Endothelial Cells and Mitochondria: Two Key Players in Liver Transplantation
Abstract
1. Introduction
2. Mitochondria and Endothelial Cells under Physiological Conditions
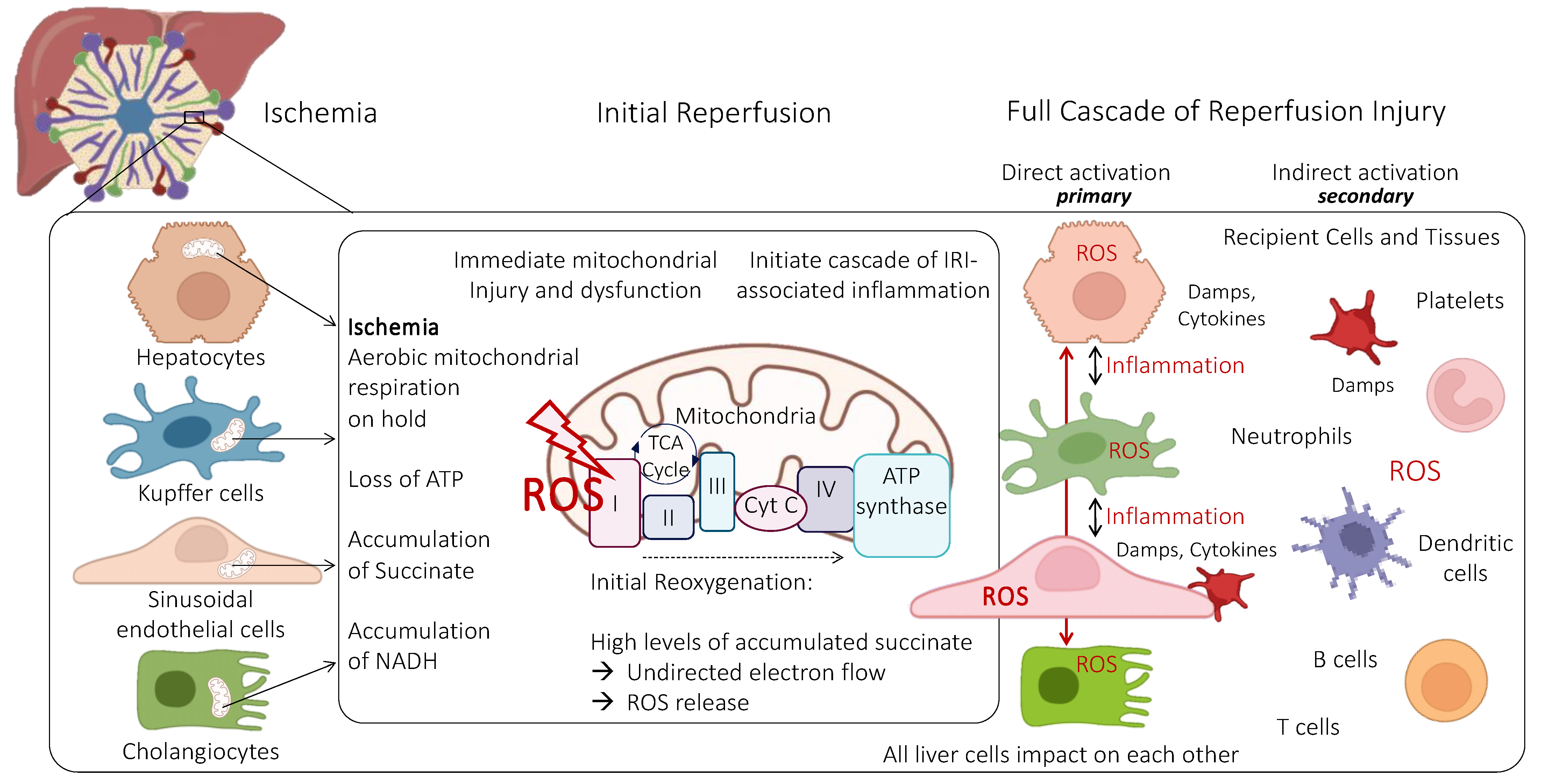
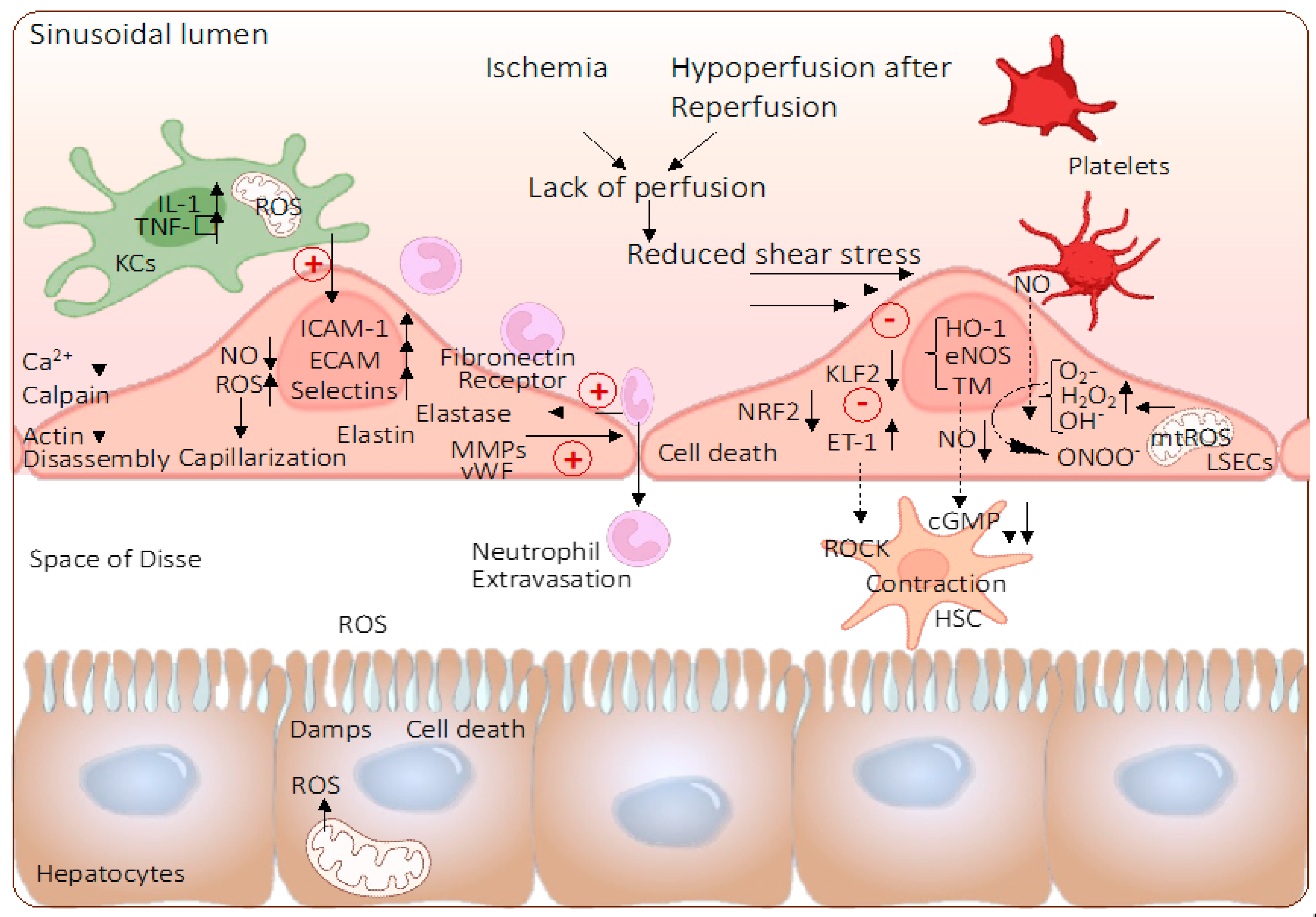
3. Role of Mitochondria and Endothelial Cells during Ischemia-Reperfusion Injury
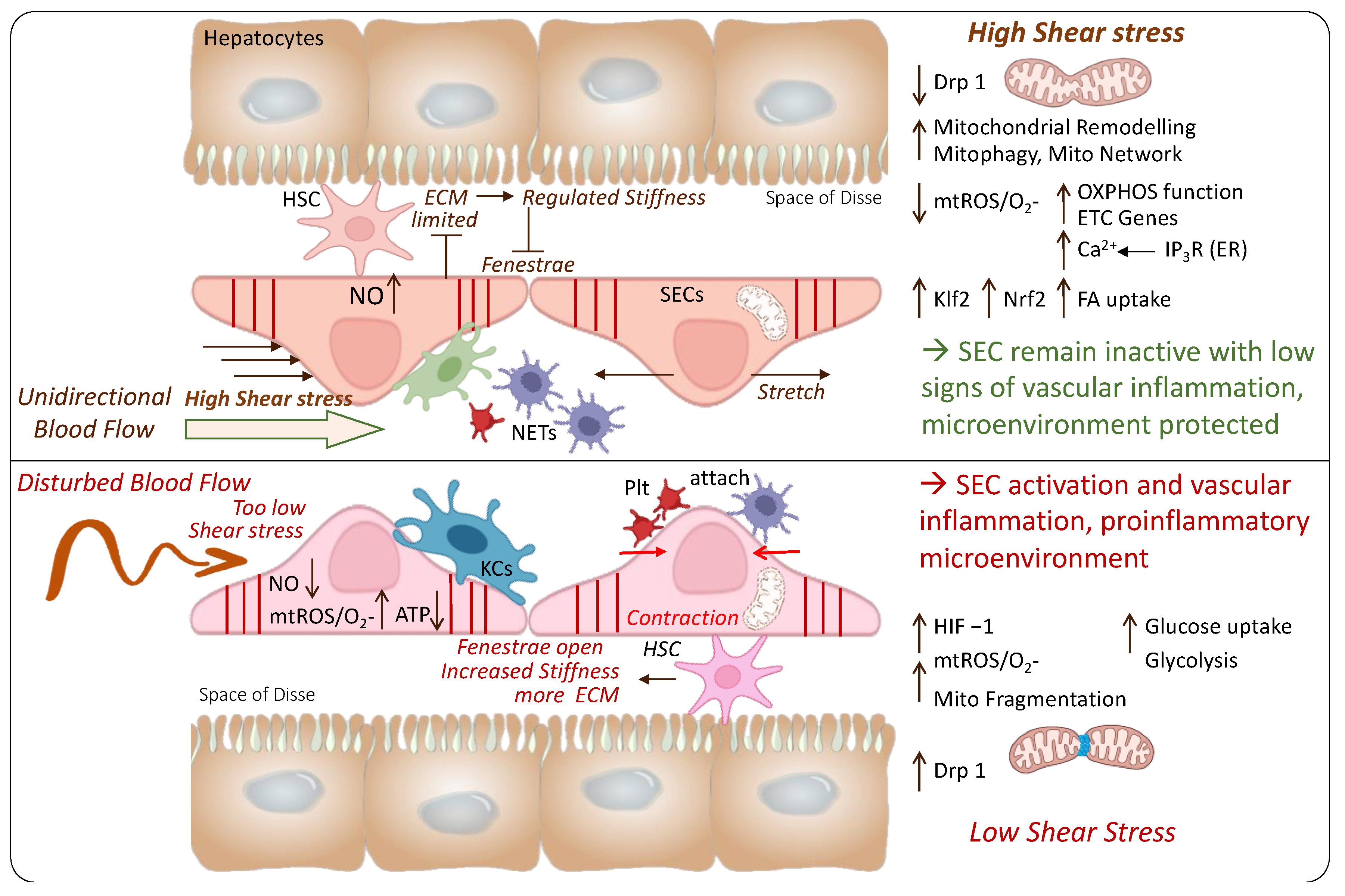
4. The Role of Endothelial Shear Stress
5. Effect of Machine Perfusion on Mitochondria and Endothelial Cells
5.1. Hypothermic Oxygenated Perfusion (HOPE)
5.1.1. Hypothermic Machine Perfusion and Mitochondria
5.1.2. The Effect of Hypothermic Machine Perfusion on Liver Endothelial Cells
5.1.3. The Role of Perfusion Pressure, Shear Stress, and Flow
5.1.4. The Role of Perfusion Duration
| Author, Year, Country | Number and Species | Liver injury Model, Study Groups | Warm Ischemia | Cold Ischemia before Perfusion | Perfusion Conditions | Active Perfusate Oxygenation | Perfusion Duration | Single/ Dual | Transplantation or NMP | Main Findings | Discussion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasuri et al., 2022, Italy [75] | Human livers, n = 47 | ECD-DBD, HOPE (n = 34), SCS only (n = 13) | none | 6.85 h | Pressure controlled Pressure: PV: 5 mmHg Belzer MP solution (modified UW) | Yes | n.a. | Single (PV) | Transplantation (samples from RCT) | Less EAD in HOPE group (8.8% vs. 30.1% after SCS), LSEC endothelization correlates with HOPE duration | ECD DBD grafts, with transplantation, no real comparison to control group, limited number of markers |
| Bochimoto et al., 2022, Japan [89] | Porcine, n = 6 | DCD, HOPE (n = 3) vs. HOPE plus rewarming to 22 °C (n = 3) | 60 min | none (minimal) | Flow controlled, Flow: PV: 0.22 mL/min/g HA: 0.06 mL/min/g, Temperature: 8 °C, Modified UW solution | Yes, pO2 200–300 mmHg | 4 h | dual | No | HOPE superior to SCS but inferior to rewarming, | Flow controlled, no cold storage control group, compared to controlled rewarming (8–22 °C), no quantitative endpoint analysis |
| De Vries et al., 2021, The Netherlands [74] | Porcine (n = 6 each group) | DCD livers, HOPE vs. D-HOPE | 30 min | SCS duration n.a. | Pressure controlled, Pressure: PV: 5 mmHg, HA: 25 mmHg, Belzer MP solution (modified UW) | Yes, 50–80 kPa (375–600 mmHg). | 2 h | Single and dual | NMP (4 h) | No difference between single and dual HOPE, both techniques protect endothelial cells and other liver tissue cells. | No transplantation |
| Brüggenwirth et al., 2020, The Netherlands [95] | Porcine (n = na), human (n = 2) | DCD, 2, 6, 24 h (pigs) DHOPE vs. 24 h SCS, human: 20 h HOPE | 30 min (pig) | 2 h (porcine), 8–11 h (human) | Pressure controlled, Pressure: PV: 5 mmHg, HA: 25 mmHg, Belzer MP solution (modified UW) | Yes, 50–80 kPa (375–600 mmHg). | 2, 6, 24 h (porcine), 20 h (human) | dual | NMP (3 or 4 h) | Prolonged D-HOPE is safe and protects endothelial cells and other liver cells, being viable after NMP following 24 h of HOPE | No transplantation, only 2 human livers |
| Kanazawa et al., 2019, Japan [79] | Porcine livers, n = 15 | DCD + SCS, HOPE (n = 5), HOPE plus rewarming (n = 5) | 60 min | None (minimal) | Pressure controlled, Pressure: PV: 3–5 mmHg; HA: 30–50 mmHg, Temperature: 8 °C | Yes, pO2 200–300 mmHg | 4 h | dual | 2 h NMP | Less intra-sinusoidal platelet aggregation, inflammation, more ATP with HOPE | No transplant model but normothermic ex-situ reperfusion |
| Kron et al., 2018, Switzerland [77] | Human, n = 6, rat, n > 32 | Severe macrosteatosis, HOPE, deoxygenated (HNPE), SCS (n = 8 each) | No | 12 h | Pressure controlled Pressure: PV: 3 mmHg Belzer MP solution (modified UW) | Yes, 60–80 kPa | 2 h | Single (PV) | Transplantation | SEC-protection through HOPE, sign. lower MMP3&8, vWF, ET-1, TIMP, CXCL5&10 expression, better survival, reduced liver injury, better graft survival (human) with HOPE | Only 6 severely steatotic human livers |
| Burlage et al., 2017, The Netherlands [80] | Discarded human livers, n = 18 | DCD (n = 15), DBD (n = 3) | n.a. | 7.18 h | Pressure controlled, pressure: PV: 5 mmHg, HA: 25 ± 5 mmHg, Belzer MP solution (modified UW) | Yes, 50–80 kPa (375–600 mmHg). | 2 h | dual | 6 h NMP | better endothelial cell function through up regulation of mechano-sensitive/cytopro-tective genes, better PV and HA flow, less expression of ET, sign. higher expression of NO, TM, eNOS, HIF-1α, VEGFa, HO-1 with HOPE; | No transplantation but reperfusion and liver evaluation during 6 h of NMP |
| Op den Dries et al., 2014, The Netherlands [78] | Porcine, n = 18 | SCS vs. HOPE (n = 9 each), DCD | 30 min asystolic DWIT | None (minimal) | Pressure controlled Pressure: PV: 5 mmHg, HA: 25 ± 5 mmHg Belzer MP solution (modified UW) | Yes, 100% | 2 h | Dual | 2 h NMP | HOPE prevents arteriolonecrosis of the peribiliary vascular plexus and protects SEC as shown with less caspase 3 positive SECs | No transplantation, 2 h NMP |
| Schlegel et al., 2014, Switzerland [83] | Rat, n = 64 | DCD with SCS and HOPE or NMP with transplantation | 30 or 60 min asystolic DWIT | 5 h | Pressure controlled Pressure: PV: 3 mmHg Belzer MP solution (modified UW), 8 °C | Yes, >80 kPa | 2 h | Single (PV) | Transplantation | SEC protection through HOPE, sign. lower MMP3&8, TIMP, CXCL10 expression, better survival, reduced liver injury, better survival (human) | Rodent model |
| Schlegel et al., 2013, Switzerland [82] | Porcine, n=46 | DCD with SCS and HOPE, low (3 mmHg) and high (8 mmHg perfusion pressure, +/−oxygen | 60 min | 7 h | Pressure controlled Pressure: PV: 3 or 8 mmHg Belzer MP solution (modified UW), 8 °C | Yes, >80 kPa (HOPE group), deoxygenated (HNPE) | 2 h | Single (PV) | Reperfusion (NMP) | More injury with high pressure HOPE, particularly liver SECs. High pO2 protected mitochondria better with more energy reloading | No transplantation, high injury, different pressures, no dual perfusion as comparator cohort |
| Henry et al., 2012, USA [76] | Human, n = 33 | ECD-DBD; SCS (n = 15), HMP (n = 18) | n.a. | 5.1 h | Flow controlled, 0.667 mL flow /g liver/min, pressure: PV: 2.9 ± 0.08 mmHg, HA; 5.5 ± 0.15 mmHg, temperature: 4–8 °C; | Not active | 4.3 ± 0.9 h | dual | Transplantation | Tissue samples (PCR) demonstrated HMP-protection including SECs, sign. Less expression of 7 adhesion molecules (seven adhesion molecules: CXCL14, CCL21, CXCL1, ICAM-1, P-Selectin, MCP-1, SDF-1a) | No active oxygenation |
| T’Hart et al., 2006, The Netherlands [81] | Rat, n = 27 | Perfusion pressure 2/12.5, 4/25 or 8/50 mmHg (PV, HA), n = 3 (1 h HOPE), n = 6 each (24 h HOPE) | DBD with SCS or HOPE (different pressures) | None | Pressure controlled Pressure: PV: 2–8; HA: 12.5–50 mmHg, Belzer MP solution | Yes, 100% | 1 h and 24 h | Dual | No | PV and HA pressure of 4 and 25 mmHg achieved good cortical perfusion, with minimal endothelial cell death & ATP reloading, higher pressures injured the organ, too low pressures resulted in hypoperfusion | Not transplanted or reperfused, DBD only, HOPE instead, of cold storage, no group with PV perfusion only |
5.2. Subnormothermic Machine Perfusion (SNMP)
5.3. Normothermic Machine Perfusion (NMP)
| Author, Year, Country | Number & Species | Liver Injury Model, Study Groups | Warm Ischemia | Cold Ischemia before Perfusion | Perfusion Conditions | Active Perfusate Oxygenation | Perfusion Duration | Single/ Dual | Transplantation | Main Findings | Discussion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kakizaki, 2018, Japan [103] | Rat, n = 20 | DCD perfused with SNMP vs. DBD | 30 min | 6 h | 23–26 °C | Oxygen pressure inflow: 500–550 mmHg, outflow 150 mmHg. | 15/30, 60, 90 min | Dual | No | Improved portal flow, bile production, decreased liver enzymes, cytokines, and increased ATP. SNMP alleviated liver SECs and hepatic microvasculature. | No transplant model, no other perfusion technique as comparison |
| Okamura 2017, Japan [104] | Rat | Induced fatty DBD | None | 10 min | 22.5 °C, pressure PV < 3 mmHg, HA 47 mmHg | Yes, pO2 150 mmHg | 4 h | Dual | No | Less release of ALT, mitochondrial glutamate dehydrogenase. ATP, bile production, HMGB1, lipid peroxidation, and glutathione significantly improved by SNMP. Electron microscopy showed improved sinusoidal microvasculature and hepatocellular mitochondria. | No transplant model, no other perfusion technique as comparison |
| Furukori, 2016, Japan [107] | Pigs, n = 8 | DCD with HMP vs. SNMP | 60 min | None | 23 °C | Yes | 4 h | Dual | No | Lower tissue IL-1b and IL-6 expression with SNMP. | No transplant model, no comparison to other perfusion technique |
| Goldaracena, 2016, Canada [102] | Pig, n = 15 | DBD with SNMP vs. NMP vs. SCS | None | 2 h | 33 °C, pressure HA 60 mmHg, PV 2–4 mmHg | Yes | 4 h | Dual | Yes | SNMP reduced IL-6, TNF-α, and galactosidase levels and increased IL-10 levels. After LT, SNMP had lower AST and bilirubin levels. Decreased hyaluronic acid, as a marker of improved endothelial cell function. | Addition of anti-inflammatory strategies improves livers during NMP, transplant model, porcine livers |
| Spetzel, 2016, Canada [101] | Pigs, n = 16 | DBD with SCS or SNMP | None | 3 h | 33 °C, HA 60 mmHg, PV pressure 2–4 mmHg | Yes | 3 h | Dual | Yes | Decreased AST, ALP and bilirubin. Improved hyaluronic acid, less apoptosis at histology | Transplant model, porcine livers, no other perfusion technique as comparison |
| Okada, 2015, Japan [105] | Pig, n = 6 | DBD splitting with SNMP | None | 100 min | 20 °C, pressure PV: 5–10 mmHg, HA: 20–30 mmHg | Yes, 160 mmHg | 100 min | Dual | Yes | No differences between groups | Liver splitting is feasible during SNMP |
| Bruinsma, 2016, US [106] | Human, n = 21 | Discarded human DCD/DBD | Variable | Variable | 21 °C, pressure PV: 4–7 mmHg, HA: 50–80 mmHg | Yes, partial O2 pressure >700 mmHg | 3 h | Dual | No | Improvement of energetic cofactors and redox and reversal of ischemia-induced alterations, including lactate metabolism and increased TCA cycle intermediates | SNMP can assess liver viability |
| Knaak, 2014, Canada [100] | Pigs, n = 10 | DCD with SCS vs. SNMP | 45 min | 4 h | 33 °C, pressure: HA: 60 mmHg, PV: 4–8 mmHg | Yes | 3 h | Dual | Yes | Decreased ALP and bilirubin, and no signs of bile duct necrosis | Transplant model, no other perfusion technique as comparison |
| Bruinsma, 2014, US [64] | Human, n = 7 | Discarded human DCD | 28 (23–34) min | 685 (473–871) min | 21 °C, pressure PV: 4–7 mmHg, HA: 50–80 mmHg | Yes, partial O2 pressure >700 mmHg | 3 h | Dual | No | Biochemical and microscopic assessment revealed minimal injury during perfusion. | No transplant model, discarded human livers, |
| Berendsen, 2012, US [99] | Rat, n = 20 | DBD/DCD with SNMP vs. SCS | 60 min | None | 21 °C, portal resistance 50&100 mMH2O | pO2 n.a. | 3 h | Dual | Yes | One-month survival was 100% in the Fresh-SNMP and UW-Control groups, 83.3% in the WI-SNMP group and 0% in the WI group | Transplant study, short cold storage, no other perfusion technique as comparison |
| Boncompagni, 2011, Italy [98] | Rat | Fatty DBD with SNMP + NMP | None | None | 20 °C | Yes, O2 concentration 550–650 mmHg | 6 h SNMP, 2 h NMP | Dual | No | Reduction of sinusoidal cell apoptosis | No transplant model, Steatotic grafts, no other perfusion technique as comparison |
| Vairetti et al., 2009 [97] | Rat | Fatty DBD with SNMP + NMP | None | None | 20 °C | Yes, O2 concentration 550–650 mmHg | 6 h SNMP, 2 h NMP | Dual | No | Higher ATP/ADP-ratio and bile production. Lower oxidative stress and biliary enzyme release with SNMP | No transplant model, Steatotic grafts, no other perfusion technique as comparison |
| Author, Year, Country | N and Species | Liver Injury Model, Study Groups | Warm Ischemia | Cold Ischemia before Perfusion | Perfusion Conditions | Active Perfusate Oxygenation | Perfusion Duration | Single/ Dual | Transplantation | Main Findings | Discussion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yang, 2020, China [115] | Rat, n = 20 | DCD + SCS or DCD + NMP or DCD + BMMSCs + NMP | 30 min | 4–8 h, none in the NMP group | PV 2 mL/g/min with 10–12 mm H2O pressure | Yes, not specified | 8 h | Single (PV) | No | Lower AST, ALT and ALP, more bile production and lactate clearance with BMMSCs + NMP; BMMScs+ NMP led to less apoptosis and mitochondrial damage with low AST levels, less nucleic swelling and mitochondrial oedema | Rodent model, no transplantation |
| Boteon, 2019, UK [114] | Discarded human livers, n = 10 | DBD/DCD + SCS and HOPE + NMP vs. HBOC + COR + NMP | 8 (7–31) for the DCD | 11 h | HOPE PV flow 0.1 mL/min/g NMP flow: PV: 0.25 mL/min/g; HA: 0.75 mL/min/g, NMP pressure HA 30–50 mmHg, PV 8–10 mmHg. | Yes, pO2 80–100 kPa (HOPE); pO2: 40 kPa for NMP | 2 h HOPE 1 h COR | Dual HOPEDual NMP | No | Downstream activation of the inflammatory cascade and less expression of CD14 in HOPE + NMP compared to cold-to-warm, decrease in CD11b in neutrophil of both groups | Discarded human livers, without transplantation, comparison of different perfusion techniques |
| Nostedt, 2019, Canada [113] | Pig, n = 24 | DCD with initial flush either AD or HTK at different temp. with NMP | 60 min | None | Pressure HA: 60 mmHg, PV weight-adjusted | Yes, not specified | 12 h | Dual | No | Histologic injury and TNF-α levels were lowest in NMP with 4 °C flush groups, no differences in lactate and biochemistry. HTK 4 °C group had the least oedema and sinusoidal dilatation. | Pigs model without transplantation. |
| Zhang, 2016, China [112] | Mini pigs, n = 24 | DBD SCS vs. NMP | None | No SCS in NMP group, SCS group 133.8 ± 21.90 min | PV flow 320–580 mL/min with pressure 8–10 mmHg; HA Flow 129–239 mL/min HA pressure 85–100 mmHg | Yes, pO2: 80–100 mmHg CO2 partial pressure 30–50 mmHg | 127.3 ± 37.12 min | Dual | Yes | No cell necrosis or hepatic sinusoids and endothelial cell damage with NMP. Less AST, ALT, lactates and LDH in NMP in postoperative days 3 and 5 (p < 0.05). | Pig model, transplant model |
| Fondevila, 2011, Spain [109] | Pig, n = 36 | DCD with SCS, NECMO + SCS, NECMO + NMP | 90 min | Directly perfused after SCS | SCS: 1L UW 60 min NECMO, 1.8 L/min, 37 °C. NMP: PV: 8 mmHg, HA: 60/40 mmHg, 35.5–37.5 °C. | Yes in NECMO 1.8 L/min NMP: pO2: 60/40 mmHg * | 60 min NECMO 4 h NMP | Dual | Yes | Lower TNF /IL-6 mRNA expression in NECMO + NMP after reperfusion. Lower endothelial cell injury with NECMO + NMP | Transplant model |
| Banan, 2016, US [111] | Pig, n = 18 | DBD with immediate NMP, or with lower temp, 20 or 30 or 60 or 120 min rewarming | None | <15 min in the immediate NMP, 4 h in other groups | Flow 0.45 ± 0.15 L/min HA: and 1.3 ± 0.2 L/min (PV) arterial pressures of 80 ± 5 mmHg and portal pressures 6 ± 1 mm Hg | Yes, 95% O2 and 5% CO2 | 8 h in immediate NMP 4 h in other groups | Dual | No | Immediate NMP had lower transaminases, b-galactosidase, less SEC damage measured with hyaluronic acid, less necrosis at biopsy | Immediate NMP vs. 20,30,60, 120 min rewarming, no transplant model. |
| Op den Dries, 2013, Netherlands [110] | Discarded human livers, n = 4 | DCD with SCS, perfused with NMP | 38 ± 11.9 min § | 7 ± 1 h # | Mean pressure HA: 50 mmHg, PV 11 mmHg. Mean HA flow of 283 ± 29 mL/min; mean PV flow: 686 ± 25 mL/min | Yes, 60 kPa | 6 h | Dual | No | ALT, gamma-GT remained stable. Lactate decreased, normal pH, no signs of sinusoidal damage. No evidence of apoptosis. Partial loss of the biliary epithelial cell layer | Discarded human livers, no transplant model |
| Liu, 2013, US [108] | Pig, n = 15 | DCD with SCS + NMP Prostacyclin or adenosine or none | 60 min | Directly NMP after warm ischemia | Mean pressure HA: 90–100 mmHg, PV 7–12 mmHg | Yes * | 10 h | Dual | No | Lower AST, ALD and LDH in prostacyclin perfused together with higher bile production. Vasodilatation is key factor for better outcomes with NMP | Pig models without transplantation. |
| Fondevilla, 2011, Spain [109] | Pig, n = 36 | DCD with SCS, NECMO + SCS, NECMO + NMP | 90 min | Directly perfused after SCS | SCS: 1 L UW 60 min of NECMO, 1.8 L/min, 37 °C. NMP: PV: 8 mmHg, HA: 60/40 mmHg, 35.5–37.5 °C. | Yes in NECMO 1.8 L/min NMP: pO2: 60/40 mmHg * | 60 min NECMO 4 h NMP | Dual | Yes | Lower TNF /IL-6 mRNA expression in NECMO+ NMP after reperfusion. Lower endothelial cell injury with NECMO + NMP | Pig Model with transplantation |
6. Summary and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hong, S.-G.; Shin, J.; Choi, S.Y.; Powers, J.C.; Meister, B.M.; Sayoc, J.; Son, J.S.; Tierney, R.; Recchia, F.A.; Brown, M.D.; et al. Flow pattern–dependent mitochondrial dynamics regulates the metabolic profile and inflammatory state of endothelial cells. J. Clin. Investig. 2022, 7, e159286. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, Z.; Wang, L.; Lin, Y.; Fang, H.; Lei, J.; Cao, T.; Wang, G.; Dang, E. Single-cell transcriptome profiling reveals vascular endothelial cell heterogeneity in human skin. Theranostics 2021, 11, 6461–6476. [Google Scholar] [CrossRef]
- Malek, A.M.; Alper, S.L.; Izumo, S. Hemodynamic Shear Stress and Its Role in Atherosclerosis. JAMA 1999, 282, 2035–2042. [Google Scholar] [CrossRef]
- Mills, E.L.; Kelly, B.; O’Neill, L.A.J. Mitochondria are the powerhouses of immunity. Nat. Immunol. 2017, 18, 488–498. [Google Scholar] [CrossRef]
- Hofmann, J.; Otarashvili, G.; Meszaros, A.; Ebner, S.; Weissenbacher, A.; Cardini, B.; Oberhuber, R.; Resch, T.; Öfner, D.; Schneeberger, S.; et al. Restoring Mitochondrial Function While Avoiding Redox Stress: The Key to Preventing Ischemia/Reperfusion Injury in Machine Perfused Liver Grafts? Int. J. Mol. Sci. 2020, 21, 3132. [Google Scholar] [CrossRef]
- Lu, J.; Sharma, L.K.; Bai, Y. Implications of mitochondrial DNA mutations and mitochondrial dysfunction in tumorigenesis. Cell Res. 2009, 19, 802–815. [Google Scholar] [CrossRef]
- Gómez, J.; Mota-Martorell, N.; Jové, M.; Pamplona, R.; Barja, G. Mitochondrial ROS production, oxidative stress and aging within and between species: Evidences and recent advances on this aging effector. Exp. Gerontol. 2023, 174, 112134. [Google Scholar] [CrossRef]
- Eelen, G.; de Zeeuw, P.; Treps, L.; Harjes, U.; Wong, B.W.; Carmeliet, P. Endothelial Cell Metabolism. Physiol. Rev. 2018, 98, 3–58. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Lee, M.D.; Buckley, C.; Zhang, X.; McCarron, J.G. Mitochondrial ATP production is required for endothelial cell control of vascular tone. bioRxiv 2021. [Google Scholar] [CrossRef] [PubMed]
- Dumas, S.J.; Meta, E.; Borri, M.; Goveia, J.; Rohlenova, K.; Conchinha, N.V.; Falkenberg, K.; Teuwen, L.-A.; de Rooij, L.; Kalucka, J.; et al. Single-Cell RNA Sequencing Reveals Renal Endothelium Heterogeneity and Metabolic Adaptation to Water Deprivation. J. Am. Soc. Nephrol. 2020, 31, 118–138. [Google Scholar] [CrossRef] [PubMed]
- Tombor, L.S.; John, D.; Glaser, S.F.; Luxán, G.; Forte, E.; Furtado, M.; Rosenthal, N.; Baumgarten, N.; Schulz, M.H.; Wittig, J.; et al. Single cell sequencing reveals endothelial plasticity with transient mesenchymal activation after myocardial infarction. Nat. Commun. 2021, 12, 681. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Nogimori, Y.; Imamura, H.; Ando, J. Shear stress activates mitochondrial oxidative phosphorylation by reducing plasma membrane cholesterol in vascular endothelial cells. Proc. Natl. Acad. Sci. USA 2020, 117, 33660–33667. [Google Scholar] [CrossRef]
- Wu, D.; Huang, R.-T.; Hamanaka, R.B.; Krause, M.; Oh, M.-J.; Kuo, C.-H.; Nigdelioglu, R.; Meliton, A.Y.; Witt, L.; Dai, G.; et al. HIF-1α is required for disturbed flow-induced metabolic reprogramming in human and porcine vascular endothelium. Elife 2017, 6, e25217. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Xu, J.; Tian, F.; Hu, S.; Chen, Y.; Fu, Z. Melatonin attenuates myocardial ischemia-reperfusion injury via improving mitochondrial fusion/mitophagy and activating the AMPK-OPA1 signaling pathways. J. Pineal Res. 2019, 66, e12542. [Google Scholar] [CrossRef]
- Ong, S.-B.; Subrayan, S.; Lim, S.Y.; Yellon, D.M.; Davidson, S.M.; Hausenloy, D.J. Inhibiting Mitochondrial Fission Protects the Heart Against Ischemia/Reperfusion Injury. Circulation 2010, 121, 2012–2022. [Google Scholar] [CrossRef]
- Hessheimer, A.J.; Vengohechea, J.B.; Fondevila, C. Metabolomic Analysis, Perfusate Composition, and Pseudo-physiology of the Isolated Liver During Ex Situ Normothermic Machine Perfusion. Transplantation 2023, 107, e125–e126. [Google Scholar] [CrossRef]
- Ramos, P.; Williams, P.; Salinas, J.; Vengohechea, J.B.; Lodge, J.P.A.; Fondevila, C.; Hessheimer, A.J. Abdominal Organ Preservation Solutions in the Age of Machine Perfusion. Transplantation 2022, 107, 326–340. [Google Scholar] [CrossRef]
- Davidson, S.M.; Duchen, M.R. Endothelial mitochondria: Contributing to vascular function and disease. Circ. Res. 2007, 100, 1128–1141. [Google Scholar] [CrossRef]
- Fujii, Y.; Johnson, M.; Gores, G. Mitochondrial dysfunction during anoxia/reoxygenation injury of liver sinusoidal endothelial cells. Hepatology 1994, 20, 177–185. [Google Scholar] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pell, V.R.; Chouchani, E.T.; Murphy, M.P.; Brookes, P.S.; Krieg, T. Moving forwards by blocking back-flow the yin and yang of MI therapy. Circ. Res. 2016, 118, 898–906. [Google Scholar] [CrossRef]
- Kim, M.; Stepanova, A.; Niatsetskaya, Z.; Sosunov, S.; Arndt, S.; Murphy, M.P.; Galkin, A.; Ten, V.S. Attenuation of oxidative damage by targeting mitochondrial complex I in neonatal hypoxic-ischemic brain injury. Free Radic. Biol. Med. 2018, 124, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Pell, V.R.; James, A.M.; Work, L.M.; Saeb-Parsy, K.; Frezza, C.; Krieg, T.; Murphy, M.P. A Unifying Mechanism for Mitochondrial Superoxide Production during Ischemia-Reperfusion Injury. Cell Metab. 2016, 23, 254–263. [Google Scholar] [CrossRef]
- Cordes, T.; Lucas, A.; Divakaruni, A.S.; Murphy, A.N.; Cabrales, P.; Metallo, C.M. Itaconate modulates tricarboxylic acid and redox metabolism to mitigate reperfusion injury. Mol. Metab. 2020, 32, 122–135. [Google Scholar] [CrossRef]
- Ali, A.; Wang, A.; Ribeiro, R.V.P.; Beroncal, E.L.; Baciu, C.; Galasso, M.; Gomes, B.; Mariscal, A.; Hough, O.; Brambate, E.; et al. Static lung storage at 10 °C maintains mitochondrial health and preserves donor organ function. Sci. Transl. Med. 2021, 13, eabf7601. [Google Scholar] [CrossRef] [PubMed]
- Malhi, H.; Gores, G.J. Cellular and Molecular Mechanisms of Liver Injury. Gastroenterology 2008, 134, 1641–1654. [Google Scholar] [CrossRef] [PubMed]
- Bhogal, R.H.; Weston, C.J.; Velduis, S.; Leuvenink, H.G.D.; Reynolds, G.M.; Davies, S.; Nyguet-Thin, L.; Alfaifi, M.; Shepard, E.L.; Boteon, Y.; et al. The Reactive Oxygen Species–Mitophagy Signaling Pathway Regulates Liver Endothelial Cell Survival During Ischemia/Reperfusion Injury. Liver Transplant. 2018, 24, 1437–1452. [Google Scholar] [CrossRef]
- Dare, A.J.; Logan, A.; Prime, T.A.; Rogatti, S.; Goddard, M.; Bolton, E.M.; Bradley, J.A.; Pettigrew, G.J.; Murphy, M.P.; Saeb-Parsy, K. The mitochondria-targeted anti-oxidant MitoQ decreases ischemia-reperfusion injury in a murine syngeneic heart transplant model. J. Heart Lung Transplant. 2015, 34, 1471–1480. [Google Scholar] [CrossRef]
- Longnus, S.L.; Rutishauser, N.B.; Gillespie, M.N.; Reichlin, T.; Carrel, T.P.; Sanz, M.N. Mitochondrial Damage-associated Molecular Patterns as Potential Biomarkers in DCD Heart Transplantation: Lessons From Myocardial Infarction and Cardiac Arrest. Transplant. Direct 2021, 8, e1265. [Google Scholar] [CrossRef]
- Zhai, Y.; Petrowsky, H.; Hong, J.C.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Ischaemia-reperfusion injury in liver transplantation—From bench to bedside. Nat. Rev. Gastroentrerol. Hepatol. 2013, 10, 79–89. [Google Scholar] [CrossRef]
- Khandoga, A.; Kessler, J.S.; Hanschen, M.; Khandoga, A.G.; Burggraf, D.; Reichel, C.; Hamann, G.F.; Enders, G.; Krombach, F. Matrix metalloproteinase-9 promotes neutrophil and T cell recruitment and migration in the postischemic liver. J. Leukoc. Biol. 2006, 79, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, A.L.; Qurashi, M.; Shetty, S. The Role of Sinusoidal Endothelial Cells in the Axis of Inflammation and Cancer Within the Liver. Front. Physiol. 2020, 11, 990. [Google Scholar] [CrossRef] [PubMed]
- Guixé-Muntet, S.; de Mesquita, F.C.; Vila, S.; Hernández-Gea, V.; Peralta, C.; García-Pagán, J.C.; Bosch, J.; Gracia-Sancho, J. Cross-talk between autophagy and KLF2 determines endothelial cell phenotype and microvascular function in acute liver injury. J. Hepatol. 2017, 66, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Peralta, C.; Jiménez-Castro, M.B.; Gracia-Sancho, J. Hepatic ischemia and reperfusion injury: Effects on the liver sinusoidal milieu. J. Hepatol. 2013, 59, 1094–1106. [Google Scholar] [CrossRef]
- Coon, B.G.; Timalsina, S.; Astone, M.; Zhuang, Z.W.; Fang, J.; Han, J.; Themen, J.; Chung, M.; Yang-Klingler, Y.J.; Jain, M.; et al. A mitochondrial contribution to anti-inflammatory shear stress signaling in vascular endothelial cells. J. Cell Biol. 2022, 221, e202109144. [Google Scholar] [CrossRef]
- Nakamura, K.; Johnson, G.L. PB1 Domains of MEKK2 and MEKK3 Interact with the MEK5 PB1 Domain for Activation of the ERK5 Pathway. J. Biol. Chem. 2003, 278, 36989–36992. [Google Scholar] [CrossRef]
- Parmar, K.M.; Larman, H.B.; Dai, G.; Zhang, Y.; Wang, E.T.; Moorthy, S.N.; Kratz, J.R.; Lin, Z.; Jain, M.K.; Gimbrone, M.A., Jr.; et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J. Clin. Investig. 2006, 116, 49–58. [Google Scholar] [CrossRef]
- Sohn, S.J.; Li, D.; Lee, L.K.; Winoto, A. Transcriptional Regulation of Tissue-Specific Genes by the ERK5 Mitogen-Activated Protein Kinase. Mol. Cell. Biol. 2005, 25, 8553–8566. [Google Scholar] [CrossRef]
- Jain, M.K.; Sangwung, P.; Hamik, A. Regulation of an inflammatory disease: Krüppel-like factors and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 499–508. [Google Scholar] [CrossRef]
- Lee, J.S.; Yu, Q.; Shin, J.T.; Sebzda, E.; Bertozzi, C.; Chen, M.; Mericko, P.; Stadtfeld, M.; Zhou, D.; Cheng, L.; et al. Klf2 Is an Essential Regulator of Vascular Hemodynamic Forces In Vivo. Dev. Cell 2006, 11, 845–857. [Google Scholar] [CrossRef]
- Lindell, S.L.; Muir, H.; Brassil, J.; Mangino, M.J. Hypothermic Machine Perfusion Preservation of the DCD Kidney: Machine Effects. J. Transplant. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Prudhomme, T.; Kervella, D.; Ogbemudia, A.E.; Gauttier, V.; Le Bas-Bernardet, S.; Minault, D.; Hervouet, J.; Cantarovich, D.; Karam, G.; Renaudin, K.; et al. Successful pancreas allotransplantations after hypothermic machine perfusion in a novel diabetic porcine model: A controlled study. Transpl. Int. 2020, 34, 353–364. [Google Scholar] [CrossRef]
- Dekker, R.J.; Van Soest, S.; Fontijn, R.D.; Salamanca, S.; De Groot, P.G.; van Bavel, E.; Pannekoek, H.; Horrevoets, A.J.G. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Krüppel-like factor (KLF2). Blood 2002, 100, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Birdsey, G.M.; Shah, A.V.; Dufton, N.; Reynolds, L.E.; Almagro, L.O.; Yang, Y.; Aspalter, I.M.; Khan, S.T.; Mason, J.C.; Dejana, E.; et al. The Endothelial Transcription Factor ERG Promotes Vascular Stability and Growth through Wnt/β-Catenin Signaling. Dev. Cell 2015, 32, 82–96. [Google Scholar] [CrossRef]
- Poisson, J.; Lemoinne, S.; Boulanger, C.; Durand, F.; Moreau, R.; Valla, D.; Rautou, P.-E. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J. Hepatol. 2017, 66, 212–227. [Google Scholar] [CrossRef]
- Mönkemöller, V.; Øie, C.; Hübner, W.; Huser, T.; McCourt, P. Multimodal super-resolution optical microscopy visualizes the close connection between membrane and the cytoskeleton in liver sinusoidal endothelial cell fenestrations. Sci. Rep. 2015, 5, 16279. [Google Scholar] [CrossRef]
- Braet, F.; Riches, J.; Geerts, W.; Jahn, K.A.; Wisse, E.; Frederik, P. Three-dimensional organization of fenestrae labyrinths in liver sinusoidal endothelial cells. Liver Int. 2009, 29, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.A.; Sporstøl, M.; Fladeby, C.; Kjeken, R.; Barois, N.; Berg, T. Receptor-mediated endocytosis of immune complexes in rat liver sinusoidal endothelial cells is mediated by FcgammaRIIb2. Hepatology 2007, 46, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Pollara, J.; Edwards, R.W.; Lin, L.; Bendersky, V.; Brennan, T.V. Circulating mitochondria in deceased organ donors are associated with immune activation and early allograft dysfunction. J. Clin. Investig. 2018, 3, e121622. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, D.; Hu, D.; Zhou, X.; Zhou, Y. The role of mitochondria in NLRP3 inflammasome activation. Mol. Immunol. 2018, 103, 115–124. [Google Scholar] [CrossRef]
- Sorbara, M.; Girardin, S. Mitochondrial ROS fuel the inflammasome. Cell. Res. 2011, 21, 558–560. [Google Scholar] [CrossRef]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Porte, R.; Dutkowski, P. Protective mechanisms and current clinical evidence of hypothermic oxygenated machine perfusion (HOPE) in preventing post-transplant cholangiopathy. J. Hepatol. 2022, 76, 1330–1347. [Google Scholar] [CrossRef]
- Nasralla, D.; for the Consortium for Organ Preservation in Europe; Coussios, C.C.; Mergental, H.; Akhtar, M.Z.; Butler, A.J.; Ceresa, C.D.L.; Chiocchia, V.; Dutton, S.J.; García-Valdecasas, J.C.; et al. A randomized trial of normothermic preservation in liver transplantation. Nature 2018, 557, 50–56. [Google Scholar] [CrossRef]
- Markmann, J.F.; Abouljoud, M.S.; Ghobrial, R.M.; Bhati, C.S.; Pelletier, S.J.; Lu, A.D.; Ottmann, S.; Klair, T.; Eymard, C.; Roll, G.R.; et al. Impact of Portable Normothermic Blood-Based Machine Perfusion on Outcomes of Liver Transplant: The OCS Liver PROTECT Randomized Clinical Trial. JAMA Surg. 2022, 157, 189–198. [Google Scholar] [CrossRef]
- Gaurav, R.M.; Butler, A.J.M.; Kosmoliaptsis, V.; Mumford, L.; Fear, C.; Swift, L.; Fedotovs, A.M.; Upponi, S.M.; Khwaja, S.M.; Richards, J.; et al. Liver Transplantation Outcomes From Controlled Circulatory Death Donors. Ann. Surg. 2022, 275, 1156–1164. [Google Scholar] [CrossRef]
- Mergental, H.; Laing, R.W.; Kirkham, A.J.; Perera, M.T.P.R.; Boteon, Y.L.; Attard, J.; Barton, D.; Curbishley, S.; Wilkhu, M.; Neil, D.A.H.; et al. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat. Commun. 2020, 11, 2939. [Google Scholar] [CrossRef]
- Schlegel, A.; Muller, X.; Dutkowski, P. Hypothermic liver perfusion. Curr. Opin. Organ Transplant. 2017, 22, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Czigany, Z.M.; Pratschke, J.; Froněk, J.; Guba, M.; Schöning, W.; Raptis, D.A.M.; Andrassy, J.; Kramer, M.M.; Strnad, P.; Tolba, R.H.M.; et al. Hypothermic Oxygenated Machine Perfusion Reduces Early Allograft Injury and Improves Post-transplant Outcomes in Extended Criteria Donation Liver Transplantation From Donation After Brain Death. Ann. Surg. 2021, 274, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Ravaioli, M.; Germinario, G.; Dajti, G.; Sessa, M.; Vasuri, F.; Siniscalchi, A.; Morelli, M.C.; Serenari, M.; Del Gaudio, M.; Zanfi, C.; et al. Hypothermic oxygenated perfusion in extended criteria donor liver transplantation—A randomized clinical trial. Am. J. Transplant. 2022, 22, 2401–2408. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Mueller, M.; Muller, X.; Eden, J.; Panconesi, R.; von Felten, S.; Steigmiller, K.; Da Silva, R.X.S.; de Rougemont, O.; Mabrut, J.-Y.; et al. A multicenter randomized-controlled trial of hypothermic oxygenated perfusion (HOPE) for human liver grafts before transplantation. J. Hepatol. 2023, 78, 783–793. [Google Scholar] [CrossRef]
- Panconesi, R.; Flores Carvalho, M.; Mueller, M.; Dutkowski, P.; Muiesan, P.; Schlegel, A. Mitochondrial Reprogramming—What Is the Benefit of Hypothermic Oxygenated Perfusion in Liver Transplantation? Transplantology 2021, 2, 149–161. [Google Scholar] [CrossRef]
- Selten, J.; Schlegel, A.; de Jonge, J.; Dutkowski, P. Hypo- and normothermic perfusion of the liver: Which way to go? Best Pract. Res. Clin. Gastroenterol. 2017, 31, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, B.G.; Yeh, H.; Özer, S.; Martins, P.N.; Farmer, A.; Wu, W.; Saeidi, N.; Dries, S.O.D.; Berendsen, T.A.; Smith, R.N.; et al. Subnormothermic Machine Perfusion for Ex Vivo Preservation and Recovery of the Human Liver for Transplantation. Am. J. Transplant. 2014, 14, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Brüggenwirth, I.M.; van Leeuwen, O.B.; Müller, M.; Dutkowski, P.; Monbaliu, D.; Martins, P.N.; Porte, R.J.; de Meijer, V.E. The importance of adequate oxygenation during hypothermic machine perfusion. JHEP Rep. 2020, 3, 100194. [Google Scholar] [CrossRef]
- van Rijn, R.; Schurink, I.J.; de Vries, Y.; Berg, A.P.V.D.; Cerisuelo, M.C.; Murad, S.D.; Erdmann, J.I.; Gilbo, N.; de Haas, R.J.; Heaton, N.; et al. Hypothermic Machine Perfusion in Liver Transplantation—A Randomized Trial. N. Engl. J. Med. 2021, 384, 1391–1401. [Google Scholar] [CrossRef]
- Patrono, D.; Cussa, D.; Sciannameo, V.; Montanari, E.; Panconesi, R.; Berchialla, P.; Lepore, M.; Gambella, A.; Rizza, G.; Catalano, G.; et al. Outcome of liver transplantation with grafts from brain-dead donors treated with dual hypothermic oxygenated machine perfusion, with particular reference to elderly donors. Am. J. Transplant. 2022, 22, 1382–1395. [Google Scholar] [CrossRef]
- Fujita, S.; Hamamoto, I.; Nakamura, K.; Tanaka, K.; Ozawa, K. Isolated perfusion of rat livers: Effect of temperature on O2 consumption, enzyme release, energy store, and morphology. Nihon Geka Hokan. Arch. Fur Jpn. Chir. 1993, 62, 58–70. [Google Scholar]
- Schlegel, A.; Muller, X.; Mueller, M.; Stepanova, A.; Kron, P.; de Rougemont, O.; Muiesan, P.; Clavien, P.-A.; Galkin, A.; Meierhofer, D.; et al. Hypothermic oxygenated perfusion protects from mitochondrial injury before liver transplantation. Ebiomedicine 2020, 60, 103014. [Google Scholar] [CrossRef]
- Abele, D.; Heise, K.; Pörtner, H.O.; Puntarulo, S. Temperature-dependence of mitochondrial function and production of reactive oxygen species in the intertidal mud clam Mya arenaria. J. Exp. Biol. 2002, 205, 1831–1841. [Google Scholar] [CrossRef] [PubMed]
- Lazeyras, F.; Buhler, L.; Vallee, J.P.; Hergt, M.; Nastasi, A.; Ruttimann, R.; Morel, P.; Buchs, J.B. Detection of ATP by ‘in line’31P magnetic resonance spectroscopy during oxygenated hypothermic pulsatile perfusion of pigs’ kidneys. Magn. Reson. Mater. Phys. Biol. Med. 2012, 25, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Lüer, B.; Koetting, M.; Efferz, P.; Minor, T. Role of oxygen during hypothermic machine perfusion preservation of the liver. Transpl. Int. 2010, 23, 944–950. [Google Scholar] [CrossRef]
- Venema, L.H.; Brat, A.; Moers, C.; Hart, N.A.; Ploeg, R.J.; Hannaert, P.; Minor, T.; Leuvenink, H. Effects of Oxygen During Long-term Hypothermic Machine Perfusion in a Porcine Model of Kidney Donation After Circulatory Death. Transplantation 2019, 103, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- de Vries, Y.M.; Brüggenwirth, I.M.A.B.; Karangwa, S.A.; von Meijenfeldt, F.A.B.; van Leeuwen, O.B.; Burlage, L.C.M.; de Jong, I.E.M.; Gouw, A.S.H.M.; de Meijer, V.E.M.; Lisman, T.; et al. Dual Versus Single Oxygenated Hypothermic Machine Perfusion of Porcine Livers: Impact on Hepatobiliary and Endothelial Cell Injury. Transplant. Direct 2021, 7, e741. [Google Scholar] [CrossRef] [PubMed]
- Vasuri, F.; Germinario, G.; Ciavarella, C.; Carroli, M.; Motta, I.; Valente, S.; Cescon, M.; D’errico, A.; Pasquinelli, G.; Ravaioli, M. Trophism and Homeostasis of Liver Sinusoidal Endothelial Graft Cells during Preservation, with and without Hypothermic Oxygenated Perfusion. Biology 2022, 11, 1329. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.D.; Nachber, E.; Tulipan, J.; Stone, J.; Bae, C.; Reznik, L.; Kato, T.; Samstein, B.; Emond, J.C.; Guarrera, J.V. Hypothermic Machine Preservation Reduces Molecular Markers of Ischemia/Reperfusion Injury in Human Liver Transplantation. Am. J. Transplant. 2012, 12, 2477–2486. [Google Scholar] [CrossRef]
- Kron, P.; Schlegel, A.; Mancina, L.; Clavien, P.-A.; Dutkowski, P. Hypothermic oxygenated perfusion (HOPE) for fatty liver grafts in rats and humans. J. Hepatol. 2018, 68, 82–91. [Google Scholar] [CrossRef]
- Dries, S.O.D.; Sutton, M.E.; Karimian, N.; de Boer, M.T.; Wiersema-Buist, J.; Gouw, A.S.H.; Leuvenink, H.G.D.; Lisman, T.; Porte, R.J. Hypothermic Oxygenated Machine Perfusion Prevents Arteriolonecrosis of the Peribiliary Plexus in Pig Livers Donated after Circulatory Death. PLoS ONE 2014, 9, e88521. [Google Scholar] [CrossRef]
- Kanazawa, H.; Obara, H.; Yoshikawa, R.; Meng, L.; Hirano, T.; Okada, Y.; Nishikawa, Y.; Matsuno, N. Impact of Machine Perfusion on Sinusoid Microcirculation of Liver Graft Donated After Cardiac Death. J. Surg. Res. 2020, 245, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Burlage, L.C.; Karimian, N.; Westerkamp, A.C.; Visser, N.; Matton, A.P.; van Rijn, R.; Adelmeijer, J.; Wiersema-Buist, J.; Gouw, A.S.; Lisman, T.; et al. Oxygenated hypothermic machine perfusion after static cold storage improves endothelial function of extended criteria donor livers. HPB 2017, 19, 538–546. [Google Scholar] [CrossRef] [PubMed]
- ‘t Hart, N.A.; Der Van Plaats, A.; Leuvenink, H.; Van Goor, H.; Wiersema-Buist, J.; Verkerke, G.J.; Rakhorst, G.; Ploeg, R.J. Determination of an adequate perfusion pressure for continuous dual vessel hypothermic machine perfusion of the rat liver. Transpl. Int. 2007, 20, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; de Rougemont, O.; Graf, R.; Clavien, P.-A.; Dutkowski, P. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts. J. Hepatol. 2013, 58, 278–286. [Google Scholar] [CrossRef]
- Schlegel, A.; Kron, P.; Graf, R.; Dutkowski, P.; Clavien, P.A. Warm vs. cold perfusion techniques to rescue rodent liver grafts. J Hepatol. 2014, 61, 1267–1275. [Google Scholar] [CrossRef]
- Van Der Plaats, A.; ’THart, N.A.; Verkerke, G.J.; Leuvenink, H.G.D.; Ploeg, R.J.; Rakhorst, G. Hypothermic machine preservation in liver transplantation revisited: Concepts and criteria in the new millennium. Ann. Biomed. Eng. 2004, 32, 623–631. [Google Scholar] [CrossRef]
- Kerkweg, U.; Jacob, M.; de Groot, H.; Mannherz, H.-G.; Rauen, U. Cold-induced apoptosis of rat liver endothelial cells: Contribution of mitochondrial alterations. Transplantation 2003, 76, 501–508. [Google Scholar] [CrossRef]
- Jain, S.; Xu, H.; Duncan, H.; Jones, J.W., Jr.; Zhang, J.X.; Clemens, M.G.; Lee, C.Y. Ex-vivo study of flow dynamics and endothelial cell structure during extended hypothermic machine perfusion preservation of livers. Cryobiology 2004, 48, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Abele, D. Toxic oxygen: The radical life-giver. Nature 2002, 420, 27. [Google Scholar] [CrossRef]
- Gallinat, A.; Fox, M.; Lüer, B.; Efferz, P.; Paul, A.; Minor, T. Role of Pulsatility in Hypothermic Reconditioning of Porcine Kidney Grafts by Machine Perfusion After Cold Storage. Transplantation 2013, 96, 538–542. [Google Scholar] [CrossRef]
- Koetting, M.; Lüer, B.; Efferz, P.; Paul, A.; Minor, T. Optimal Time for Hypothermic Reconditioning of Liver Grafts by Venous Systemic Oxygen Persufflation in a Large Animal Model. Transplantation 2011, 91, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Bochimoto, H.; Ishihara, Y.; Zin, N.K.M.; Iwata, H.; Kondoh, D.; Obara, H.; Matsuno, N. Ultrastructural changes in porcine liver sinusoidal endothelial cells of machine perfused liver donated after cardiac death. World J. Gastroenterol. 2022, 28, 2100–2111. [Google Scholar] [CrossRef] [PubMed]
- Brüggenwirth, I.M.; Mueller, M.; Lantinga, V.A.; Camagni, S.; De Carlis, R.; De Carlis, L.; Colledan, M.; Dondossola, D.; Drefs, M.; Eden, J.; et al. Prolonged preservation by hypothermic machine perfusion facilitates logistics in liver transplantation: A European observational cohort study. Am. J. Transplant. 2022, 22, 1842–1851. [Google Scholar] [CrossRef] [PubMed]
- Boteon, A.P.C.S.; Schlegel, A.; Carvalho, M.F.; Boteon, Y.L. Hypothermic oxygenated machine perfusion as a tool to facilitate liver transplantation in the acute-on-chronic liver failure scenario. Liver Transplant. 2022, 28, 1678–1680. [Google Scholar] [CrossRef]
- Pavicevic, S.; Uluk, D.; Reichelt, S.; Fikatas, P.; Globke, B.; Raschzok, N.; Schmelzle, M.; Öllinger, R.; Schöning, W.; Eurich, D.; et al. Hypothermic oxygenated machine perfusion for extended criteria donor allografts: Preliminary experience with extended organ preservation times in the setting of organ reallocation. Artif. Organs 2022, 46, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Brüggenwirth, I.M.A.; Lantinga, V.A.; Rayar, M.; Berg, A.P.V.D.; Blokzijl, H.; Reyntjens, K.M.E.M.; Porte, R.J.; de Meijer, V.E. Prolonged dual hypothermic oxygenated machine preservation (DHOPE-PRO) in liver transplantation: Study protocol for a stage 2, prospective, dual-arm, safety and feasibility clinical trial. BMJ Open Gastroenterol. 2022, 9, e000842. [Google Scholar] [CrossRef]
- Brüggenwirth, I.M.; van Leeuwen, O.B.; de Vries, Y.; Bodewes, S.B.; Adelmeijer, J.; Wiersema-Buist, J.; Lisman, T.; Martins, P.N.; de Meijer, V.E.; Porte, R.J. Extended hypothermic oxygenated machine perfusion enables ex situ preservation of porcine livers for up to 24 hours. JHEP Rep. 2020, 2, 100092. [Google Scholar] [CrossRef] [PubMed]
- Post, I.C.; de Boon, W.M.; Heger, M.; van Wijk, A.C.; Kroon, J.; van Buul, J.D.; van Gulik, T.M. Endothelial cell preservation at hypothermic to normothermic conditions using clinical and experimental organ preservation solutions. Exp. Cell Res. 2013, 319, 2501–2513. [Google Scholar] [CrossRef] [PubMed]
- Vairetti, M.; Ferrigno, A.; Carlucci, F.; Tabucchi, A.; Rizzo, V.; Boncompagni, E.; Neri, D.; Gringeri, E.; Freitas, I.; Cillo, U. Subnormothermic machine perfusion protects steatotic livers against preservation injury: A potential for donor pool increase? Liver Transpl. 2009, 15, 20–29. [Google Scholar] [CrossRef]
- Boncompagni, E.; Gini, E.; Ferrigno, A.; Milanesi, G.; Gringeri, E.; Barni, S.; Cillo, U.; Vairetti, M.; Freitas, I. Decreased apoptosis in fatty livers submitted to subnormothermic machine-perfusion respect to cold storage. Eur. J. Histochem. 2011, 55, e40. [Google Scholar] [CrossRef]
- Berendsen, T.A.; Bruinsma, B.G.; Lee, J.; D’andrea, V.; Liu, Q.; Izamis, M.-L.; Uygun, K.; Yarmush, M.L. A simplified subnormothermic machine perfusion system restores ischemically damaged liver grafts in a rat model of orthotopic liver transplantation. Transplant. Res. 2012, 1, 6. [Google Scholar] [CrossRef]
- Knaak, J.M.; Spetzler, V.N.; Goldaracena, N.; Boehnert, M.U.; Bazerbachi, F.; Louis, K.S.; Adeyi, O.A.; Minkovich, L.; Yip, P.M.; Keshavjee, S.; et al. Subnormothermic ex vivo liver perfusion reduces endothelial cell and bile duct injury after donation after cardiac death pig liver transplantation. Liver Transplant. 2014, 20, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Spetzler, V.N.; Goldaracena, N.; Echiverri, J.; Kaths, J.M.; Louis, K.S.; Adeyi, O.A.; Yip, P.M.; Grant, D.R.; Selzner, N.; Selzner, M. Subnormothermic ex vivo liver perfusion is a safe alternative to cold static storage for preserving standard criteria grafts. Liver Transplant. 2016, 22, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Goldaracena, N.; Echeverri, J.; Spetzler, V.N.; Kaths, J.M.; Barbas, A.S.; Louis, K.S.; Adeyi, O.A.; Grant, D.R.; Selzner, N.; Selzner, M. Anti-inflammatory signaling during ex vivo liver perfusion improves the preservation of pig liver grafts before transplantation. Liver Transplant. 2016, 22, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Kakizaki, Y.; Miyagi, S.; Shimizu, K.; Miyazawa, K.; Nakanishi, W.; Tokodai, K.; Hara, Y.; Nakanishi, C.; Unno, M.; Kamei, T.; et al. The Effects of Short-term Subnormothermic Perfusion After Cold Preservation on Liver Grafts From Donors After Circulatory Death: An Ex Vivo Rat Model. Transplantation 2018, 102, e147–e154. [Google Scholar] [CrossRef] [PubMed]
- Okamura, Y.; Hata, K.; Tanaka, H.; Hirao, H.; Kubota, T.; Inamoto, O.; Kageyama, S.; Tamaki, I.; Yermek, N.; Yoshikawa, J.; et al. Impact of Subnormothermic Machine Perfusion Preservation in Severely Steatotic Rat Livers: A Detailed Assessment in an Isolated Setting. Am. J. Transplant. 2017, 17, 1204–1215. [Google Scholar] [CrossRef]
- Okada, N.; Mizuta, K.; Oshima, M.; Yamada, N.; Sanada, Y.; Ihara, Y.; Urahashi, T.; Ishikawa, J.; Tsuji, T.; Hishikawa, S.; et al. A Novel Split Liver Protocol Using the Subnormothermic Oxygenated Circuit System in a Porcine Model of a Marginal Donor Procedure. Transplant. Proc. 2015, 47, 419–426. [Google Scholar] [CrossRef]
- Bruinsma, B.G.; Sridharan, G.V.; Weeder, P.D.; Avruch, J.H.; Saeidi, N.; Özer, S.; Geerts, S.; Porte, R.J.; Heger, M.; van Gulik, T.M.; et al. Metabolic profiling during ex vivo machine perfusion of the human liver. Sci. Rep. 2016, 6, 22415. [Google Scholar] [CrossRef]
- Furukori, M.; Matsuno, N.; Meng, L.; Shonaka, T.; Nishikawa, Y.; Imai, K.; Obara, H.; Furukawa, H. Subnormothermic Machine Perfusion Preservation with Rewarming for Donation after Cardiac Death Liver Grafts in Pigs. Transplant. Proc. 2016, 48, 1239–1243. [Google Scholar] [CrossRef]
- Liu, Q.; Nassar, A.; Farias, K.; Buccini, L.; Mangino, M.J.; Baldwin, W.; Bennett, A.; O’rourke, C.; Iuppa, G.; Soliman, B.G.; et al. Comparing Normothermic Machine Perfusion Preservation with Different Perfusates on Porcine Livers From Donors After Circulatory Death. Am. J. Transplant. 2016, 16, 794–807. [Google Scholar] [CrossRef]
- Fondevila, C.; Hessheimer, A.J.; Maathuis, M.-H.J.; Muñoz, J.; Taurá, P.; Calatayud, D.; Leuvenink, H.; Rimola, A.; Ploeg, R.J.; García-Valdecasas, J.C. Superior Preservation of DCD Livers with Continuous Normothermic Perfusion. Ann. Surg. 2011, 254, 1000–1007. [Google Scholar] [CrossRef]
- Dries, S.O.D.; Karimian, N.; Sutton, M.E.; Westerkamp, A.C.; Nijsten, M.W.N.; Gouw, A.S.H.; Wiersema-Buist, J.; Lisman, T.; Leuvenink, H.G.D.; Porte, R.J. Ex vivo Normothermic Machine Perfusion and Viability Testing of Discarded Human Donor Livers. Am. J. Transplant. 2013, 13, 1327–1335. [Google Scholar] [CrossRef]
- Banan, B.; Xiao, Z.; Watson, R.; Xu, M.; Jia, J.; Upadhya, G.A.; Mohanakumar, T.; Lin, Y.; Chapman, W. Novel strategy to decrease reperfusion injuries and improve function of cold-preserved livers using normothermic ex vivo liver perfusion machine. Liver Transplant. 2016, 22, 333–343. [Google Scholar] [CrossRef]
- Zhang, Z.-B.; Gao, W.; Shi, Y.; Liu, L.; Ma, N.; Chen, J.; Zhu, Z.-J. Protective role of normothermic machine perfusion during reduced-size liver transplantation in pigs. Liver Transplant. 2016, 22, 968–978. [Google Scholar] [CrossRef]
- Nostedt, J.J.; Churchill, T.; Ghosh, S.; Thiesen, A.; Hopkins, J.; Lees, M.C.; Adam, B.; Freed, D.H.; Shapiro, A.M.J.; Bigam, D.L. Avoiding initial hypothermia does not improve liver graft quality in a porcine donation after circulatory death (DCD) model of normothermic perfusion. PLoS ONE 2019, 14, e0220786. [Google Scholar] [CrossRef]
- Boteon, Y.L.; Laing, R.W.; Schlegel, A.; Wallace, L.; Smith, A.; Attard, J.; Bhogal, R.H.; Reynolds, G.; Perera, M.T.P.; Muiesan, P.; et al. The impact on the bioenergetic status and oxidative-mediated tissue injury of a combined protocol of hypothermic and normothermic machine perfusion using an acellular haemoglobin-based oxygen carrier: The cold-to-warm machine perfusion of the liver. PLoS ONE 2019, 14, e0224066. [Google Scholar] [CrossRef]
- Yang, L.; Cao, H.; Sun, D.; Hou, B.; Lin, L.; Shen, Z.Y.; Song, H.L. Bone marrow mesenchymal stem cells combine with normothermic machine perfusion to improve rat donor liver quality-the important role of hepatic microcirculation in donation after circulatory death. Cell Tissue Res. 2020, 381, 239–254. [Google Scholar] [CrossRef]
- Eshmuminov, D.; Becker, D.; Borrego, L.B.; Hefti, M.; Schuler, M.J.; Hagedorn, C.; Muller, X.; Mueller, M.; Onder, C.; Graf, R.; et al. An integrated perfusion machine preserves injured human livers for 1 week. Nat. Biotechnol. 2020, 38, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Lau, N.; Ly, M.; Dennis, C.; Liu, K.; Kench, J.; Crawford, M.; Pulitano, C. Long-term normothermic perfusion of human livers for longer than 12 days. Artif. Organs 2022, 46, 2504–2510. [Google Scholar] [CrossRef] [PubMed]
- Eshmuminov, D.; Becker, D.; Hefti, M.L.; Mueller, M.; Hagedorn, C.; Dutkowski, P.; von Rohr, P.R.; Halbe, M.; Segerer, S.; Tibbitt, M.W.; et al. Hyperoxia in portal vein causes enhanced vasoconstriction in arterial vascular bed. Sci. Rep. 2020, 10, 20966. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.-A.; Dutkowski, P.; Mueller, M.; Eshmuminov, D.; Borrego, L.B.; Weber, A.; Muellhaupt, B.; Da Silva, R.X.S.; Burg, B.R.; von Rohr, P.R.; et al. Transplantation of a human liver following 3 days of ex situ normothermic preservation. Nat. Biotechnol. 2022, 40, 1610–1616. [Google Scholar] [CrossRef]
- Mueller, M.; Hefti, M.; Eshmuminov, D.; Schuler, M.J.; Da Silva, R.X.S.; Petrowsky, H.; De Oliveira, M.L.; Oberkofler, C.E.; Hagedorn, C.; Mancina, L.; et al. Long-term Normothermic Machine Preservation of Partial Livers: First Experience with 21 Human Hemi-livers. Ann. Surg. 2021, 274, 836–842. [Google Scholar] [CrossRef]
- Parente, A.; Carvalho, M.F.; Eden, J.; Dutkowski, P.; Schlegel, A. Mitochondria and Cancer Recurrence after Liver Transplantation—What Is the Benefit of Machine Perfusion? Int. J. Mol. Sci. 2022, 23, 9747. [Google Scholar] [CrossRef] [PubMed]
- von Horn, C.; Lüer, B.; Malkus, L.; Minor, T. Comparison Between Terminal or Preterminal Conditioning of Donor Livers by Ex Situ Machine Perfusion. Transplantation 2023, 107, 1286–1290. [Google Scholar] [CrossRef] [PubMed]
- Eden, J.; Da Silva, R.X.S.; Cortes-Cerisuelo, M.; Croome, K.; De Carlis, R.; Hessheimer, A.J.; Muller, X.; de Goeij, F.; Banz, V.; Magini, G.; et al. Utilization of livers donated after circulatory death for transplantation – An international comparison. J. Hepatol. 2023, 78, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parente, A.; Flores Carvalho, M.; Schlegel, A. Endothelial Cells and Mitochondria: Two Key Players in Liver Transplantation. Int. J. Mol. Sci. 2023, 24, 10091. https://doi.org/10.3390/ijms241210091
Parente A, Flores Carvalho M, Schlegel A. Endothelial Cells and Mitochondria: Two Key Players in Liver Transplantation. International Journal of Molecular Sciences. 2023; 24(12):10091. https://doi.org/10.3390/ijms241210091
Chicago/Turabian StyleParente, Alessandro, Mauricio Flores Carvalho, and Andrea Schlegel. 2023. "Endothelial Cells and Mitochondria: Two Key Players in Liver Transplantation" International Journal of Molecular Sciences 24, no. 12: 10091. https://doi.org/10.3390/ijms241210091
APA StyleParente, A., Flores Carvalho, M., & Schlegel, A. (2023). Endothelial Cells and Mitochondria: Two Key Players in Liver Transplantation. International Journal of Molecular Sciences, 24(12), 10091. https://doi.org/10.3390/ijms241210091







