materials. J. Mater. Chem. A 2013, 1, 3561–3564. [Google Scholar] [CrossRef]
- Turhan, H.; Tukenmez, E.; Karagoz, B.; Bicak, N. Highly fluorescent sensing of nitroaromatic explosives in aqueous media using pyrene-linked PBEMA microspheres. Talanta 2018, 179, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Bal, M.; Köse, A.; Özpaça, Ö.; Köse, M. Pyrene, Anthracene, and Naphthalene-Based Azomethines for Fluorimetric Sensing of Nitroaromatic Compounds. J. Fluoresc. 2023; ahead of print. [Google Scholar] [CrossRef]
- Zyryanov, G.V.; Kopchuk, D.S.; Kovalev, I.S.; Nosova, E.V.; Rusinov, V.L.; Chupakhin, O.N. Chemosensors for detection of nitroaromatic compounds (explosives). Russ. Chem. Rev. 2014, 83, 783–819. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Baranova, A.A.; Lugovik, K.I.; Shafikov, M.Z.; Khokhlov, K.O.; Cheprakova, E.M.; Rusinov, G.L.; Chupakhin, O.N.; Charushin, V.N. Detection of nitroaromatic explosives by new D–π–A sensing fluorophores on the basis of the pyrimidine scaffold. Anal. Bioanal. Chem. 2016, 408, 4093–4101. [Google Scholar] [CrossRef] [PubMed]
- Kovalev, I.S.; Sadieva, L.K.; Taniya, O.S.; Yurk, V.M.; Minin, A.S.; Santra, S.; Zyryanov, G.V.; Charushin, V.N.; Chupakhin, O.N.; Tsurkan, M.V. Computer vision vs. spectrofluorometer-assisted detection of common nitro-explosive components with bola-type PAH-based chemosensors. RSC Adv. 2021, 11, 25850–25857. [Google Scholar] [CrossRef]
- Khasanov, A.F.; Kopchuk, D.S.; Kovalev, I.S.; Taniya, O.S.; Giri, K.; Slepukhin, P.A.; Santra, S.; Rahman, M.; Majee, A.; Charushin, V.N.; et al. Extended cavity pyrene-based iptycenes for the turn-off fluorescence detection of RDX and common nitroaromatic explosives. New J. Chem. 2017, 41, 2309–2320. [Google Scholar] [CrossRef]
- Kovalev, I.S.; Taniya, O.S.; Sadieva, L.K.; Volkova, N.N.; Minin, A.S.; Grzhegorzhevskii, K.V.; Gorbunov, E.B.; Zyryanov, G.V.; Chupakhin, O.N.; Charushin, V.N.; et al. Bola-type PAH-based Fluorophores/Chemosensors: Synthesis via an Unusual Clemmensen Reduction and Photophysical Studies. J. Photochem. Photobiol. A Chem. 2021, 420, 113466. [Google Scholar] [CrossRef]
- Sun, H.; Chen, S.; Zhong, A.; Sun, R.; Jin, J.; Yang, J.; Liu, D.; Niu, J.; Lu, S. Tuning Photophysical Properties via Positional Isomerization of the Pyridine Ring in Donor–Acceptor-Structured Aggregation-Induced Emission Luminogens Based on Phenylmethylene Pyridineacetonitrile Derivatives. Molecules 2023, 28, 3282. [Google Scholar] [CrossRef]
- Fu, Y.; Finney, N.S. Small-molecule fluorescent probes and their design. RSC Adv. 2018, 8, 29051–29061. [Google Scholar] [CrossRef] [Green Version]
- Jensen, E.C. Use of Fluorescent Probes: Their Effect on Cell Biology and Limitations. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2012, 295, 2031–2036. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, N.I.; Bakov, V.V.; Anichina, K.K.; Bojinov, V.B. Fluorescent Probes as a Tool in Diagnostic and Drug Delivery Systems. Pharmaceuticals 2023, 16, 381. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Yang, M.; Zhang, C.; Ma, Y.; Qin, Y.; Lei, Z.; Chang, L.; Lei, L.; Wang, T.; Yang, Y. Aggregation-induced emission (AIE)-active fluorescent probes with multiple binding sites toward ATP sensing and live cell imaging. J. Mater. Chem. B 2017, 5, 8525–8531. [Google Scholar] [CrossRef]
- Yang, Q.; Wen, Y.; Zhong, A.; Xu, J.; Shao, S. An HBT-based fluorescent probe for nitroreductase determination and its application in Escherichia coli cell imaging. New J. Chem. 2020, 44, 16265–16268. [Google Scholar] [CrossRef]
- Zhao, D.; Han, H.-H.; Zhu, L.; Xu, F.-Z.; Ma, X.-Y.; Li, J.; James, T.D.; Zang, Y.; He, X.-P.; Wang, C. Long-Wavelength AIE-Based Fluorescent Probes for Mitochondria-Targeted Imaging and Photodynamic Therapy of Hepatoma Cells. ACS Appl. Bio Mater. 2021, 4, 7016–7024. [Google Scholar] [CrossRef]
- Du, X.; Wang, J.; Qin, A.; Tang, B. Application of AIE-active probes in fluorescence sensing. Chin. Sci. Bull. 2020, 65, 1428–1447. [Google Scholar] [CrossRef]
- Ma, J.; Gu, Y.; Ma, D.; Lu, W.; Qiu, J. Insights into AIE materials: A focus on biomedical applications of fluorescence. Front. Chem. 2022, 10, 985578. [Google Scholar] [CrossRef]
- Terai, T.; Nagano, T. Fluorescent probes for bioimaging applications. Curr. Opin. Chem. Biol. 2008, 12, 515–521. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Q.; Pan, X.; Lu, W.; Zhang, J. Development and Challenge of Fluorescent Probes for Bioimaging Applications: From Visualization to Diagnosis. Top. Curr. Chem. (Z) 2022, 380, 22. [Google Scholar] [CrossRef]
- Gao, L.; Wang, W.; Wang, X.; Yang, F.; Xie, L.; Shen, J.; Brimble, M.A.; Xiao, Q.; Yao, S.Q. Fluorescent probes for bioimaging of potential biomarkers in Parkinson’s disease. Chem. Soc. Rev. 2021, 50, 1219–1250. [Google Scholar] [CrossRef]
- Liu, A.; Liu, H.; Peng, X.; Jia, J.; Fu, Y.; He, Q.; Cao, H.; Cheng, J. Direct and ultrasensitive fluorescence detection of PETN vapor based on a fuorene-dimer probe via a synergic backbone and side-chain tuning. Anal. Methods 2018, 10, 2567–2574. [Google Scholar] [CrossRef]
- Ganiga, M.; Cyriac, J. Detection of PETN and RDX using a FRET-based fluorescence sensor system. Anal. Methods 2015, 7, 5412–5418. [Google Scholar] [CrossRef]
- Wang, C.; Huang, H.; Bunes, B.R.; Wu, N.; Xu, M.; Yang, X.; Yu, L.; Zang, L. Trace Detection of RDX, HMX and PETN Explosives Using a Fluorescence Spot Sensor. Sci. Rep. 2016, 6, 25015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrew, T.L.; Swager, T.M. A Fluorescence Turn-On Mechanism to Detect High Explosives RDX and PETN. J. Am. Chem. Soc. 2007, 129, 7254–7255. [Google Scholar] [CrossRef] [PubMed]
- Vovusha, H.; Sanyal, B. DFT and TD-DFT studies on the electronic and optical properties of explosive molecules adsorbed on boron nitride and graphene nano flakes. RSC Adv. 2015, 5, 4599–4608. [Google Scholar] [CrossRef]
- Cawkwell, M.J.; Zecevic, M.; Luscher, D.J.; Ramos, K.J. Dependence of the Elastic Stiffness Tensors of PETN, α-RDX, γ-RDX, ϵ-RDX, ϵ-CL-20, DAAF, FOX-7, and β-HMX on Hydrostatic Compression. Propellants Explos. Pyrotech. 2022, 47, e202100281. [Google Scholar] [CrossRef]
- Gruzdkov, Y.A.; Dreger, Z.A.; Gupta, Y.M. Experimental and Theoretical Study of Pentaerythritol Tetranitrate Conformers. J. Phys. Chem. A 2004, 108, 6216–6221. [Google Scholar] [CrossRef]
- Liu, S.; Ess, D.H.; Schauer, C.K. Density Functional Reactivity Theory Characterizes Charge Separation Propensity in Proton-Coupled Electron Transfer Reactions. J. Phys. Chem. A 2011, 115, 4738–4742. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, S.; Rong, C.; Zhong, A.; Liu, S. Toward Understanding the Isomeric Stability of Fullerenes with Density Functional Theory and the Information-Theoretic Approach. ACS Omega 2018, 3, 17986–17990. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, J.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Schlegel, H.B.; Scalmani, G.; Barone, V.; Mennucci, B.; et al. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Doose, S.; Neuweiler, H.; Sauer, M. Fluorescence Quenching by Photoinduced Electron Transfer: A Reporter for Conformational Dynamics of Macromolecules. ChemPhysChem 2009, 10, 1389–1398. [Google Scholar] [CrossRef]
- Akbar, R.; Baral, M.; Kanungo, B.K. Photoluminescence and Coordination Behaviour of Lanthanide Complexes of Tris (Aminomethyl)Ethane-5-Oxine in Aqueous Solution. J. Fluoresc. 2017, 27, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Thongyod, W.; Buranachai, C.; Pengpan, T.; Punwong, C. Fluorescence quenching by photoinduced electron transfer between 7-methoxycoumarin and guanine base facilitated by hydrogen bonds: An in silico study. Phys. Chem. Chem. Phys. 2019, 21, 16258–16269. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21. [Google Scholar] [CrossRef]

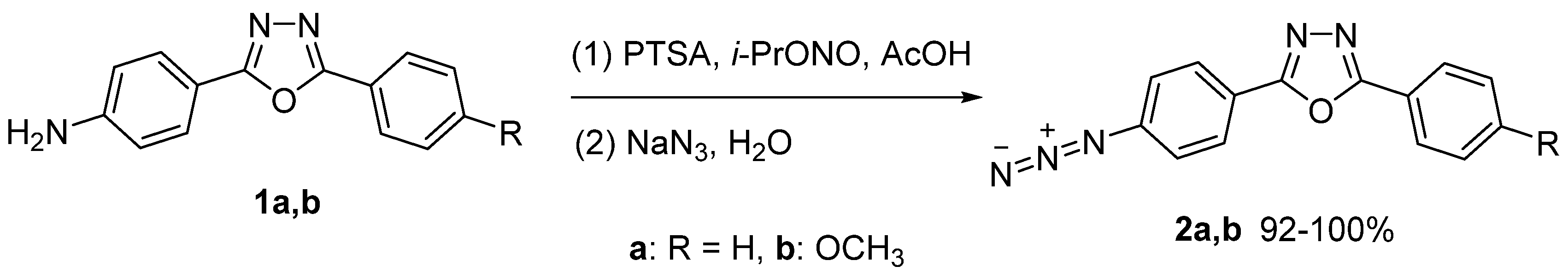
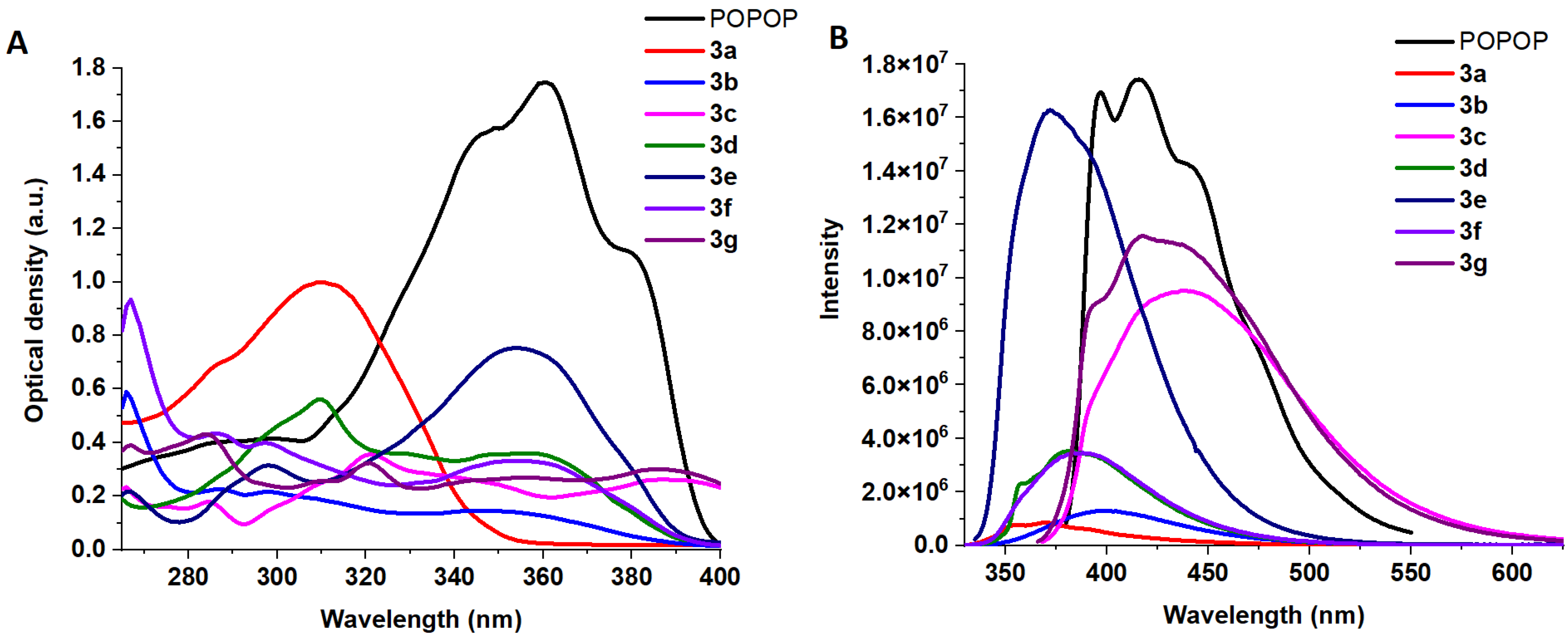
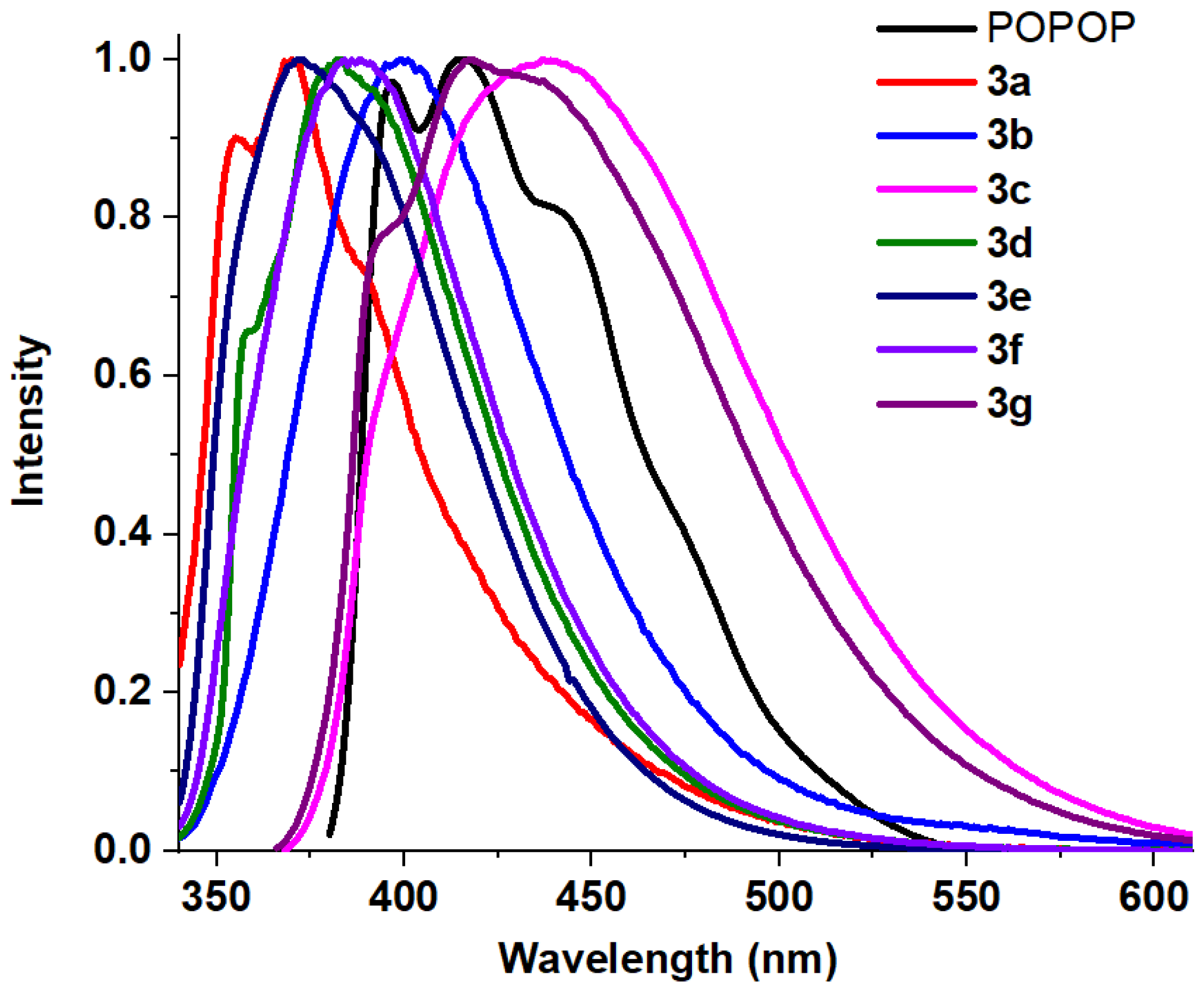
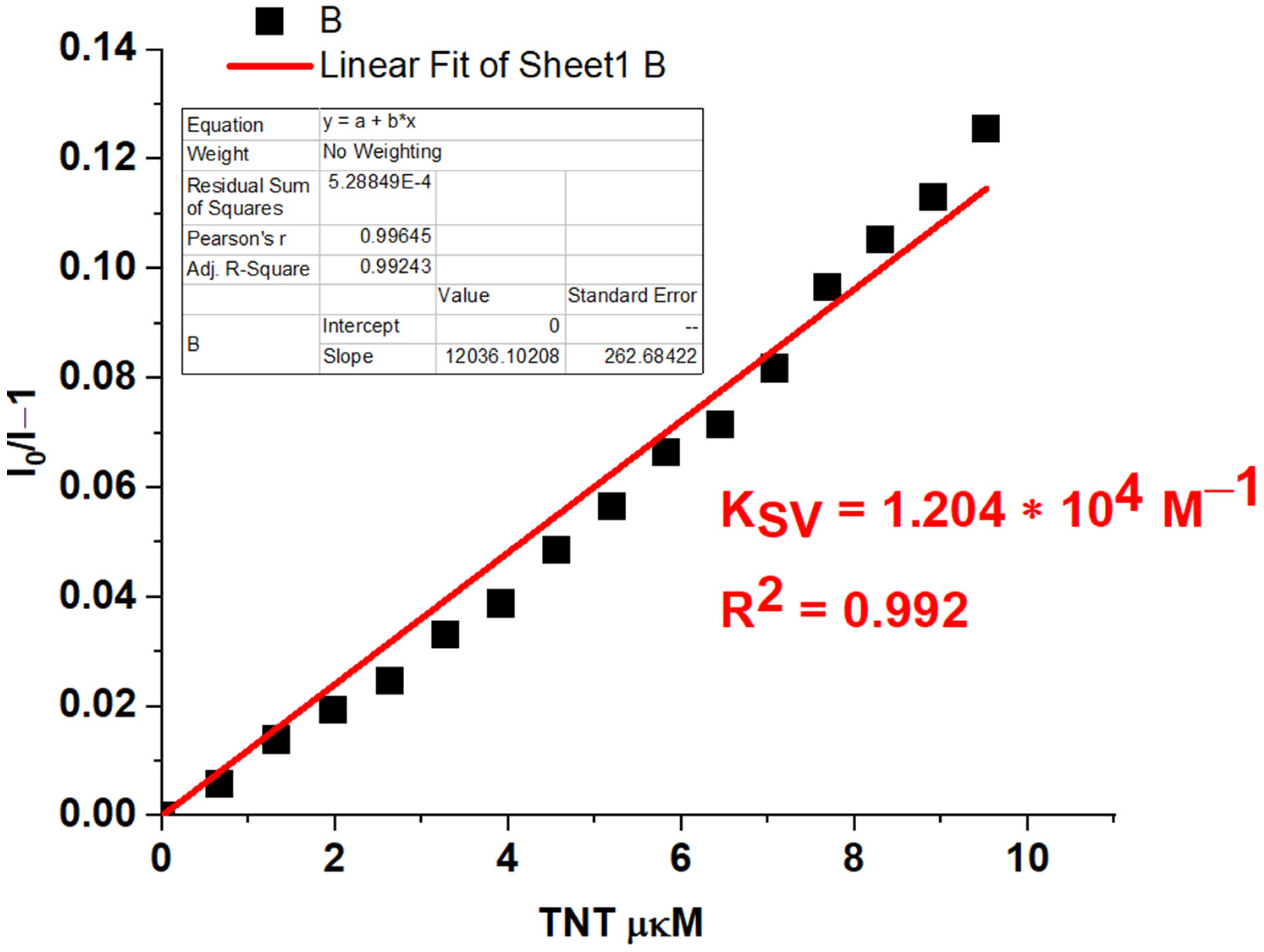


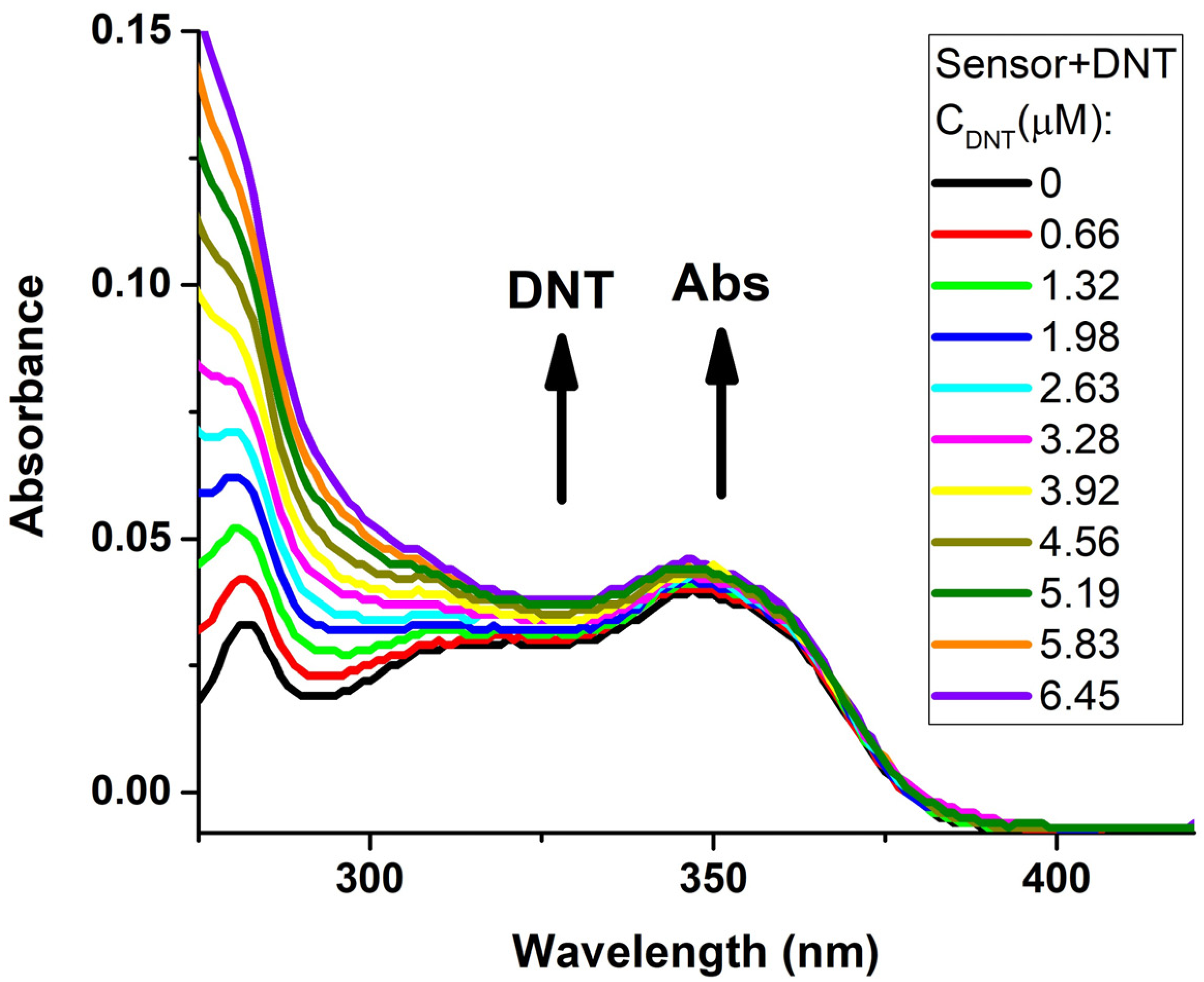
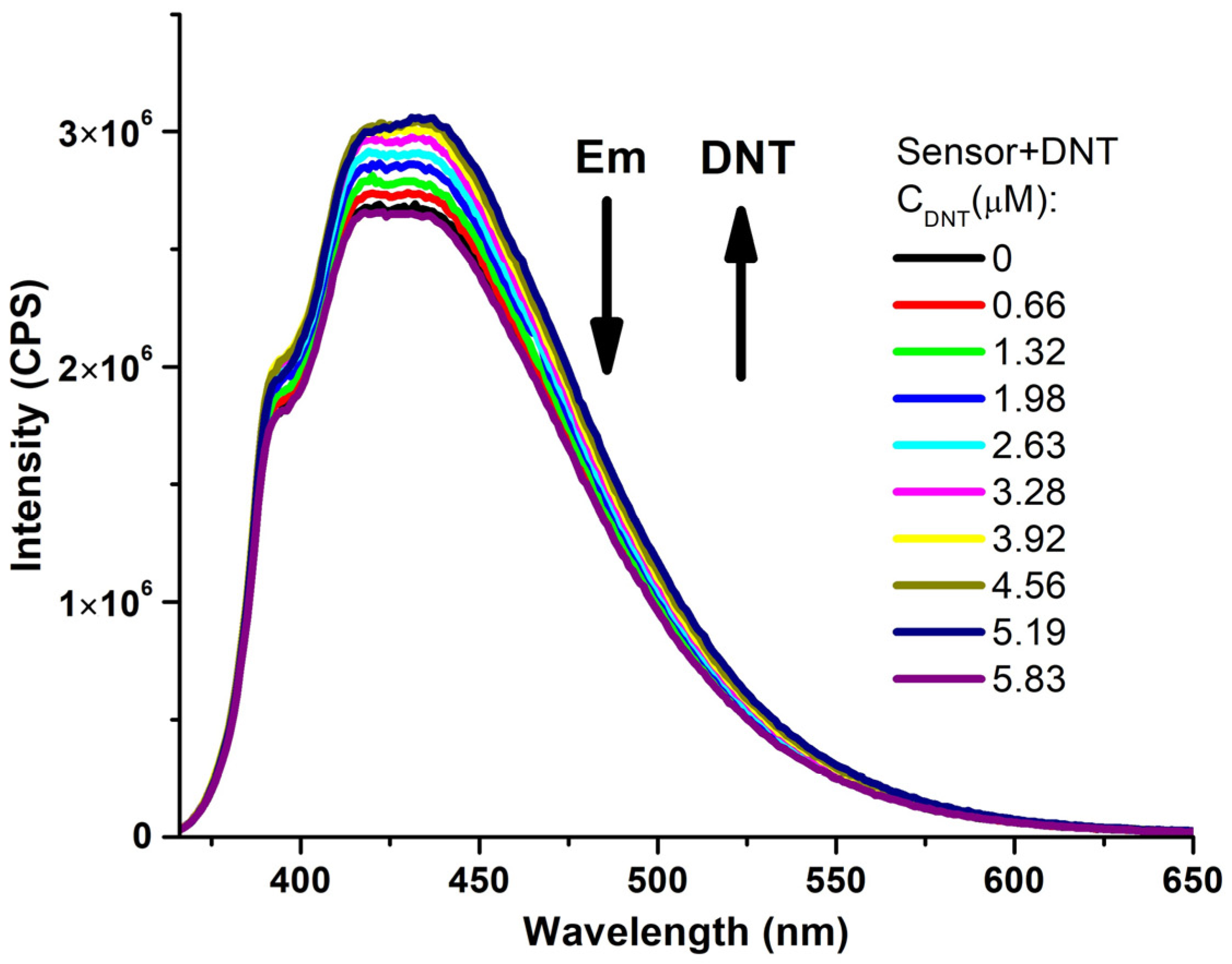

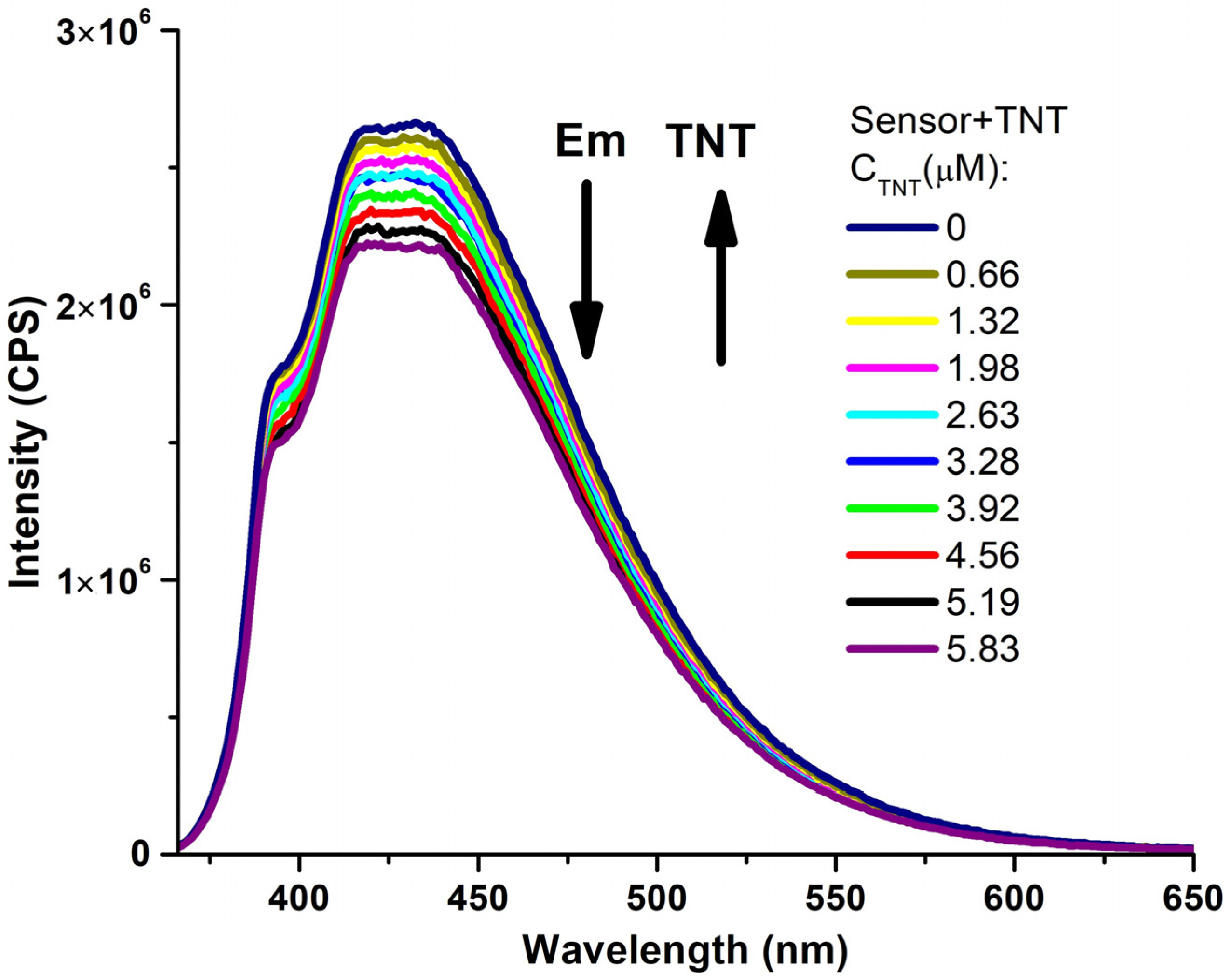
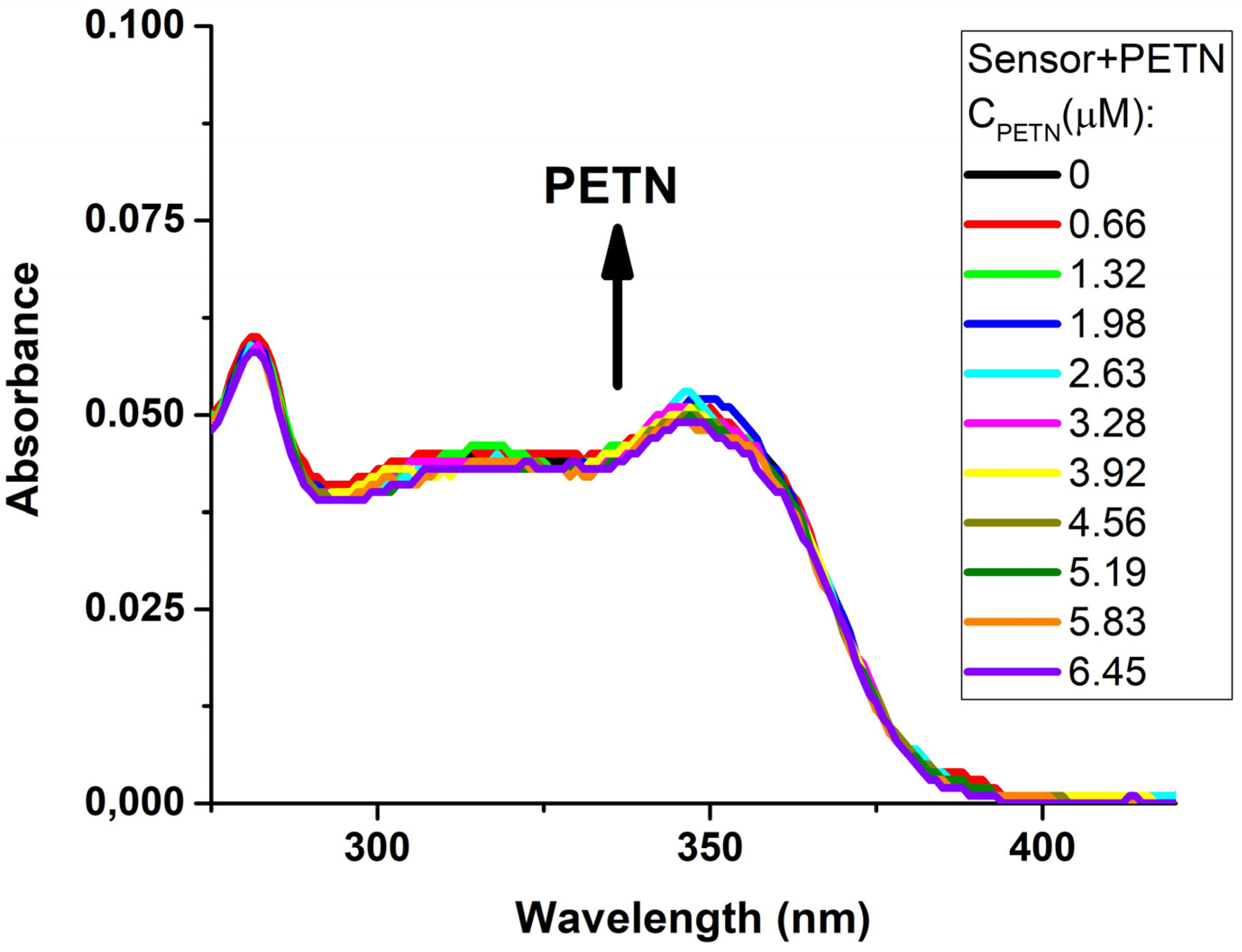
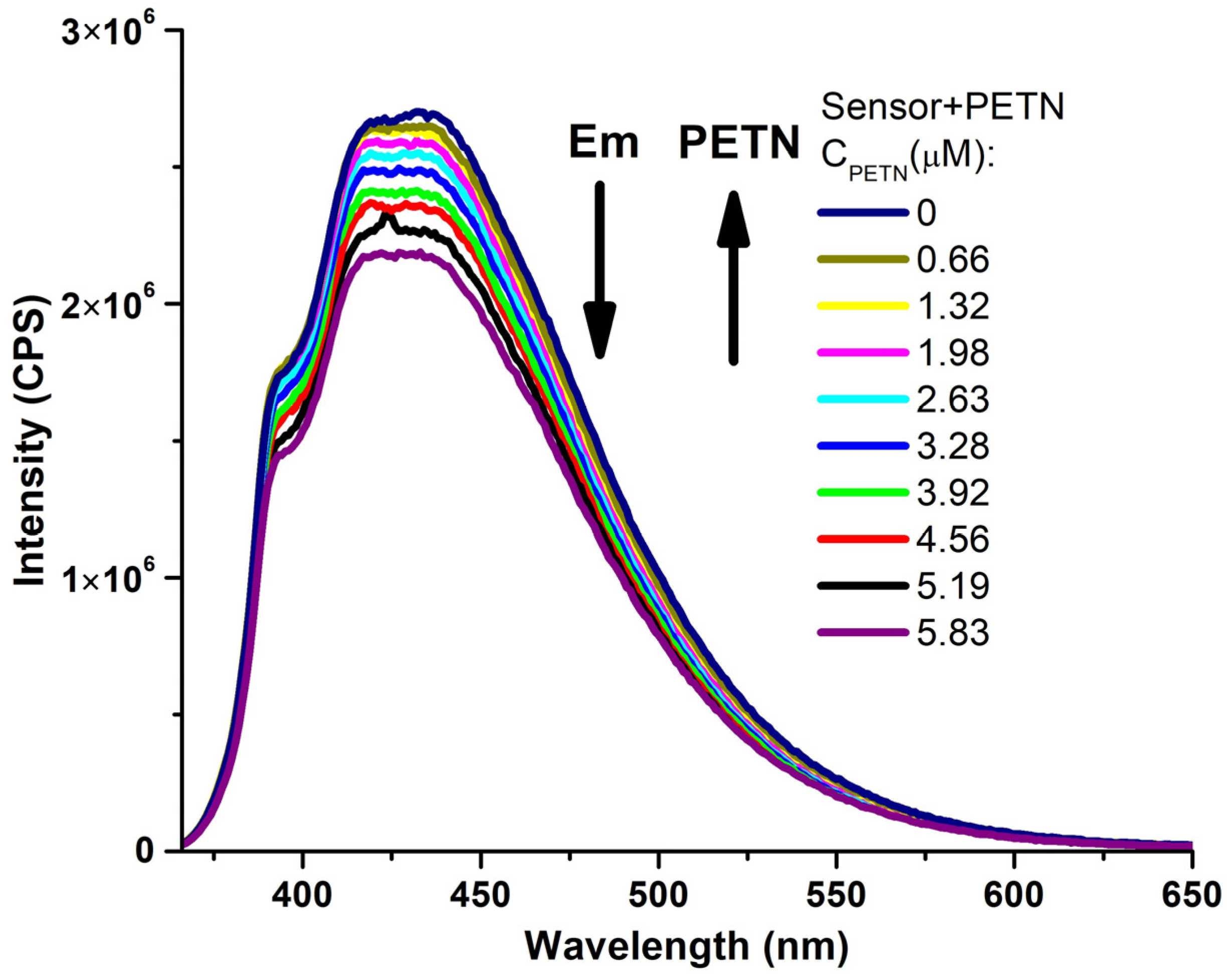
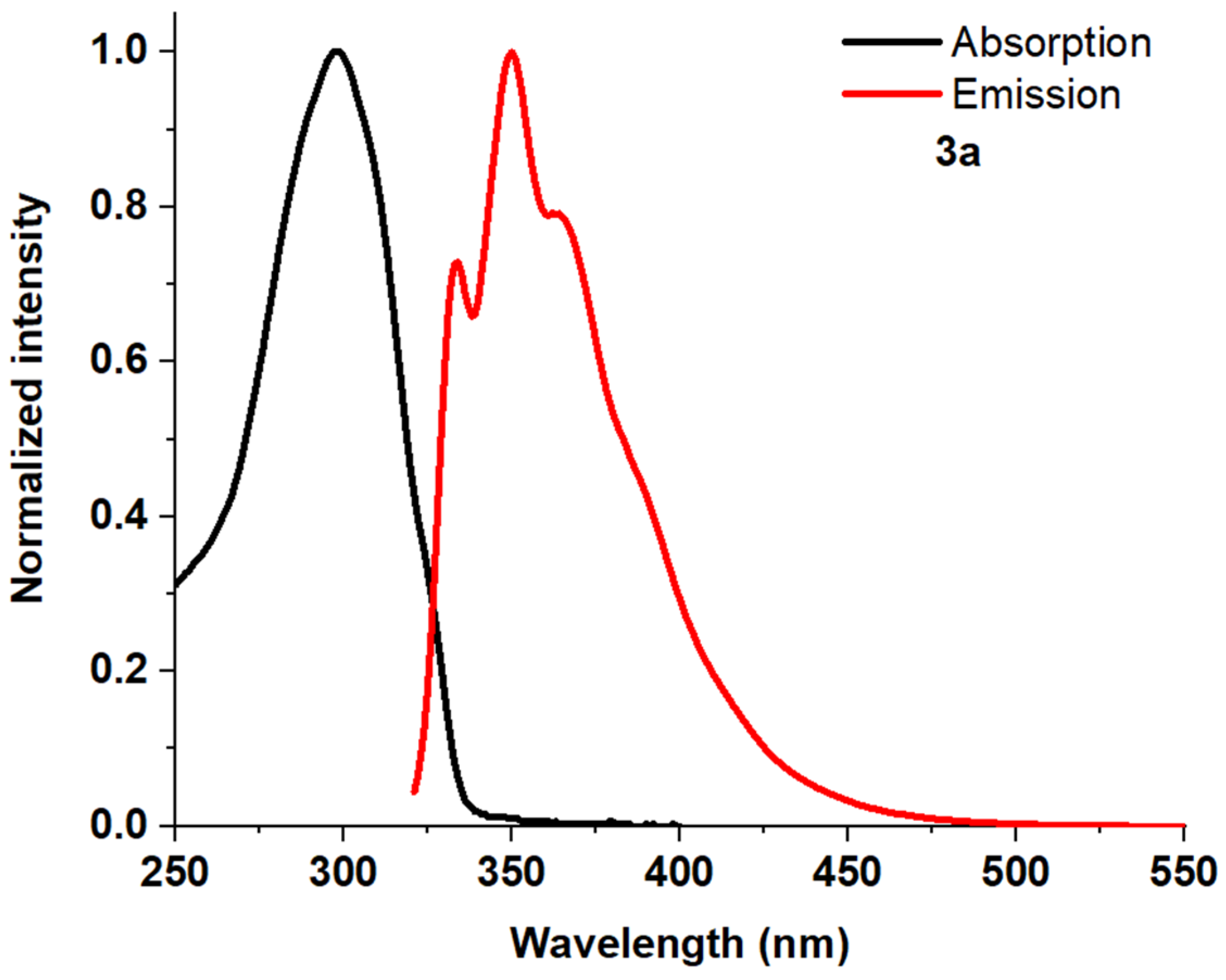
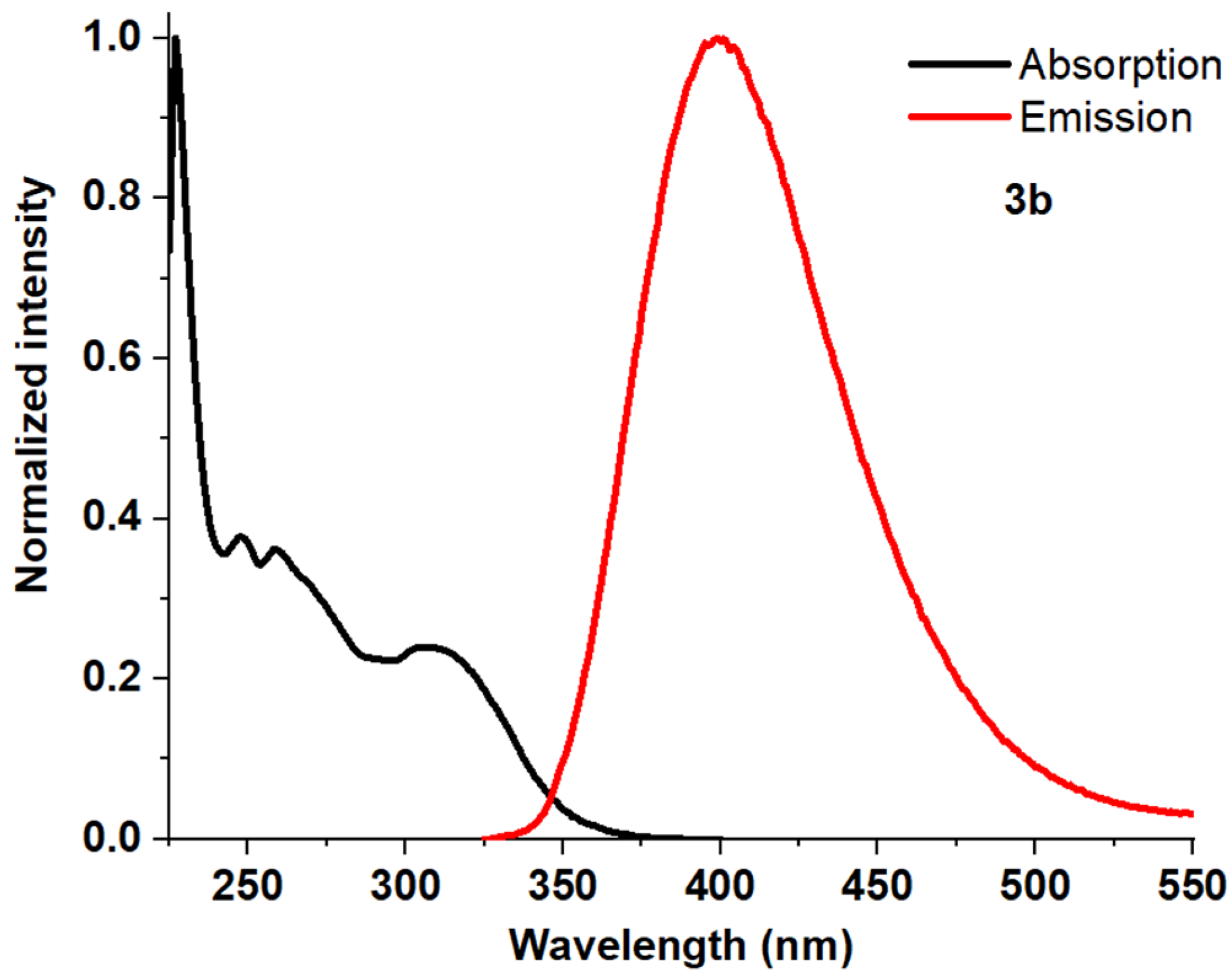
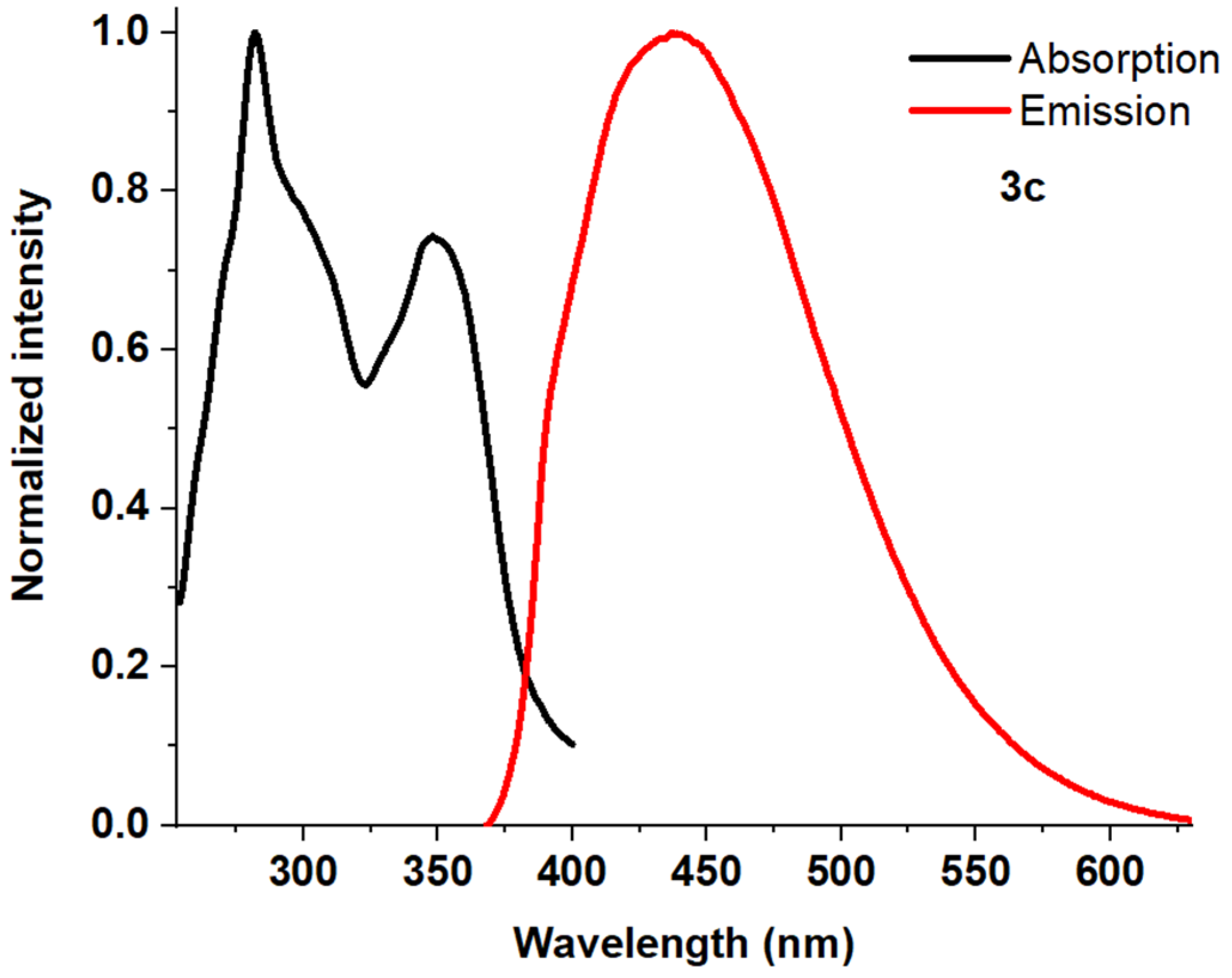
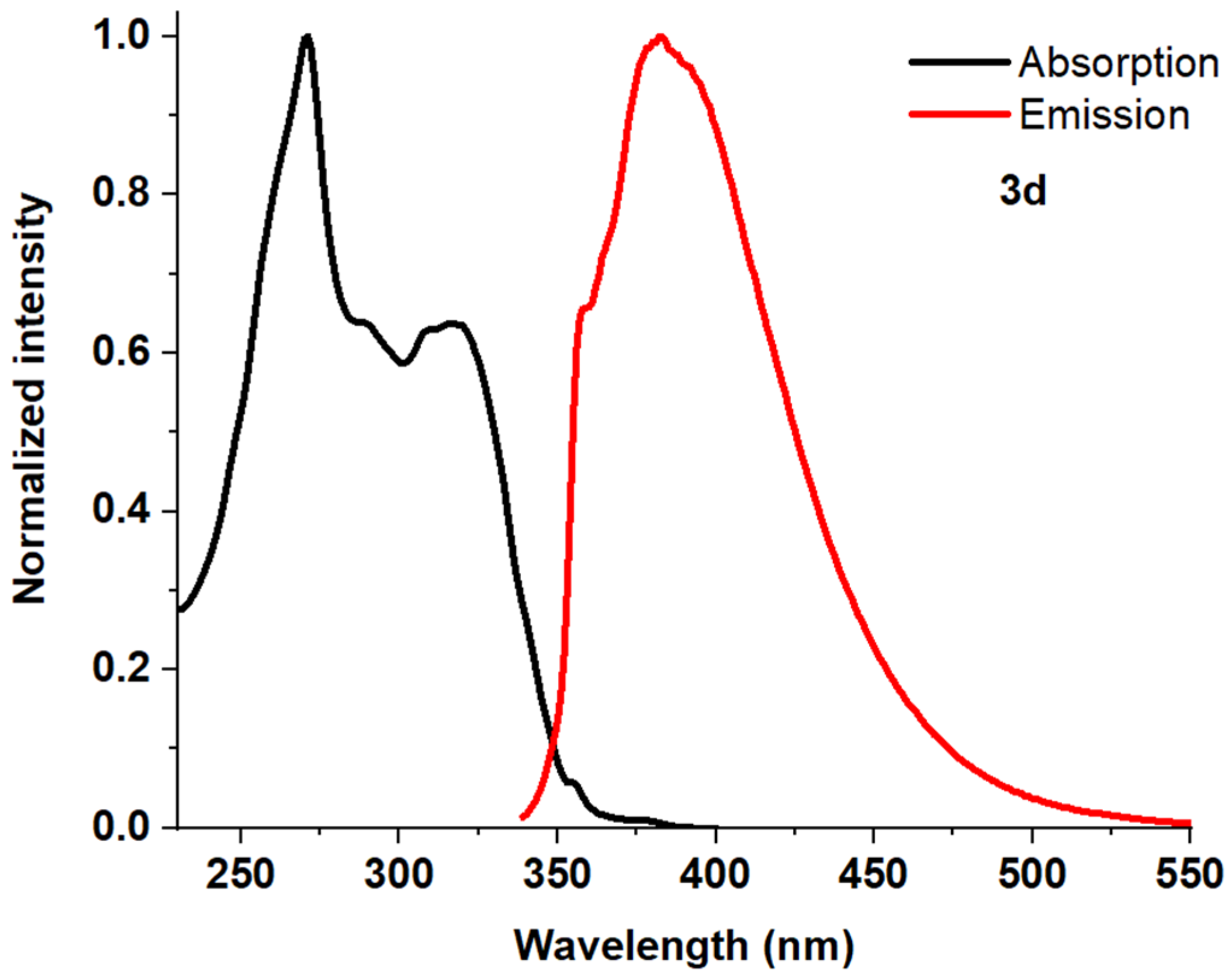
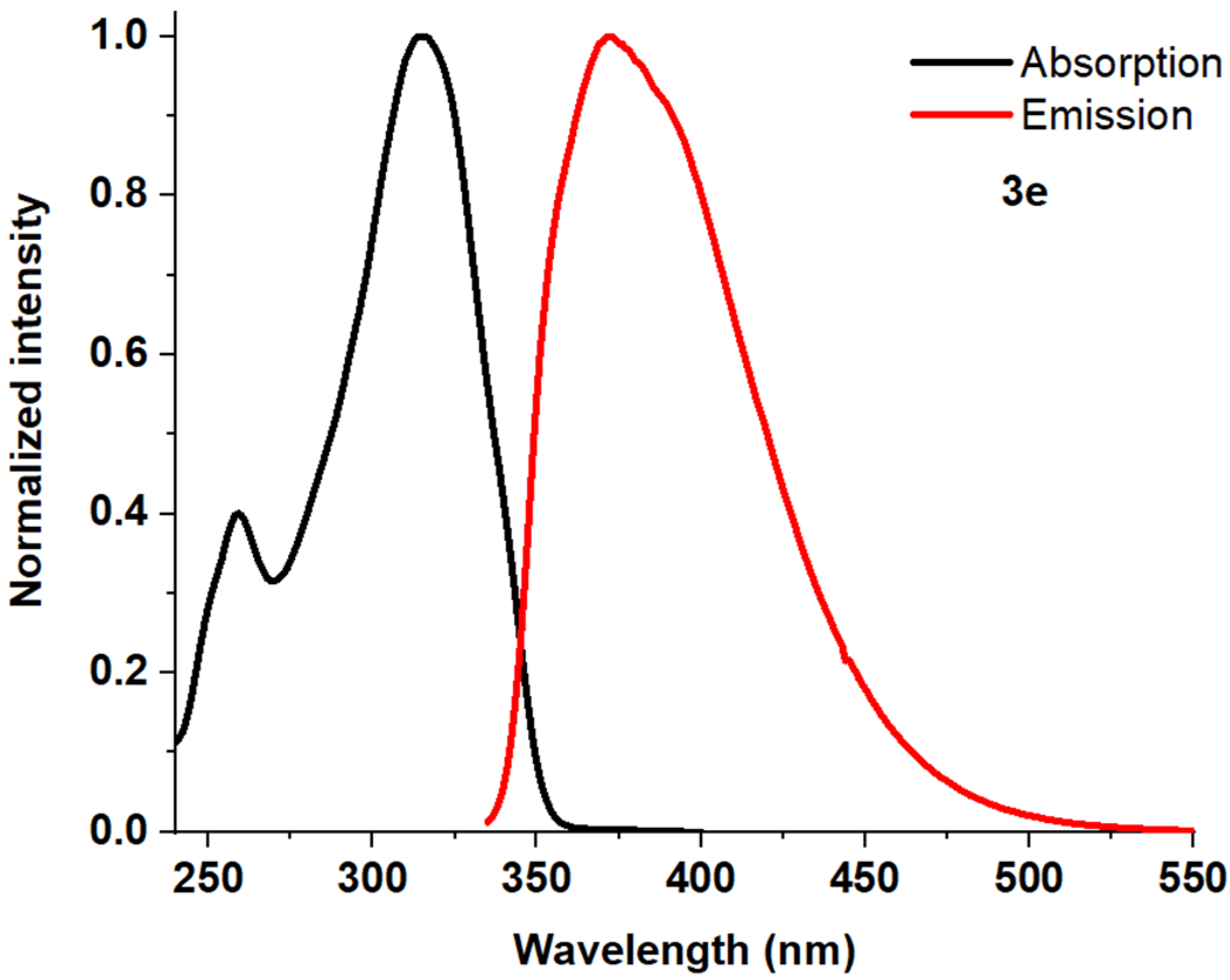
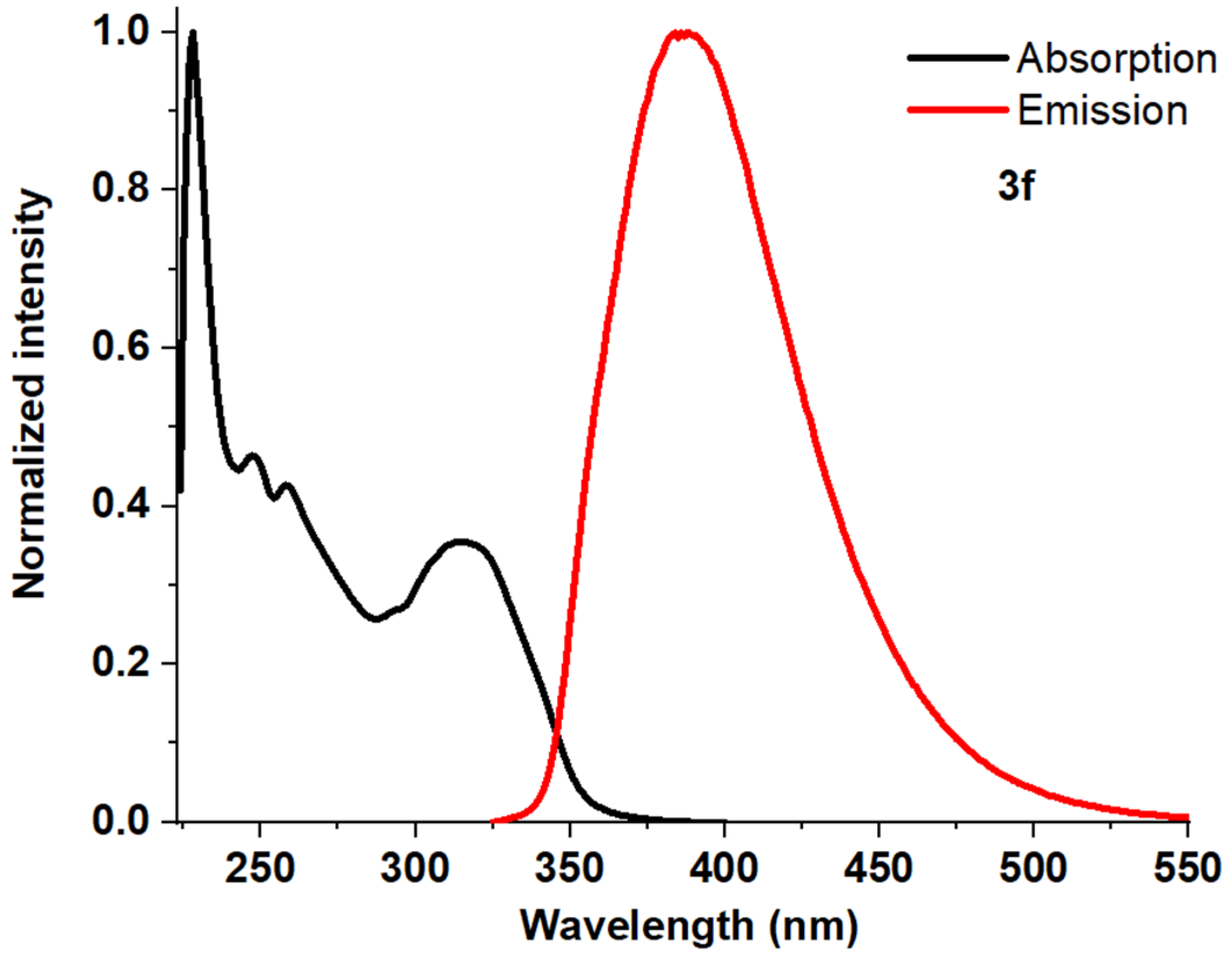

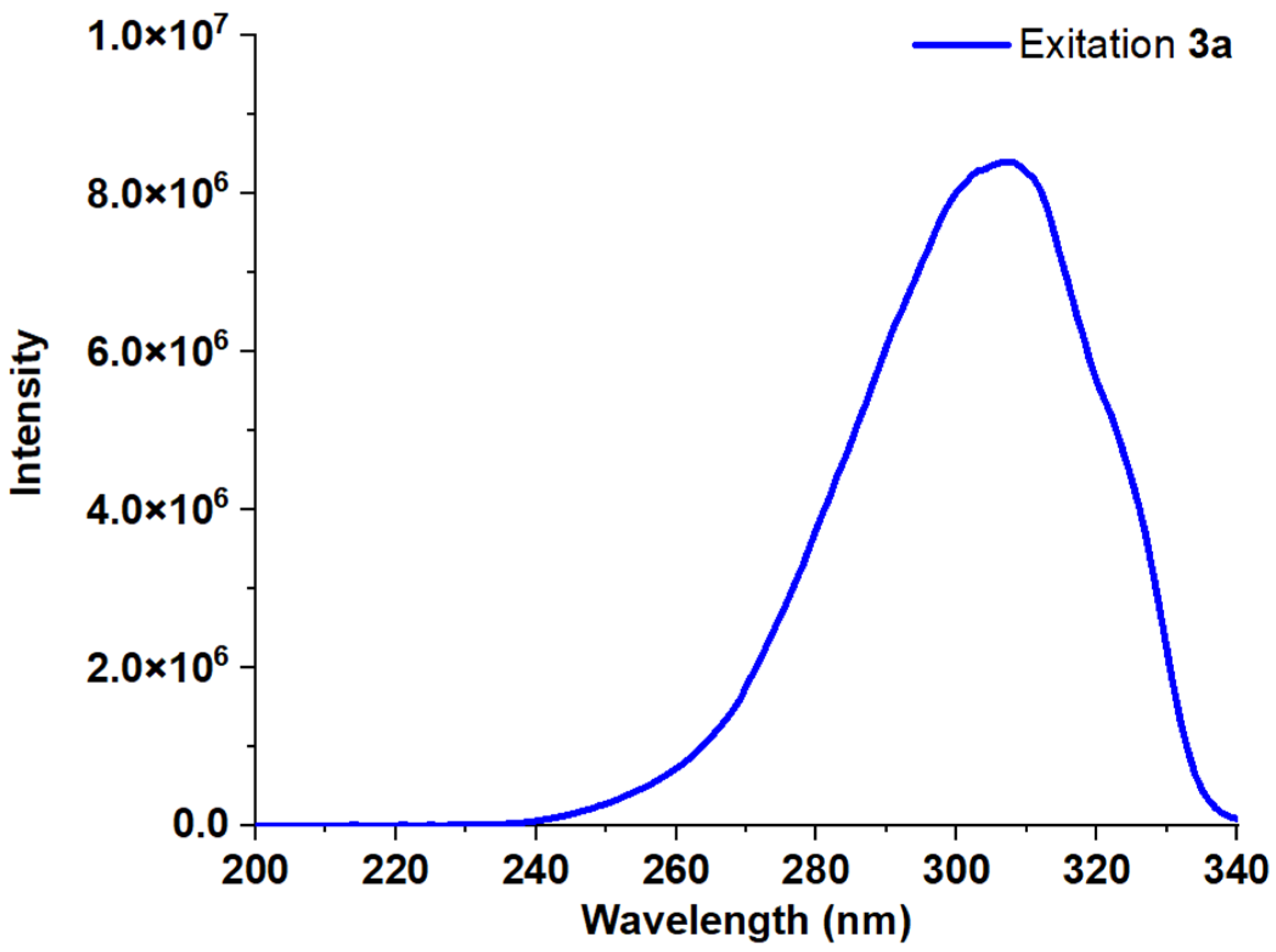
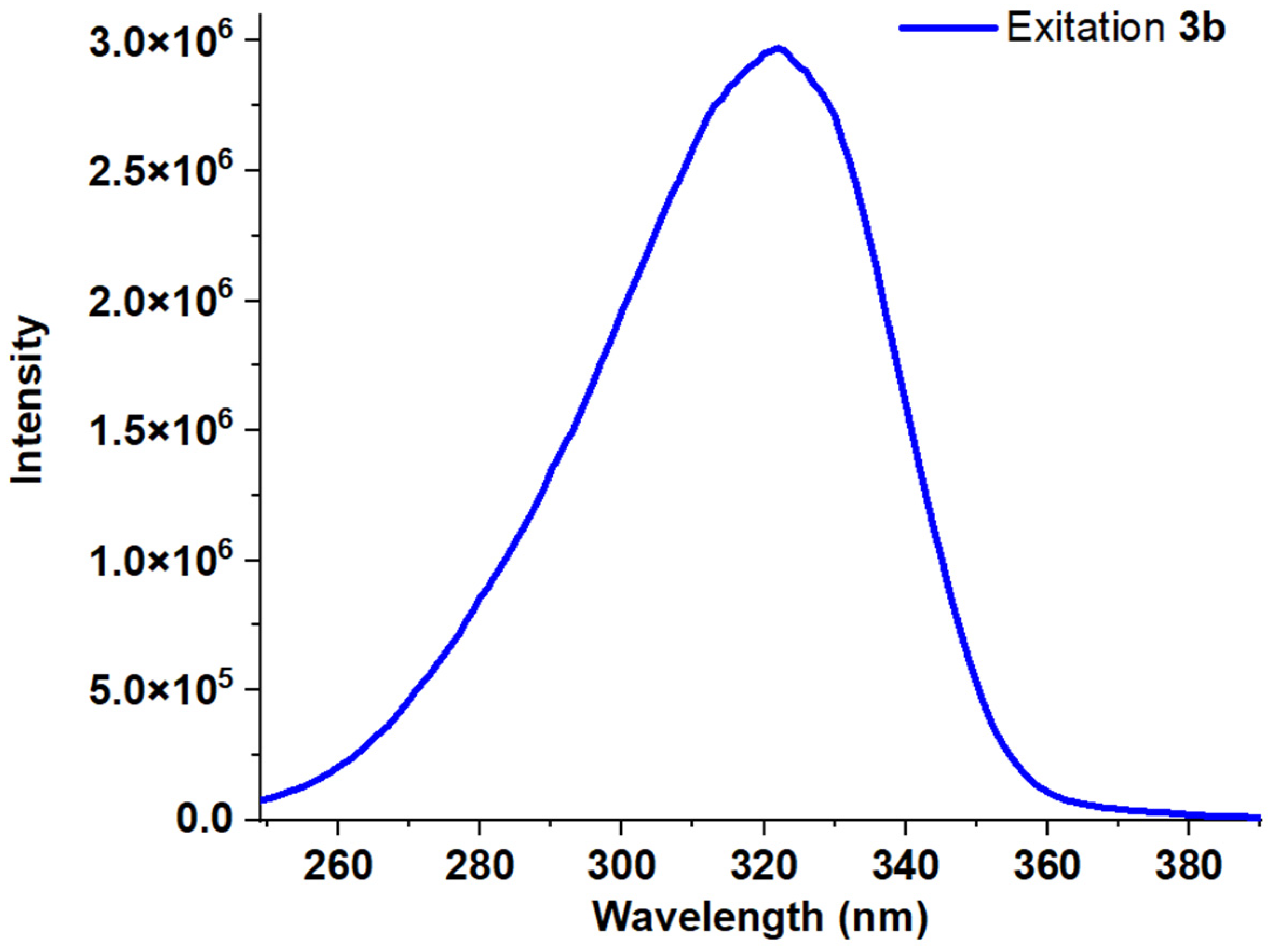
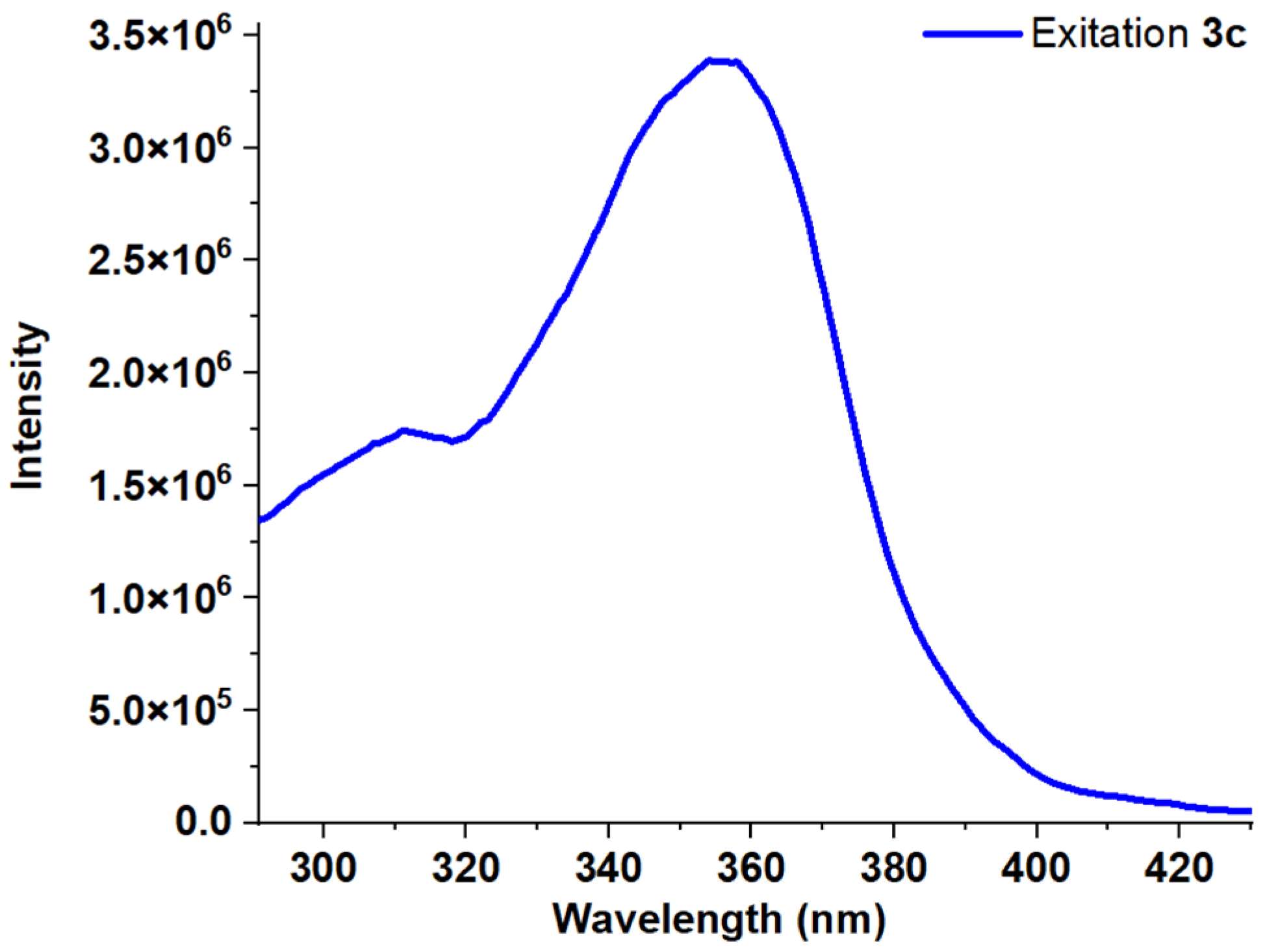
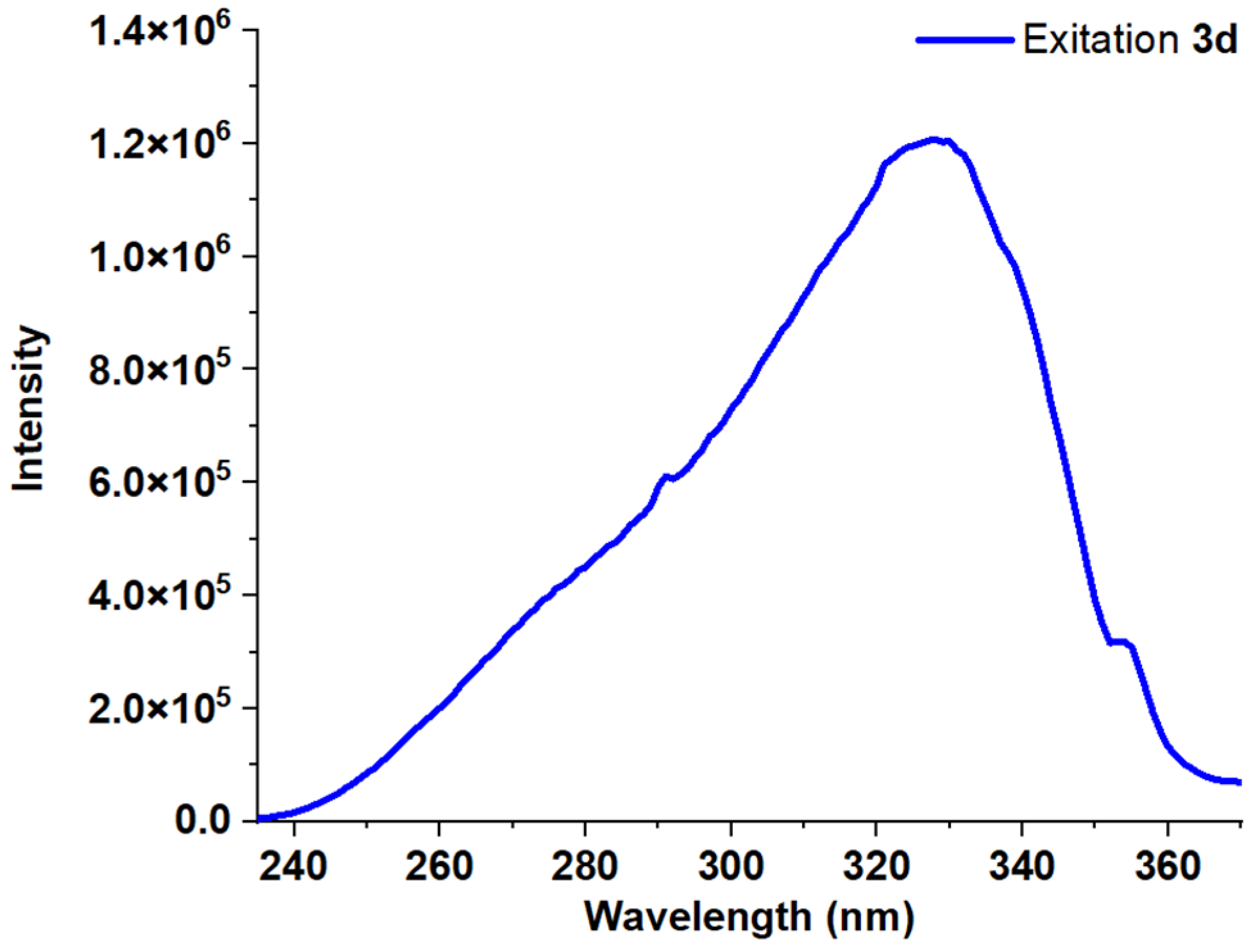


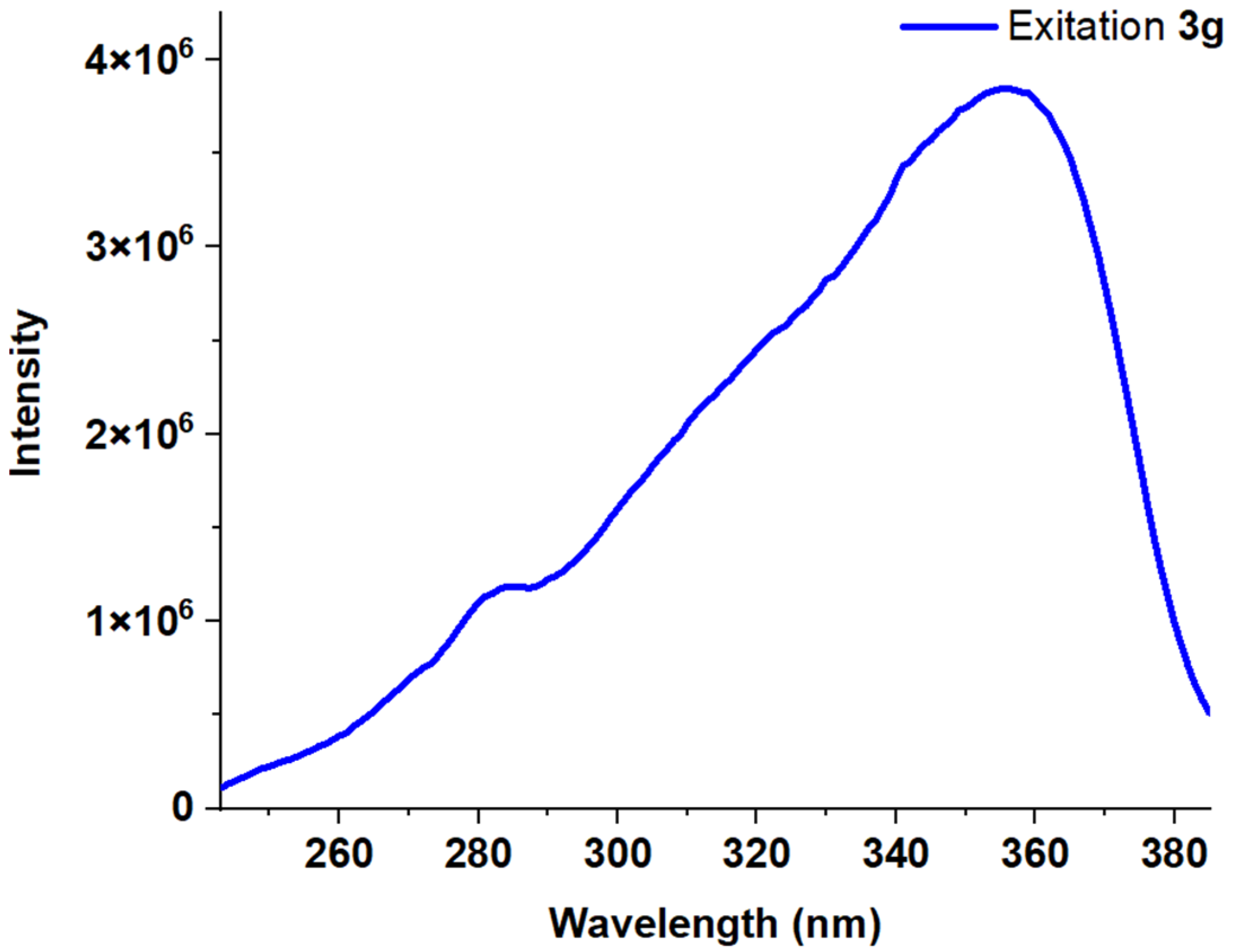
| Entry | Comp. | λabs max a, nm (εM, M−1 cm−1) | λem max b, nm | Stokes Shift, nm | τ, ns c | Φf, (%) d |
|---|---|---|---|---|---|---|
| 1 | POPOP | 346 (99,000) 361 (111,400) 384 (68,000) | 416 443 | 97 | 97.5 e | |
| 2 | 3a | 248 (43,300) 309 (61,800) | 356 370 | 47 | 17 | |
| 3 | 3b | 248 (22,500) 259 (21,600) 307 (14,500) | 398 | 150 | 0.66 | 48 |
| 4 | 3c | 245 (17,800) 282 (35,600) 348 (26,300) | 441 | 159 | 4.52 | 23 |
| 5 | 3d | 271 (56,200) 317 (35,900) | 382 | 111 | 1.22 | 30 |
| 6 | 3e | 259 (31,500) 315 (75,200) | 373 | 58 | 0.72 | 98.13 f |
| 7 | 3f | 228 (93,400) 247 (43,400) 258 (39,900) 315 (33,200) | 388 | 160 | 0.49 | 71 |
| 8 | 3g | 228 (39,100) 245 (43,200) 282 (32,400) 319 (26,900) 346 (29,900) | 393 (sh) 417 | 172 | 4.34 | 84 |
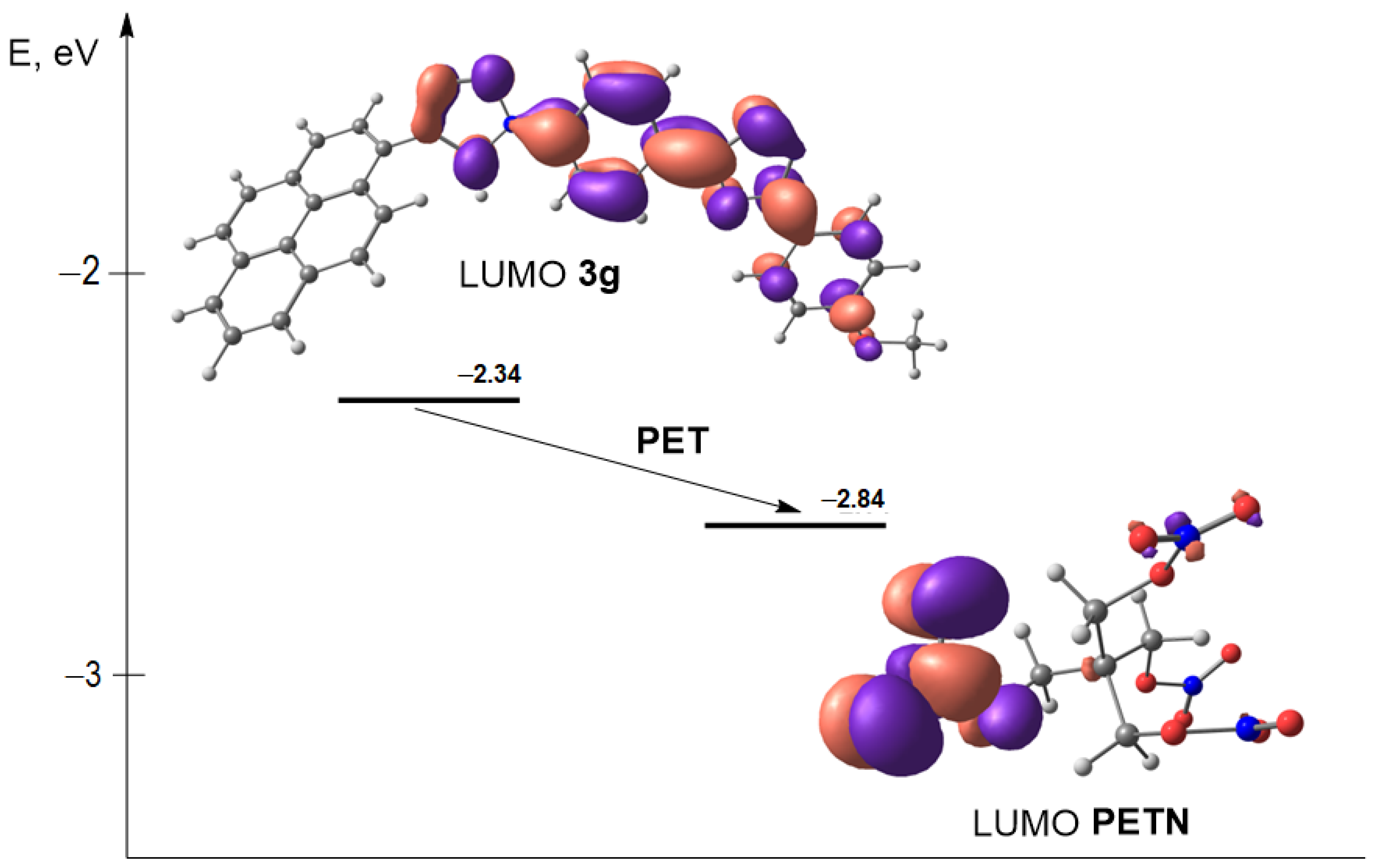
| Structure | HOMO Energy | LUMO Energy |
|---|---|---|
| 3g | −5.71 | −2.34 |
| PETN | −9.14 | −2.84 |
| Entry | Compound | τ1, ns a | α1 b | τ2, ns a | α2 b | τav c, ns a | χ2 d |
|---|---|---|---|---|---|---|---|
| 1 | 3a | 0.97 | 1.0000 | - | - | 0.97 | 1.08 |
| 2 | 3b | 0.54 | 0.8942 | 1.64 | 0.1058 | 0.66 | 1.22 |
| 3 | 3c | 1.55 | 0.1114 | 4.89 | 0.8886 | 4.52 | 1.15 |
| 4 | 3d | 0.89 | 0.9092 | 4.56 | 0.0908 | 1.22 | 1.23 |
| 5 | 3e | 0.72 | 1.0000 | - | - | 0.72 | 1.15 |
| 6 | 3f | 0.42 | 0.9458 | 1.68 | 0.0542 | 0.49 | 1.18 |
| 7 | 3g | 1.08 | 0.0672 | 4.58 | 0.9328 | 4.34 | 1.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, M.S.; Kovalev, I.S.; Slovesnova, N.V.; Sadieva, L.K.; Platonov, V.A.; Novikov, A.S.; Santra, S.; Morozova, J.E.; Zyryanov, G.V.; Charushin, V.N.; et al. Polyaromatic Hydrocarbon (PAH)-Based Aza-POPOPs: Synthesis, Photophysical Studies, and Nitroanalyte Sensing Abilities. Int. J. Mol. Sci. 2023, 24, 10084. https://doi.org/10.3390/ijms241210084
Mohammed MS, Kovalev IS, Slovesnova NV, Sadieva LK, Platonov VA, Novikov AS, Santra S, Morozova JE, Zyryanov GV, Charushin VN, et al. Polyaromatic Hydrocarbon (PAH)-Based Aza-POPOPs: Synthesis, Photophysical Studies, and Nitroanalyte Sensing Abilities. International Journal of Molecular Sciences. 2023; 24(12):10084. https://doi.org/10.3390/ijms241210084
Chicago/Turabian StyleMohammed, Mohammed S., Igor S. Kovalev, Natalya V. Slovesnova, Leila K. Sadieva, Vadim A. Platonov, Alexander S. Novikov, Sougata Santra, Julia E. Morozova, Grigory V. Zyryanov, Valery N. Charushin, and et al. 2023. "Polyaromatic Hydrocarbon (PAH)-Based Aza-POPOPs: Synthesis, Photophysical Studies, and Nitroanalyte Sensing Abilities" International Journal of Molecular Sciences 24, no. 12: 10084. https://doi.org/10.3390/ijms241210084
APA StyleMohammed, M. S., Kovalev, I. S., Slovesnova, N. V., Sadieva, L. K., Platonov, V. A., Novikov, A. S., Santra, S., Morozova, J. E., Zyryanov, G. V., Charushin, V. N., & Ranu, B. C. (2023). Polyaromatic Hydrocarbon (PAH)-Based Aza-POPOPs: Synthesis, Photophysical Studies, and Nitroanalyte Sensing Abilities. International Journal of Molecular Sciences, 24(12), 10084. https://doi.org/10.3390/ijms241210084









